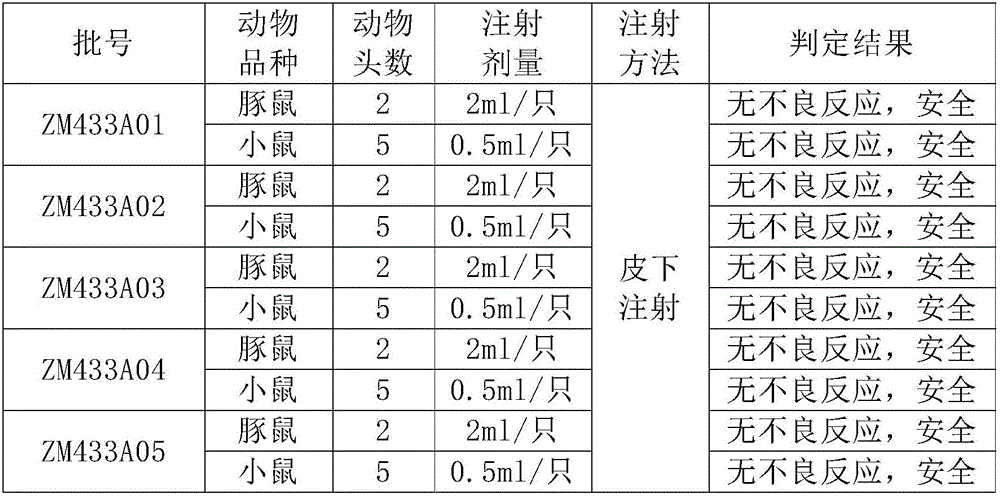
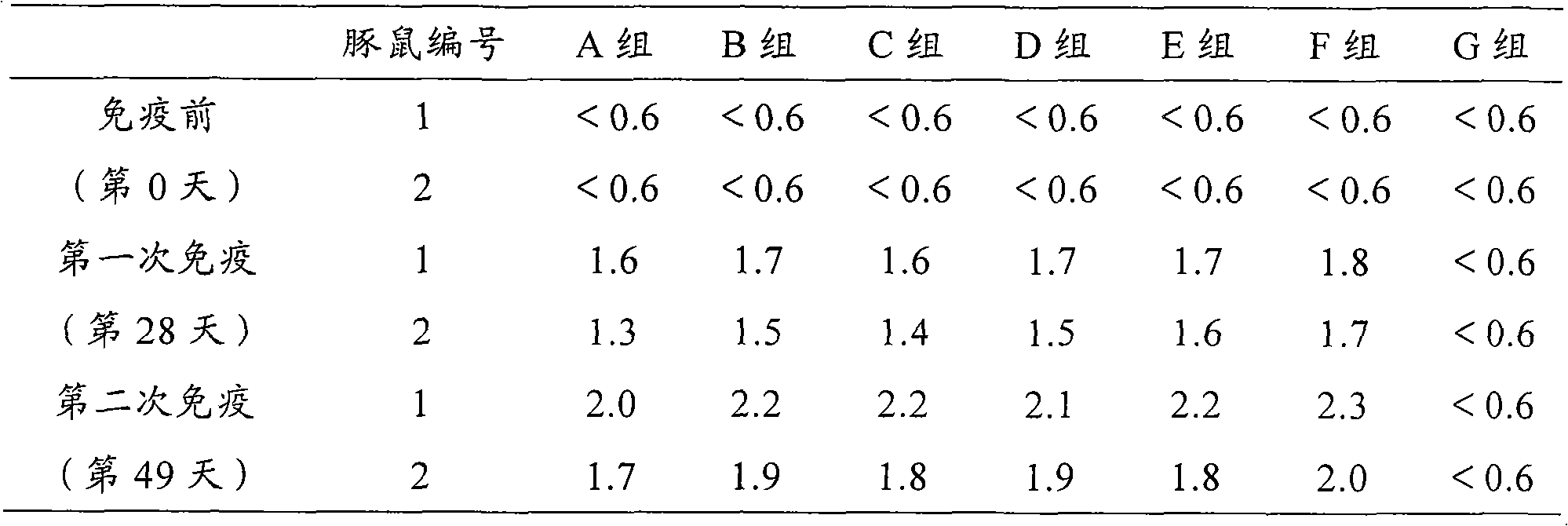
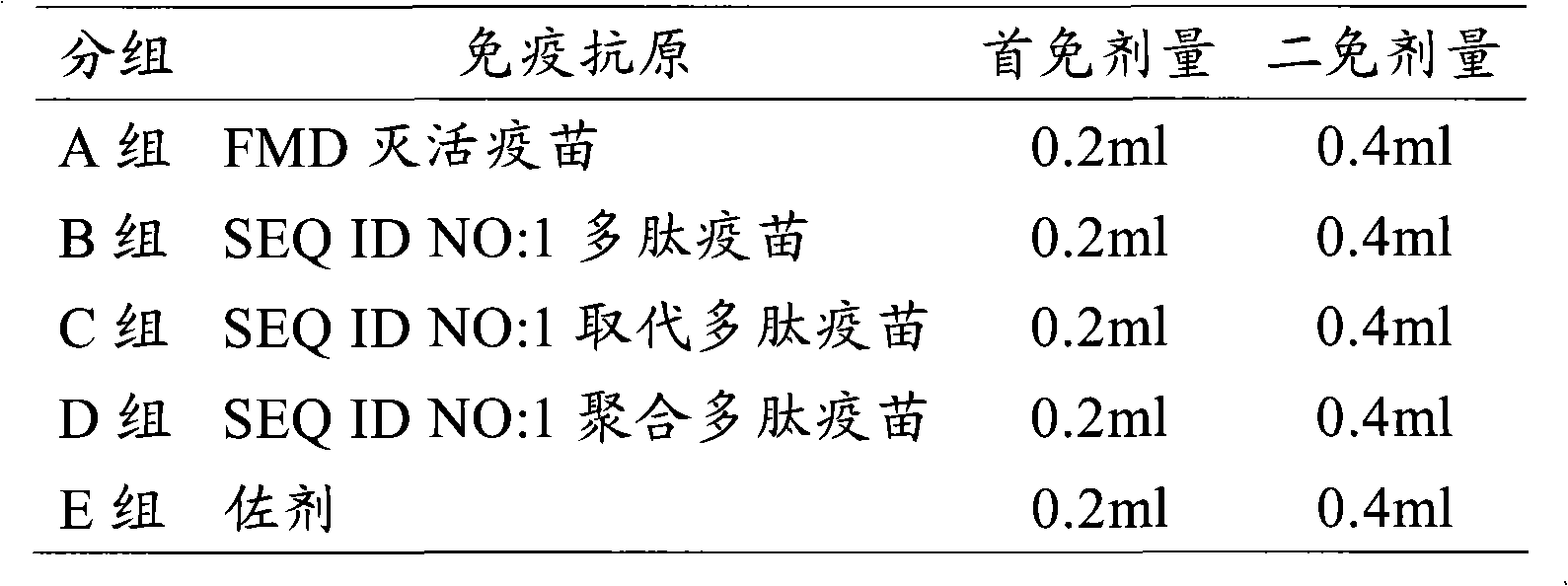
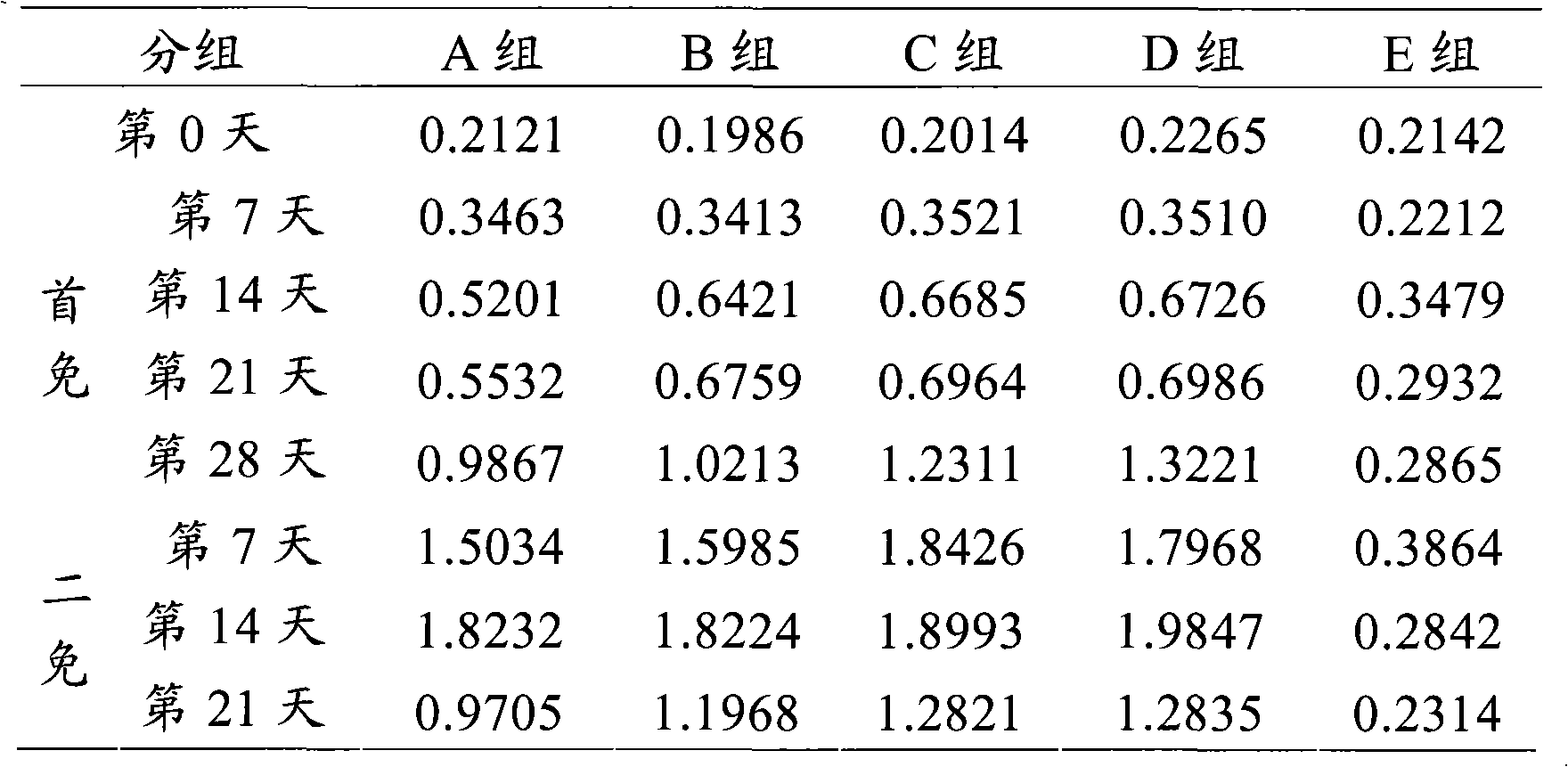
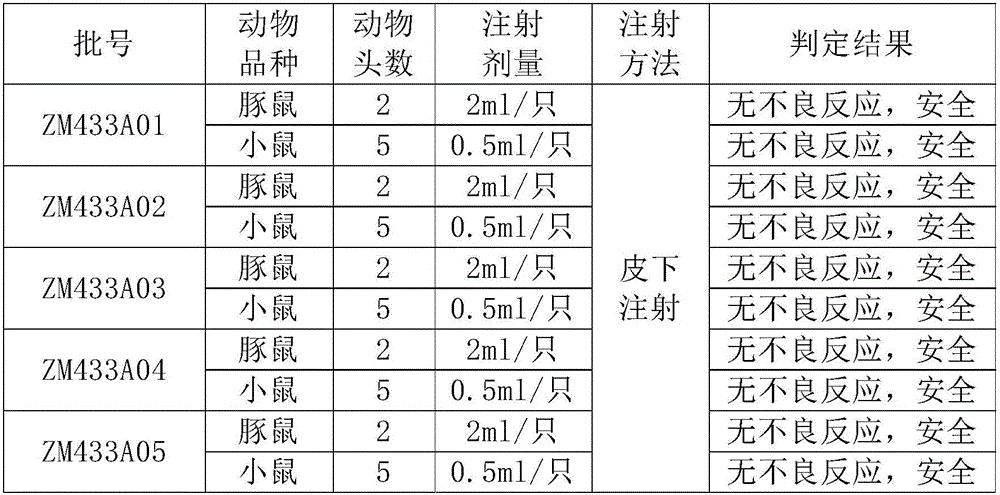
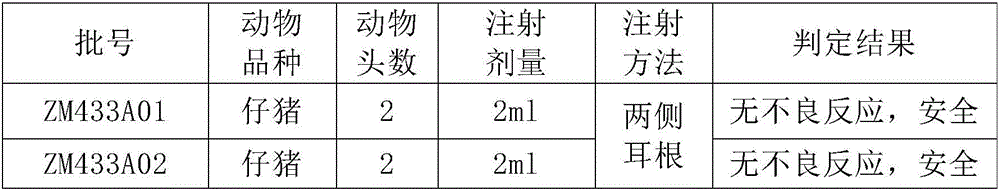
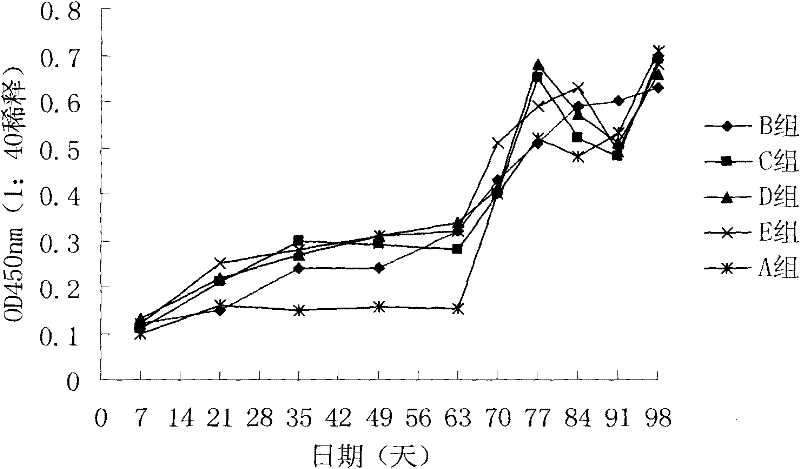
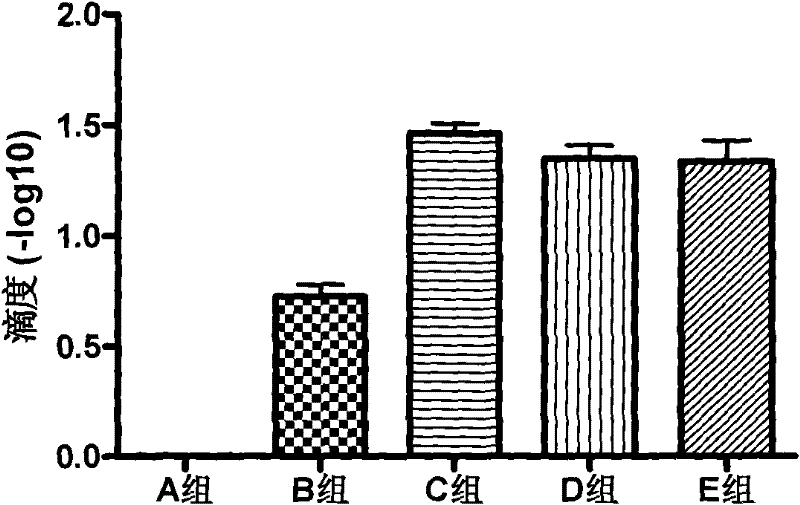
Patents
Literature
49 results about "Antigenic variation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antigenic variation or antigenic alteration refers to the mechanism by which an infectious agent such as a protozoan, bacterium or virus alters the proteins or carbohydrates on its surface and thus avoids a host immune response. It is related to phase variation. Antigenic variation not only enables the pathogen to avoid the immune response in its current host, but also allows re-infection of previously infected hosts. Immunity to re-infection is based on recognition of the antigens carried by the pathogen, which are "remembered" by the acquired immune response. If the pathogen's dominant antigen can be altered, the pathogen can then evade the host's acquired immune system. Antigenic variation can occur by altering a variety of surface molecules including proteins and carbohydrates. Antigenic variation can result from gene conversion, site-specific DNA inversions, hypermutation, or recombination of sequence cassettes. The result is that even a clonal population of pathogens expresses a heterogeneous phenotype. Many of the proteins known to show antigenic or phase variation are related to virulence.
Breaking immunological toterance with a genetically encoded unnatural amino acid
InactiveUS20090263376A1Improving immunogenicityStrong immune responseAntibacterial agentsNervous disorderDiseaseAntigenic variation
The present invention comprises methods and compositions for producing and / or enhancing an immunological response in a subject against a target moiety such as a disease-related moiety by administration of an antigenic version of the target moiety having one or more unnatural amino acid and / or by administration of an antibody against a version of a target moiety having one or more unnatural amino acid which antibody is cross reactive with the natural target moiety.
Owner:THE SCRIPPS RES INST
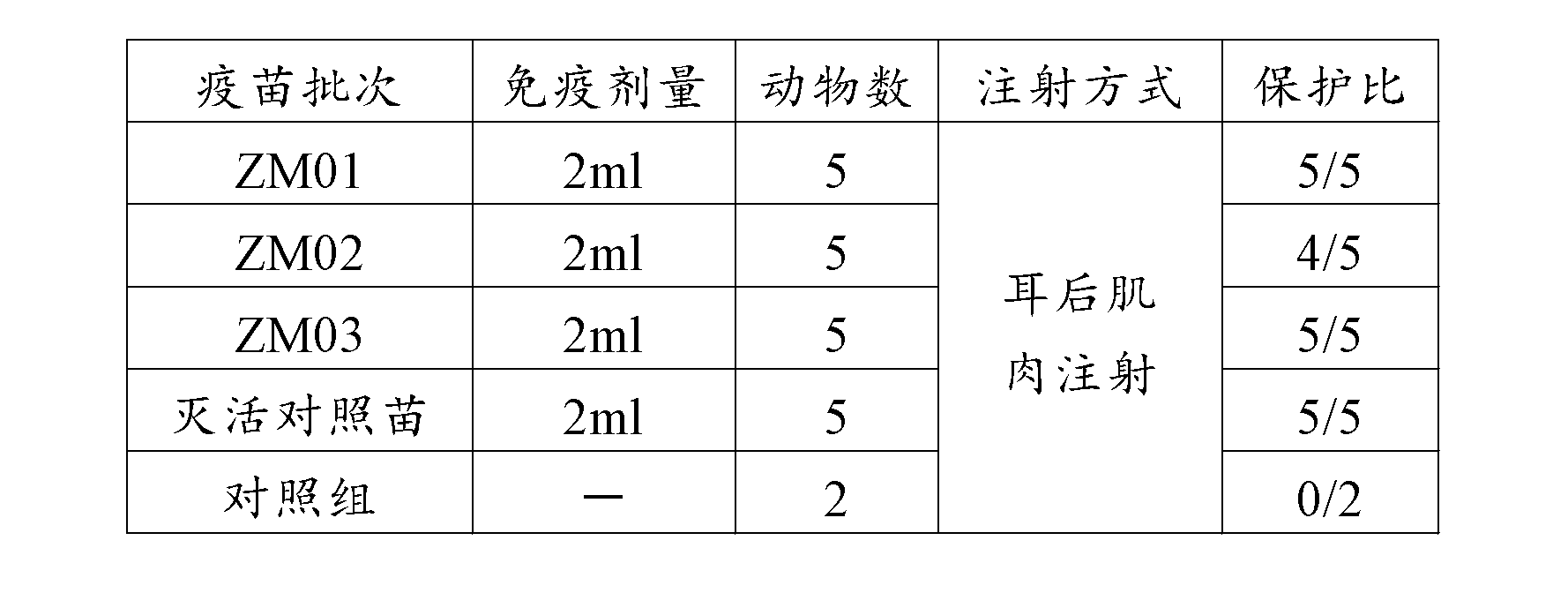
H9N2 avian influenza virus vaccine strain and application of H9N2 avian influenza virus vaccine strain in immune protection
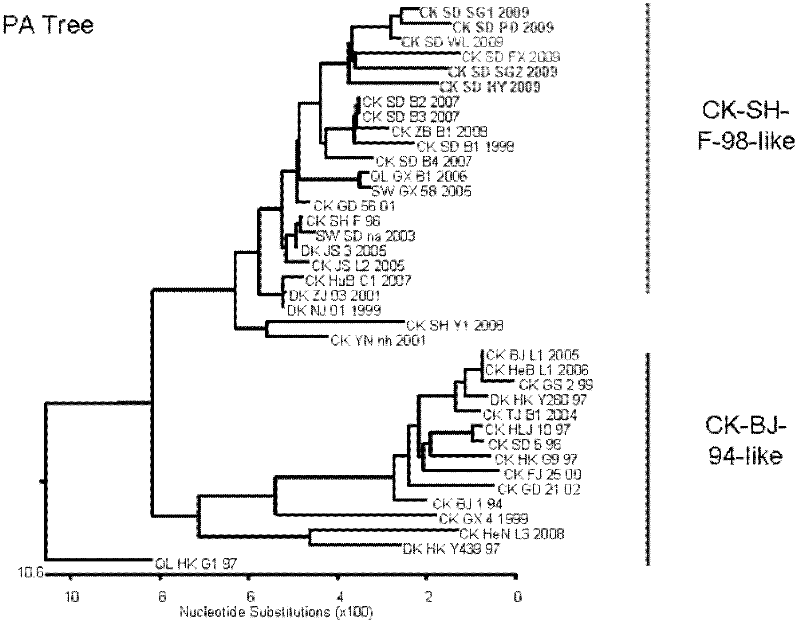
The present invention relates to the field of animal virology, and provides a recombinant chicken-origin H9N2 avian influenza virus vaccine strain and a method for isolation, identification and purification of the strain. The invention further relates to a research of biological characteristics of the strain, especially to a research of characteristics of the strain adopted as the vaccine strain,and an evaluation of immune effects of the strain on SPF chickens. The preservation number of the strain is CCTCCNO:V201030. According to the present invention, the antigen variation conditions of the virus strain and other isolated virus strains are represented from the molecular level; after the virus strain is prepared into the vaccine, the prepared vaccine is adopted to immunize the 4 week old SPF chickens, with the protection effect analysis of the homologous H9 influenza wild virus strain and the heterologous H9 influenza wild virus strain, the results show that the influenza virus strain can be adopted as the spare vaccine strain of H9 subtype avian influenza. With the present invention, the spare vaccine strain is provided for prevention of the avian influenza outbreak by using the vaccine, the molecular biology technology program is provided for screen of the avian influenza virus vaccine strain, the molecular biology background is provided for study of the mechanism of animal infection by the avian influenza, and the important public health significance is provided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Separation identification and purification process for chicken source H9N2 avian influenza virus strain and uses thereof
The invention relates to the field of animal virology, and provides separation identification and purification methods, biological characteristics and applications in biology of a recombinant fowl H9N2 avian influenza virus strain with preservation number being CCTCC NO:V200811; the differences between the virus and other separation strains are explained in term of molecular level; and the virus strain is proved to be capable of being used as candidate strain of avian influenza vaccines and as antigen of H9 subtype AIV hemoagglutination and hemoagglutination inhibition test (HA-HI). The strain has significance in knowing the molecular evolution and antigenic variation of fowl H9N2 avian influenza virus in China.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Synthetic peptide vaccine for treating porcine reproductive and respiratory syndrome and preparation method thereof
InactiveCN101845083AImprove securityImprove efficiencyViral antigen ingredientsVirus peptidesMedicinePorcine reproductive and respiratory syndrome virus
The invention provides a synthetic peptide vaccine for treating porcine reproductive and respiratory syndrome and a preparation method thereof, in particular relates to polypeptide of the synthetic peptide vaccine for treating the porcine reproductive and respiratory syndrome and a vaccine which contains the polypeptide and a method for preparing the polypeptide and the vaccine. The amino acid sequence of the polypeptide is an amino acid sequence shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 or SEQ ID No.4. The vaccine prepared from the polypeptide can effectively cope with the antigenic variation of a porcine reproductive and respiratory syndrome virus, ensures biological safety, is easy for large-scale synthesis and has good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
O-type aftosa synthetic peptide vaccine
ActiveCN101659695AImprove protectionFree from attackAntiviralsPeptide preparation methodsChemical synthesisPeptide vaccine
The invention provides O-type aftosa synthetic peptide vaccine, and in particular relates to polypeptide or polypeptide polymer thereof used in the vaccine as well as the vaccine containing the polypeptide or the polypeptide polymer thereof and a preparation method thereof. The polypeptide has amino acid sequences shown in SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3. The O-type aftosa synthetic peptide vaccine carries out chemical synthesis of potential antigen site peptide segments by carrying out sequencing of domestic aftosa epidemic strains to study the variation of the main antigen sites ofaftosa and combining with computer assistant to carry out antigen site analytical prediction. Candidate polypeptide antigens are screened out by carrying out large numbers of animal experiments and aftosa virus antigen sites are optimized according to the screening result; and T cell epitope and B cell epitope are effectively combined to improve the immune effects of the polypeptide antigens. TheO-type aftosa synthetic peptide vaccine can effectively cope with the antigen variation of aftosa virus and has ideal biosafety and easy large-scale synthesis, thereby having a good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
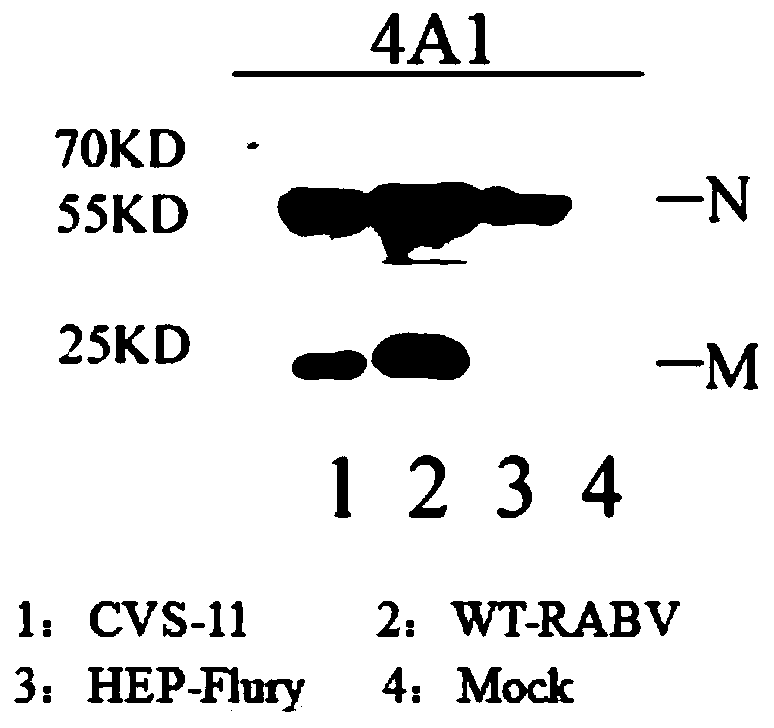
Hybridoma cell strain to secrete anti-Rabies virus M protein monoclonal antibody and application thereof
ActiveCN109970852AGood biological propertiesImmunoglobulins against virusesTissue cultureEpitopeBiological property
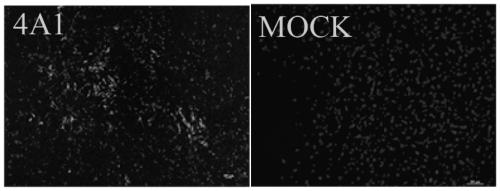
The invention discloses a hybridoma cell strain to secrete anti-Rabies virus M protein monoclonal antibody and application thereof and belongs to the technical field of biology. The hybridoma cell strain has a category name of hybridoma cell strain 4A1, collected in China Center for Type Culture Collection, Wuhan University, Wuhan, China under CCTCC NO: C201947. A monoclonal antibody prepared withthe hybridoma cell strain has high titer, good specificity and good biological features; fine antigen variant epitope of the monoclonal antibody in recognizing RABV M protein is identified, so that the monoclonal antibody is suitable for distinguishing vaccine Flury virus strains and other RABV strains; when applied to the preparation of kits to detect RABV (Rabies virus), the monoclonal antibodyis capable of detecting RABV infections and identifying and diagnosing vaccine Flury virus strain and other RABV strains.
Owner:ZHEJIANG UNIV
Peptide vaccine for animal and preparation method thereof
InactiveCN101565458AImprove protectionImprove immunityVirus peptidesAntiviralsChemical synthesisPeptide vaccine
Owner:CHINA ANIMAL HUSBANDRY IND
Monoclonal antibody IE5 for detecting new castle disease virus variation strain
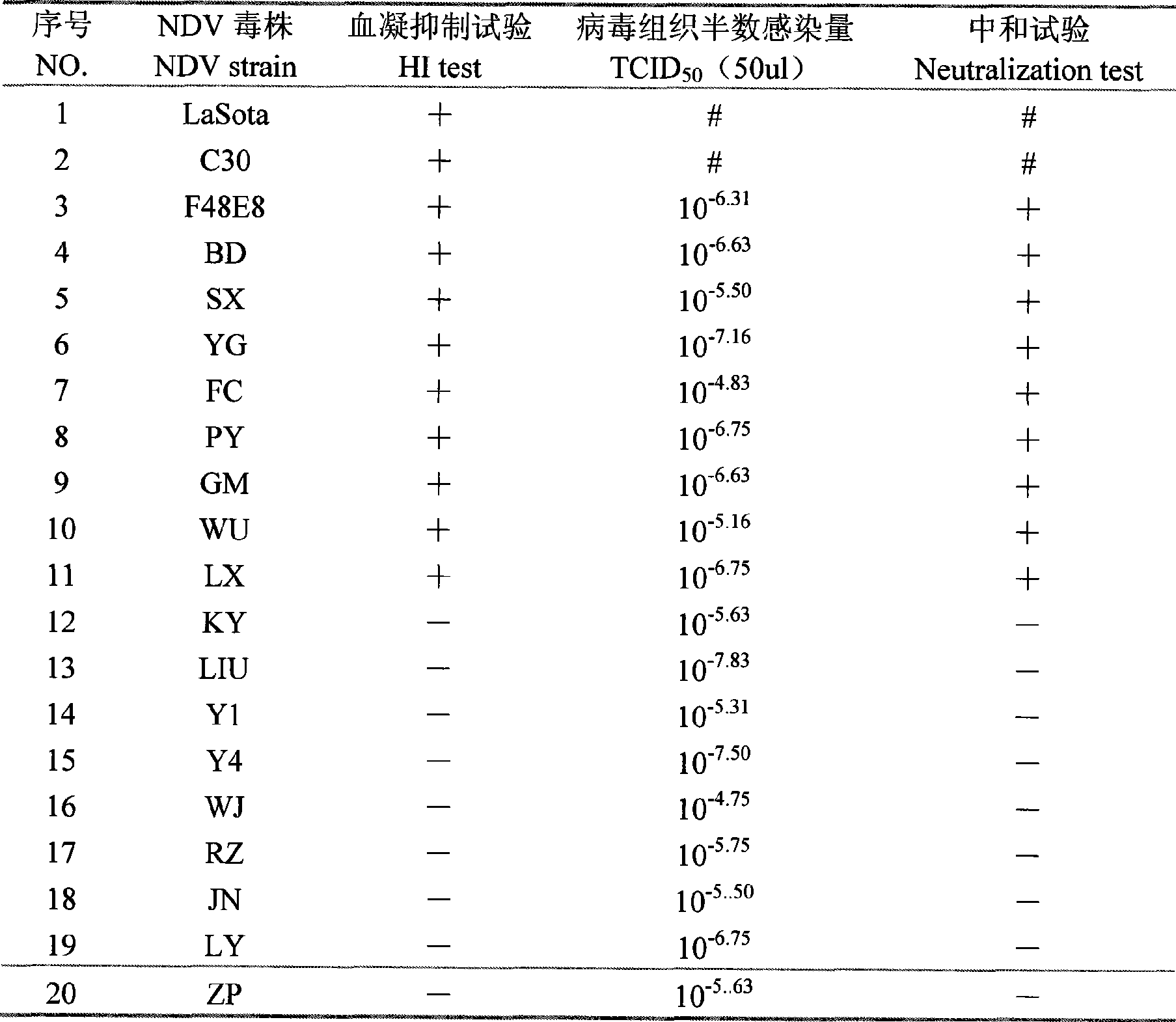
The invention provides a monoclonal antibody for detecting new-castle disease virus variant. The preparation method comprises the following steps: immunizing a BALB / C mouse of eight ages using purified new-castle disease virus NDV LaSota strain as immunogen, boostering immnunization, standing for three days, and taking mouse splenic cells to fuse with myeloma cells SP2 / 0 in a logarithmic growth phase. The purified NDV LaSota strain is antigen coated enzyme marking board for screening positive Hybridoma cell, and the monoclone 1E5 is positive via ELISA detection, and is subcloned, and then injected into an abdominal cavity of a sensitized BALB / C mouse of 8 to 10 ages for preparing ascites. The mab bioactivity identification result indicates that mab 1E5 ascites has ELISA titer of 100 multiply 2<10>, immunoglobulin subclass of IgG2a, HI titer of 14log2, and cell neutralization titer for NDV standard virulent strain F48E8 of 320. The monoclonal antibody can be used for antigenic variation research of the new-castle disease virus to monitor and forecast epidemic situation of new castle disease, and provide scientific theoretical basis for the effective prevention and control of the new castle disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Synthetic peptide vaccine for resisting porcine circovirus and preparation method thereof
The invention provides a synthetic peptide vaccine for resisting porcine circovirus and a preparation method thereof, in particular relates to a polypeptide of the synthetic peptide vaccine for resisting porcine circovirus and a polymer thereof as well as a vaccine containing the polypeptide and a preparation method thereof. The amino acid sequence of the polypeptide is shown by SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3. The vaccine prepared by the polypeptide can effectively deal with antigenic variation of the porcine circovirus, has no biological safety problem, is easy to synchronize in a large scale and has favorable application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Polypeptide used for preparing O type peptide vaccine of cattle foot-and-mouth disease and preparation methods and applications thereof
ActiveCN103183728AImprove immune efficiencyDoes not cause feverVirus peptidesAntiviralsMedicinePeptide vaccine
The invention provides polypeptide used for preparing O type peptide vaccine of cattle foot-and-mouth disease. The polypeptide comprises an amino acid sequence presented by SEQ ID NO.1 and the peptide vaccine containing the polypeptide. The O type synthetic peptide vaccine of cattle foot-and-mouth disease provided by the invention has excellent immune efficacy, and cannot cause the problems of fever, swelling and the like existing in traditional vaccines, so that the vaccine can effectively respond to antigenic variation of current foot-and-mouth disease virus, prevents biosecurity problems, is easy to synthesize in a large scale, and has an excellent application prospect. The invention further provides preparation methods and pharmaceutical applications of the polypeptide and the peptide vaccine.
Owner:中国牧工商集团有限公司 +1
Mdck-derived cell strain suspension-cultured in protein-free medium and method for proliferating virus using cell strain
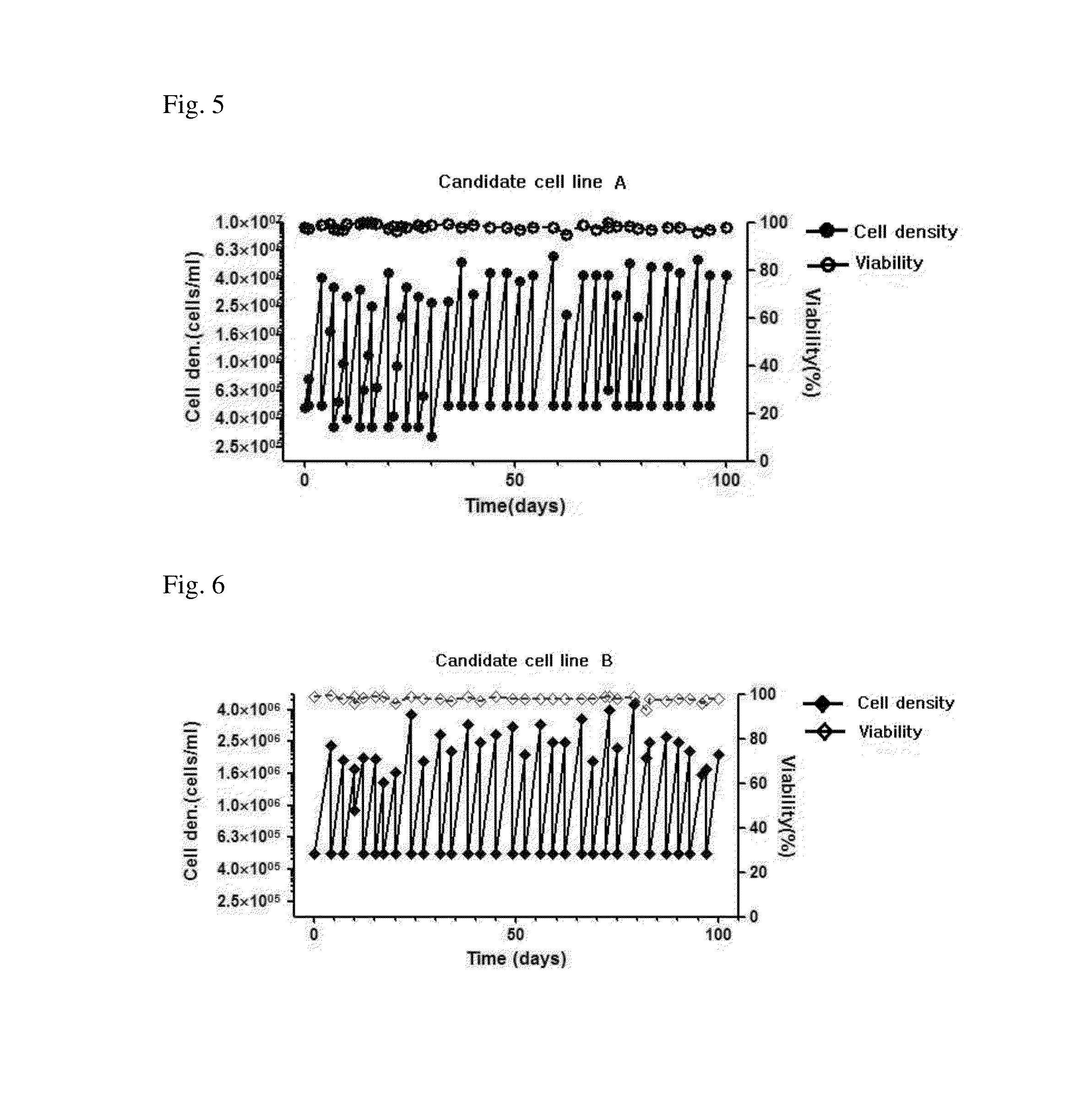
InactiveCN105378072ALess antigenic variationHigh and uniform productivitySsRNA viruses negative-senseViral antigen ingredientsVaccine virusVirus antigenic variation
The present invention relates to a novel MDCK-derived cell strain capable of being suspension-cultured in a protein-free medium and a method for proliferating virus using the MDCK cell strain to produce a vaccine. The novel MDCK-derived cell strain according to the present invention exhibits high and uniform productivity with respect to various viruses, and has a low tumorigenic capacity while causing less viral antigenic variation, and thus can be useful in producing vaccine viruses.
Owner:MOGAM BIOTECH RES INST
Mdck-derived cell strain suspension-cultured in protein-free medium and method for proliferating virus using cell strain
ActiveUS20160108367A1High and uniform productivityLess viral antigenic variationSsRNA viruses negative-senseViral antigen ingredientsVirus antigenic variationCell strain
The present invention relates to a novel MDCK-derived cell line capable of being suspension-cultured in a protein-free medium and a method for proliferating a virus using the MDCK-derived cell line to produce a vaccine. The novel MDCK-derived cell line exhibits high and uniform productivity for various viruses, while causing less viral antigenic variations with low tumorigenicity, and thus can be useful in producing viruses used for vaccines.
Owner:MOGAM BIOTECH RES INST
Bovine foot and mouth disease A-type polypeptide vaccine
ActiveCN104672312AImprove immune efficiencyEffective response to antigenic variationSsRNA viruses positive-senseVirus peptidesPeptide vaccineAntigenic variation
The invention discloses an antigen polypeptide, a polypeptide composition and a vaccine for preparing a bovine foot and mouth disease A-type polypeptide vaccine. The polypeptide composition comprises a polypeptide obtained by connecting the polypeptide fragment shown in sequence 1 with the polypeptide fragment shown in sequence 5. The vaccine provided by the invention comprises the polypeptide composition. The bovine foot and mouth disease A type polypeptide vaccine provided by the invention has good immune potency, so that the problems of fever, red and swollen caused by the traditional vaccine cannot be triggered. The vaccine provided by the invention can effectively response the antigenic variation of the current foot and mouth disease virus, is free of biological safety, easy for large-scale synthesize and has a good application future. The invention additionally discloses a preparation method of the polypeptide and the polypeptide vaccine and a pharmaceutical application thereof.
Owner:CHINA ANIMAL HUSBANDRY IND
Hn epitope recognized by avian immune system and antigenic variant newcastle disease viruses carrying changes in the epitope
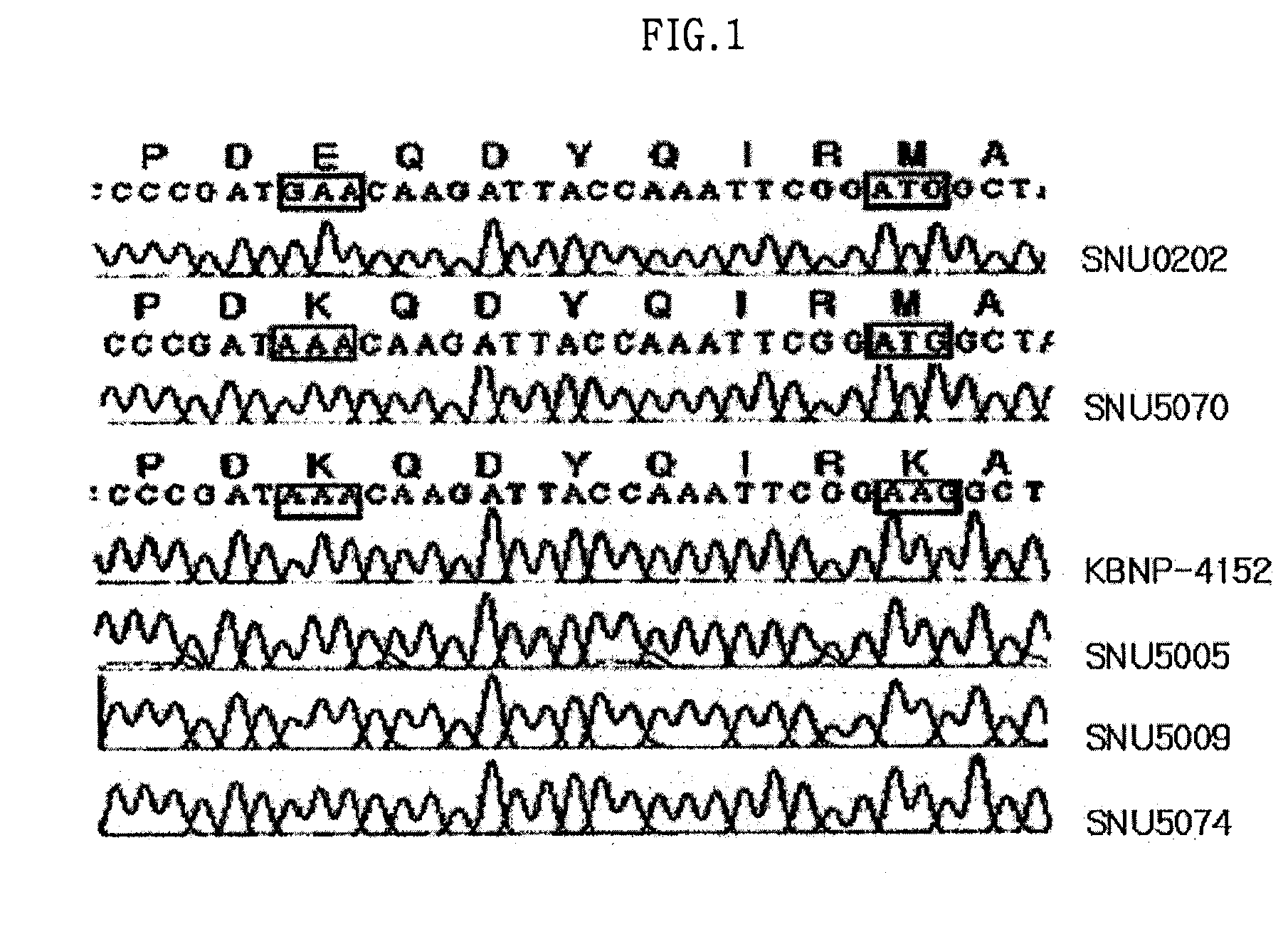
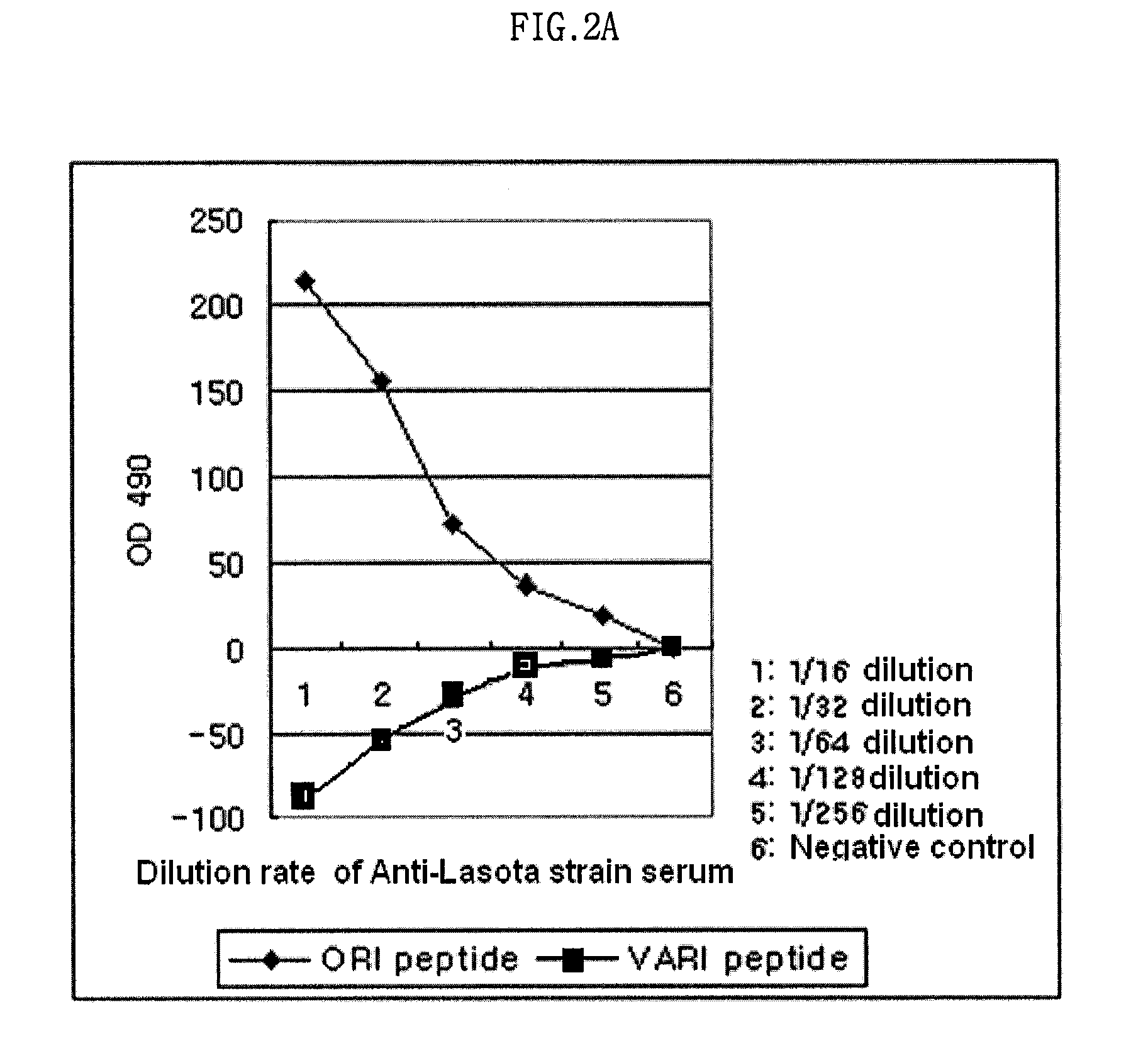
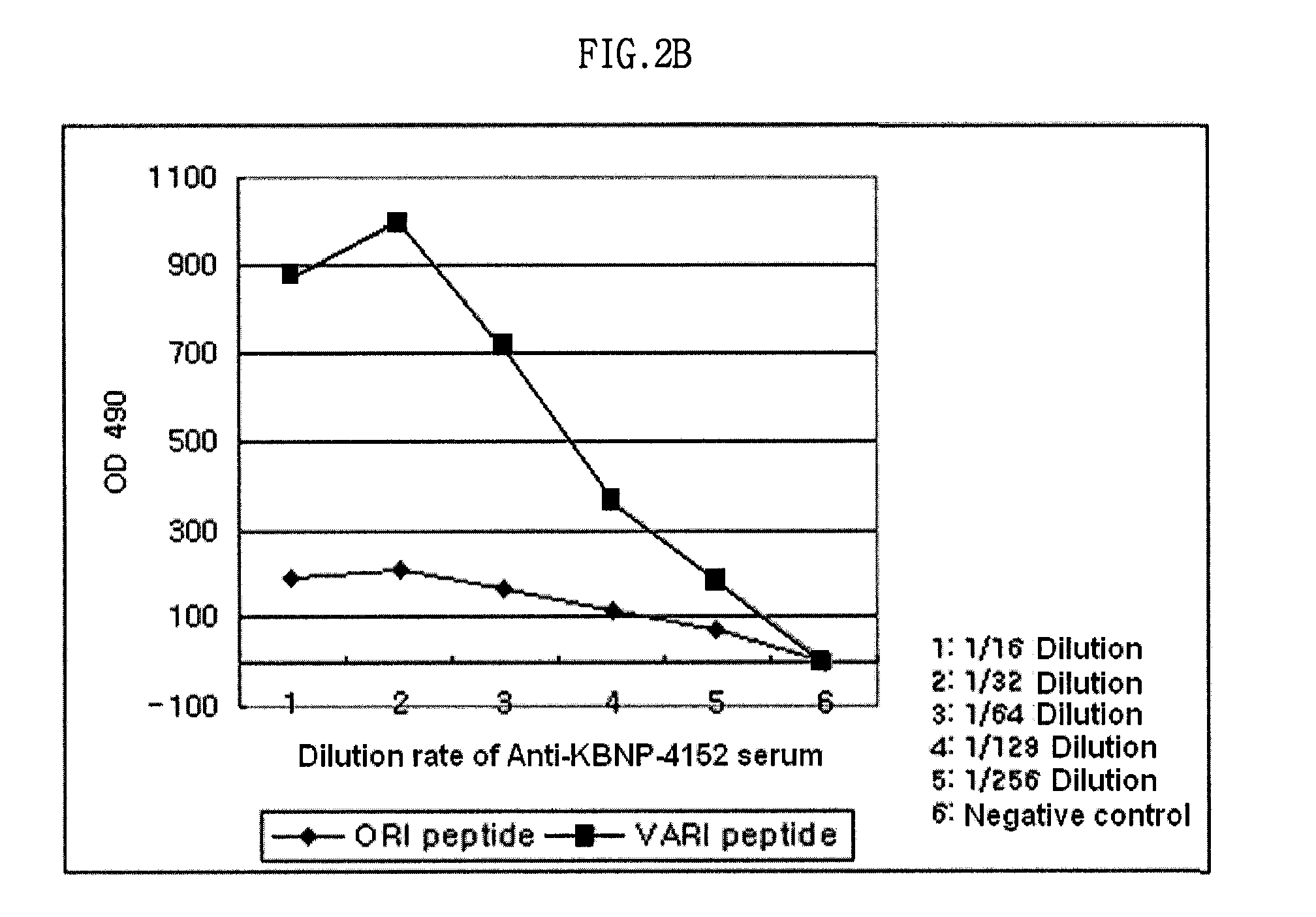
The present invention relates to an epitope of HN protein in Newcastle disease virus which can be recognized by an avian immune system and an antibody against the epitope, a method for detecting a Newcastle disease virus by using the antibody, and an antigenic variant of Newcastle disease virus carrying changes in the epitope. The epitope of HN protein and the antigenic variant of Newcastle disease virus can be used for developing efficient vaccines, and further, in diagnosing the Newcastle disease virus rapidly and exactly.
Owner:KBNP +1
Synthetic peptide vaccine as well as preparation method and application thereof
ActiveCN105906693AImprove protectionImprove immunitySsRNA viruses positive-senseViral antigen ingredientsPeptide vaccinePolymer
The invention provides a synthetic peptide vaccine for a foot-and-mouth disease, and particularly relates to a polypeptide for an O-type synthetic peptide vaccine for the foot-and-mouth disease, a polypeptide polymer of the polypeptide, a vaccine containing the polypeptide or the polypeptide polymer, and preparation methods of the polypeptide or the polypeptide polymer and the vaccine. The polypeptide has an amino acid sequence shown in SEQ ID NO.3. The candidate polypeptide antigens are screened by a lot of animal tests, the antigen site of the foot and mouth disease virus is optimized according to the screening result, and T cell epitope and B cell epitope are effectively combined, so that the immune effect of the polypeptide antigen is enhanced. The O-type synthetic peptide vaccine for the foot-and-mouth disease can be used for effectively processing antigenic variation of foot-and-mouth disease viruses, is good in biosecurity and easy to synthetize at a large scale and has good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Synthetic peptide vaccine and preparation method thereof
ActiveCN103848902AImprove protectionImprove immunitySsRNA viruses positive-senseViral antigen ingredientsPeptide vaccineAmino acid
The invention provides a synthetic peptide vaccine for a foot-and-mouth disease, and particularly relates to a polypeptide for an O-type synthetic peptide vaccine for a foot-and-mouth disease and a polypeptide polymer thereof, a vaccine containing the polypeptide or the polypeptide polymer thereof, and preparation methods thereof. The polypeptide comprises an amino acid sequence shown in SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3. The candidate polypeptide antigens are screened by a lot of animal tests, the antigen site of a foot and mouth disease virus is optimized according to the screening result, T cell epitope and B cell epitope are effectively combined, and the immune effect of the polypeptide antigen is enhanced. The antigenic variation of the foot-and-mouth virus can be effectively coped with by the O-type synthetic peptide vaccine for the foot-and-mouth disease, and the synthetic peptide vaccine is good in biosecurity, and easy to synthetize at a large scale, and has good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Polypeptide fragment composition and application in preparation of porcine reproductive and respiratory syndrome vaccine thereof
InactiveCN102321180AEffective response to antigenic variationNo biosecurity issuesAntiviralsAntibody medical ingredientsAgricultural scienceActive component
The invention discloses a polypeptide fragment composition and an application in preparation of a porcine reproductive and respiratory syndrome vaccine thereof. The polypeptide fragment composition provided by the invention is a polypeptide shown in sequence 1 of the sequence table, a polypeptide shown in sequence 2 of the sequence table, a polypeptide shown in sequence 3 of the sequence table, apolypeptide shown in sequence 4 of the sequence table, a polypeptide shown in sequence 5 of the sequence table, and a polypeptide shown in sequence 6 of the sequence table. The polypeptide fragment composition provided by the invention can be used as an active component of a porcine reproductive and respiratory syndrome vaccine, and has great application value for the prevention and treatment of porcine reproductive and respiratory syndrome. The vaccine provided by the invention can effectively cope with antigenic variation of porcine reproductive and respiratory syndrome virus, has no biological safety problem, is suitable for large-scale synthesis, and has good application prospects.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Asia I-type aftosa synthetic peptide vaccine
InactiveCN101659696AFree from attackHigh level of immune responseAntiviralsPeptide preparation methodsChemical synthesisComputer aid
The invention provides Asia I-type aftosa synthetic peptide vaccine, and in particular relates to polypeptide or polypeptide polymer thereof used in the vaccine as well as the vaccine containing the polypeptide or the polypeptide polymer thereof and a preparation method thereof. The polypeptide has amino acid sequences shown in SEQ ID No.1, SEQ ID No.2 and SEQ ID NO.3. The Asia I-type aftosa synthetic peptide vaccine carries out chemical synthesis of potential antigen site peptide segments by carrying out sequencing of domestic aftosa epidemic strains to study the variation of the main antigen sites of aftosa and combining with a computer-aided method to carry out aftosa antigen site analytical prediction; and candidate polypeptide antigens are screened out by carrying out large numbers of animal experiments to obtain polypeptide antigens which have high immunoreaction level and can protect animals from the attack of aftosa epidemic strains. The Asia I-type aftosa synthetic peptide vaccine prepared by the polypeptide antigens can effectively cope with the antigen variation of aftosa virus without causing biosafety problems and has easy large-scale synthesis, thereby having a good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Bird flu synthetic peptide vaccine and preparation method thereof
ActiveCN101628932ANo biosecurity issuesEase of large-scale synthesisVirus peptidesAntiviralsBird fluPeptide vaccine
The invention provides a bird flu synthetic peptide vaccine and a preparation method thereof, in particular to polypeptide used for the bird flu synthetic peptide vaccine or a polymer thereof, as well as a vaccine containing the polypeptide or the polymer thereof and both preparation methods thereof, wherein an amino acid sequence of the polypeptide is an amino acid sequence showed as SEQ ID NO.1 or SEQ ID NO.2. The vaccine made from the polypeptide or the polymer thereof can effectively deal to antigenic variation of bird flu virus, has no biological safety problem, can be synthesized easily in large scale and has favorable application prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
Asia I synthetic peptide vaccine of foot and mouth disease
ActiveCN101643500AFree from attackHigh level of immune responseVirus peptidesAntiviralsChemical synthesisAnimal testing
The invention provides an Asia I synthetic peptide vaccine of a foot and mouth disease, in particular to a polypeptide or a polypeptide polymer thereof for the Asia I synthetic peptide vaccine of thefoot and mouth disease, the vaccine containing the polypeptide or the polypeptide polymer thereof, and a preparation method of both, wherein, the amino acid sequence of the polypeptide is shown as SEQID NO:1. In the invention, variation situation of main antigen sites of the foot and mouth disease is researched by determining a sequence of an epidemic strain of the foot and mouth disease, and meanwhile analysis prediction of the antigen sites of the foot and mouth disease is performed followed by chemical synthesis on possible peptide segments of the antigen sites combined with a computer auxiliary method; and candidate polypeptide antigens are screened through a large quantity of animal experiments to finally obtain the polypeptide antigens with high immune reaction level which can protect well an animal from being attacked by the epidemic strain of the foot and mouth disease. The vaccine prepared from the polypeptide antigens has the advantages of no biological safety problem, easylarge-scale synthesis, good application prospect and good ability of effectively dealing with antigen variation of foot and mouth disease virus.
Owner:CHINA ANIMAL HUSBANDRY IND
A kind of synthetic peptide vaccine and preparation method thereof
ActiveCN103848902BImprove protectionImprove immunitySsRNA viruses positive-senseViral antigen ingredientsPeptide vaccineScreening Result
The invention provides a synthetic peptide vaccine for a foot-and-mouth disease, and particularly relates to a polypeptide for an O-type synthetic peptide vaccine for a foot-and-mouth disease and a polypeptide polymer thereof, a vaccine containing the polypeptide or the polypeptide polymer thereof, and preparation methods thereof. The polypeptide comprises an amino acid sequence shown in SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3. The candidate polypeptide antigens are screened by a lot of animal tests, the antigen site of a foot and mouth disease virus is optimized according to the screening result, T cell epitope and B cell epitope are effectively combined, and the immune effect of the polypeptide antigen is enhanced. The antigenic variation of the foot-and-mouth virus can be effectively coped with by the O-type synthetic peptide vaccine for the foot-and-mouth disease, and the synthetic peptide vaccine is good in biosecurity, and easy to synthetize at a large scale, and has good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Synthetic peptide vaccine and preparation method thereof
ActiveCN105820217AImprove protectionImprove immunitySsRNA viruses positive-senseViral antigen ingredientsPeptide antigenPeptide vaccine
The invention provides a synthetic peptide vaccine and particularly relates to polypeptide or a polypeptide polymer thereof used for the synthetic peptide vaccine for O type foot-and-mouth disease, the vaccine containing the polypeptide or the polypeptide polymer thereof and preparation methods of the polypeptide or the polypeptide polymer thereof and the vaccine, wherein the polypeptide has the amino acid sequence as indicated in SEQ ID No .2 .According to the synthetic peptide vaccine and the preparation method thereof, candidate peptide antigen is screened through a great number of animal tests, foot and mouth disease virus antigen sites are optimized according to the screening result, T cell epitope and B cell epitope are effectively combined, and the immune effect of the polypeptide antigen is enhanced .The synthetic peptide vaccine for O type foot-and-mouth disease can effectively cope with antigen variation of foot-and-mouth disease virus, biosecurity is good, large-scale synthesis can be conducted easily, and good application prospect is achieved.
Owner:CHINA ANIMAL HUSBANDRY IND
Synthetic peptide vaccine for resisting porcine circovirus and preparation method thereof
The invention provides a synthetic peptide vaccine for resisting porcine circovirus and a preparation method thereof, in particular relates to a polypeptide of the synthetic peptide vaccine for resisting porcine circovirus and a polymer thereof as well as a vaccine containing the polypeptide and a preparation method thereof. The amino acid sequence of the polypeptide is shown by SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3. The vaccine prepared by the polypeptide can effectively deal with antigenic variation of the porcine circovirus, has no biological safety problem, is easy to synchronize in a large scale and has favorable application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Bird flu synthetic peptide vaccine and preparation method thereof
ActiveCN101628932BNo biosecurity issuesEase of large-scale synthesisVirus peptidesAntiviralsBird fluAvian influenza virus
The invention provides a bird flu synthetic peptide vaccine and a preparation method thereof, in particular to polypeptide used for the bird flu synthetic peptide vaccine or a polymer thereof, as well as a vaccine containing the polypeptide or the polymer thereof and both preparation methods thereof, wherein an amino acid sequence of the polypeptide is an amino acid sequence showed as SEQ ID NO.1or SEQ ID NO.2. The vaccine made from the polypeptide or the polymer thereof can effectively deal to antigenic variation of bird flu virus, has no biological safety problem, can be synthesized easilyin large scale and has favorable application prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
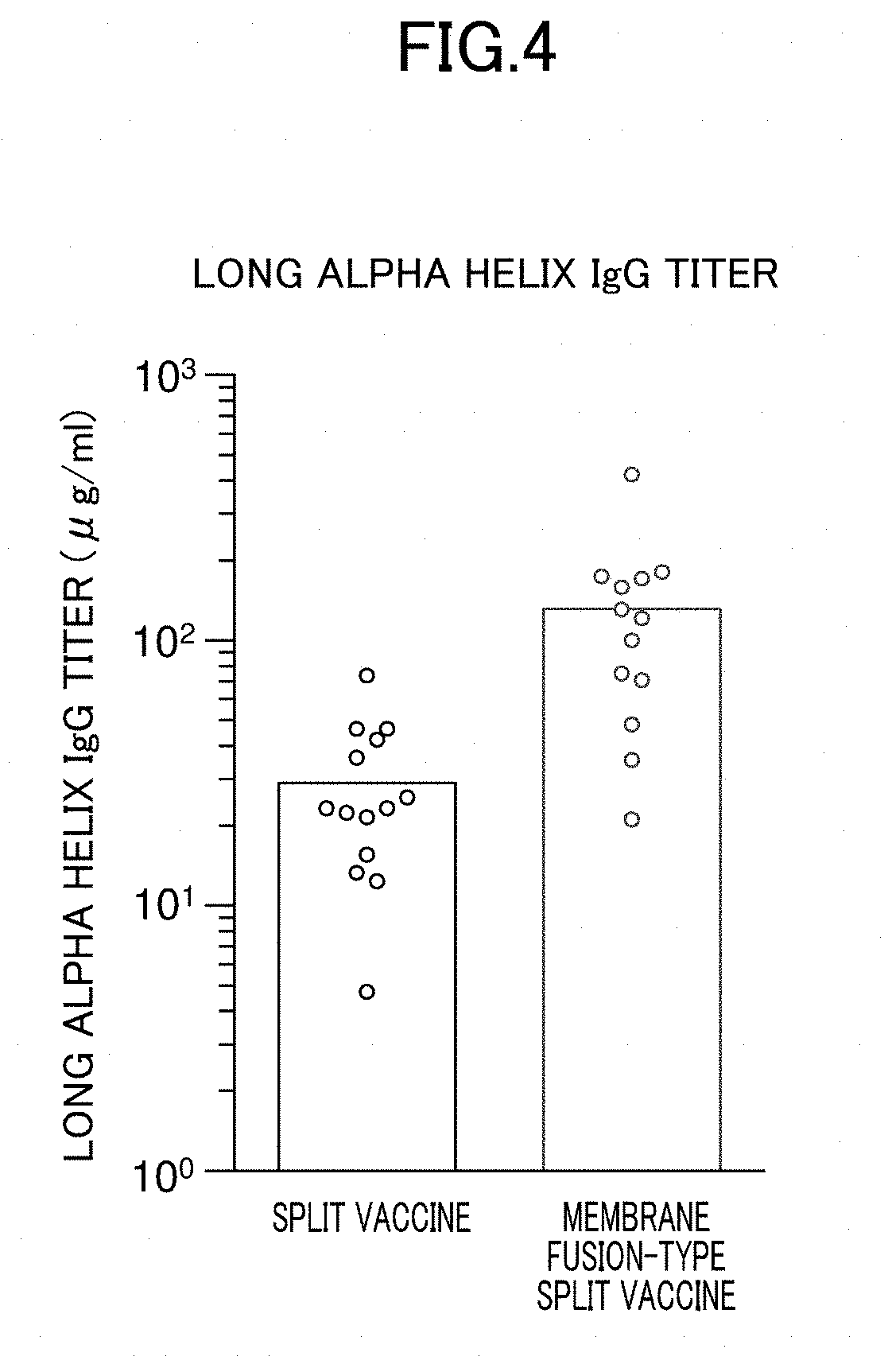
Method for producing influenza HA split vaccine
ActiveUS20190345231A1SsRNA viruses negative-senseViral antigen ingredientsAntigenic variationAntigenicity
Provided is a method for producing an influenza HA split vaccine which produces an antibody that binds to a HA stem region of influenza, the HA stem region being less likely to cause antigenic variation, An influenza HA split vaccine is subjected to an acidic treatment. Through the acidic treatment, an influenza HA split vaccine which produces an antibody that binds to a LAH of the HA stem region is obtained. This influenza HA split vaccine has an excellent protective ability against infection of other influenza viruses of different antigenicity.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES
Synthetic peptide vaccine and preparation method thereof
InactiveCN101565457BImprove protectionImprove immunityVirus peptidesAntiviralsChemical synthesisAnimal testing
Owner:CHINA ANIMAL HUSBANDRY IND
Porcine circovirus synthetic peptide vaccine and preparation method and applications thereof
ActiveCN107056897AImprove securityImprove featuresViral antigen ingredientsVirus peptidesPolypeptide compositionAntigenic variation
The invention discloses an antigen polypeptide for preparing a porcine circovirus synthetic peptide vaccine, a polypeptide composition and a vaccine. The antigen polypeptide is a polypeptide with sequence 1 or sequence 2. The vaccine comprises the polypeptide or the polypeptide composition. The porcine circovirus synthetic peptide vaccine provided by the invention has good immune potency, and does not induce fever, swelling and other problems like traditional vaccines. Therefore, the vaccine of the invention can effectively deal with the antigenic variation of porcine circovirus, has good biosafety, is easy for large-scale synthesis, and has good application prospects. The invention also provides a preparation method and pharmaceutical applications of the polypeptide and the polypeptide vaccine.
Owner:CHINA ANIMAL HUSBANDRY IND
A kind of bird flu synthetic peptide vaccine and preparation method thereof
ActiveCN109206492BSynthetic Effective CopingNo biosecurity issuesSsRNA viruses negative-senseViral antigen ingredientsChemical synthesisEngineering
Owner:CHINA ANIMAL HUSBANDRY IND
Method for predicting flu antigen through model and application thereof
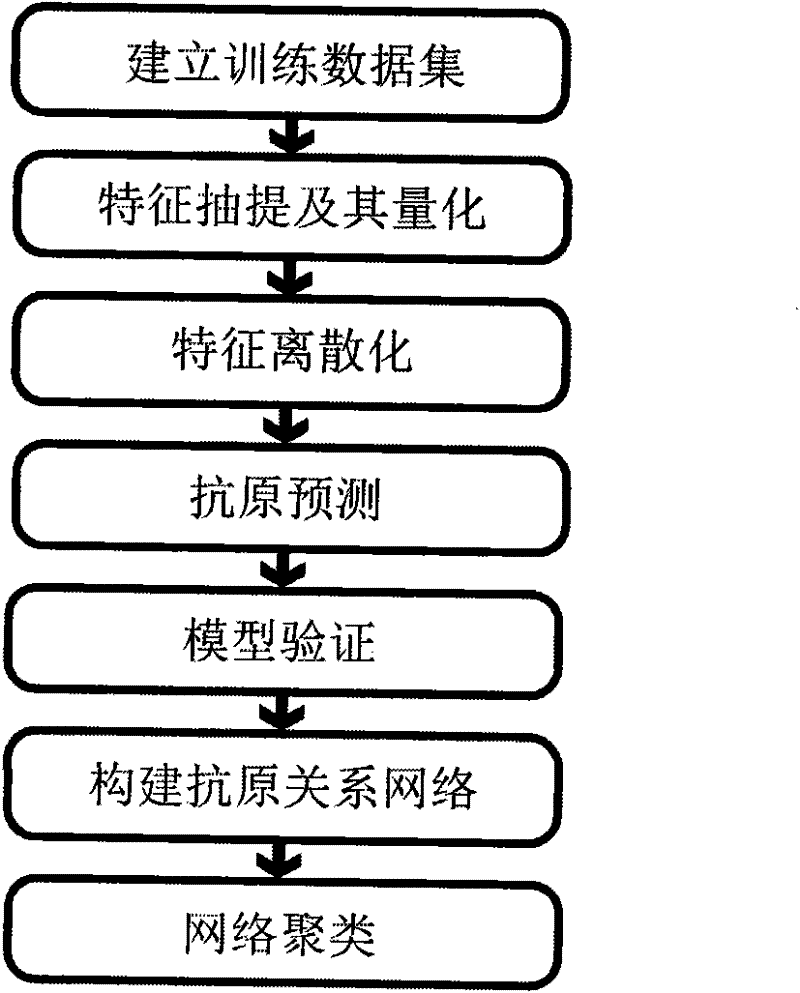
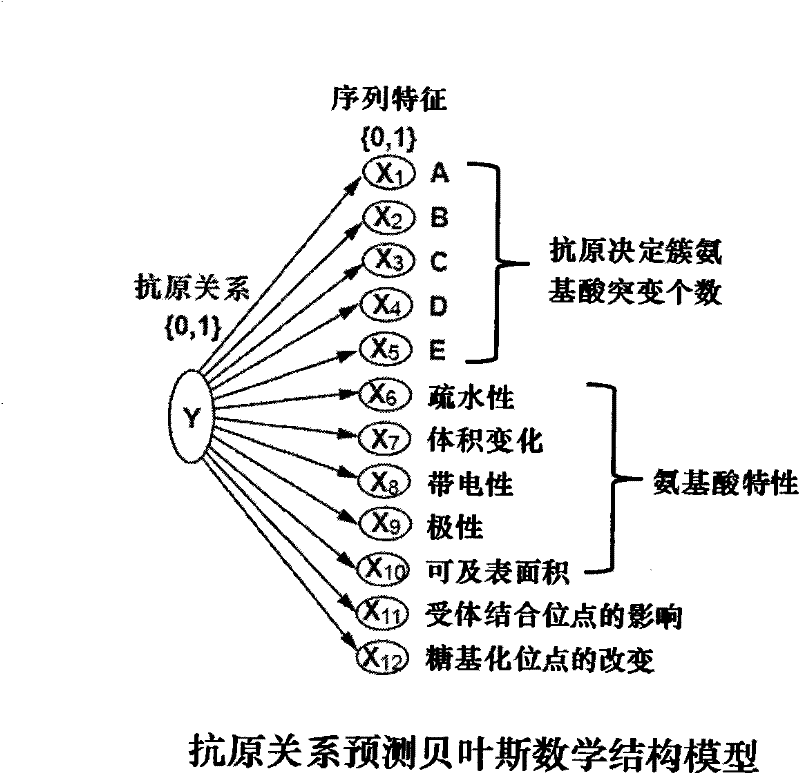
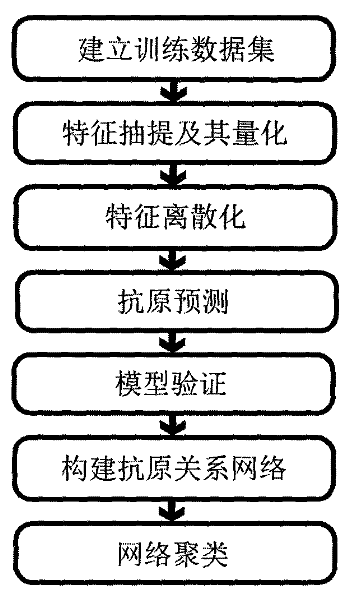
ActiveCN101847179BAntigen relationship is simpleAntigen relationship is convenientSpecial data processing applicationsReceptorProteinogenic amino acid
The invention discloses a method for predicting flu antigen through a model and an application thereof. The method comprises the following steps: extracting 12 features affecting the flu antigen, wherein the 12 features include mutation numbers of five antigenic determinant amino acid, five physical and chemical properties of HA protein amino acid, factor affecting receptor, and number of glycosylated site, the five physical and chemical properties of amino acid include hydrophobicity, volume change, electriferous property, polarity and integrable surface area; and making statistics on the 12features of 3681 virus pairs similar to known antigen and 1720 virus pairs variant from antigen, thereby establishing a prediction model on antigen relation. On the basis of sequence, the method can present the antigen relation among viruses, and thus having the advantages of simpleness, convenience and high sensitivity. The process of antigen evolution can be visually displayed through the network. The invention is used for revealing flu spreading rules, screening vaccine candidate strains and the like.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT +1
Method for preparing influenza ha split vaccine
PendingCN114096273ASsRNA viruses negative-senseViral antigen ingredientsTGE VACCINEAntigenic variation
Provided is a method for preparing an influenza HA split vaccine which produces an antibody that binds to an HA stem region of influenza, wherein it is difficult for the HA stem region to produce an antigenic variant. Acidic treatment is performed on the influenza HA split vaccine. By performing the acidic treatment, the influenza HA split vaccine is obtained which produces an antibody that binds to an LAH of an HA stem region. This influenza HA split vaccine has good protective ability against infection from other influenza viruses having different antigenicities.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com