Method for preparing influenza ha split vaccine
A technology for vaccines and influenza, applied in biochemical equipment and methods, microbes, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
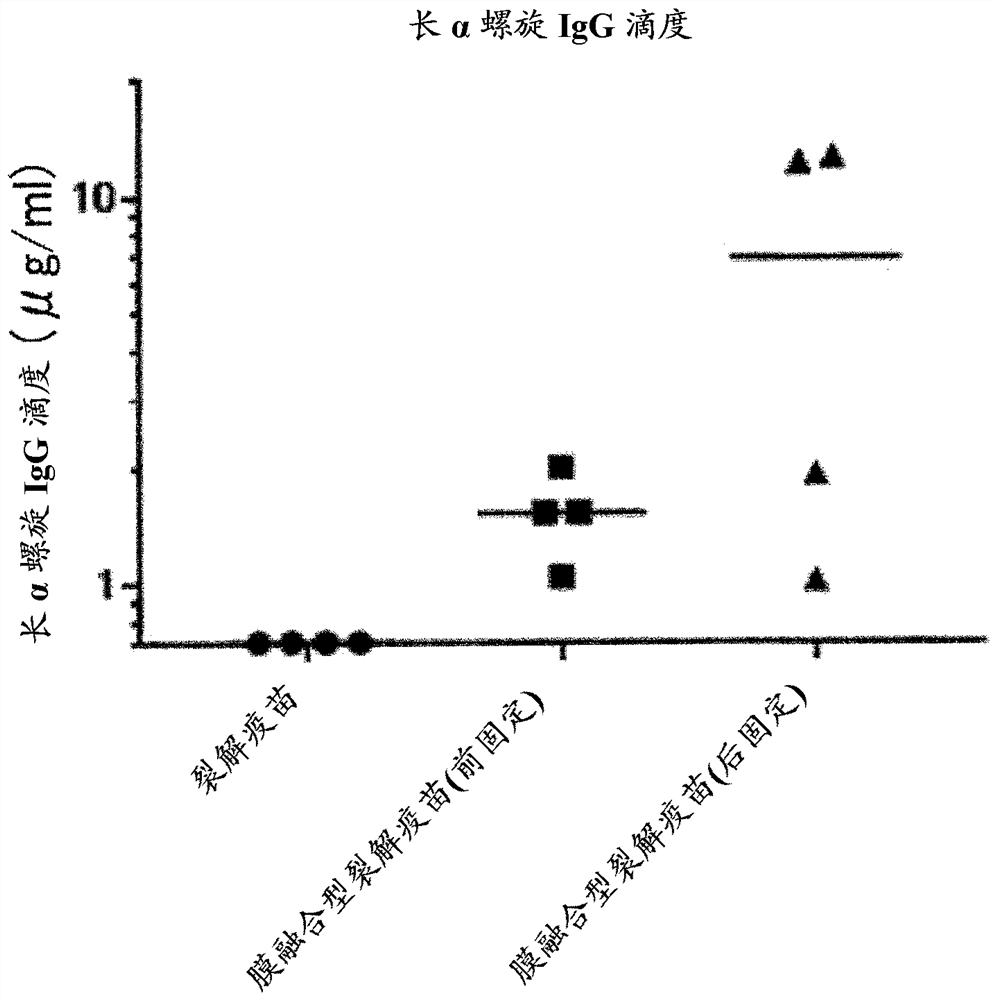
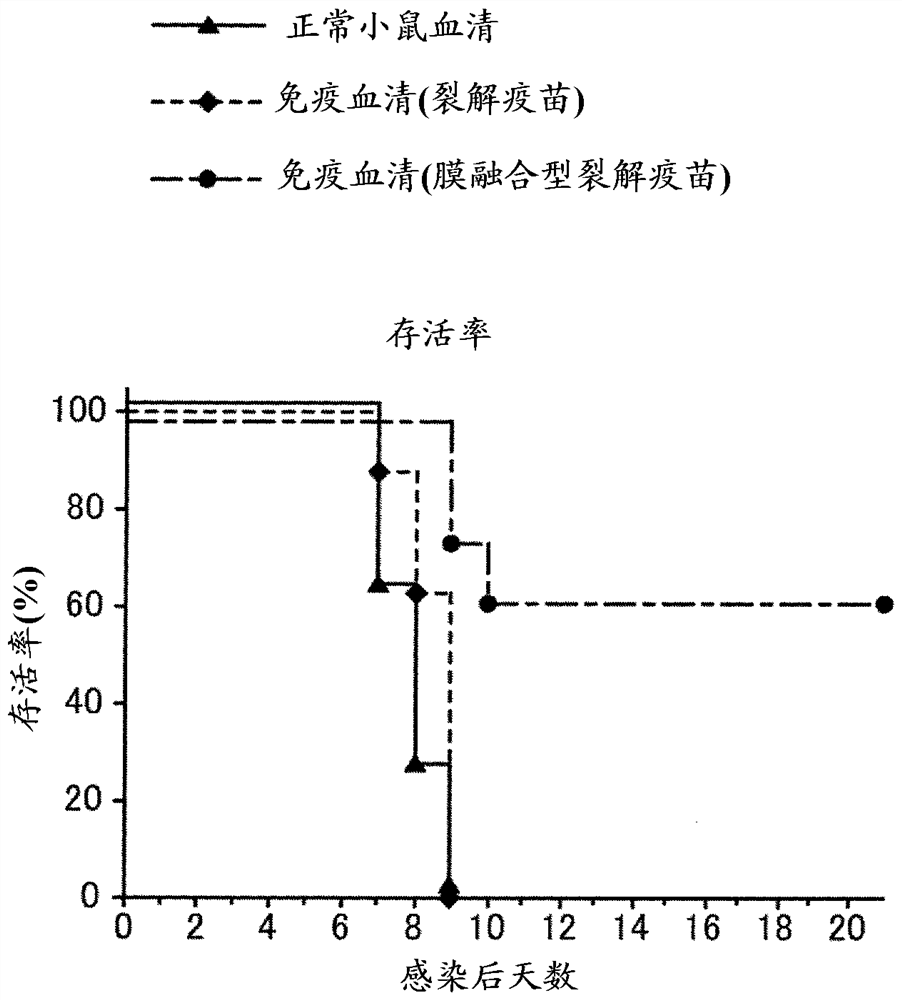
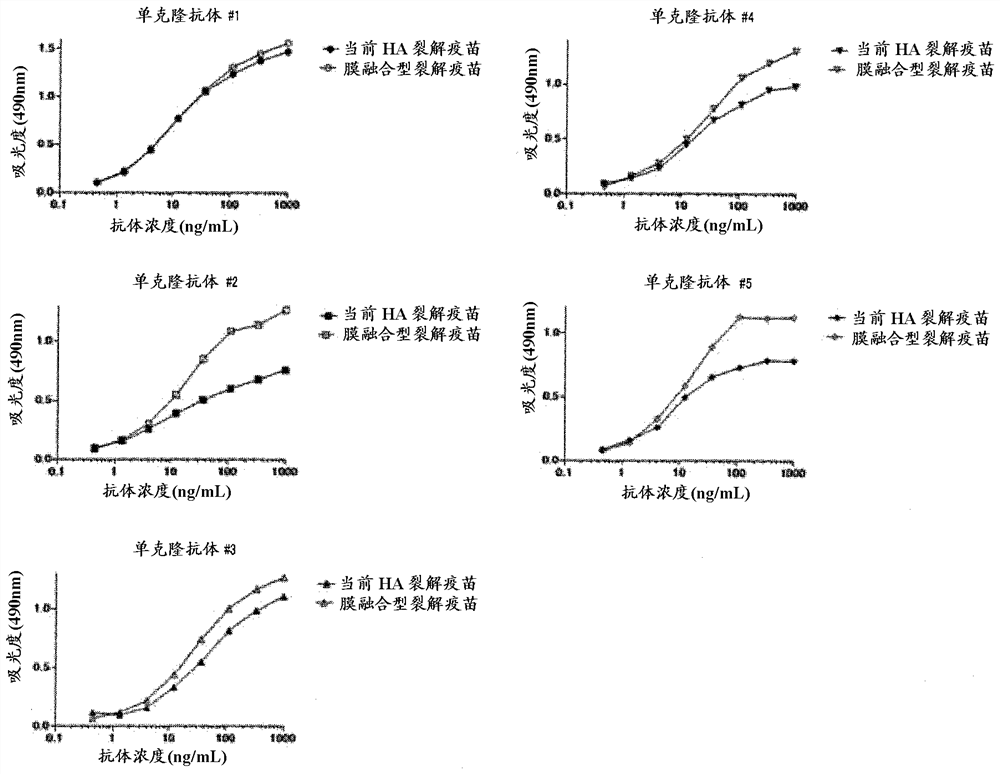
[0113] 1. Preparation of HA Split Vaccine
[0114]Add Tween 80 to H3N2 influenza virus particles (X31 strain) or H1N1 influenza virus particles (A / Puerto Rico / 8 / 34 strain) suspended in phosphate buffered saline to a final concentration of 0.1 v / v% and suspend in it . Diethyl ether was added and suspended, and the suspension was left standing until the water layer and ether layer were completely separated, and then the ether layer was removed. After repeating this ether extraction, diethyl ether remaining in the recovered aqueous layer was distilled off at normal pressure to obtain a HA split vaccine.
[0115] 2. Acid treatment
[0116] The HA split vaccine was suspended in phosphate-buffered saline, and then subjected to acid treatment by adding 0.15 M citrate buffer (pH 3.5) to bring the pH to 5.0. After standing at room temperature for 30 minutes, 1 M Tris buffer (pH 8.0) was added to restore the pH to 7.3. Thereafter, centrifugation was performed to obtain a membrane-fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com