Synthetic peptide vaccine for resisting porcine circovirus and preparation method thereof
A technology for synthesizing peptide vaccines and porcine circoviruses, which is applied in the field of vaccines and their preparation, and can solve the problems of inability to induce cellular immune responses and weak protein immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 : Solid Phase Synthesis of Polypeptide Antigens for Synthetic Peptide Vaccines
[0042] This example is the solid-phase synthesis of the polypeptide antigen of the synthetic peptide vaccine provided by the present invention. The polypeptide antigen of the present invention can be prepared by ABI 433A automatic polypeptide synthesizer by Merrifield solid-phase synthesis method, wherein 9-fluorenylmethoxycarbonyl (Fmoc) modified amino acid is used, and the solid phase carrier is Rink Amide MBHA resin. The production process usually includes solid-phase synthesis of polypeptide antigens, cleavage of polypeptides, purification of antigens and sterilized storage.
[0043] 1. Preparation of synthetic raw materials
[0044] The peptide antigen sequences of the synthetic peptide vaccine are respectively the amino acid sequences shown in SEQ ID NO.1, SEQ ID NO.2, SEQ INNO.3 and (SEQ ID NO.2) 2 -K-εK-CONH 2 Polymerized peptides of the indicated amino acid sequence,...
Embodiment 2
[0056] Example 2 : Formulation of Synthetic Peptide Vaccines
[0057] This example is the formulation of the synthetic peptide vaccine provided by the present invention.
[0058] Dilute the peptide solution to 50 μg / ml with water for injection to prepare the antigen aqueous phase; Seppicmontanide ISA 50V oil adjuvant was sterilized at 121°C for 30 minutes, and used as the oil phase for later use. Under the condition of 22°C, according to the volume ratio of the antigen water phase and the sterilized oil phase 50V as 1:1, the oil phase was first added to the emulsification tank, stirred at a slow speed of 100 rpm for 2 minutes, and then the water phase was slowly added, and the Stir for 30 minutes after completion, then stir at a high speed of 9000 rpm for 20 minutes, then let stand for 5 minutes, and then obtain a synthetic peptide vaccine against porcine circovirus after subpackaging, which has the amino acid sequence shown in SEQ ID NO.1. A peptide vaccine, a synthetic pe...
Embodiment 3
[0059] Example 3 : Efficacy Test of Synthetic Peptide Vaccines
[0060] In this example, the efficacy of the synthetic peptide vaccine provided by the present invention is evaluated by the following different methods.
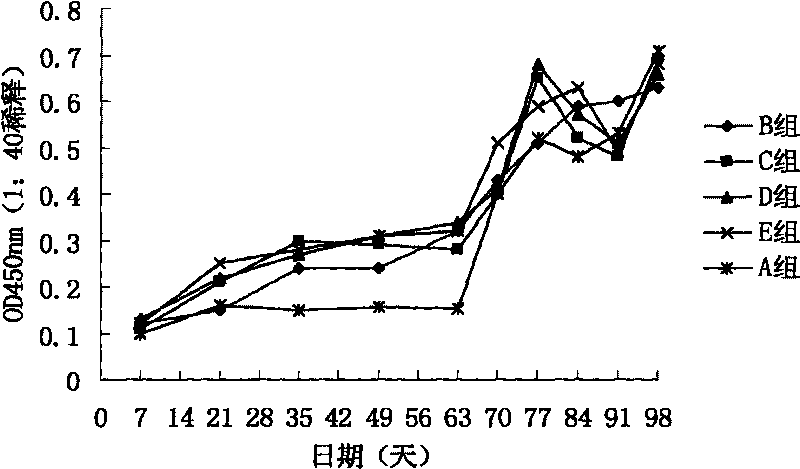
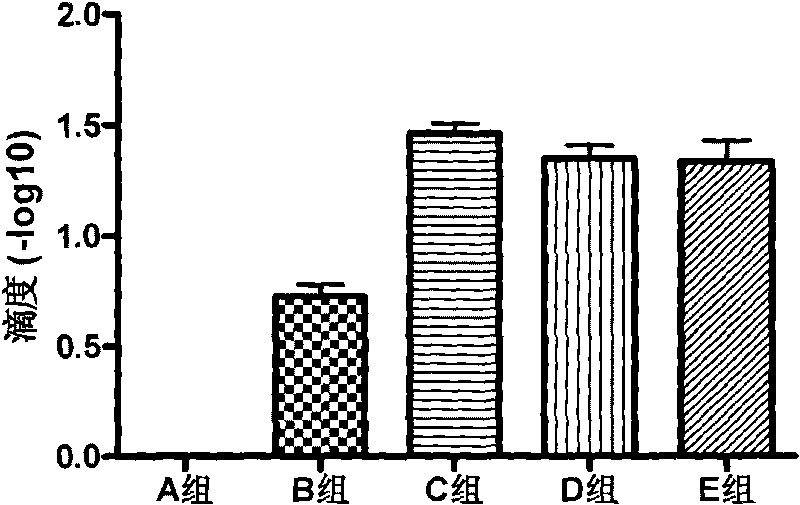
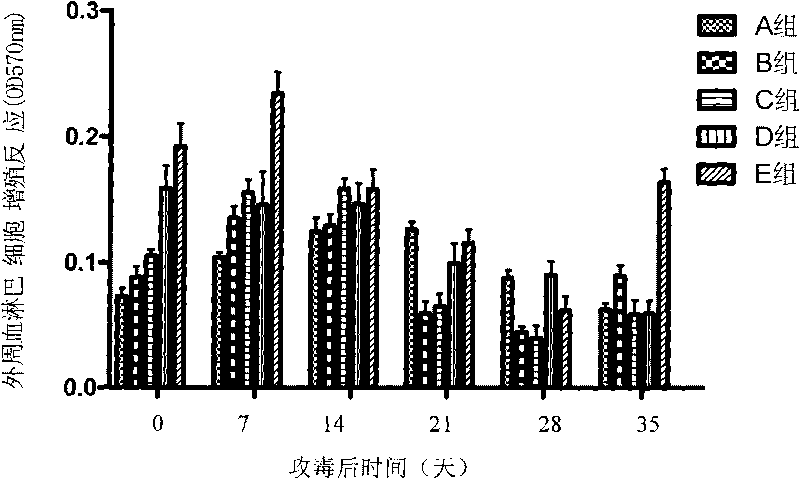
[0061] Divide the 7-day-old healthy piglets into 5 groups, see Table 1 for the grouping information, 3 pigs in each group, inject 1ml of synthetic peptide vaccine into the muscles of the left and right sides of the neck, and inject 3 times intramuscularly, with an interval of two weeks between each time, and the third immunization Infection by oral and nasal routes after 4 weeks10 6.22 TCID 50 / ml of PCV2 tissue virus (BJ-HB strain) (gifted by China Agricultural University), each head was inoculated with 5ml.
[0062] Table 1 Grouping of experimental animals and immune challenge dose
[0063]
group
Number of test animals
(head)
immune dose
(ml)
(ml)
Group A
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com