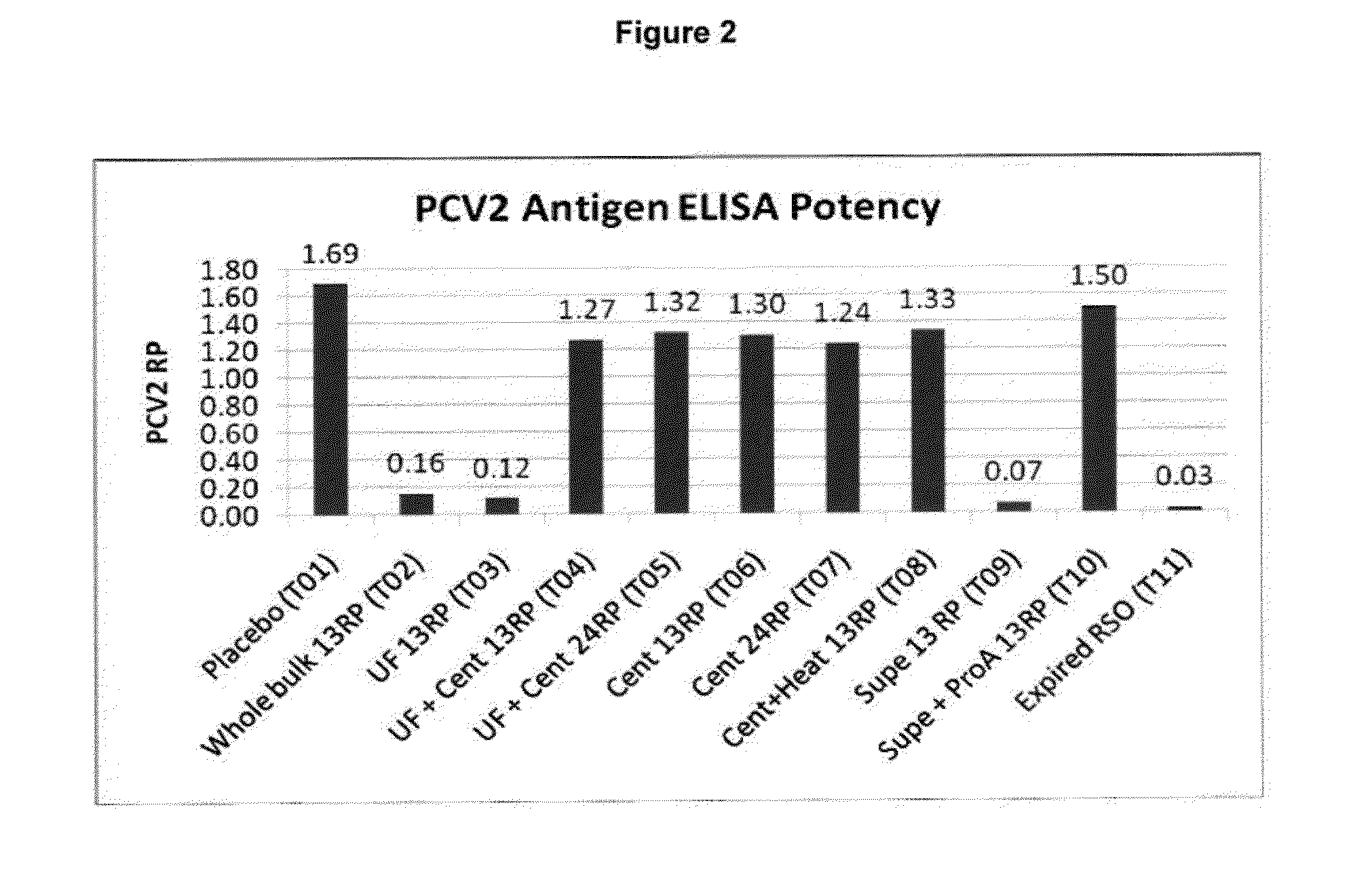
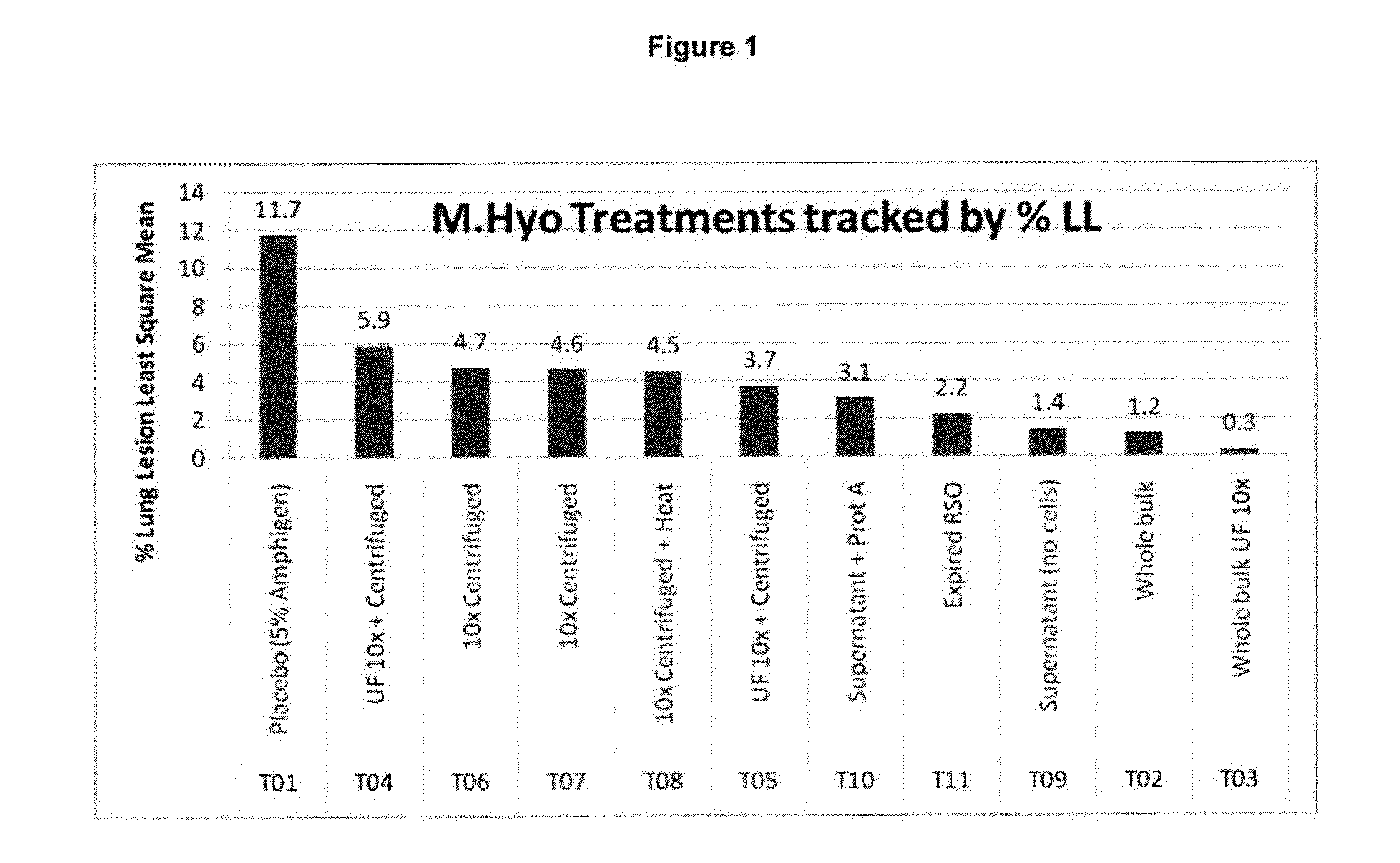
Patents
Literature
216 results about "Porcine Circoviruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Porcine circovirus (PCV) is a single-stranded DNA virus (class II), that is nonenveloped with an unsegmented circular genome. The viral capsid is icosahedral and approximately 17 nm in diameter.
Methods of overexpression and recovery of porcine circovirus type 2 ORF2
ActiveUS20090042245A1SsRNA viruses positive-senseInorganic active ingredientsOpen reading frameIntracellular
An improved method for recovering the protein expressed by open reading frame 2 from PCV2 is provided. The method generally involves the steps of transfecting recombinant virus containing open reading frame 2 coding sequences into cells contained in growth media, causing the virus to express open reading frame 2, and recovering the expressed protein in the supernate. This recovery should take place beginning approximately 5 days after infection of the cells in order to permit sufficient quantities of recombinant protein to be expressed and secreted from the cell into the growth media. Such methods avoid costly and time consuming extraction procedures required to separate and recover the recombinant protein from within the cells.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Porcine circovirus and Helicobacter combination vaccines and methods of use
InactiveUS20060029617A1Viral antigen ingredientsMicrobiological testing/measurementDiseasePorcine Circoviruses
The present invention is based on the discovery of novel species of the genus Helicobacter that are associated with gastro-esophageal ulceration in pigs. In particular, a novel species, H. cerdo, has been used as a source of antigenic material for the development of vaccine for the treatment of the gastro-esophageal disorders. Most advantageously, the novel Helicobacter and the porcine circoviruses associated with PMWS in pigs are useful for providing combination vaccines whereby immunogens derived from both types of pathogens may be codelivered to the target animal to stimulate the generation of protective antibodies and immunity. The invention, therefore, provides vaccines that are useful for the tratment of gastro-esophageal ulceration and PMWS in porcines. The present invention includes, therefore, multivalent immunogenic compositions and vaccines, multivaccine kits, and combined immunization or vaccination methods which make it possible to use such combined immunization or vaccination programmes.
Owner:MERIAL LTD
PCV2 immunogenic compositions and methods of producing such compositions
ActiveUS7700285B1Reduce the impactReduce severityViral antigen ingredientsMicrobiological testing/measurementOpen reading frameCell culture media
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. The method generally involves the steps of transfecting recombinant virus containing open reading frame 2 coding sequences into cells contained in growth media, causing the virus to express open reading frame 2, and recovering the expressed protein in the supernate. This recovery should take place beginning approximately 5 days after infection of the cells in order to permit sufficient quantities of recombinant protein to be expressed and secreted from the cell into the growth media. Such methods avoid costly and time consuming extraction procedures required to separate and recover the recombinant protein from within the cells.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Multivalent pcv2 immunogenic compositions and methods of producing such compositions
InactiveUS20080226669A1Lowered viral titerImprove the immunityAntibacterial agentsCompounds screening/testingDiseaseOpen reading frame
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. Also provided is recombinant PCV2 ORF2 protein, and immunogenic compositions comprising PCV2 ORF2 protein. Moreover, multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of PCV2 infection, preferably PCV2 ORF2 protein, or an immunogenic composition comprising PCV2 ORF2 protein, and at least one immunogenic active component of another disease-causing organism in swine,
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Mycoplasma Hyopneumoniae Avirulent Adjuvanted Live Vaccine
ActiveUS20090117152A1Preventing and minimize severityElicit immune responseAntibacterial agentsBacterial antigen ingredientsViral antigensImmunogenicity
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS SERVICE LLC
Pcv2 immunogenic compositions and methods of producing such compositions
InactiveUS20090022751A1Reduce severitySsRNA viruses positive-senseViral antigen ingredientsOpen reading frameIntracellular
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. The method generally involves the steps of transfecting recombinant virus containing open reading frame 2 coding sequences into cells contained in growth media, causing the virus to express open reading frame 2, and recovering the expressed protein in the supernate. This recovery should take place beginning approximately 5 days after infection of the cells in order to permit sufficient quantities of recombinant protein to be expressed and secreted from the cell into the growth media. Such methods avoid costly and time-consuming extraction procedures required to separate and recover the recombinant protein from within the cells.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Porcine circovirus type II inactivated vaccine of and method for preparing same
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
Preparation of PCV2 ORF2 capsid protein virus-like particles derived from escherichia coli
InactiveCN103173470AKeep natural activityHigh activityBacteriaViral antigen ingredientsEscherichia coliOrf2 gene
The invention relates to porcine circovirus PCV2 ORF2 gene optimized by using escherichia coli expression codon and a preparation method of PCV2 ORF2 capsid protein virus-like particles derived from the escherichia coli.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Recombinant baculovirus strain of porcine circovirus type 2 Cap protein expression, construction method and application thereof
InactiveCN101358182AImprove immune activityViral antigen ingredientsAntiviralsMicroorganism preservationImmunocompetence
The present invention discloses a recombinant baculovirus strain rBac / PCV2Cap (microorganism preservation number: CGMCC NO.2083) efficiently expressing Porcine circovirus type 2 Cap protein and applications thereof. The recombinant baculovirus strain rBac / PCV2Cap constructed by the present invention can efficiently express recombinant PCV2-Cap protein in insect cells, and the expressed recombinant Cap protein, which has good immunocompetence and antigenicity, can serve as a subunit vaccine used to prevent the related plague caused by Porcine circovirus type 2 infection as well as a detecting and diagnostic antigen for Porcine circovirus type 2 serum antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Porcine Circovirus Type 2 (PCV2), Immunogenic Composition Containing the Same, Test Kit, and Application Thereof
ActiveUS20120164170A1Improve immunityReduce severityAntibacterial agentsVirus peptidesPorcine circovirusImmunogenicity
The invention relates to a novel porcine circovirus type 2 (PCV2) strain. The invention also relates to immunogenic compositions containing the novel PCV2 strain, PCV2 test kits, and applications of the novel PCV2 strain.
Owner:VIRBAC H K TRADING LTD
Multivalent pcv2 immunogenic compositions and methods of producing such compositions
ActiveUS20090092636A1Improve the immunityReduce severityAntibacterial agentsCompounds screening/testingDiseaseOpen reading frame
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. Also provided is recombinant PCV2 ORF2 protein, and immunogenic compositions comprising PCV2 ORF2 protein. Moreover, multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of PCV2 infection, preferably PCV2 ORF2 protein, or an immunogenic composition comprising PCV2 ORF2 protein, and at least one immunogenic active component of another disease-causing organism in swine,
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
PCV/Mycoplasma Hyopneumoniae/PRRS Combination Vaccine
ActiveUS20130266603A1Protective immune responseAntibacterial agentsBacterial antigen ingredientsImmune complexVirus antigen
This invention provides a trivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; a porcine circovirus type 2 (PCV2) antigen; and a PRRS virus antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Recombination virus particles for expressing 2-typed porcine circovirus nucleocapsid protein Cap gene
InactiveCN101289658ANon-pathogenicImprove replication efficiencyViral antigen ingredientsAntiviralsAntigenShuttle vector
The invention relates to construction and application of recombinant nucleocapsids of Cap genes of porcine circovirus expression type 2 nucleocapsid protein, belonging to the genetic engineering bacterin field. C-terminal gene fragments of porcine parvovirus (PPV) VP2 genes are cloned into a type 5 adenovirus shuttle vector of the human beings, and recombinant adenovirus rAd-deltaVP2 is obtained; deltaVP2 proteins are expressed successfully and highly efficiently and can be self-assembled into the nucleocapsids [PPV:VLPs]; the PPV VP2 nucleocapsids are used as antigen transport vectors and 165 to 200 sites of amino acid (deltaCap) genes of the porcine circovirus type 2 (PCV2) nucleocapsid proteins (Cap) are embedded into an N-terminal (deltaVP2) of the PPV VP2, and then recombinant adenovirus rAd-deltaCap-deltaVP2 is obtained; embedded VP2 (deltaCap-deltaVP2) proteins are expressed successfully and highly efficiently and can be self-assembled into nucleocapsids [PPV:VLP(PCV2)]. The invention also relates to application of the recombinant virus and recombinant PPV VP2 nucleocapsids of the expression Cap genes of the recombinant virus in the aspects of bacterin immunity and so on.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
RPA (recombinase polymerase amplification) primer and detection kit for rapidly detecting African swine fever viruses
PendingCN109797246AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesQuarantinePorcine reproductive and respiratory syndrome virus
The invention discloses an RPA (recombinase polymerase amplification) primer and a detection kit for rapidly detecting African swine fever viruses. Target genes can be effectively amplified, specificity is 100%, detection sensitivity is 102 copy / reaction, and sensitivity is equivalent to that of fluorescent quantitative PCR (polymerase chain reaction). Cross reaction between the RPA amplificationprimer and one of classical swine fever viruses, vesicular exanthema swine viruses I, porcine reproductive and respiratory syndrome viruses, porcine circoviruses and the like is omitted. A RPA isothermal amplification system is rapidness in reaction and wide in temperature range, effective amplification of the target genes can be achieved at the temperature of 38-46 DEG C, and the detection kit can rapidly, efficiently and sensitively detect the African swine fever viruses and has the advantages that the kit is simple to operate, high in specificity, safe and free from pollution, reaction results are easily observed and the like. Effective technical means are provided for on-site rapidness detecting and screening of infection nucleic acid of the African swine fever viruses, and the RPA amplification primer has great significance for control of infection spreading of the African swine fever viruses in China and inspection and quarantine in infected areas and entry and exit ports.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Codon optimized porcine circovirus type 2 Cap protein coding gene and application thereof
InactiveCN103451196AStructuredImproving immunogenicityBacteriaViral antigen ingredientsPorcine CircovirusesImmunogenicity
The invention provides a codon optimized porcine circovirus type 2 Cap protein coding gene and application thereof, and belongs to the field of the molecular biology. The Cap protein coding gene is as shown in SEQ ID NO: 1. The invention also provides a recombinant vector containing the gene and recombinant bacteria containing the gene. A method for preparing porcine circovirus type 2 virus-like particles comprises the steps of inducing the recombinant bacteria to express a porcine circovirus type 2 Cap protein, lysing the recombinant bacteria, and taking the lysate for purification to obtain the porcine circovirus type 2 virus-like particles. The invention also provides a gene engineering subunit vaccine with the virus-like particles as an active ingredient. The codon optimized porcine circovirus type 2 Cap is capable of realizing abundant soluble expression. The virus-like particles (VLP) are assembled from the Cap proteins and have excellent immunogenicity. A vaccine prepared from the virus-like particles provided by the invention is capable of preventing porcine circovirus type 2 infection and has the advantages of good immunogenicity, high antibody level and effective protection after challenge.
Owner:JIANGSU ACAD OF AGRI SCI
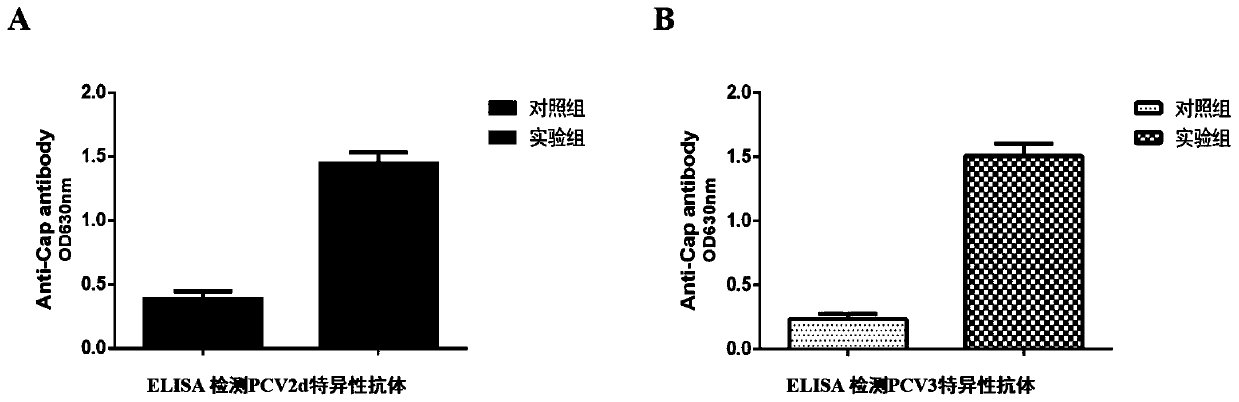
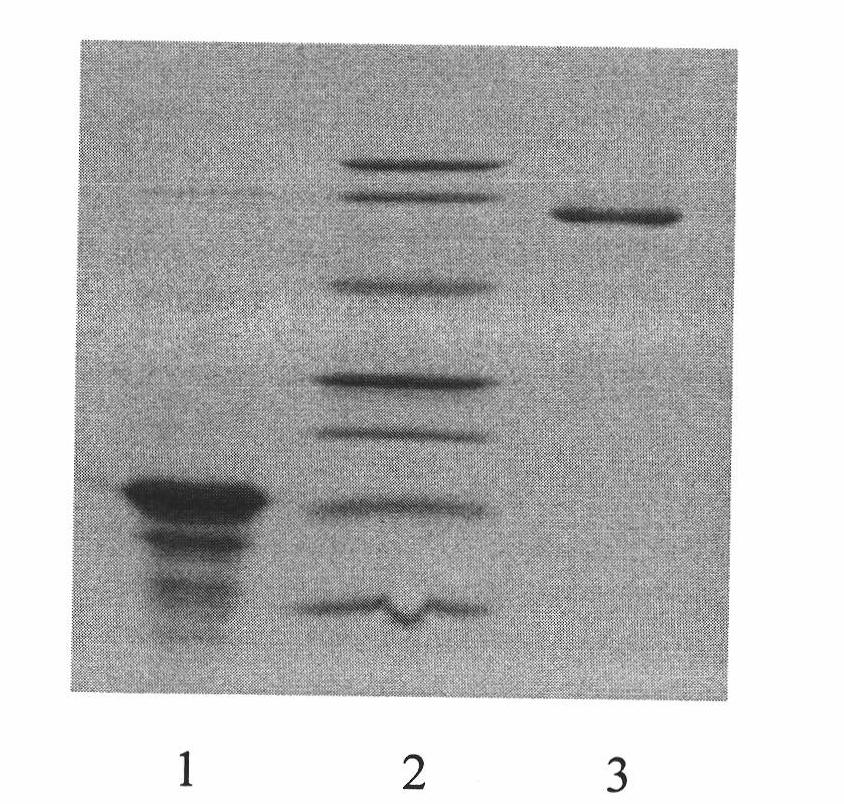
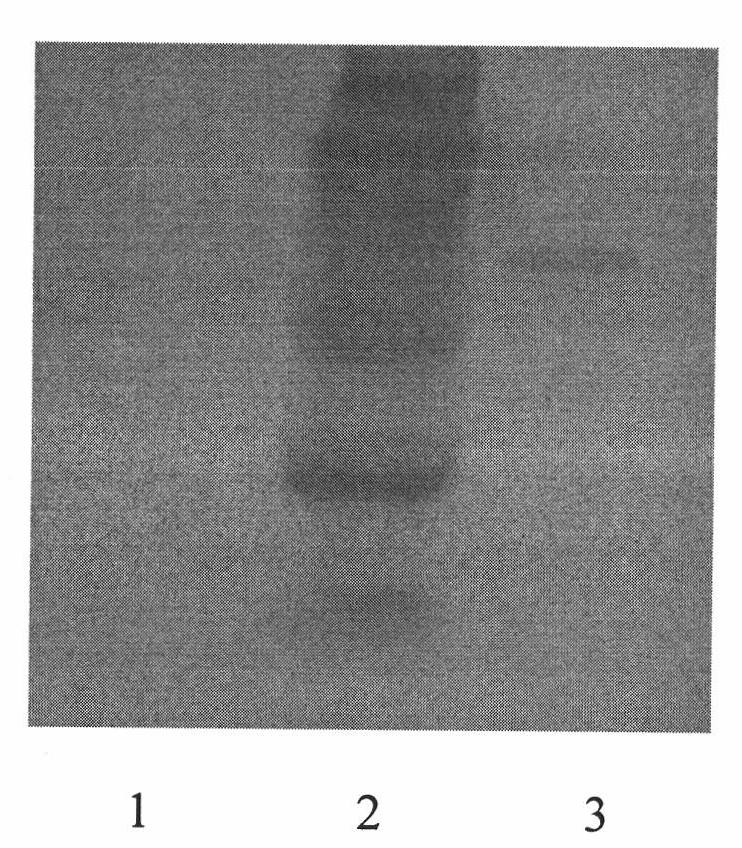
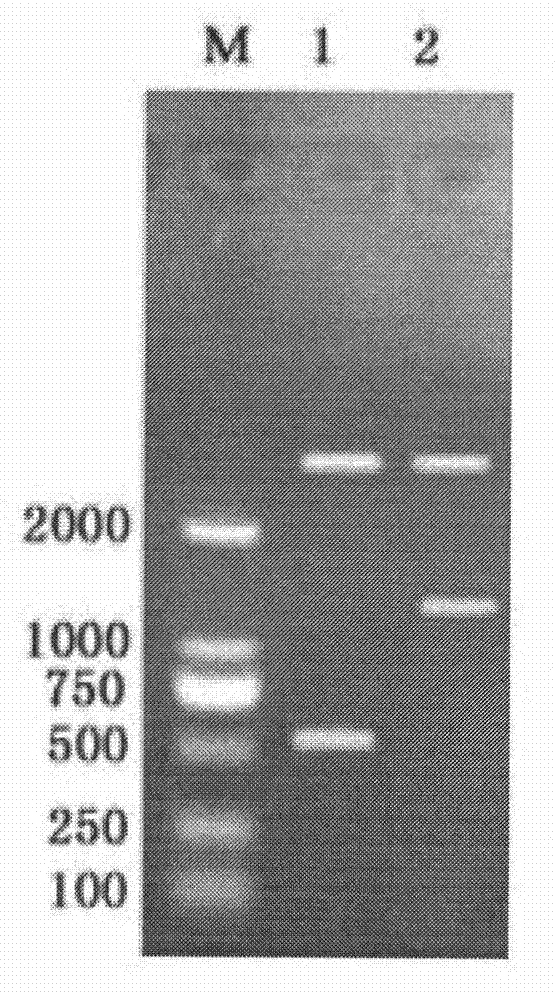
Porcine circovirus 2d-type and 3-type Cap protein bivalent subunit vaccine and preparation method and application thereof
ActiveCN110606873AEasy to operatePreserve immunogenicityVirus peptidesAntiviralsEpitopeBaculovirus expression vector system
The invention provides a porcine circovirus 2d-type and 3-type Cap protein bivalent subunit vaccine and a preparation method and application thereof. By cloning porcine circovirus 2d-type and 3-type Cap protein epitope genes, an insect-baculovirus expression vector system is adopted to successfully express and obtain high-purity 2d-type and 3-type Cap proteins. The porcine circovirus 2d-type and 3-type Cap proteins bivalent subunit vaccine is developed for the first time by utilizing the two expressed proteins. The prepared bivalent vaccine is strong in immunity and high in safety, can achievean ideal immune protection effect on the 2d type and 3 type porcine circoviruses, and provides an effective means for prevention and control of the porcine circoviruses.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Prokaryotic expression protein of VP73 gene from African swine fever virus and preparation method thereof
InactiveCN102101889ANo risk of poisoningHigh detection sensitivityMicroorganism based processesPeptide preparation methodsAfrican swine fever virus AntibodySwine Fever Virus
The invention relates to a method for preparing a genetic engineering product, in particular to a prokaryotic expression protein of VP73 gene from African swine fever virus (ASFV) and a preparation method thereof. The preparation method comprises: artificially synthesizing the whole-length sequence of VP73 gene according to the sequence of the VP73 gene from ASFV in GenBank, constructing a recombinant expression vector pET32a-VP73, sequencing, verifying, transforming prokaryotic expression recipient bacteria E.coli BL21(DE3), and inducing expression by isopropyl-1-thio-beta-d-galactopyranoside (IPTG), wherein the molecular weight of the recombinant fusion protein is about 65KD. Protein purified by nickel column affinity chromatography can undergo a specific immune imprinting reaction with ASFV positive serum and avoid cross reaction with viruses such as swine fever virus, hog cholera virus, porcine circovirus, porcine reproductive and respiratory syndrome virus, swine influenza virus and pseudorabiesvirus. Experiments show the expressed protein has high detection sensitivity, and high specificity. When the antigen is used for detection, risk of spreading poison is avoided. And the antigen can be used as a detection antigen for use in an enzyme-linked immuno sorbent assay (ELISA) method for identifying an ASFV antibody.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
ELISA kit for porcine circovirus type 2 antibody detection
InactiveCN103033614AAntigen space structure is goodHigh sensitivityMaterial analysisSerum igeElisa kit
The invention discloses an ELISA (enzyme linked immunosorbent assay) kit for porcine circovirus type 2 (PVC2) antibody detection. The kit includes an antibody detection board, a sample diluting solution, an enzyme-labeled antigen, a washing solution, a substrate color developing solution, a stop solution, a positive serum and a negative serum. The Cap protein coated with antibody detection board of the kit is PCV2 eukaryotic expression nucleocapsid Cap protein, and the non-specific reactions are greatly reduced by using the Cap protein coupled enzyme-labeled antigen, so that the antibody detection accuracy is improved and the kit is more favorable for large-scale popularization and use.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD +1
New mycoplasma hyopneumoniae strain and vaccine composite of new mycoplasma hyopneumoniae
ActiveCN104450559AImprove immunityDoes not affect weight gainAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaMycoplasma
The invention provides a mycoplasma hyopneumoniae strain HN0613 which has better immunogenicity proved through isolation and identification, also provides a mycoplasma hyopneumoniae antigen prepared by using the mycoplasma hyopneumoniae strain HN0613 and a mycoplasma hyopneumoniae vaccine containing the mycoplasma hyopneumoniae antigen. The invention further provides a porcine circovirus type 2 / mycoplasma hyopneumoniae bicombinant vaccine, containing a PCV2 antigen (inactivated PCV2 antigen or PCV2ORF2 protein), an inactivated mycoplasma hyopneumoniae and vaccine adjuvants. Through injection of the bicombinant vaccine, the aim of preventing PCV and mycoplasma hyopneumoniae can be achieved and the effect of prevention and protection from mycoplasma hyopneumoniae infection can be achieved further.
Owner:PU LIKE BIO ENG +1
PCV/mycoplasma hyopneumoniae/PRRS combination vaccine
Owner:ZOETIS SERVICE LLC
Pcv2 immunogenic compositions and methods of producing such compositions
ActiveUS20150190497A1Reduce severitySsRNA viruses positive-senseVirus peptidesOpen reading frameIntracellular
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. The method generally involves the steps of transfecting recombinant virus containing open reading frame 2 coding sequences into cells contained in growth media, causing the virus to express open reading frame 2, and recovering the expressed protein in the supernate. This recovery should take place beginning approximately 5 days after infection of the cells in order to permit sufficient quantities of recombinant protein to be expressed and secreted from the cell into the growth media. Such methods avoid costly and time consuming extraction procedures required to separate and recover the recombinant protein from within the cells.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Vaccine composition, and preparation method and application thereof
InactiveCN104288760AHigh titer contentImprove immunityViral antigen ingredientsAntiviralsImmune effectsAntigen
The invention provides a vaccine composition comprising an immune amount of a porcine circovirus type 2 antigen, an immune amount of porcine reproductive and respiratory syndrome virus antigen, a porcine parvovirus antigen and a carrier acceptable in veterinary medicine. Antigens in the vaccine composition have no mutual interference on each other, even show some immune synergism; the composition causes small stress after immunization, is more safe, has immune effect better than that of a single vaccine, avoids the stress response caused by multiple inoculation, simplifies the complex immune procedure, and reduces the cost of epidemic prevention.
Owner:PU LIKE BIO ENG
Porcine circovirus 2 type ELISA antibody detection kit
InactiveCN104725490AIncreased sensitivityStrong specificityVirus peptidesBiological testingSerum igePositive control
The invention discloses a preparation method of a porcine circovirus 2 type (PVC2) total-length Cap protein and an ELISA antibody detection kit taking the Cap protein as an antigen. The ELISA antibody detection kit contains an elisa plate coated with the porcine circovirus 2 type nucleocapsid protein, and also contains sample diluent, a confining liquid, a washing liquid, an enzyme conjugate, enzyme substrate solution, stop solution and negative and positive control serum. The ELISA antibody detection kit has strong specificity and high sensitivity when being used for detecting PCV2 and can be popularized and applied in a large scale.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Traditional Chinese medicine composition for treating porcine circovirus disease and preparation method thereof
The invention discloses a traditional Chinese medicine composition for treating porcine circovirus disease and a preparation method thereof. The traditional Chinese medicine composition comprises the following traditional Chinese medicines: astragalus root, iastis root, forsythia fruit, honeysuckle, cordyceps polysaccharide, codonopsitis, atractylodes rhizome, angelica, fresh rehmannia root, Chinese goldthread, grifola umbrellata, tuckahoe, white atractylodes rhizome, tangerine peel, and epimedium. The preparation method comprises the following steps: 1) respectively attritioning and screening various components except the cordyceps polysaccharide; 2) uniformly mixing the astragalus root, codonopsitis, forsythia fruit, atractylodes rhizome, angelica, grifola umbellate, tuckahoe with the cordyceps polysaccharide; 3) uniformly mixing the iastis root, honeysuckle, fresh rehmannia root, Chinese goldthread, white atractylodes rhizome, tangerine peel, and the epimedium; 4) uniformly mixing the medicine power obtained from the step 2) and the step 3), so as to obtain the traditional Chinese medicine composition. The traditional Chinese medicine composition is simply prepared, safe and reliable, and has no toxic and side effect, high bioavailability, and reliably and effectively prevents and treats the porcine circovirus disease, and moreover promotes the growth of the pit and is widely used in clinical.
Owner:河南后羿制药有限公司
PCV/Mycoplasma hyopneumoniae combination vaccine
ActiveUS9125885B2Antibacterial agentsBacterial antigen ingredientsImmunglobulin eMycoplasma hyopneumoniae
This invention provides a multivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; and a porcine circovirus type 2 (PCV2) antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
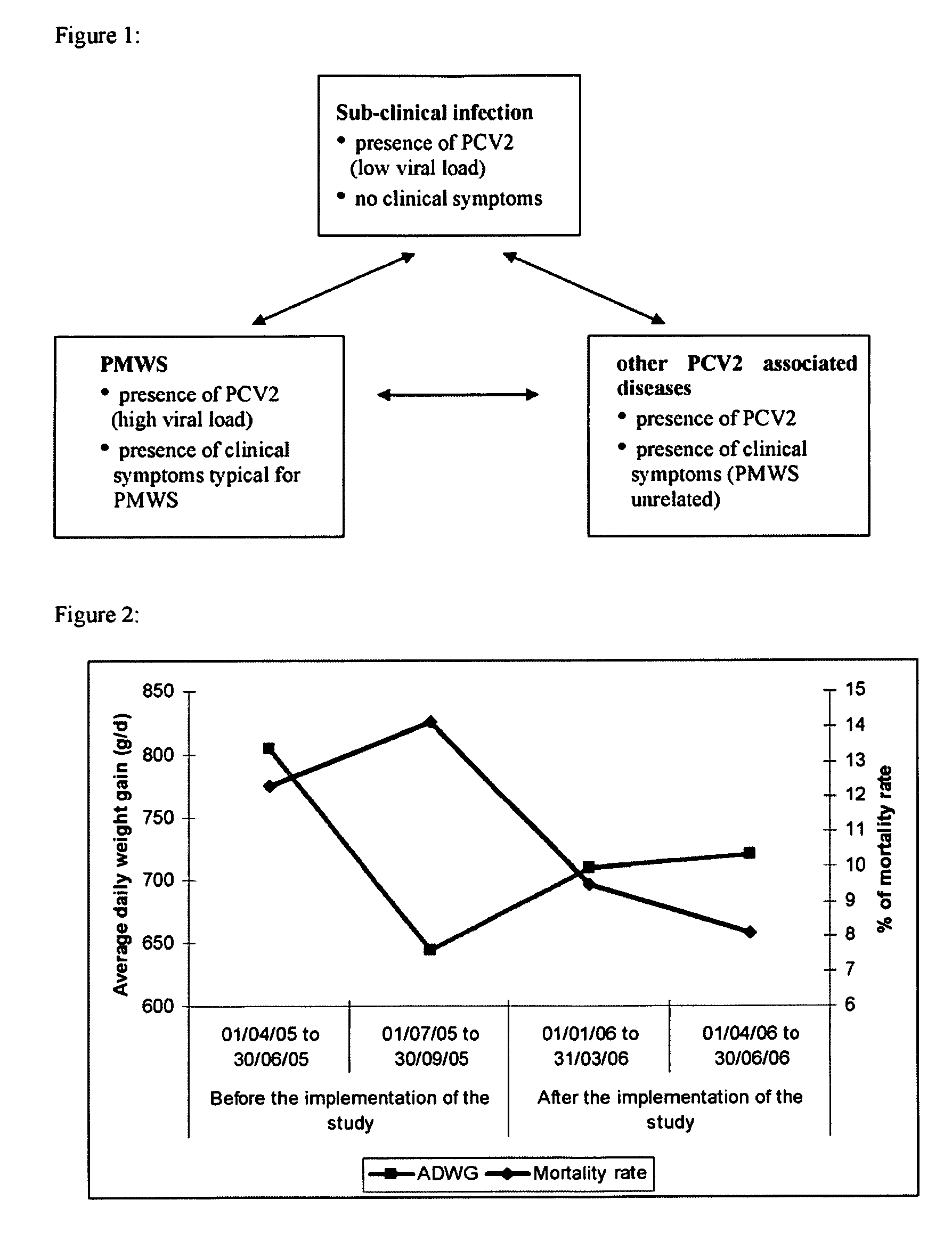
Prevention and treatment of sub-clinical pcvd
ActiveUS20090010954A1Reduce in quantityReduce percentageMetabolism disorderViral antigen ingredientsSub clinicalImmunogenicity
The present invention relates to the use of an immunogenic composition comprising a porcine circovirus type 2 (PCV2) antigen for the prevention and treatment of sub-clinical PCV2 infection in animals, preferably in pigs.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Pharmaceutical Compositions Comprising A Pancreatic Enzyme Preparation With Viral Infectivity Reduced Below A Significant Level And Methods Of Preparing And Using The Same
InactiveUS20140017223A1Reduce alkylation activitySure easySsRNA viruses positive-sensePeptide/protein ingredientsAmylasePorcine circovirus
The present invention provides for pharmaceutical compositions comprising pancreatic enzyme preparations (PEPs) with viral infectivity reduced below significant levels and having high enzymatic activity. The PEPs can comprise lipases, proteases, amylases, non-enveloped viruses (e.g., porcine parvovirus (PPV), porcine circovirus type 2 (PCV-2), porcine encephalomyocarditis virus (EMCV)), and enveloped viruses (e.g., vesicular stomatitis virus (VSV), and influenza A (IFA)). The present invention also includes methods of treating pancreatic insufficiency by administering these pharmaceutical compositions and methods of making the same by treating the PEP with beta-propiolactone (BPL) to reduce viral infectivity.
Owner:APTALIS PHARMA CANADA
Method for synchronously detecting porcine circovirus type 2 (PCV2), classical swine fever virus (CSFV) and porcine reproductive and respiratory syndrome virus (PRRSV)
InactiveCN102382905ASingle detection backgroundHigh sensitivityMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVDisease
The invention discloses a method for synchronously detecting a porcine circovirus type 2 (PCV2), a classical swine fever virus (CSFV) and a porcine reproductive and respiratory syndrome virus (PRRSV). The method comprises the following steps of: designing three pairs of specific probes by the PRRSV nucleic acid, PCV2 nucleic acid and CSFV nucleic acid, preparing reporter genes of probe markers by using polymerase chain reaction (PCR), and implementing loop-mediated indirect PCR detection of three viruses by cyclization and reverse PCR amplification of the reporter genes. the pathogens of PRRSV, PCV2 and CSFV in sample tissues can be synchronously detected by designing three pairs of specific probes aiming at three viruses, marking the same reporter genes, designing a pair of reverse PCR amplification primers and establishing a loop-mediated indirect PCR detection system, so that certain reference is provided for scientifically preventing and controlling porcine diseases. Clinical tests show that the method has the advantages of single detection background, high sensitivity, strong specificity, good stability and the like, can be used for single-virus detection, can also be used for synchronous detection of multiple viruses, and provides a tool for viral clinical diagnosis and epidemiological study of PRRSV, PCV2 and CSFV.
Owner:ZHENGZHOU COLLEGE OF ANIMAL HUSBANDRY ENG
Multiplex PCR detection kit for identifying porcine circovirus
InactiveCN108456747ARapid identificationConvenient for clinical operationMicrobiological testing/measurementGenotypeEpidemiologic survey
The invention discloses a multiplex PCR detection kit for identifying porcine circovirus (PCV). The kit comprises the following three pairs of multiplex PCR detection primers which can quickly distinguish genotype PCV1, genotype PCV2 and genotype PCV3: PCV1-F and PCV1-R, PCV2-F and PCV2-R, and PCV3-F and PCV3-R.The multiplex PCR detection kit disclosed by the invention can be used for detecting the PCV and distinguishing different genotypes, has the characteristics of being quick, simple, convenient, high in specificity and high in sensibility, and can perform large batches of sample detectionat the same time; only one strand displacement amplification is needed, so that the situation whether a sample is infected by PCV1, PCV2 or PCV3, or is jointly infected by two or three of the PCV1, the PCV2 and the PCV3 can be identified; and the multiplex PCR detection kit can provide technical support for epidemic situation surveillance, epidemiologic investigation and comprehensive preventingand controlling of the PCV, and has a favorable application prospect.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Pcv 2-based methods and compositions for the treatment of pigs
The present invention relates to methods and compositions for vaccinating pigs against porcine circovirus type 2 (PCV2) associated diseases. In particular, the invention relates to recombinant expression vectors allowing for secretion or cell membrane expression of a truncated form of the PCV2 open reading frame 2 (ORF2) protein.
Owner:VECTOGEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com