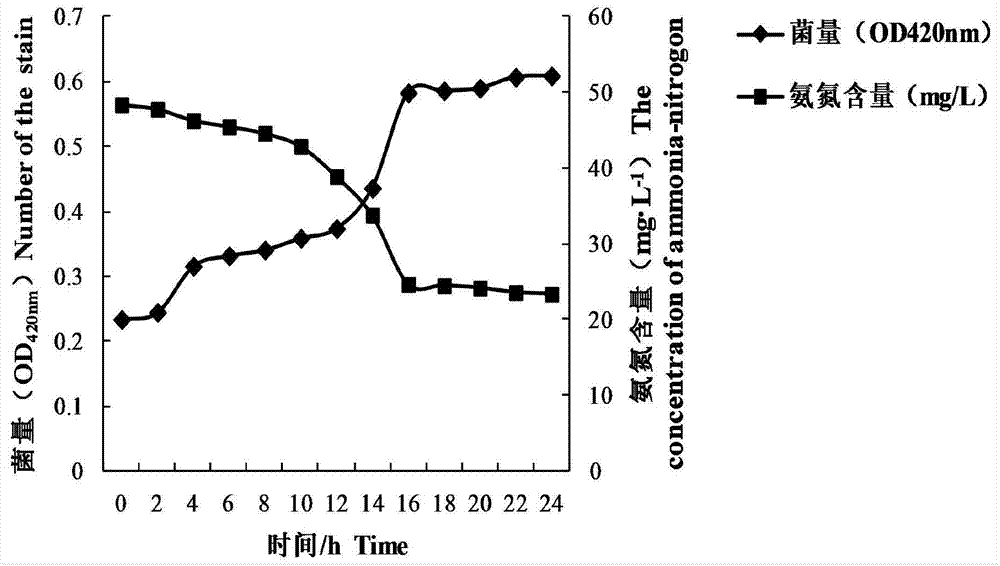
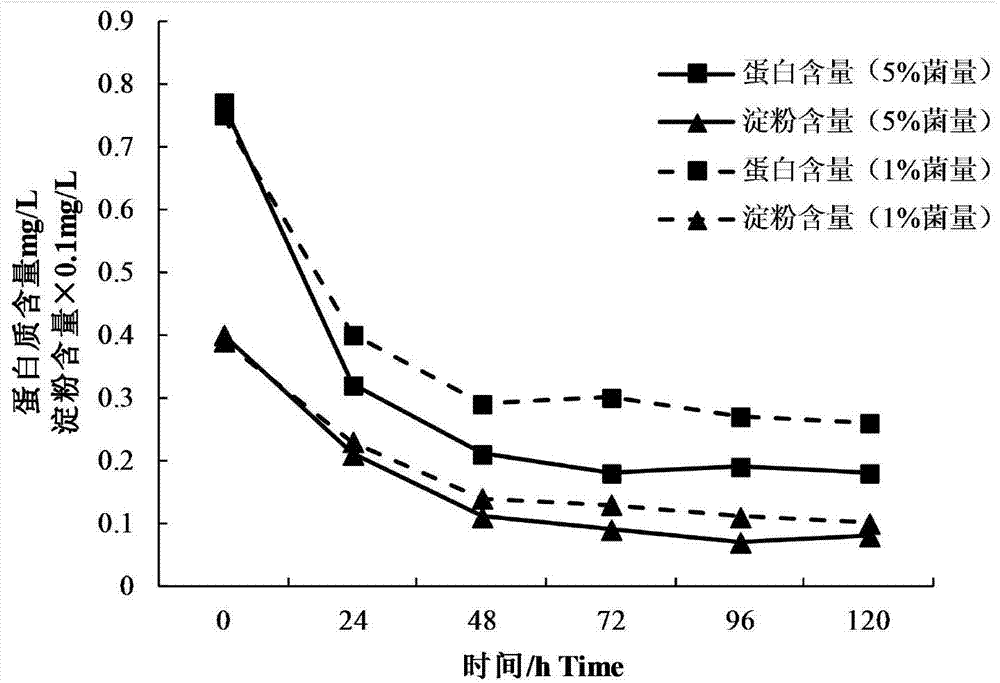
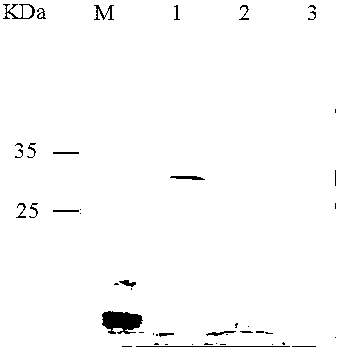
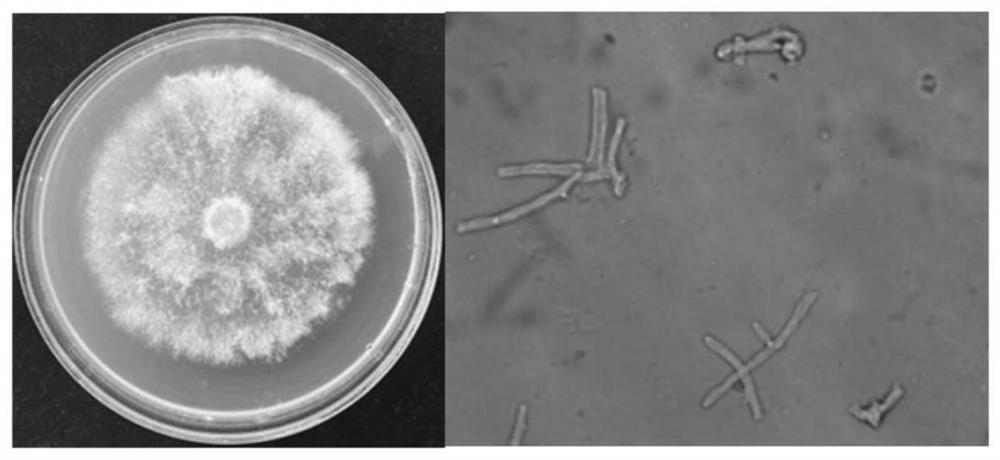
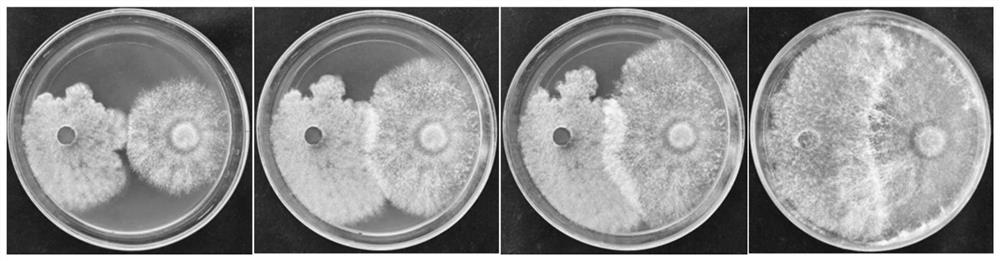
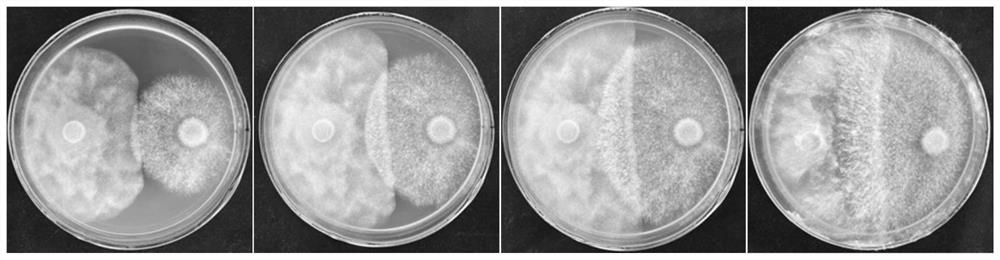
Patents
Literature
182results about How to "Non-pathogenic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bacillus amyloliquefaciens strain and application thereof
InactiveCN101985608AGood control effectControl epidemicBiocideBacteriaEcological environmentOrganism
The invention relates to a bacillus amyloliquefaciens strain, which is characterized in that the collection No. of the strain is CGMCC No.3789; and the 16S rRNA of the strain accesses to GenBank, with accession No. of HQ179100. The strain is used for controlling rice bacterial leaf streak. The strain has the following advantages: the strain has good control effect on the rice bacterial leaf streak, is free of toxin and pathogenicity, is harmless to people and livestock and is environment-friendly; the prepared biocontrol bacteria or the compound biocontrol bacteria formed by compounding the prepared biocontrol bacteria and bismerthiazol are sprayed after being diluted, so that reasonable distribution of the microfloras in the ecological environment around the root leaves of the rice can be controlled and managed and the living environment of the pathogenic bacteria is worsened to form a rice ecosystem with biodiversity, thus effectively and enduringly controlling prevalence of the rice bacterial leaf streak.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Bacillus amyloliquefaciens and application thereof
The invention provides bacillus amyloliquefaciens SZ-60 which is characterized in that the collection number is CGMCC No.8277. The bacterial colony formed by the strain on a beef-extract peptone (NB) medium by virtue of single-cell reproduction is irregular with oyster white color, slight upheaval at the center and wet and semitransparent surface; the microscopy is of a rod shape, and the size is (0.3-0.4)*(3.2-3.3)microns; with flagella, G- and spores, the strain can grow on the beef-extract peptone (NB) medium containing 2-5% of NaCl; the formula of the beef-extract peptone (NB) medium contains 3.0g of beef extract, 10.0g of peptone, 5.0g of NaCl, 17g of agar and 1,000ml of water, and the pH is 6.8-7.2. The bacillus amyloliquefaciens realizes a remarkable antagonistic action on the main pathogenic bacteria causing ginseng root rot, epidemic diseases, sclerotinia sclerotiorum, cylindrocarpon destructans, black spot and damping off. The invention also provides an application of the bacillus amyloliquefaciens SZ-60 in preventing plant fungal diseases, or application in preparing a microbial preparation for preventing plant fungal diseases.
Owner:JILIN AGRICULTURAL UNIV
Method of producing composite and highly effective microorganism preparation for waste water treatment
InactiveCN101033450ANo toxicityNon-pathogenicMicroorganismsTreatment with aerobic and anaerobic processesEffective microorganismSludge
This invention relates to a preparation method for a compound high efficient microbe preparation used in processing waste water including: A, selecting and cultivating, B, fermenting and cultivating the preparation of oxygen and anaerobic compound microbes, C, fermenting and cultivating the preparation of aerobic microbes, D, mixing them. 1, the preparation prepared in this invention is avirulent and will not cause second time pollution, 2, microbes in this preparation can degradate organics and ammonia nitrogen pollutant factors thoroughly due to multikind of microbes and quick speed to degradate pollutants in waste water, 3, output of mud is less, the life time is long and its settlement is good, 4, the preparation method is simple.
Owner:赵志龙 +1
Bacillus velezensis capable of inhibiting virus and promoting plant growth and application of bacillus velezensis
ActiveCN109868250APromote growthNon-pathogenicBiocidePlant growth regulatorsBacillus cereusHigh stress
The invention provides bacillus velezensis for inhibiting virus and promoting plant growth and application of a bacillus velezensis SNB55 strain in prevention and treatment of plant virus diseases andplant fungal diseases and promotion of plant growth. The bacillus velezensis for inhibiting virus and promoting plant growth and the application of the bacillus velezensis have the beneficial effectsthat the bacillus velezensis has no toxicity and pathogenicity, is safe to human and livestock, is environment-friendly, and has high stress resistance and stable antibacterial activity; the bacillusvelezensis can inhibit phytopathogens such as bacillus cereus, tobacco mosaic virus, tomato yellow leaf curl virus, fusarium wilt of cucumbers, tobacco target spot pathogens, phytophthora capsici, rice sheath blight disease and botrytis cinerea, can be secrete indoleacetic acid to promote seed germination and plant growth; and the strain is a multifunctional biocontrol bacterium and has a wide application prospect.
Owner:SHENYANG AGRI UNIV
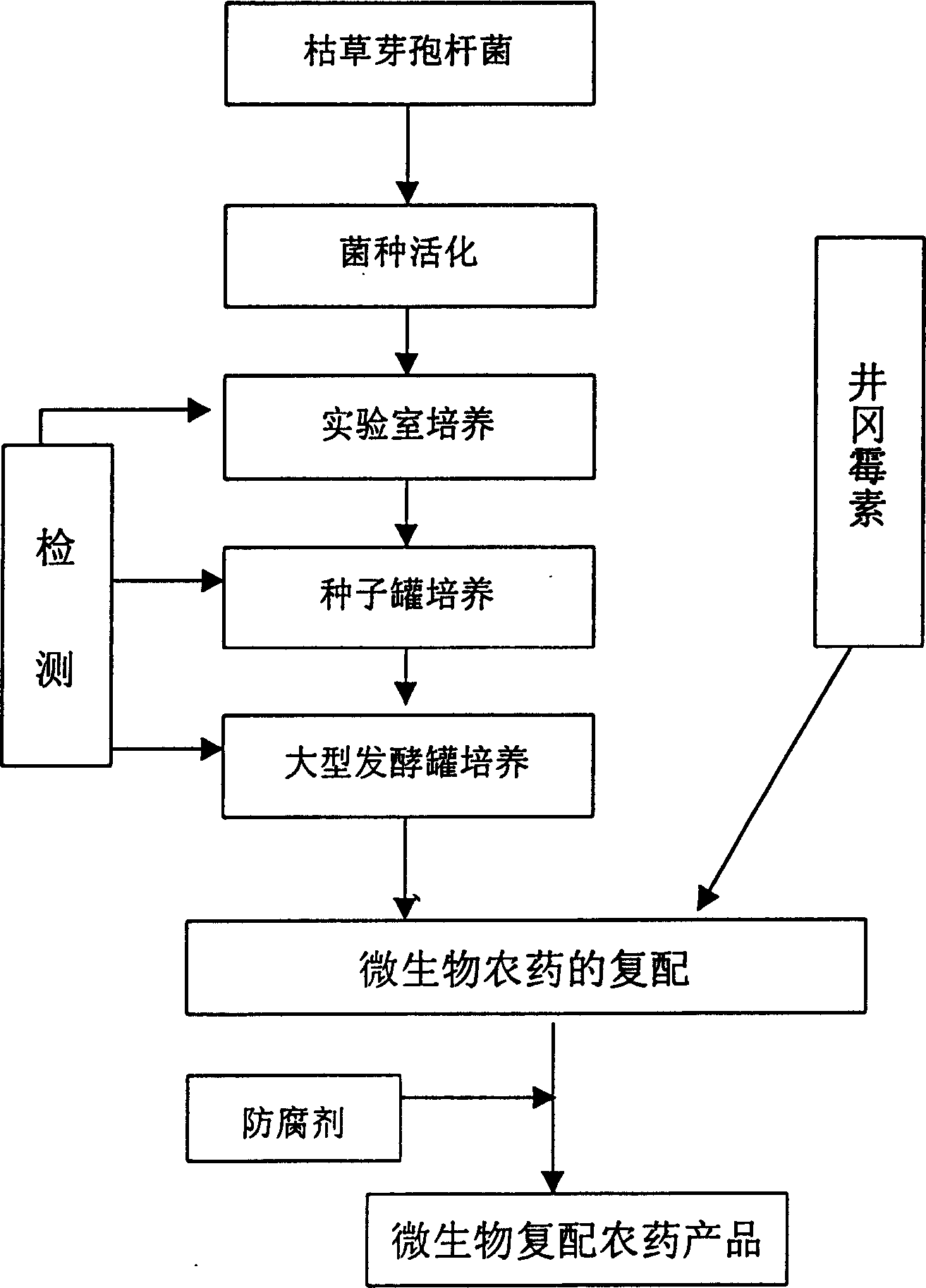
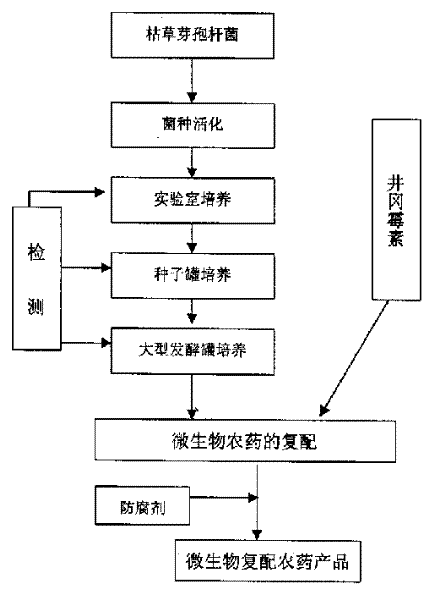
Recompounded microbe germicide for preventing and controlling banded sclerotial blight and green smut of rice
InactiveCN1468524AControl epidemicEnhance colonization abilityBiocideAnimal repellantsMicroorganismBacillus subtilis
The present invention is recompounded microbe germicide for preventing and controlling banded sclerotial blight and green smut of rice. The recompounded microbe germicide is recompounded through thefermentation culture of Bacillus subtilis and recompounding the fermented liquid with certain content of Bacillus subtilis and Jinggangmeisu. It is used in preventing and controlling banded sclerotial blight and green smut of rice in field via utilizing the complementary superiority of Bacillus subtilis and Jinggangmeisu. It has high functions of preventing and controlling banded sclerotial blight and green smut of rice, less influence on rice quality, stable germicidal effect and long preservation period.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Chaetomium globosum and application thereof
ActiveCN104694397AField disease preventionHave a growth-promoting effectBiocideFungiBiotechnologyMicrobial agent
The invention discloses a chaetomium globosum FSR-74. The collection unit of the chaetomium globosum FSR-74 is China General Microbiological Culture Collection Center, the collection date of the chaetomium globosum FSR-74 is September 19, 2014 and the collection number of the chaetomium globosum FSR-74 is CGMCC No. 9690. The strain is relatively slow in growth and has light brown colonies and an irregular wave-like edge when subjected to plate culture at 25 DEG C in a potato dextrose agar (PDA) culture medium. FSR-74 ascocarps are supergene and fixed on the surface of a matrix by rhizoids; top hair is wavy, brown and has intervals, and dense wart points are arranged on the surface; 8 ascospores are arranged in an ascus; the ascospores are shaped like lemons and have single apical bud holes. The chaetomium globosum FSR-74 has an efficient broad-spectrum bacteriostatic effect against main pathogenic bacteria causing ginseng rust rot, blight, sclerotinia rot, rust rot, blackspot, sheath blight and gray mold. The invention further provides a biological control mechanism and application of the chaetomium globosum FSR-74 in prevention and treatment of plant fungal diseases, as well as application in preparation of a microbial agent for preventing and treating the plant fungal diseases.
Owner:JILIN AGRICULTURAL UNIV
Bacillus methylotrophicus and application thereof
The invention provides bacillus methylotrophicus SZ-3 and SZ-81, with collection numbers of CGMCC No.8275 and CGMCC No.8276 respectively. The bacillus methylotrophicus SZ-3 has obvious antagonistic effects on pathogenic bacteria causing root rot, black spot and sclerotinia sclerotiorum of ginseng. The bacillus methylotrophicus SZ-81 has obvious antagonistic effects on pathogenic bacteria causing epidemic diseases, root rust rot and black spot of panax ginseng. The invention also provides an application of the bacillus methylotrophicus SZ-3 and SZ-81 to control of fungal diseases of plants or an application to preparation of microbial preparations for controlling fungal diseases of plants.
Owner:JILIN AGRICULTURAL UNIV
Polysporus trichoderma and application thereof
The invention discloses a polysporus trichoderma (Hypocrea pachybasioide) FSR-97 strain, wherein the preservation unit is China General Microbiological Culture Collection Center, the preservation date is 19, September, 2014 and the preservation number is CGMCC No.9691. The strain is cultured on a potato dextrose agar culture medium (PDA) plate at 25 DEG C and rapidly grows, and a monospore is germinated to form a white bacterial colony; the colour on the back surface of the bacterial colony is transited from colourless to yellow. The polysporus trichoderma (Hypocrea pachybasioide) FSR-97 has an effective broad-spectrum antibacterial effect on the main pathogenic bacteria causing the panaxginsen fusarium rot, epidemic disease, sclerotiniose, root rust rot, black spot, rhizoctonia solani, and botrytis. The invention further discloses a plurality of biocontrol mechanisms of the polysporus trichoderma (Hypocrea pachybasioide) FSR-97 for preventing and controlling the plant fungous disease, and an application for preparing a microbial preparation to prevent and control the plant fungous disease.
Owner:四川臻润农业科技有限公司
Recombinant low-virulent vaccine strain of chicken infectious bursal disease viruses (IBDV) and application thereof
ActiveCN101935637ANon-pathogenicGood spiritsViral antigen ingredientsMicroorganism based processesProtective antigenOrganism
The invention discloses a recombinant low-virulent vaccine strain of chicken infectious bursal disease viruses (IBDV) and application thereof. In the invention, a major protective antigen gene VP2 of an epidemic superhigh virulent strain is cloned, the nucleotide of the gene VP2 is modified by mutation and then used for replacing a corresponding segment of a Gt genome of a low-virulent strain of the IBDV, so that the infectious clone of a recombinant genome of the IBDV is constructed, and the recombinant low-virulent vaccine strain is saved and identified by using an IBDV reverse genetic operation system. The microbial collection number of the vaccine strain is CGMCC No.3749. The recombinant low-virulent vaccine strain of the invention has high replicability, genetic stability and safety. The immune effect of the low-virulent vaccine strain of the invention is as good as that of the medium-virulent vaccine strain, but is superior to that of the low-virulent vaccine strain. The biological safety of the low-virulent vaccine strain of the invention is superior to that of the medium-virulent vaccine strain. As the vaccine strain, the recombinant low-virulent vaccine strain of the invention has the characteristics of high efficiency and low toxicity, is a good candidate vaccine strain and can be used for controlling chicken infectious bursal disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Bacillus amyloliquefaciens B-1619 strain and application in preventing and controlling soil-borne disease of nightshade vegetables thereof
InactiveCN102719382AGood control effectControl happensBiocideBacteriaBiotechnologyBacillus amyloliquefaciens
The invention relates to a bacillus amyloliquefaciens B-1619 strain and application in preventing and controlling soil-borne disease of nightshade vegetables thereof. The preservation number of the strain is CGMCC No. 4671. When the strain is applied, biocontrol strain powder is mixed with soil before seeding, and soil with biocontrol strain is covered on seeds after seeding; the biocontrol strain powder is firstly planted on the surface of the soil before seed separation; diluted biocontrol strain liquid is aligned to seeds of nightshade vegetables to be planted before planting, and the diluted biocontrol strain liquid is irrigated to the roots of the plants of nightshade vegetables during the planting; the diluted biocontrol strain liquid is irrigated to the roots of the plants of nightshade vegetables periodically after the planting; or, the application ways in each period from the seeding to the harvesting period of the nightshade vegetables are combined.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Brevibacillus laterosporus strain and application thereof
The invention discloses a Brevibacillus laterosporus strain N106-2 of which the collection number is CGMCC No.8482. The strain has strong indoor flat inhibiting actions on multiple plant pathogenic fungi (fussarium wilt of tomato, root rot of chili, gray mold of tomato, Rhizoctonia solani, black spot of cabbage, anthracnose of pear and black rot of pear) and plant pathogenic bacteria (bacterial wilt of tomato, fire blight of pear and streak of rice); and the strain can secrete proteinase, cellulase and surfactant antimicrobial related substances with hemolytic activity. The Brevibacillus laterosporus can be prepared into a biocontrol aqua; the biocontrol aqua has certain preventing and treating effects on fussarium wilt of tomato, fusarium rot of chili, anthracnose of pome and eyespot of pome. The Brevibacillus laterosporus N106-2 has the advantages of no toxicity, no pathogenicity and high safety for human and livestock, is environment-friendly, is a useful resource for development of microbial pesticides, and has potential application prospects.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Recombination virus particles for expressing 2-typed porcine circovirus nucleocapsid protein Cap gene
InactiveCN101289658ANon-pathogenicImprove replication efficiencyViral antigen ingredientsAntiviralsAntigenShuttle vector
The invention relates to construction and application of recombinant nucleocapsids of Cap genes of porcine circovirus expression type 2 nucleocapsid protein, belonging to the genetic engineering bacterin field. C-terminal gene fragments of porcine parvovirus (PPV) VP2 genes are cloned into a type 5 adenovirus shuttle vector of the human beings, and recombinant adenovirus rAd-deltaVP2 is obtained; deltaVP2 proteins are expressed successfully and highly efficiently and can be self-assembled into the nucleocapsids [PPV:VLPs]; the PPV VP2 nucleocapsids are used as antigen transport vectors and 165 to 200 sites of amino acid (deltaCap) genes of the porcine circovirus type 2 (PCV2) nucleocapsid proteins (Cap) are embedded into an N-terminal (deltaVP2) of the PPV VP2, and then recombinant adenovirus rAd-deltaCap-deltaVP2 is obtained; embedded VP2 (deltaCap-deltaVP2) proteins are expressed successfully and highly efficiently and can be self-assembled into nucleocapsids [PPV:VLP(PCV2)]. The invention also relates to application of the recombinant virus and recombinant PPV VP2 nucleocapsids of the expression Cap genes of the recombinant virus in the aspects of bacterin immunity and so on.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
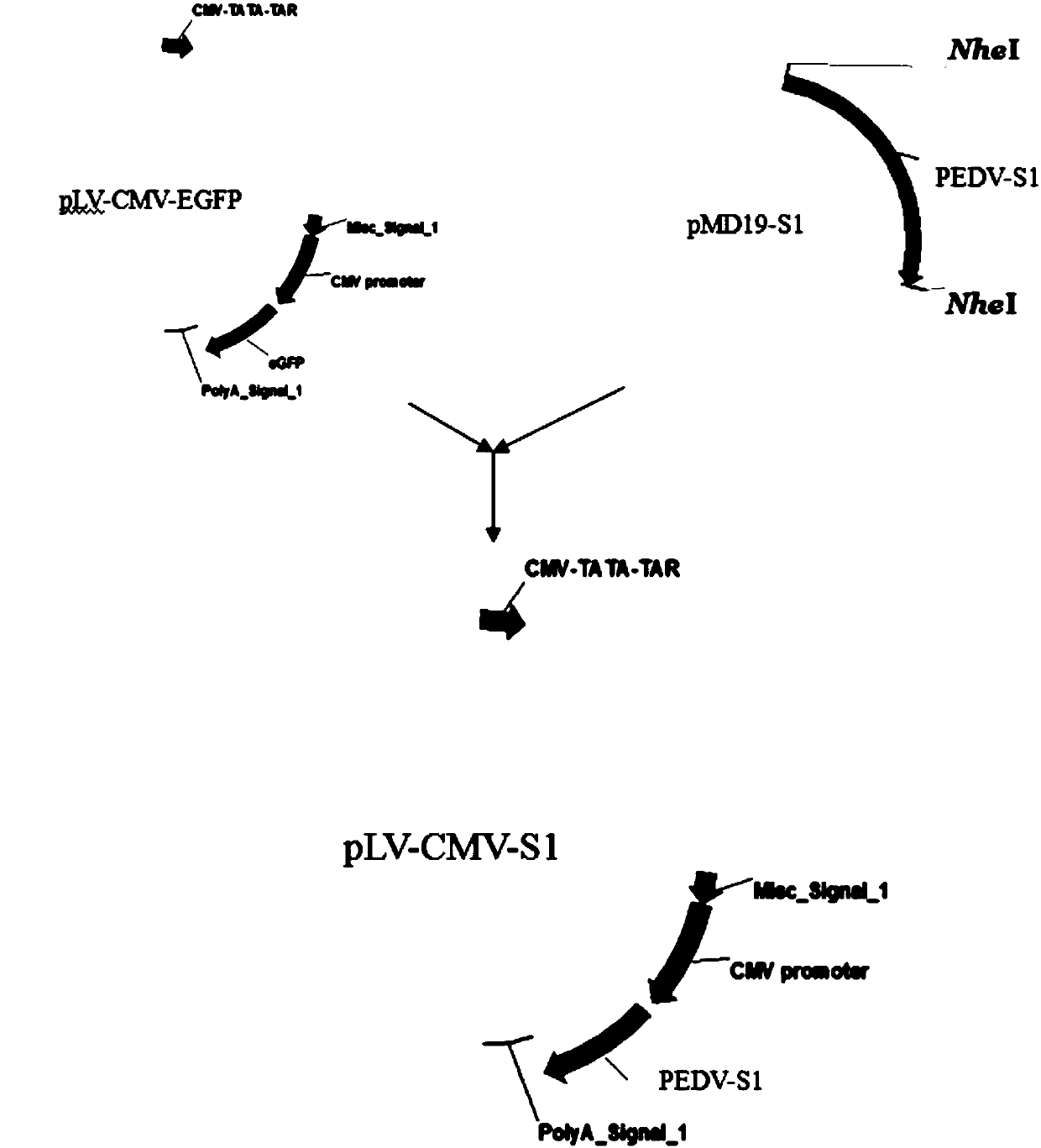
Recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, vaccine and application
InactiveCN107619819AEase of mass productionGood antigenicityAntiviralsAntibody medical ingredientsEpidemic diarrheaAdjuvant
The invention discloses a recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, a vaccine and an application. The recombinant cell line is constructed by transfecting HEK-293T cells by virtue of recombinant plasmid which is constructed by carrying a target gene on a lentiviral vector, and then transfecting the HEK-293T cells by virtue of generated high-titer virus particles; and the recombinant cell line, which can achieve stable expression, can still keep an excellent protein expression level after several passages. The recombinant cell line for stable expression of the porcine epidemic diarrhea virus S1 protein provided by the invention has the characteristics of being easy for culture, rapid in proliferation, unlimited in expansion, stable in property and high in protein expression amount; and when the vaccine, which is prepared from the expression protein and adjuvants, is used for immunizing pigs, the generation of a high-titer porcine epidemicdiarrhea virus neutralizing antibody can be induced from animal bodies, and the piglets (the pigs) can resist strong attack of porcine epidemic diarrhea viruses.
Owner:GUANGZHOU BONIZZI BIOTECH CO LTD
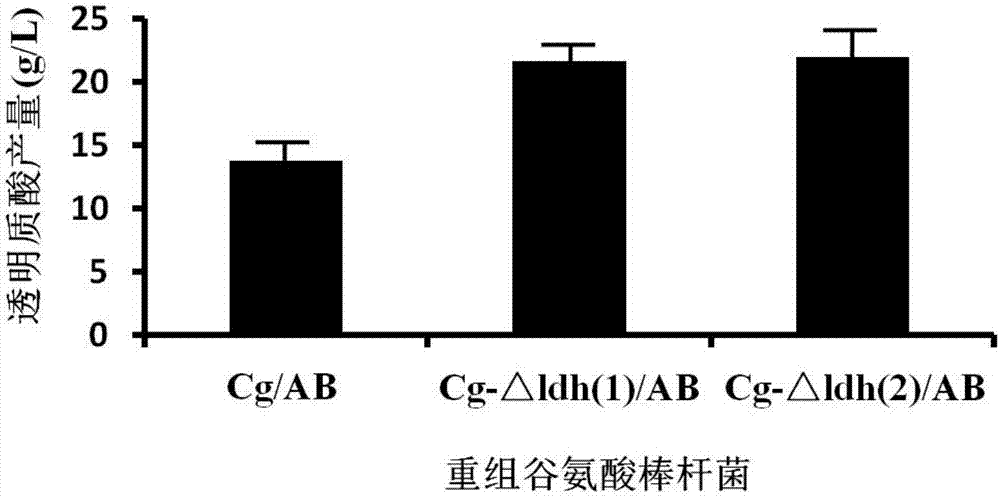
Genetically-engineered bacterium realizing high production of hyaluronic acid and application thereof
ActiveCN103937734AIncrease productionNon-pathogenicBacteriaMicroorganism based processesMicroorganismNucleotide
The invention discloses genetically-engineered bacterium realizing high production of hyaluronic acid and application of the genetically-engineered bacterium, belonging to the field of biotechnology and biochemical industry. The genetically-engineered bacterium is formed by transforming corynebacterium glutamicum by use of a recombinant vector containing a hyaluronic acid synthetase gene, and the nucleotide sequence of the hyaluronic acid synthetase gene is as shown in a sequence table SEQ ID No:1. The genetically-engineered bacterium is used for producing hyaluronic acid, high-yield hyaluronic acid can be obtained by independently expressing hyaluronic acid synthetase, and CgHasB and CgHasC do not become choke points which limit the high production of recombined bacterium. According to the method provided by the invention, a corynebacterium glutamicum host is free of pathogenicity to both humans and animals and is food-grade safe microorganism, the yield of the synthesized hyaluronic acid is high and above 6.0g / L, so the genetically-engineered bacterium has good industrial application prospect.
Owner:TSINGHUA UNIV
Bacillus pumilus shou002 and application thereof
InactiveCN103937701AEnhanced inhibitory effectNon-pathogenicBacteriaAnimal feeding stuffEcological environmentZoology
The present invention provides Bacillus pumilus shou002 and an application thereof, wherein the Bacillus pumilus shou002 is preserved in the China Center for Type Culture Collection on November 3, 2013, has the preservation number of CCTCC NO:M2013536, provides good inhibition effects for aquatic pathogenic bacteria, has characteristics of no pathogenicity, safety, reliability, effective degradation of organic matters, ammonia nitrogen, nitrite nitrogen and other pollutants in breeding water bodies, breeding environment optimizing, and promotion of water environment ecology beneficial cycle, and can be used as the feed additive and the water body ecology environment modifier so as to be used in aquaculture.
Owner:SHANGHAI OCEAN UNIV
Actinomycete capable of inhibiting phytophthoramelonis and screening method thereof
InactiveCN103013852AGood application prospectNon-pathogenicBiocideBacteriaLaboratory cultureColletotrichum
The invention relates to actinomycete capable of inhibiting phytophthoramelonis and a screening method of the actinomycete. Streptomyces purpeofuscus with preservation number CGMCC No.6191 is preserved in China general microbiological culture collection center. The streptomyces purpeofuscus is safe, nontoxic and effective antagonistic actinomycete capable of inhibiting phytophthoramelonis. The actinomycete has no pathogenicity to people and livestock, can be safely used for biological prevention and control of phytophthora melonis katsura and also has certain inbibitional effect on pyricularia grisea, phytophthora capsici leonian, phytophthora parasitica var nicotianae and colletotrichum glecosporioides. At present, few reports on phytophthoramelonis antagonistic bacteria are few in China, particularly reports on inhibiting action of the streptomyces purpeofuscus on phytophthoramelonis do not exist, and therefore, the antagonistic strain has a good application prospect.
Owner:HUNAN AGRICULTURAL UNIV
Gene engineering bacterium for high-yield hyaluronic acid and construction method and application of gene engineering bacteria
PendingCN107354119AIncrease productionNon-pathogenicBacteriaMicroorganism based processesLactate dehydrogenaseGene engineering
The invention discloses a gene engineering bacterium for high-yield hyaluronic acid and a construction method and application of the gene engineering bacteria, and belongs to the field of gene engineering and biochemical engineering. The gene engineering bacterium for high-yield hyaluronic acid is constructed through the steps that a lactate dehydrogenase gene in corynebacterium glutamicum is inactivated, and then a hyaluronan synthase gene is transferred into the corynebacterium glutamicum. According to the gene engineering bacterium for high-yield hyaluronic acid, a corynebacterium glutamicum host has no pathogenicity to both humans and animals and is a food-grade safe microorganism; and the recombinant corynebacterium glutamicum for high-yield hyaluronic acid is high in hyaluronic acid yield which is up to 22g / L or above and three times the level of existing industrial production, and a new bacterial strain has good industrialization application prospects.
Owner:TSINGHUA UNIV
Biological bactericide for controlling paddy rice diseases
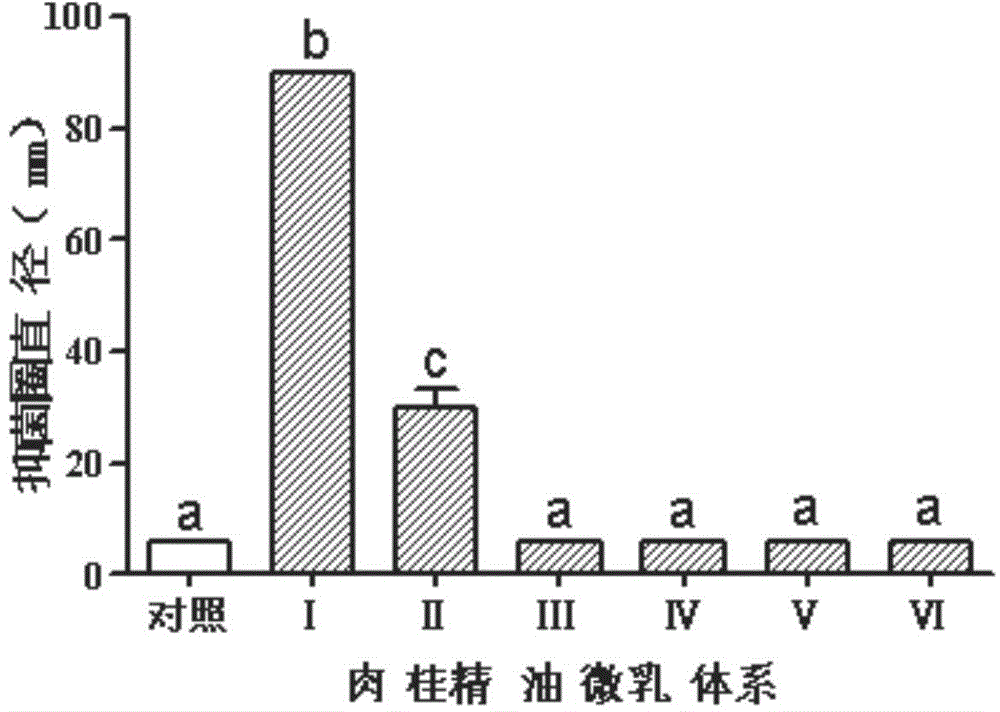
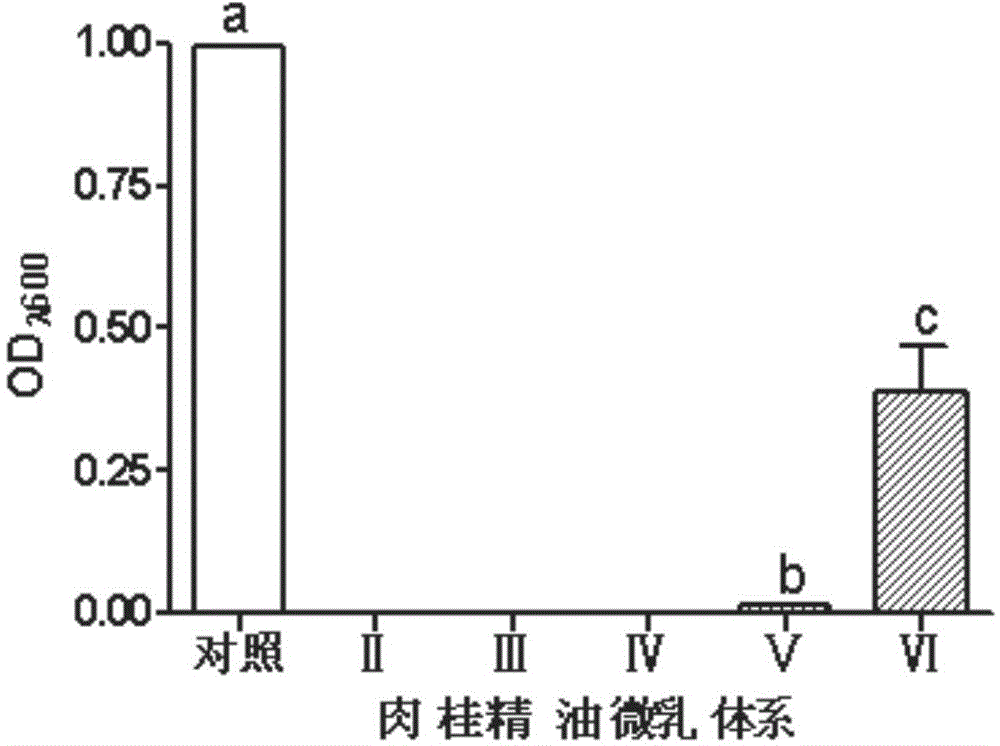
A disclosed biological bactericide for controlling paddy rice diseases employs cinnamon essential oil as the active component. Specifically, the biological bactericide is composed of cinnamon essential oil, a surfactant, a co-emulsifier and water, and per Kg of the biological bactericide contains 0.75-75000 mg of cinnamon essential oil, 1.75-175000 mg of the surfactant, 0.75-75000 mg of the co-emulsifier and the balance water. The biological bactericide is mainly used for controlling xanthomonas oryzae pv.oryzae.
Owner:海宁市袁花镇工业投资有限公司
Grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine
InactiveCN107760716AInducible propertiesImproving immunogenicityViral antigen ingredientsAntiviralsEscherichia coliDisease
The invention discloses a grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine, and belongs to the technical field of gene engineering and molecular immunology. The preparation method disclosed by the invention is characterized by comprising the steps of extracting viral genome RNA, performing reverse transcription to convert the viral genome RNA into cDNA, amplifying a corresponding DNA sequence out, constructing the DNA sequence to pcDNA-3.1(+) plasmid, converting Escherichia coli DH5alpha, screening out positive clone bacteria containing recombinant plasmid, culturing a lot of the positive bacteria and extracting recombinant plasmid S11-pcDNA3.1 contained in the bacteria. The recombinant plasmid is utilized as nucleic acid vaccine to perform intramuscular injection on the grass carps, and the nucleic acid vaccine enters the muscle cells and expresses VP35 protein of the grass carp reovirus in the muscle cells; thus, fish body immune cell proliferation is stimulated, antiviral related gene expression is up regulated, fish bodies are stimulated to generate antiviral antibodies, and capability of grass carps in resisting grass carp reovirus infection is effectively improved; furthermore, the grass carp reovirus S11 gene eukaryotic expression recombinant plasmid can be used for preventing a grass carp hemorragic disease caused by the grass carp reovirus in aquaculture.
Owner:HENAN NORMAL UNIV
Irpex lacteus LL210, application thereof and biocontrol microbial agent
The invention relates to irpex lacteus LL210, application thereof and a biocontrol microbial agent. The irpex lacteus provided by the invention is classified and named as Irpex lacteus, and the preservation number of the Irpex lacteus is CGMCC No.21057. A strain is separated from tomato leaves, and is non-toxic and free of pathogenicity. A plate confrontation test shows that the strain can effectively inhibit mycelial growth and spore germination of tomato botrytis cinerea and tomato late blight, and can be used for antagonizing plant pathogenic fungi tomato botrytis cinerea and / or phytophthora. The biocontrol microbial agent prepared from the irpex lacteus LL210 is used for preventing and treating tomato diseases, the prevention and treatment effects on tomato botrytis cinerea and late blight reach 81.35% and 87.68%, the biocontrol microbial agent has a good disease prevention effect and good compatibility with the environment, and a new microbial resource is provided for biological prevention and treatment of diseases of plants such as tomatoes.
Owner:HENAN UNIV OF SCI & TECH
Recombinant BHK cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the same in preparation of vaccines and diagnosis reagents of classical swine fever
ActiveCN103751773AFully emulsifiedAvoid infectionAntiviralsBiological testingStructural proteinMicrobiological culture
The present invention discloses a recombinant cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the recombinant cell line in preparation of vaccines and diagnosis reagents of classical swine fever, wherein the recombinant cell line is BCSFV-E012, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7720. In addition, the present invention further discloses an establishment method for the cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and a method for preparing a classical swine fever prevention vaccine composition by using the cell line. The present invention further discloses applications of the E0-E1-E2 protein stably expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents. The classical swine fever vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no classical swine fever virus non-structural protein antibody production so as to identify the vaccinated animal and the virus infected animal.
Owner:HARBIN WEIKE BIOTECH DEV +1
Influenza B virus Vero cell productive adaptive strain and preparation and application thereof
ActiveCN101619306AImprove protectionOvercome deficienciesInactivation/attenuationMicroorganism based processesTiterInfluenza vaccine
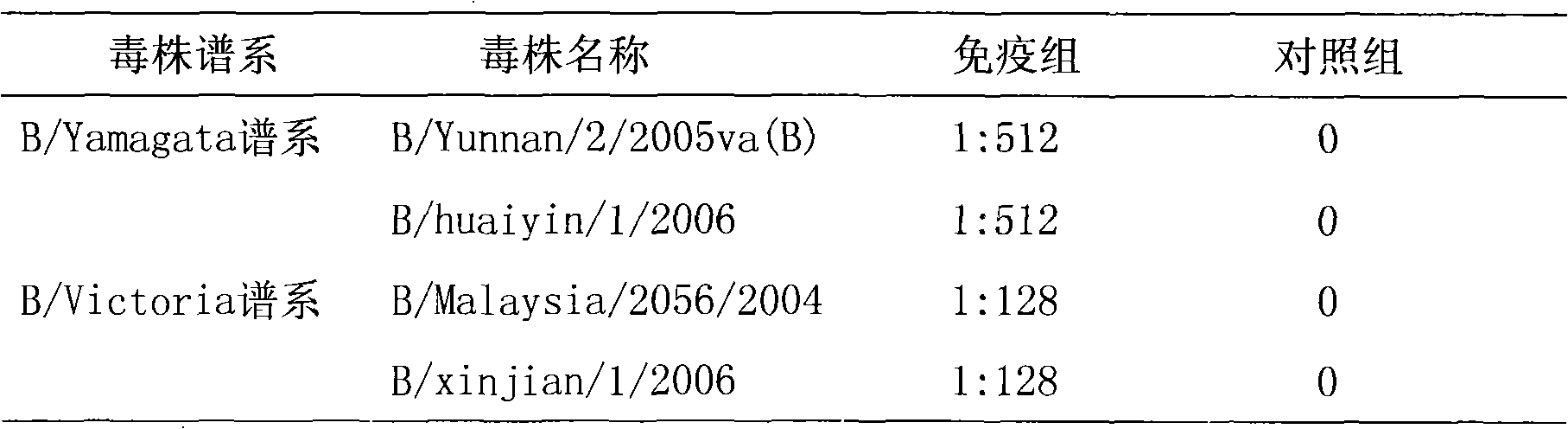
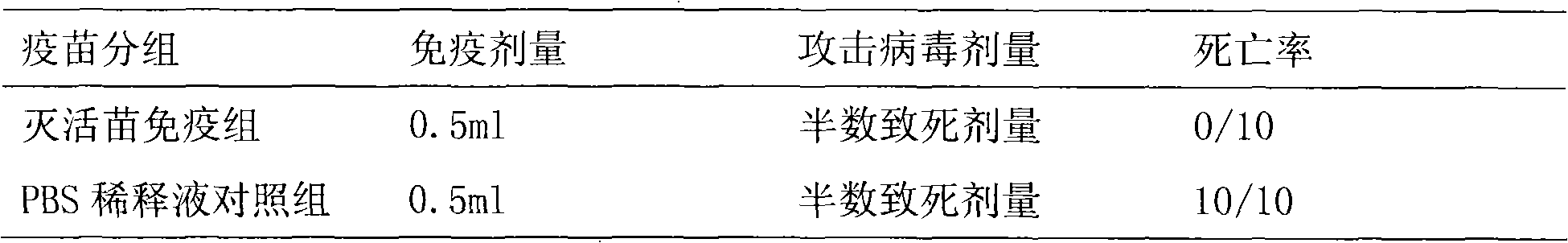
The invention provides an influenza B virus Vero cell productive adaptive strain and a preparation method and application thereof. The influenza B virus Vero cell productive adaptive strain is named as B / Yunnan / 2 / 2005va(B) with a preserved number of CGMCC No.2931. The virus strain can be continuously subcultured on the Vero cell, and a clotting titer can be kept over 1: 512. Through detecting, the virus strain is the influenza B virus, belonging to a Yamagata pedigree. The virus strain includes eight genetic fragments of the influenza B virus and has the characteristic of continuously producing the Vero cells. On the one hand, the virus strain is inoculated in the Vero cell, the supernate of a culture is collected to obtain a influenza B virus vaccine which has favorable immunogenicity and proactive effects through an experiment; on the other hand, the virus strain also can be used as a donor virus strain which is reassorted through a natural selective pressure or a reverse genetics to obtain the adaptive strain of an epidemic strain on the Vero cell, thereby an epidemic strain Vero cell influenza vaccine is prepared.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Plant endophyte and application thereof
ActiveCN103224900APromote rice growthIncreased available zinc contentBiocidePlant growth regulatorsSedum alfrediiFertilizer
The invention discloses a plant endophyte with a category name of Enterobacter sp. and a complete name of Enterobacter sp. SaCS20. The strain is preserved at China General Microbiological Culture Collection Center (CGMCC) on March 28th, 2013, and has a preservation number of CGMCC NO: 7381. The invention also discloses the application of the plant endophyte in promoting paddy rice growth, improving paddy rice yield, or promoting trace element zinc accumulation in paddy rice. The Enterobacter sp. CGMCC 7381 provided by the invention is separated from the root system of a zinc hyperaccumulator sedum alfredii. The plant endophyte has no pathogenicity upon paddy rice, and can be successfully colonized into paddy rice root systems. With the plant endophyte, paddy rice growth can be promoted, rhizosphere soil available zinc content can be increased, and grain zinc enrichment can be promoted. The application provided by the invention is simple to operate, and is safe and economical. With the plant endophyte and the application, soil micronutrient fertilizer investment can be reduced, and crop yield can be increased.
Owner:ZHEJIANG UNIV
Application of inhibin recombinant fusion protein to preparing medicines for promoting oestrus and hybridization of sows
InactiveCN102166348ANo toxicityNon-pathogenicPeptide/protein ingredientsRecombinant DNA-technologyMedicinePharmaceutical drug
The invention discloses an application of an inhibin recombinant fusion protein to preparing medicines for promoting oestrus and hybridization of sows. When inhibin is used for the sows: 0.5-1mg of the inhibin recombinant protein is used for initial immunization; and after 15-21 days, 0.25-0.5mg of the inhibin recombinant protein is used for second immunization. The immune inhibin can increase the estrus rate and the litter sizes of the sows. The invention has obvious effects on overcoming anestrus of the sows, caused by heat stress in summer, promoting the breeding activities of breeding sows, and particularly on promoting oestrus of breeding sows and back-up sows and increasing litter sizes. Thus, the non-productive time of the breeding sows is shortened, the breeding performance of thebreeding sows is improved, and the economic benefit of sow raising is increased.
Owner:SOUTH CHINA AGRI UNIV
Cyprinid herpesvirus II DNA vaccine based on baculovirus vector as well as building method and application thereof
ActiveCN108728490ANon-pathogenicHigh biosecurityViral antigen ingredientsVirus peptidesDiseaseDna transfection
The invention relates to a cyprinid herpesvirus II DNA vaccine based on a baculovirus vector as well as a building method and application thereof. Codons of regional sequences 1-186, 993-1197, 603-783and 85-186 nt of cyprinid herpesvirus II ORF72, ORF66, ORF81 and ORF82 are optimized; then, ORF72-ORF66-ORF81-ORF82 fusion sequences are chemically synthesized; next, the promoter control is performed; the clone into the baculovirus transfer vector pFSATBacTMDual is performed to build recombinant plasmids pFSATBacTMDual-FA-D4ORF; after the plasmids are used for converting DH10 / Bac competent cells, Bacmid-FA-D4ORF is obtained; after the Bacmid-FA-D4ORF DNA is used for transfecting silkworm culture cells, BmNPV-FA-D4ORF is obtained and is the cyprinid herpesvirus II DNA vaccine based on the baculovirus vector. The DNA vaccine can realize the immune on carassius auratus gibelio by using an injection or oral administration or soaking method; the gill bleeding diseases of the carassius auratusgibelio can be reduced.
Owner:苏州培恩特生物科技有限公司
Lactobacillus gasseri and application thereof to preparation of fermented milk
The invention belongs to the field of fermented milk products, and discloses lactobacillus gasseri and application thereof to preparation of fermented milk. A strain is the lactobacillus gasseri LGZ 1029 which is preserved in the Guangdong Microbial Culture Collection Center, short for GDMCC on April 16, 2019, and the strain preservation number is GDMCC No:60641. The probiotic lactobacillus gasseri LGZ 1029 is used as a fermentation agent, and compounded with a traditional fermented milk fermentation stain to conduct fermented milk liquid fermentation, and the lactobacillus gasseri fermented milk is prepared. A process is simple, the fermentation time is greatly shortened, the prepared fermented milk is moderate in acidity and high in viable count, no whey is separated out, better rheological properties and water-retaining capability are achieved, the taste is stick slip and delicate, aroma components are strong, and the viable count is still 10<7> CFU / mL or above after storage at 4 DEG C for 28 d.
Owner:SOUTH CHINA UNIV OF TECH
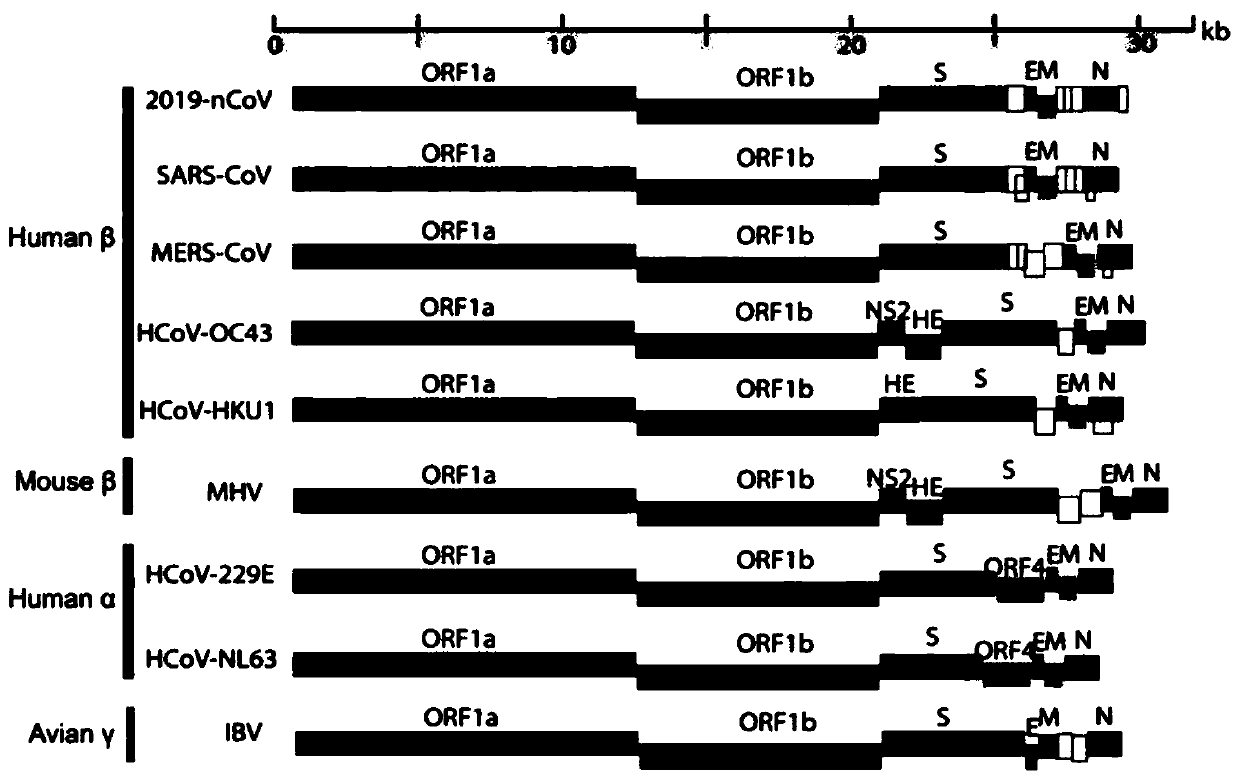
Pseudo-virus standard substance for nucleic acid diagnosis of novel coronavirus 2019-nCov and application thereof
PendingCN111378785AMeet the performance evaluation index requirementsOvercome the problem of CT value judgmentSsRNA viruses positive-senseMicrobiological testing/measurementGene synthesisBiological safety
The invention relates to a pseudo-virus standard substance for nucleic acid diagnosis of novel coronavirus 2019-nCov and application thereof. The pseudo-virus standard substance is prepared through the steps of gene synthesis, plasmid extraction, pseudo-virus packaging and the like. Wherein the synthesized gene comprises a novel coronavirus 2019-nCov gene RdRp, a Gene E and a Gene N; the 2019-nCovpseudo-virus standard substance is provided for the first time, is different from RNA synthesized in vitro and clinical positive living virus in the past, perfectly overcomes the defects of RNA and clinical positive living virus, is really suitable for performance evaluation of test kit, comprises limit of detection, specificity, repeatability and the like, and is further applied to clinical diagnosis of 2019-nCov; the pseudo-virus standard substance has the advantages of no pathogenicity, reproducibility, reliable quality control method and stable batch-to-batch, can be stably prepared and supplied for a long time, requires the biological safety level of P2 in a laboratory, and meets the safety requirements of many units.
Owner:仁宽(上海)生物科技有限公司
Method for constructing genetic engineering strains for producing (R)-acetoin and application of genetic engineering strains
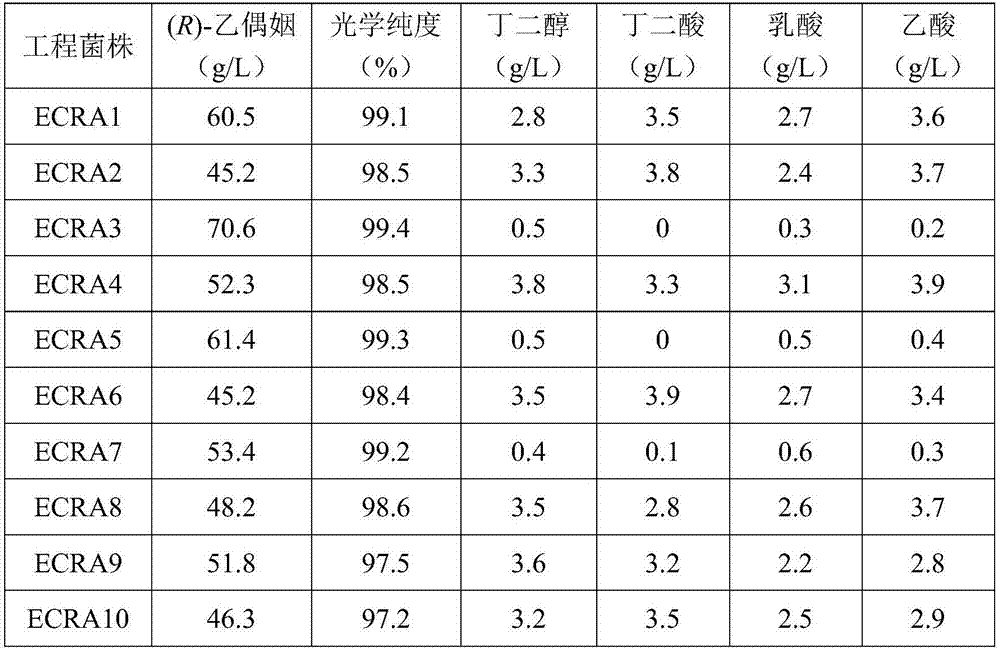
ActiveCN107129959AReduce pathogenicityNon-pathogenicBacteriaTransferasesBiotechnologyAcetolactate synthase
The invention discloses a method for constructing genetic engineering strains for producing (R)-acetoin and application of the genetic engineering strains. The method includes optimizing codons of nucleotide sequences of alpha-acetolactate synthase genes, alpha-acetolactate decarboxylase genes and NADH (reduced form of nicotinamide adenine dinucleotide) oxidase genes and acquiring each gene cluster with three genes by the aid of artificial synthesis processes; inserting the gene clusters into expression vectors to obtain polycistron recombinant plasmids; introducing the polycistron recombinant plasmids into host bacteria E. coli and knocking out key genes of main byproduct synthesis paths to obtain the genetic engineering strains for producing the (R)-acetoin. The method and the application have the advantages that raw materials for the genetic engineering strains can come from wide sources and are low in cost, the strains are free of pathogenicity, oxidized form coenzymes NAD+ (nicotinamide adenine dinucleotide+) can be effectively regenerated, the strains are high in (R)-acetoin yield and production efficiency, the maximum yield can reach 72.1 g / L, and the optical purity can reach 99% at least; the (R)-acetoin is produced by the aid of non-grain cassava flour and inexpensive nitrogen sources which are used as fermentation raw materials, and accordingly the production cost can be reduced.
Owner:GUANGXI ACAD OF SCI
Method for preparing cyprini herpesvirus II antigen coated polyhedrosis based on baculovirus expression system
ActiveCN106834352AImprove biological activityReduce manufacturing costAntibody mimetics/scaffoldsVirus peptidesAntigenStructural protein
The invention relates to the technology of antigen protein expression, and particularly relates to a method for preparing a cyprini herpesvirus II antigen coated polyhedrosis based on a baculovirus expression system. According to the method, by designing and recombining bombyx mori nuclear polyhydrosis virus BmNPV-VP3-cyHV-polh, 1-186, 993-1197, 603-783 and 85-186 regional sequences of ORF72, ORF66, ORF81 and ORF82 and the coding sequence of the 1-279 region of a VP3 gene of bombyx mori cytoplasmic polyhedrosis virus structural protein are connected in series to form a fused sequence which is controlled by baculovirus P10 promoter; and the bombyx mori cytoplasmic polyhedrosis protein gene is controlled by a polyhedrin gene promoter of baculovirus. The virus is used for inoculating bombyx mori or bombyx mori culture cells, the recombinant virus expressed cyprini herpesvirus II antigen protein can be coated in bombyx mori cytoplasmic polyhedrosis; the formed polyhedrosis can be purified by simple differential centrifugation; the purified polyhedrosis is cracked under a basic condition, the polyhedrosis protein can be precipitated by centrifuging, and the cyprini herpesvirus II antigen is reserved in supernatant, so that the cyprini herpesvirus II antigen can be quickly and conveniently obtained.
Owner:苏州培恩特生物科技有限公司
Bacillus amyloliquefaciens and preparation method of exopolysaccharides thereof
ActiveCN106520641ANon-irritatingAntigenicBacteriaMicroorganism based processesChromatographic separationBacillus amyloliquefaciens
The invention provides bacillus amyloliquefaciens EZ99. The bacillus amyloliquefaciens is gram positive bacteria, exists in plant rhizosphere soil, has no pathogenicity for people and plants, is collected from an orchard county, in the west of a Qilihe district in the Lanzhou city of the Gansu province, and has been identified, registered and preserved in the China common microorganism strain preservation center, and the preservation number is CGMCC No.13267. Component analysis is performed on bacterial colonies formed by the bacillus amyloliquefaciens on a PMA and MS culture medium, and it is found that the bacillus amyloliquefaciens has the characteristic of synthesizing and secreting exopolysaccharides. Optimal culture medium formulas and fermentation optimal conditions based on fermentation production of the exopolysaccharides of the strains are built, a fermentation solution is subjected to centrifuging, alcohol precipitation, dialysis and chromatographic separation, four purification polysaccharide components are obtained, and four-polysaccharide-component monosaccharide composition and monomer molecular weight are determined. The exopolysaccharides obtained through the strain and the method have the various applications of medicines, cosmetics, food additives, biological film raw materials and the like.
Owner:甘肃尚农生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com