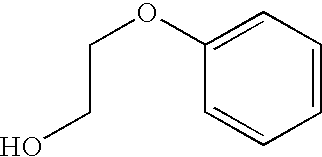
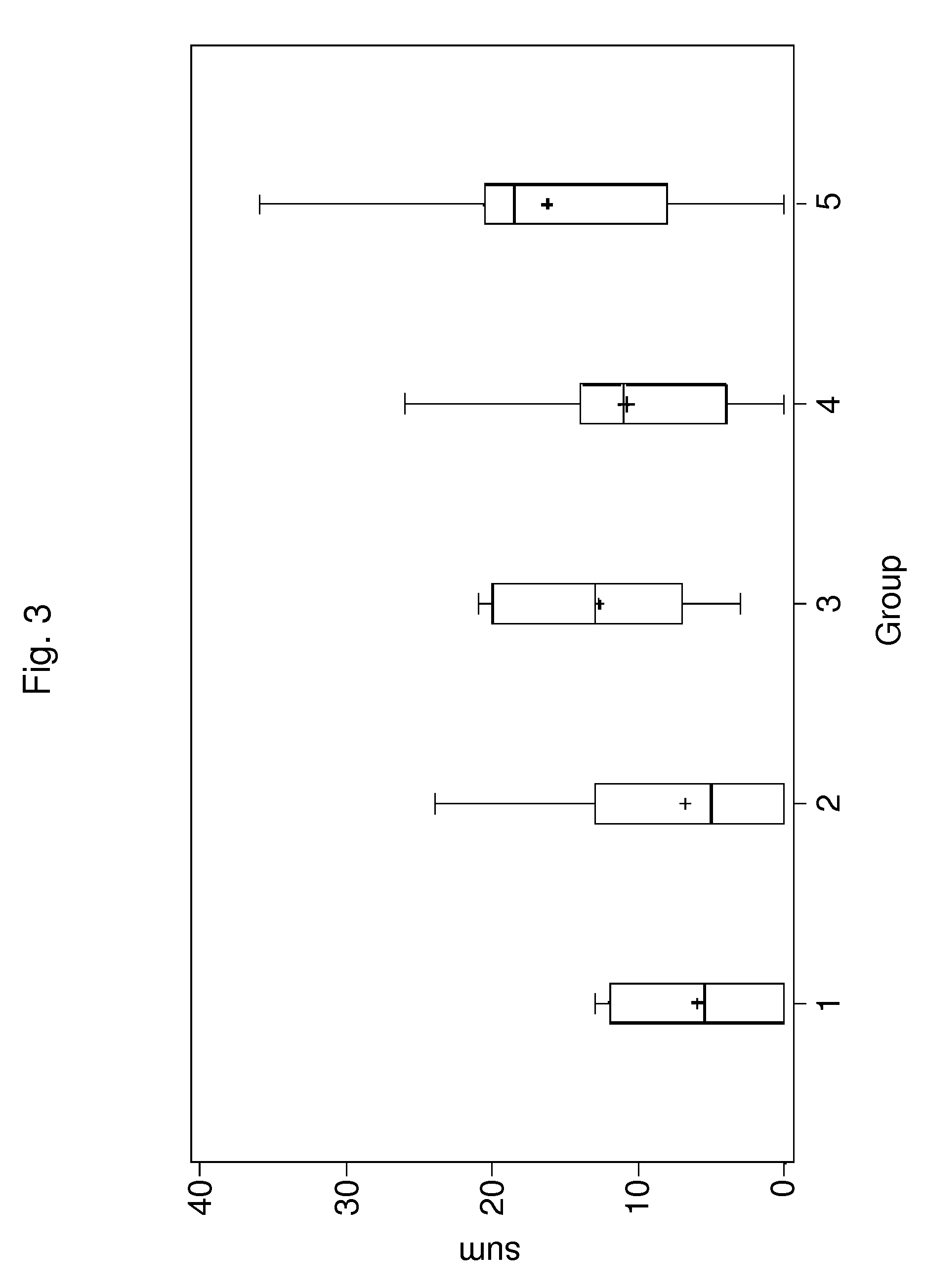
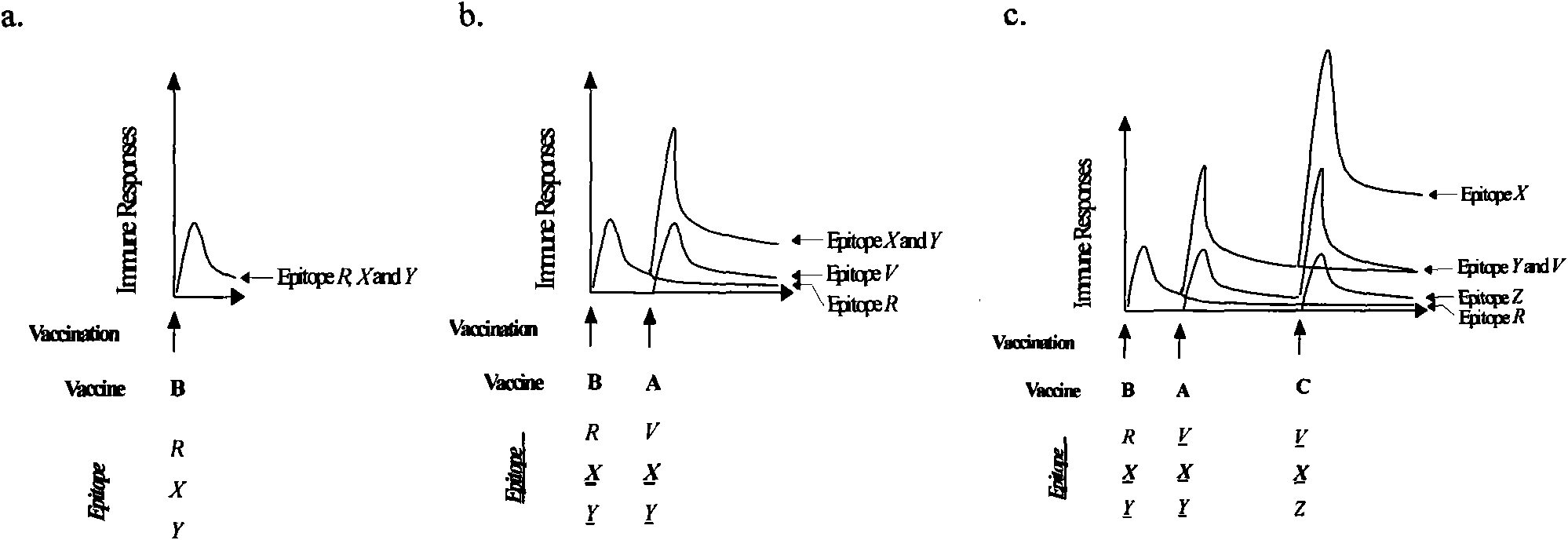
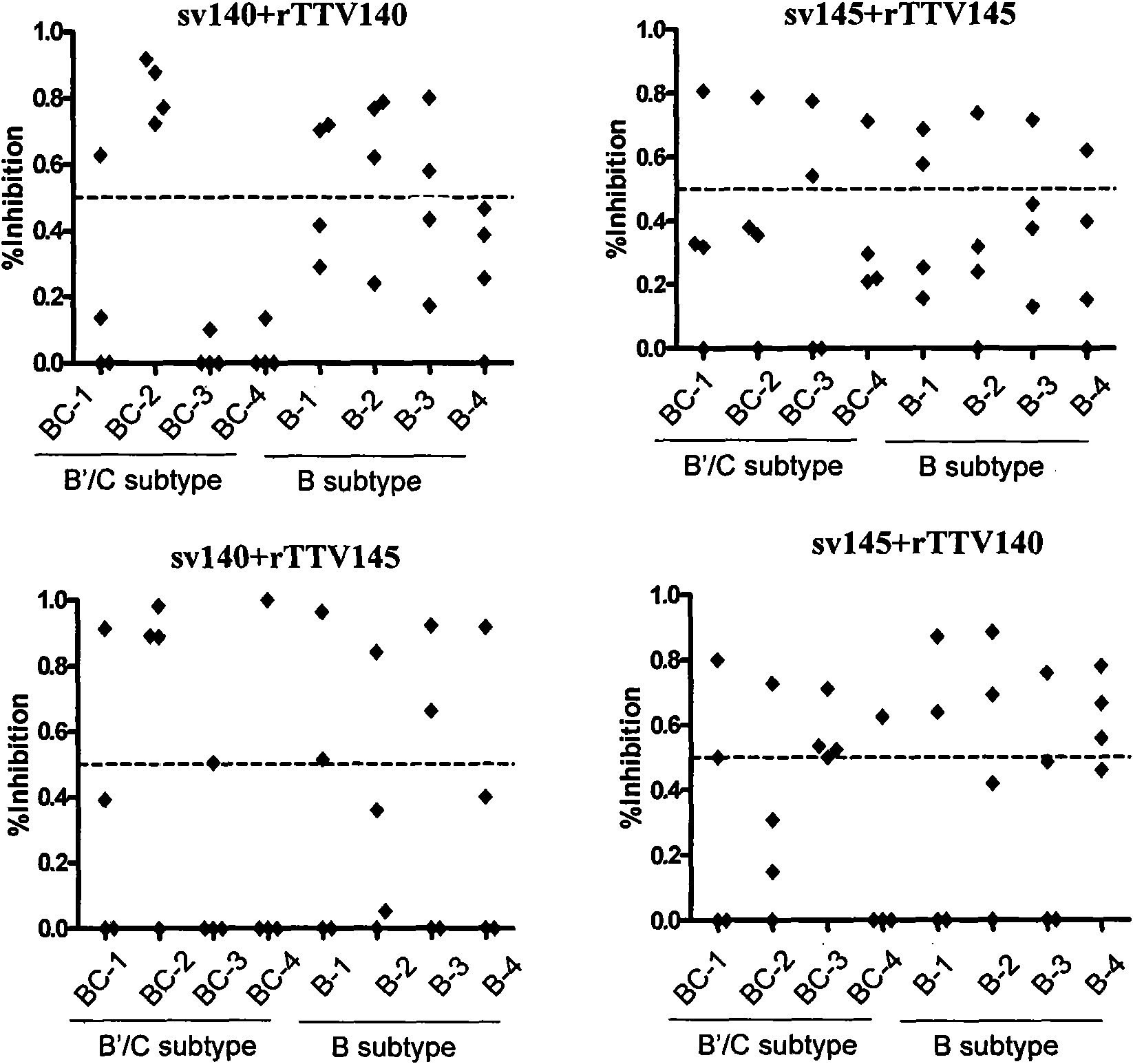
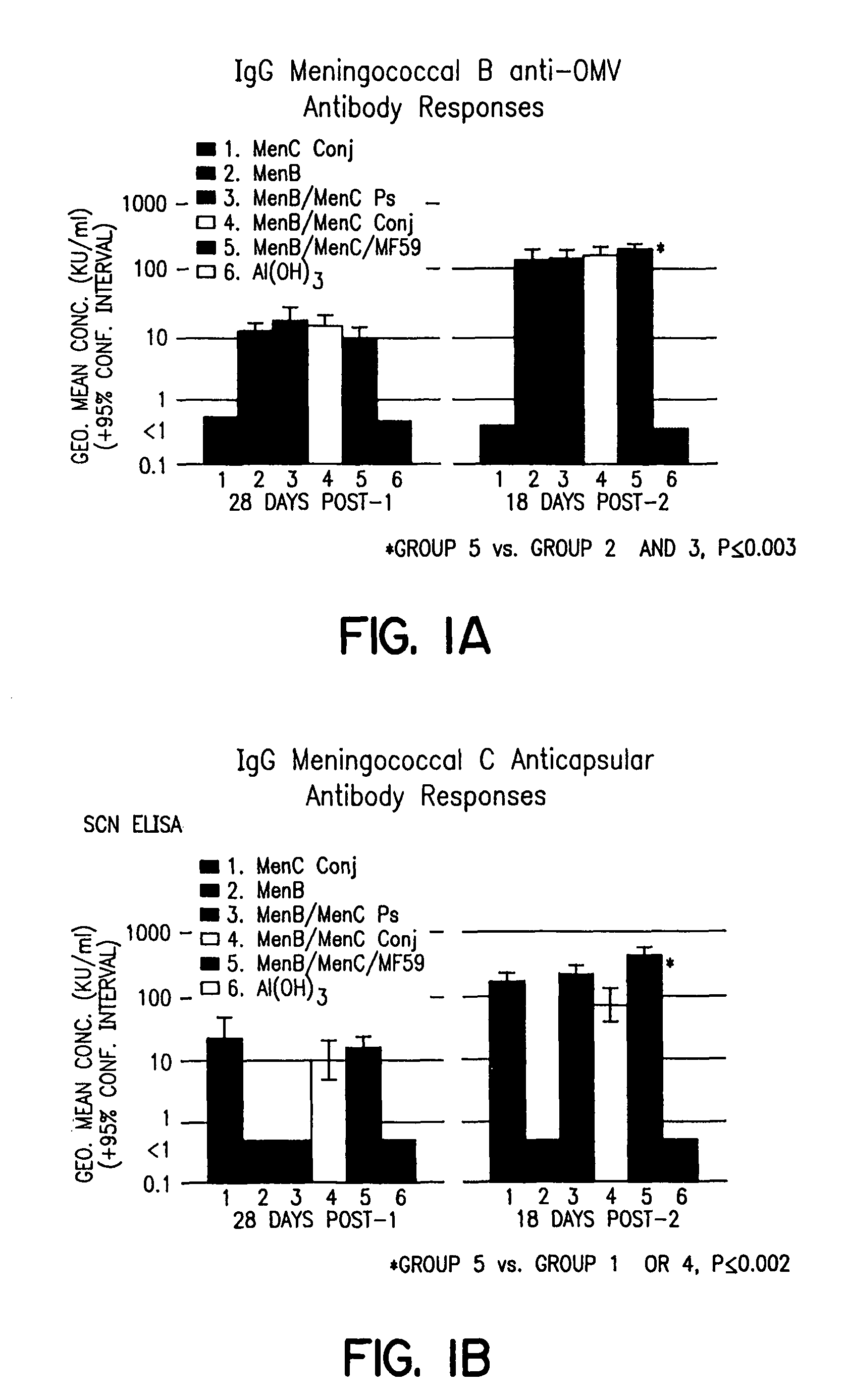
Patents
Literature
126 results about "Combination vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combination vaccines available for many years include diphtheria and tetanus toxoids and whole-cell pertussis vaccine (DTwP); measles-mumps-rubella vaccine (MMR); and trivalent inactivated polio vaccine (IPV).
Combination vaccine
ActiveUS20160166678A1SsRNA viruses negative-senseSugar derivativesHemagglutininFamily Orthomyxoviridae
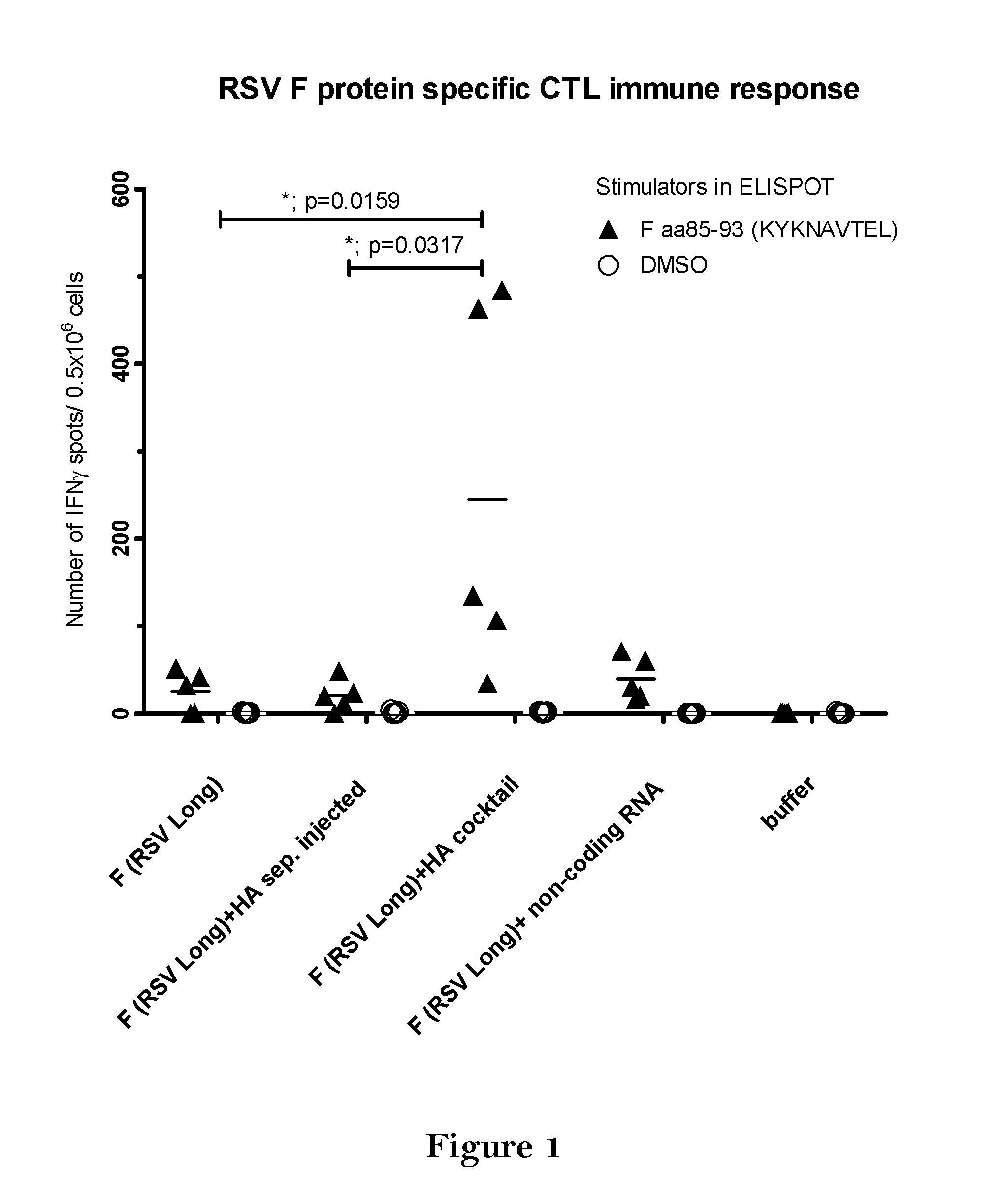
The present invention relates to a vaccine, especially a combination vaccine providing at least a first and a second antigenic function, the combination vaccine comprising at least one RNA encoding at least one or more proteins or fragments, variants or derivatives of proteins awarding antigenic function, wherein the first antigenic function being a Fusion (F) protein or a fragment, variant or derivative of a Fusion (F) protein derived from the virus family Paramyxoviridae and the second antigenic function being an Hemagglutinin (HA) protein or a fragment, variant or derivative of an Hemagglutinin (HA) protein derived from the virus family Orthomyxoviridae. Furthermore, the present invention is directed to a kit or kit of parts comprising the components of said combination vaccine and to said combination vaccine for use in a method of prophylactic or therapeutic treatment of diseases, particularly in the prevention or treatment of infectious diseases like RSV and influenza.
Owner:CUREVAC SE
Synthetic nanocarrier combination vaccines
Disclosed are dosage forms and related methods, that include a first population of synthetic nanocarriers that have one or more first antigens coupled to them, one or more second antigens that are not coupled to the synthetic nanocarriers, and a pharmaceutically acceptable excipient.
Owner:SELECTA BIOSCI
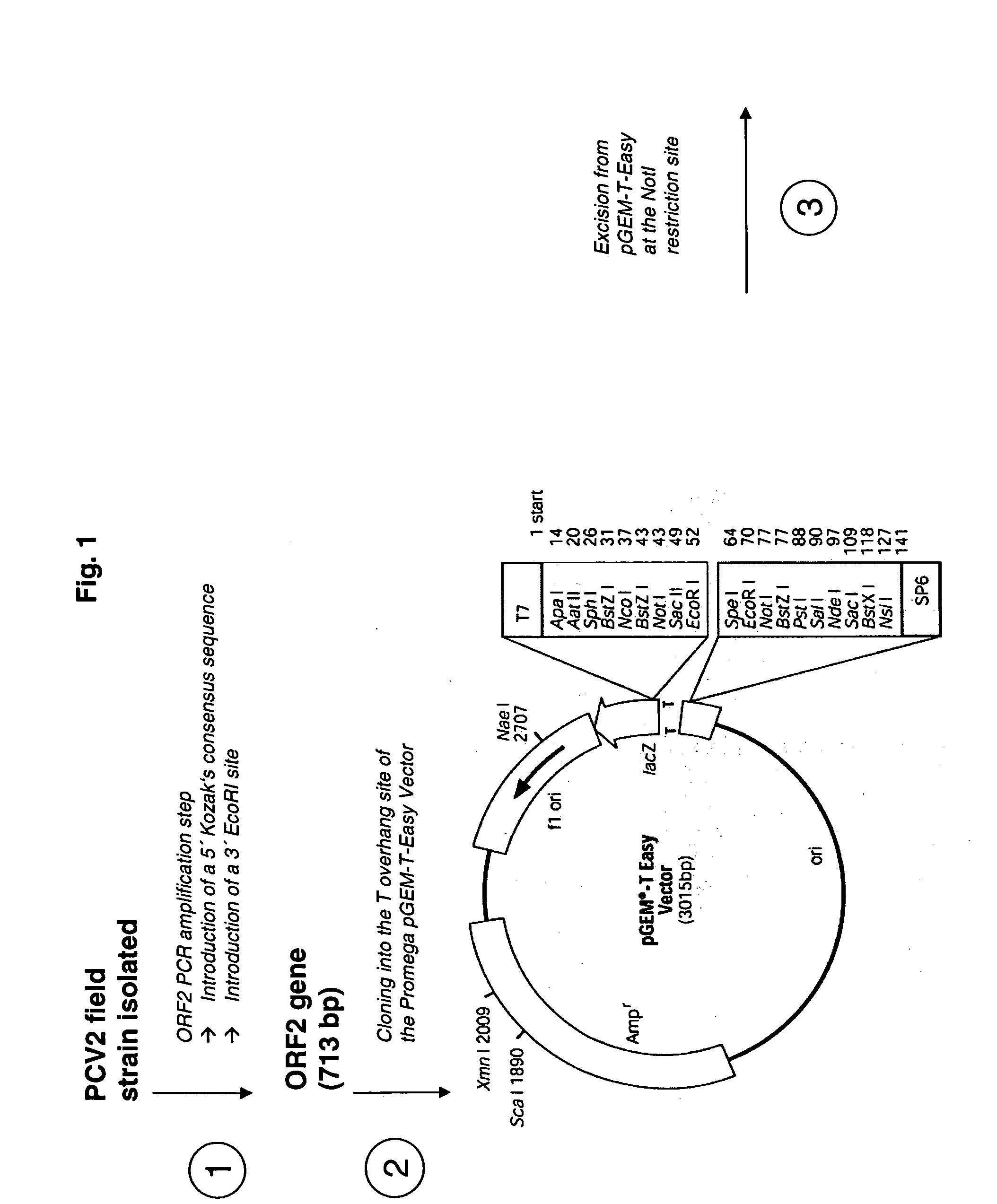
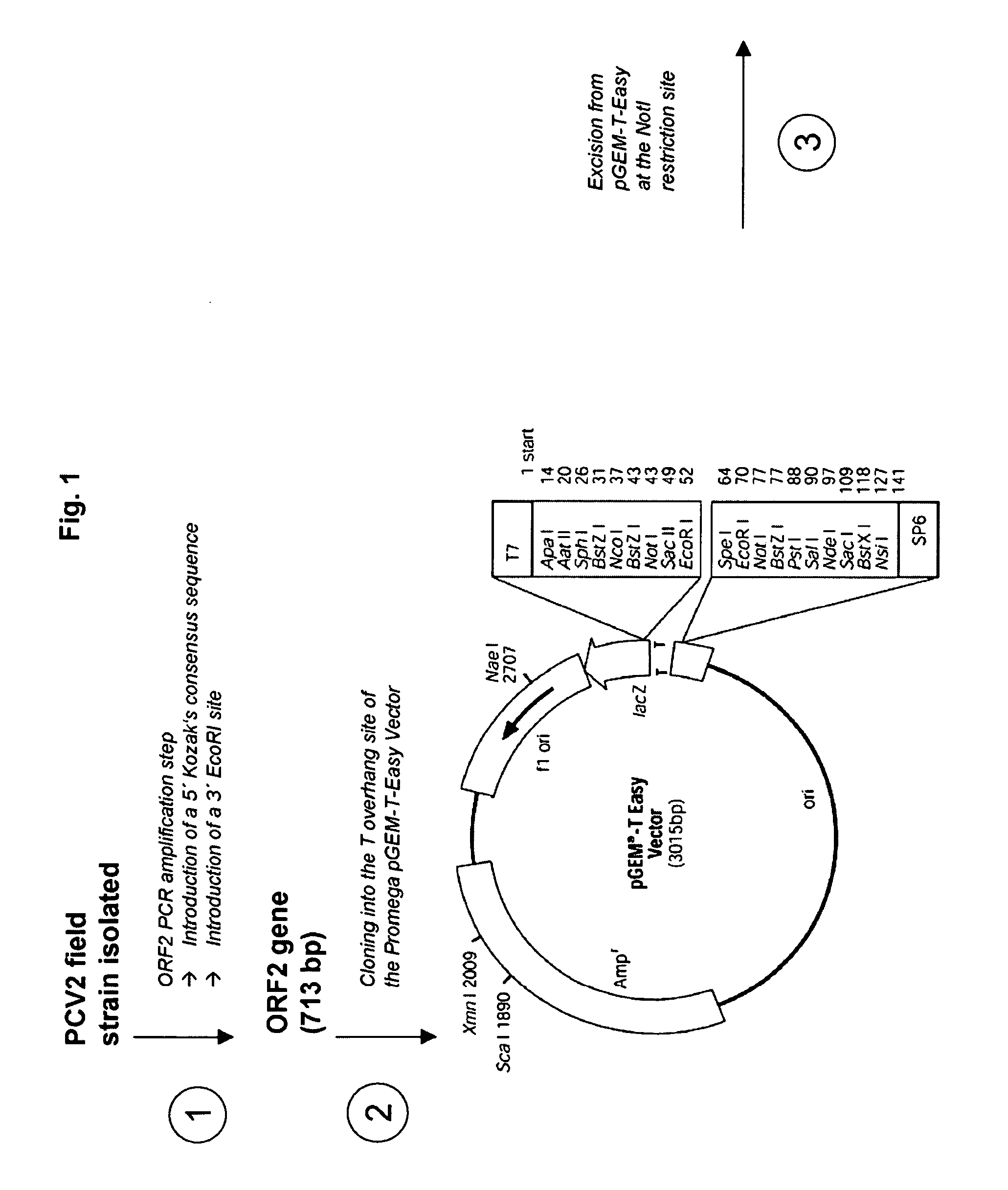
Porcine circovirus and Helicobacter combination vaccines and methods of use
InactiveUS20060029617A1Viral antigen ingredientsMicrobiological testing/measurementDiseasePorcine Circoviruses
The present invention is based on the discovery of novel species of the genus Helicobacter that are associated with gastro-esophageal ulceration in pigs. In particular, a novel species, H. cerdo, has been used as a source of antigenic material for the development of vaccine for the treatment of the gastro-esophageal disorders. Most advantageously, the novel Helicobacter and the porcine circoviruses associated with PMWS in pigs are useful for providing combination vaccines whereby immunogens derived from both types of pathogens may be codelivered to the target animal to stimulate the generation of protective antibodies and immunity. The invention, therefore, provides vaccines that are useful for the tratment of gastro-esophageal ulceration and PMWS in porcines. The present invention includes, therefore, multivalent immunogenic compositions and vaccines, multivaccine kits, and combined immunization or vaccination methods which make it possible to use such combined immunization or vaccination programmes.
Owner:MERIAL LTD
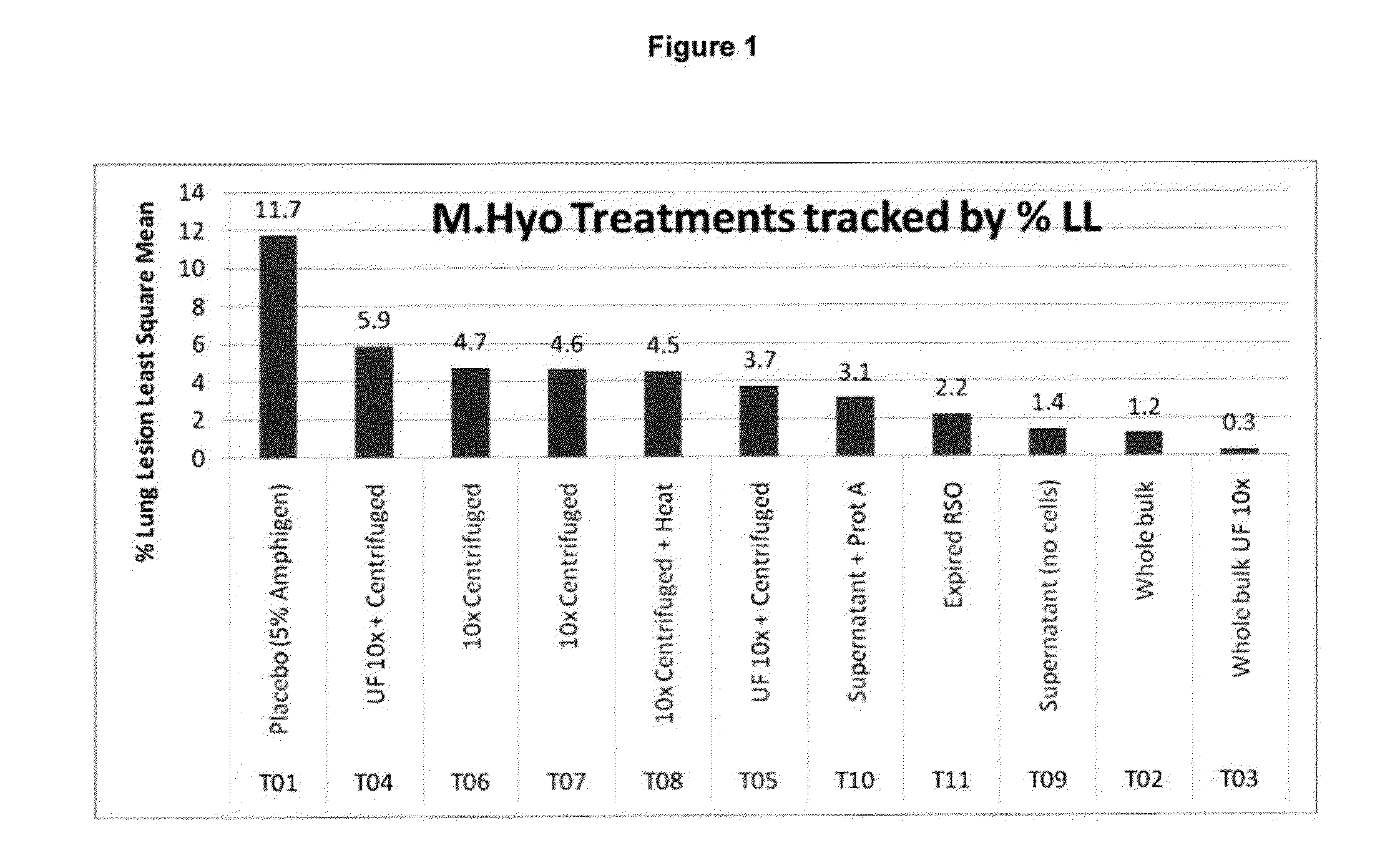
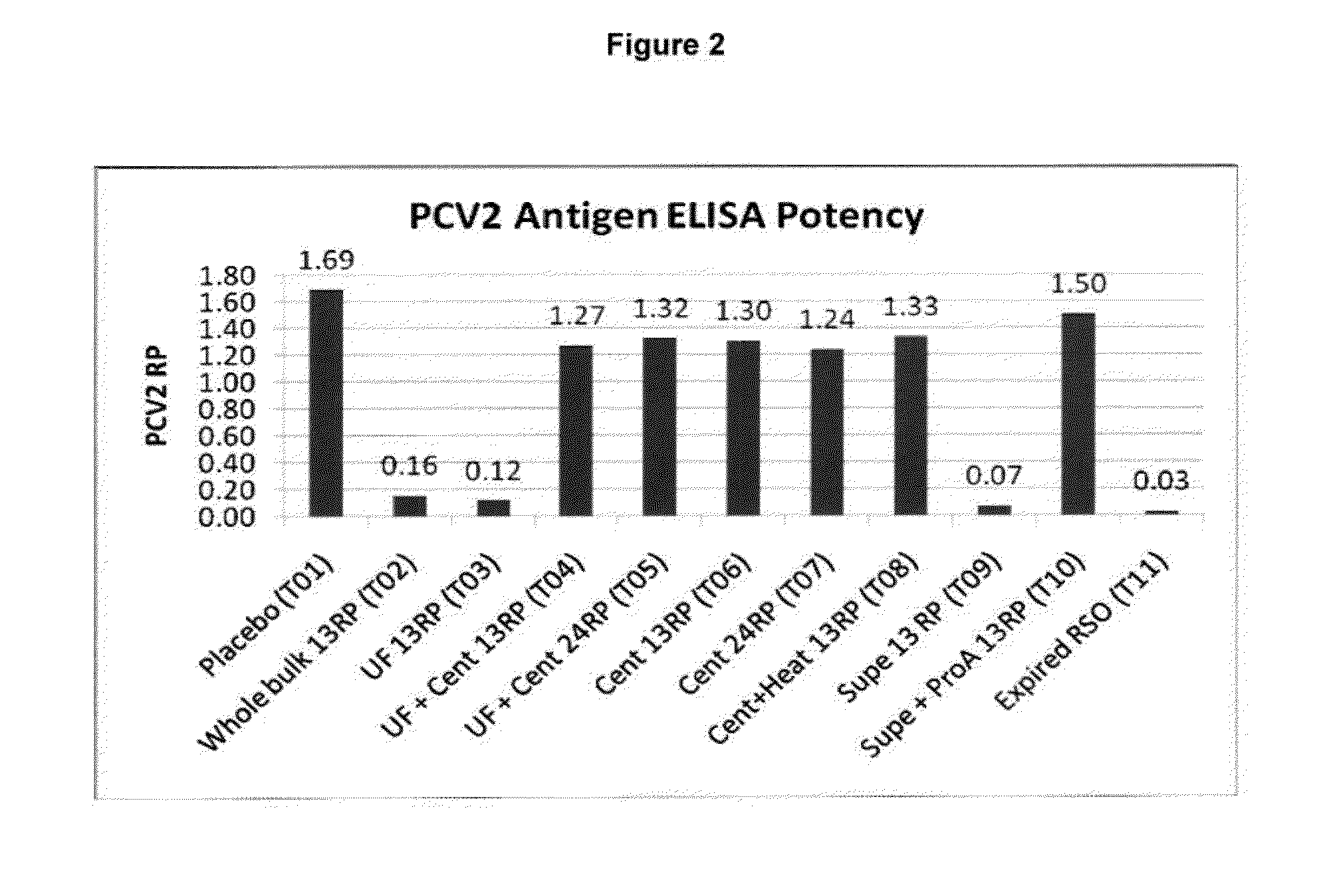
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
InactiveUS20090317423A1Reduce incidenceEffective immunityViral antigen ingredientsAntiinfectivesDiseaseActive component
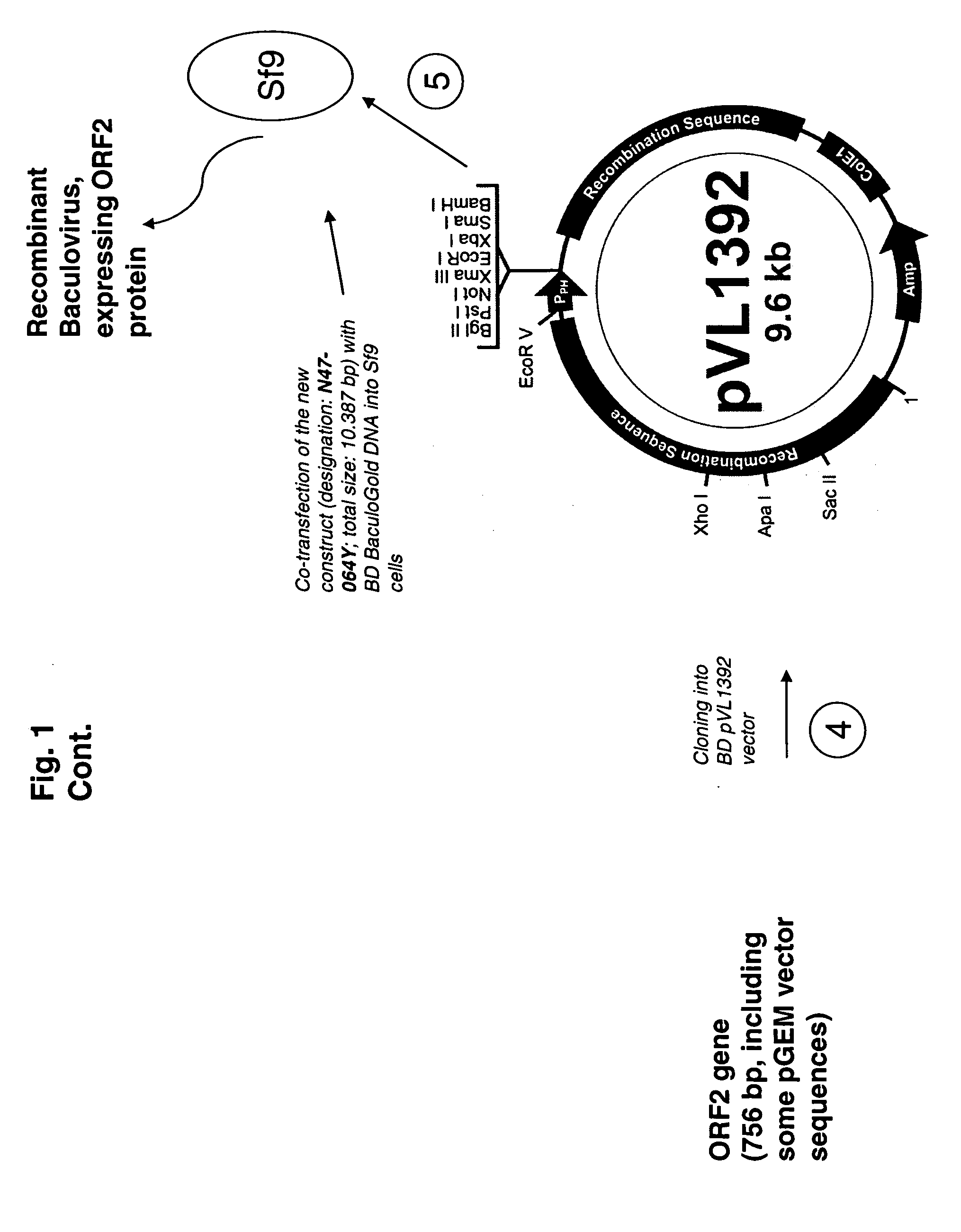
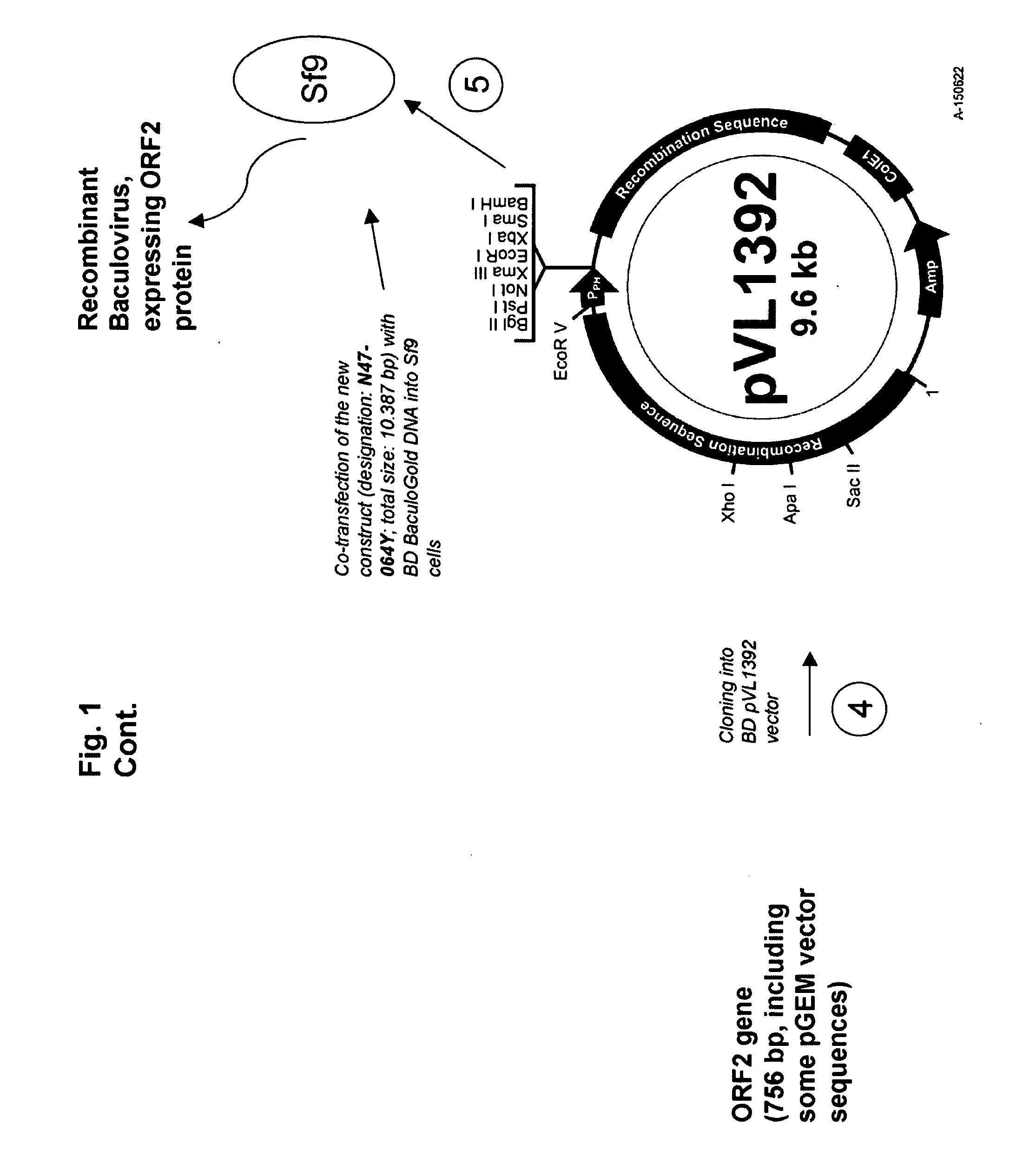
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
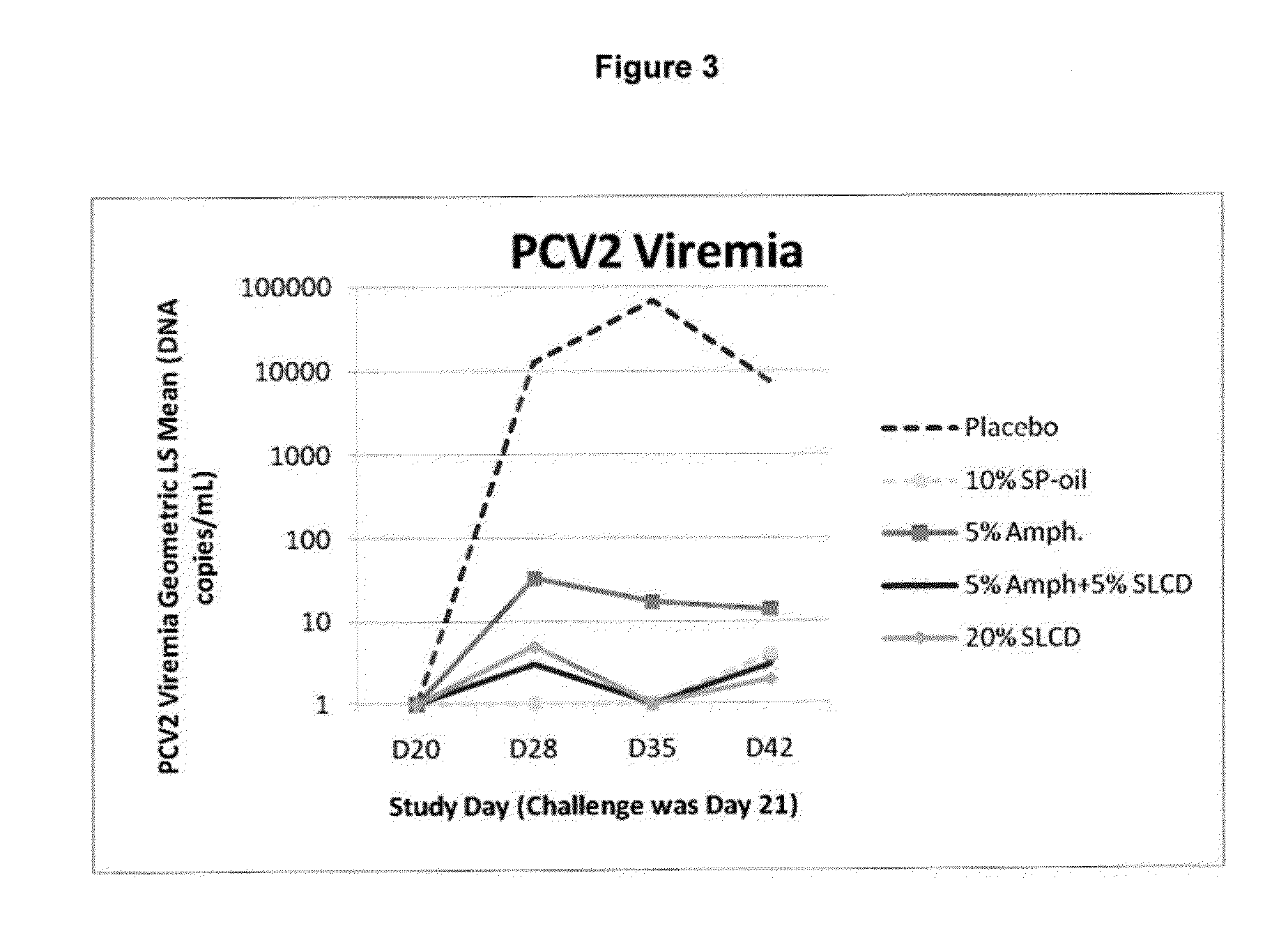
Multivalent pcv2 immunogenic compositions and methods of producing such compositions
InactiveUS20080226669A1Lowered viral titerImprove the immunityAntibacterial agentsCompounds screening/testingDiseaseOpen reading frame
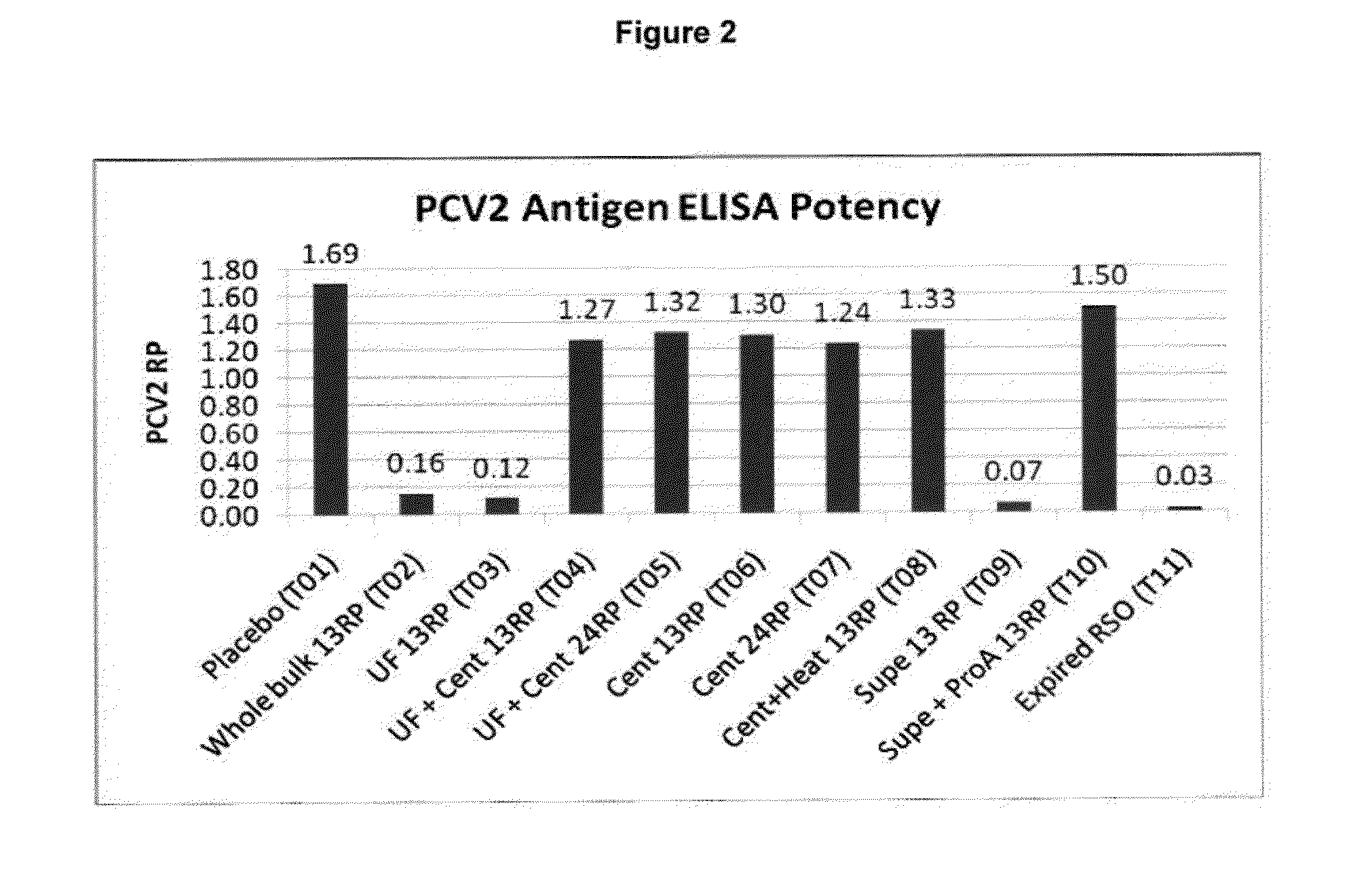
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. Also provided is recombinant PCV2 ORF2 protein, and immunogenic compositions comprising PCV2 ORF2 protein. Moreover, multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of PCV2 infection, preferably PCV2 ORF2 protein, or an immunogenic composition comprising PCV2 ORF2 protein, and at least one immunogenic active component of another disease-causing organism in swine,
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Poly saccharide-protein combination vaccine
InactiveCN1425465AImproving immunogenicityReduce the number of vaccinationsViral antigen ingredientsKetone active ingredientsMedicineNeisseria
Group A and Group C epidemuic cerebrospinal meningitis Neisseria capsular polysaccharide and type-B influenza Hemophilus polysaccharide are combined onto effective protein carrier chemically via covalent bond, so that combined polysacchairde-protein vaccine for immunologically inocualting human body is prepared to prevent infection caused by Group A and Group C epidemic cerebrospinal meningitis neisseria and type-B influenza Hemophilus.
Owner:BEIJING LUZHU BIOTECH +1
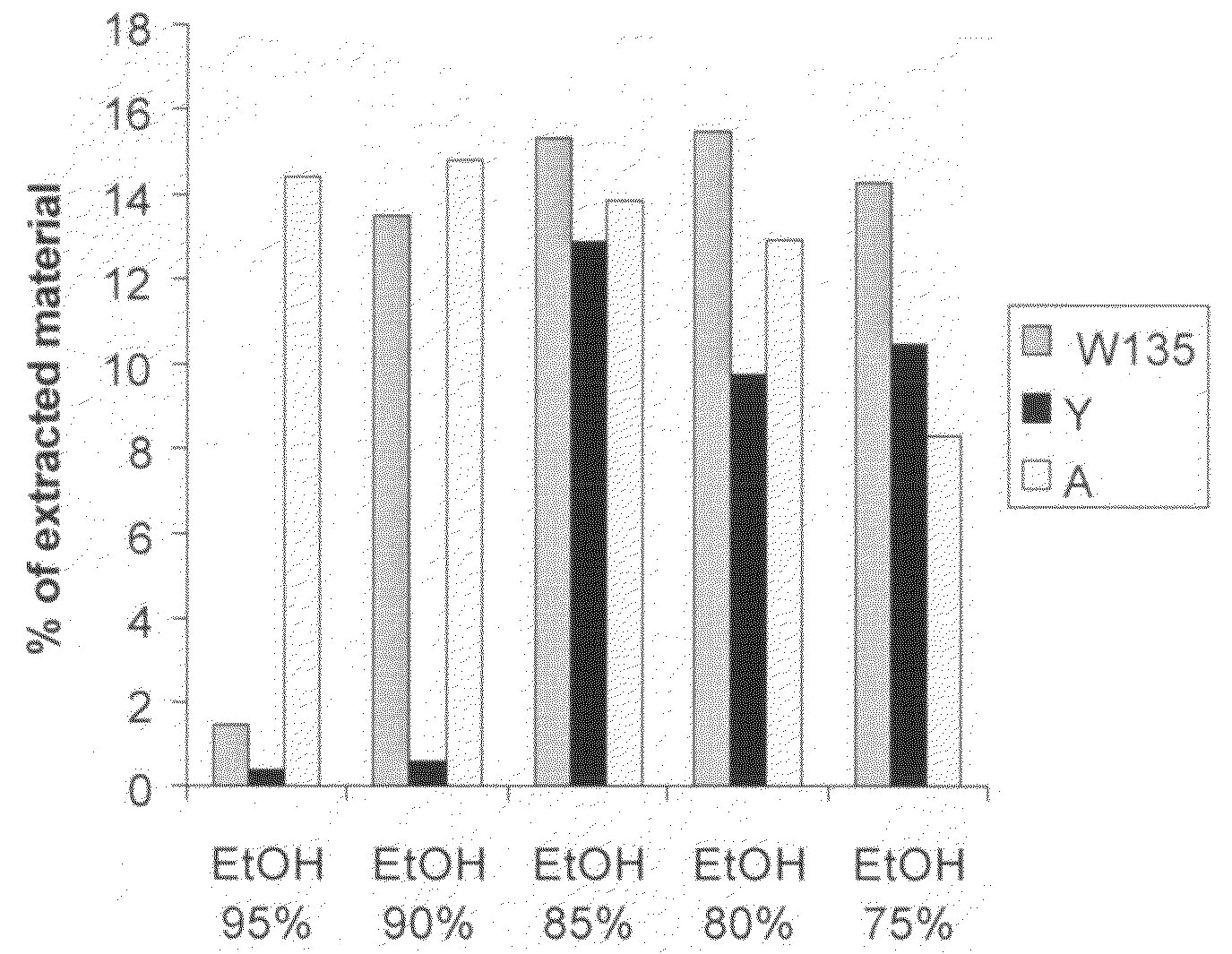
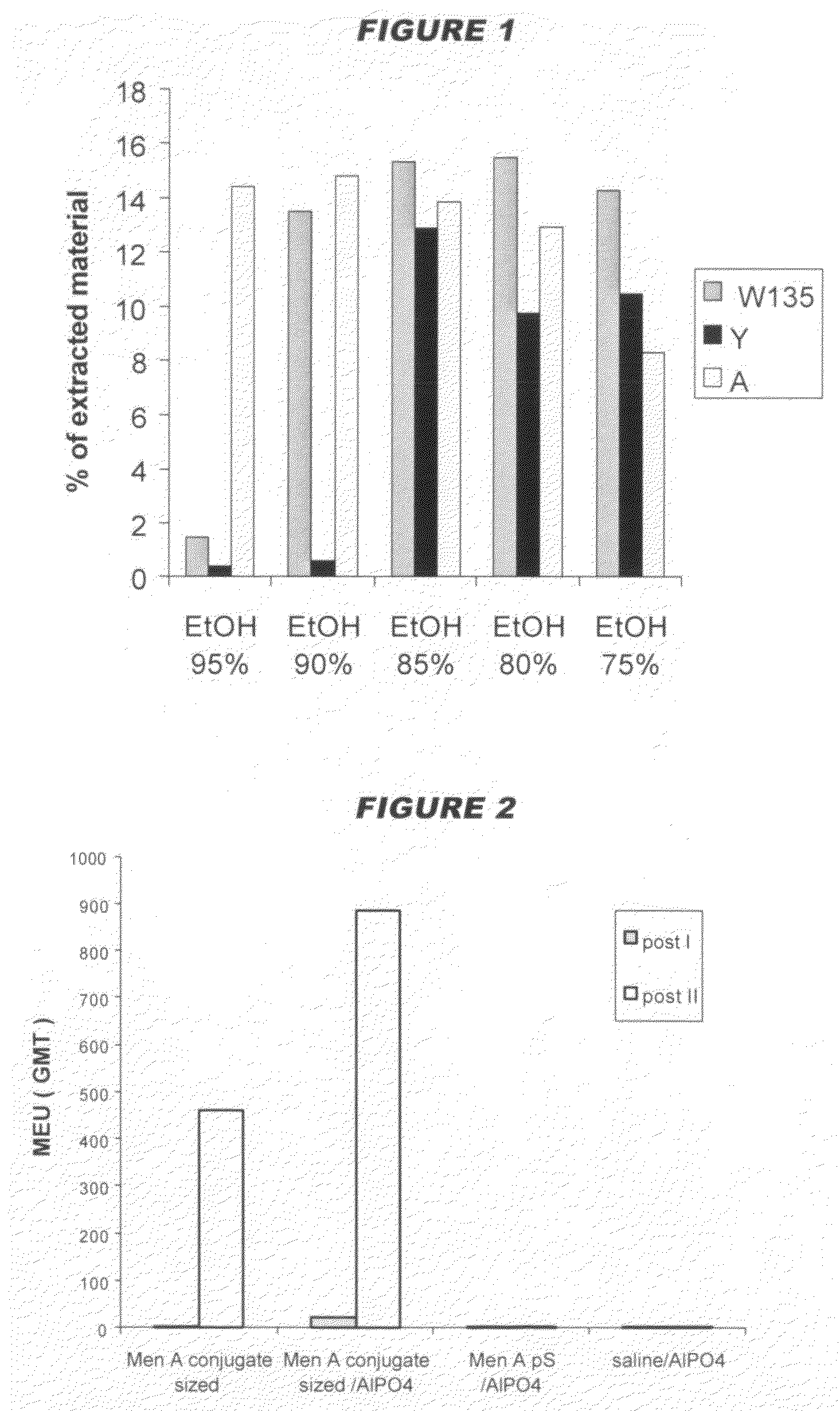
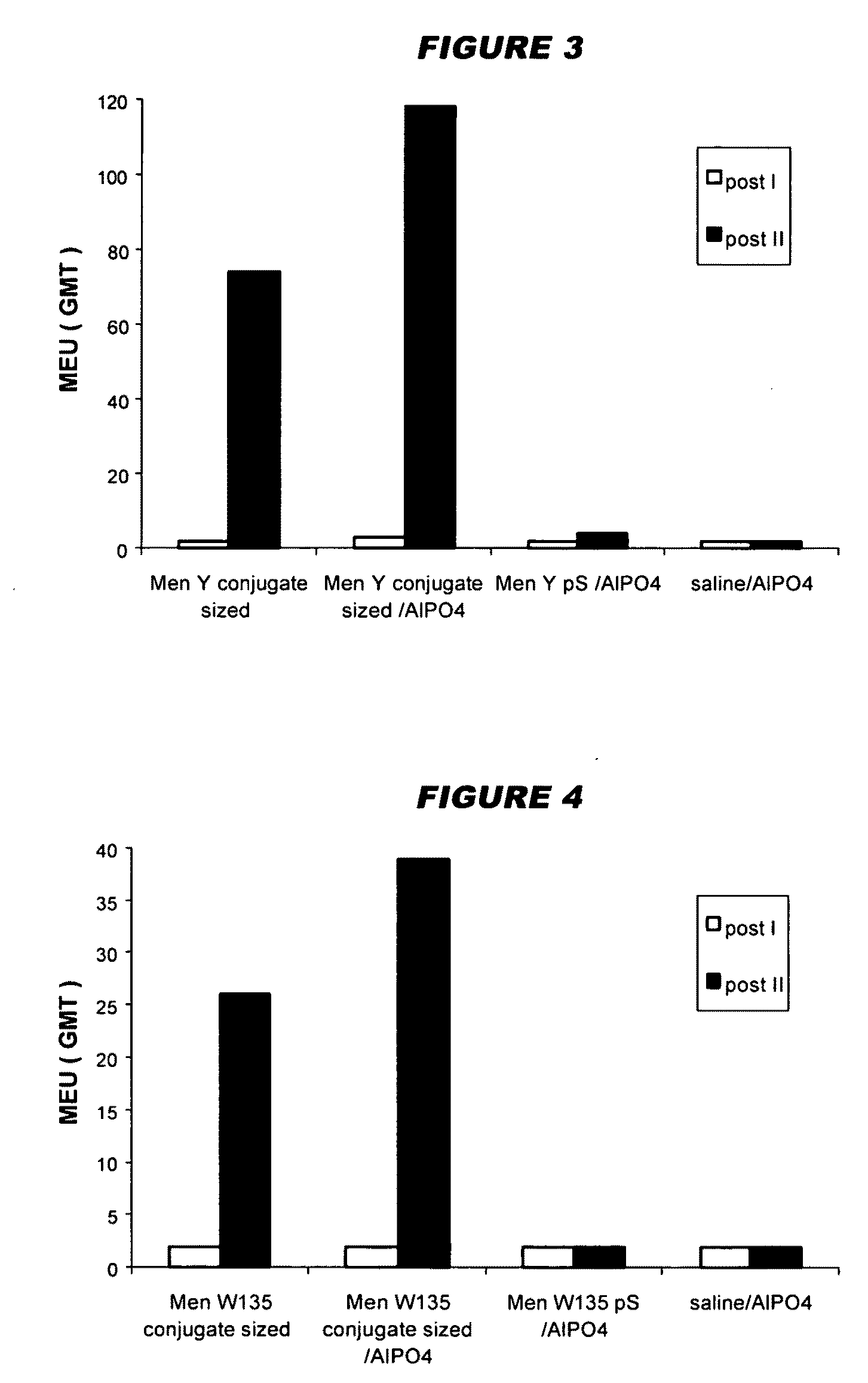
Capsular polysaccharide solubilisation and combination vaccines
ActiveUS20050106181A1Improving immunogenicityW13 dosProtein composition from fishAntibacterial agentsAlcohol ethylMicrobiology
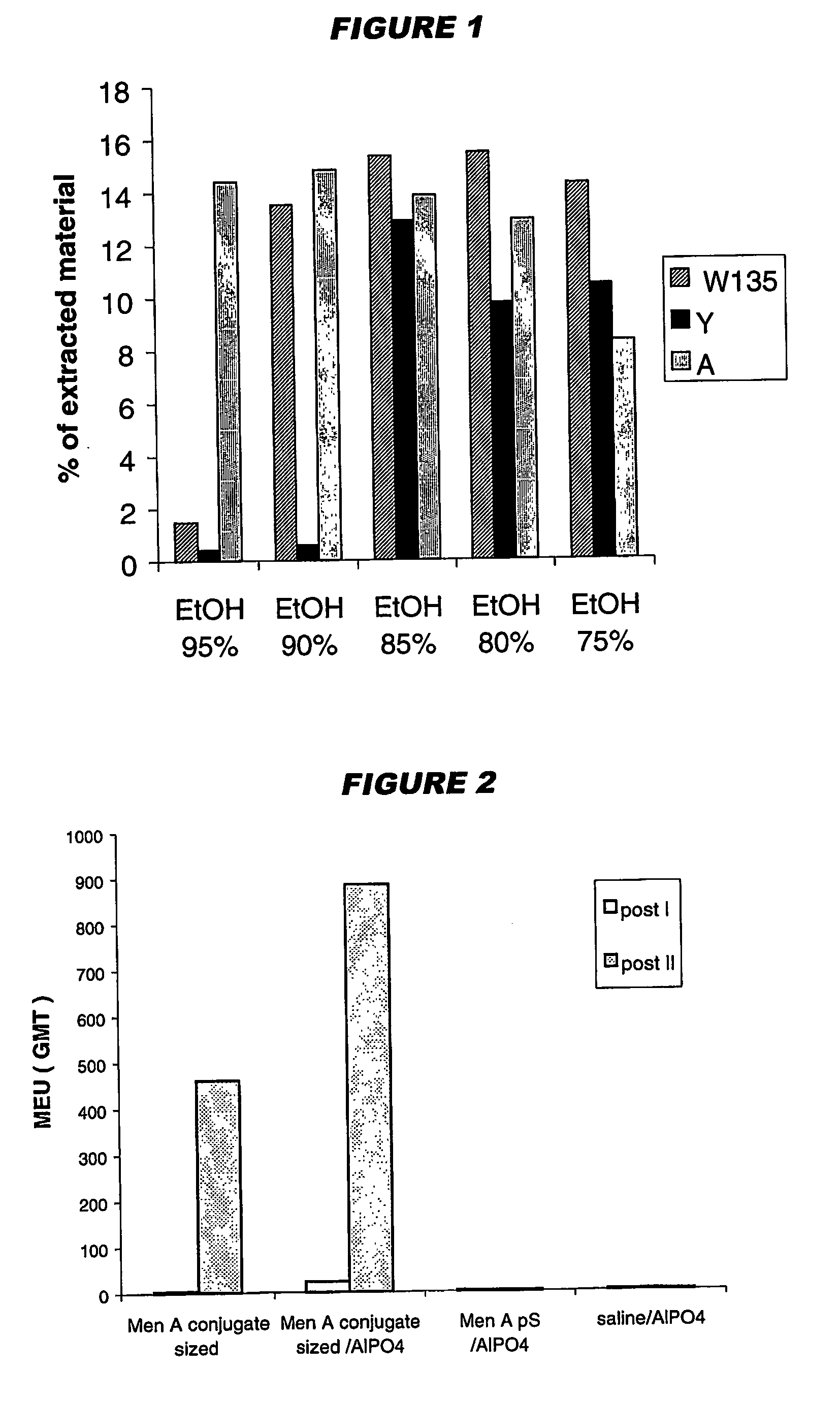
Precipitated bacterial capsular polysaccharides can be efficiently re-solubilised using alcohols as solvents. The invention provides a process for purifying a bacterial capsular polysaccharide, comprising the steps of (a) precipitation of said polysaccharide, followed by (b) solubilisation of the precipitated polysaccharide using ethanol. CTAB can be used for step (a). The material obtained, preferably following hydrolysis and sizing, can be conjugated to a carrier protein and formulated as a vaccine. Also, in vaccines comprising saccharides from the serogroups A and C, the invention provides that the ratio (w / w) of MenA saccharide:MenC saccharide is >1.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel mycoplasma hyopneumoniae bacterial strain and vaccine composition thereof
ActiveCN103031258AEffective preventionEffective therapeuticAntibacterial agentsBacterial antigen ingredientsCircovirusPneumonia mrsa
The invention provides a mycoplasma hyopneumoniae strain HN0613 which is isolated and identified to have better immunogenicity, and further provides a mycoplasma hyopneumoniae antigen prepared from the mycoplasma hyopneumoniae strain HN0613 and mycoplasma pneumonia pneumovax containing the mycoplasma hyopneumoniae antigen. The invention further provides a bivalent combined vaccine of porcine circovirus II and mycoplasma hyopneumoniae. The duplex combination vaccine comprises PCV (Porcine Circovirus) antigen II (inactivated porcine circovirus antigen II or PCV20 RF2 protein), inactivated mycoplasma hyopneumoniae and vaccine adjuvant. The combination vaccine can achieve the purpose of preventing the porcine circovirus disease and the mycoplasma pneumonia swine by injection once, and has a protective effect of preventing mycoplasma hyopneumoniae infection.
Owner:PU LIKE BIO ENG
Vaccine
InactiveUS20100074918A1Lower immune responseAntibacterial agentsSsRNA viruses positive-senseCo administrationCombination vaccines
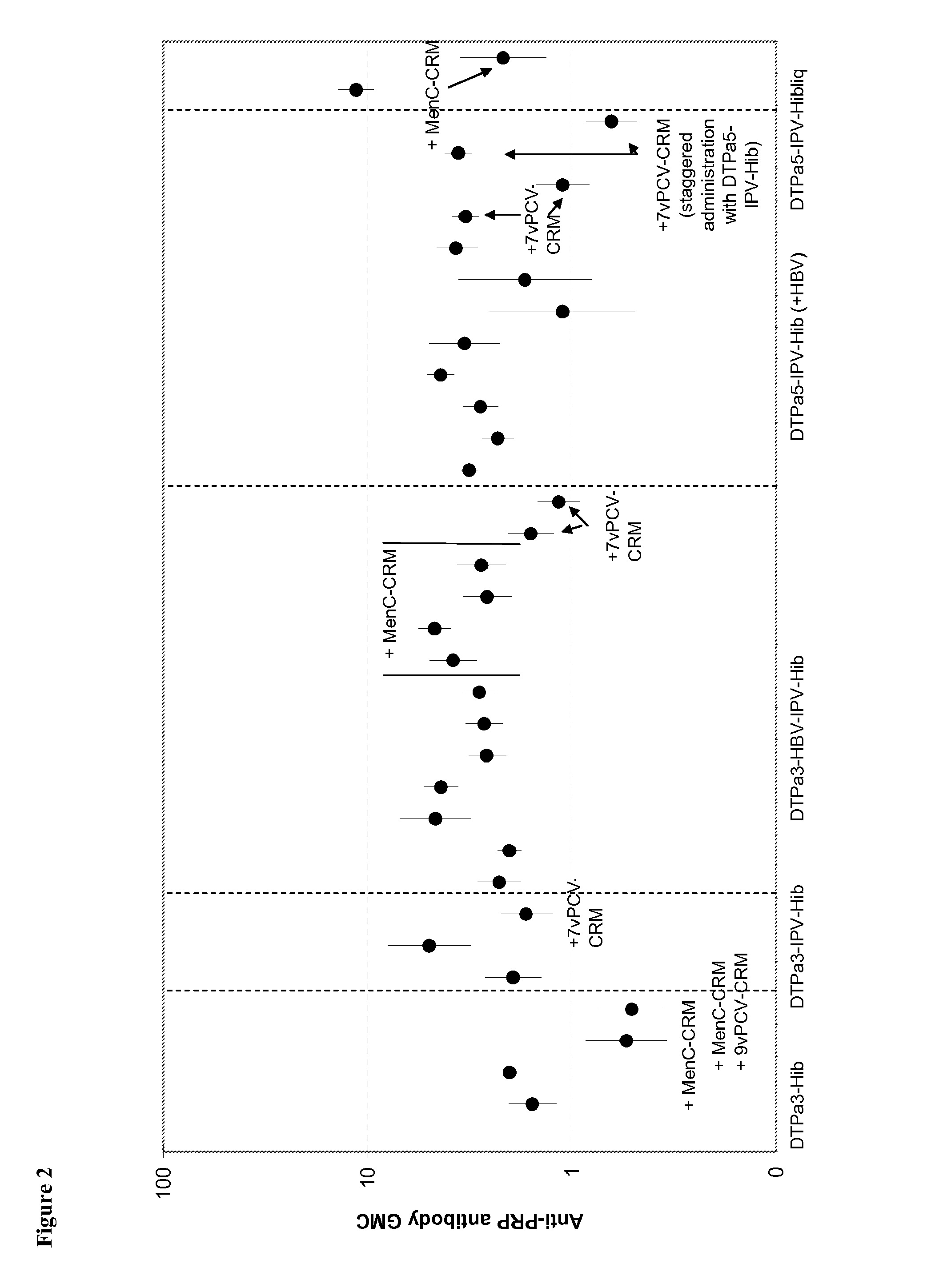
The present invention relates to the field of vaccines and in particular to combination vaccines and co-administration schedules. The present inventor discloses that overuse of CRM in paediatric vaccines can result in bystander immune interference to certain antigens and provide solutions to this problem.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent pcv2 immunogenic compositions and methods of producing such compositions
ActiveUS20090092636A1Improve the immunityReduce severityAntibacterial agentsCompounds screening/testingDiseaseOpen reading frame
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. Also provided is recombinant PCV2 ORF2 protein, and immunogenic compositions comprising PCV2 ORF2 protein. Moreover, multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of PCV2 infection, preferably PCV2 ORF2 protein, or an immunogenic composition comprising PCV2 ORF2 protein, and at least one immunogenic active component of another disease-causing organism in swine,
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
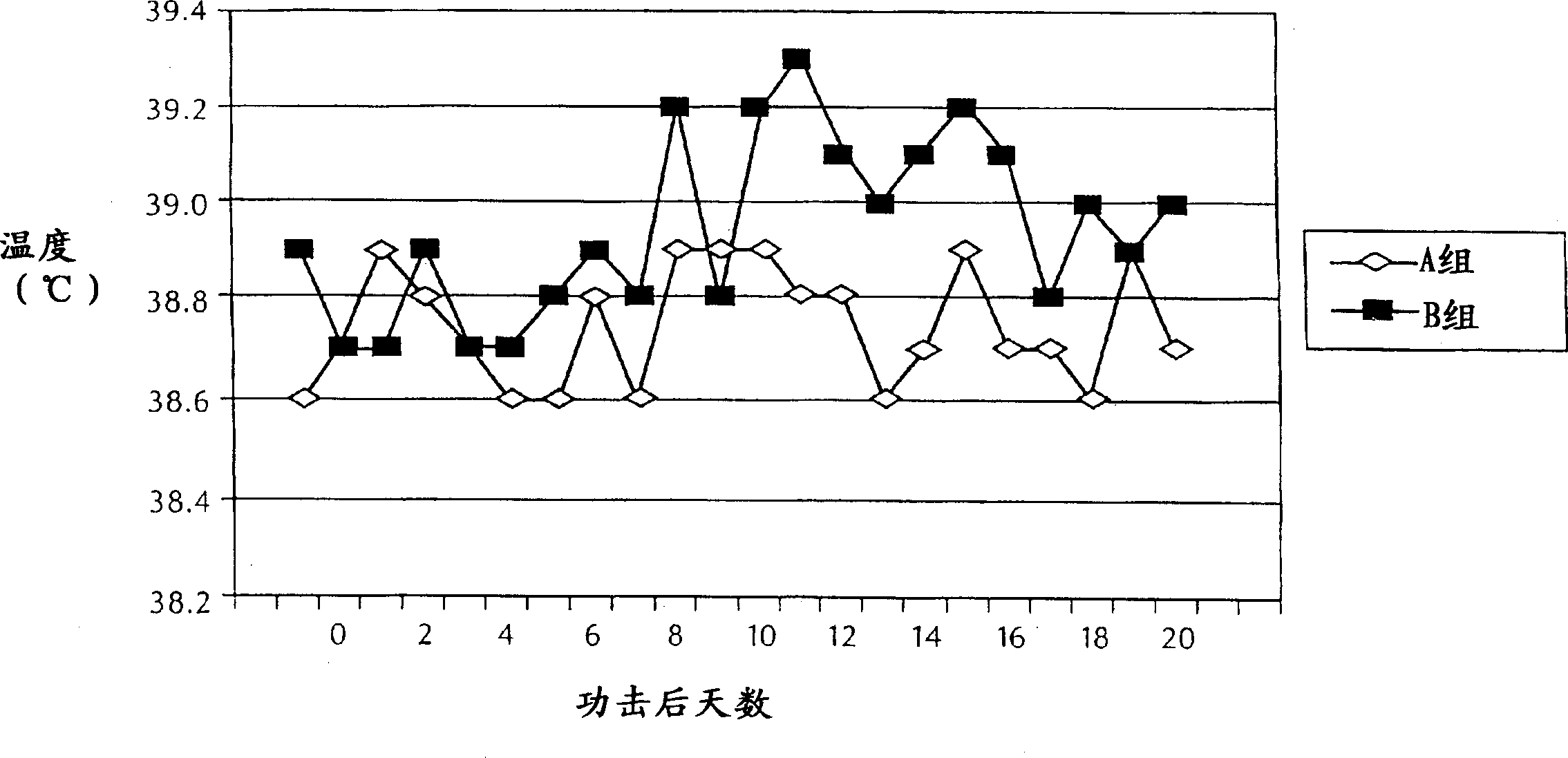
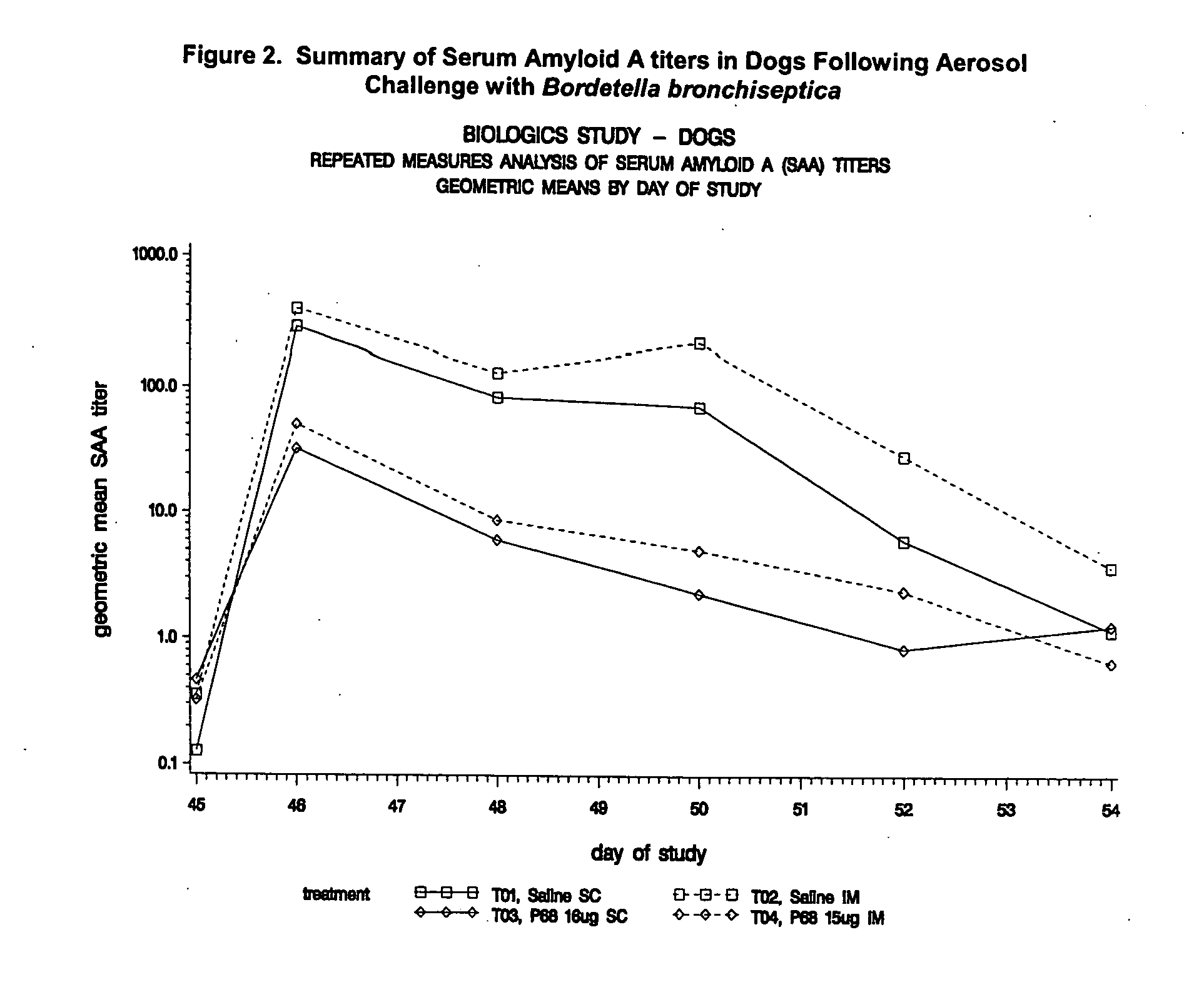
Canine combination vaccines
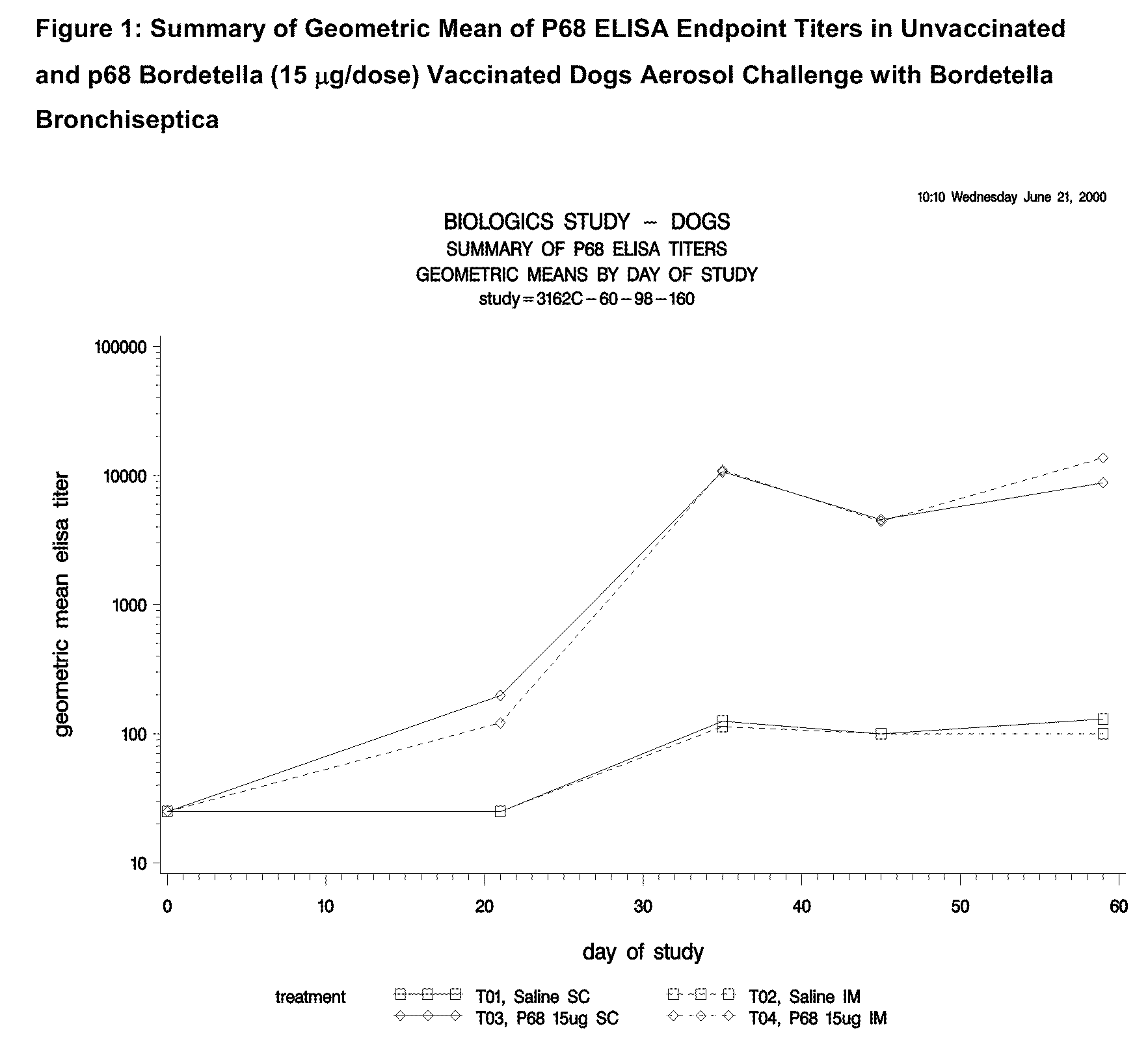
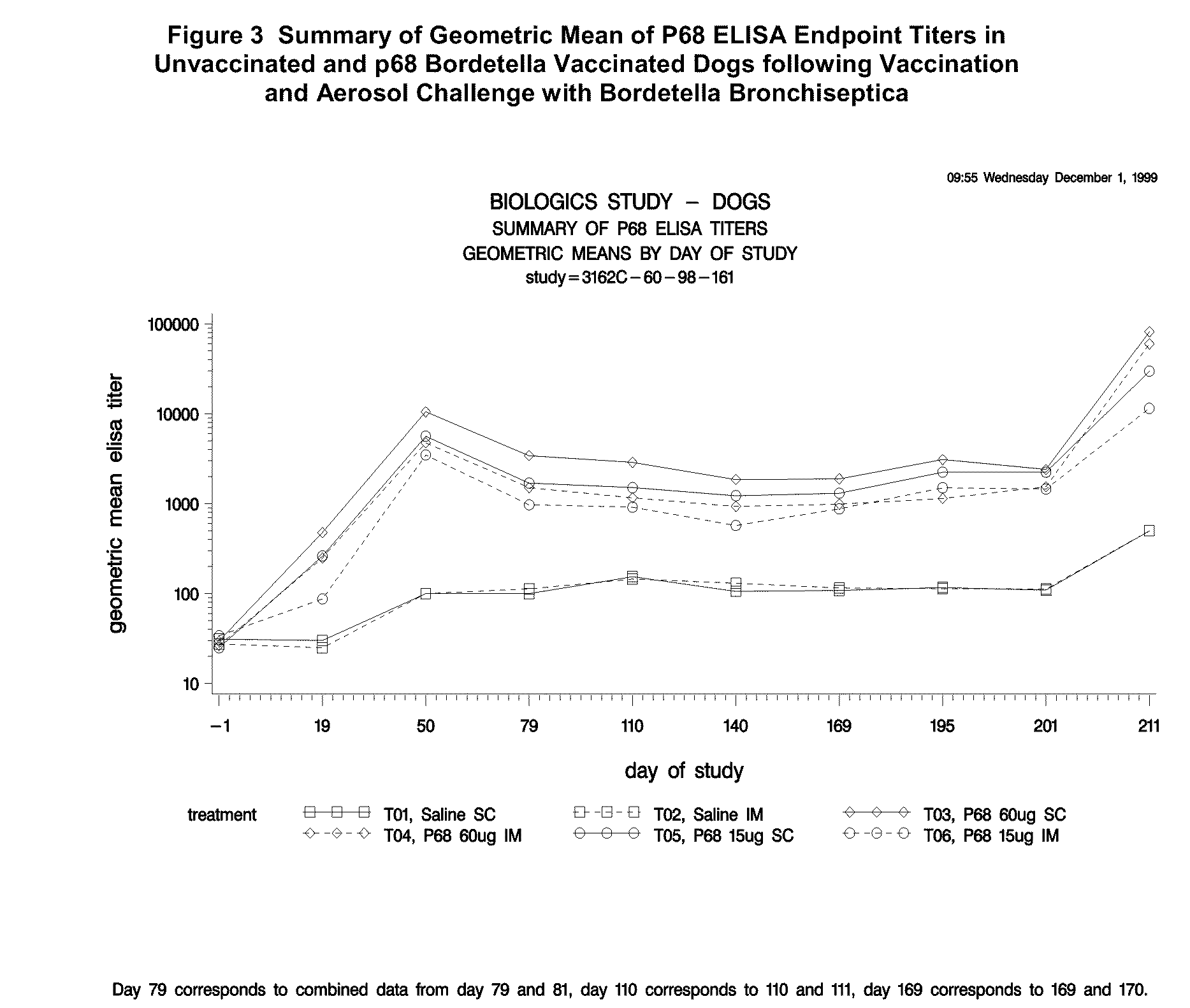
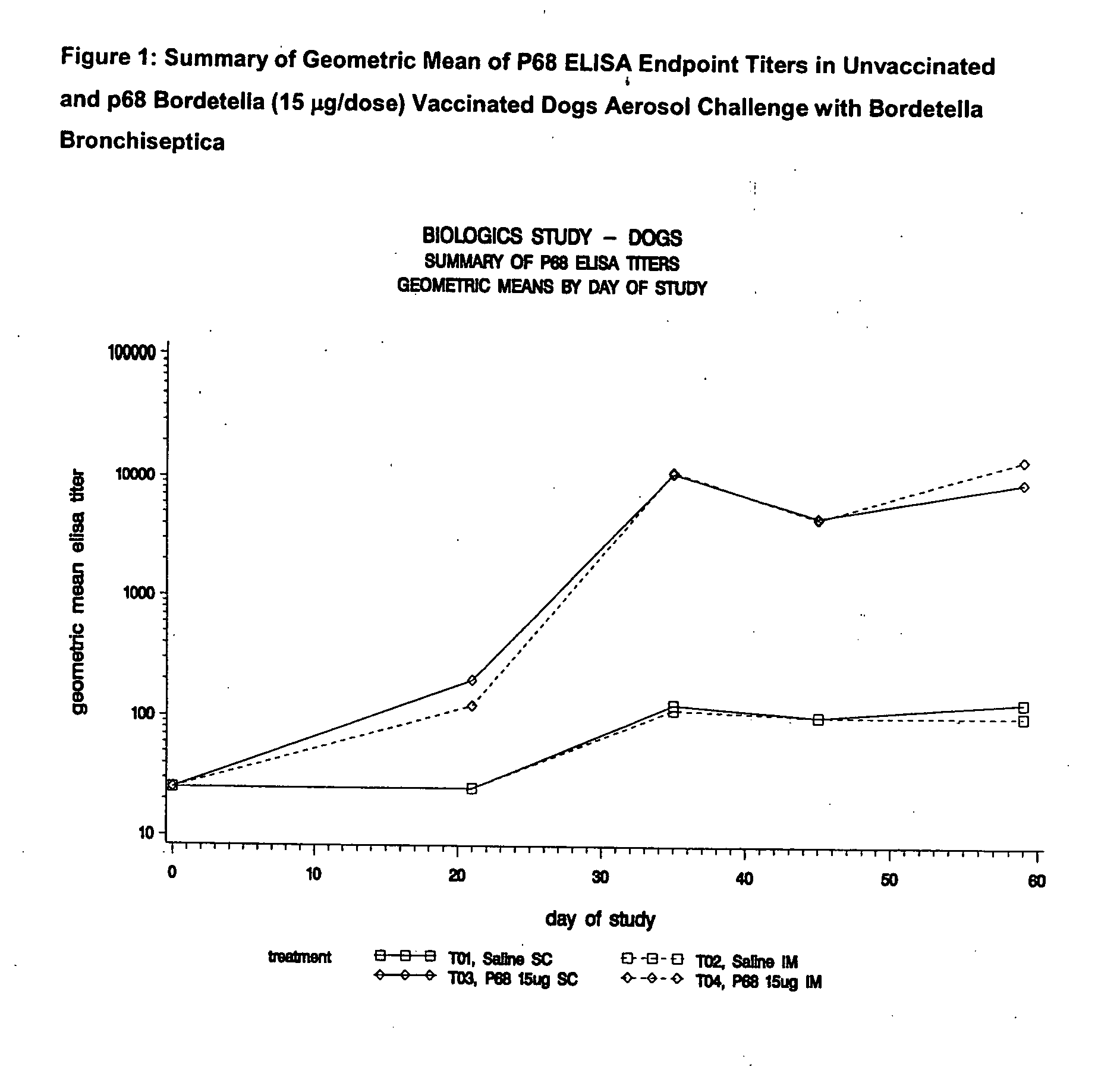
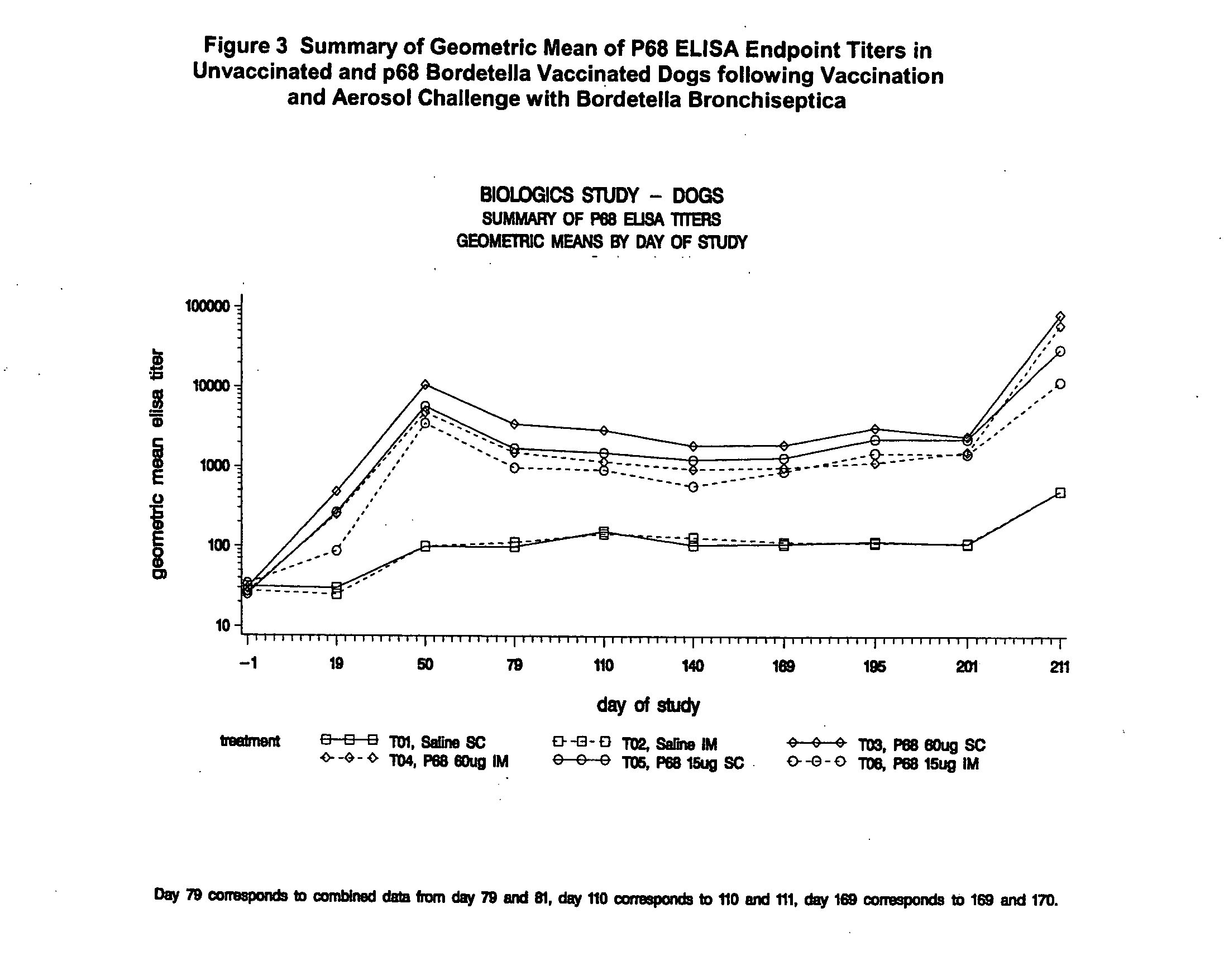
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
PCV/Mycoplasma Hyopneumoniae/PRRS Combination Vaccine
ActiveUS20130266603A1Protective immune responseAntibacterial agentsBacterial antigen ingredientsImmune complexVirus antigen
This invention provides a trivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; a porcine circovirus type 2 (PCV2) antigen; and a PRRS virus antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
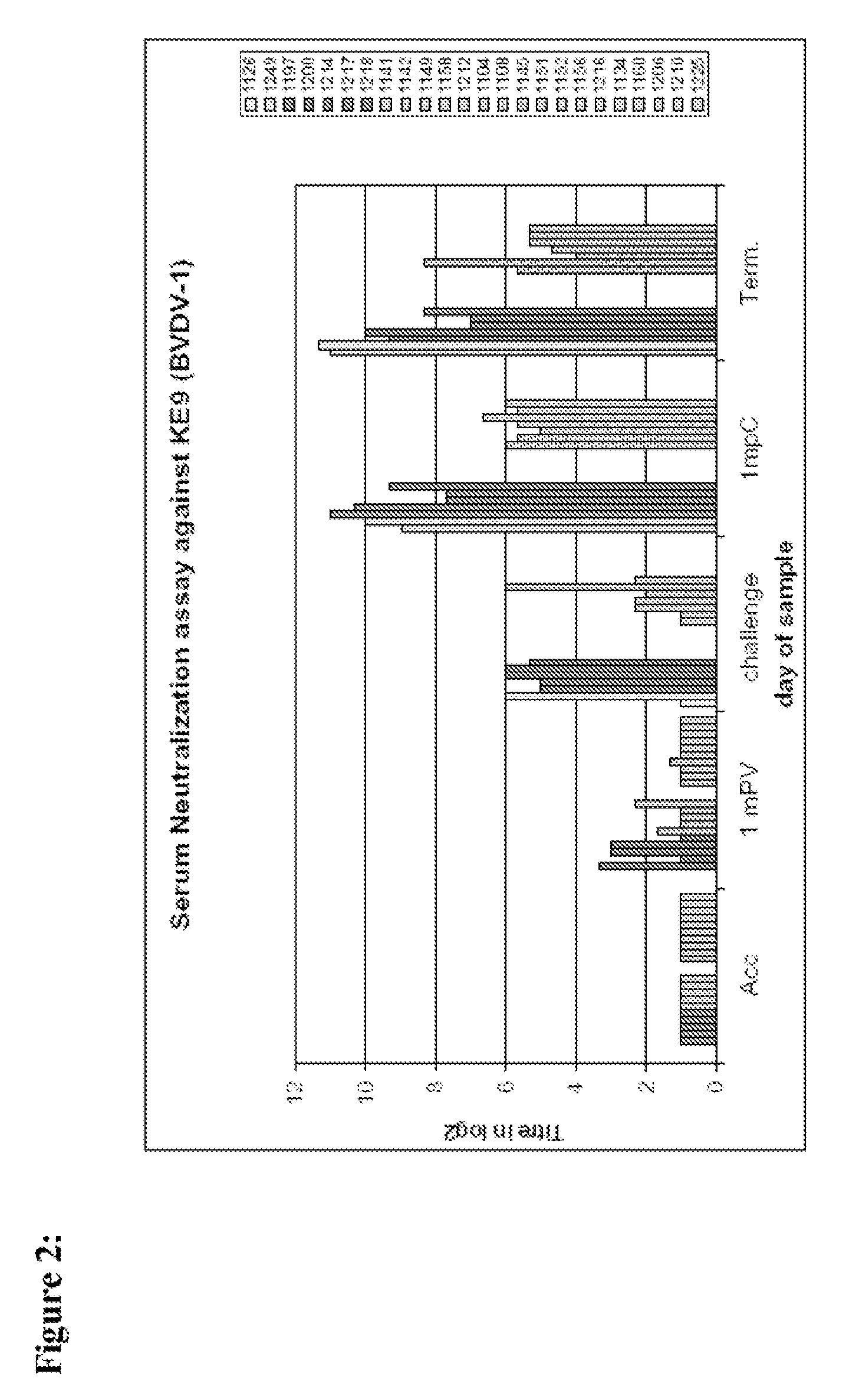
Combination vaccine comprising an attenuated bovine viral diarrhea virus
InactiveUS20090068223A1SsRNA viruses negative-senseSsRNA viruses positive-senseBovine Viral Diarrhea VirusesActive component
The present invention relates to combination vaccines for the prophylaxis and treatment of microbiological infections in cattle which comprise an attenuated bovine viral diarrhea virus (BVDV) for the prophylaxis and treatment of BVDV caused infections, and a further immunological active component for the prophylaxis and treatment of microbiological infections other than BVDV.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
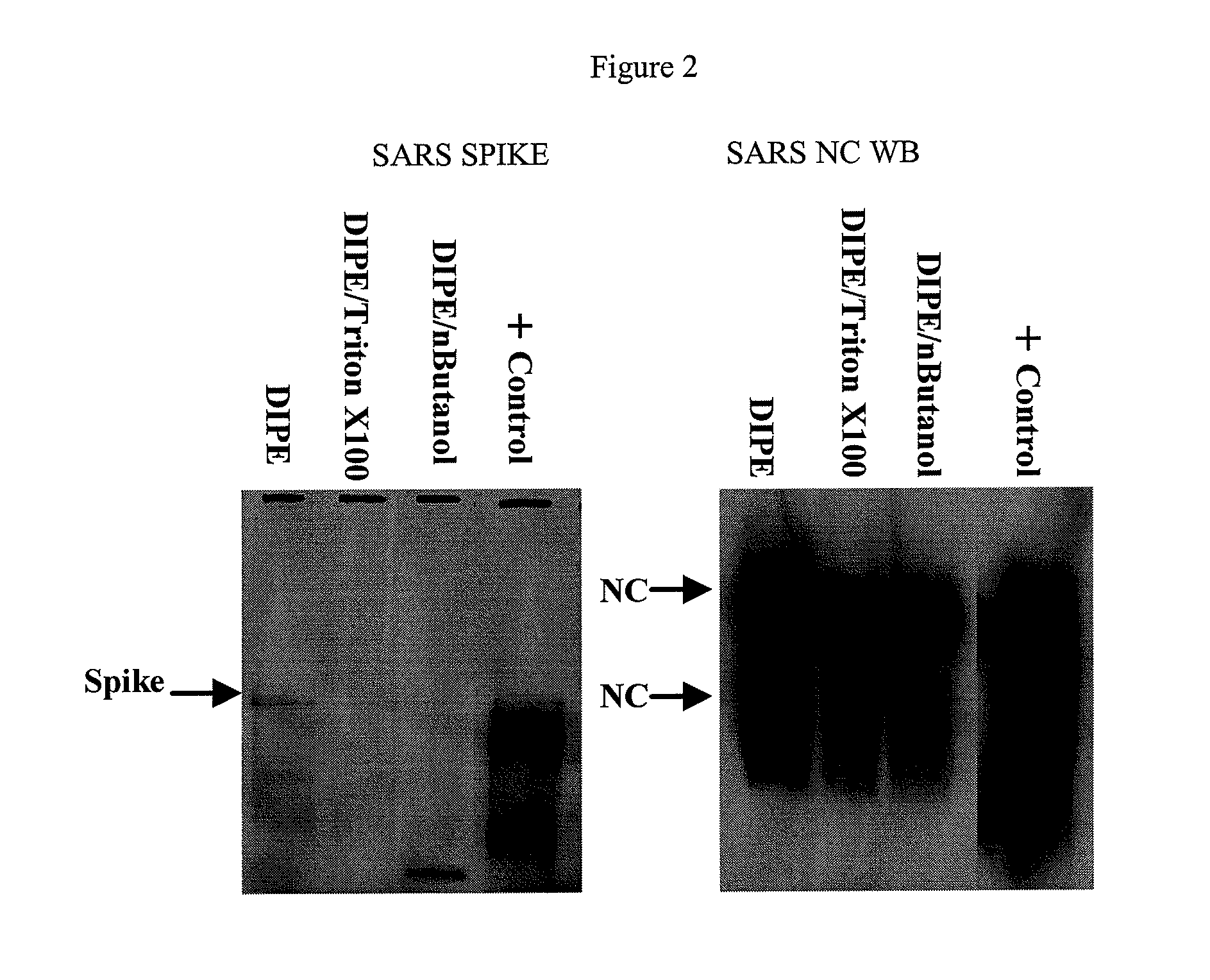
SARS Vaccine Compositions and Methods of Making and Using Them
InactiveUS20090017069A1The process is simple and effectiveThe method is simple and efficientSsRNA viruses positive-senseViral antigen ingredientsLipid formationViral Vaccine
Owner:ELI LILLY & CO +1
Combination vaccines with 1-hydroxy-2-phenoxyethane preservative
Processes for preparing combination vaccines that include diphtheria and tetanus toxoids, where these two toxoids are used in the processes as a single component containing both toxoids, and also containing 1-hydroxy-2-phenoxyethane.
Owner:NOVARTIS AG
PCV/Mycoplasma Hyopneumoniae Combination Vaccine
This invention provides a multivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; and a porcine circovirus type 2 (PCV2) antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Capsular polysaccharide solubilisation and combination vaccines
InactiveUS20090130147A1Improving immunogenicityAntibacterial agentsPeptide/protein ingredientsAlcohol ethylMicrobiology
Precipitated bacterial capsular polysaccharides can be efficiently re-solubilised using alcohols as solvents. The invention provides a process for purifying a bacterial capsular polysaccharide, comprising the steps of (a) precipitation of said polysaccharide, followed by (b) solubilisation of the precipitated polysaccharide using ethanol. CTAB can be used for step (a). The material obtained, preferably following hydrolysis and sizing, can be conjugated to a carrier protein and formulated as a vaccine. Also, in vaccines comprising saccharides from both serogroups A and C, the invention provides that the ratio (w / w) of MenA saccharide:MenC saccharide is >1.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Combination vaccine
InactiveUS20090130146A1Effective seroprotectionGood immune protectionAntibacterial agentsBacterial antigen ingredientsBacteroidesDisease
The present invention relates to the combination of antigens directed against bacteria and viruses, their uses and the preparation of medicaments in order to confer protection against infectious diseases. In particular, the invention relates to a combination vaccine comprising at least one antigen of Clostridium tetani, at least one antigen from Corynebacterium diphtheriae, and at least one antigen from the TBE-flavivirus suitable to confer seroprotection against diseases and medical conditions caused by these pathogenic organisms.
Owner:NOVARTIS AG
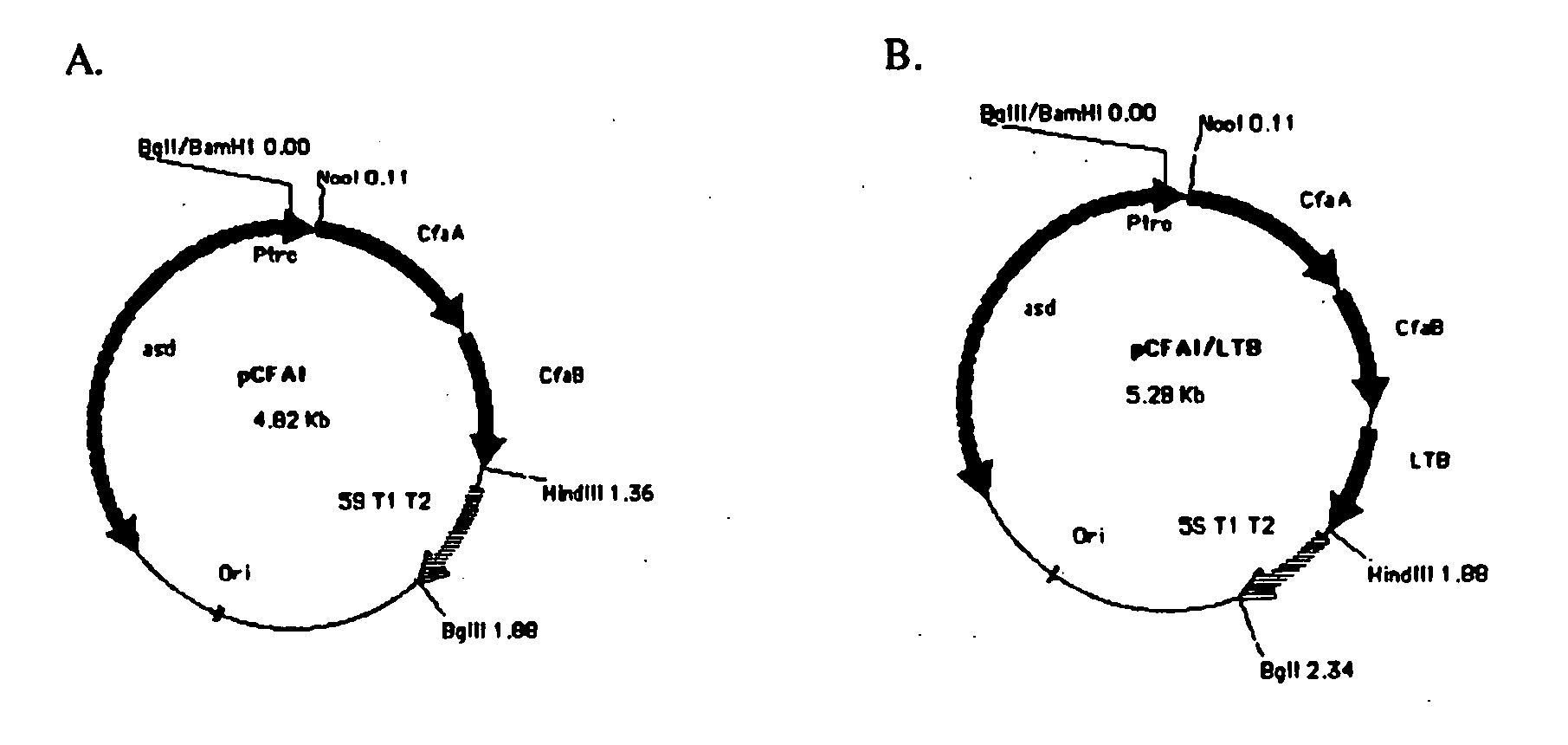
Construction of live attenuated Shigella vaccine strains that express CFA/I antigens (cfaB and CfaE) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic E.coli
ActiveUS20070237791A1Reduce intrusionBacterial antigen ingredientsBacteriaHeterologousMucosal Immune Responses
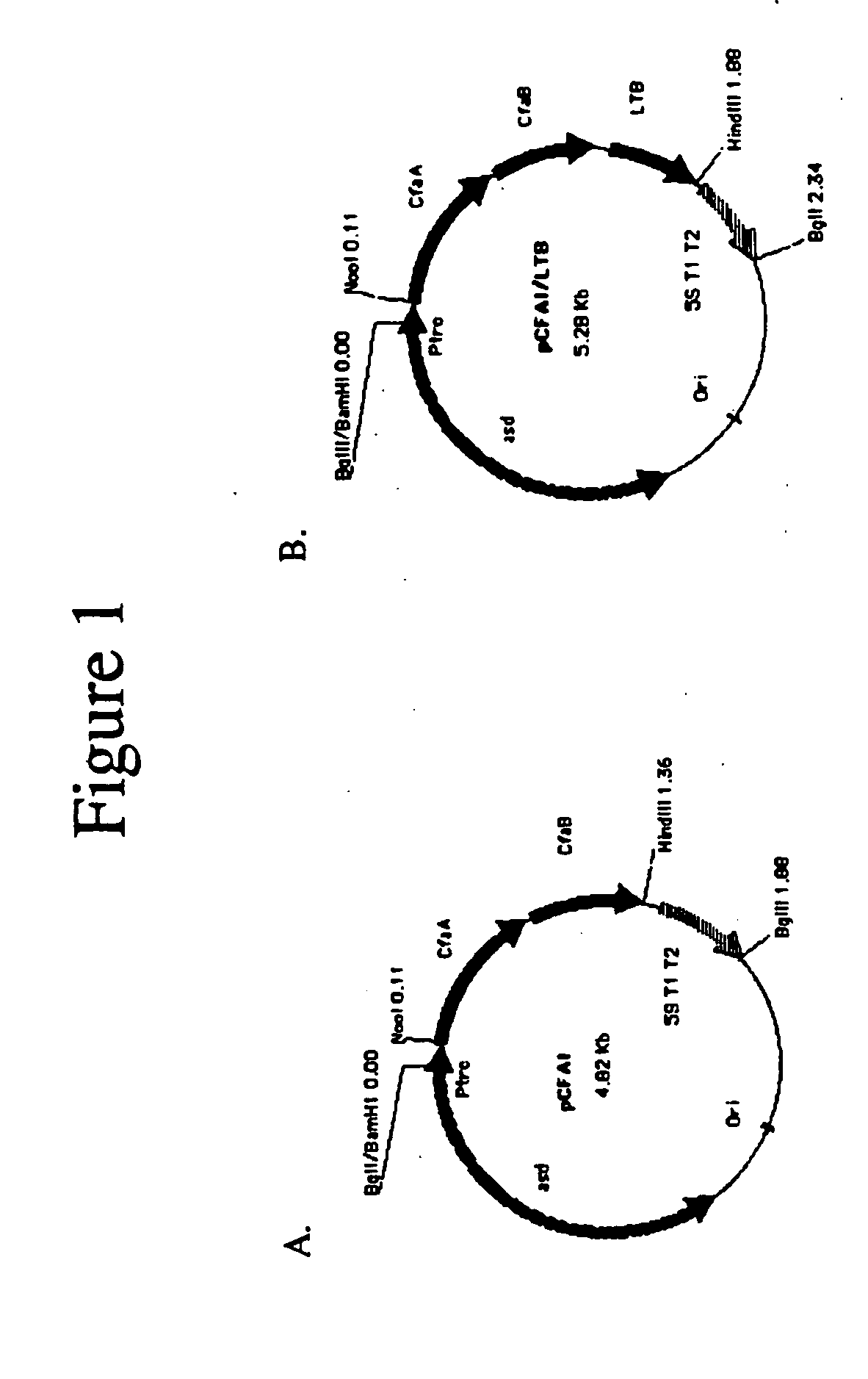
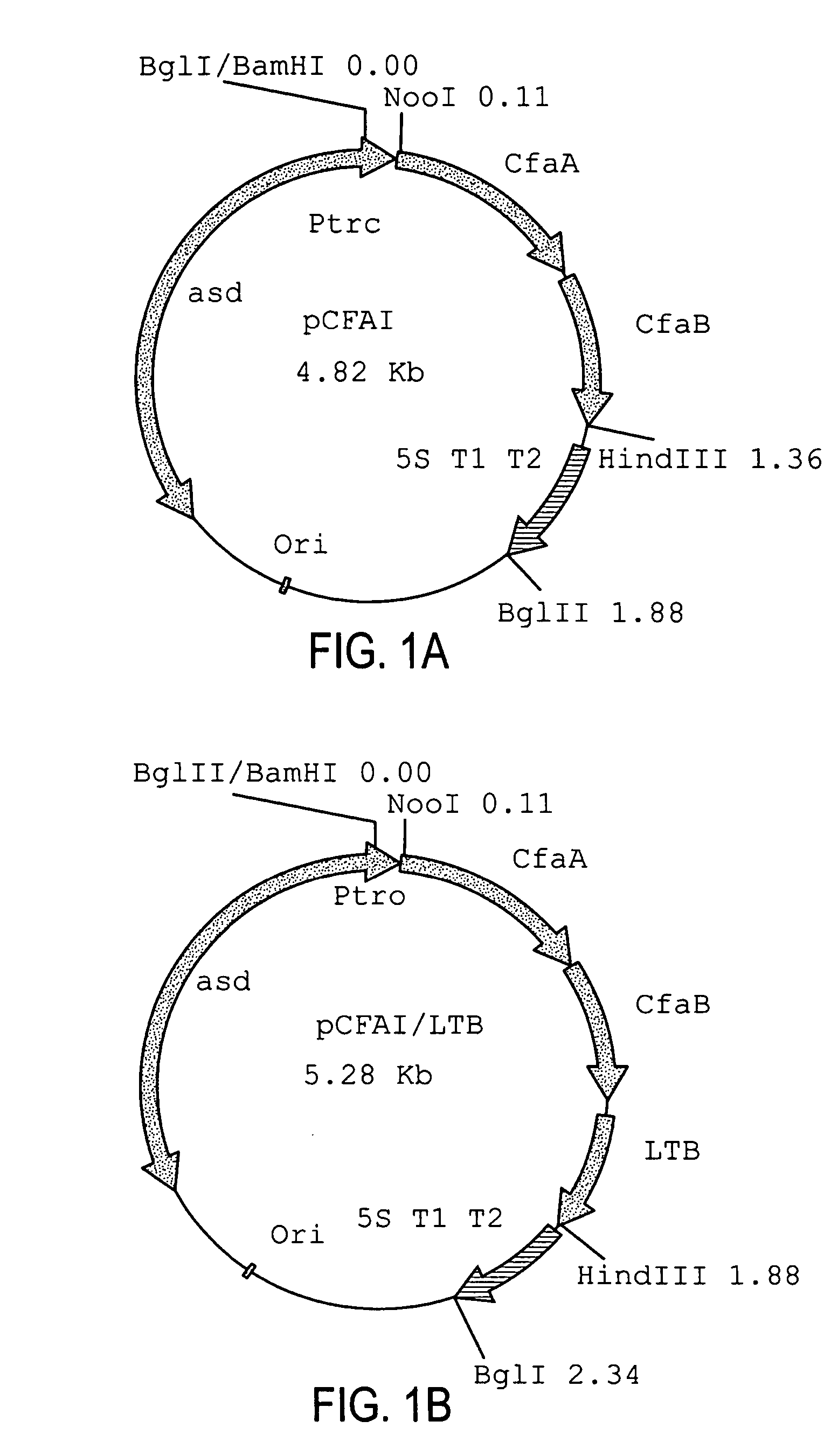
With the goal of creating a combination vaccine against Shigella and other diarrheal pathogens we have constructed a prototype vaccine strain of Shigella flexneri 2a (SC608) that can serve as a vector for the expression and delivery of heterologous antigens to the mucosal immune system. SC608 is an asd derivative of SC602, a well-characterized vaccine strain, which has recently undergone several phase 1 and 2 trials for safety and immunogenicity. Using non-antibiotic asd-based plasmids, we have created novel constructs for the expression of antigens from enterotoxigenic E. coli (ETEC), including CFA / I (CfaB and CfaE) and the B-subunit from heat-labile enterotoxin (LTB) in Shigella vaccine strain SC608. Heterologous protein expression levels and cellular localization are critical to immune recognition and have been verified by immunoblot analysis. Following intranasal immunization (SC608(CFAI) and SC608(CFAI / LTB) of guinea pigs, serum IgG and IgA immune responses to both the Shigella LPS and ETEC antigens can be detected by ELISA. In addition, ELISPOT analysis for ASCs from cervical lymph nodes and spleen showed similar responses. All vaccine strains conferred high levels of protection against challenge with wild-type S. flexneri 2a using the Sereny test. Furthermore, serum from guinea pigs immunized with SC608 expressing CfaB and LTB contained antibodies capable of neutralizing the cytological affects of heat-labile toxin (HLT) on Chinese Hamster Ovary (CHO) cells. These initial experiments demonstrate the validity of a multivalent invasive Shigella strain that can serve as a vector for the delivery of pathogen-derived antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Polyvalent pneumococcal polysaccharide combination vaccine
InactiveCN1566145AEasy to operateLow costBacterial antigen ingredientsNitro compound active ingredientsMass ratioProtomer
The invention provides a coupling substance of pneumococcoal polysaccharide carrier protein, vaccines containing the coupling substance and process for preparation, wherein the coupling substance has the structural formula of Pr-L-Ps, wherein Pr is carrier protein, Ps is pneumococcoal polysaccharide, L is covalent bond or connection group, and the mass ratio of Ps:Pr=0.5-3.5:1. The coupling substance can form protection to the homogeneous pneumococcosis protomer which produces capsular polysaccharide compositions.
Owner:SHANGHAI JIANYI SCI & TECH DEV
Modified live vaccine of mycoplasma bovis, methods of producing modified live mycoplasma bovis vaccines, combination vaccines and methods of treatment
ActiveUS20100272759A1Reduce incidenceReduce severityAntibacterial agentsBacterial antigen ingredientsBacteroidesMycobacterium Infections
The present invention relates to new attenuated M. bovis bacteria strains passaged at least 110 times. Moreover, the present invention also provides immunogenic compositions comprising live bacteria of any of those attenuated M. bovis bacteria strain, their manufacture and use for the treatment and prophylaxis of M. bovis infections and combinations with other veterinary vaccines or medicaments.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Combination vaccine against various HIVs and combination method thereof
InactiveCN101569745ANew Vaccine FeaturesGenetic material ingredientsAntiviralsHIV vaccineCell Membrane Proteins
The invention discloses a combination vaccine against various HIVs and a combination method thereof. The combination vaccine consists of two or more HIV vaccines, different AIDS vaccines contain different HIV membrane proteins or membrane protein encoding genes, and each HIV vaccine is independently inoculated and inoculated at least one time, namely the total times of inoculation are at least twice. The core of the technology is that the different HIV membrane proteins are used to carry out sequential immunization, namely in sequential repeated immune processes of the vaccines, the HIV membrane proteins used in the immunization at different times are different so as to obtain a broad-spectrum neutral antibody against the HIVs with high titer. The HIV preventing vaccine developed by the method can be used for preventing various subtype HIV infections.
Owner:VACDIAGN BIOTECH
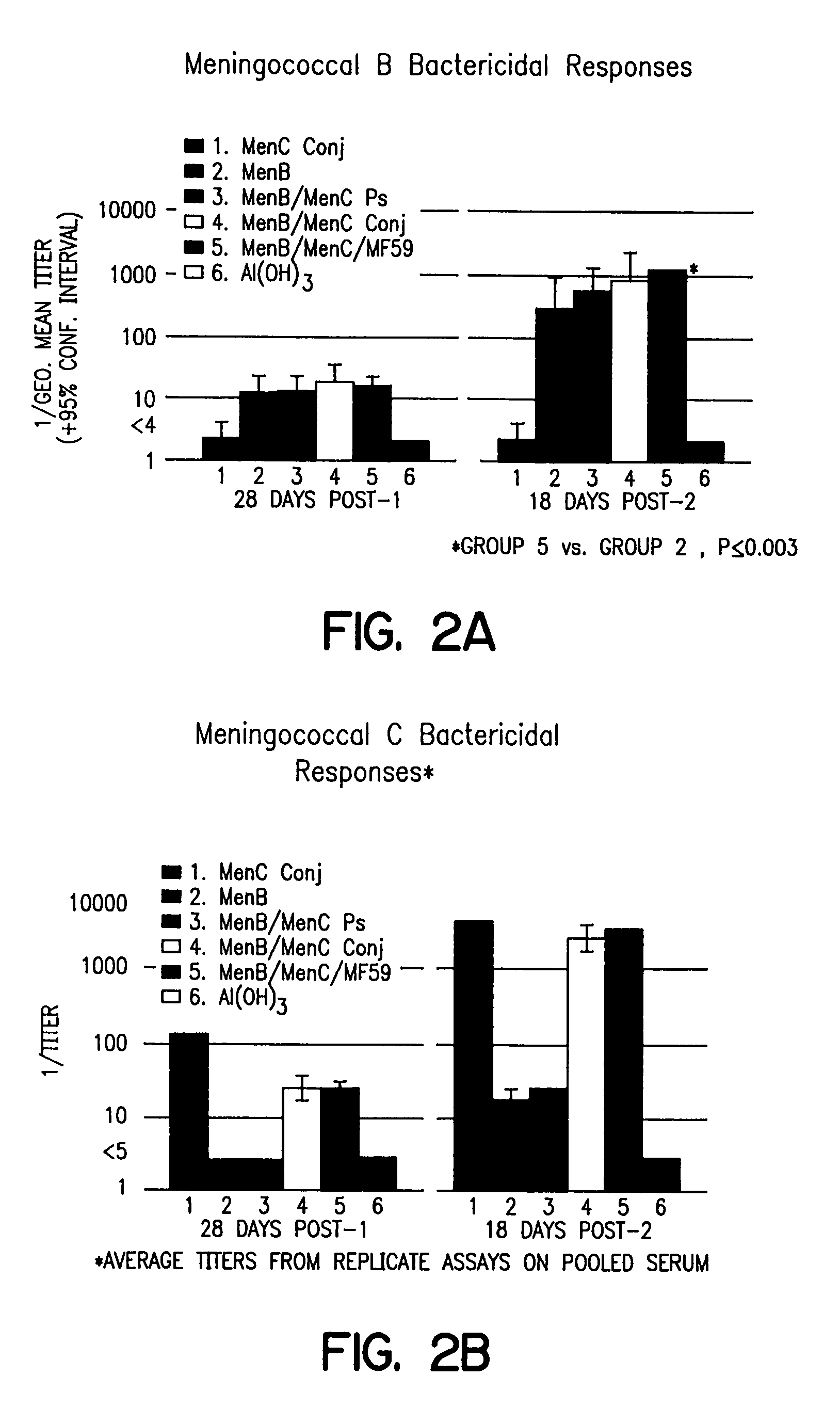
Combination meningitidis B/C vaccines
InactiveUS8007815B1Antibacterial agentsBacterial antigen ingredientsLiposome VesicleNeisseria meningitidis
Combination vaccines for treating or preventing Neisseria meningitidis infection are described. The vaccines include Neisseria meningitidis serogroup B proteoliposomic vesicles and Neissera meningitidis serogroup C conjugated oligosaccharides.
Owner:NOVARTIS AG +2
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
ActiveUS20150174233A1Reduce incidenceReduce severityBacterial antigen ingredientsViral antigen ingredientsDiseaseMultivalent Vaccine
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
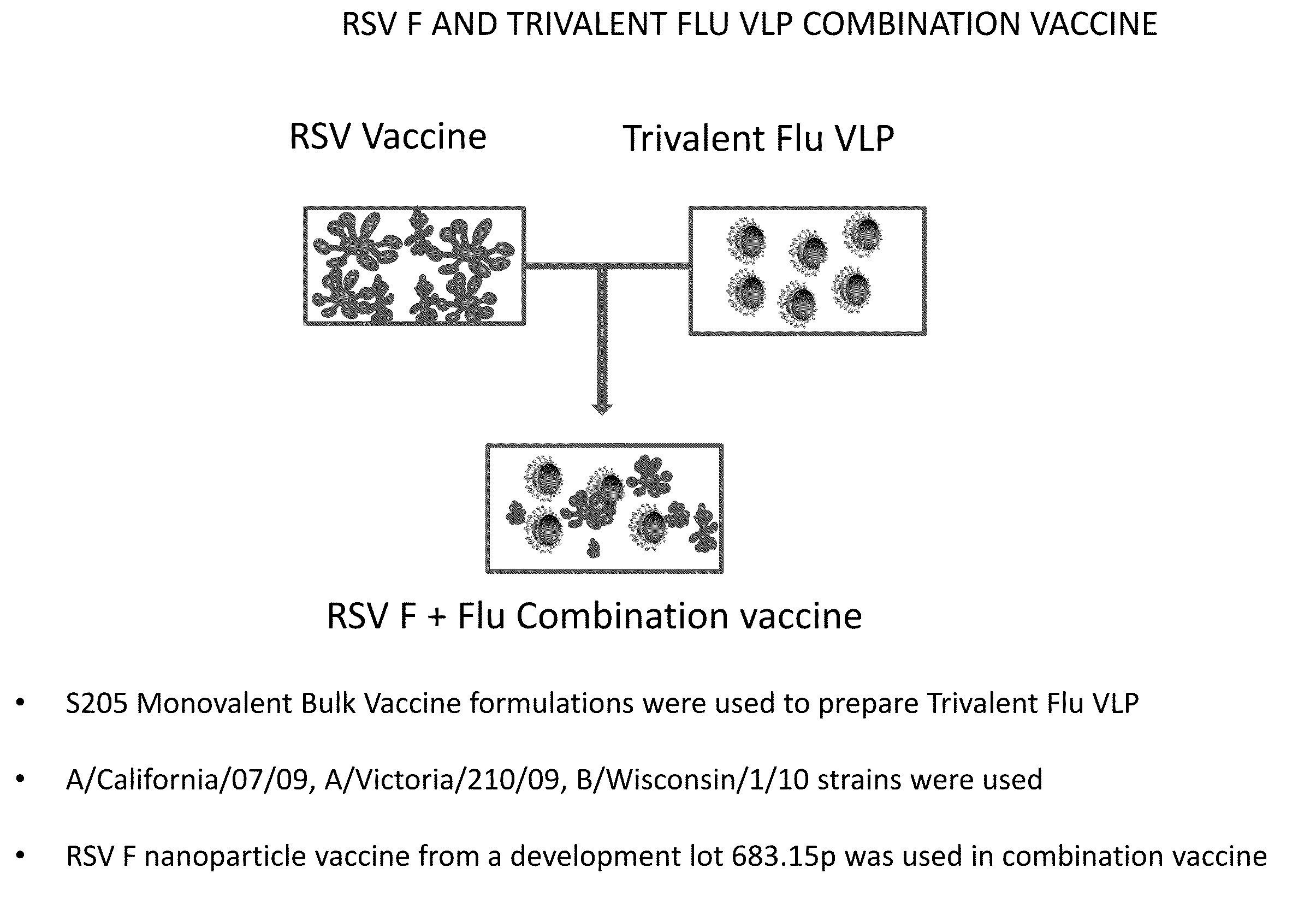
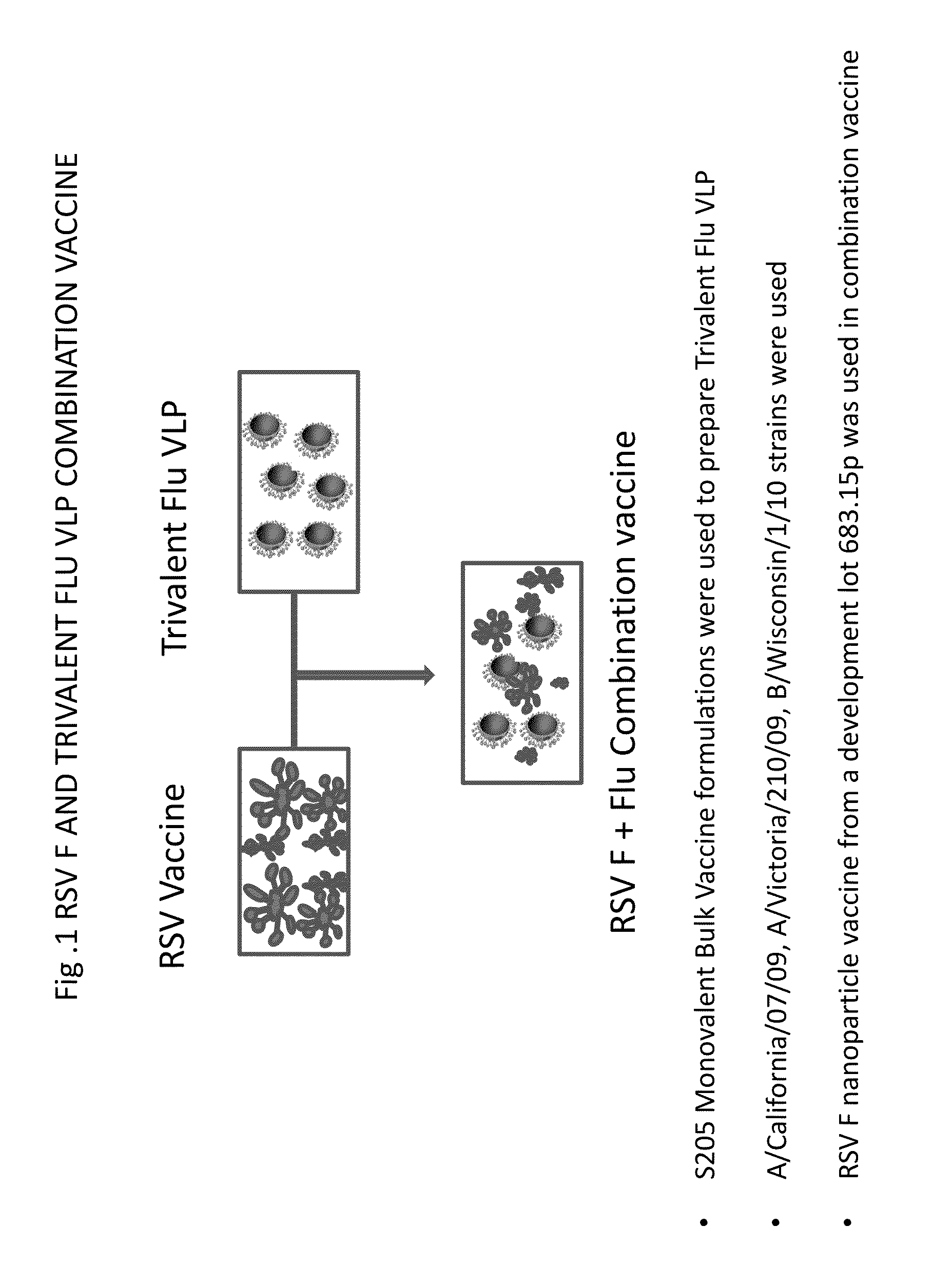
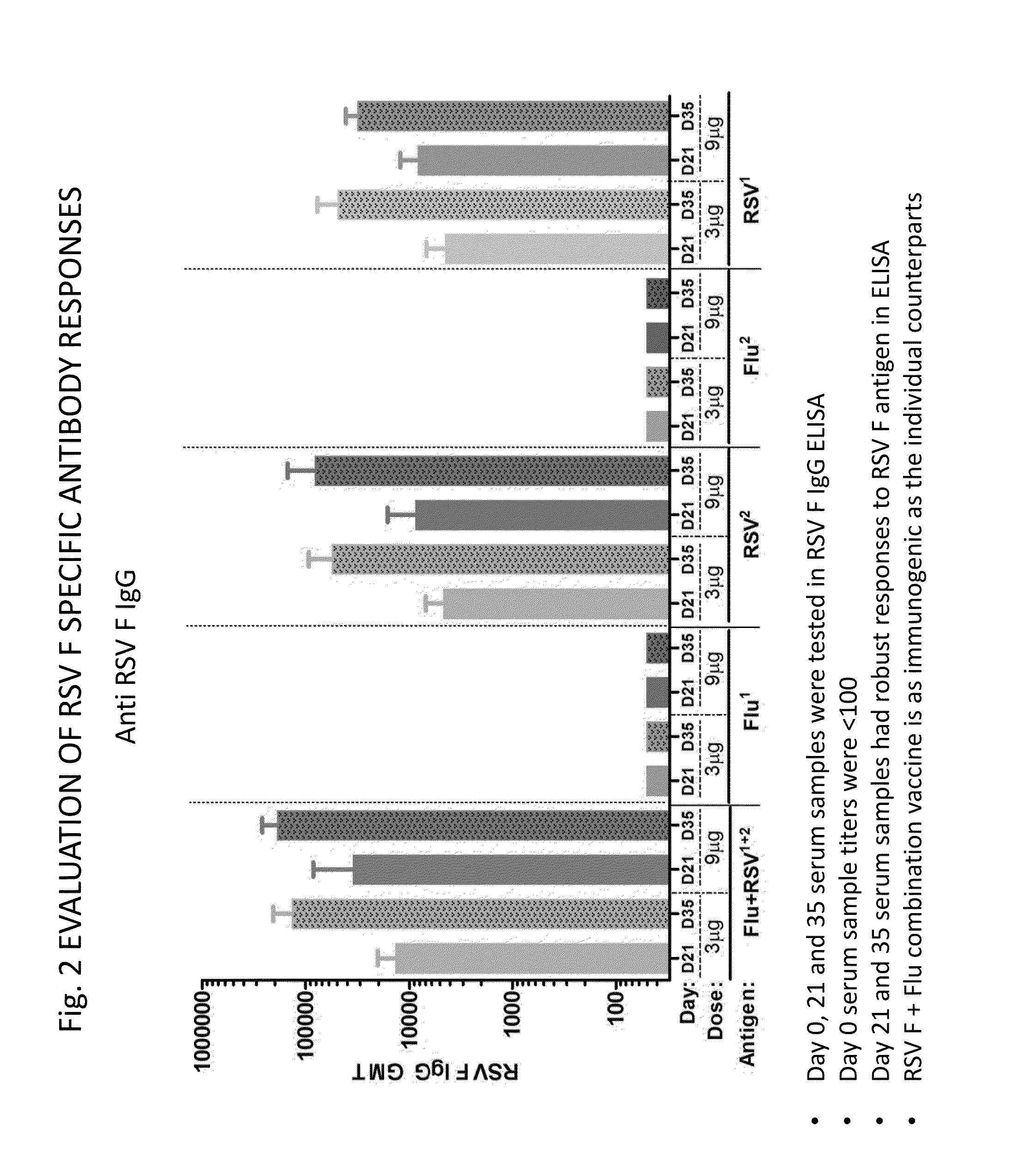
Combination vaccine for respiratory syncytial virus and influenza
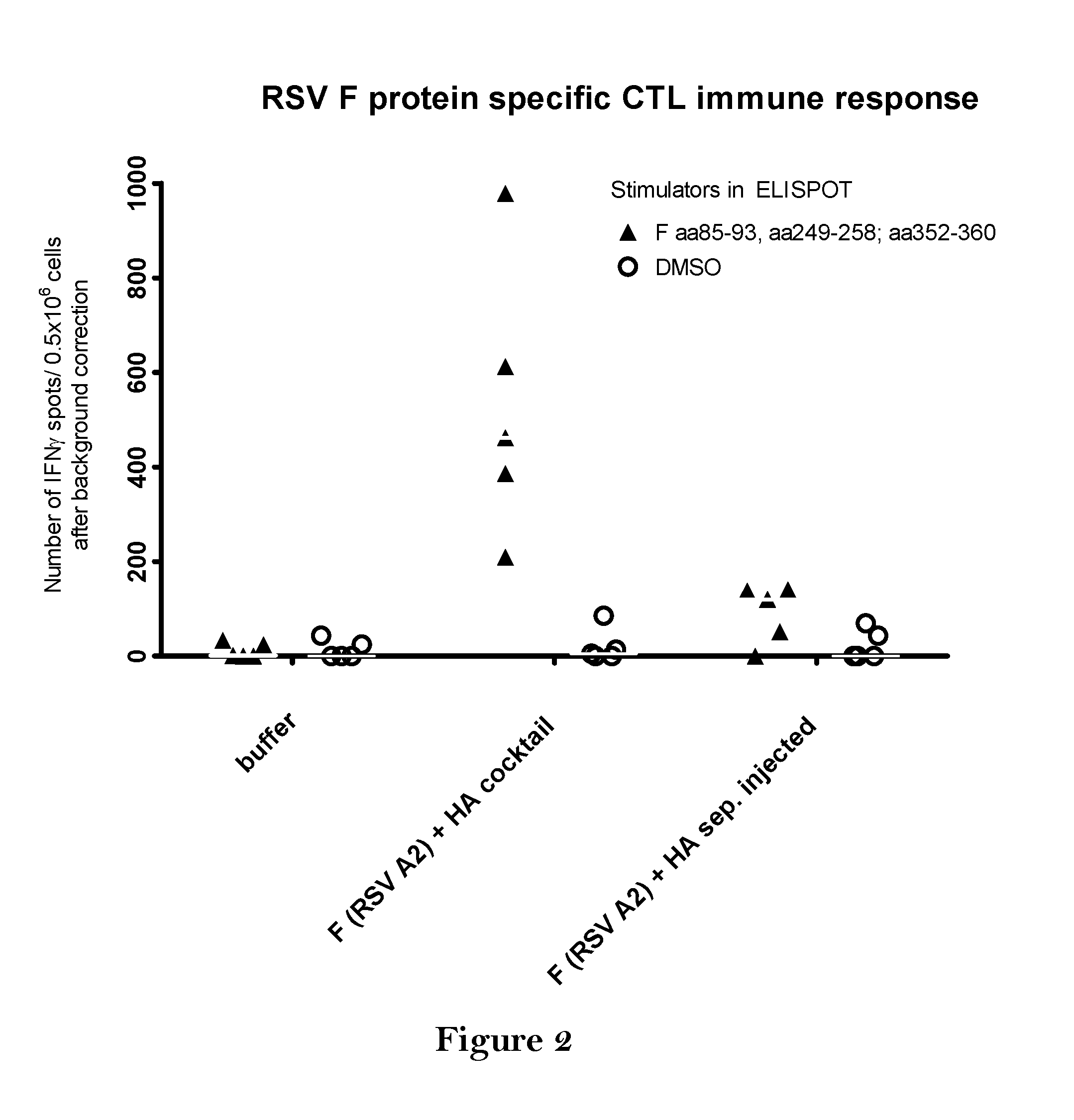
InactiveUS20140227309A1Enhance immune responseEnhanced RSV F responseSsRNA viruses negative-senseViral antigen ingredientsImmunogenicityInfluenza a
The present disclosure is directed to compositions and methods for raising immune responses against influenza and respiratory synctial virus by administering combination immunogenic composition against both viruses at the same time. The combination compositions contain an RSV component and one, two, three, four, or more influenza components. The combination compositions provide a greater immune response than that obtained by separately administering the RSV and influenza components.
Owner:NOVAVAX
Mycoplasma bovis vaccine and methods of reducing pneumonia in animals
The present invention relates to Mycoplasma bovis vaccines and methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma bovis by administering to the animal an effective amount of a Mycoplasma bovis vaccine. The Mycoplasma bovis vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The Mycoplasma bovis vaccine administered in accordance with the present can be synthesized or recombinantly produced. The invention also relates to combination vaccines, methods of preparing Mycoplasma bovis vaccines and kits.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Canine vaccines against Bordetella bronchiseptica
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention relates to vaccines and methods for protecting dogs against disease caused by Leptospira bratislava. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona.
Owner:PFIZER INC +1
Combination vaccine with acellular pertussis
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, acellular pertussis, and infections caused by Haemophilus influenzae and polio viruses. The present invention also relates to inclusion of antigens for protection against infections caused Hepatitis virus and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
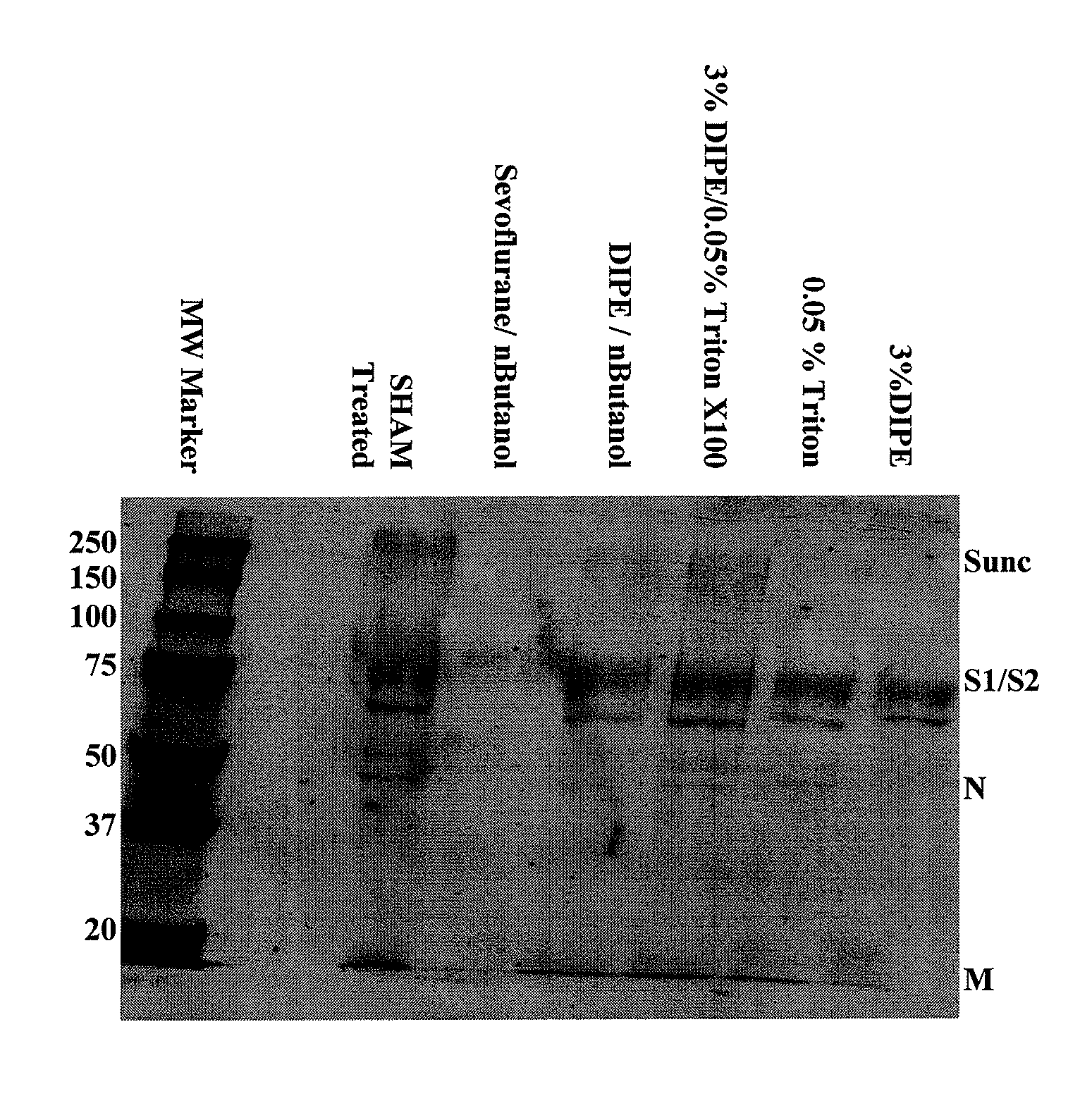
Method of making modified immunodeficiency virus particles
InactiveUS7439052B2The process is simple and effectivePositive immunologic responseSsRNA viruses positive-senseViral antigen ingredientsLipid formationImmunodeficiency virus
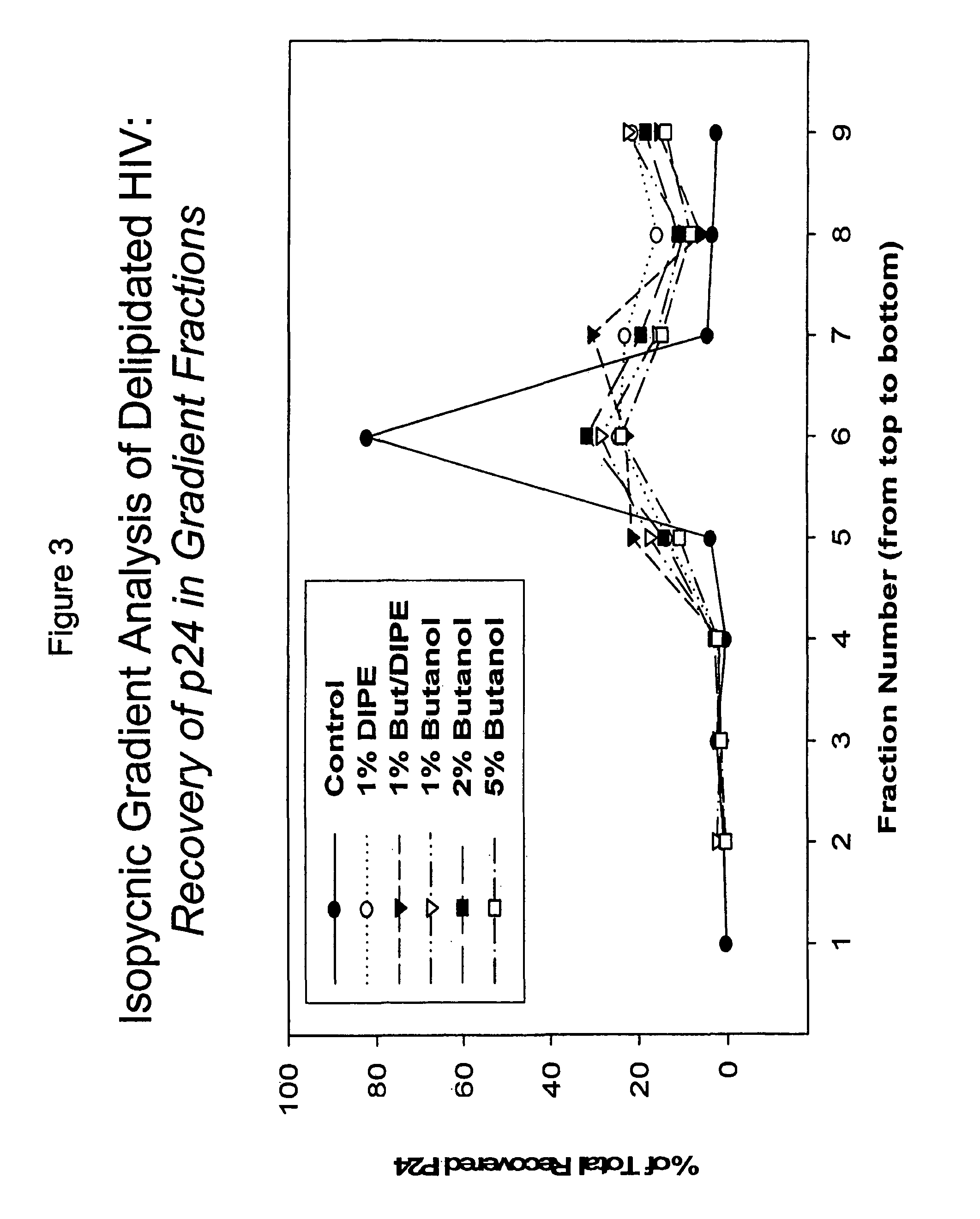
Described are a composition and method for reducing the occurrence and severity of infectious diseases, especially infectious diseases in which lipid-containing infectious viral organisms are found in biological fluids, such as blood. Solvents useful for extracting lipids from lipid-containing infectious viral organisms are employed thereby creating immunogenic modified, partially delipidated viral particles with reduced infectivity. Provided are delipidated viral vaccine compositions, such as therapeutic vaccine compositions, comprising these modified, partially delipidated viral particles with reduced infectivity, optionally combined with a pharmaceutically acceptable carrier or an immunostimulant. The vaccine composition is administered to a patient to provide protection against a lipid-containing infectious viral organism or, as a therapeutic vaccine, to treat or alleviate infection by the lipid-containing infectious viral organism. The vaccine compositions of the present invention include combination vaccines of modified viral particles obtained from one or more strains of a virus and / or one or more types of virus.
Owner:LIPID SCI +1
Combination Vaccines With Whole Cell Pertussis Antigen
InactiveUS20090214586A1Speeding drying processImproving immunogenicityAntibacterial agentsViral antigen ingredientsMeningococcal carriageTime of use
Vaccines have been studied that comprise (a) D-T-Pw-HepB-Hib antigens and (b) one or more meningococcal conjugate antigens. A number of improvements and variations of these vaccines have been discovered. The vaccines can be prepared extemporaneously at the time of use by mixing together two components: (a) a first component comprising D, T, wP and HBsAg antigens; and (b) a second component comprising a Hib conjugate and one or more meningococcal conjugates.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com