Capsular polysaccharide solubilisation and combination vaccines
a polysaccharide and solubilisation technology, applied in the field of vaccines, can solve the problems of short protection time, poor immune response, and inability to use infants, and achieve the effect of improving the immunogenicity of the mena component, enhancing the immunogenicity of the menw135 saccharide, and enhancing the ability of the menw135 antigen to elicit an immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
A. Production and Purification of Meningococcal Polysaccharides
[0128]Meningococci of serogroups A, W135 and Y were grown in 500 ml flasks containing 150 ml of Franz A as medium, for 12 hours at 35±1° C. Agitation was set at 150 rpm using a 35 mm throw Shaker. 85 ml of the culture was then inoculated in 20 L fermentor containing Watson as medium.
[0129]After 18.5 hours (W135 and Y) or 16.5 hours (A), when OD=10 was reached, the fermentation was interrupted by adding 300 ml of formalin and then, after 2 hours of incubation, and the fermentor was cooled to 10° C. The supernatant was collected by centrifugation followed by filtration (0.22 μm), and ultrafiltration with a 30 kDa membrane.
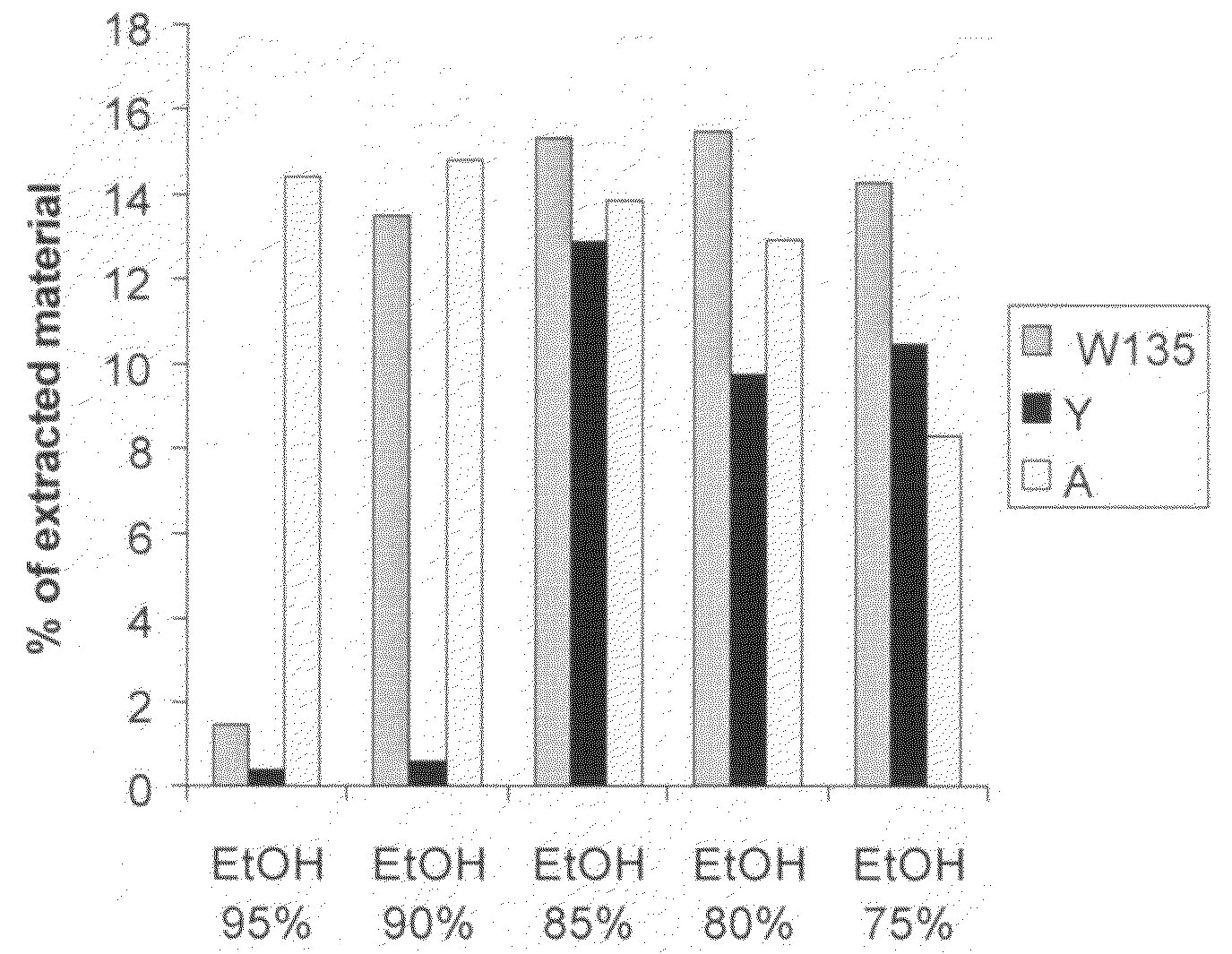
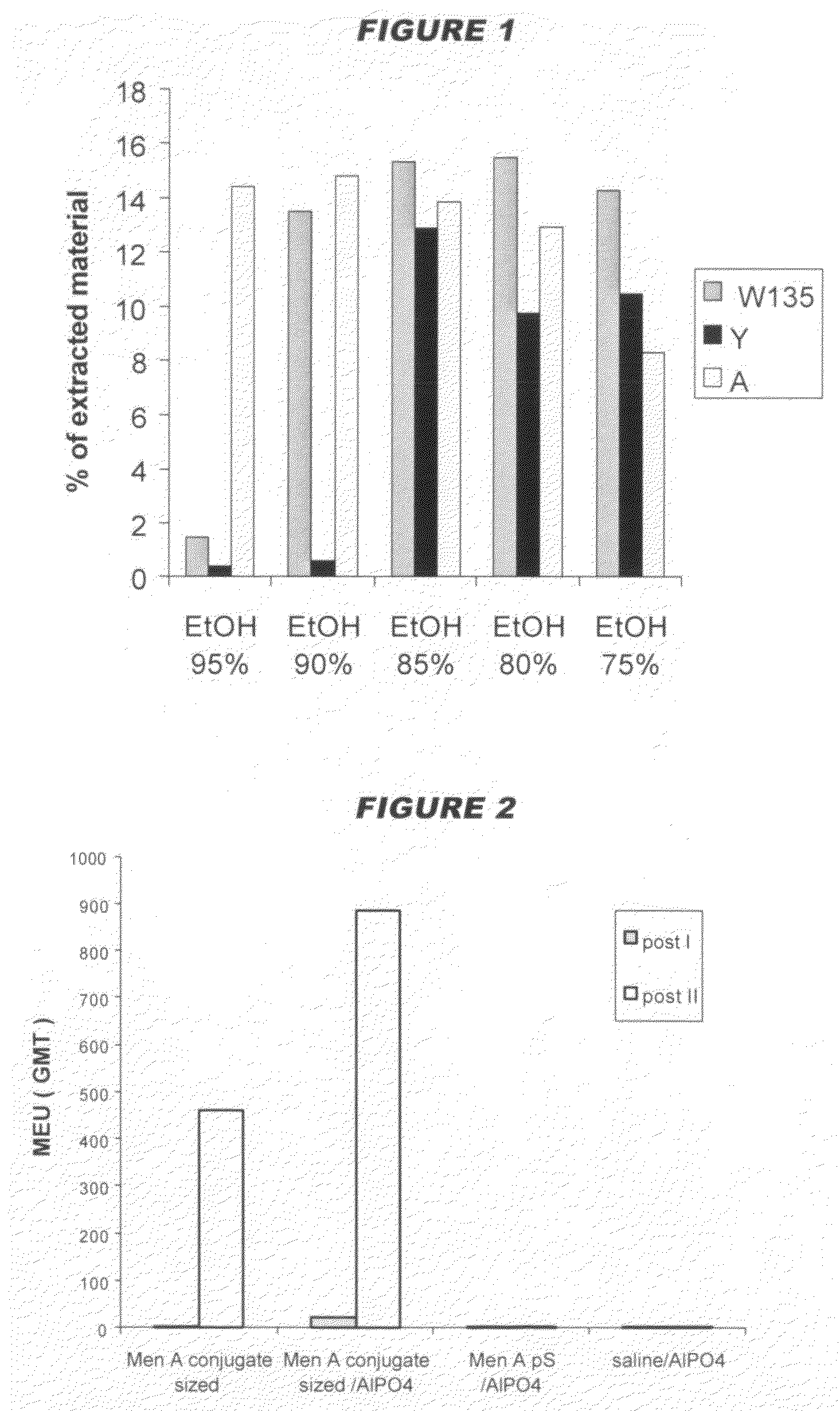
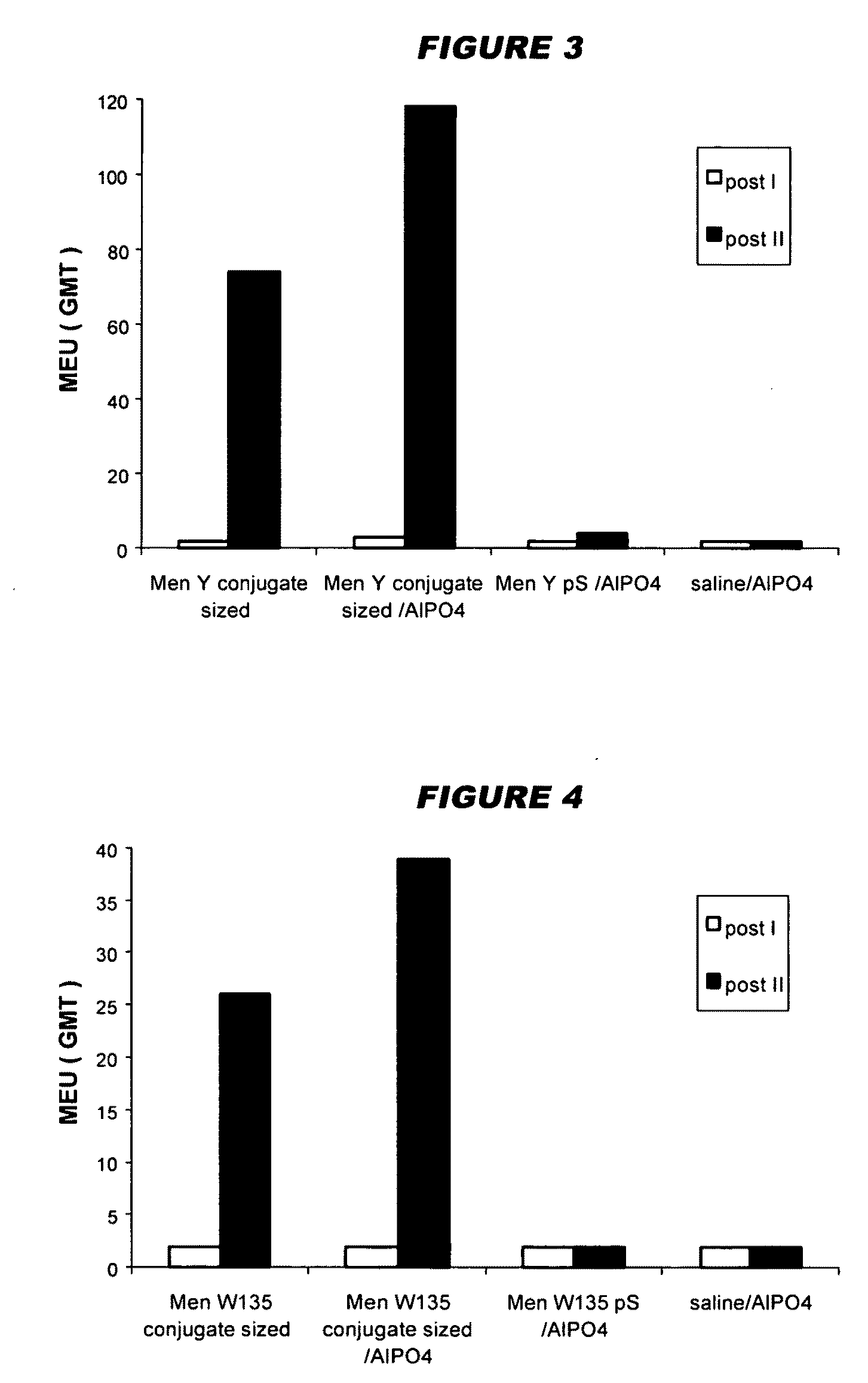
[0130]The crude concentrated polysaccharide was then precipitated by addition of CTAB as a 100 mg / ml water solution. The volumes added are shown in the following table. After 12 hours at room temperature, the CTAB complexes were recovered by centrifugation. The CTAB complex was extracted by adding a 95% e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com