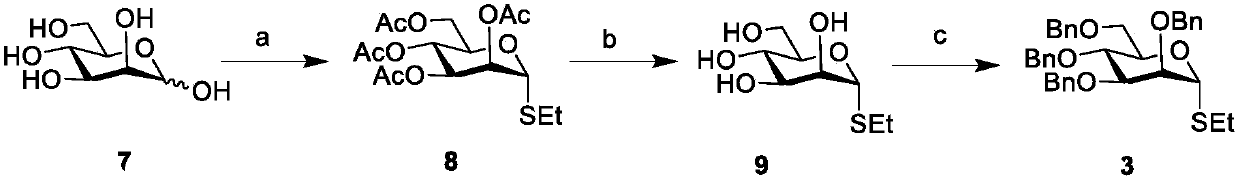
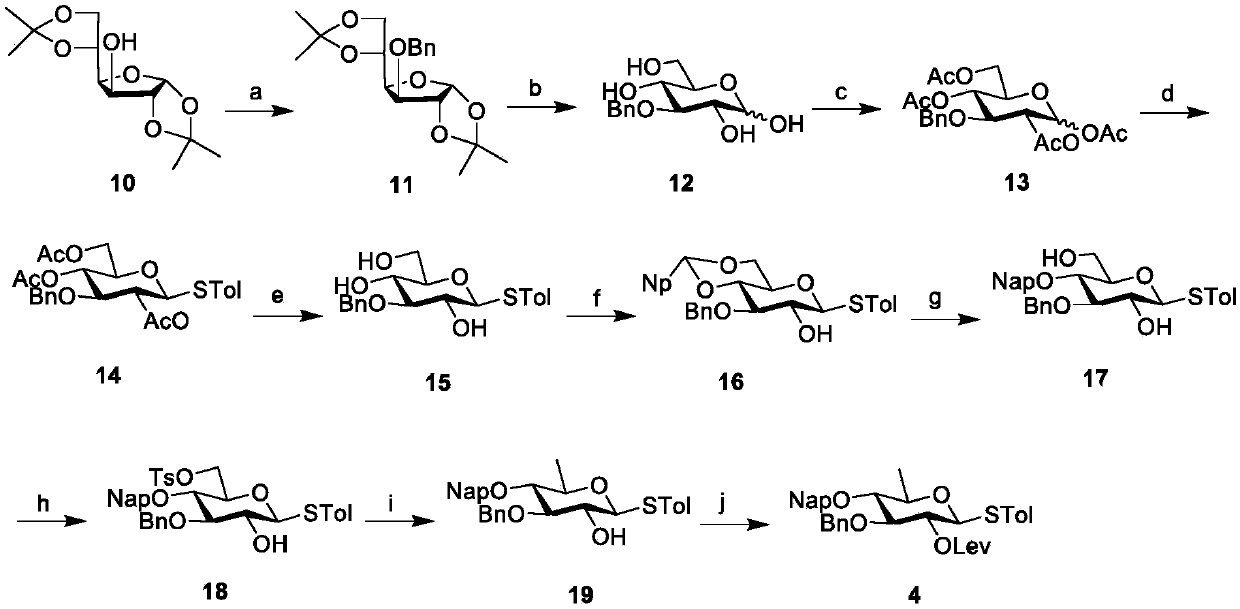
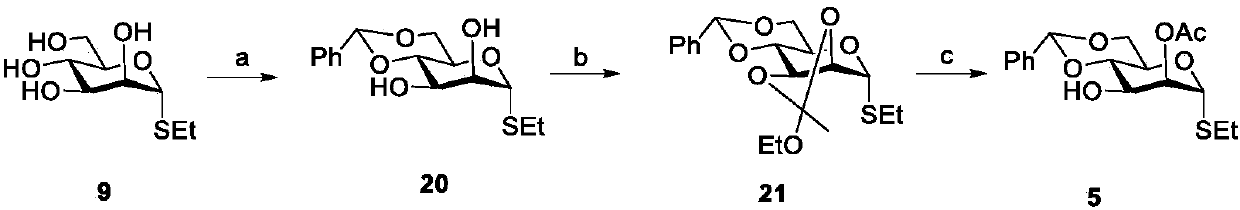
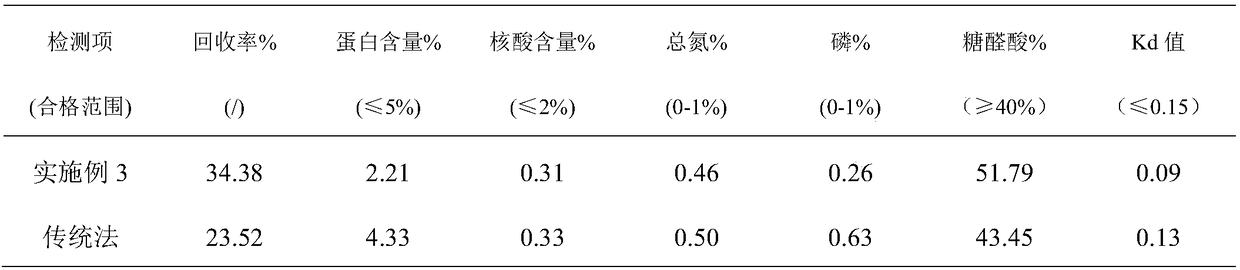
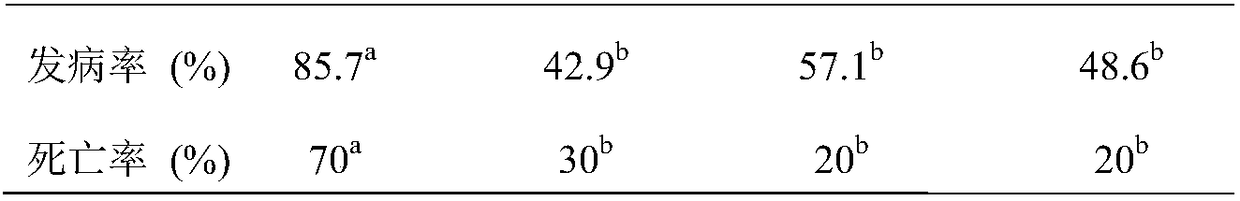
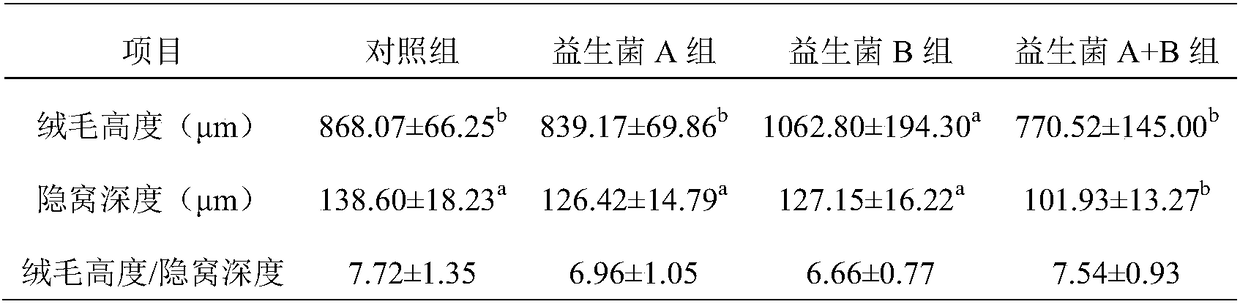
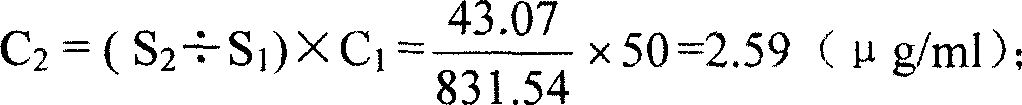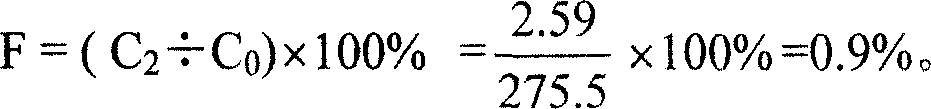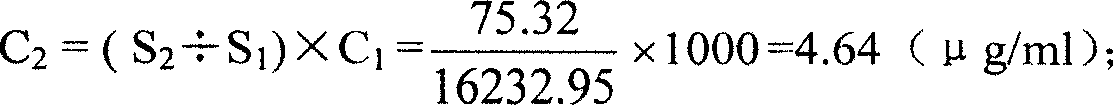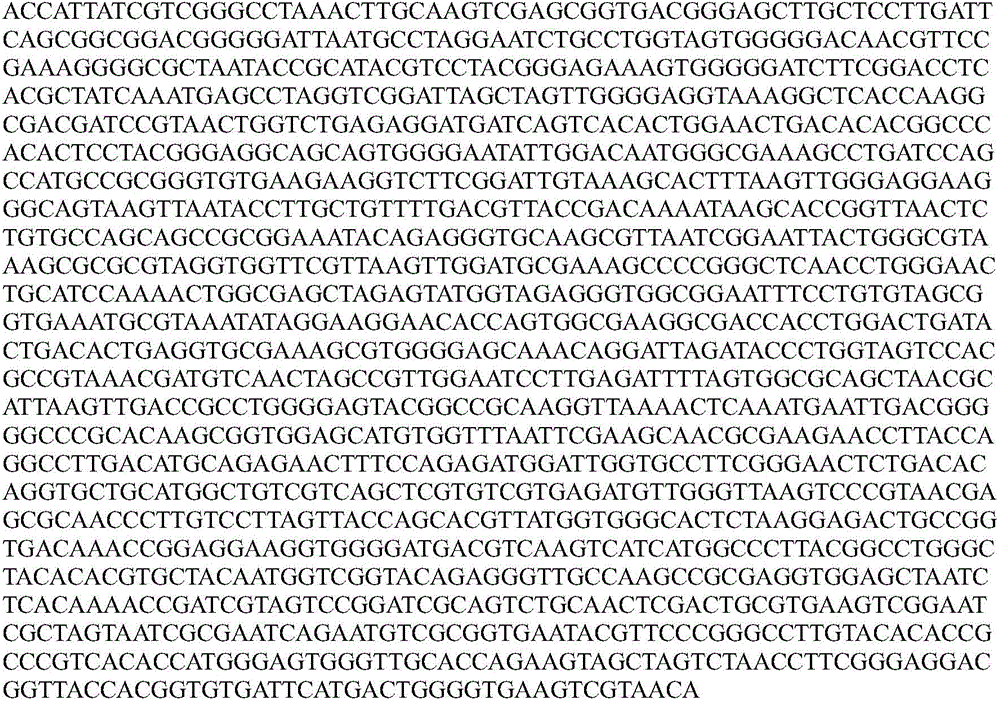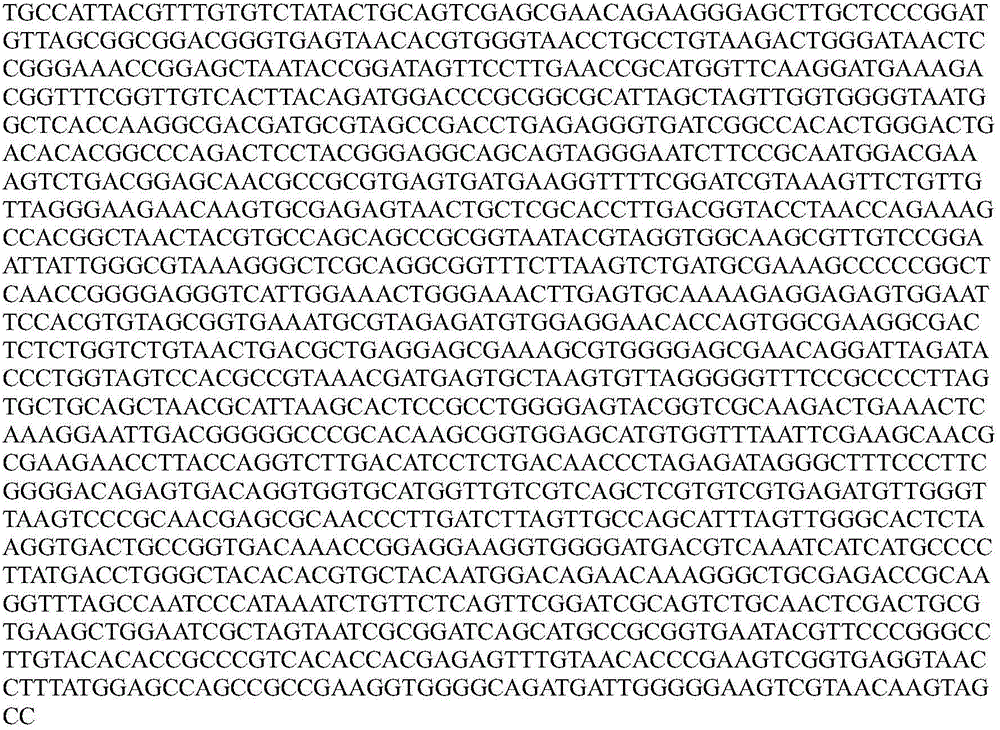Patents
Literature
65 results about "Bacterial capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
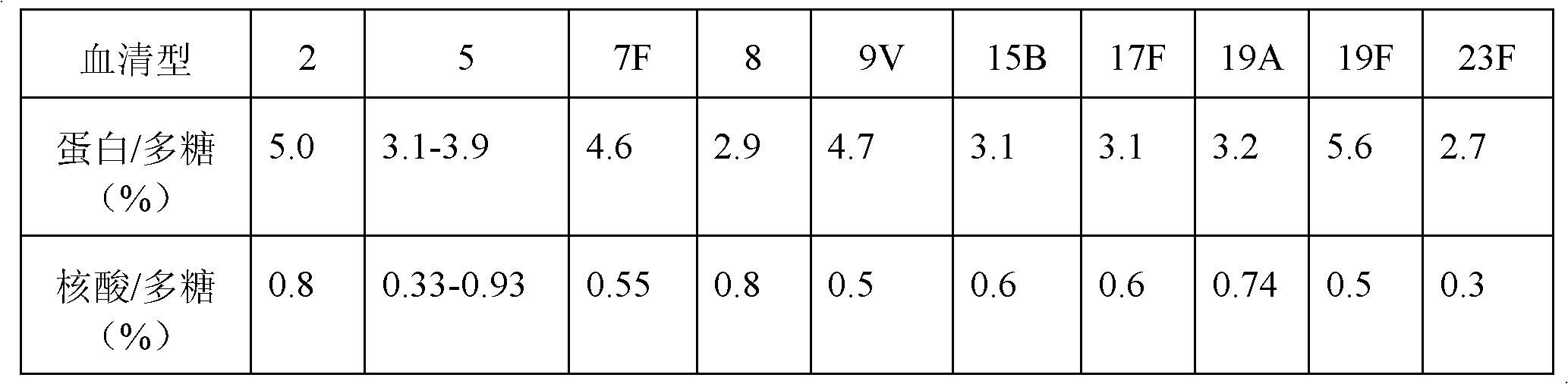
The bacterial capsule is a very large structure of many bacteria. It is a polysaccharide layer that lies outside the cell envelope, and is thus deemed part of the outer envelope of a bacterial cell. It is a well-organized layer, not easily washed off, and it can be the cause of various diseases.
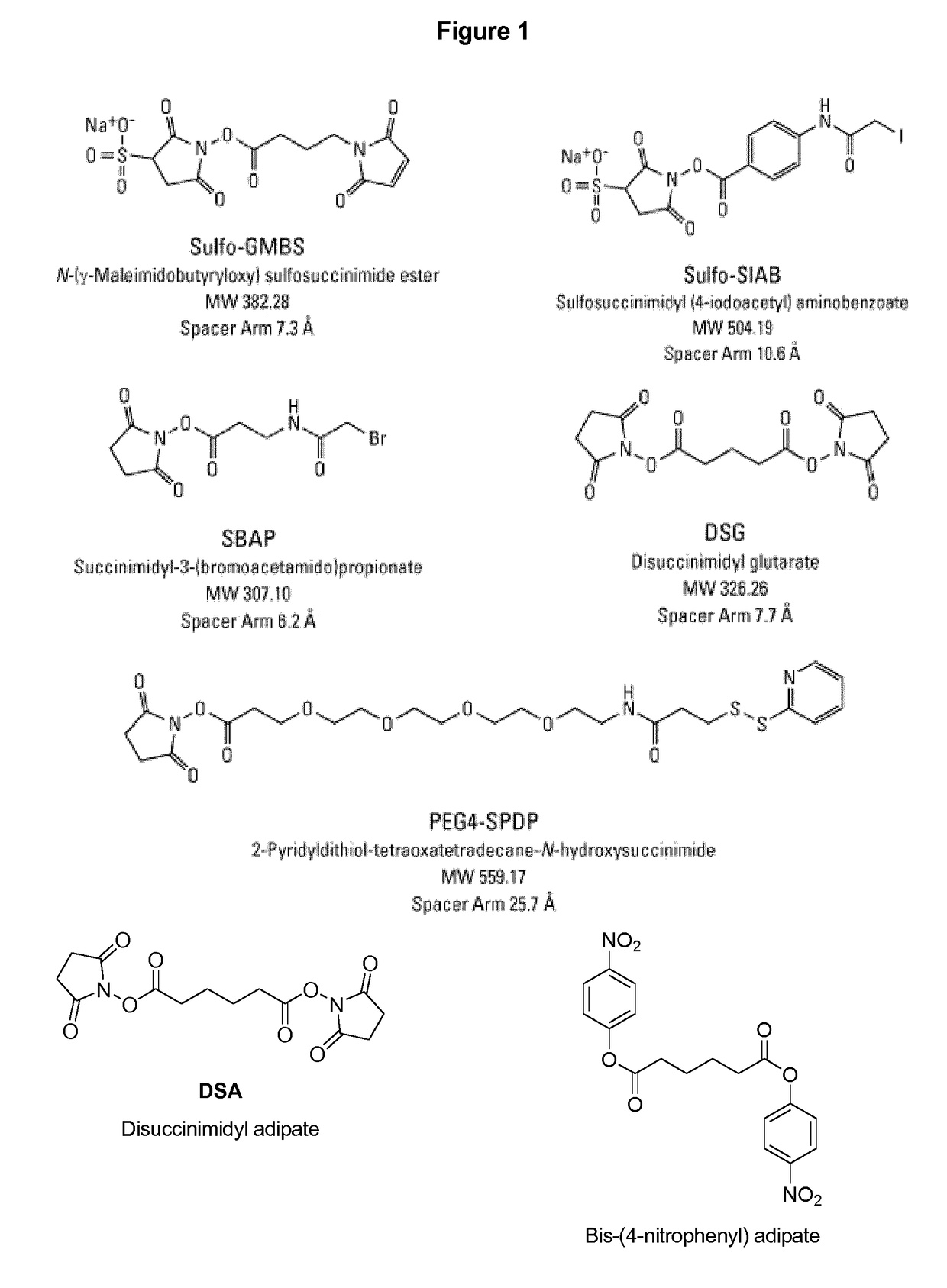
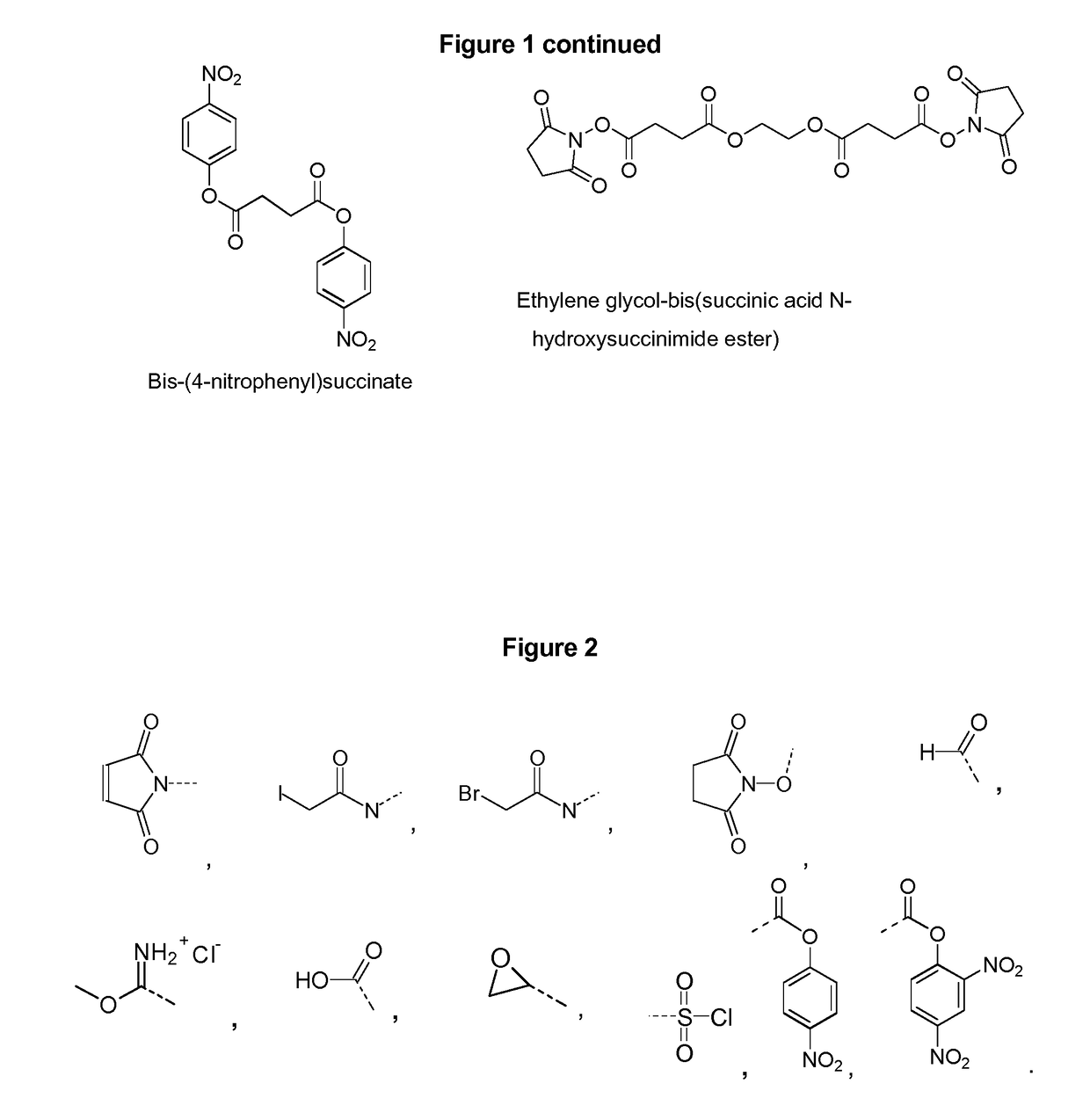
Capsular polysaccharide solubilisation and combination vaccines
ActiveUS20050106181A1Improving immunogenicityW13 dosProtein composition from fishAntibacterial agentsAlcohol ethylMicrobiology
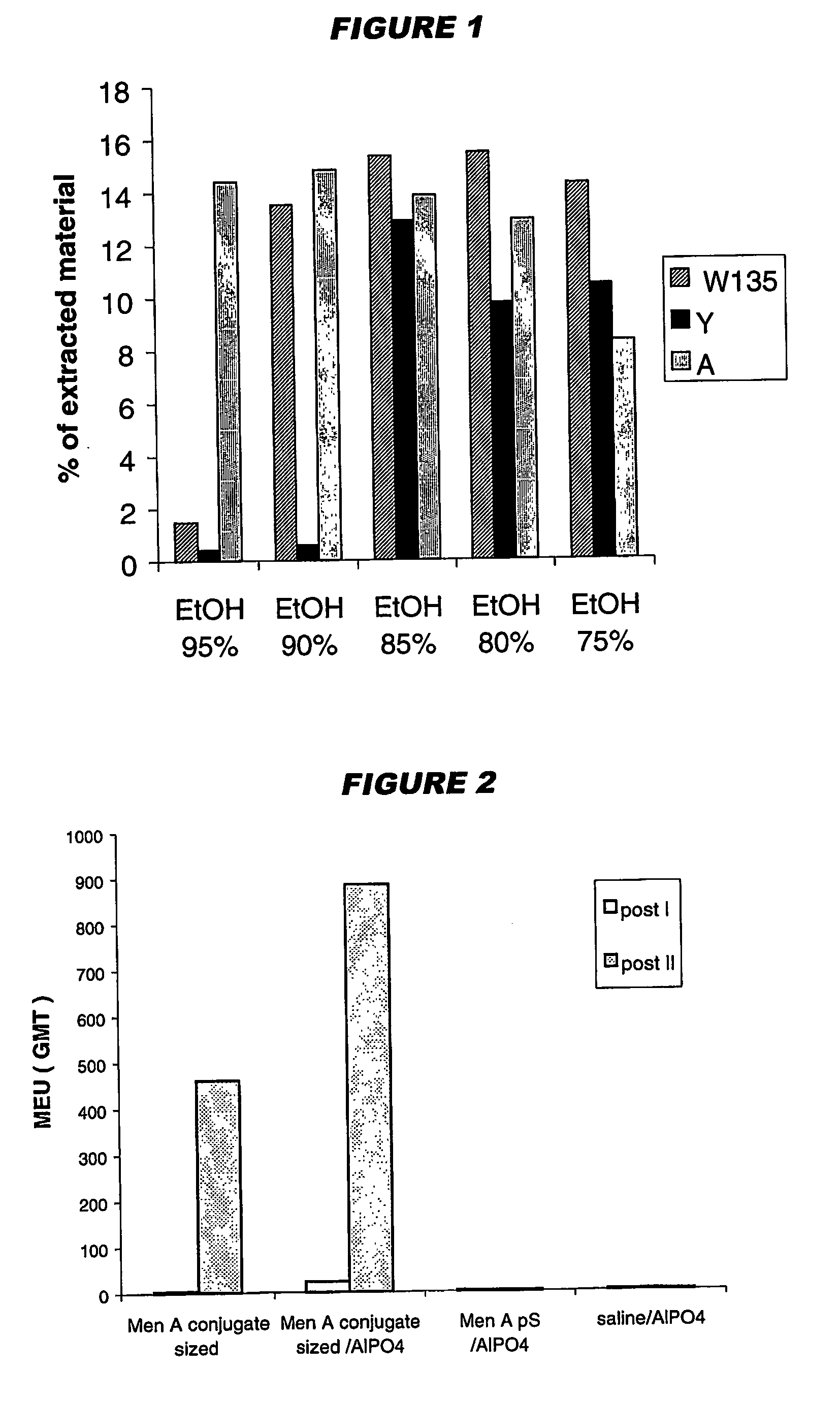
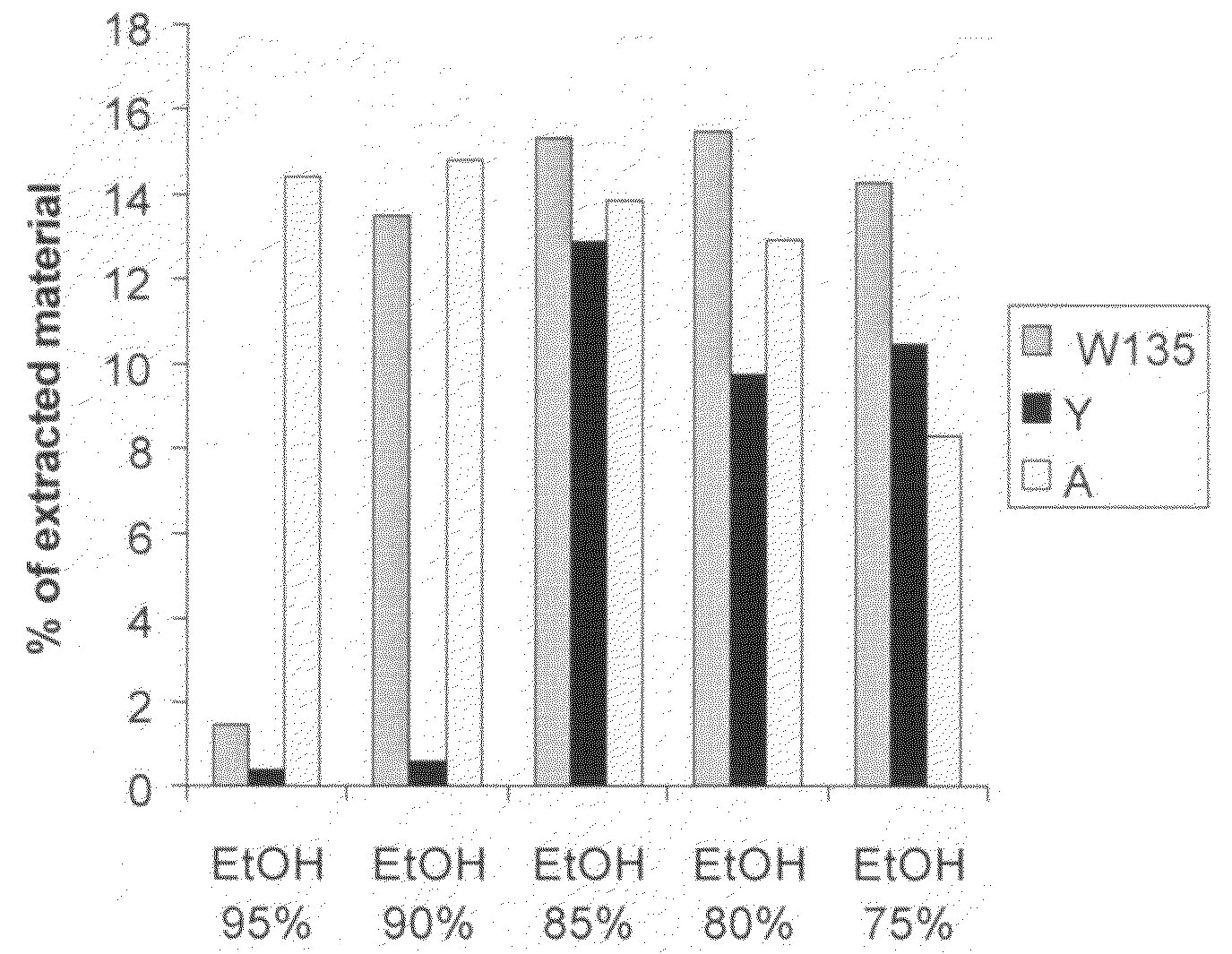
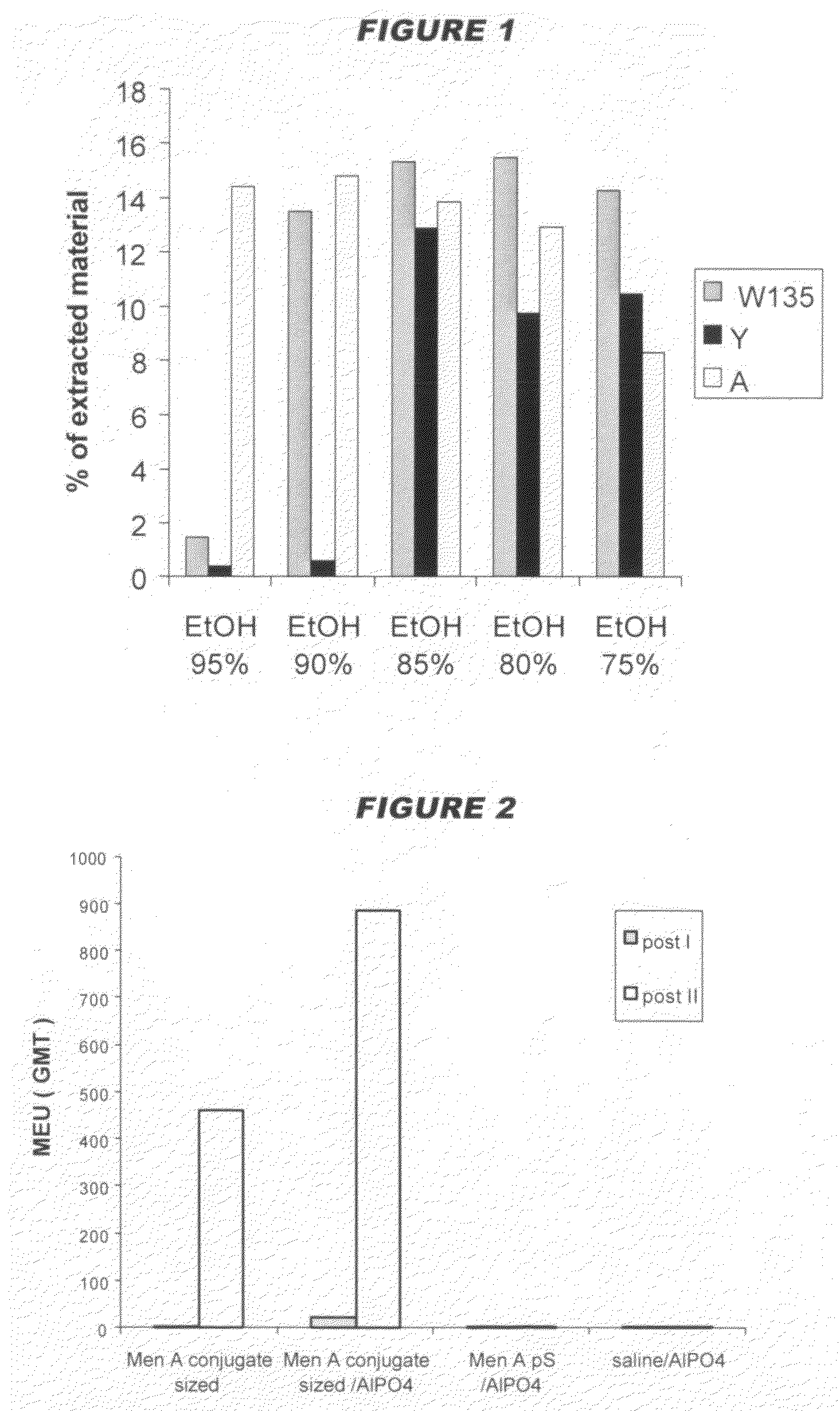
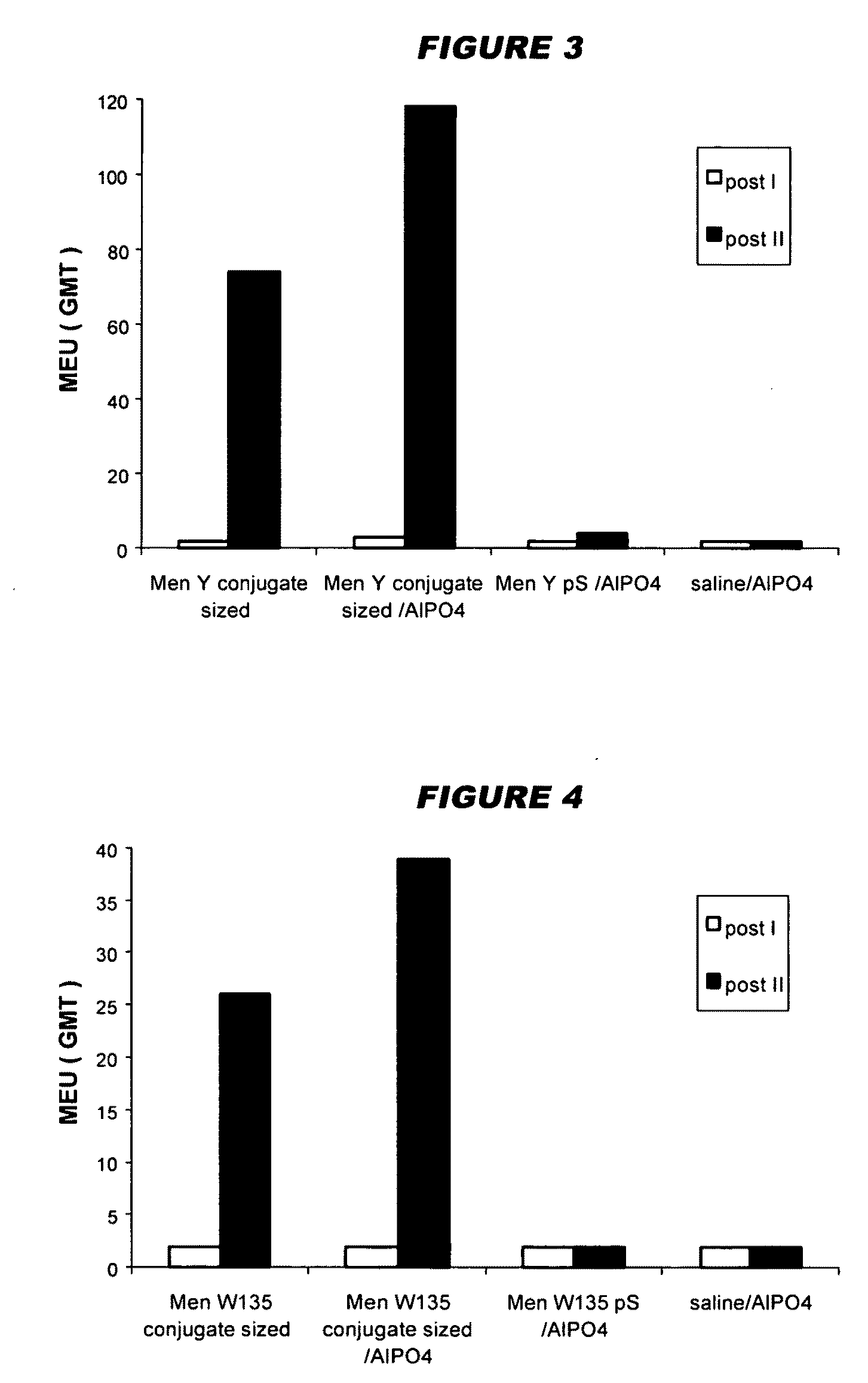
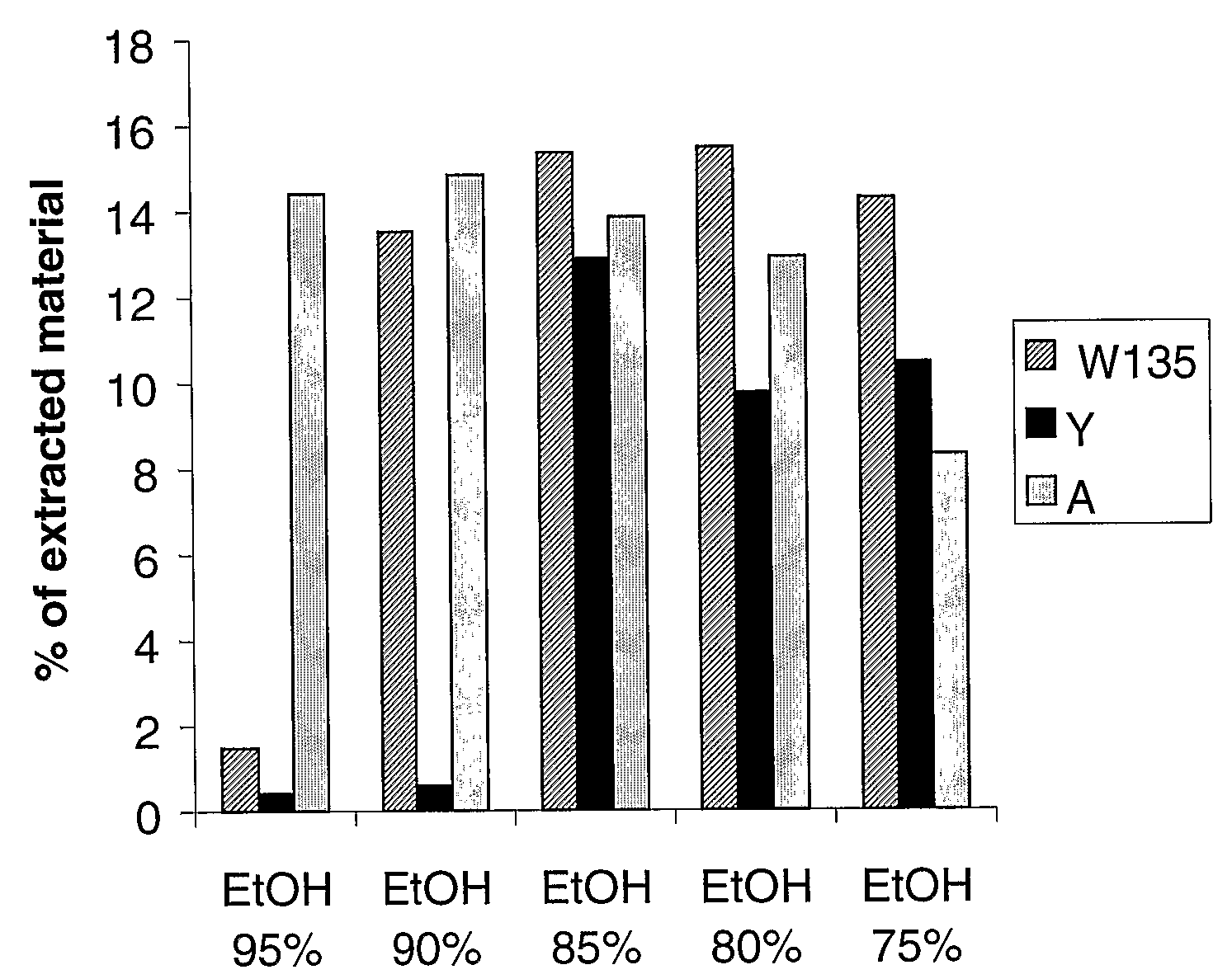
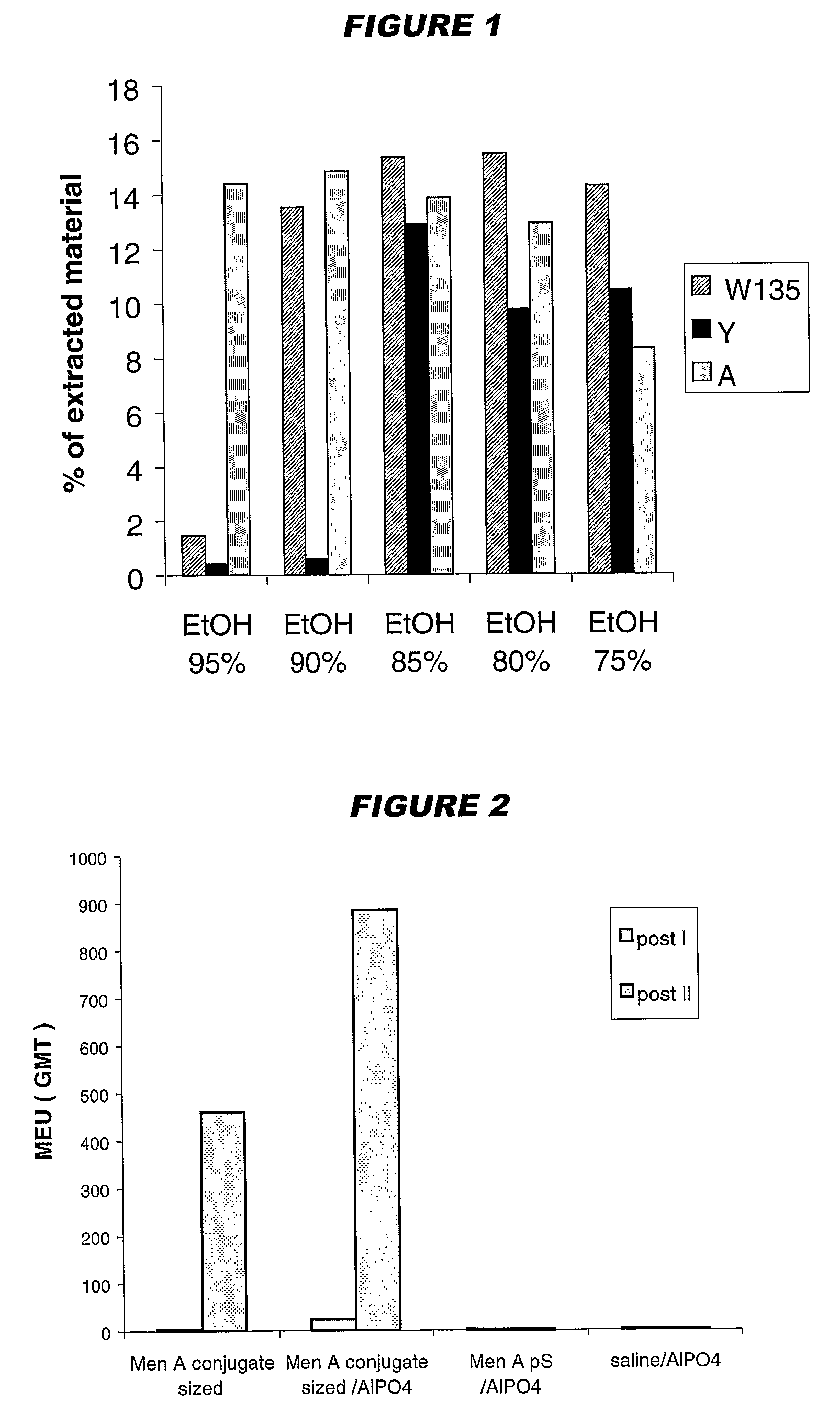
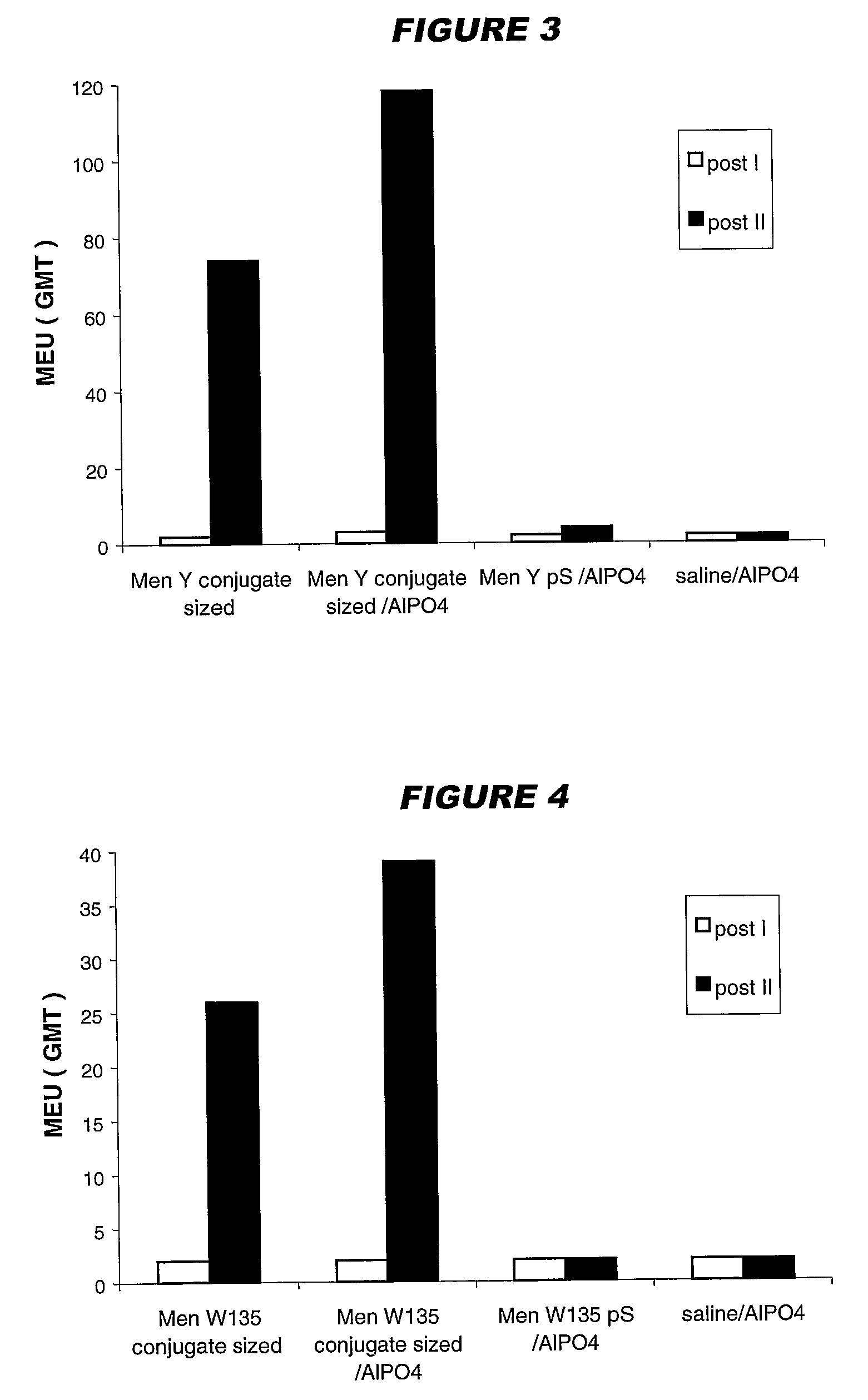
Precipitated bacterial capsular polysaccharides can be efficiently re-solubilised using alcohols as solvents. The invention provides a process for purifying a bacterial capsular polysaccharide, comprising the steps of (a) precipitation of said polysaccharide, followed by (b) solubilisation of the precipitated polysaccharide using ethanol. CTAB can be used for step (a). The material obtained, preferably following hydrolysis and sizing, can be conjugated to a carrier protein and formulated as a vaccine. Also, in vaccines comprising saccharides from the serogroups A and C, the invention provides that the ratio (w / w) of MenA saccharide:MenC saccharide is >1.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Polyvalent bacteria capsule polysaccharide-protein conjugate combined vaccine
ActiveCN1709505AImproving immunogenicityReduce the number of vaccinationsBacterial antigen ingredientsHaemophilusMedicine
The present invention relates to a polyvalent bacterial capsule polysaccharide-protein conjugate combined vaccine preparation, in particular, it is a combined vaccine containing group A, group C, group Y and group W135 epidemic cerebrospinal meningitis coccal capsule polysaccharide-protein conjugate and b type haemophilus influenzal capsule polysaccharide-protein conjugate.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM
Pneumo-streptococcal-polysaccharide adventitia jointed vaccine and preparing method
InactiveCN101024079AEasy to manufactureImprove immunityAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The present invention relates to a streptococcus pneumoriae polysaccharide-outer membrane protein combined vaccine and its preparation method, belonging to the field of streptococcus pneumoriae vaccine. The main antigen component of said vaccine is a streptococcus pneumoriae capsular polysaccharide-outer membrane protein combined product obtained by covalently connecting the capsular polysaccharide produced by streptococcus pneumoriae with its outer membrane protein. Said capsular polysaccharide is the capsular polysaccharide of one or several kinds of streptococcus pneumoriae, its molecular weight is about 200-500 KDa, every polysaccharide molecule has about 300-700 repeating units. The outer membrane protein is the outer membrane protein of one or several kinds of streptococcus pneumoriae, its molecular weight is about 30-100 Kda. Said vaccine can be used for preventing or curing the diseases induced by streptococcus pneumoriae.
Owner:CHANGHUI BIOLOGICAL ENG FUZHOU
Therapeutic nucleic acid-3' -conjugates
InactiveUS20050191680A1Efficient killingPrevent breakdownMaterial nanotechnologyActivity regulationHalf-lifeWhite blood cell
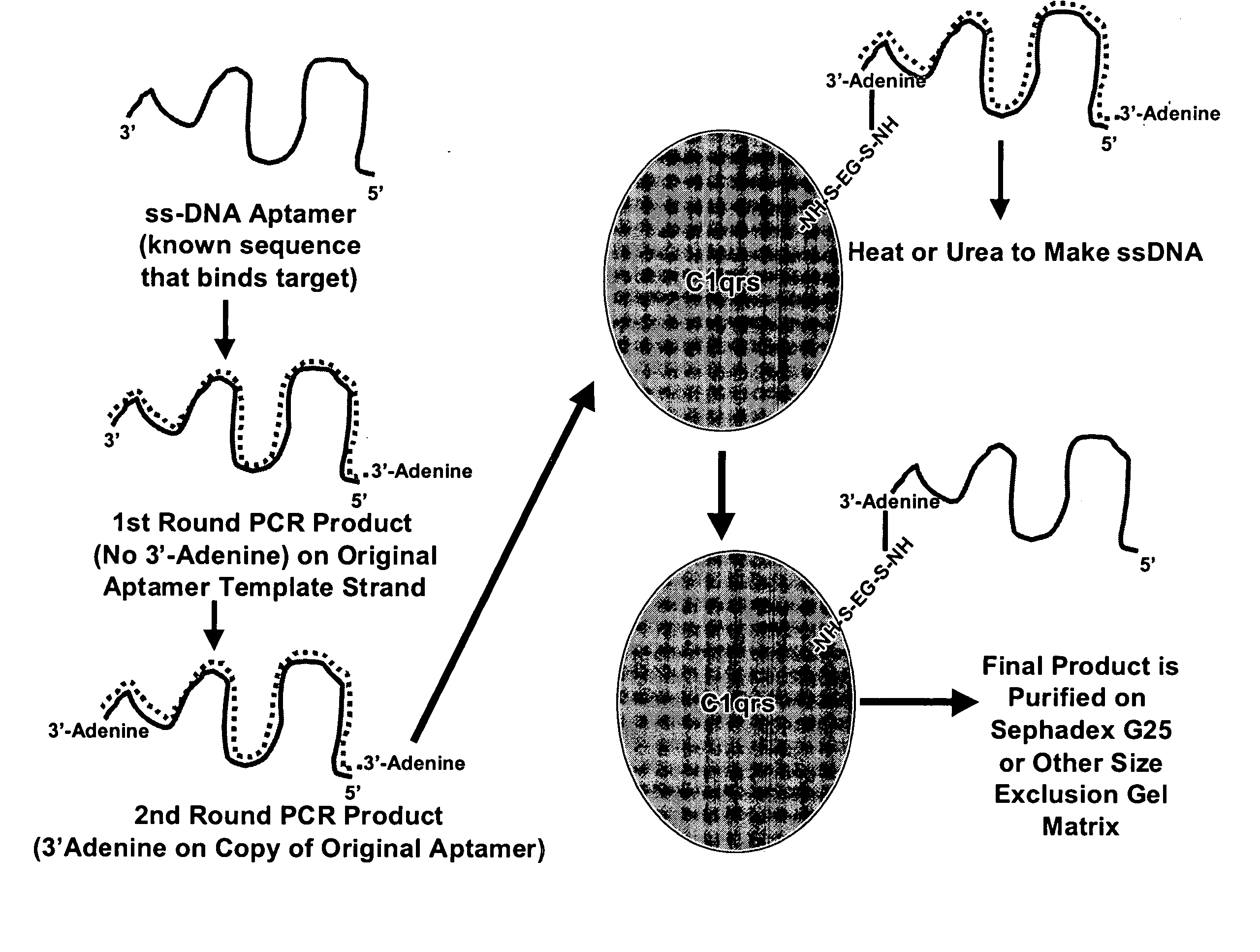
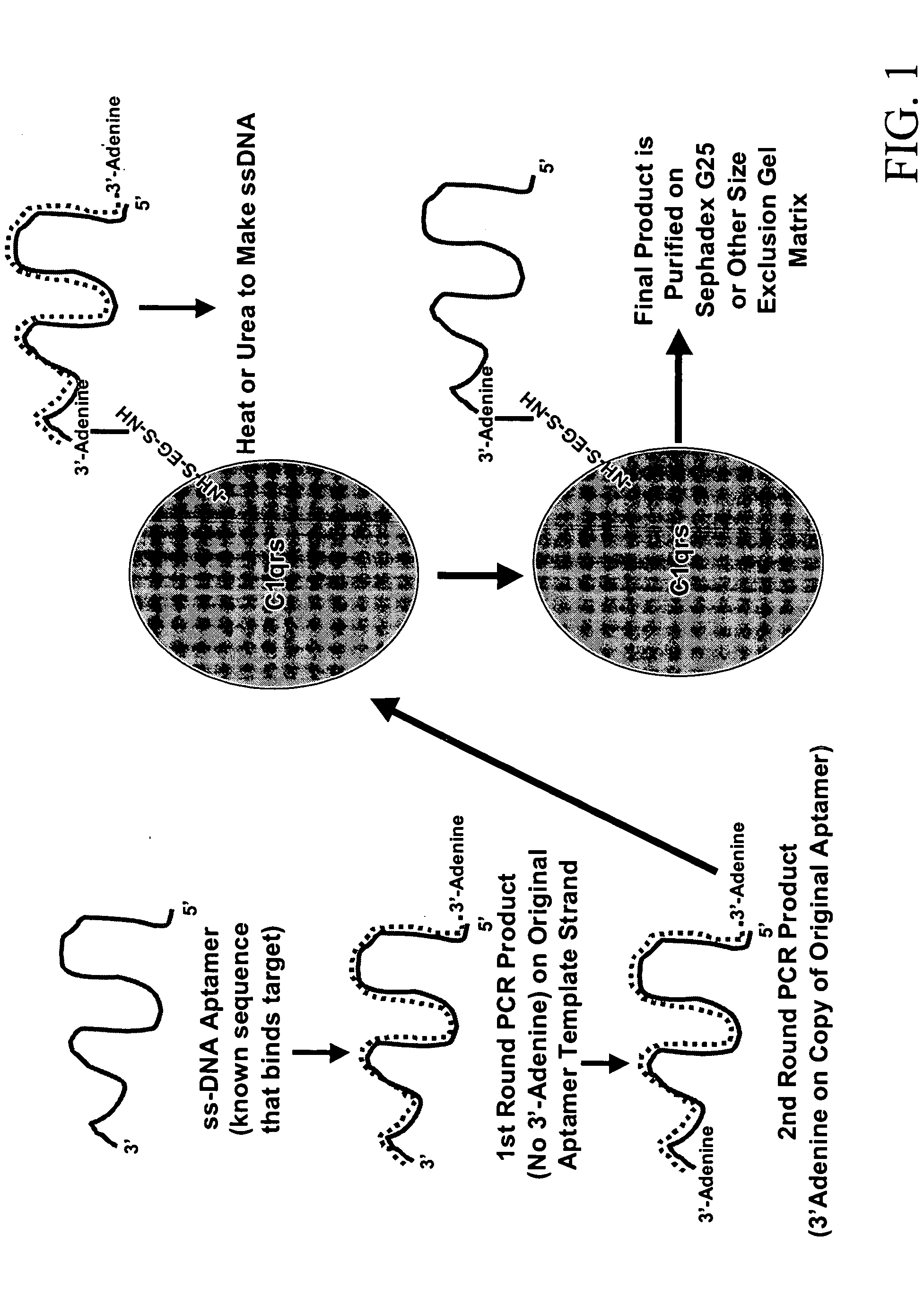
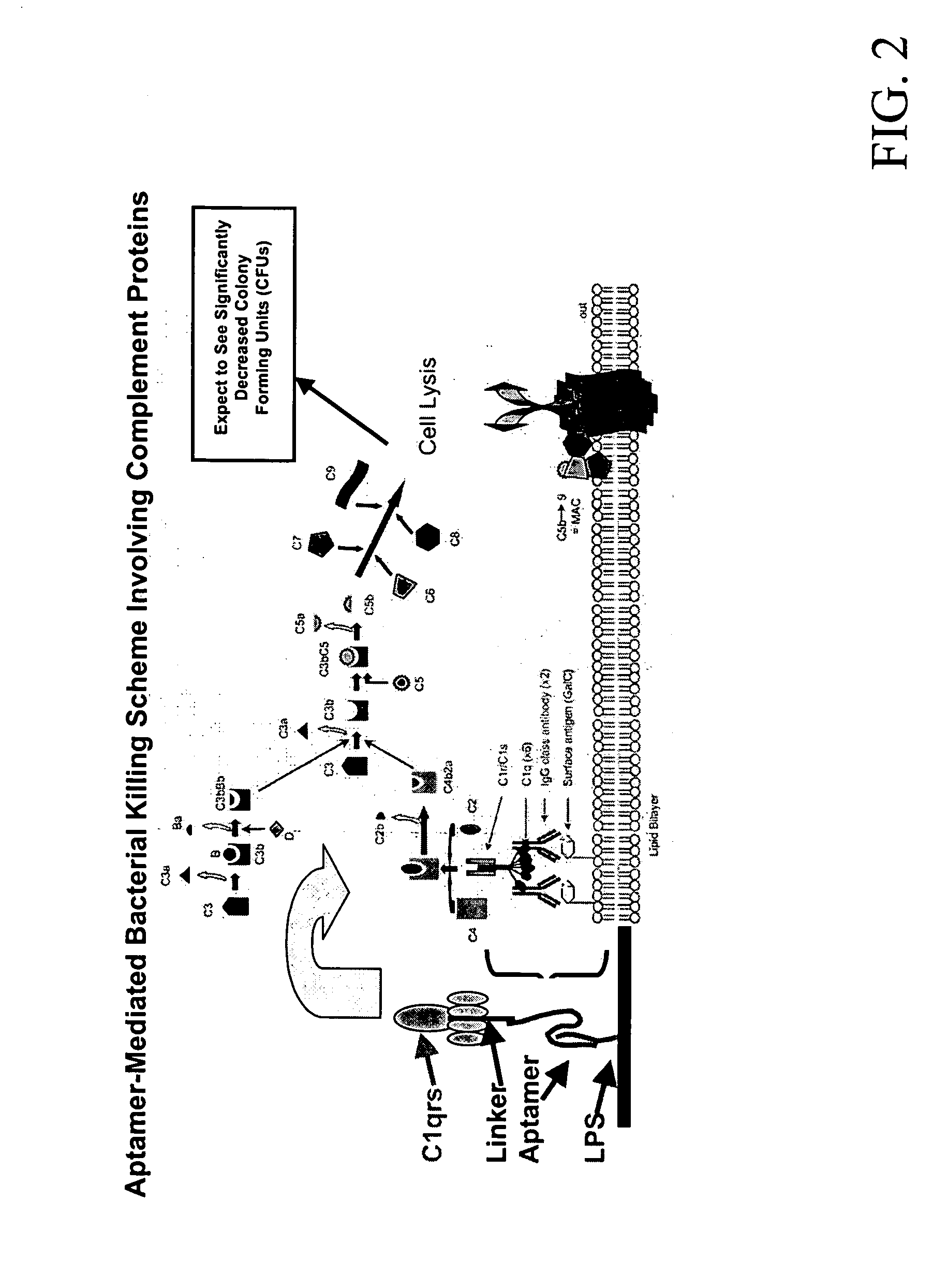
Methods are described for improvement of the serum half life of therapeutic nucleic acids by 3′ conjugation to useful target proteins, or other large molecules with useful function. In one embodiment, a 3′ A, C or G overhang is added to ds-DNA and the primary amines conjugated using biocompatible bifunctional linkers to proteins. The resulting nucleic acid-3′-conjugates are serum nuclease-resistant and retained in vivo for long periods without rapid kidney clearance. Further, the choice of conjugate imparts additional functionality to the nucleic acid-3-conjugate. For example, if the protein in the DNA-protein conjugate is the first component of the complement cascade (Clq or Clqrs) and the DNA aptamer has been developed against surface components of a target cell, it can be used to treat bacterial or parasitic infections and cancers. If the protein is serum albumin or another common (nonimmunogenic) blood protein and the aptamer is directed against a toxin or venom, the aptamer-protein conjugate can be used as an antidote that binds and neutralizes the toxin or venom. Similar DNA (aptamer)-nanotube, -enzyme, and -toxin conjugates could also be used to target and selectively kill bacteria, parasites, and cancer cells in vivo. If the protein is an Fc antibody fragment or C3b protein from the complement system and the aptamer is developed against a bacterial cell capsular material, other cell surface component or viral cell surface component, then the aptamer-3′-protein conjugate can aid in opsonization of the target cells or viruses by phagocytic leukocytes.
Owner:OTC BIOTECH
Capsular polysaccharide solubilisation and combination vaccines
InactiveUS20090130147A1Improving immunogenicityAntibacterial agentsPeptide/protein ingredientsAlcohol ethylMicrobiology
Precipitated bacterial capsular polysaccharides can be efficiently re-solubilised using alcohols as solvents. The invention provides a process for purifying a bacterial capsular polysaccharide, comprising the steps of (a) precipitation of said polysaccharide, followed by (b) solubilisation of the precipitated polysaccharide using ethanol. CTAB can be used for step (a). The material obtained, preferably following hydrolysis and sizing, can be conjugated to a carrier protein and formulated as a vaccine. Also, in vaccines comprising saccharides from both serogroups A and C, the invention provides that the ratio (w / w) of MenA saccharide:MenC saccharide is >1.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Capsular Polysaccharides Solubilisation and Combination Vaccines
InactiveUS20090117148A1Protective efficacyImproving immunogenicityAntibacterial agentsProtein composition from fishAlcoholSalmonella serotype typhi
Precipitated bacterial capsular polysaccharides can be efficiently re-solubilised using alcohols as solvents. The invention provides a process for purifying a bacterial capsular polysaccharide, comprising the steps of (a) precipitation of said polysaccharide, followed by (b) solubilisation of the precipitated polysaccharide using ethanol. CTAB can be used for step (a). The material obtained, preferably following hydrolysis and sizing, can be conjugated to a carrier protein and formulated as a vaccine. Also, in vaccines comprising saccharides from both serogroups A and C, the invention provides that the ratio (w / w) of MenA saccharide:MenC saccharide is >1.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Methods for the treatment of bacterial infections
This invention relates generally to the discovery of a method of inhibiting, preventing or treating bacterial infections. The invention also relates to a method of inhibiting bacterial capsule biogenesis.
Owner:UNIVERSITY OF KANSAS +2
Capsule staining of bacteria
InactiveCN101255458AIntegrity guaranteedImprove reliabilityMicrobiological testing/measurementBacteroidesAcid fuchsin
The invention relates to a bacterial capsule staining method, comprising the steps: 1) dropping a droplet of sterile water on a glass slide; 2) bacteria culturing for 24 to 48 hours, selecting the bacteria with a transfering loop, and slowly coating the glass slide in water moisture state spirally from the center to the outside to keep the capsule complete; 3) natural drying or blow drying with electric wind; 4) dropping 2-3 droplets of pure methanol, fixing for 1-2 minutes, and inclining to remove the methanol; 5) dropping carbolic acid fuchsin to stain for 1-3 minutes, and covering a cover glass; and 6) absorbing the liquid surrounding the cover glass with a piece of absorbent paper, inclining the slide glass to absorb partial staining liquid under the cover glass. By improvement on the slide making method, the staining method and the operation steps, the completeness of bacterial capsule is effectively kept, the growing state of all the bacterial capsule in the specimen can be clearly displayed, the operation difficulty of capsule staining is obviously reduced, and the reliability of bacterial capsule recognition is improved.
Owner:ZHEJIANG UNIV
Ethoxyphenyl thienyl compounds and methods for the treatment of bacterial infections
This invention relates generally to the discovery of a method of inhibiting, preventing or treating bacterial infections. The invention also relates to a method of inhibiting bacterial capsule biogenesis.
Owner:DUKE UNIV +2
Hyaluronic acid binding peptides enhance host defense against pathogenic bacteria
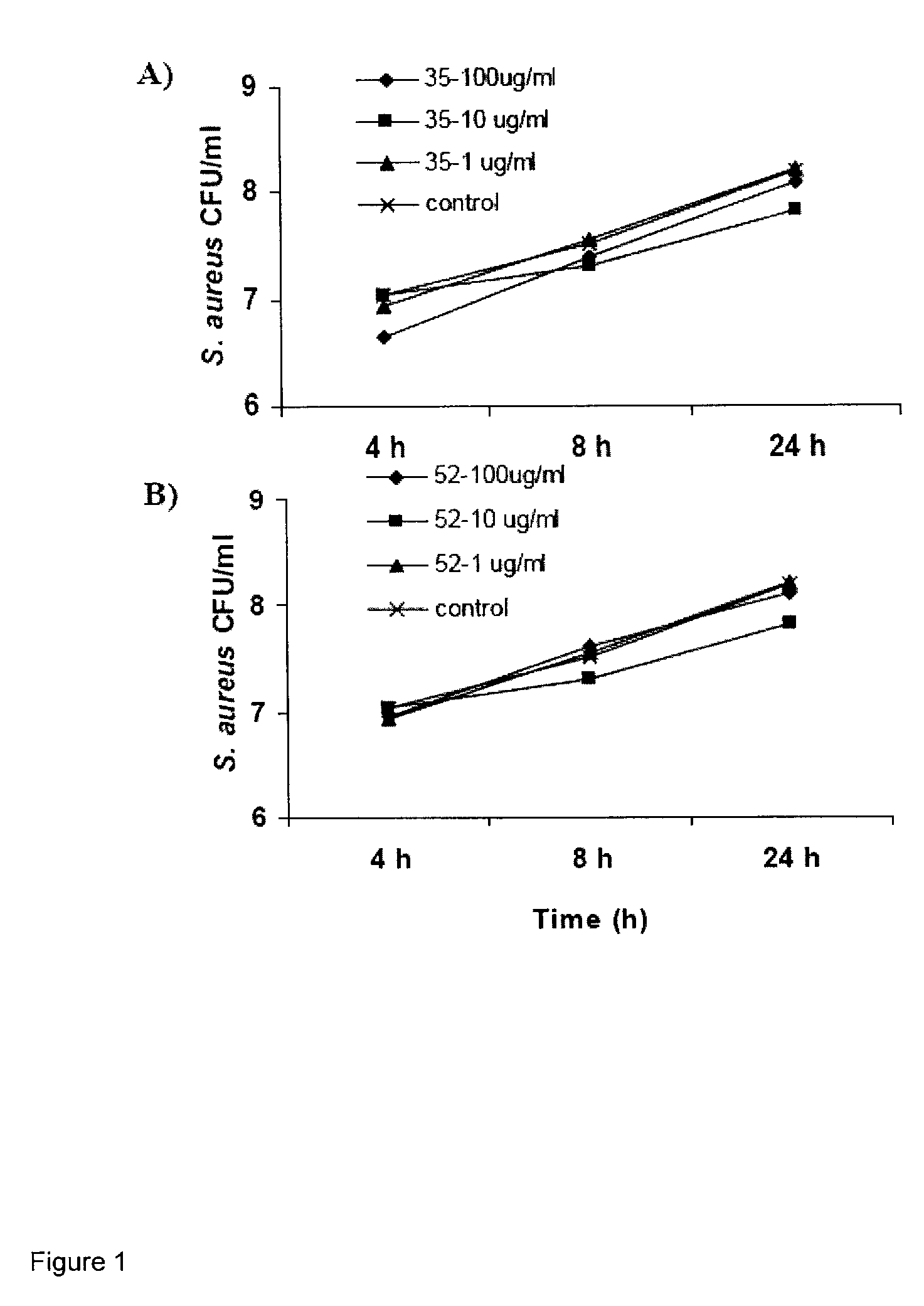
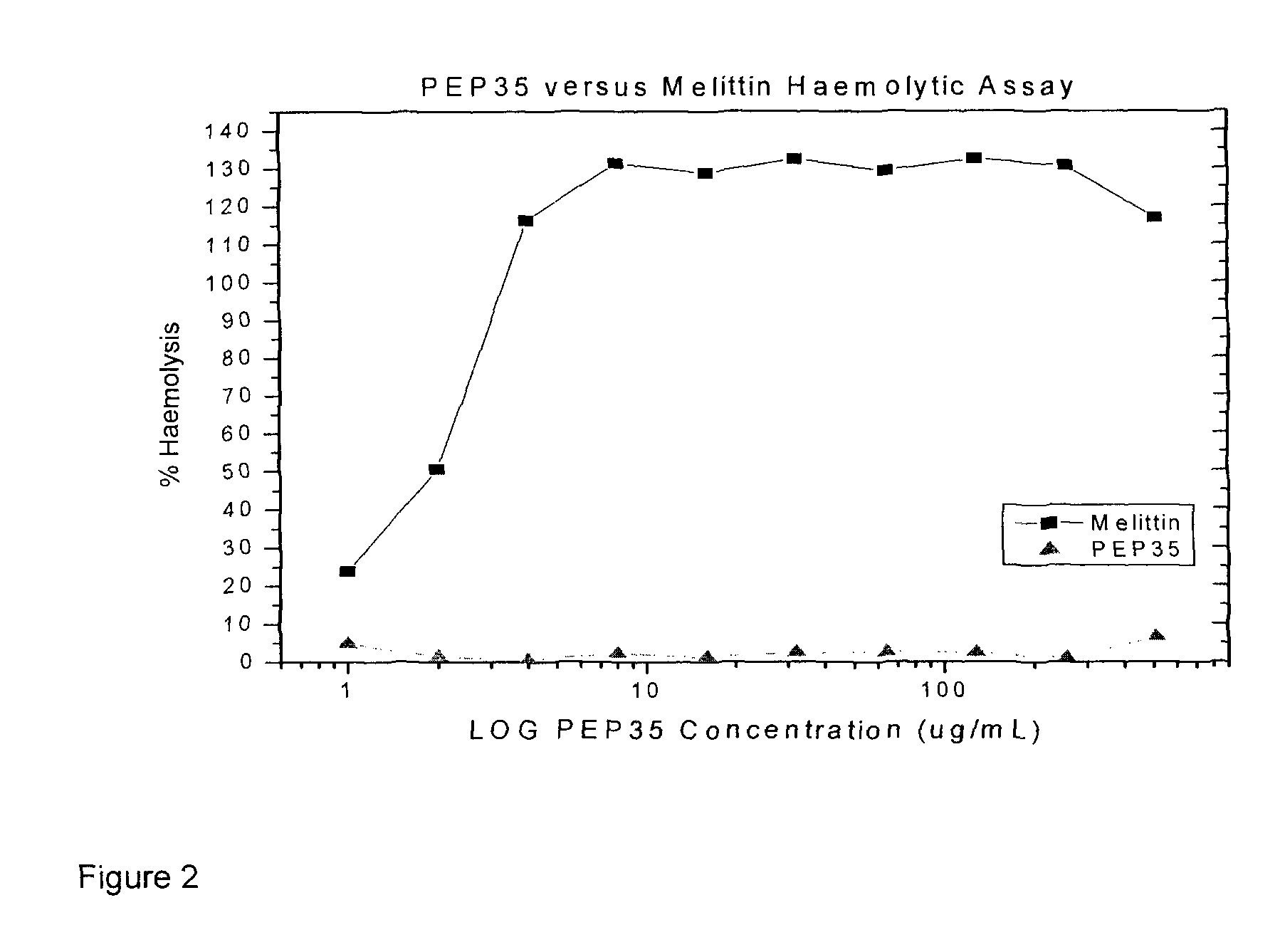
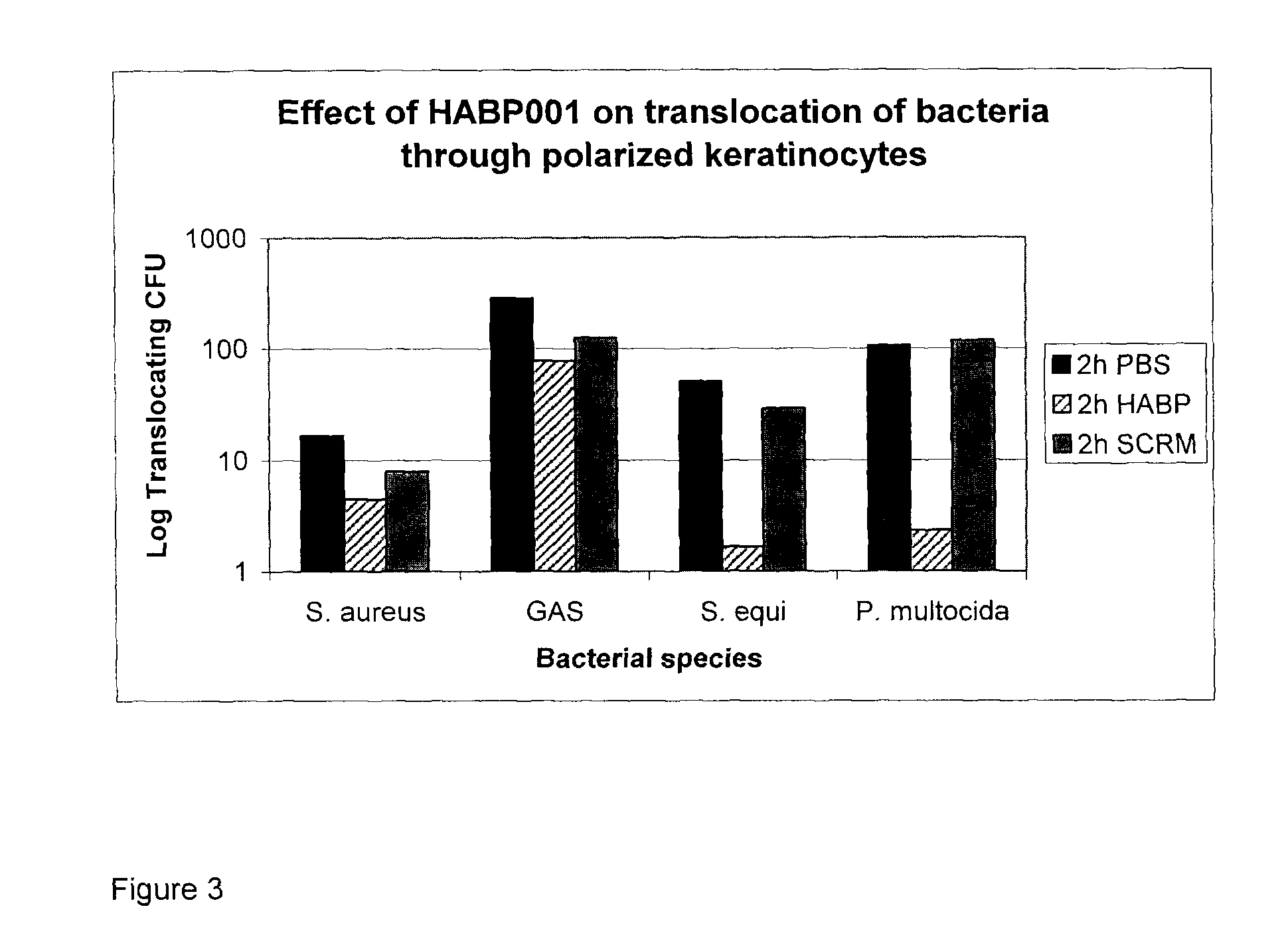
Several species of bacteria capable of invasive infections, such as S. pyogenes, S. equi and P. multocida, contain hyaluronic acid (HA) in their capsules. Bacterial species such as Staphylococcus aureus and related Staphylococci have capsules that contain acidic polysaccharides. Bacterial capsule or bacterial surface binding peptides were synthesized and tested in a culture model of invasive bacterial infections, specifically translocation through polarized keratinocyte cultures. The peptides reduced the translocation of a variety of bacterial species, with a concomitant increase in bacterial internalization by the keratinocytes. In vivo, subcutaneous inoculation of encapsulated GAS treated with peptides delayed bacterial dissemination. In a mouse surgical wound model infected with S. aureus, treatment with peptides reduced the numbers of bacteria and inflammation at the wound site.
Owner:CANGENE CORP
Method for measuring content of free protein in bacterial capsule protein-polysaccharide ligature
InactiveCN101000351AAccurate measurementAccurate Free Protein ContentComponent separationPreparing sample for investigationFree proteinCarrier protein
A method for measuring content of free protein in solder of bacterial capsule protein-polysaccharide includes determining out carrier protein concentration in bacterial capsule protein-polysaccharide solder to be detected by utilizing Lowry manner, using carrier protein corresponding to solder to be detected as sample one and using solder to be detected as sample two, using high efficiency gel chromatography to obtain chromatogram of sample one and two separately then applying external-labeling means to calculate out content of free protein.
Owner:云南沃森生物技术股份有限公司
Cryptococcus Neoformans capsular polysaccharide preparation method
InactiveCN102952201AHigh purityReduce usageMicroorganism based processesFermentationEthanol precipitationChemistry
The invention relates to the fungal polysaccharide preparation field, and concretely relates to a preparation method of Cryptococcus Neoformans capsular polysaccharide. The invention provides a method for preparing the Cryptococcus Neoformans capsular polysaccharide through utilizing cetyl trimethyl ammonium bromide. The method comprises the following steps: 1, processing a secretion supernatant containing the capsular polysaccharide through using calcium acetate and glacial acetic acid, and precipitating through using ethanol to prepare a crude capsular polysaccharide; and 2, processing a solution of the crude capsular polysaccharide through using the cetyl trimethyl ammonium bromide to obtain the purified capsular polysaccharide. The method has the advantages of simplicity, high efficiency, low cost, and effective avoiding of the use of toxic and harmful chemical reagents.
Owner:天津贻诺琦生物工程有限公司
Method for constructing klebsiella with deleted capsula
ActiveCN101397547ALow viscosityReduce pathogenicityBacteriaMicroorganism based processesPathogenicityViscosity
The invention discloses a method for constructing Klebsiella without capsules, pertaining to the technical field of biochemical engineering. In PDO producing bacteria, a gene knockout method is adopted to knock out the promoter of the capsule protosome or integrate a terminator behind the promoter so as to stop the transcription of the capsule protosome, thus leading the capsules of the Klebsiella to be partially or completely deleted. Therefore, the fermentation liquor viscosity of the Klebsiella is reduced, the separation and extraction difficulty of POD products is reduced, simultaneously, the thalli pathogenicity is lowered, thereby being favorable to the PDO industrial production application of the Klebsiella.
Owner:TSINGHUA UNIV +1
Compositions and methods for detection, prevention, and treatment of anthrax and other infectious diseases
ActiveUS20090280513A1High molecular weightEasy to produceAntibacterial agentsBacterial antigen ingredientsSerum igeAgonist
Compositions and methods for the detection, prevention, or treatment of anthrax or other infectious diseases. In one aspect, the present invention provides methods of immunizing humans or animals against Bacillus anthracis or other capsulated pathogens. The methods include administering a capsular polypeptide of a pathogen of interest and a CD40 agonist to a human or animal. The capsular polypeptide or the CD40 agonist is administered in such an amount or frequency that an immunoprotective response can be elicited in the human or animal against the pathogen of interest. In another aspect, the present invention provides methods of using passive immunization with anti-capsular polypeptide antibodies to prevent or treat infections caused by Bacillus anthracis or other pathogens. In yet another aspect, the present invention provides methods useful for diagnosis of anthrax by detection of capsular polypeptide in serum or other biological samples.
Owner:STC UNM +1
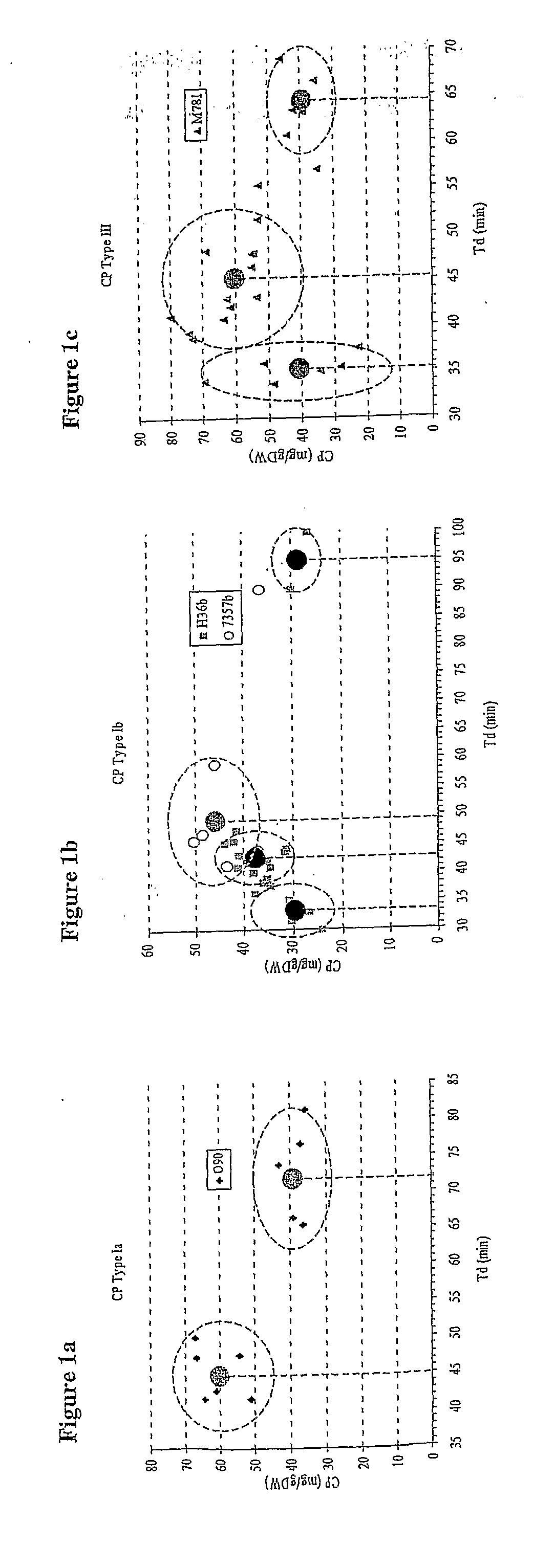
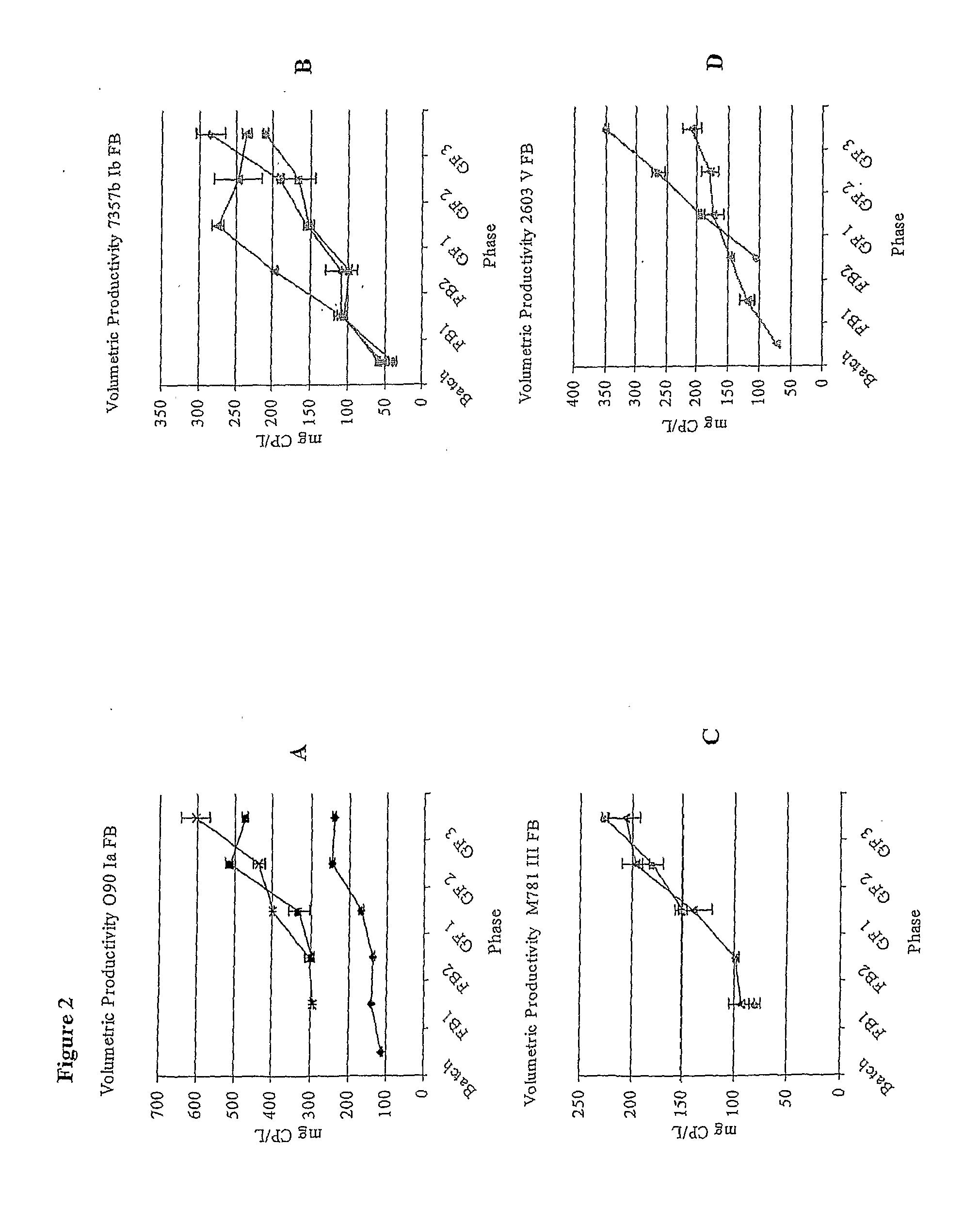
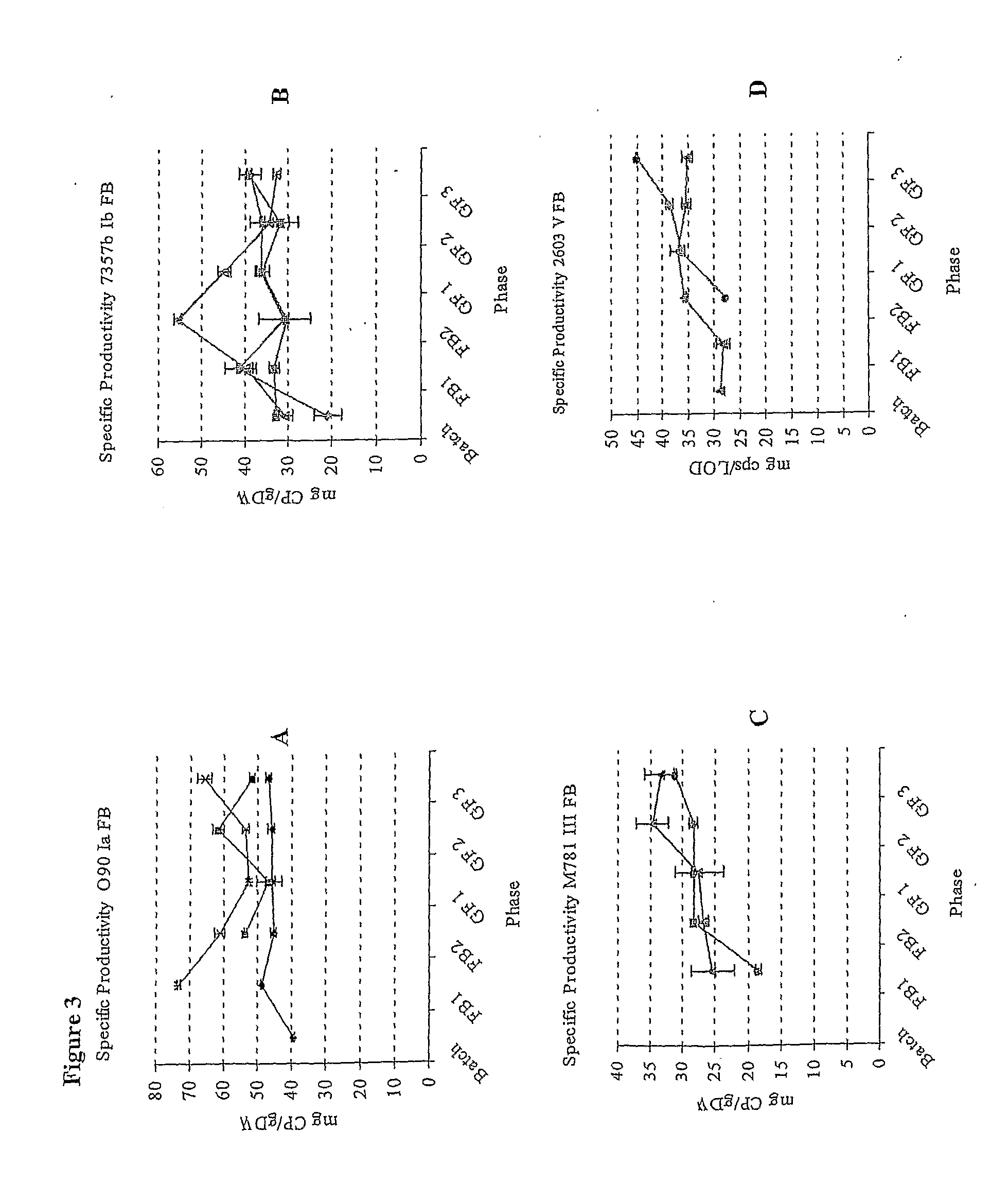
Fed Batch Culture Methods for Streptococci
InactiveUS20080312137A1High yieldAntibacterial agentsBacterial antigen ingredientsMicrobiologyPolysaccharide
This invention relates to the optimization of culture conditions to improve the production of bacterial capsular polysaccharides from Streptococcus strains in fed-batch culture.
Owner:NOVARTIS AG
Method for rapid purification of bacterial capsular polysaccharide
The invention discloses a method for rapid purification of bacterial capsular polysaccharide. The method can fast remove pollutants comprising proteins and nucleic acid from a specific bacterial fermentation broth and retains purified capsular polysaccharide. The method comprises the following steps of fermentation of capsular polysaccharide-containing bacteria, acid adjustment of a pH value of a fermentation broth, precipitation of thalli and impurities of the fermentation broth, centrifugation, microfiltration, ultrafiltration condensation and filter wash so that a crude bacterial capsular polysaccharide solution is obtained. A basic principle of the method comprises that through acid adjustment, a pH value of a fermentation broth is in a range of 3 to 5 so that thalli having whole shapes and other impurities are removed and capsular polysaccharide is retained in the fermentation broth and thus purification of capsular polysaccharide is realized. The method can be used for purification of capsular polysaccharide of pneumococcocci, haemophilus influenzae type b, epidemic meningococcocci and typhoid bacillus.
Owner:KANVAX BIOPHARM
Vaccines against Streptococcus pneumoniae serotype 8
ActiveUS10220083B2Improving immunogenicityImprove flow characteristicsAntibacterial agentsOrganic active ingredientsProtection sexAntibody
The present invention relates to synthetic saccharides of general formula (I) that are related to Streptococcus pneumoniae serotype 8 capsular polysaccharide, conjugates thereof and the use of said saccharides and conjugates for raising a protective immune response in a human and / or animal host. Furthermore, the synthetic saccharide structures of general formula (I) are useful as marker in immunological assays for detection of antibodies against Streptococcus pneumoniae bacteria.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Sedum rhizosphere cadmium-resistant bacillus sp. strain, screening method and application thereof
InactiveCN105018375AIncreased resistance to heavy metal cadmiumBacteriaContaminated soil reclamationHigh resistanceScreening method
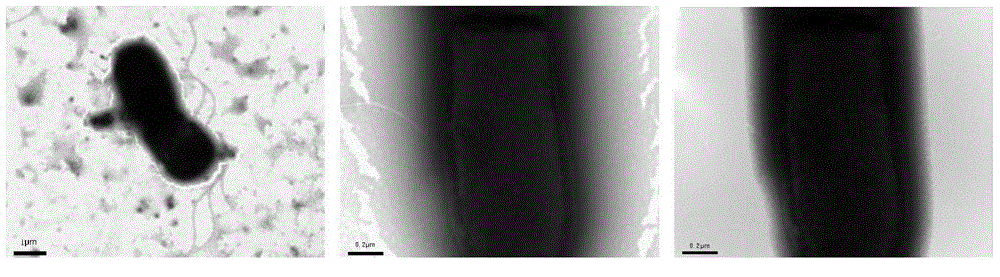
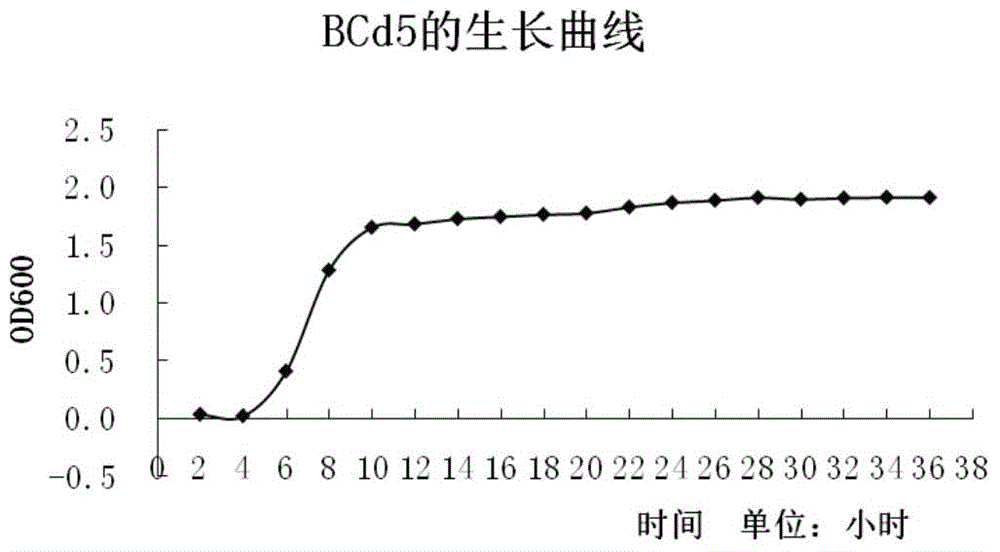
The invention relates to a sedum rhizosphere cadmium-resistant bacillus sp. BCd5 strain and its screening method. The invention specifically relates to a sedum rhizosphere cadmium-resistant bacillus sp. BCd5 strain which has been preserved in the China General Microbiological Culture Collection Center (CGMCC) on December 1st, 2014 with the preservation number being CGMCC NO. 10077. 16S rDMA gene sequence of the strain is as shown in the Figure 1. The strain is a Gram-negative bacterium, aerobasilus having flagellum and capsule. The strain has high resistance to cadmium and has practical significance and important engineering application value in plant-microbial remediation of cadmium-contaminated soil.
Owner:CHINA THREE GORGES CORPORATION +1
Sedum rhizosphere lead-resistant strain pseudomonas as well as screening method and application thereof
InactiveCN104974960AIncreased tolerance to heavy metal leadBacteriaContaminated soil reclamationHigh resistanceScreening method
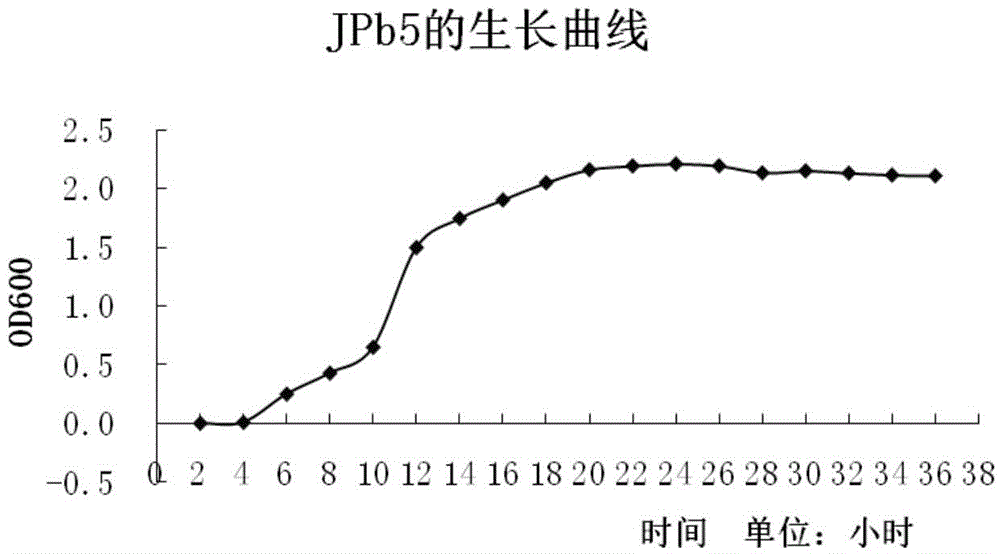
The invention relates to sedum rhizosphere lead-resistant strain pseudomonas sp JPb5, in particular to a lead-resistant strain pseudomonas sp JPb5 separated from sedum rhizosphere. The lead-resistant strain pseudomonas sp JPb5 is preserved in the China General Microbiological Culture Collection Center with the preservation number of CGMCC No.10087 on December 1, 2014. The lead-resistant strain pseudomonas sp JPb5 is gram-negative bacteria or aerobasilus, and has flagella and capsules. The strain has relatively high resistance to heavy metal lead, and has practical significance and important engineering application value on the aspect of remediation of heavy metal lead contaminated soil through combination of plants and microorganisms.
Owner:CHINA THREE GORGES CORPORATION +1
Preparation method of clostridium bolteae surface capsular polysaccharide structure derivative
ActiveCN108329362ASimple stepsLow costEsterified saccharide compoundsSugar derivativesGlycoconjugateOligosaccharide
The invention discloses a preparation method of a clostridium bolteae surface capsular polysaccharide structure derivative and belongs to the field of sugar chemistry. The preparation method comprisesthe following steps: taking glucose as a glycosyl donor to obtain a target beta-glycosidic bond; then successfully synthesizing a disaccharide building block through an oxidization-reduction glucoseC-2 site method; then synthesizing a target oligosaccharide structure which takes the disaccharide building block as a repeating unit, such as the gram-positive bacterium surface capsular polysaccharide structure derivative [arrow3]-alpha-D-Manp-(1arrow4)-beta-D-Rhap-(1arrow]5-Linker. A reducing end of decaose is connected with a connecting arm and is used for connecting protein to form a glycoconjugate for carrying out immunology researches. The method provided by the invention has the advantages of simplicity, time saving, labor saving and low cost; the obtained clostridium bolteae surface capsular polysaccharide structure derivative is possibly used for developing and preparing medicine related to autism.
Owner:JIANGNAN UNIV
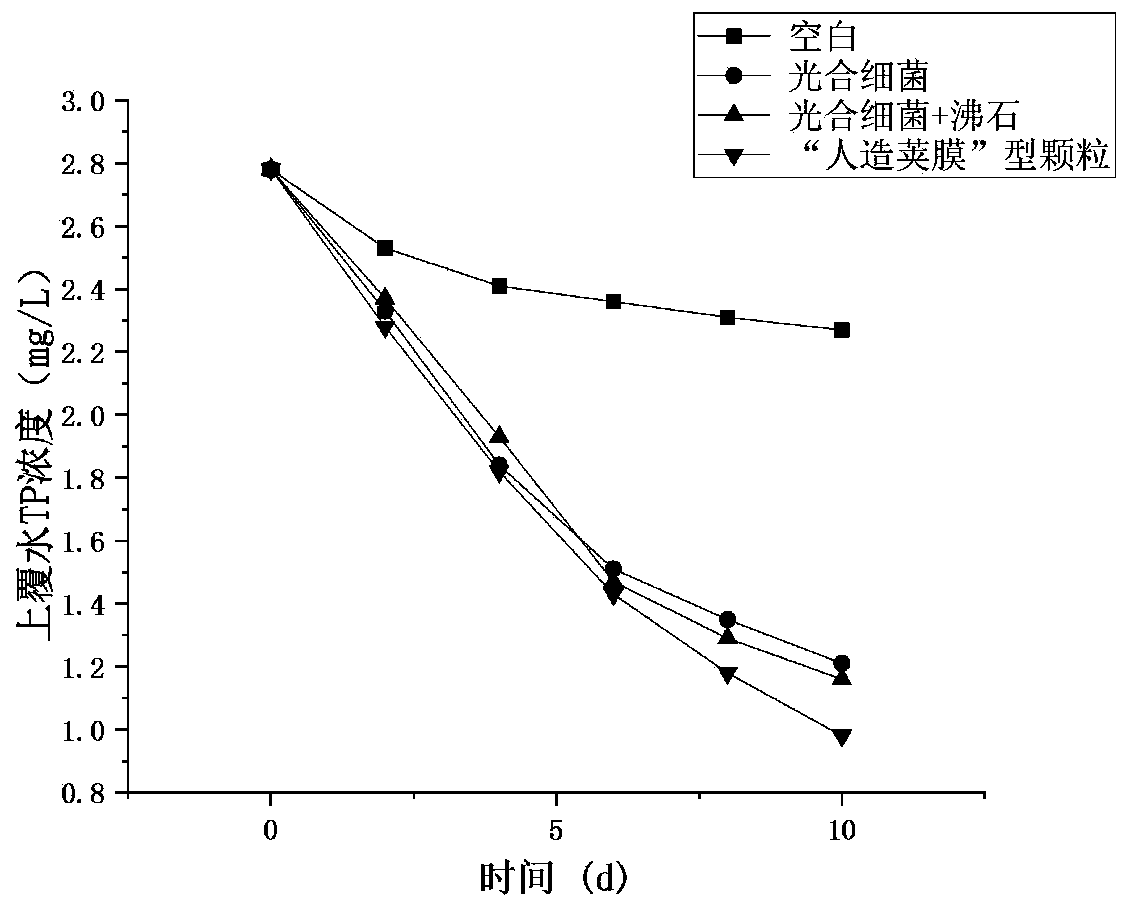
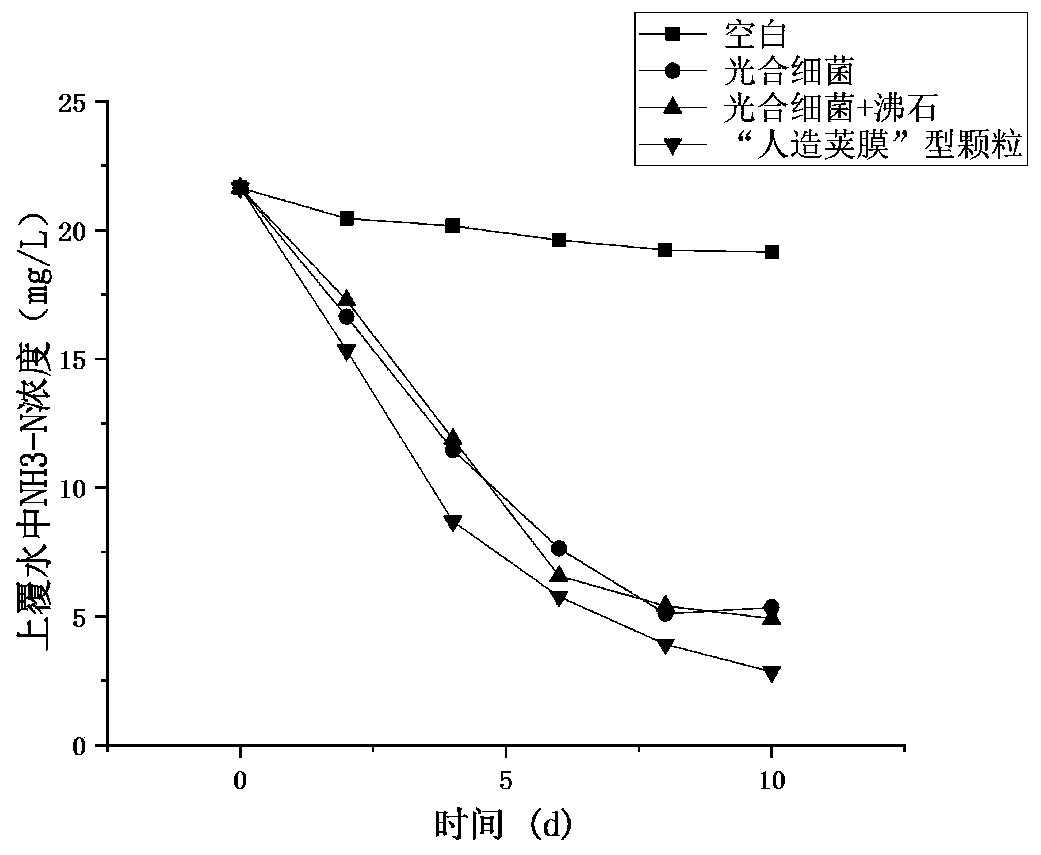
Artificial capsule type multifunctional particle as well as preparation method and application thereof
ActiveCN110655202AImprove abilitiesImprove purification effectWater/sewage treatment by flocculation/precipitationBiological water/sewage treatmentMicroorganismMineral particles
The invention provides a preparation method of artificial capsule type multifunctional particles, and belongs to the field of surface water pollution treatment. According to the preparation method, ore is used as a carrier, microorganisms are loaded on the ore by adding a binder and a flocculant, and meanwhile, mineral particles are coated with a binder layer, a flocculant layer and a high-efficiency degrading bacterium layer to form structures similar to bacterial capsules. The capsule structure not only can protect microorganisms in mineral particles, but also has the capability of immobilizing external efficient degrading bacteria. Minerals, a binding-flocculating material and microorganisms in the multifunctional particles coordinate with one another, comprehensive utilization is realized, the advantages of each components are maintained, optimal adjustment is performed according to different pollution characteristics of water body, the floating water purification capacity is effectively improved, the effects of energy conservation, emission reduction and resource utilization are achieved, and the method can be widely applied to pollution treatment of rivers, lakes and reservoirs and has good market application prospects.
Owner:四川千路环保科技有限责任公司
Method for preparing pneumococcal capsular polysaccharide by viscosity control
The invention belongs to the field of biological products and relates to a preparation method of pneumococcal capsular polysaccharide. According to the preparation method, pneumococcus inactivated andclarified fermentation liquid as an initial feed liquid is used for purification and preparation by steps as follows: ultrafiltering and concentrating the clarified fermentation liquid, adding CTAB to form a precipitate with polysaccharide, performing centrifugation to collect the precipitate, and performing depolymerization by sodium chloride and chromatography purification to finally obtain pneumococcal capsular polysaccharide; viscosity control comprises steps of ultrafiltering, clarifying and filtering, precipitation by CTAB, depolymerization by sodium chloride and chromatography.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Screening method of feed probiotics capable of preventing chicken's infection caused by clostridium perfringens
InactiveCN108165619AAvoid discomfortAvoid blindnessMicrobiological testing/measurementMicroorganism based processesIntestinal microorganismsScreening method
The invention discloses a screening method of feed probiotics capable of preventing chicken's infection caused by clostridium perfringens. The method comprises following steps: screening chickens infected by clostridium perfringens in clinic and disease resistant chickens; collecting the intestinal chyme samples; carrying out high throughput sequencing on microbial floras in the intestinal tractsof infected chickens and disease resistant chickens; comparing and analyzing the microbes in the intestinal tracts of infected chickens and disease resistant chickens through a metagenomics method; and screening and identifying probiotics capable of preventing infection caused by clostridium perfringens. According to the method, the metagenomics big data of floras in the intestinal tracts of infected chickens and disease resistant chickens is analyzed; probiotics capable of preventing infection caused by clostridium perfringens are selected from massive microbial floras in the intestinal tracts of disease resistant chickens, the screened probiotics are from same animals, the rejection between probiotics and a host is avoided, moreover, the screening is targeted, aimless screening is avoided, and a large amount of labor, resources, and time is saved.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Biological demanganization functional bacterium
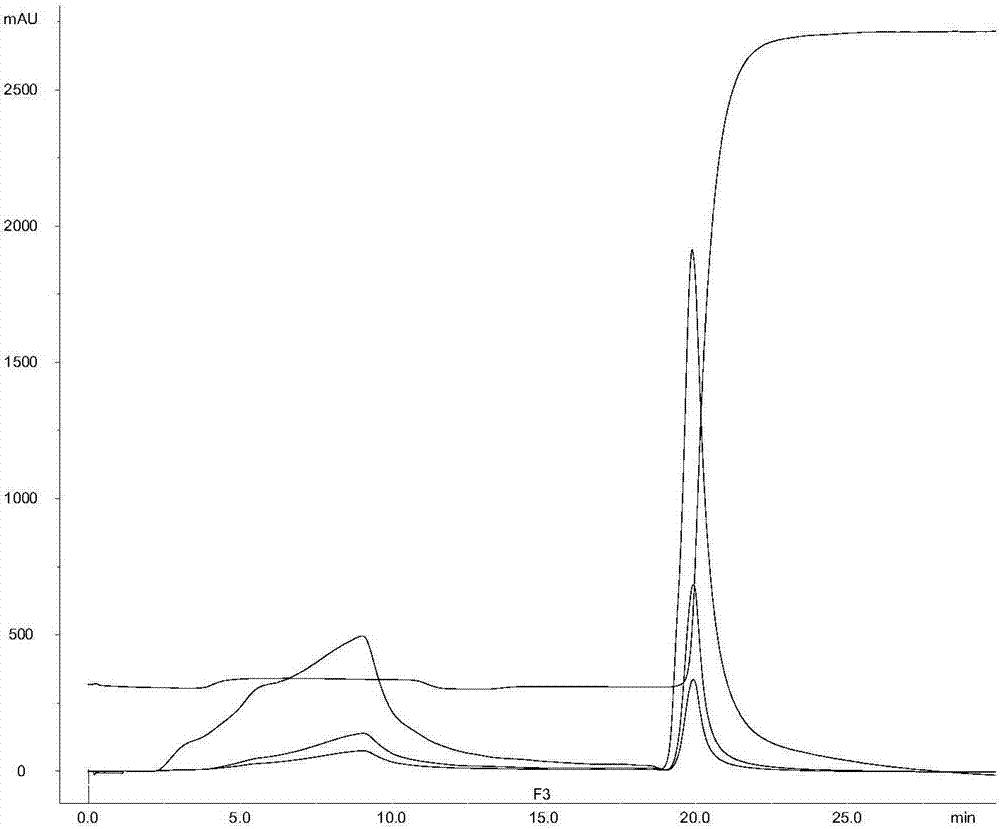
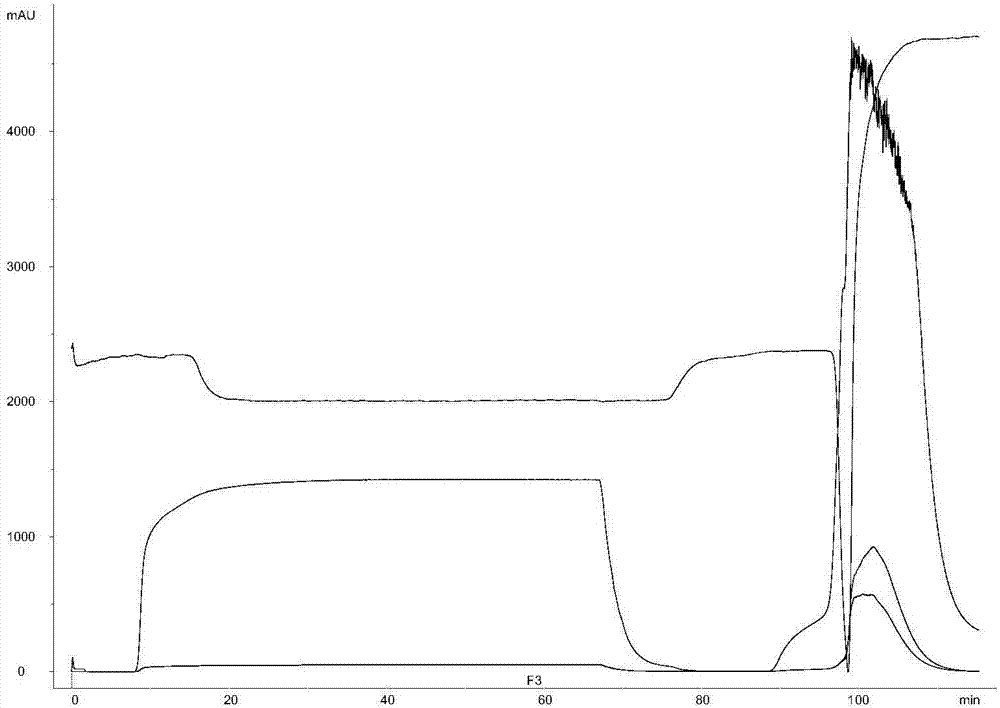
A biological demanganization function strain is disclosed, in particular to a microbe. The invention solves the establishment of the prior biological oxidation demanganization function strain which depends on natural selection, weak biochemical capability and bad demanganization effect and long cultured, tamed, and starting time of the water disposal demanganization biological filter tank establishment and long restoration period after the function strain is broken. The biological demanganization function strain MB4 (Exiguobacterium sp.) belongs to the microminiature Bacterium and is preserved in the Chinese microbe bacterial preservation management committee common microbe center and the preservation date is Oct. 30, 2007 and the preservation number is CGMCC No.2237. The bacterial MB4 is Gram-positive microaerobic bacteria which is bacillus brevis and small in length and is 1-1.5 mum in length, 0.5-0.8 mum in breadth. Two ends of the cell are blunt circle and form brown colony on the culture medium without transparent and the central color is deeper and the peripheral color is lighter. The entire colony is round shape. The strain MB4 has power storage particle without spore and capsule. The dyeing of N, N, N', N'-tetramethyl p-phenylene diamine is positive. The biological demanganization function strain MB4 of the invention is provided with Mn 2+ removing ability and the Mn 2+ clearance is over 91%.
Owner:HARBIN INST OF TECH
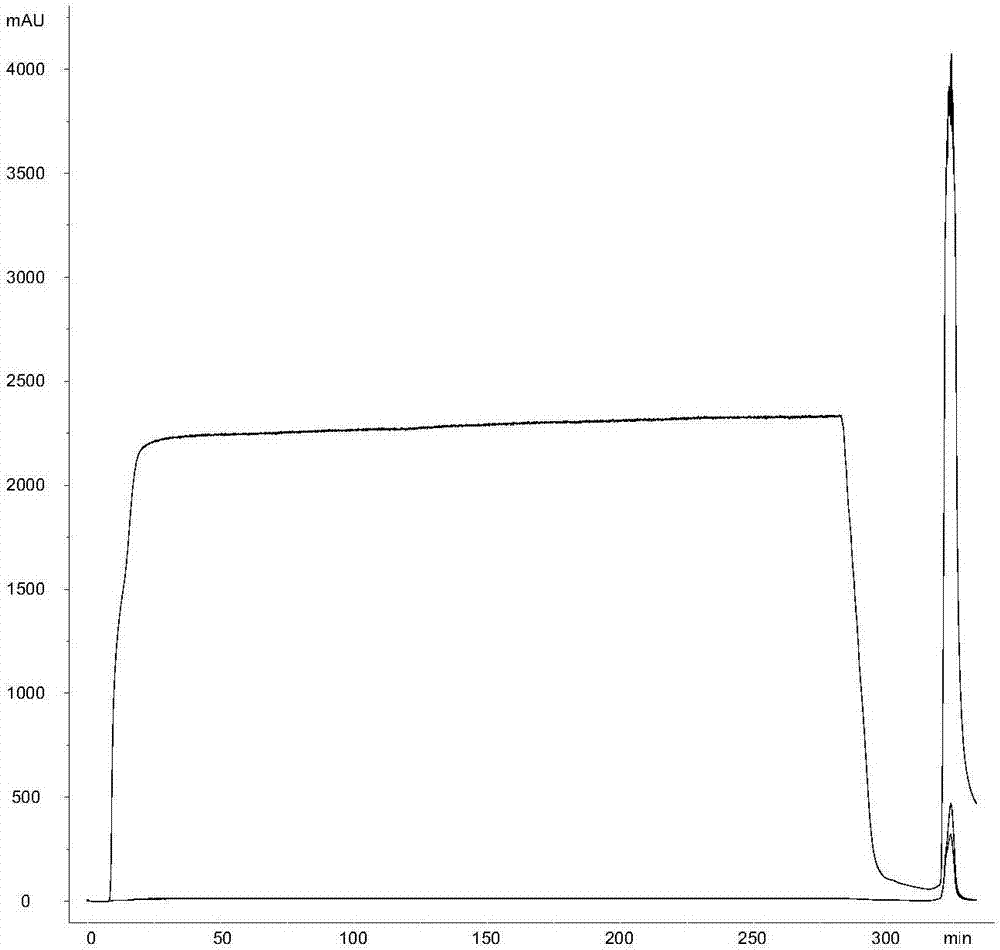
Method for purifying bacterial capsular polysaccharide
The invention discloses a method for purifying bacterial capsular polysaccharide. The method comprises the following steps: treating a streptococcus pneumonia fermentation culture solution with an inactivator, adding acid for regulating the pH value to be less than or equal to 6.5, precipitating impurities, centrifuging, and carrying out microfiltration for removing precipitates, so that polysaccharide clarified liquor is obtained; carrying out ultrafiltration liquid change on the polysaccharide clarified liquor by adopting a membrane with the molecular weight cut-off of 10-100KDa and concentrating, and carrying out chromatography on the obtained capsular polysaccharide solution by combining compound type ion exchange chromatography and hydroxyl phosphate grey salt chromatography, so that the purified capsular polysaccharide solution is obtained; and concentrating and carrying out ultrafiltration on the capsular polysaccharide solution, and carrying out sterile filtration and storing. By combining the compound type ion exchange chromatography and the hydroxyl phosphate grey salt chromatography, the contents of protein and nucleic acid impurities can be effectively reduced to be lower than 1%, the quality of a purified polysaccharide product is higher than European Union pharmacopoeia standard requirements, and the recovery rate is more than 60%. The method disclosed by the invention has the advantages that the technology is simple and rapid, the enlargement is easy, and industrial production can be realized.
Owner:SHANGHAI RUIZHOU BIOTECH CO LTD
High throughput screening method for salt resistant mutation bacterial strain of colloid bacillus cereus
InactiveCN101314789AReduce screening workloadImprove screening efficiencyMicrobiological testing/measurementHigh-Throughput Screening MethodsPhosphate
The invention discloses a high-throughput screening method of a colloid bacillus salt tolerant mutant strain, which comprises the following steps: firstly, after being activated in nitrogenfree medium, colloid bacillus is placed in a liquid culture medium for restraining the growth of capsula, and cultured to the logarithmic growth phase, thallus is centrifuged and collected, physiological saline or phosphate buffer is used for suspending the thallus, and the concentration of the thallus is adjusted to 10<4>-10<5>cfu ml<-1>; secondly, Co<60> is used for inducing the thallus suspending liquid, and the amount of a mutagenic agent is 1-8kGy; the thallus suspending liquid after being induced is centrifuged to obtain the induced thallus; thirdly, after being diluted to 10<3>-10<4>cfu ml <-1> by using the liquid screening culture medium, the mutate thallus is cultured after being separately packaged into a culture plate with 96 holes for the high-throughput screening, after the secondary screening is performed, the salt tolerant mutant strain is obtained. In the invention, nuclear radiation inducement and the high-throughput screening method are used for obtaining the salt tolerant mutant strain of the colloid bacillus, so as to provide the strain for the rehabilitation of greenhouse soil.
Owner:ZHEJIANG SCI-TECH UNIV
Chromium-resisting strain separated from rhizosphere of red-spotted stonecrop, selecting method, and application of shromium-resisting strain
InactiveCN104974958AIncreased resistance to heavy metal chromiumBacteriaContaminated soil reclamationScreening methodMicrobiological culture
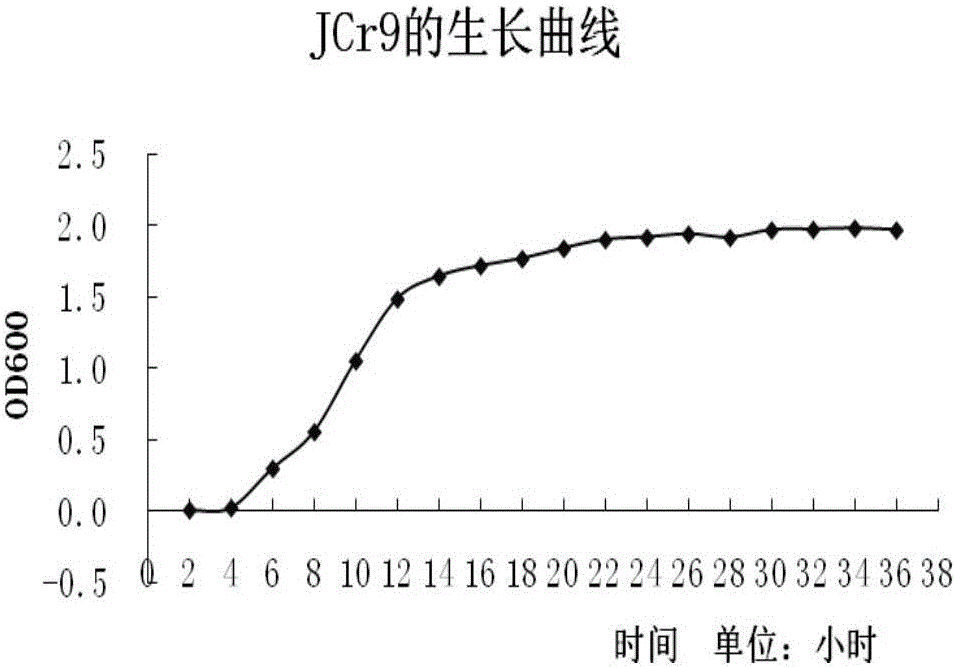
The invention relates to a chromium-resisting bacillus separated from rhizosphere of red-spotted stonecrop (pumilus bacillus pumilus JCr9) which is preserved in China General Microbiological Culture Collection Center (CGMCC) on Dec, 1, 2014, with the preservation number of CGMCC No.10078, wherein the 16S rDNA gene sequence is shown in figure 1. The pumilus bacillus pumilus JCr9 is gram-negative bacterium and aerobic corynebacterium, has no atrichia or capsule, has strong tolerance for heavy metal chromium, and has practical significance and important engineering application value for plant-microbial remediation of soil polluted by heavy metal chromium.
Owner:CHINA THREE GORGES CORPORATION +1
Immunogenic protein conjugates and method for making and using the same
InactiveUS20140302084A1Maximum protectionEnhance immune responseBacterial antigen ingredientsAntibody mimetics/scaffoldsProtective antigenConjugate vaccine
Production of protein conjugate vaccines by use of transpeptidase enzymes, such as sortase enzymes. For example, homogenous immunoconjugates (e.g., a population of molecules having the same structure) formed by conjugating an antigenic polypeptide and a bacterial capsule component are provided. In certain aspects, methods for generating an immune response to B. anthracis by use of protective antigen-PDGA immunoconjugates are provided.
Owner:UNIVERSITY OF CHICAGO
Sulfate reduction bacterium with polyacrylamide degradation function and application thereof
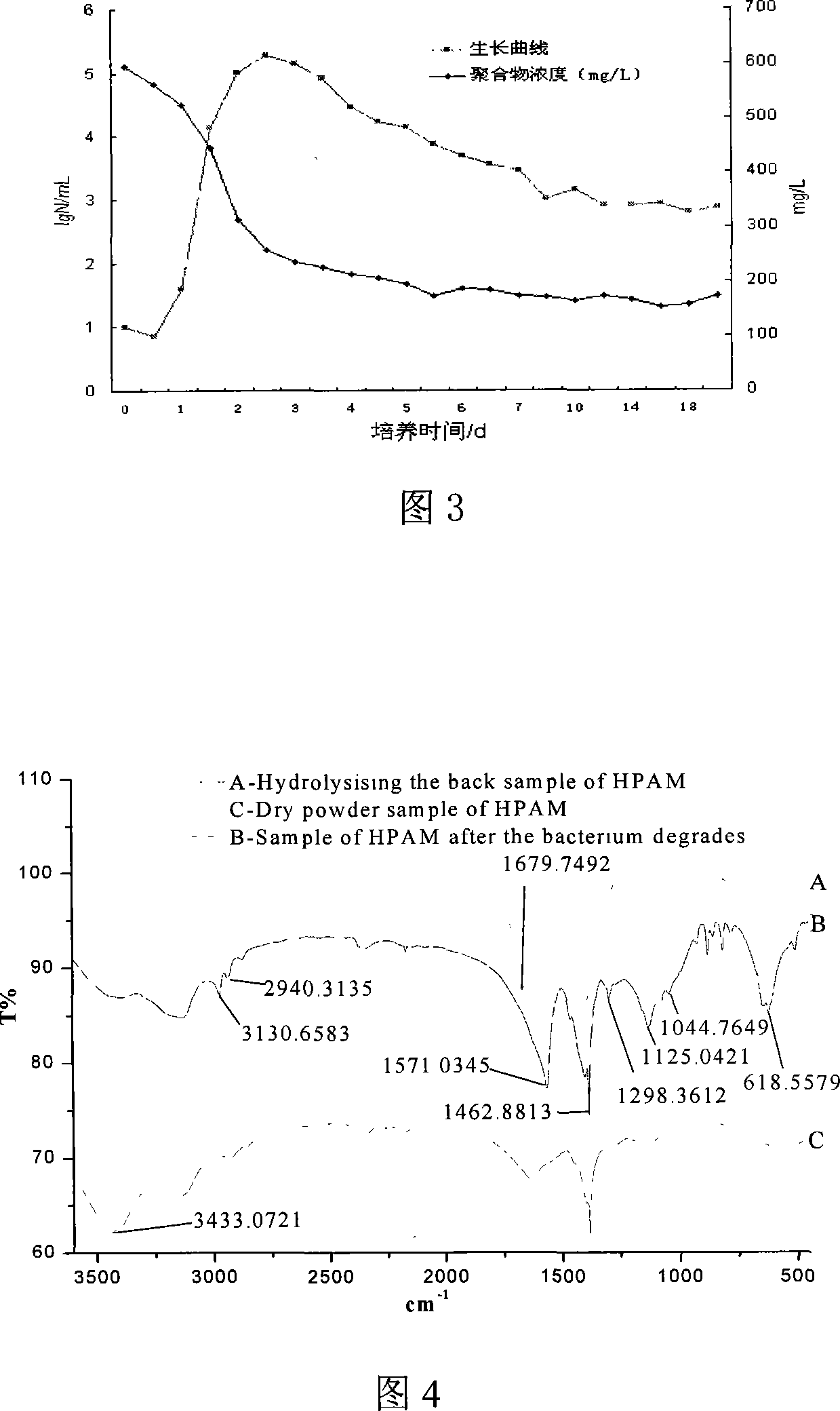
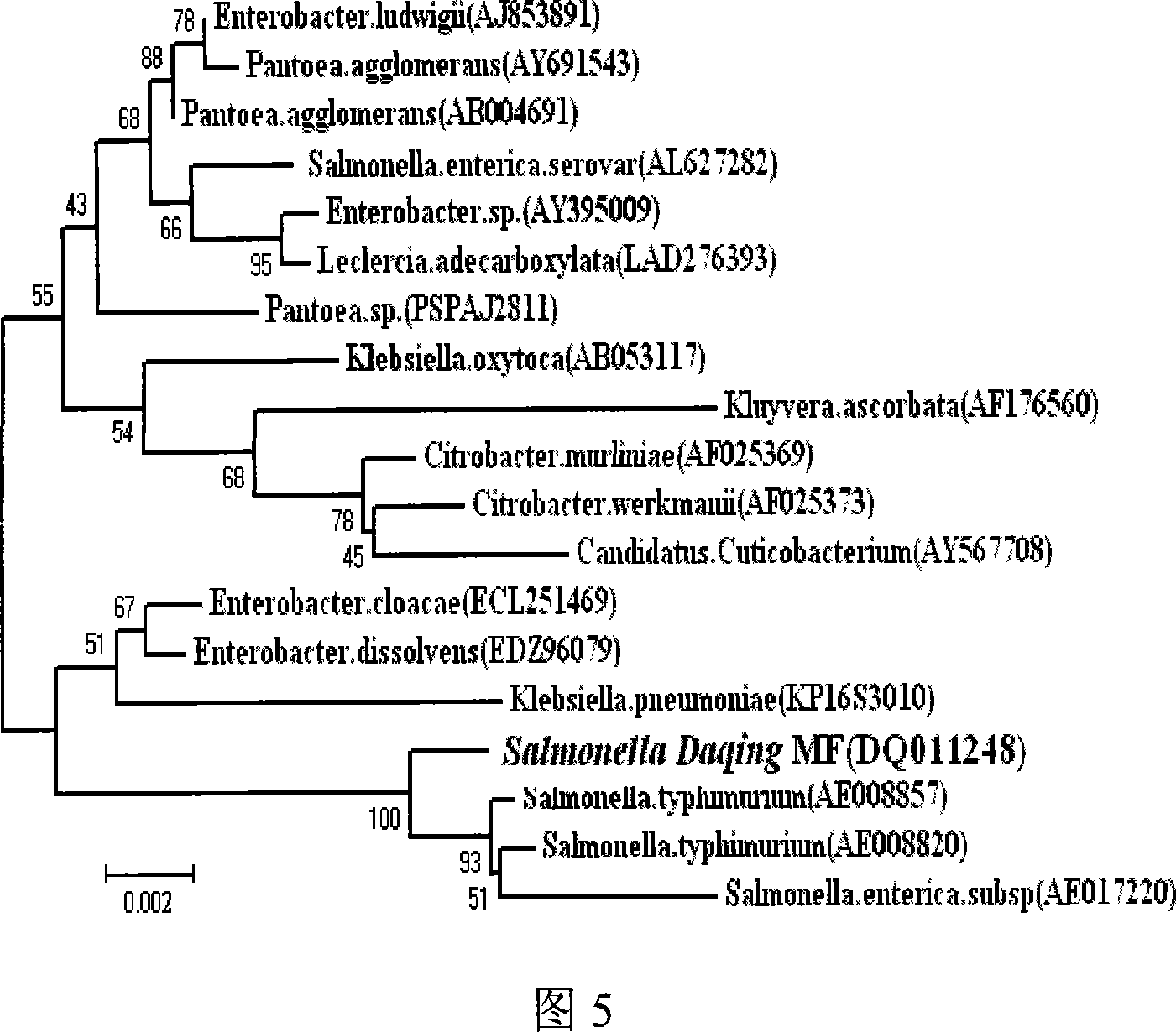
Disclosed are a sulfate reducing bacterium with a polyacylamide degradation function and applications of the bacterium. The invention relates to a microorganism and applications of the microorganism. The invention firstly put forward the sulfate reducing bacterium with the polyacylamide degradation function, the bacterium is named as Salmonella Daqing MF, namely, Salmonella Daqing MF (preservation code: CGMCC No.1910), preserved in the China General Microbiological Culture Collection Center, and belonging to the Salmonella Genera and the Salmonella Daqing Species in taxonomy. The bacterium belonging to the Gram negative short-bacilli is covered by a capsule and is obligate anaerobic, variable in size bound in 0.35Mu-0.7Mum width multiplied by 0.7Mum-1.6Mum length, without flagella on the bacterium body; when growing on a solid medium, the bacterium develops into a black round colony, smooth in surface and irregular in edge.
Owner:江苏哈宜环保研究院有限公司
Hyaluronic Acid Binding Peptides Enhance Host Defense Against Pathogenic Bacteria
ActiveUS20090030180A1Avoid infectionAvoid bacterial infectionAntibacterial agentsPeptide-nucleic acidsBacteroidesCulture model
Several species of bacteria capable of invasive infections, such as S. pyogenes, S. equi and P. multocida, contain hyaluronic acid (HA) in their capsules. Bacterial species such as Staphylococcus aureus and related Staphylococci have capsules that contain acidic polysaccharides. Bacterial capsule or bacterial surface binding peptides were synthesized and tested in a culture model of invasive bacterial infections, specifically translocation through polarized keratinocyte cultures. The peptides reduced the translo-cation of a variety of bacterial species, with a concomitant increase in bacterial internalization by the keratinocytes. In vivo, subcutaneous inoculation of encapsulated GAS treated with peptides delayed bacterial dissemination. In a mouse surgical wound control model infected with S. aureus, treatment with peptides reduced the numbers of bacteria and inflammation at the wound site.
Owner:CANGENE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com