Preparation method of clostridium bolteae surface capsular polysaccharide structure derivative
A technology for gram-positive bacteria and derivatives, applied in the field of carbohydrate chemistry, can solve the problems of long time, high cost, difficulty in vaccine preparation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
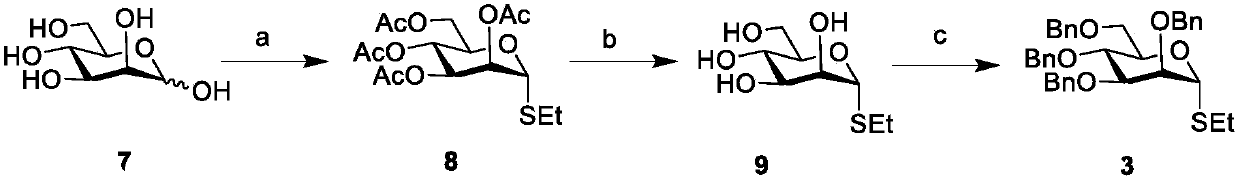
[0069] The synthesis of embodiment 1 sugar block 3 is as follows figure 1 :
[0070] Specific test operation and steps:
[0071] Compound 8: Under argon protection, commercial D-mannose (40g, 222mmol) was dissolved in acetic anhydride (228mL), sodium acetate (24g, 288.6mmol) was added, stirred at 80°C for two hours, TLC After monitoring the complete reaction of the raw materials, extract with dichloromethane, wash with saturated sodium bicarbonate solution, collect the organic phase, dry over anhydrous sodium sulfate, filter out sodium sulfate with filter paper, and remove the solvent by rotary evaporation to obtain a brown syrup, which is directly put into the next reaction. Dissolve peracetylated mannose in anhydrous dichloromethane (1000 mL), add Molecular sieves, add ethanethiol (25mL), cool to 0°C, add boron trifluoride ether solution (55mL) dropwise, stir and react in ice bath for 30 minutes, rise to room temperature and stir for 32 hours, then add 0.5 equivalent Tri...
Embodiment 2
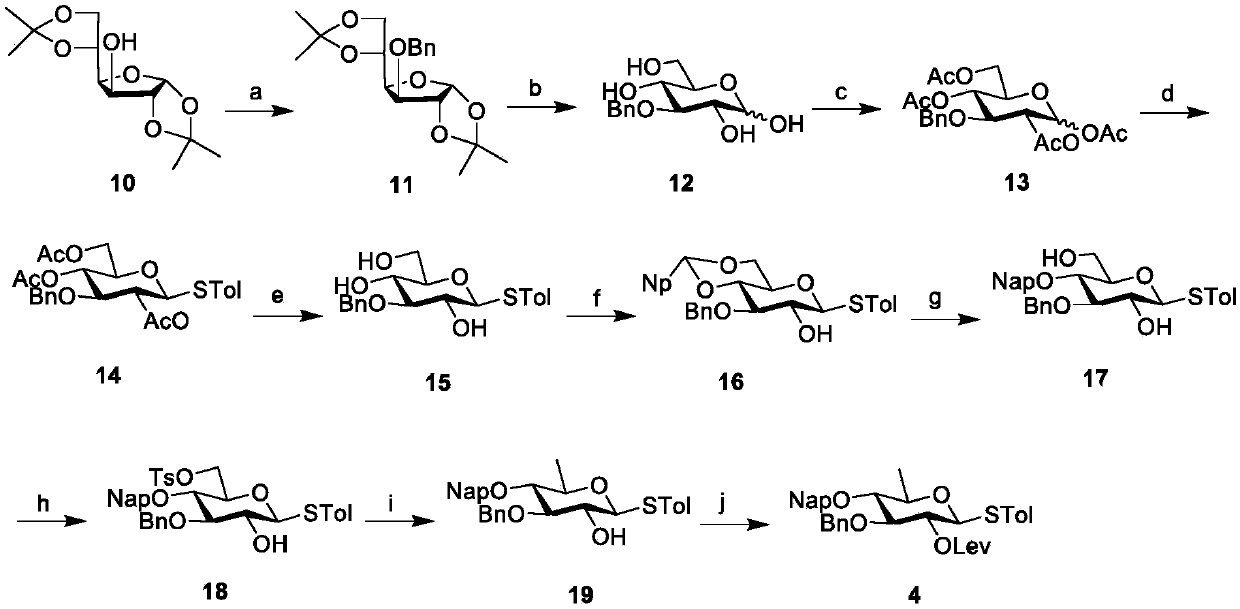
[0073] The synthesis of embodiment 2 sugar block 4 is as follows figure 2 :
[0074] Specific test operation and steps:
[0075] Compound 11: Under argon protection, commercial diacetone glucose 10 (50g, 191mmol) was dissolved in dimethylformamide (490mL), cooled to 0°C, and sodium hydride (15.4g, 382mmol ), added benzyl bromide (35mL) dropwise, reacted in an ice bath for 30 minutes, raised to room temperature and reacted for 5 hours, after the TLC monitoring of the raw material reaction was complete, added ice water to terminate the reaction, extracted with dichloromethane (3 × 250mL), collected The organic phase was dried with anhydrous sodium sulfate, and filtered to remove sodium sulfate with filter paper to obtain syrup 11 (66.2 g, 189.1 mmol, 99%). 1 H NMR (400MHz, CDCl 3)δ: 7.61~7.08(m,5H,Ph),5.90(d,J=3.7Hz,1H,1-H),4.68(d,J=11.8Hz,1H,PhCH),4.63(d,J= 11.8Hz, 1H, PhCH), 4.58(d, J=3.7Hz, 1H, 2-H), 4.37(dt, J=7.7, 6.1Hz, 1H, 5-H), 4.15(dd, J=7.8, 3.2Hz, 1H, 6-H), 4.11...
Embodiment 3
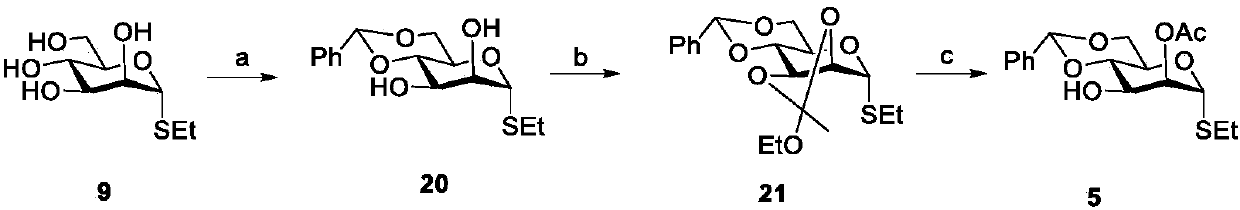
[0083] The synthesis of embodiment 3 sugar block 5 is as follows image 3 :
[0084] Compound 20: Under the protection of argon, dissolve compound 8 (11.3g, 28.7mmol) in methanol (250mL), add a catalytic amount of sodium methoxide, and react at room temperature for 12 hours. After TLC monitors that the reaction of the raw materials is complete, add a cation exchange resin Adjust the pH to 5-6, filter the resin with filter paper, and remove the solvent by rotary evaporation to obtain a brown syrup, which is directly used for the next reaction. The obtained syrup was dissolved in dimethylformamide (90mL), stirred at room temperature, p-toluenesulfonic acid (735mg, 3.9mmol) and benzaldehyde dimethyl acetal (5mL, 32.8mmol) were added, and the temperature was raised to 60°C for reaction 7 Hours, TLC monitors that after the reaction of the raw material is complete, triethylamine (5 mL) is added to stop the reaction, extracted with dichloromethane (3 × 150 mL), washed with saturated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com