Method for rapid purification of bacterial capsular polysaccharide
A technology of bacterial capsular polysaccharide and capsular polysaccharide, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problem of high C-polysaccharide content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
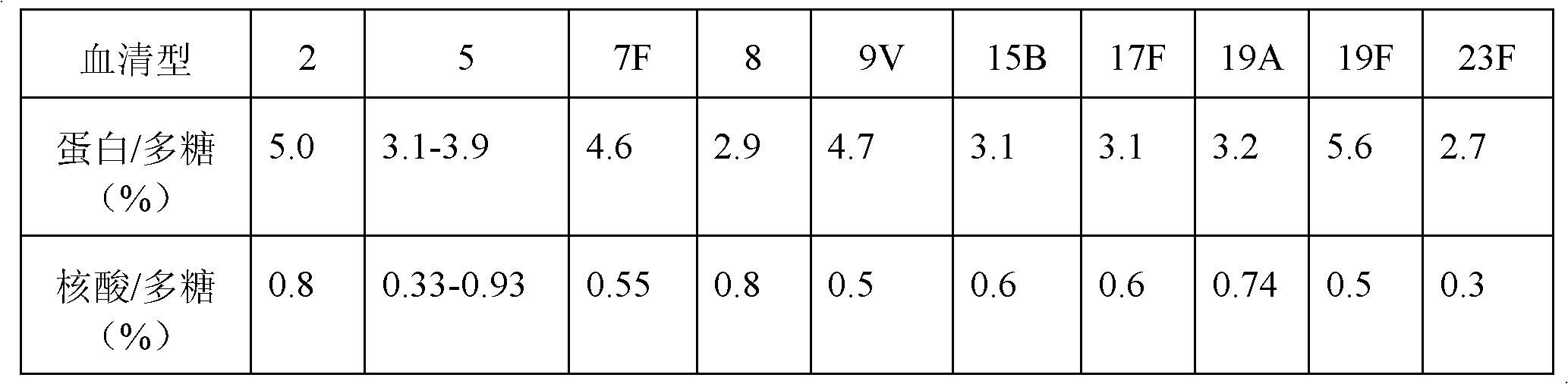
[0057] Purify the capsular polysaccharide from the fermentation broth of pneumococcal serotype 19F, the specific method steps are as follows:
[0058] Step 1, fermentation of capsular polysaccharide bacteria: ferment specific capsular polysaccharide bacteria in a fermenter equipped with bacterial culture medium;
[0059] (1), main seed and working seed preparation
[0060] Pneumococcal serotype 19F was obtained from the American Type Culture Collection, inoculated the strains in the freeze-dried seed tube into 5ml of yeast-acid hydrolyzed casein broth, and cultured at 36°C±2°C for 12-24 hours , when the bacteria grow to OD 600 When the reading is 0.6-2.0, inoculate the bacterial solution into 150ml of fresh yeast-acid hydrolyzed casein culture solution, and cultivate at 36°C±2°C for 5-20 hours to the exponential growth phase, stop the cultivation, and pack Freeze-dried and stored at 2-8°C for main seeds.
[0061] Inoculate the strains of the freeze-dried tube of the main se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com