Catalytic adsorbents obtained from municipal sludges, industrial sludges, compost and tobacco waste and process for their production
a technology of catalysts and adsorbents, which is applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, and separation processes, etc., can solve the problems of weak dispersion interaction with the carbon surface, the chemical state of these species and their dispersion on the surface, and the potency of activated carbons. to retain certain molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
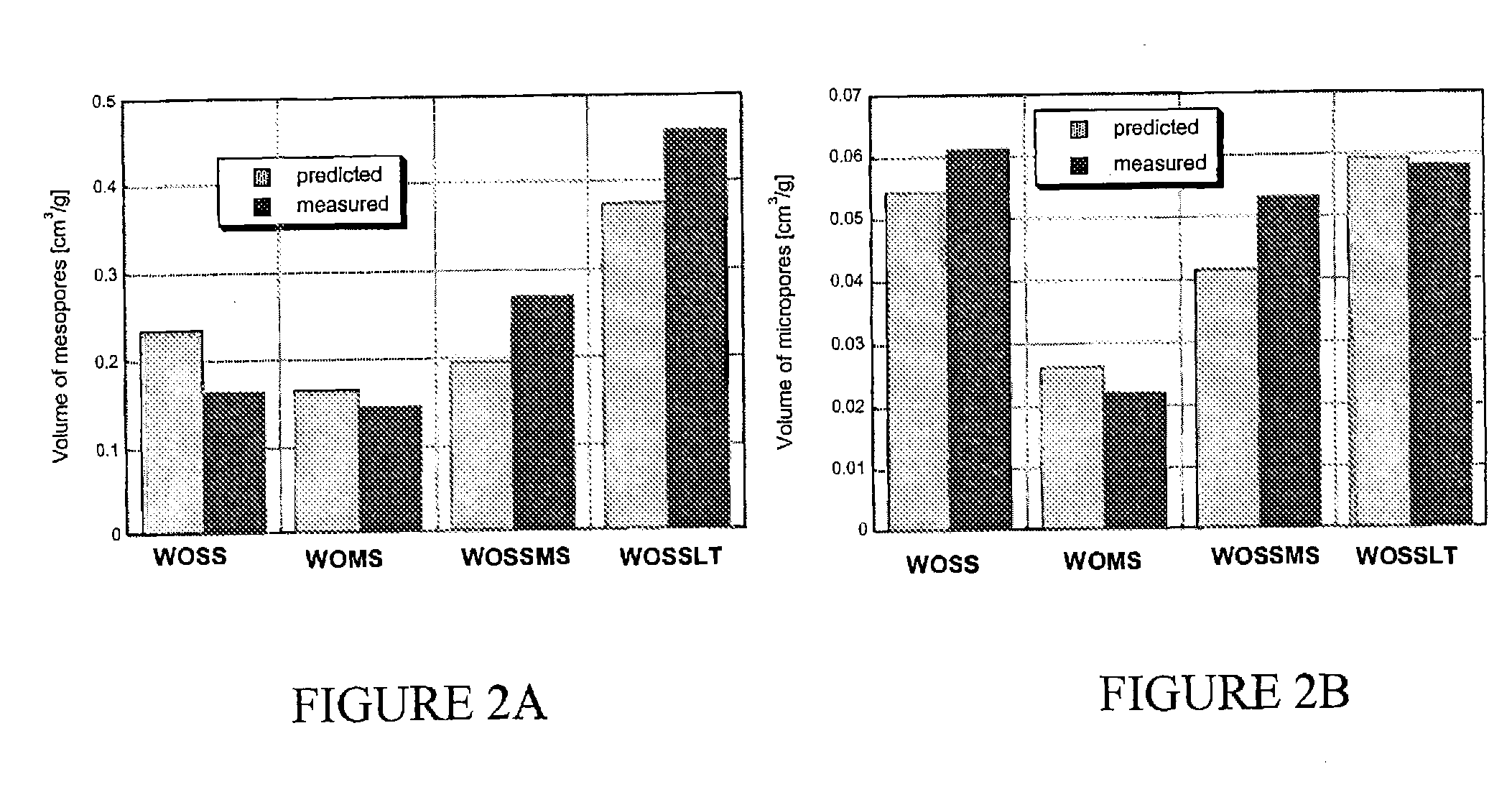
[0081] The homogeneous mixtures of waste sludges were prepared as listed in Table 3 and dried at 120° C. The dried samples were then crushed and pyrolized in a horizontal furnace at 950° C. for 30 min. The temperature ramp was 10 degrees / minute. An inert atmosphere was provided by 10 ml / min. flow of nitrogen. The yields, ash content and densities of materials are listed in Table 3.
TABLE 3Adsorbents' composition, yields, ash content and densities.WetDryYieldcompo-Solidcompo-(dryAshγSamplesitioncontentsitionmass)content*[g / cm3]WOWO:23.6WO:29920.48100%100%SSSS: 100%24.6SS: 100%45800.46MSMS: 100%23.4MS: 100%47@0.85WOSSWO: 50%—WO: 49%34@0.46SS: 50%SS: 51%WOMSWO: 50%—WO: 50%50@0.47MS: 50%MS: 50%WOSSMSWO: 40%—WO: 46%41@0.46SS: 40%SS: 31%MS 10%MS 23%
*Determined as mass left at 950° C. after in TA run in air.
@ - not determined due to reaction with air during burning
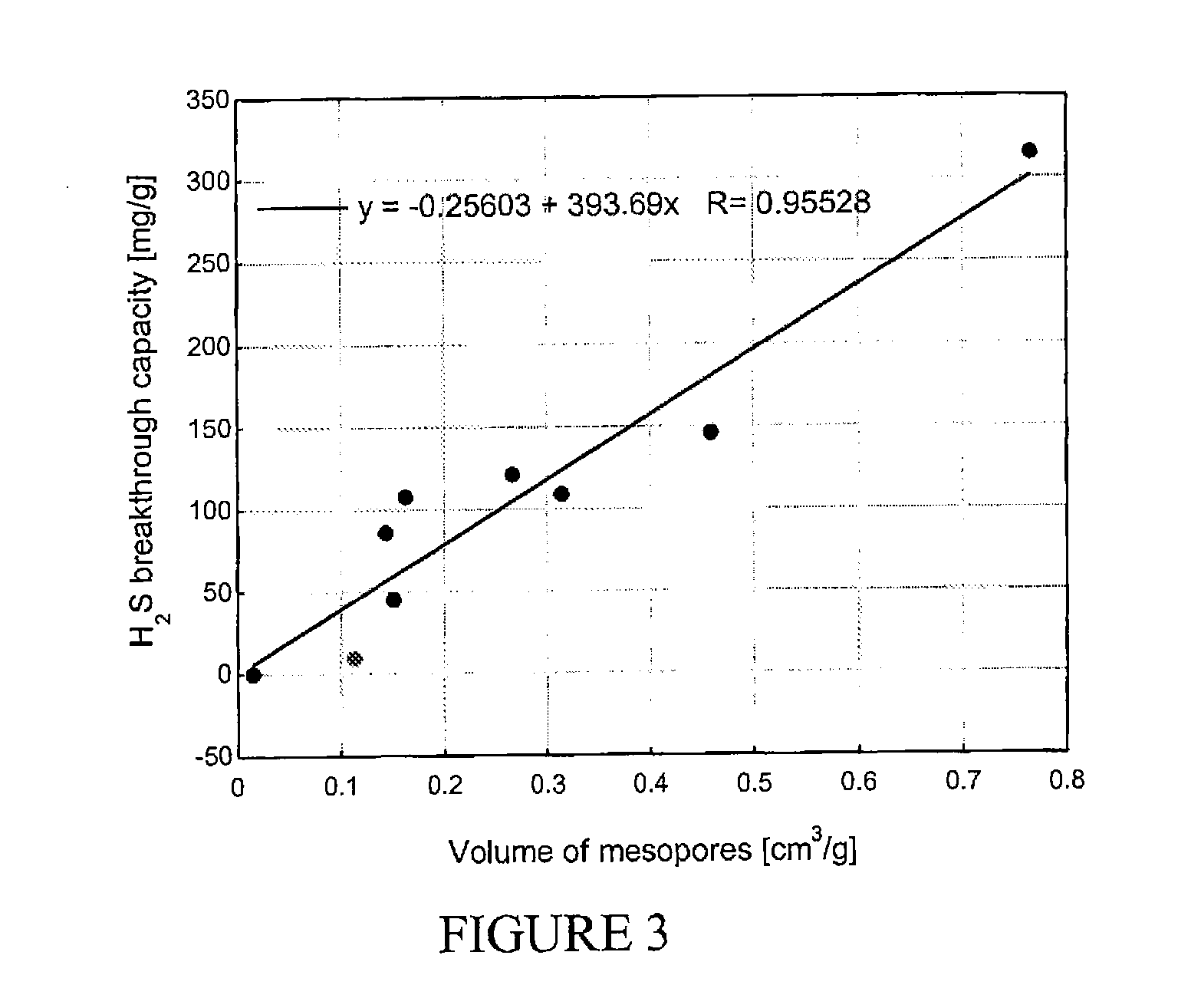
[0082] The performance of materials as sorbents for hydrogen sulfide was evaluated using lab developed breakthrough tests. A...
example 2
[0086] The homogeneous mixtures of waste sludges were prepared as listed in Table 8 and dried at 120° C. The dried samples were then crushed and pyrolized in a horizontal furnace at 650° C. for 30 min. The temperature ramp was 10 degrees / minute. An inert atmosphere was provided by 10 ml / min flow of nitrogen. The yields, ash content and densities of materials are listed in Table 8.
TABLE 8Adsorbents' composition, yield, and densitiesYieldWetSolidDry(dryγSamplecompositioncontentcompositionmass)[g / cm3]WOLTWO: 100%23.6WO: 100%320.26SSLTSS: 100%24.6SS: 100%470.52MSLTMS: 100%23.4MS: 100%0.47WOSSLTWO: 50%—WO: 49%0.36SS: 50%SS: 51%WOMSLTWO: 50%—WO: 50%580.38MS: 50%MS: 50%WOSSMSLTWO: 40%—WO: 46%460.38SS: 40%SS: 31%MS 10%MS 23%
*Determined as mass left at 950° C. after thermol analyses run in air.
LT—low temperature, 650° C.
[0087] The performance of materials as sorbents for hydrogen sulfide was evaluated using lab developed breakthrough tests. Adsorbent samples were packed into a column (le...
example 3
[0091] The homogeneous mixtures of waste sludges were prepared as listed in Table 12 and dried at 120° C. The dried samples were then crushed and pyrolyzed in a horizontal furnace at 950° C. for 60 min. The temperature ramp was 10 deg / min. An inert atmosphere was provided by 10 ml / min flow of nitrogen. The yields and densities of the materials are listed in Table 12.
TABLE 12Adsorbents' composition and their densitiesWetSolidDryγSamplecompositioncontentcomposition[g / cm3]WO60WO: 100%23.6WO: 100%0.47SS60SS: 100%24.6SS: 100%0.46MS60MS: 100%23.4MS: 100%0.84WOSS60WO: 50%—WO: 49%0.41SS: 50%SS: 51%WOMS60WO: 50%—WO: 50%0.46MS: 50%MS: 50%WOSSMS60WO: 40%—WO: 46%0.45SS: 40%SS: 31%MS 10%MS 23%
[0092] The performance of materials as sorbents for hydrogen sulfide was evaluated using lab developed breakthrough tests. Adsorbent samples were packed into a column (length 60 mm, diameter 9 mm, bed volume 6 cm3) and prehumidified with moist air (relative humidity 80% at 25° C.) for an hour. The amount ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com