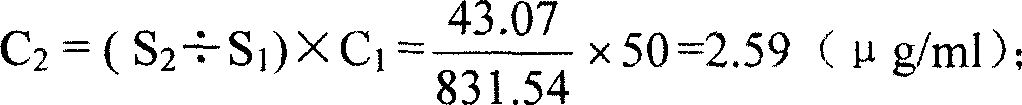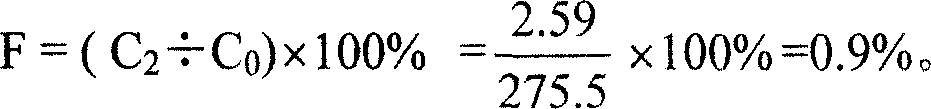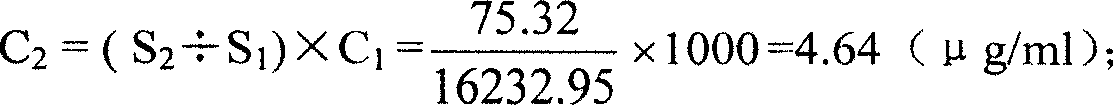Method for measuring content of free protein in bacterial capsule protein-polysaccharide ligature
A technology for bacterial capsular polysaccharide and protein content, applied in the biological field, can solve problems such as large differences in properties, and achieve good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Utilize the Lowry method to measure the total protein concentration in the meningococcal group A polysaccharide tetanus toxoid conjugate to be tested, it is 275.5 μ g / ml (C 0 ); get the tetanus toxoid solution corresponding to the conjugate, and dilute to 50 μg / ml with normal saline according to its protein concentration (C 1 ), as sample 1; take the meningococcal group A polysaccharide tetanus toxoid conjugate to be tested, as sample 2;
[0021] Load sample 1 and sample 2 respectively on a high-efficiency gel chromatography column TSKgel G5000PW (30cm×7.5mm) fully balanced with physiological saline, with a sample volume of 20μl, an elution flow rate of 0.3ml / min, and record the chromatogram at a wavelength of 214nm; The time corresponding to the top of the carrier protein elution peak was 27.89 minutes, and the corresponding time to the end of the peak was 34.76 minutes;
[0022] Integrate the sample 1 chromatogram, adjust the integration window to be the second half ...
Embodiment 2
[0028] Utilize the Lowry method to measure the total protein concentration in the meningococcal group C polysaccharide tetanus toxoid conjugate to be tested, it is 320.4 μ g / ml (C 0 ); get the tetanus toxoid solution corresponding to the conjugate, and dilute to 1000 μg / ml with normal saline according to its protein concentration (C 1 ), as sample 1; take the meningococcal group C polysaccharide tetanus toxoid conjugate to be tested, as sample 2;
[0029] Load sample 1 and sample 2 respectively on a high-efficiency gel chromatographic column TSKgelG5000PWxl (30cm×7.8mm) fully balanced with physiological saline, with a sample volume of 200μl, an elution flow rate of 1.0ml / min, and record the chromatogram at a wavelength of 214nm; carrier The corresponding time of protein elution peak peak is 8.37min, and the corresponding time of peak end is 10.22min;
[0030] Integrate the chromatogram of sample 1, and adjust the integration window to be the second half peak from the correspo...
Embodiment 3
[0036] Utilize the Lowry method to measure the total protein concentration in the Haemophilus influenzae type b polysaccharide tetanus toxoid conjugate to be tested, it is 183.8 μ g / ml (C 0 ); get the tetanus toxoid solution corresponding to the conjugate, and dilute to 500 μg / ml with normal saline according to its protein concentration (C 1 ), as sample 1; take the polysaccharide tetanus toxoid conjugate of Haemophilus influenzae type b to be tested, as sample 2;
[0037]Load sample 1 and sample 2 respectively on a high-efficiency gel chromatographic column TSKgelG4000PWxl (30cm×7.8mm) fully balanced with physiological saline, with a sample volume of 100μl, an elution flow rate of 0.5ml / min, and record the chromatogram at a wavelength of 214nm; carrier The corresponding time of protein elution peak peak is 16.75min, and the corresponding time of peak end is 20.46min;
[0038] Integrate the chromatogram of sample 1, and adjust the integration window to be the second half peak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com