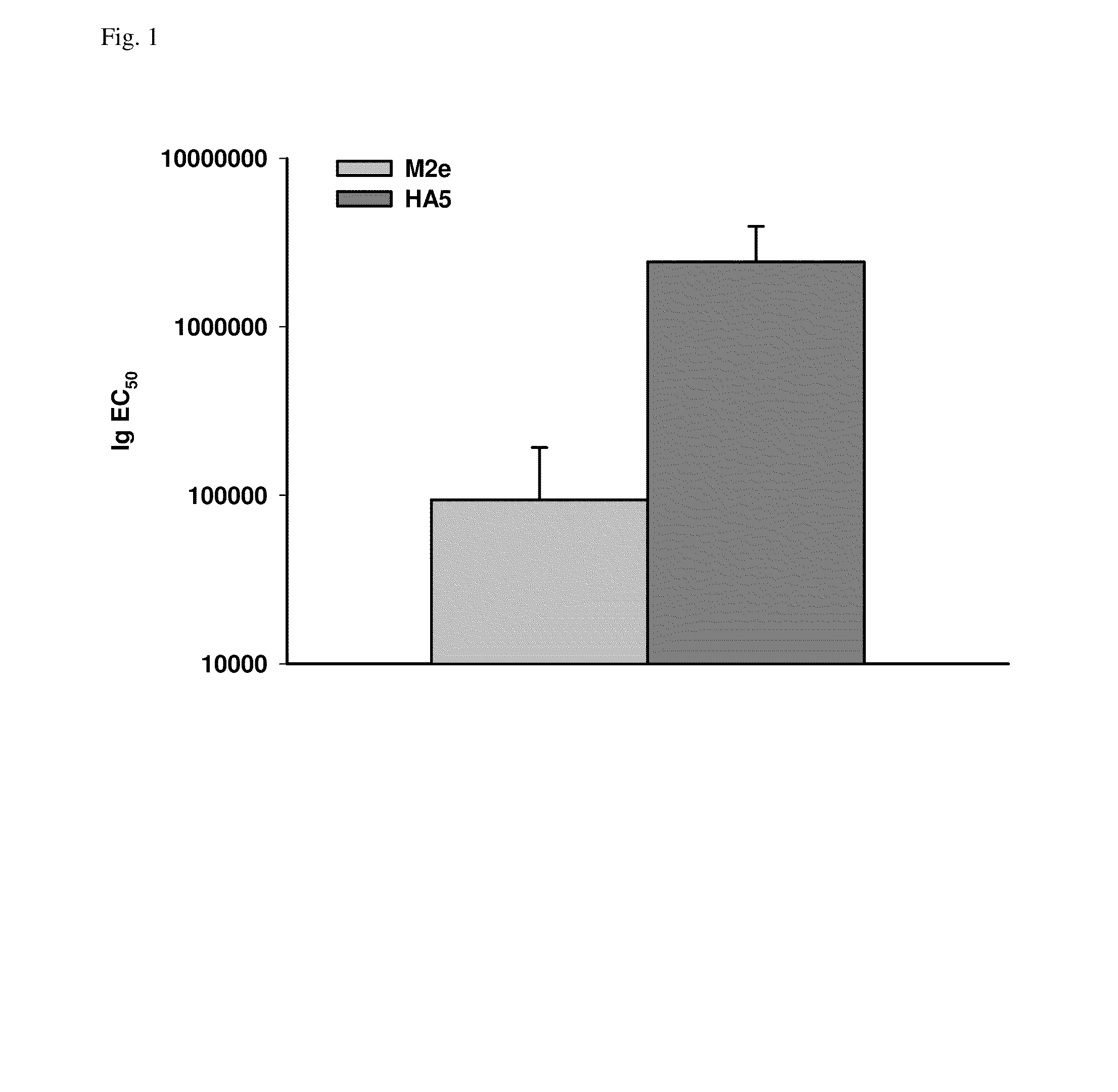
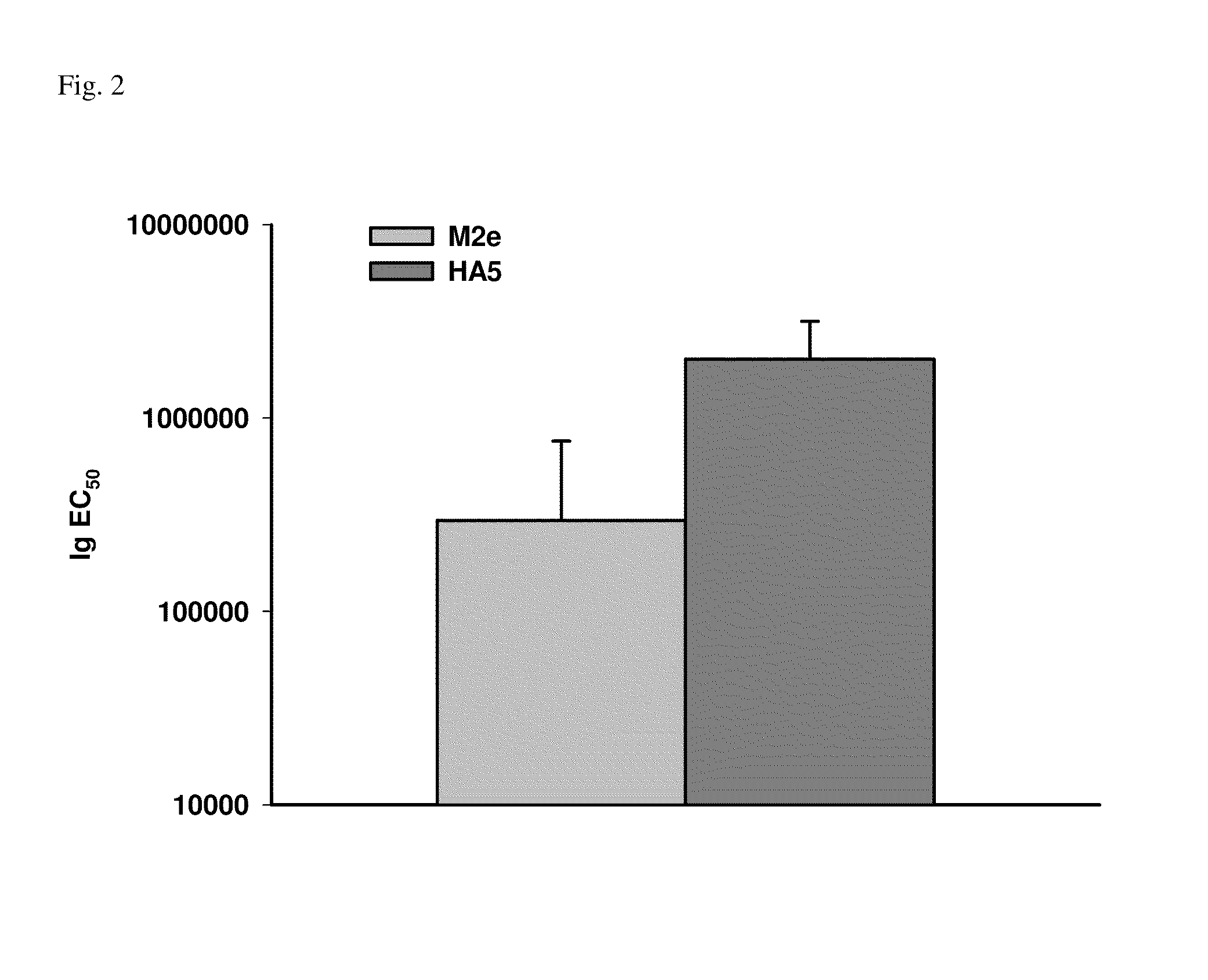
Synthetic nanocarrier combination vaccines
a technology of synthetic nanocarriers and vaccines, applied in the direction of viruses, powder delivery, inorganic non-active ingredients, etc., can solve the problems of inability to comfortably and/or safely combine the number of antigens in a single dosage form, the total liquid volume of the dosage form becomes too large to comfortably and/or safely, and the existing vaccine formulation is limited in coverage or physical incompatibility with one another
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthetic Nanocarriers with Covalently Coupled Adjuvant
[0134]Nanocarriers comprising PLGA-R848, PLA-PEG-N3, and ova peptide were prepared via double emulsion method wherein the ova peptide was encapsulated in the nanocarriers.
[0135]The polyvinyl alcohol (Mw=11 KD-31 KD, 87-89% partially hydrolyzed) was purchased from JT Baker. Ovalbumin peptide 323-339 was obtained from Bachem Americas Inc. (3132 Kashiwa Street, Torrance Calif. 90505. Part # 4065609). PLGA-R848, and PLA-PEG-N3 conjugates were synthesized and purified.
[0136]The above materials were used to prepare the following solutions:
[0137]1. PLGA-R848 conjugate in methylene chloride @ 100 mg / mL
[0138]2. PLA-PEG-N3 in methylene chloride @ 100 mg / mL
[0139]3. Ovalbumin peptide 323-339 in 0.13N HCl @ 70 mg / mL
[0140]4. Polyvinyl alcohol in 100 mM pH 8 phosphate buffer @50 mg / mL
[0141]Solution #1 (0.75 mL) and solution #2 (0.25 mL) were combined and solution #3 (0.1 mL) or 0.13N HCl (0.1 mL) was added in a small vessel and the mixture was...
example 2
Composition with Synthetic Nanocarriers and Uncoupled Antigen (Prophetic)
[0144]A 4 mL portion of the synthetic nanocarrier suspension from Example 1 containing 8 mg of L2 substituted nanocarriers is centrifuged to settle the particles. The supernatant is discarded and a 0.5-mL suspension of Gardasil®, Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine containing purified virus-like particles (VLPs) of the major capsid (L1) protein of HPV Types 6, 11, 16, and 18 is added. The combination vaccine is agitated to re-suspend the nanocarriers and the resulting suspension is stored at −20° C. prior to use.
example 3
Synthetic Nanocarriers with Non-Covalently Coupled Adjuvant [Prophetic]
[0145]DNA containing cationic disulfide PRINT nanocarriers are produced by the method described in the patent application of DeSimone, WO2008118861, example 16 with the exception that the ssDNA-fluorescein of example 16 is replaced by the phosphorothioated DNA CpG 7909. After isolation, the cationic nanocarriers are suspended in 1.0 mL of PBS solution containing 10 mg / mL of heparin. After stirring at room temperature for 2 hours, the nanocarriers are isolated by centrifugation and are washed twice with PBS by centrifugation and decantation. The nanocarriers containing CpG 7909 with surface adsorbed heparin are re-suspended in 1.0 mL of PBS and are stored at −20° C. prior to use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com