Patents
Literature
419 results about "Virus antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A virus antigen is a toxin or other substance given off by a virus which causes an immune response in its host.
Method for large scale production of virus antigen
The present invention provides improved methods of production of viral antigen on a culture of adherent cells bound to a microcarrier, wherein the methods provide for increased viral antigen yield per culture medium volume. The invention is also directed to a cell culture biomass of adherent cells having increased cell density and microcarrier concentration compared to the respective confluent cell culture.
Owner:OLOGY BIOSERVICES INC
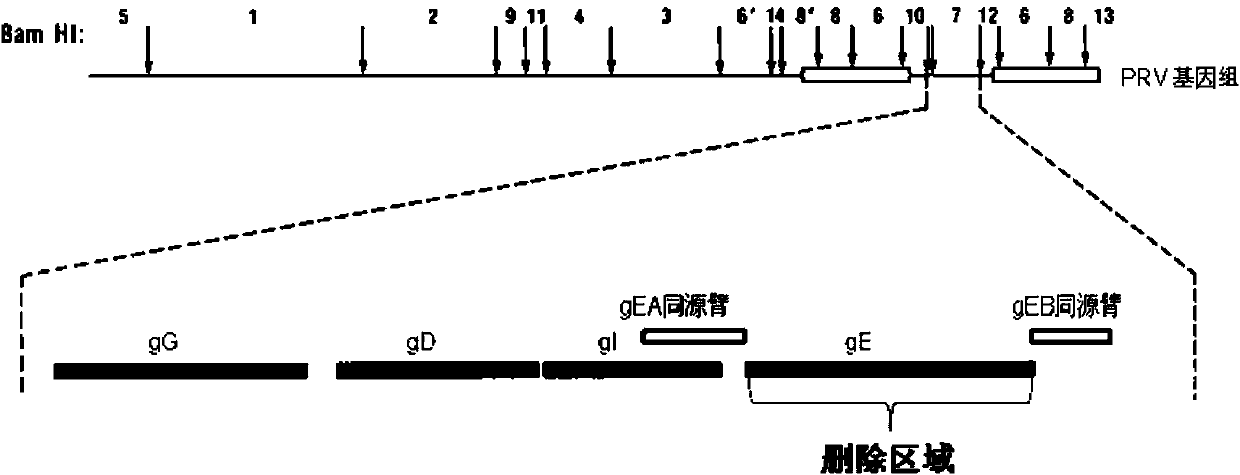
Porcine pseudorabies virus gene deletion strain, vaccine composition, and preparation method and application of vaccine composition
ActiveCN103923884ASymptoms relieved or improvedMicroorganism based processesAntiviralsVirus antigenTGE VACCINE
The invention provides a porcine pseudorabies virus gene deletion strain, a vaccine composition, and a preparation method and an application of the vaccine composition. The vaccine composition comprises an immunizing dose of an attenuated livetotivirus antigen and an inactivated totivirus antigen of the porcine pseudorabies virus gene deletion strain or its culture. The vaccine composition can effectively induce the antibody production, can effectively protect pigs, and can be used as a marking vaccine to effectively differentiate wild strains and vaccine strains.
Owner:PU LIKE BIO ENG
Veterinarian virus kind biological product heat resisting freeze drying protective agent and its preparation technique
A freeze-dried high-temp. resistant protecting agent for virus-type biologic products for veterinary medicine is prepared from several components through proportionally mixing. It features that each of its components is sterilized separately. For the components which can be sterilized under high temp., they are dissolved in distilled water according to the proportion of formulation and are sterilized at 116 deg.C for 30-40 min; and for those which do not resist the high temp., they are also dissolved in distilled water according the proportion of formulation and are filtered with 0.22 micron pore diameter filter membrane to remove bacteria; and then the two parts are mixed to obtain the freeze-dried high-temp. resistant, protecting agent. The mentioned two parts are added to the virus antigen liquid according to a proportion of 1:1 and after packaging, they are freeze-dried. The product can be stored at 2-8 deg.C for 24 months.
Owner:卫广森
Mycoplasma Hyopneumoniae Avirulent Adjuvanted Live Vaccine
ActiveUS20090117152A1Preventing and minimize severityElicit immune responseAntibacterial agentsBacterial antigen ingredientsViral antigensImmunogenicity
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS SERVICE LLC
Measles subunit vaccine
Compositions and methods for making and using therapeutic formulations of measles virus antigens with a Proteosome-based adjuvant are provided. The measles virus antigens may be derived from a variety of sources, such as from recombinant production or from a split antigen preparation. The measles vaccine formulations may be used, for example, in methods for treating or preventing a measles virus infection and eliciting a protective immune response.
Owner:ID BIOMEDICAL CORP LAVAL +1
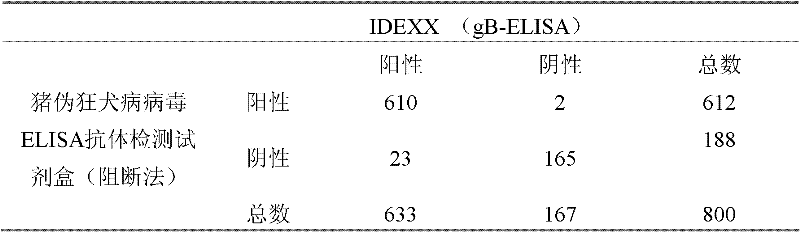
Kit for detecting pig pseudorabies virus antibodies and block enzyme-linked immuno sorbent assay (ELISA) method
The invention discloses a kit for detecting pig pseudorabies virus antibodies and a block enzyme-linked immuno sorbent assay (ELISA) method. The kit for detecting pig pseudorabies virus antibodies comprises pig pseudorabies virus monoclonal antibodies which are labelled by horseradish peroxidase, wherein the pig pseudorabies virus monoclonal antibodies are monoclonal antibodies obtained by pig pseudorabies viruses as immunogens and the pig pseudorabies viruses are pseudorabies virus strain Ea. The kit for detecting pig pseudorabies virus antibodies also comprises an enzyme label plate, a sample diluent, negative and positive contrasts, a coloured solution, a washing solution, and a stopping solution. The block ELISA method comprises the following steps of 1, taking out a detection plate pre-coated with virus antigens from the kit for detecting pig pseudorabies virus antibodies, adding diluted blood serum needing to be detected into the detection plate pre-coated with the virus antigens, and simultaneously, setting negative and positive contrast apertures, 2, shaking up the diluted blood serum in the negative and the positive contrast apertures, shaking off a solution in the negative and the positive contrast apertures, and washing the detection plate by the washing solution, and 3, adding the pig pseudorabies virus monoclonal antibodies labelled by horseradish peroxidase into the negative and the positive contrast apertures, washing, adding the colored solution into the negative and the positive contrast apertures to carry out room-temperature coloration in the dark, adding the stopping solution into the negative and the positive contrast apertures, and determining OD630nm values of the negative and the positive contrast apertures by an ELISA apparatus. The block ELISA method has the advantages of good singularity, high sensitivity, short detection time, and high accuracy because of utilization of an S / N ratio method in result determination.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
IgG kit for detecting streetvirus of dogs using indirect enzyme immunosorbent assay and preparation method thereof
The invention refers to a kind of detecting reagent box and the manufacturing method, concretely refers to the reagent which is indirect enzyme immune sorption experiment for detecting rabies virus IgG and the manufacturing method. The reagent box compositions are: beforehand enclosed rabies virus antigen enzyme label board, sample diluting solution, HRP-rabies resisting IgG enzyme compound, condensed washer solvent, substrate and stopping liquid. The specificity of the reagent can reach 100%; the sensitivity is 1:640; the accuracy (the variation coefficient) is 6.98%. The reagent uses indirect ELISA to detect the rabies virus IgG antibody.
Owner:湖北省预防医学科学院
PCV/Mycoplasma Hyopneumoniae/PRRS Combination Vaccine
ActiveUS20130266603A1Protective immune responseAntibacterial agentsBacterial antigen ingredientsImmune complexVirus antigen
This invention provides a trivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; a porcine circovirus type 2 (PCV2) antigen; and a PRRS virus antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Method of treating viral infections
InactiveUS20050053625A1Reduce antigen/glycoprotein secretionLimiting reinfection mediated spreadPeptide/protein ingredientsViral antigen ingredientsCompound (substance)Virus antigen
The present invention relates to the treatment of viral infections, particularly HBV and HCV infections, with a combination comprising a vaccine against a virus antigen and compounds that inhibit glucosidase activity in the host cell.
Owner:BLOCK TIMOTHY M +2
Methods for detection or measurement of viruses
InactiveUS20110262892A1Improve automationMicrobiological testing/measurementBiological material analysisViral antibodyActive agent
A method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) an anionic surfactant and (2) an amphoteric surfactant, nonionic surfactant or protein denaturant; a virus assay method using said treating method; a method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) a chaotropic ion and (2) an acidifying agent; a virus assay method using said treating method; a virus assay method, characterized in that a virus antigen and a virus antibody are measured based on their binding to their probe in the presence of a surfactant with an alkyl group of 10 or more carbon atoms and a secondary, tertiary or quaternary amine, or a nonionic surfactant, or of both of them; and a monoclonal antibody and a hybridoma producing the same for carrying out said method.
Owner:AOYAGI KATSUMI +4
Bactrian camel heavy-chain (HC) variable-domain antibody resisting porcine circovirus 2 as well as preparation method and application thereof
ActiveCN102766207AMicrobiological testing/measurementMicroorganism based processesSingle-domain antibodyAmino acid
The invention discloses a bactrian camel heavy-chain (HC) variable-domain antibody resisting a porcine circovirus 2 as well as a preparation method and an application thereof, and belongs to the field of a biotechnology. A PCV2 (Porcine Circovirus Virus) inactivated vaccine immunized bactrian camel is firstly utilized and a phage display technology is utilized to establish a PCV2 immune VHH antibody base; and a high-specificity variable domain antibody of heavy chains of HCAbs (VHH) which has a good affinity with the PCV, particularly a PCV2 virus antigen; and the antibody disclosed by the invention has an amino acid sequence shown as SEQ (sequence) ID NO.1 or an amino acid sequence which is shown as SEQ ID NO.2 and has the sequence isogeny being more than 95%. The PCV2 heavy-chain variable-domain antibody disclosed by the invention has the very important meanings on researching porcine circovirus, particularly researching a porcine circovirus 2-type infection mechanism and establishing rapid antigen detection with high sensitivity and specificity or diagnosing a reagent.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Sample pad processing liquid, H7 subtype avian influenza virus colloidal gold test strip and preparation method thereof
The invention discloses a sample pad processing liquid, a H7 subtype avian influenza virus colloidal gold test strip and a preparation method thereof, the processing liquid is prepared as follows: taking 8-20g of PEG(polyethylene glycol)-200, 11-25g of PVP (polyvinyl pyrrolidone), 10-45g of BSA (bovine serum albumin), 0.5-30ml of Triton X - 100, and 0.8-15ml of Twain-20, then using 5mM-10mM phosphate buffer solution to fix the volume to 1L, and adjusting the pH value to 9.5; and the colloidal gold test strip is formed by overlapping and pasting in turn a sample pad, a glass fiber membrane, a cellulose nitrate membrane and absorbent paper on a bottom plate, and the sample pad is processed by the processing liquid. The processing liquid can increase the sample pad hydrophilicity, contributes to the sample pad fast wetting, and promotes the chromatographic effect, aqueous solution formed by dissolving large molecules in water has suspension and dispersion effect, and can protect virus antigen in a sample. The test strip has the advantages of safe and simple operation, strong specificity, high sensitivity, and low rate of cross infection.
Owner:GUANGZHOU WONDFO BIOTECH
Preparation method and application of electrochemical immunosensor based on HS-beta-CD-Ag-GOD conjugate
ActiveCN104459124ACatalytic reductionDoubly magnifiedBiological material analysisMaterial analysis by electric/magnetic meansImmune profilingBovine serum albumin
The invention belongs to the technical fields of immunoassay and biosensing and discloses a preparation method and application of an electrochemical immunosensor based on an HS-beta-CD-Ag-GOD conjugate. The electrochemical immunosensor is used for rapidly detecting a hog cholera virus antigen CSFV. The manufacturing scheme comprises the following steps: modifying a working electrode a bare platinum carbon electrode by using MWCNTs-CD-Fc-Ab1, and sequentially adding bovine serum albumin, hog cholera virus antigen CSFV and an Ab2-HS-beta-CD-Ag-GOD conjugate. The HS-beta-CD-Ag-GOD conjugate can convert glucose into gluconic acid, two protons and two electrons are transferred to oxygen molecules, hydrogen peroxide is generated, and an HS-beta-CD-Ag nanometer material serves as a mimic enzyme, the reduction of the hydrogen peroxide is catalyzed, the dual amplification of an electrochemical signal is realized, the high sensitivity is realized, and the detection limit can be low to 0.33pg / mL.
Owner:UNIV OF JINAN
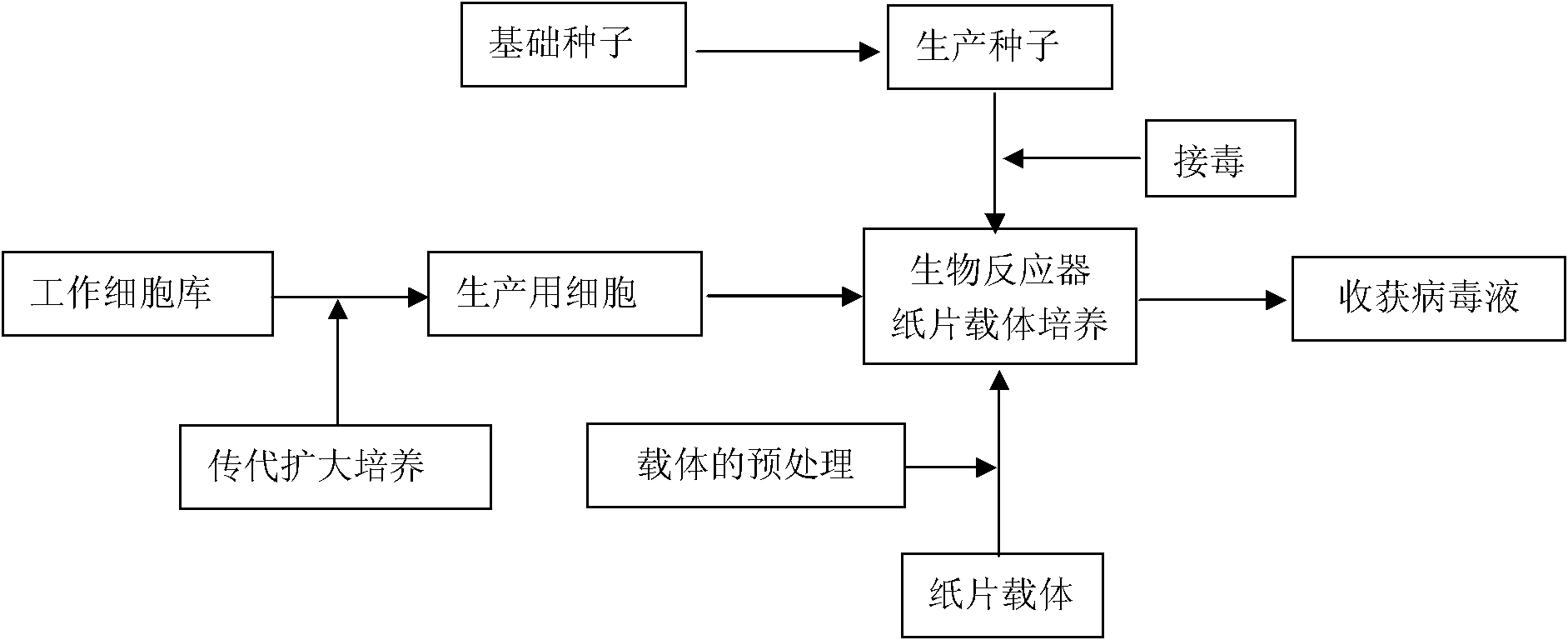
Method for production of porcine epidemic diarrhea virus
The invention discloses a technology for the production of porcine epidemic diarrhea virus by means of the microcarrier culture of VREO cells using a bioreactor, and comprises the technology for the production of different porcine epidemic diarrhea virus strains. The technology comprises the following technical steps: (1) selection of VERO cells as cell line for vaccine; (2) passage and culture of cells for vaccine; (3) propagation of seed culture of the porcine epidemic diarrhea virus; (4) microcarrier suspension culture of the VERO cells in the bioreactor; (5) propagation of porcine epidemic diarrhea virus antigen; and (6) treatment of acquired virus antigen liquid. The production method can remarkably lower production cost and enhance output-input ratio by 5 to 10 times, and has the advantages of short production period, small occupied space, great easiness for enlarging production scale rapidly, little environmental pollution, easy processing, high automation degree, a small number of staff, easy implementation of even and stable quality, obviously lowered production cost and enhanced yield and quality of vaccine.
Owner:成都史纪生物制药有限公司
Combined detection test paper of influenza A virus antigen and influenza B virus antigen and preparation method thereof
InactiveCN102445537AEnhanced signalHigh detection sensitivityMaterial analysisInfluenza B virus antigenPHA granule
The invention discloses combined detection test paper of influenza A virus antigen and influenza B virus antigen and a preparation method thereof. The test paper comprises a sample pad, a fiberglass membrane containing a colloidal gold particle label, a nitrocellulose membrane and water absorbing paper, wherein the nitrocellulose membrane comprises a detection area which is coated with an influenza A virus antibody, a detection area which is coated with an influenza B virus antibody and a control area which is coated with an goat anti-rabbit antibody; the colloidal gold particle label comprises a micro signal amplification system and a colloidal gold labeled rabbit IgG antibody; and the micro signal amplification system is a colloidal gold particle-avidin-biotin-influenza A / B virus antibody. According to the invention, an avidin-biotin microsignal amplification system is added in a double-antibody sandwich detection system, the signal of a target antibody is enlarged, the detection sensitivity is increased, false negative or detection omission due to weak signals can be avoided, simultaneously combined detection can be carried out on the influenza A and B virus antigens, and the detection time, sample and cost can be saved.
Owner:GUANGZHOU WONDFO BIOTECH
Method for preparing anti-duck viral hepatitis transfer factor
InactiveCN101953849AFree from infectionSimple manufacturing methodImmunoglobulins against virusesAntiviralsDuck viral hepatitisHepatitis
The invention discloses a method for preparing an anti-duck viral hepatitis transfer factor, which comprises the following steps of: firstly, inoculating duck viral hepatitis in a chick embryo allantoic cavity for multiplication and extracting a virus antigen from the chick embryo allantoic cavity; secondly, immunizing a healthy pig body with the extracted virus antigen; and thirdly, extracting the anti-duck viral hepatitis transfer factor from the spleen of the immune pig. In the invention, specific transfer factor is extracted from duck viral hepatitis viruses and can effectively suppress the duck viral hepatitis viruses and protect the cells of a normal body from being infected with the duck viral hepatitis viruses, so that the duck viral hepatitis is prevented and treated radically. Moreover, the preparation method of the invention is simple and feasible, short in production time and low in cost and has a promising application prospect.
Owner:河南后羿生物工程股份有限公司
Double-antibody biotin-Avidin ELISA (enzyme-linked immuno sorbent assay) detection kit for cattle viral diarrhea virus and application method thereof
InactiveCN102023217AAvoid missing detectionStrong specificityMaterial analysisBovine virus diarrhea virus AntigenAssay
The invention relates to a double-antibody biotin-Avidin ELISA (enzyme-linked immuno sorbent assay) detection kit for cattle viral diarrhea virus antigen and an application method thereof. The kit comprises washing liquid for ELISA detection, developing liquid, stopping liquid, Streptavidin-HRP, positive control and negative control and is characterized by further comprising an ELISA plate coating cattle viral diarrhea virus single antibody 3D8 and a cattle viral diarrhea virus single antibody 3F9 marked by biotin. The detection without cross reaction is performed by using the kit, thereby being capable of preventing leak detection caused by low titer in the antibody and having high specificity; a biotin-affine sensitizer is used for amplification, thereby increasing the flexibility, improving the detection rate, and simultaneously reducing the amount of second antibody; and the kit is suitable for being used widely.
Owner:YANGZHOU UNIV
Deer epidemic hemorrhage competitive enzyme-linked immunosorbent assay kit and preparation method and use thereof
InactiveCN101672849AStrong specificityIncreased sensitivityMaterial analysisSerum igeEpizootic haemorrhagic disease virus
The invention relates to a deer epidemic hemorrhage competitive enzyme-linked immunosorbent assay kit and a preparation method and a user thereof, the kit comprises an EHDV antigen-coated ELISA plate,a monoclonal antibody IgG-HRP enzyme conjugate, positive serum, negative serum, 20 times of concentrated washing liquid, 10 times of concentrated dilution buffer, a substrate I, a substrate II, a substrate III and stop solution. The preparation method comprises the following steps: a, preparing a deer epidemic hemorrhage virus ELISA-coated antigen, and detecting the safety of the deer epidemic hemorrhage virus ELISA-coated antigen; b, preparing a monoclonal antibody of deer epidemic hemorrhage virus and carrying out preparation and identification on the antibody-horseradish peroxidase (HRP) enzyme conjugate; c, preparing the positive serum and the negative serum; d, preparing the deer epidemic hemorrhage virus antigen-coated ELISA plate; e, preparing the 20 times of the concentrated washing liquid and the 10 times of the concentrated dilution buffer, and preparing the substrate I, the substrate II, the substrate III and the stop solution; and f, assembling the kit. The kit is used fordiagnosis, quarantine, detection and epidemiological investigation of deer epidemic hemorrhage and is characterized by strong specificity, high sensitivity and the like.
Owner:花群义
EV71 virus neutralization epitope detection kit or reagent and preparation method thereof
The invention relates to an EV71 virus neutralization epitope detection kit or a reagent and a preparation method thereof. Particularly, the invention performs quantitative detection on EV71 virus antigen by adopting an HD6 monoclonal antibody with spectral activity. The kit or the reagent has the advantages of easy operation, high sensitivity and the like.
Owner:SINOVAC BIOTECH
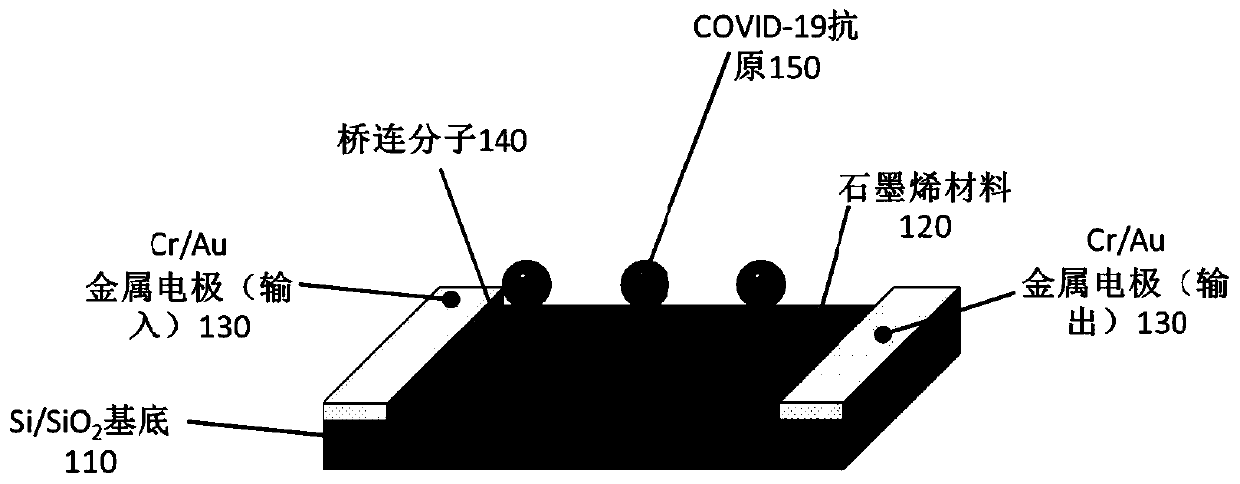
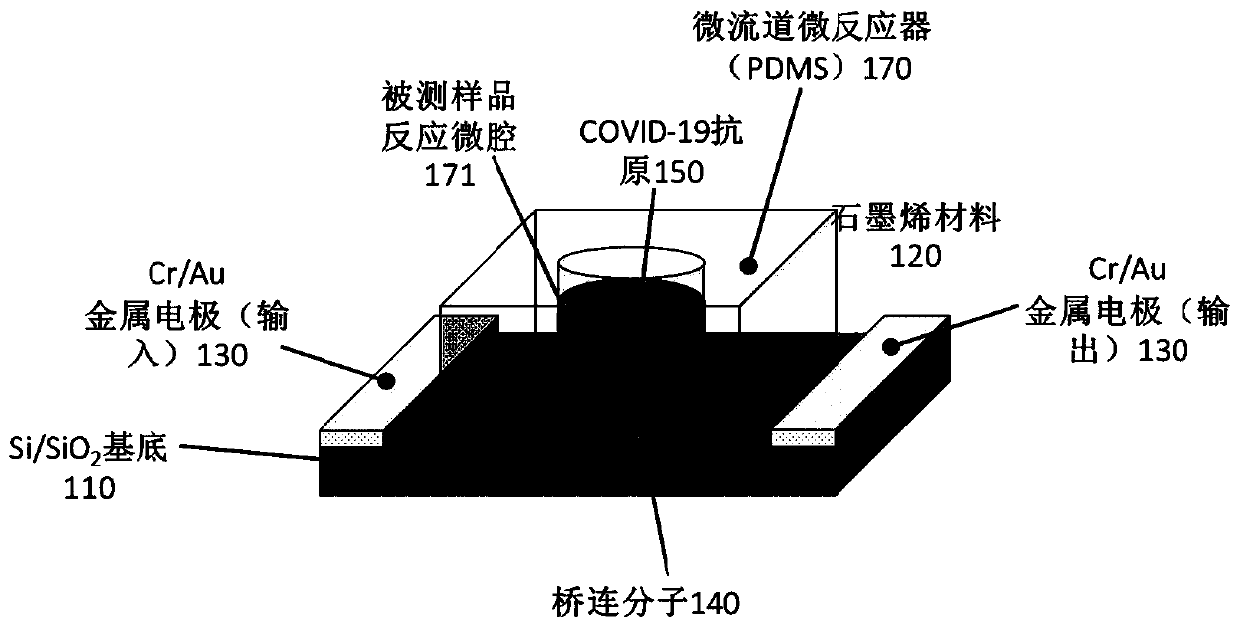
Biosensor, preparation method and virus detection system and method
ActiveCN111474365ARealize detectionMicrobiological testing/measurementBiological testingViral antibodyNucleic Acid Probes
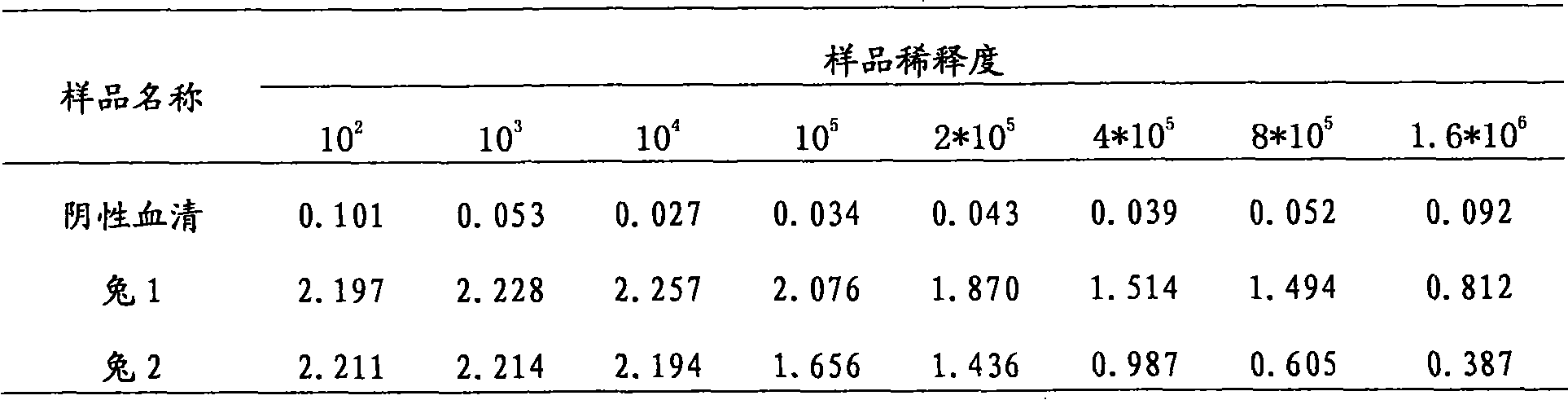
The embodiment of the invention provides a biosensor, a preparation method, a virus detection system and method. The method comprises the following steps: modifying a virus antigen or a virus antibodyor a nucleic acid probe for detecting viruses on a biosensor through bridging molecules capable of connecting various biomacromolecules, inputting a to-be-detected sample into the biosensor, and analyzing current signals before and after reaction, so as to detect whether viruses exist in the to-be-detected sample or not. The bridging molecule of the biosensor provided by the embodiment of the invention can be connected with various biomacromolecules, so that different viruses can be detected.
Owner:PEKING UNIV
Monoclonal antibody of membrane protein E for resisting West Nile virus and application thereof
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Method for producing PRRS (Porcine Reproductive and Respiratory Syndrome) viruses
ActiveCN102002482AQuality improvementIncrease productionRecovery/purificationPerfusion bioreactorPerfusion Culture
The invention discloses a method for producing PRRS (Porcine Reproductive and Respiratory Syndrome) viruses through culturing Marc-145 cells by applying a torrent-perfusion bioreactor. The method comprises the following steps of: (1) selecting Marc-145 cells as cells for culturing seedlings; (2) subculturing the Marc-145 cells; (3) reproducing virus seeds for production; (4) perfusing and culturing the Marc-145 cells on a paper carrier in the torrent-perfusion bioreactor; (5) producing the PRRS viruses in the torrent-perfusion bioreactor; and (6) treating the obtained virus antigen solution. The invention can greatly decrease the production cost, improve the input-output ratio by more than 10 times and expand the production scale easily and rapidly, has short production preparation cycle and high degree of automation, occupies less area, causes less environmental pollution which is easy to treat, consumes less labor, is easy to realize balanced and stable virus quality and can obviously improve the yield and the quality of the viruses.
Owner:成都史纪生物制药有限公司
Methods for the detection of hepatitis B and C viruses
InactiveUS7776542B1Simple and sensitive detectionSimple and sensitive and quantitationMicrobiological testing/measurementBiological material analysisViral antibodyMonoclonal antibody
A method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) an anionic surfactant and (2) an amphoteric surfactant, nonionic surfactant or protein denaturant; a virus assay method using said treating method; a method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) a chaotropic ion and (2) an acidifying agent; a virus assay method using said treating method; a virus assay method, characterized in that a virus antigen and a virus antibody are measured based on their binding to their probe in the presence of a surfactant with an alkyl group of 10 or more carbon atoms and a secondary, tertiary or quaternary amine, or a nonionic surfactant, or of both of them; and a monoclonal antibody and a hybridoma producing the same for carrying out said method.
Owner:ADVANCED LIFE SCI INST
A kind of lipid microsphere composition
The invention relates to a lipid microsphere composition. The lipid microsphere composition is characterized by comprising a lipid microsphere emulsifying agent and an immunogenic composition, wherein the lipid microsphere emulsifying agent comprises 1 to 30 weight percent of pharmaceutical grade grease, 0.1 to 10 weight percent of zwitterionic / non-ionic surfactant composition, 0.01 to 5 weight percent of stabilizing agent and the balance of aqueous solution; and the immunogenic composition is a univalent to multivalent composition and usually a trivalent composition, and contains a virus antigen or antigenic preparation from one or more pandemic influenza epidemic related influenza virus strains or with one or more pandemic influenza epidemic related influenza virus strains. The lipid microsphere composition not only can serve as an excellent aid of a vaccine, but also can serve as a conveying system of the vaccine. The lipid microsphere composition is applicable to different administration routes of the vaccine, improves compliance, diversity and selectivity of clinical application, and achieves wide, safe and effective immune effect.
Owner:LIAONING CHENGDA BIOTECH
O-type aftosa synthetic peptide vaccine
ActiveCN101659695AImprove protectionFree from attackAntiviralsPeptide preparation methodsChemical synthesisPeptide vaccine
The invention provides O-type aftosa synthetic peptide vaccine, and in particular relates to polypeptide or polypeptide polymer thereof used in the vaccine as well as the vaccine containing the polypeptide or the polypeptide polymer thereof and a preparation method thereof. The polypeptide has amino acid sequences shown in SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3. The O-type aftosa synthetic peptide vaccine carries out chemical synthesis of potential antigen site peptide segments by carrying out sequencing of domestic aftosa epidemic strains to study the variation of the main antigen sites ofaftosa and combining with computer assistant to carry out antigen site analytical prediction. Candidate polypeptide antigens are screened out by carrying out large numbers of animal experiments and aftosa virus antigen sites are optimized according to the screening result; and T cell epitope and B cell epitope are effectively combined to improve the immune effects of the polypeptide antigens. TheO-type aftosa synthetic peptide vaccine can effectively cope with the antigen variation of aftosa virus and has ideal biosafety and easy large-scale synthesis, thereby having a good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
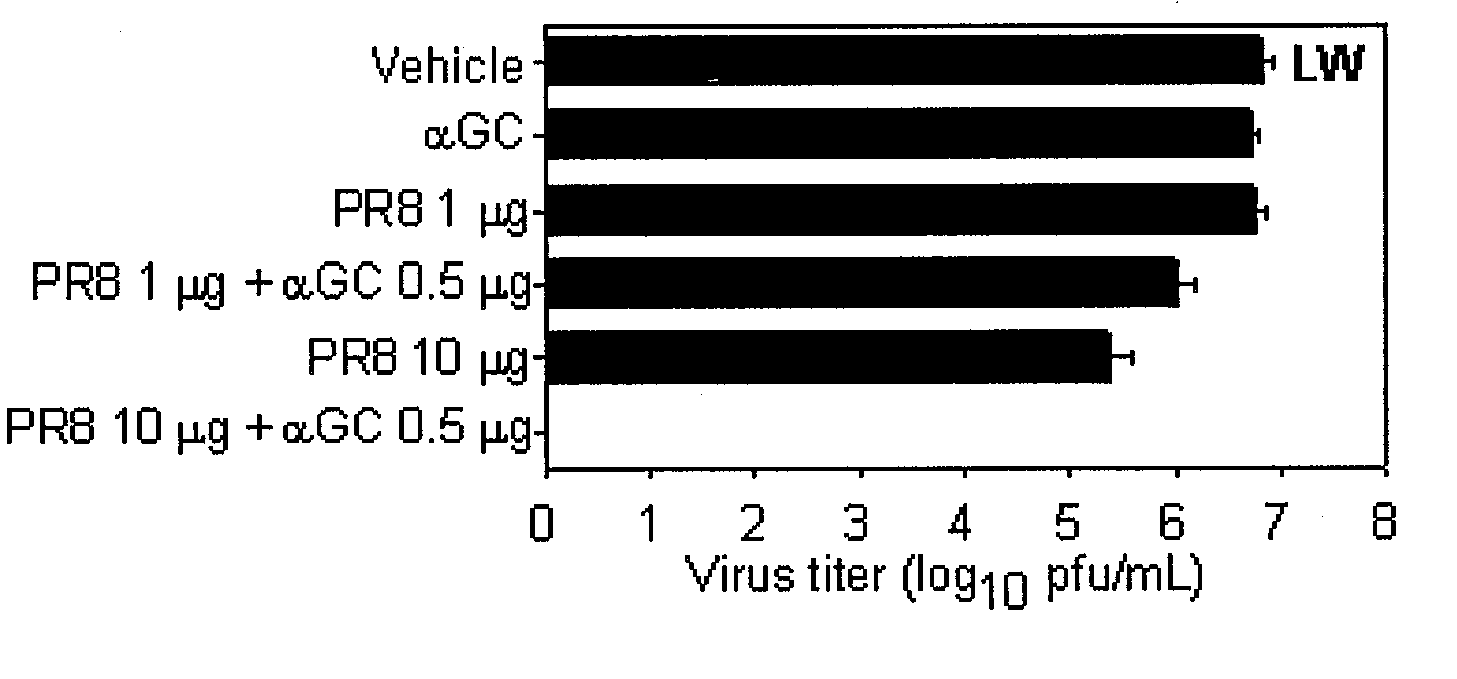
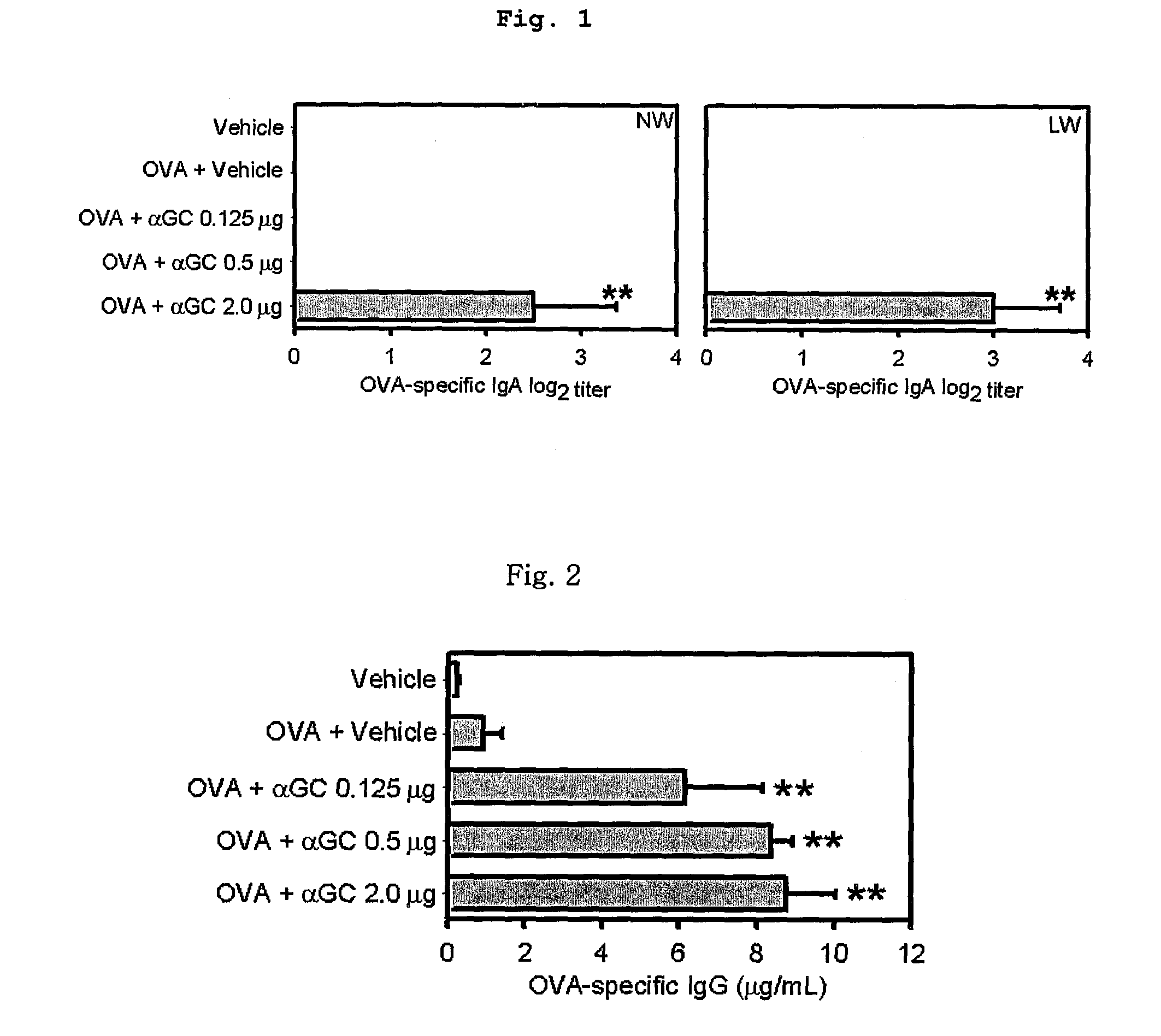
Vaccine Composition Comprising Alpha-Galactosylceramide as an Adjuvant For Intranasal Administration
InactiveUS20080317769A1Improve responseViral antigen ingredientsSnake antigen ingredientsNasal cavityAdjuvant
The present invention related to a vaccine composition comprising alpha-galactosylceramide (αGalCer) as an adjuvant for the intranasal administration. The present inventors administered αGalCer together with a tumor cell antigen or a virus antigen to the nasal cavity of a mouse and then confirmed that the αGalCer effectively induced not only humoral immunity but also cell-mediated immunity. Thus, the αGalCer can be effectively used as an adjuvant for a vaccine by the intranasal administration for the prevention and treatment of virus infection and cancer.
Owner:SEOUL NAT UNIV R&DB FOUND
Foot and mouth disease virus antigen polypeptide, fusion antigen polypeptide and vaccine
InactiveCN102180952AImproving immunogenicityImprove securityVirus peptidesAntiviralsVariant strainImmunogenicity
The invention provides a foot and mouth disease virus antigen polypeptide, a foot and mouth disease virus fusion antigen polypeptide and a foot and mouth disease virus vaccine containing the antigen polypeptide and / or the fusion antigen polypeptide. The invention further provides application of the antigen polypeptide, the fusion antigen polypeptide and the vaccine in prevention and control of foot and mouth disease virus infection. The foot and mouth disease virus antigen polypeptide, the fusion antigen polypeptide and the vaccine have broad-spectrum immunogenicity, and can generate good immunogenicity on different foot and mouth disease viruses and variant strains thereof.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Freeze-dried rabies vaccine for humans and preparation method of vaccine
ActiveCN104826101AThe process steps are simpleEasy to operateInactivation/attenuationAntiviralsHuman useSide effect
The invention relates to a freeze-dried rabies vaccine for humans and a preparation method of the vaccine, relates to the field of vaccine production preparation technologies and aims at solving the problems that effective virus antigen expression content is low, the side effect rate of a vaccinator is high and vaccine yield and quality can not meet standard requirements as only a biological reactor is adopted for producing a rabies vaccine. The freeze-dried rabies vaccine for humans is obtained by inoculating aG strain rabies virus on Vero cells and sequentially carrying out ultrafiltration and concentration, separation and purification as well as freeze drying, wherein the packing volume of the freezed-dried rabies vaccine for human use is 0.5ml / dose, and during freeze drying, the adopted vaccine freeze-drying protecting agent comprises the following ingredients: 60-90g / l of trehalos, 6-14g / l of sodium glutamate, 3-6g / l of urea, 2-3g / l of L-arginine and 10g / l of 199 culture medium, and the vaccine freeze-drying protecting agent does not contain gelatin, human serum albumin or dextran. The freeze-dried rabies vaccine for humans has the advantages that cost is low, operation is easy, pollution is hardly produced, vaccine quality and yield are greatly improved, the content of impurities in a vaccine is reduced, allergy reactions are hardly caused, and vaccine safety is greatly improved.
Owner:江生(深圳)生物技术研发中心有限公司
Influenza A,B virus antigen colloidal gold combined detection test paper
InactiveCN1904615ASave the procedure of secondary inspectionThe result is clear and easy to distinguishMaterial analysis by observing effect on chemical indicatorTest sampleColloid
The invention supplies a rapid testing tape that could simultaneously test A, B virus antigen. It covers FluA-McAb, FluB-McAb and IgG on NC film, compounds FluA-McAb, FluB-McAb marked by colloidal gold, simultaneously tests A, B virus antigen in tested sample by using film chromatography double antibody cream filling method. The invention could decrease cockamamie process, and could gain result in 10-15 minutes. It is suitable to hospital and the research for large scale epidemiology to handle sporadic affair.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
Novel vaccine adjuvant and application thereof in novel coronavirus pneumonia vaccine and other vaccines
ActiveCN111956797ASsRNA viruses positive-senseViral antigen ingredientsAntiendomysial antibodiesCoronavirus vaccination
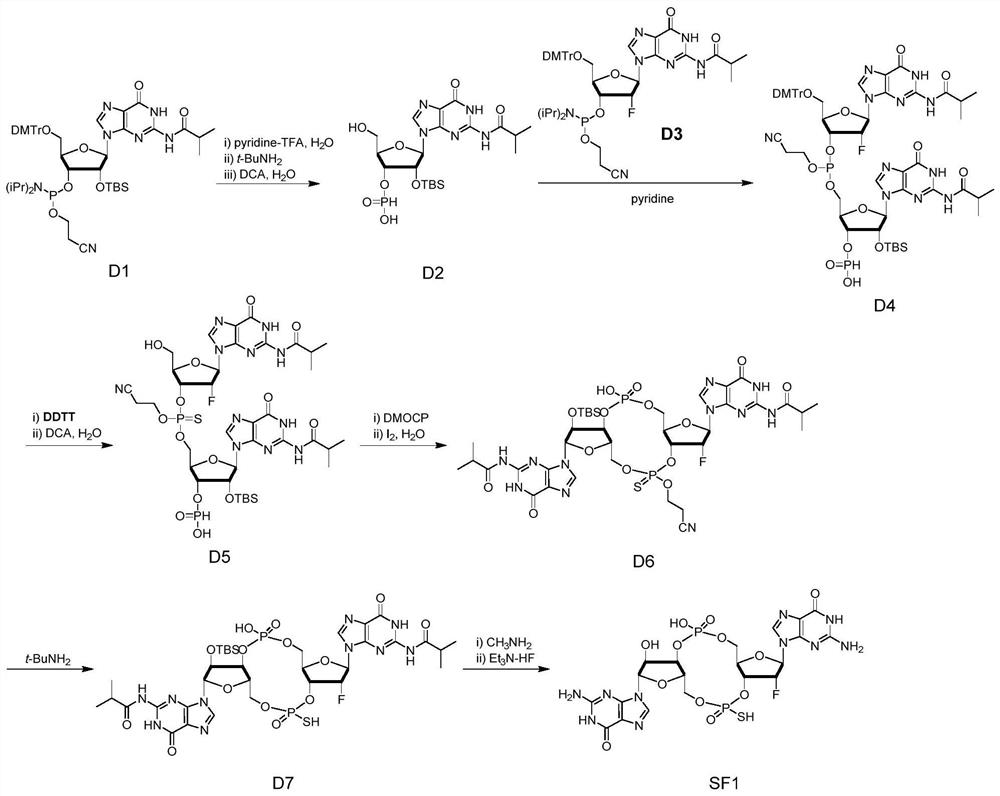
The invention relates to the field of biological medicines, in particular to a novel vaccine adjuvant and an application thereof in a novel coronavirus pneumonia vaccine and other vaccines. Chemicallymodified cyclic dinucleotide, namely an SF compound, is used as the vaccine adjuvant and is used in cooperation with the novel coronavirus vaccine, so that the SARS-CoV-2 virus antigen specific antibody titer and the generation of T cells can be remarkably improved; and the SF compound as the vaccine adjuvant is obviously superior to an aluminum adjuvant in immunopotentiation effect.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com