Monoclonal antibody of membrane protein E for resisting West Nile virus and application thereof
A monoclonal antibody, West Nile virus technology, applied in antiviral immunoglobulin, biochemical equipment and methods, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Obtaining of Monoclonal Antibody WNV-2F5' or WNV-4B3'
[0026] The murine hybridoma cell line WNV-2F5 or WNV-4B3 capable of secreting the monoclonal antibody WNV-2F5' or WNV-4B3' was prepared by the inventors according to the following method: 1) express West Nile virus in vitro using the Escherichia coli expression system The third domain protein of membrane protein E is purified to obtain recombinant EDIII protein, and the recombinant protein is refolded to refold the protein; 2) the recombinant protein is used as an antigen to immunize mice to prepare fused hybridoma cells. Cells secreting monoclonal antibodies against West Nile virus were obtained by limiting dilution.
[0027] The specific experimental methods and experimental results are as follows:
[0028] 1. Recombinant expression and renaturation of WNV membrane protein EDIII
[0029] 1. Construction of prokaryotic expression vector pET-EDIII
[0030] In order to express the WNV EDIII recombinant...
Embodiment 2
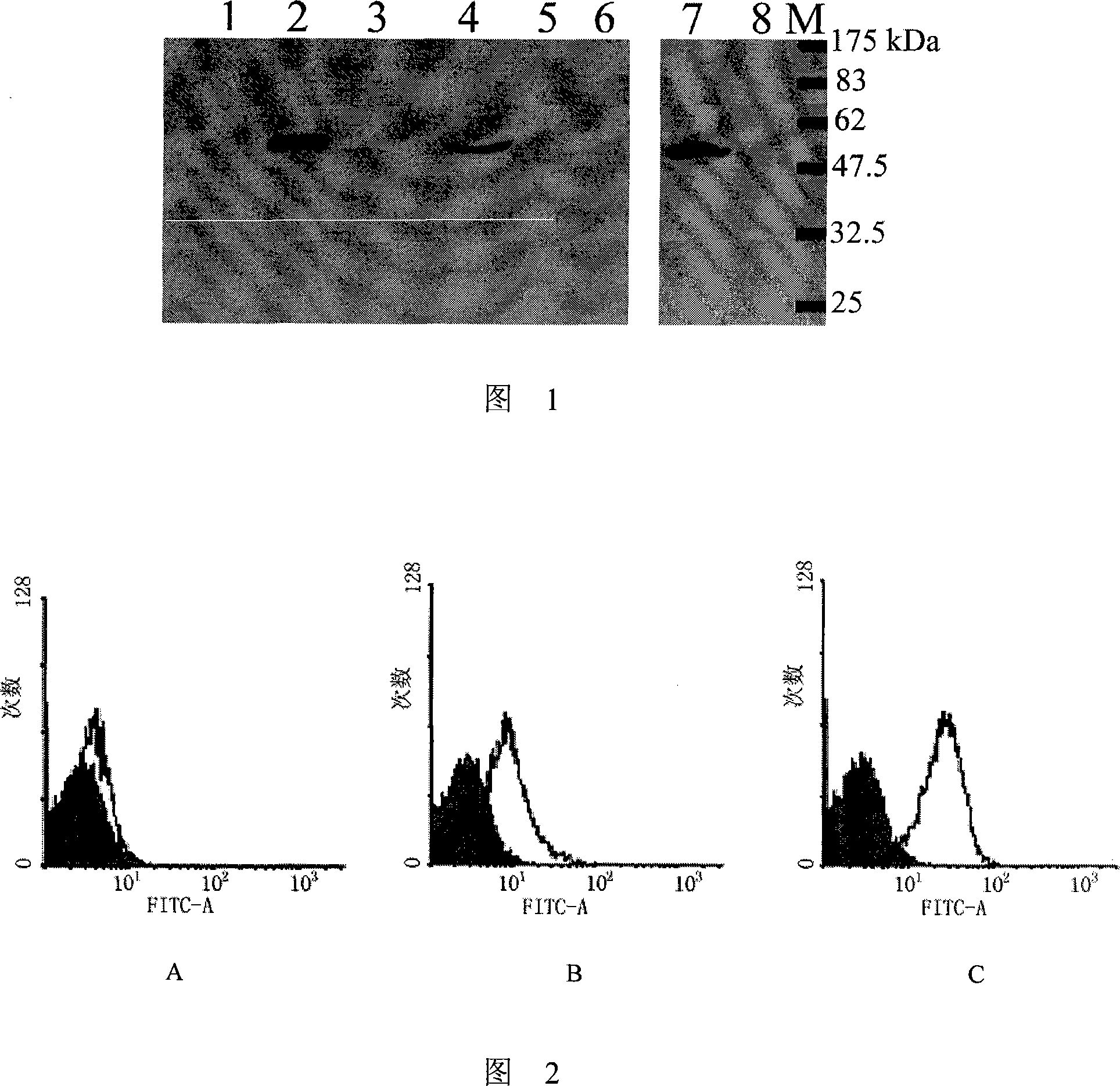
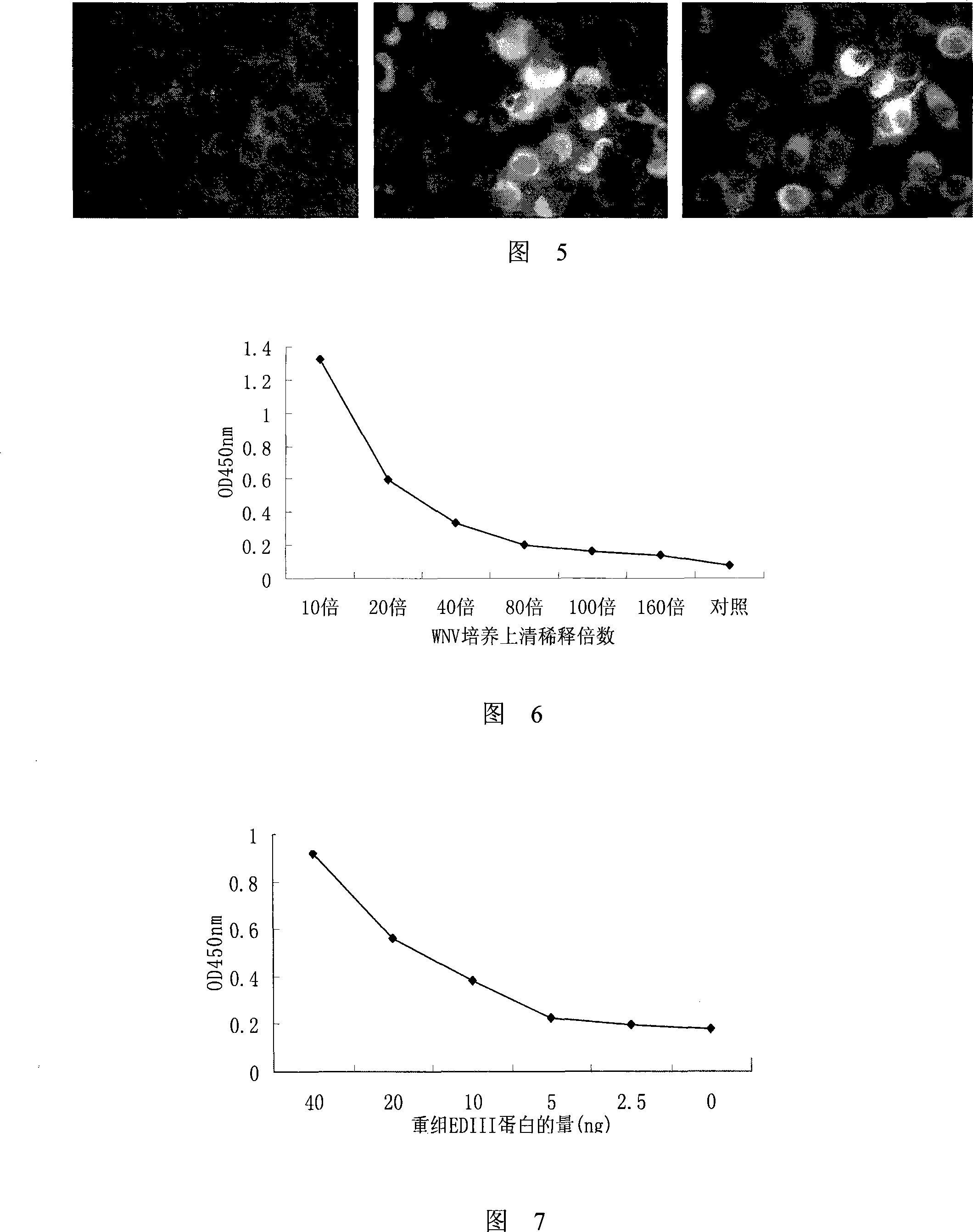
[0038] Example 2. Indirect ELISA detection of binding of purified antibody to coated antigen EDIII
[0039] With the recombinant WNV EDIII protein purified in Example 1 (dissolve 1 μg of recombinant EDIII protein in 5ml of pH9.6 carbonate buffer) to coat the microtiter plate, 20ng / hole, overnight at 4°C, use the same concentration of bovine Serum albumin (BSA) coated, as a negative control. Block with PBS solution containing 3% BSA at 37°C for 1 hour, wash with PBST solution (PBS containing 0.05% (volume percentage) Tween 20) for 4 times, and add serial dilutions with a concentration of 0.002-200 μg / ml Purified antibody, 100 μl / well, incubated at 37°C for 1 hour. After washing with PBST for 4 times, HRP-labeled anti-mouse secondary antibody (1:2000 dilution, Santa Cruz, Inc.) was added, 100 μl / well, and incubated at 37°C for 40 minutes. After washing 4 times with PBST, add a mixture of TMB (tetramethylbenzidine) and hydrogen peroxide (mixed in a volume ratio of 1:1) for colo...
Embodiment 3
[0044] Embodiment 3, immunoblotting method detects the specific reaction of antibody and denatured antigen
[0045] 1. Construction of WNV E protein expression vector pcDNA-WNV E
[0046] In order to express WNV E recombinant protein in eukaryotic cells, the following primers were designed and synthesized: P1: GGGGTACCACCATGTTCAACTGCCTTGG (Sequence 2), P2: CCGCTCGAGCGCATGCACGTTCAC (Sequence 3).
[0047] WNV virus preserved at -80°C (Bird 5810 strain, Institute of Microbiology and Epidemiology, Academy of Military Medical Sciences, references are Davis CT, Li L, May FJ, Bueno R Jr, Dennett JA, Bala AA, GuzmanH, Quiroga-Elizondo D , Tesh RB, Barrett AD. Genetic stasis of dominant West Nilevirus genotype, Houston, Texas. Emerg Infect Dis. 2007, 13 (4): 601-4.), by extracting viral RNA (QIAmp Viral RNA Mini Kit, CAT.No .52904), and then reverse transcription (TaKaRa RNA PCRKit (AMV) Ver.3.0, Code No.DRRO19A) to obtain viral cDNA, using the following PCR system (25 μl) to amplify ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com