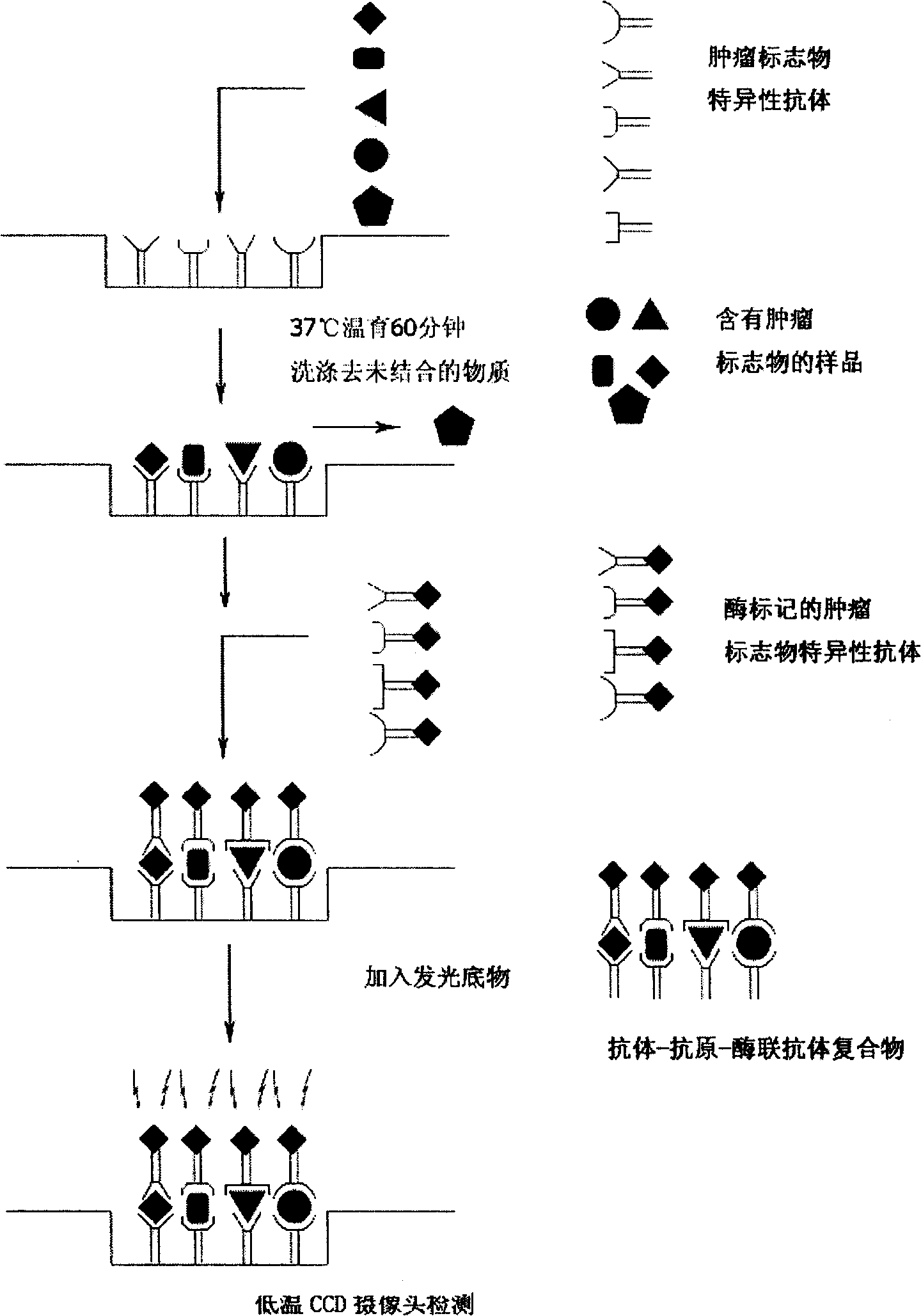
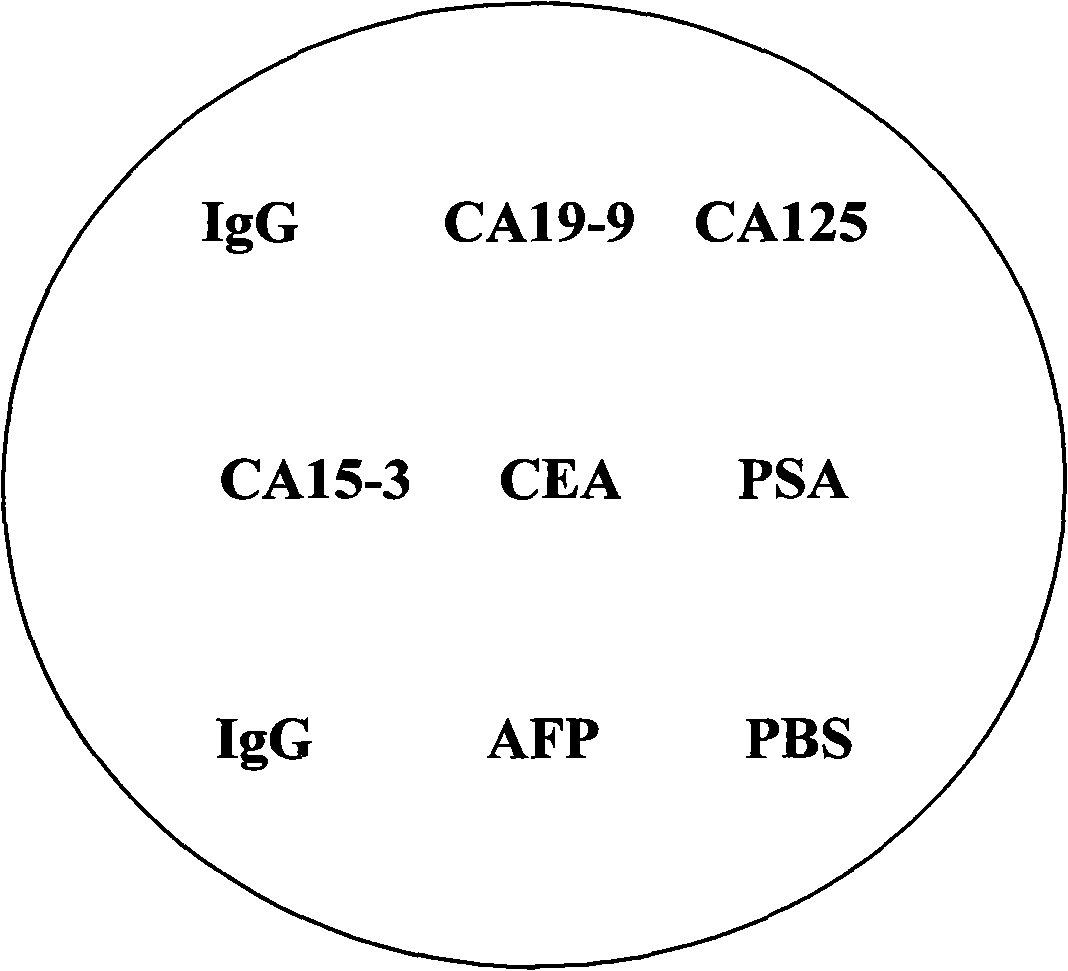
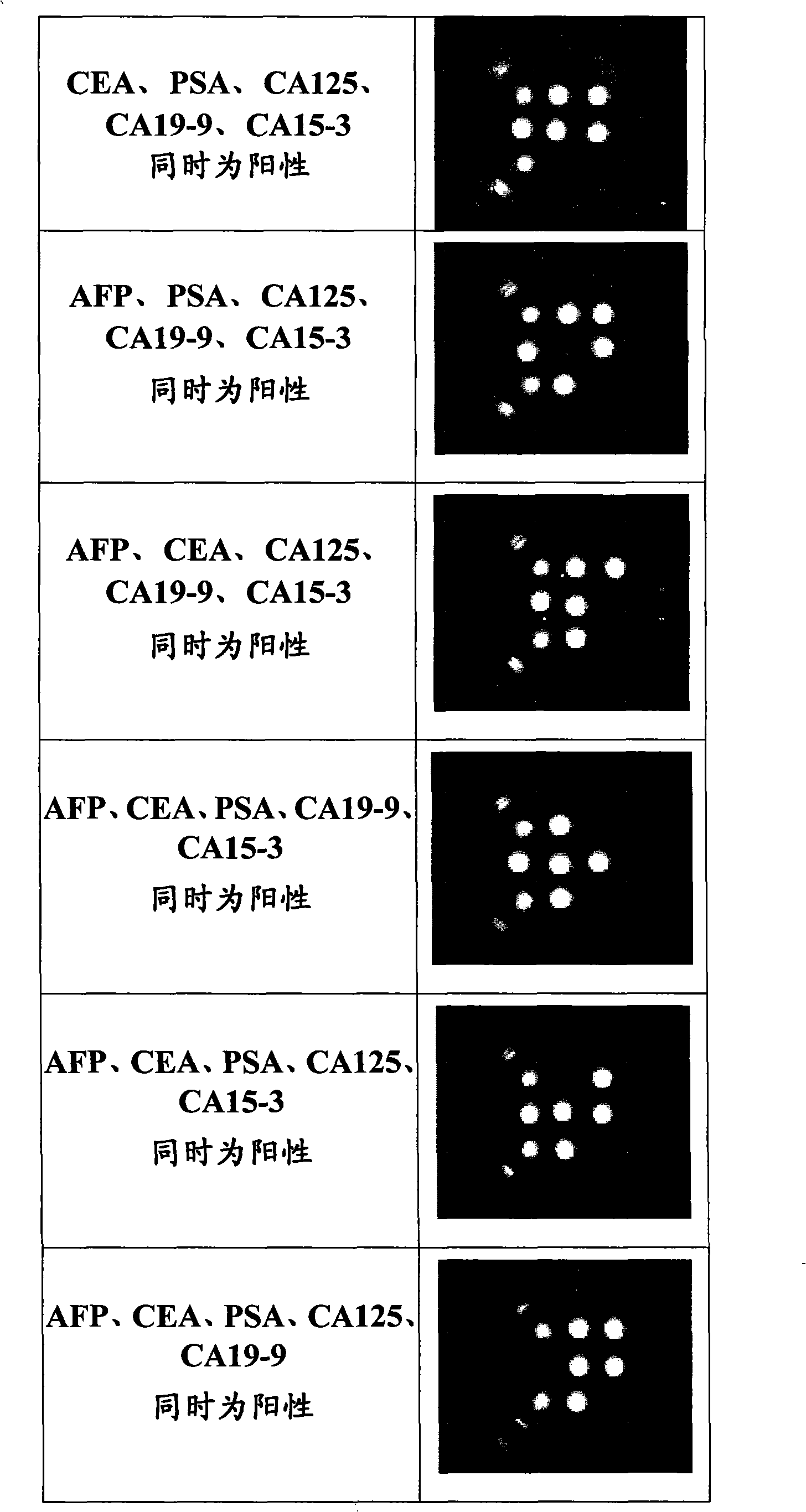
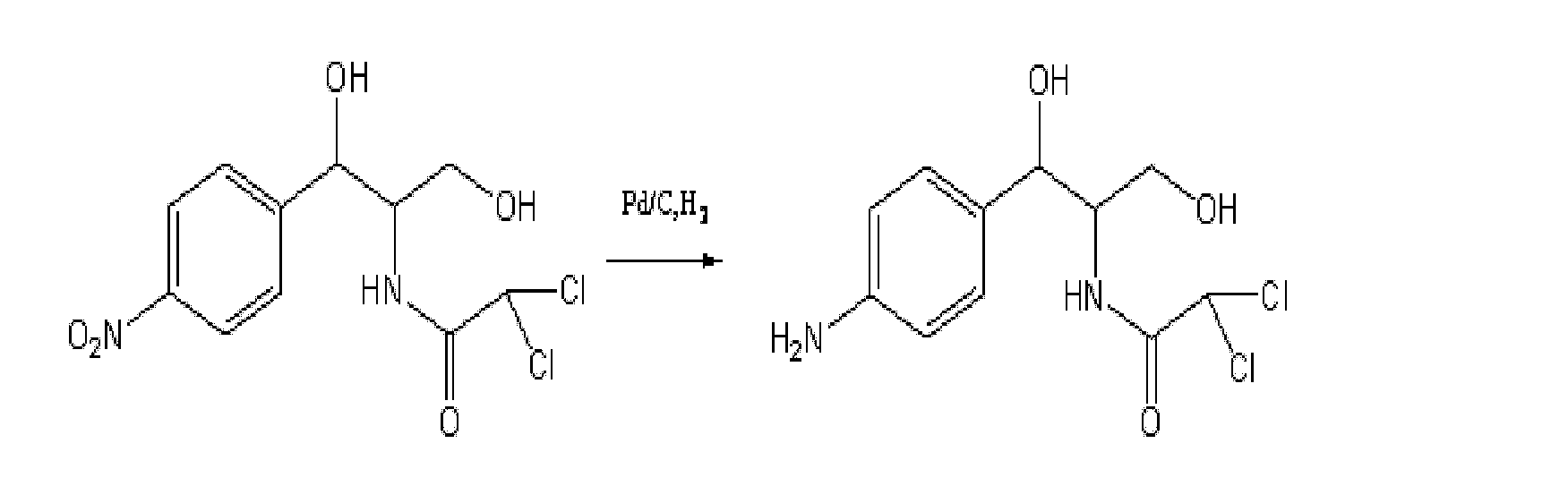
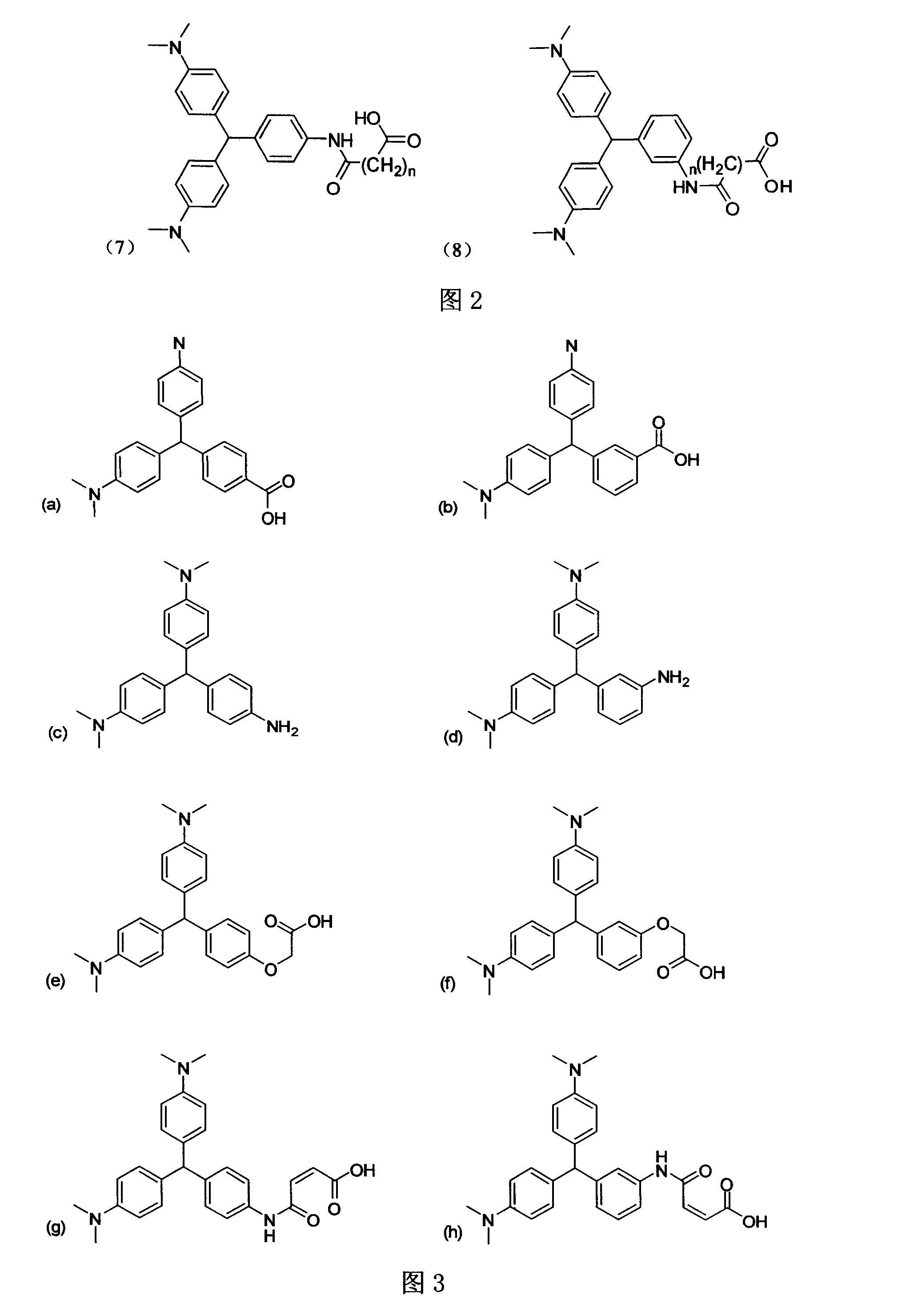
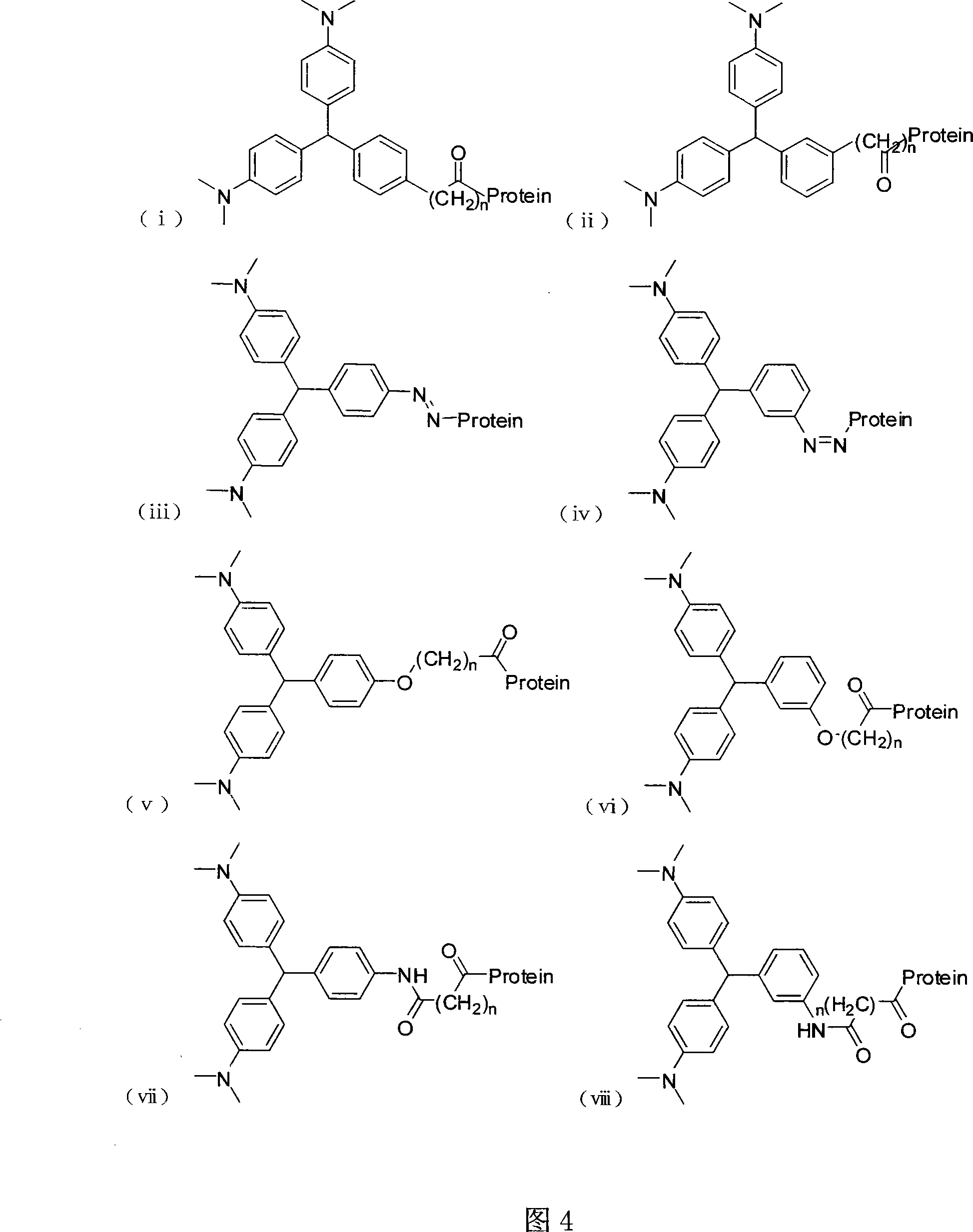
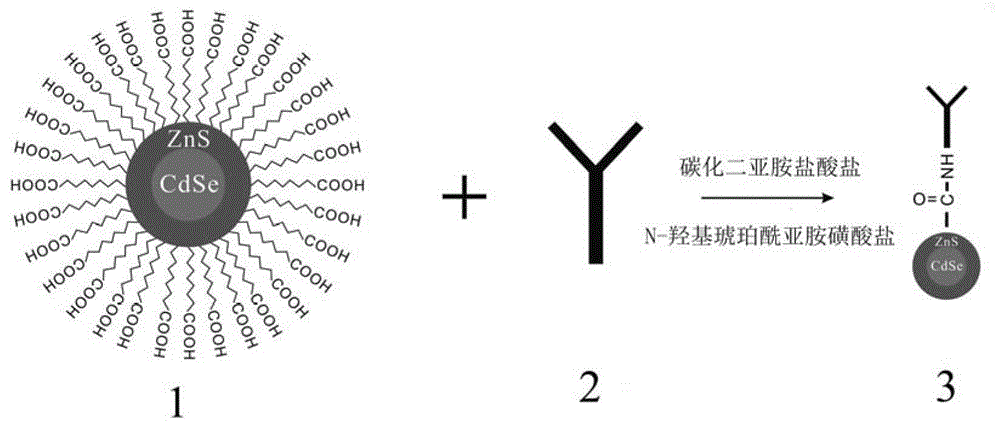
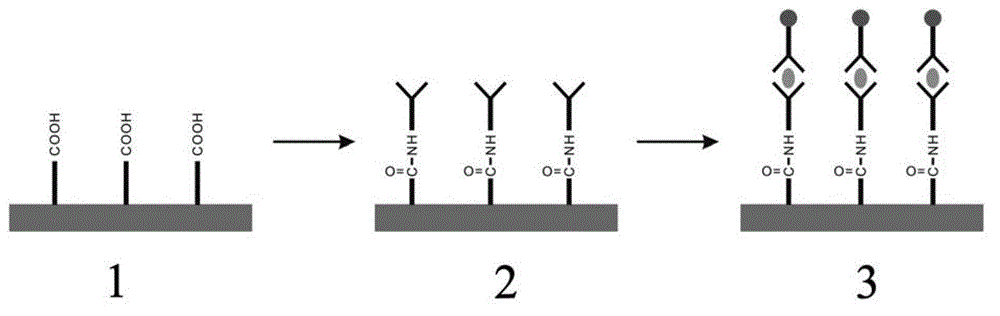
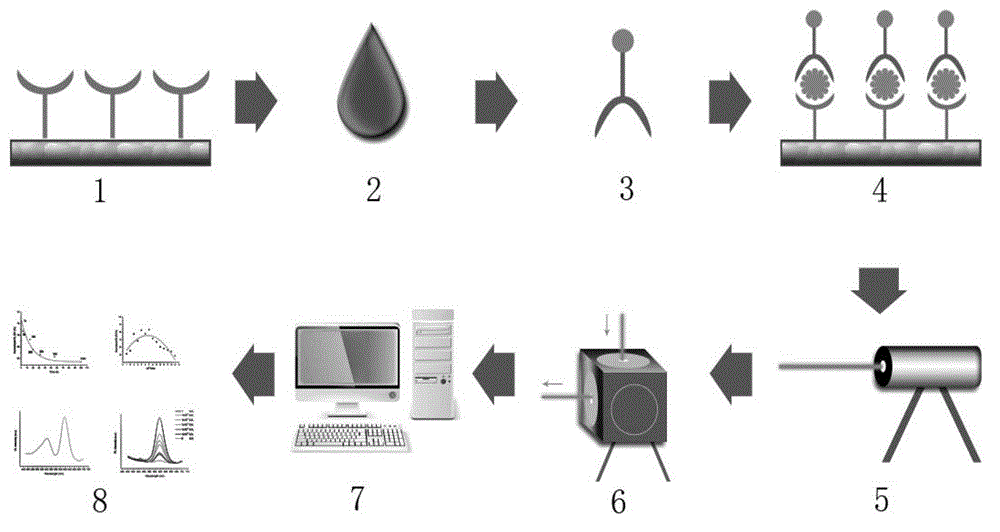
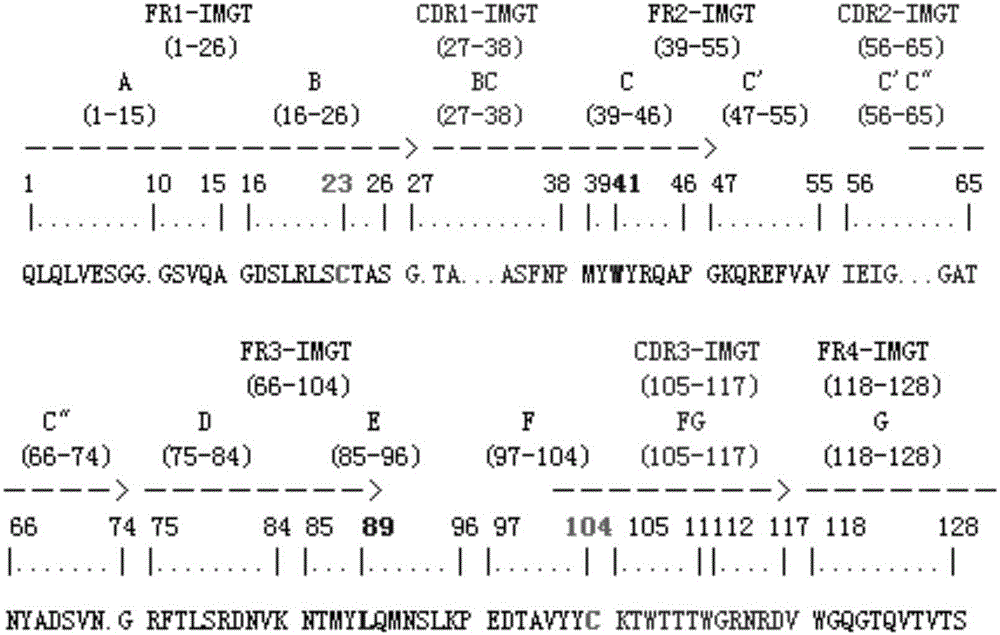
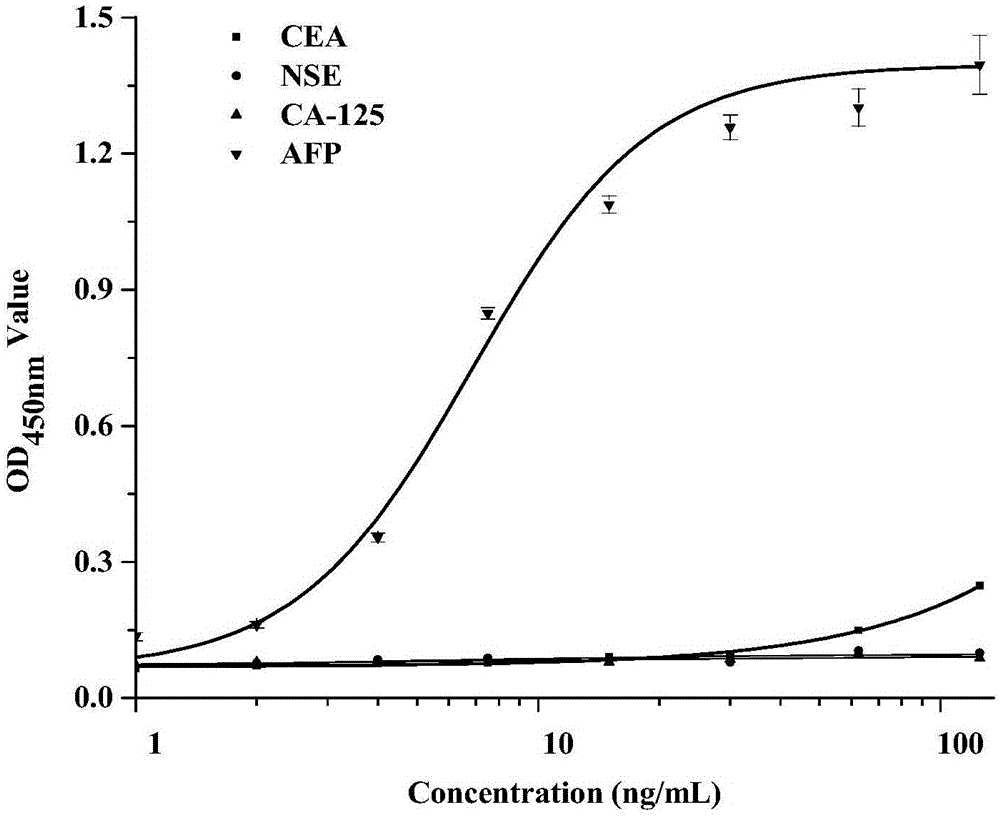
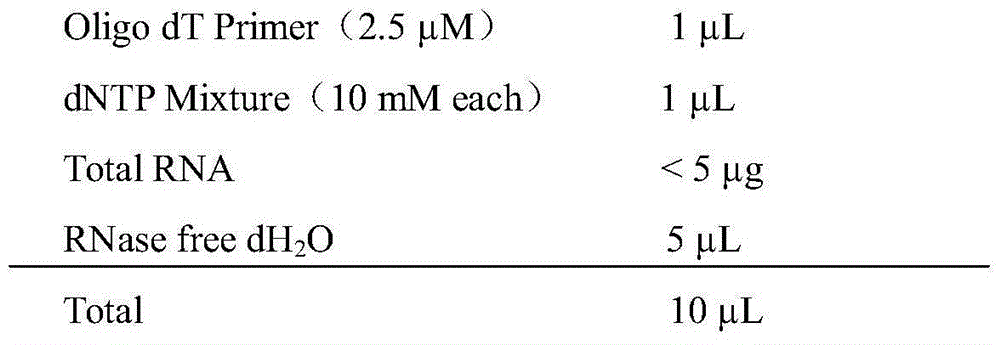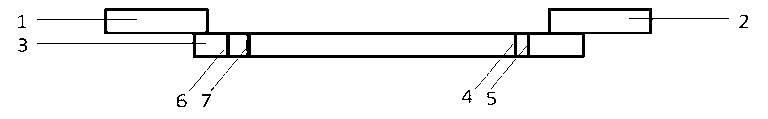Patents
Literature
2055 results about "Immuno detection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Detection strip for Zika virus detection by means of fast immunochromatography method
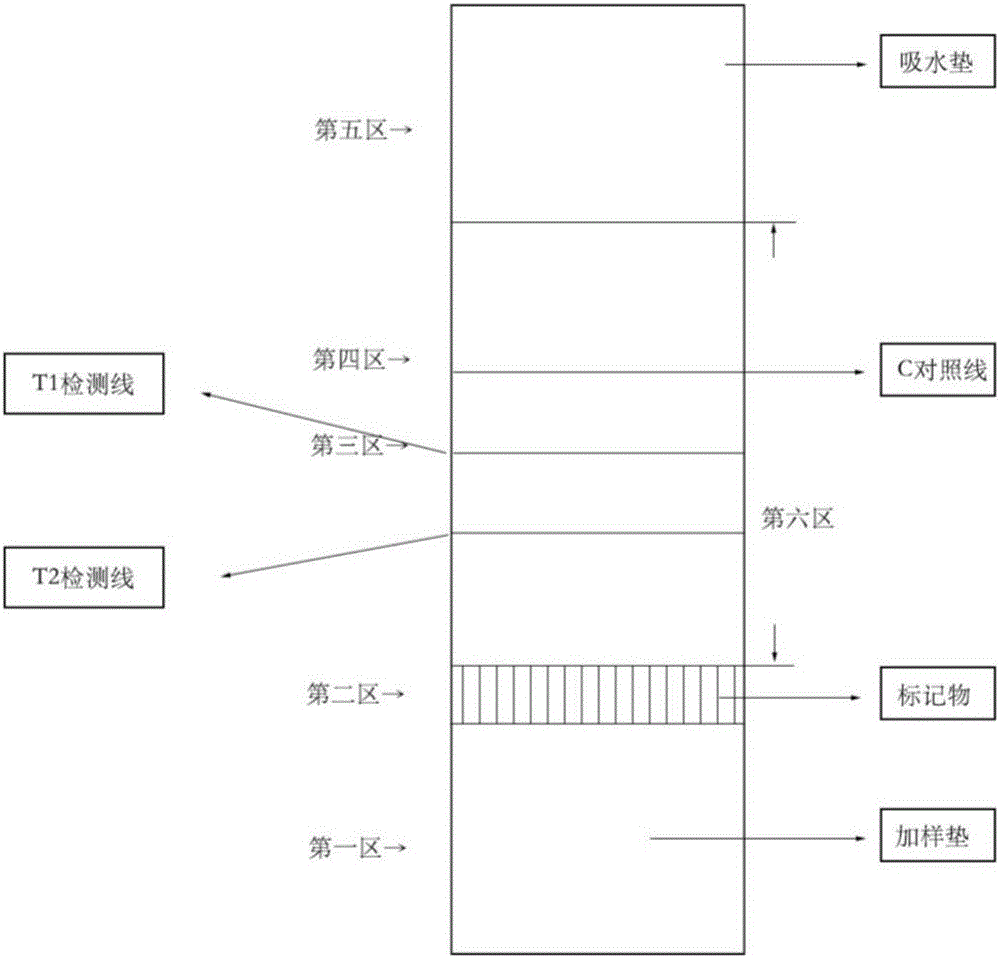
The invention provides a detection strip or kit for Zika virus detection by means of the fast immunochromatography method. The immunodetection strip has six areas. The first area is a sample adding pad; the second area is an immobilized Zika antigen marker area or anti-Zika virus antigen specific antibody marker; the third area is a detection area T and a coated antibody spray area, and the area T is matched with the second area; the fourth area is a contrast area used for immobilization of non-specific antibodies; the fifth area is a water absorber; the sixth area is a nitro fiber chromatography film. According to the detection strip or kit, only a small number of samples to be tested are needed, no equipment is required, a detection result can be obtained within ten minutes, detection is easy and fast, and the detection strip or kit can be purchased from a pharmacy so that patients can conduct detection by themselves.
Owner:卢氏实验室公司 +1
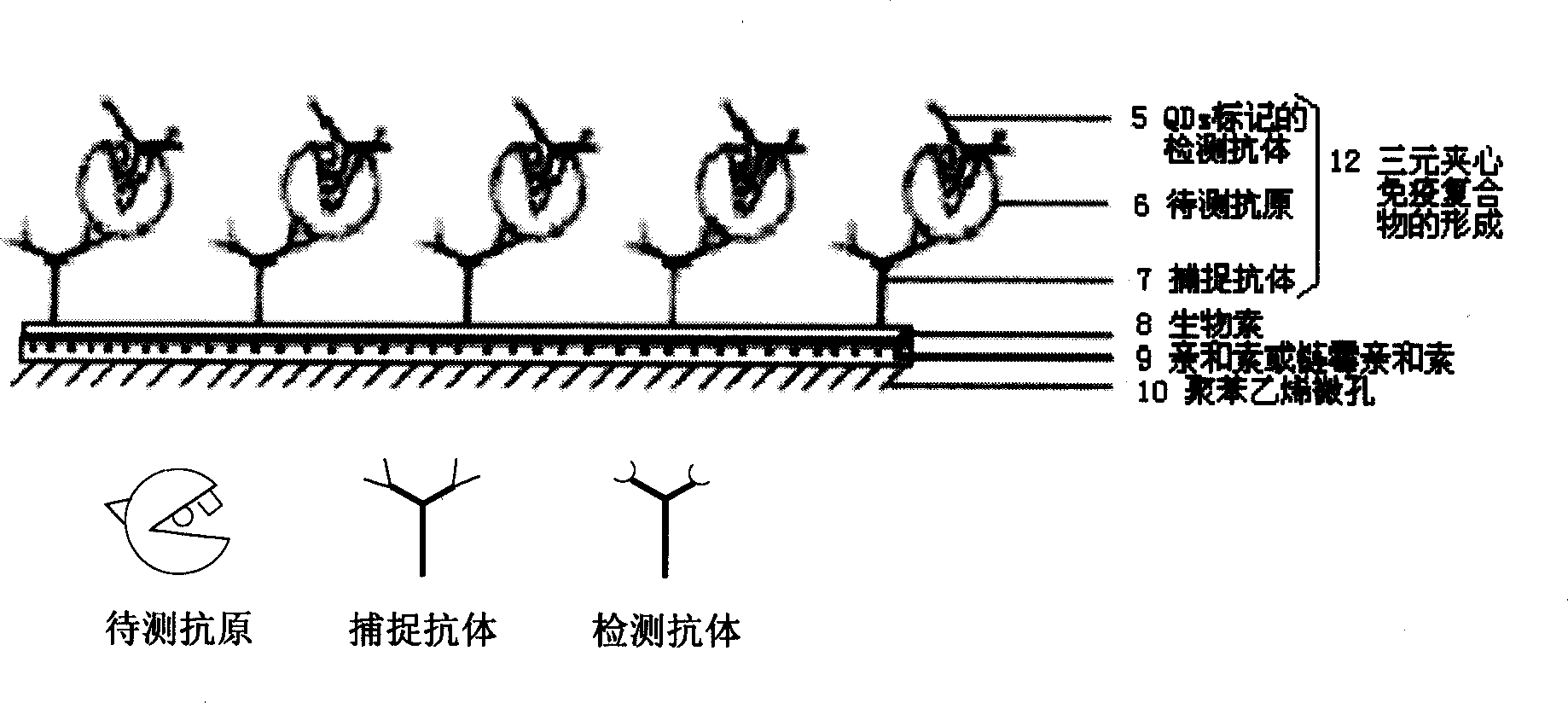
Quantum point marker sandwich immunodetection method and its diagnosis kit
InactiveCN1515909AOvercoming single color renderingNarrow Symmetrical Fluorescence PeaksBiological testingImmune complex depositionPolystyrene
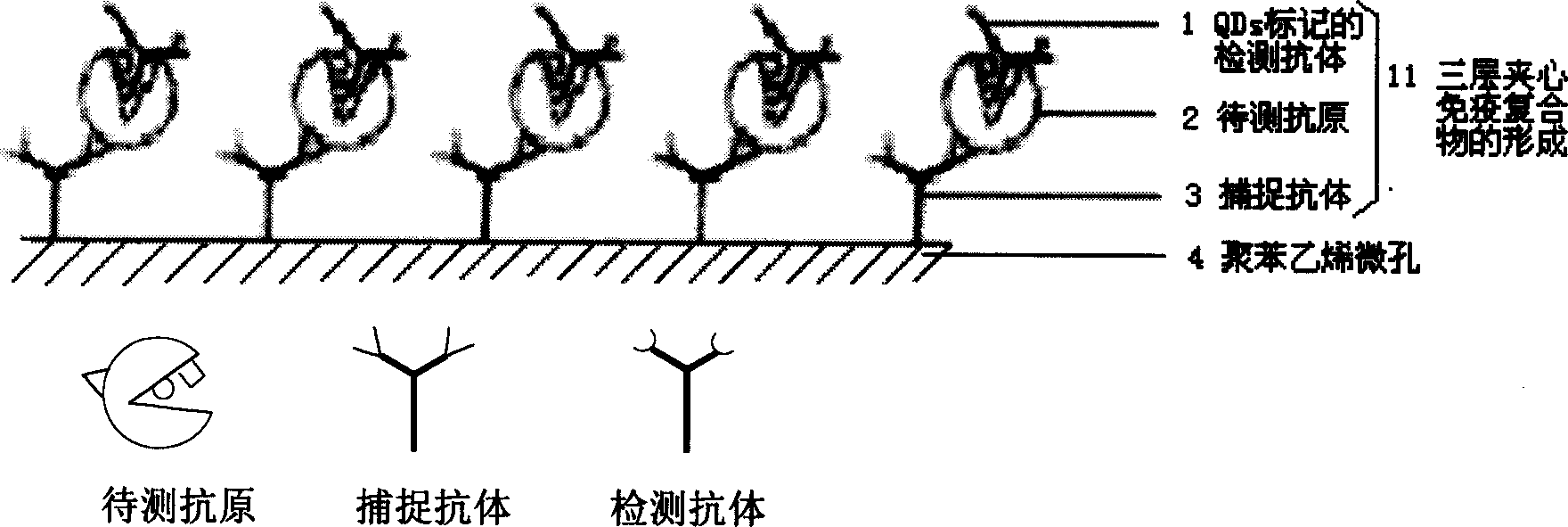
The present invention discloses a quantum point labeled sandwich immunodetection method and its diagnosis kit. It is a new type sandwich immunodetection method using QDs nano particle as label to make antigen antibody specificity sandwich reaction. It includes the following processes: firstly, directly or indirectly enveloping captured antibody in microwell of polystyrene plate, forming captured antibody-antigen-detection antibody three-layer sandwich luminescent immune complex and fluorescence intensity detection. According to that every QD has narrow and symmetrical fluorescence spectral peak it can select and use quantum point label needle sending different light to simultaneously detect several antigens to be tested in same sample.
Owner:魏景艳
Anti-mullerian hormone chemiluminescence immunoassay kit and preparation method and application thereof
InactiveCN106053791AHigh detection sensitivityImprove detection accuracyMaterial analysisMonoclonal antibodyAssay sensitivity
The invention discloses an anti-mullerian hormone chemiluminescence immunoassay kit and a preparation method and application thereof. The anti-mullerian hormone chemiluminescence immunoassay kit comprises a solid-phase carrier enveloped by an anti-mullerian hormone monoclonal antibody, and an anti-mullerian hormone monoclonal antibody marked by a chemiluminescence marker. The anti-mullerian hormone chemiluminescence immunoassay kit can complete the assay of anti-mullerian hormone by taking a full-automatic chemiluminescence immunoassay analyzer as an assay tool. Through experiments, the assay sensitivity of the anti-mullerian hormone chemiluminescence immunoassay kit can reach 0.01ng / mL, and is improved by at last ten times compared with the traditional anti-mullerian hormone assay method, and the assay precision of the anti-mullerian hormone chemiluminescence immunoassay kit is higher.
Owner:SHENZHEN YHLO BIOTECH +1
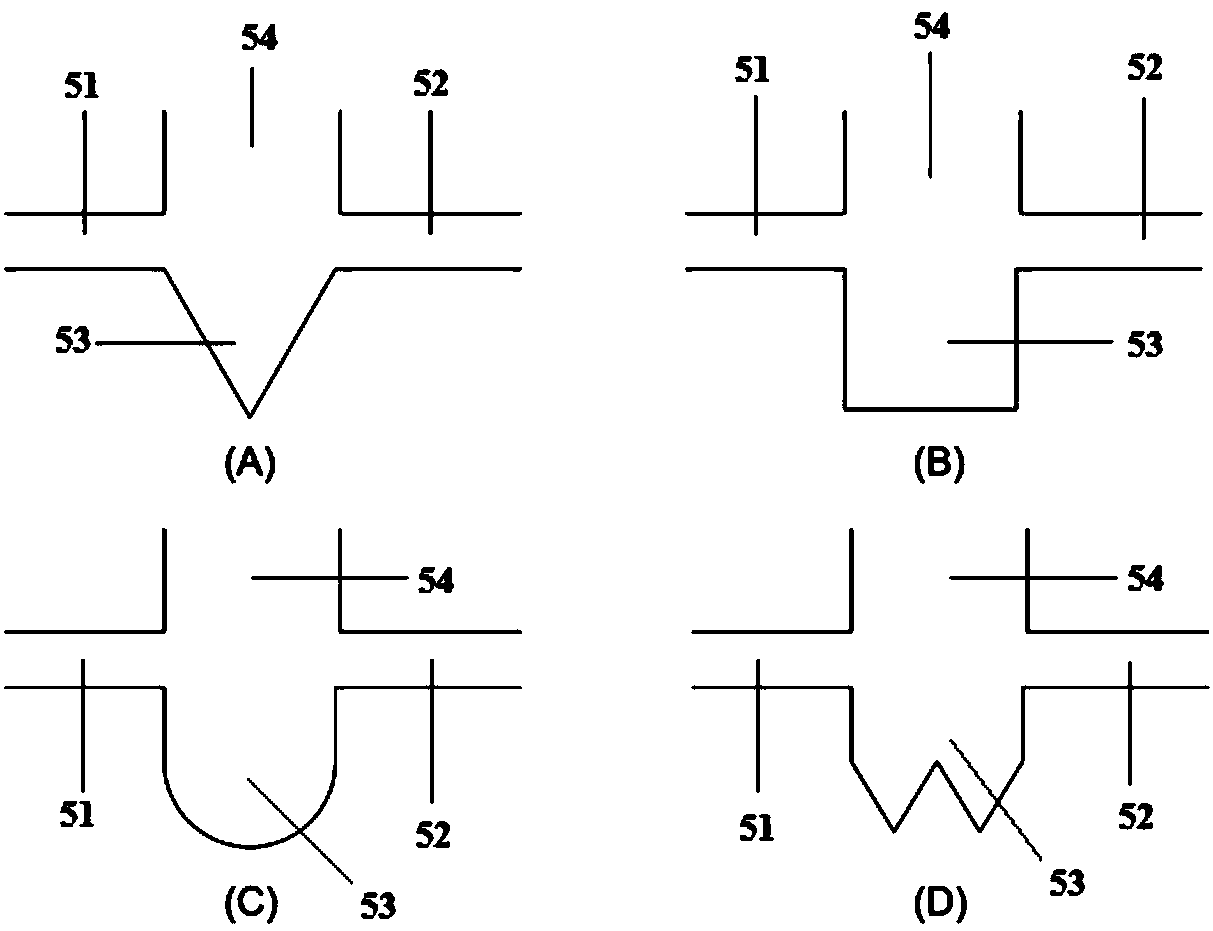
Chemiluminescent detection microfluidic chip and chemiluminescent detection microfluidic chip system and application thereof
PendingCN107930710ALow detection optical backgroundHigh detection sensitivityChemiluminescene/bioluminescenceLaboratory glasswaresChemiluminescent immunoassayImmuno detection
The invention relates to the technical field of microfluidic chip chemiluminescent immunoassay, in particular to a chemiluminescent detection microfluidic chip and a chemiluminescent detection microfluidic chip system and application thereof. The chemiluminescent detection microfluidic chip comprises a sample feeding unit (1), a liquid storage unit (2), a reaction unit (3) and a waste liquid unit(4), and further comprises a quantifying unit (5) for quantifying a sample to be detected, wherein the quantifying unit (5) comprises a liquid inlet (51) connected with the sample feeding unit, a liquid outlet (52) connected with the waste liquid unit, a quantifying structure (53) for quantifying, and a temporary storage structure (54) for temporarily storing excess liquid, which is connected withthe reaction unit. The chemiluminescent detection microfluidic chip system consists of three layers, namely an upper layer, a middle layer and a lower layer; the middle layer is the chip; the upper layer and the lower layer are used for closing the middle layer; the upper layer is provided with a sample feeding hole connected with the sample feeding unit and a yielding hole corresponding to the liquid storage unit. The chemiluminescent detection microfluidic chip is simple in detection process, can quantify the reaction sample and has high sensitivity and strong repeatability.
Owner:丁锐
Micro array-ELISA detecting kit for detecting six tumor markers
The present invention relates to a six-item tumor labeling material micro array (Array)-enzyme linked immunosorbent assay (ELISA) detection kit, in particular to a six-item tumor labeling material micro array (Array)-enzyme linked immunosorbent assay (ELISA) detection kit which is used for detecting the characteristic protein which has an indicating function to the liver cancer, lung cancer, colorectal cancer, stomach cancer, pancreatic cancer, breast cancer, cervical cancer, ovarian cancer, prostate cancer and other tumor diseases.
Owner:BEIJING BGI GBI BIOTECH +1
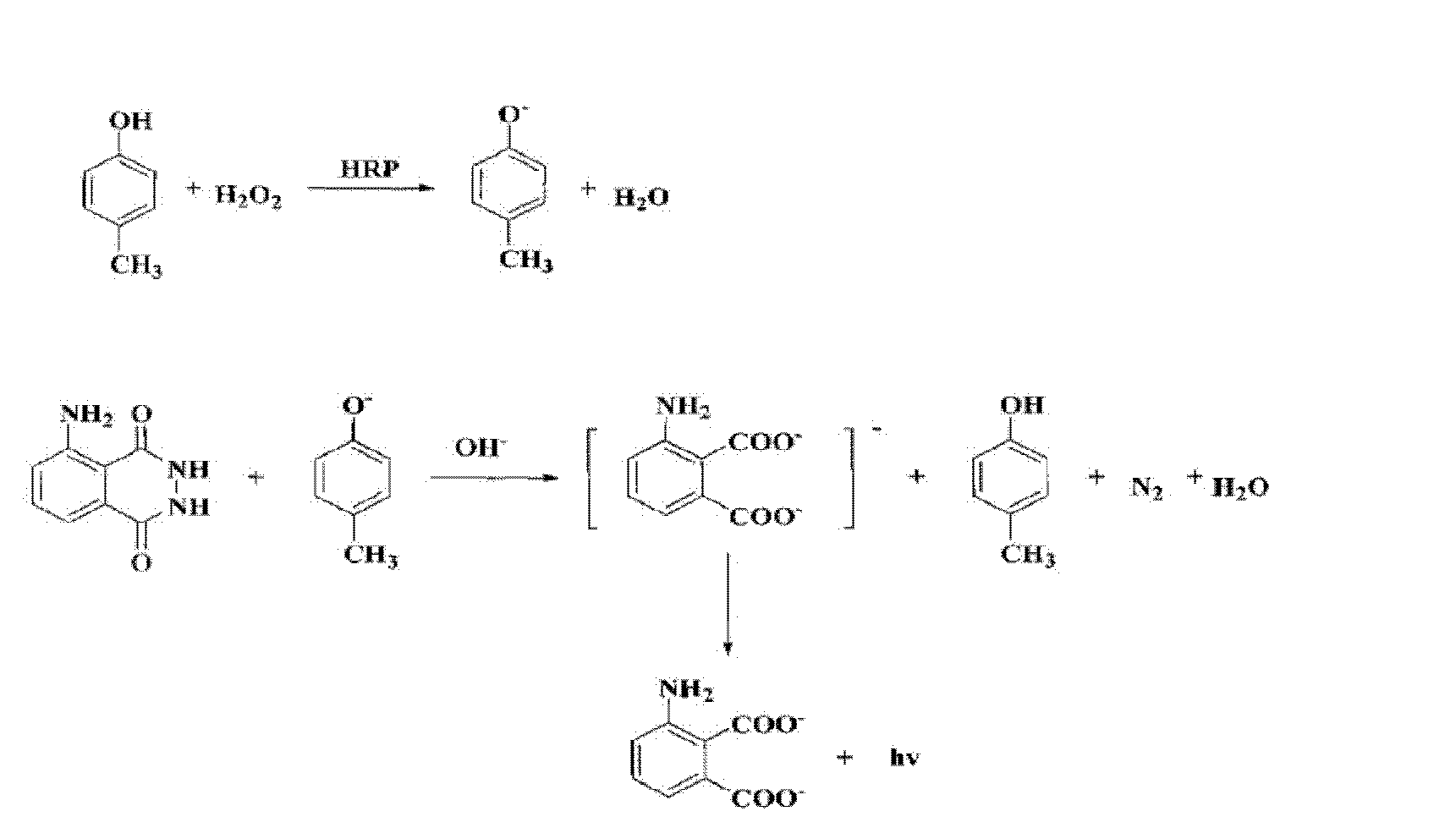
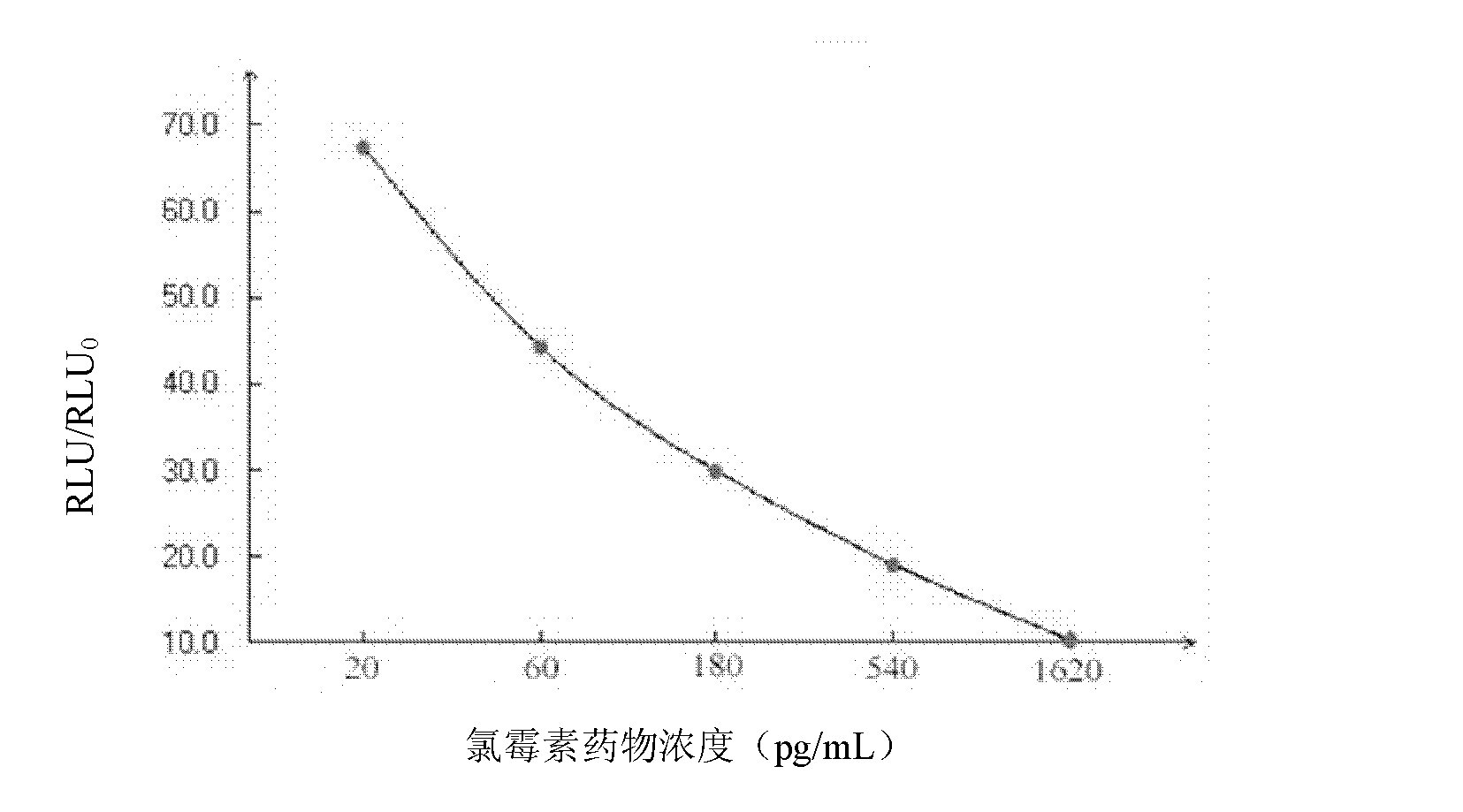
Chloramphenicol chemiluminescence enzyme-linked immunodetection kit
The present invention discloses a chloramphenicol chemiluminescence enzyme-linked immunodetection kit, which comprises a kit body, an enzyme label plate placed inside the kit body, and reagents placed inside the kit body, and is characterized in that every hole of the enzyme label plate is coated with coating antigen, the coating antigen is a chloramphenicol and carrier protein conjugate, and the reagents comprise horseradish peroxidase-labeled chloramphenicol monoclonal antibody, a series of chloramphenicol standard solutions, a concentrated phosphate buffer, a concentrated washing solution and a chemiluminescence solution. The chloramphenicol chemiluminescence enzyme-linked immunodetection kit has characteristics of high sensitivity, simple and rapid detection, and high accuracy, provides a substantially reduced operation time compared to the conventional colorimetric ELISA method, and can be used for detection of chloramphenicol residues in animal tissues (pork, chicken, pork liver and chicken liver), aquatic products (fish and shrimp) and milk.
Owner:BEIJING KWINBON BIOTECH
Synchronous quantum dot fluorescence immunological detection method and kit of multiple small molecular compounds
InactiveCN102411050AHigh detection throughputHigh sensitivityFluorescence/phosphorescenceImmune complex depositionMicrosphere
The invention provides an indirect competitive quantum dot fluorescence immunological detection method for synchronously detecting multiple small molecular compounds and detection kit, wherein the immunological detection method is a liquid phase immunological detection method which uses an encoded microsphere as a solid phase carrier and a quantum dot as a fluorescent marker and is used for competitive specificity reactions of a small molecular compound antigen. c Firstly, a captured antigen is covalently bound to the surface of the encoded microsphere, an indirect competitive fluorescent immune complex of microsphere-captured antigen-detected antibody-second antibody-quantum dot is formed in a filter film plate reaction hole through capturing the antigen, detecting the antibody and binding the second antibody specificity, and then the indirect competitive fluorescent immune complex flows across a suspension chip or a detection region of a flow analysis system one by one under the restraint of the sheath fluid, recognizes different encoded microspheres and detects the fluorescent intensity (or average fluorescence intensity) of the quantum dot to complete the detection. A plurality of small molecule compounds in the same sample can be synchronously detected, and one or more small molecule compounds in different samples can also be detected. The invention has the advantages of fast speed and high flux.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Preparation method of immune base and antigen or antibody immunoassay method
ActiveCN103116019AThe process is simpleLow costRaman scatteringImmune complex depositionRaman scattering
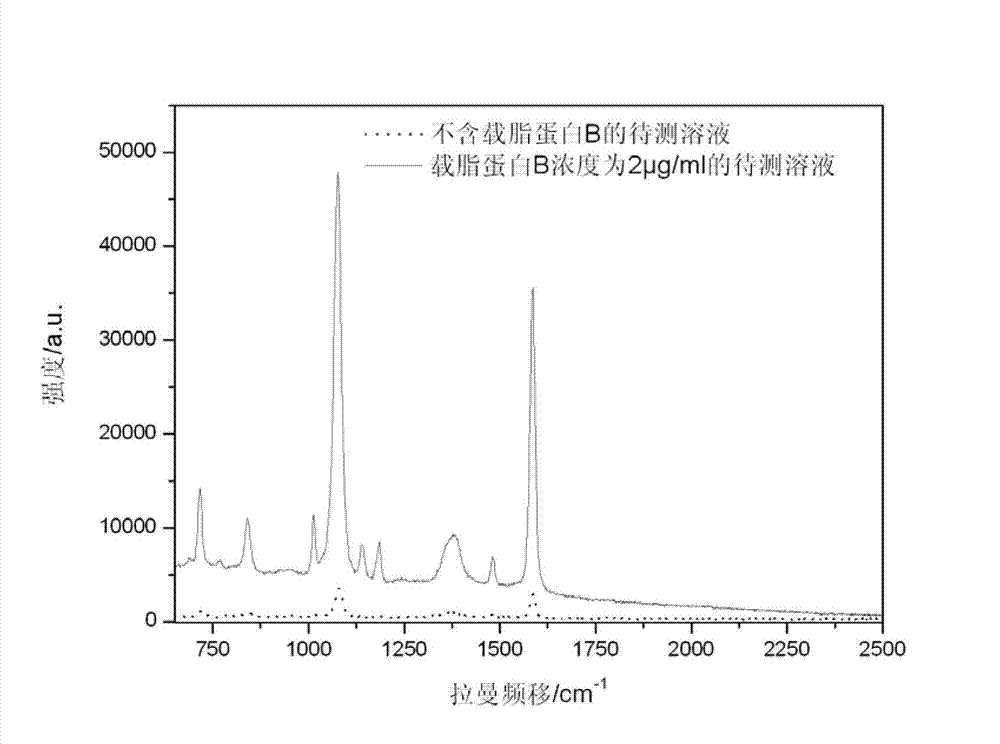
The invention discloses an immune base preparation method and an antigen or antibody immunoassay method. According to the immune base preparation method, a silicon slice is modified by using gold or silver nanoparticles, and antibodies / antigens are modified on the surfaces of the gold or silver nanoparticles, thereby being used for capturing antigens / antibodies to be assayed. According to the antigen or antibody immunoassay method, an immune base which is prepared by using the immune base preparation method and gold or silver nanoparticle immunoprobes is used, an immune base-antigen / antibody-immunoprobe three-layer sandwich structure is formed through an antigen-antibody immune complex reaction, and the assay on the antigens / antibodies to be assayed is realized in a manner that the characteristic dactylograms of Raman markers on the surfaces of the immunoprobes are assayed by using a surface-enhanced Raman scattering effect of the gold or silver nanoparticles. The method has the advantages that the sensitivity of the immunoassay can be greatly improved and the assay on high-flux multiple antigens / antibodies can be carried out.
Owner:NINGBO UNIV
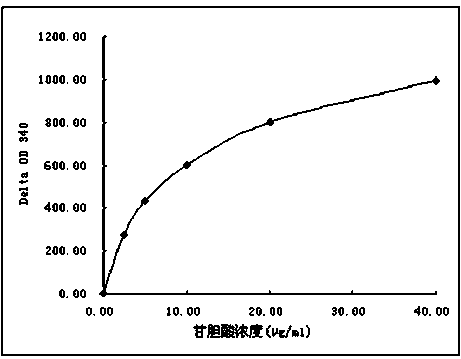
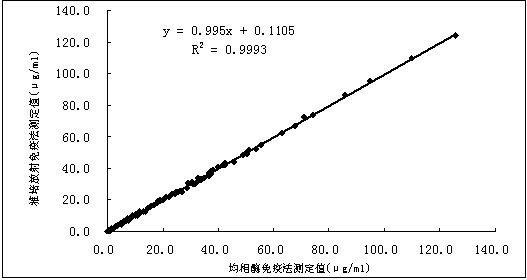
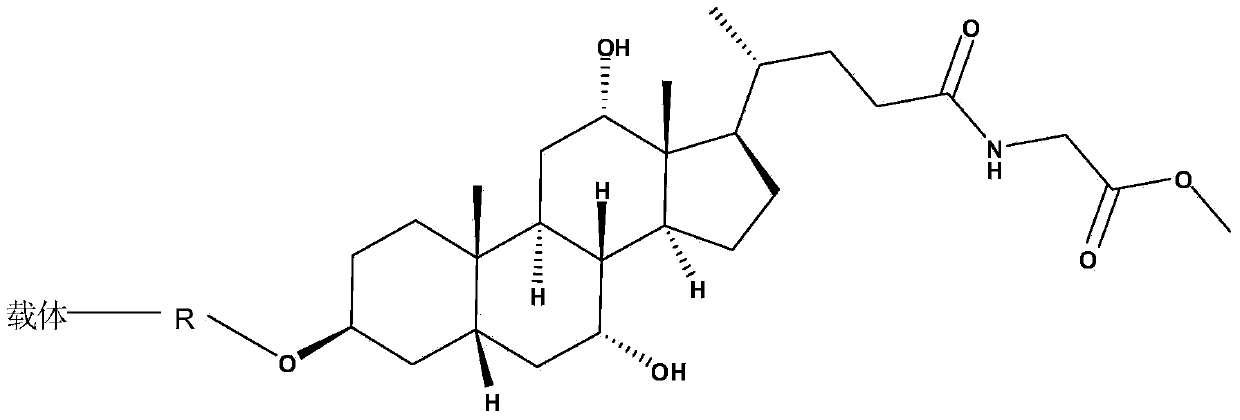
Glycocholic acid immunodetection reagent and preparing method and detecting method thereof
ActiveCN103760348AStrong immunogen specificityStrong specificityDisease diagnosisAntiendomysial antibodiesPharmaceutical drug
The invention relates to a glycocholic acid detecting reagent and a preparing method and a detecting method thereof, in particular to a glycocholic acid immunodetection reagent and a preparing method and a detecting method thereof. The glycocholic acid immunodetection reagent comprises a glycocholic acid specificity resisting antibody and an indicating reagent, wherein the indicating reagent is used for detecting the glycocholic acid specificity resisting antibody and a glycocholic acid composite; the glycocholic acid specificity resisting antibody is obtained from glycocholic acid immunogen immune animals. The glycocholic acid immunodetection reagent has the benefits that the glycocholic acid immunogen specificity is strong, the immunogenicity is high, and the prepared glycocholic acid specificity resisting antibody has strong specificity and high valence and does not have any cross reaction with 45 common drugs; a homogeneous enzyme immunodetection reagent containing the glycocholic acid specificity resisting antibody can conveniently, rapidly and accurately determine the content of glycocholic acid in a sample and can simultaneously test multiple samples on a fully-automatic biochemical analysis instrument; the high-throughout rapid measurement of the glycocholic acid is realized, the accuracy is high, the specificity is strong, and both the precision and the detection efficiency are greatly improved.
Owner:苏州博源医疗科技有限公司
Manufacture method of silver hybridization mesoporous ferroferric oxide antibiotic immunosensor and application thereof
ActiveCN102749442AHigh sensitivitySimple preparation processMaterial analysis by electric/magnetic meansBiological testingCross-linkBovine serum albumin
The invention discloses a manufacture method of a silver hybridization mesoporous ferroferric oxide antibiotic immunosensor and the application thereof. The manufacture method of the electrochemical immunosensor includes modifying thionine-graphene mixed solution on the surface of a glassy carbon electrode, conducting cross linking on an antibiotic antibody incubated by Ag-Fe3O4 mesoporous nanometer particles, closing non-specificity active sites by bovine serum albumin to manufacure the antibiotic electrochemical immunosensor. A detection method of antibiotic is that a reference electrode-saturated calomel electrode, an electrode-platinum filament electrode and a working electrode are correctly connected on an electrochemical working station, and immunodetection is conducted through the square wave voltammetry. The antibiotic electrochemical immunosensor has high sensitivity and selectivity, is simple in detection method and has the advantages of being quick, high in efficiency, goodin specificity, low in cost, convenient to operate and the like. It takes to 2-3 minutes to finish one detection process.
Owner:UNIV OF JINAN
Homogeneous immunoassay kit, detection method and application thereof
ActiveCN108051585AReduce difficultyAvoid cross reactionChemiluminescene/bioluminescenceBiological material analysisBiotin-streptavidin complexAntigen
The invention relates to a homogeneous immunoassay kit, detection method and application thereof in the technical field of biology. The kit comprises a reagent I, a reagent II, a reagent III and a reagent IV, wherein the reagent I contains a first counterpart, and the first counterpart is a known antigen or a known antibody specially combined with an antigen to be detected in a sample to be detected; the reagent II contains a receptor which can react with singlet oxygen to generate a detectable signal and a second counterpart combined with the receptor; the reagent III contains a third counterpart specially combined with the first counterpart; the reagent IV contains a donor which can generate the singlet oxygen in an excitation state; the surface of the third counterpart is coated with biotin, and the surface of the donor is coated with streptavidin. By utilizing combined detection of the kit and the homogeneous immunoassay method of the antigen and the antibody, the screening difficulty of raw materials is reduced, and the detection sensitivity is promoted at the same time.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
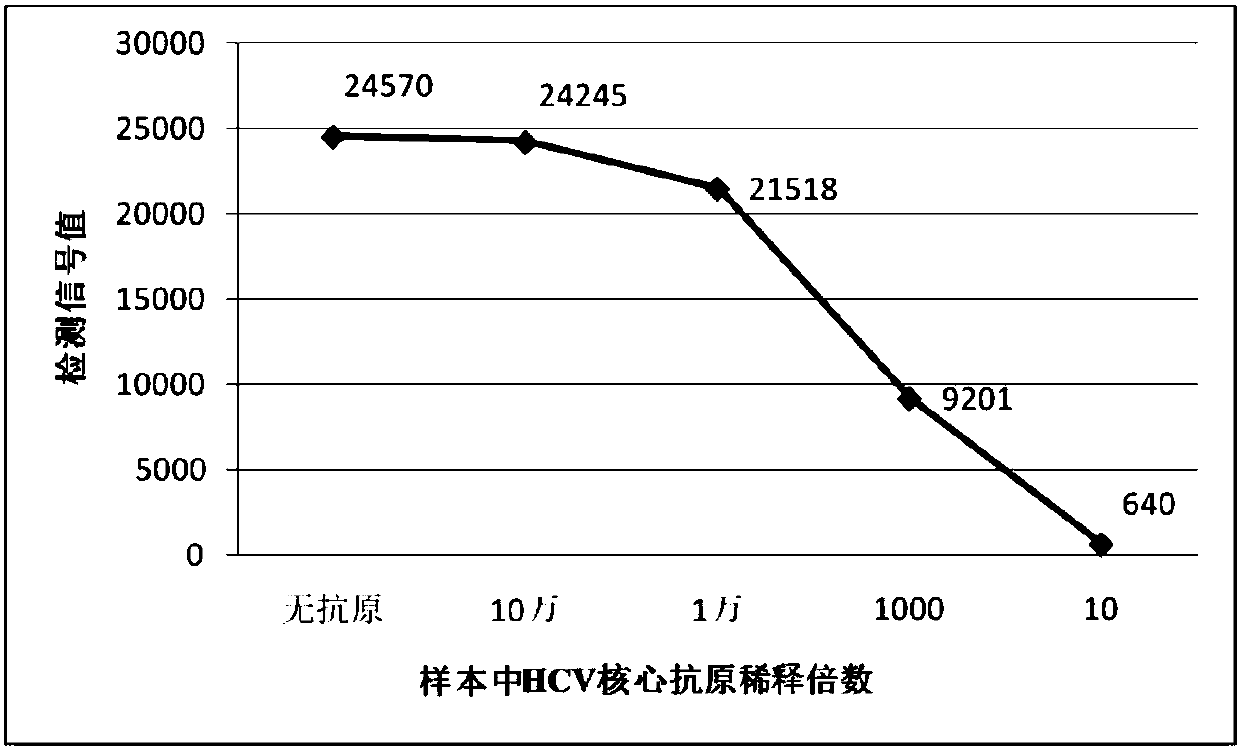
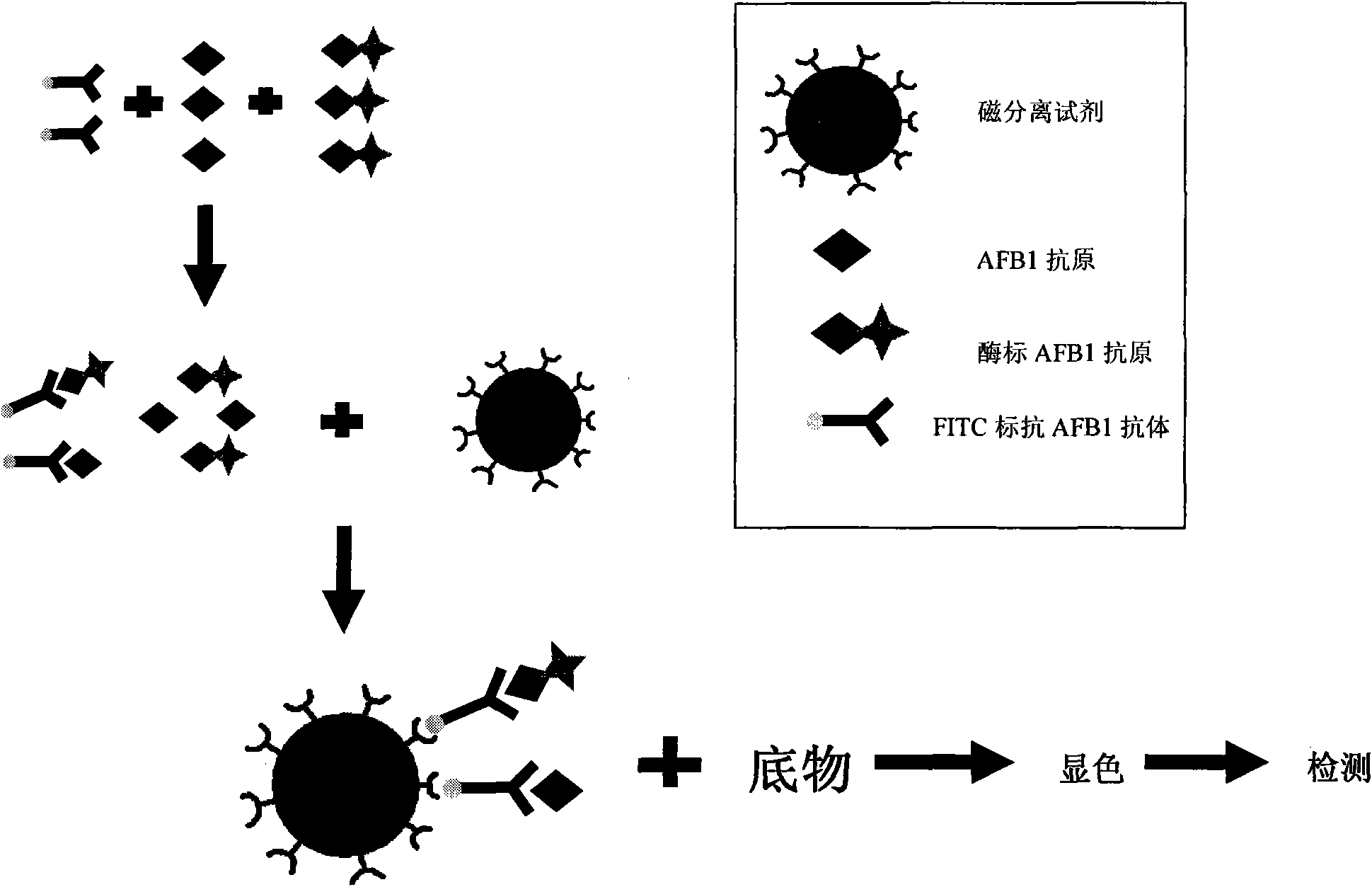
Aflatoxin B1 magnetic particle separation enzyme-linked immunoassay
InactiveCN101661036ASimple processing methodHigh detection sensitivityColor/spectral properties measurementsFluorescence/phosphorescenceSorbentFluorescein isothiocyanate
The invention provides an aflatoxin B1 (AFB1) magnetic separation enzyme-linked immunity quantitative detection method, belonging to the field of food safety immunoassay technique. The method adopts the immuno-detection principle of competition law; and AFB1 is connected with biological enzyme to prepare enzyme-labeled antigen reagent, anti-fluorescein isothiocyanate (FITC) antibody is absorbed onthe surfaces of magnetic particles to prepare magnetic separation reagent, and the FITC is connected with the AFB1 antibody to prepare anti-reagent. In a sample, the AFB1 competes with the enzyme-labeled AFB1 and is combined with a small amount of FITC-labeled anti-AFB1 antibody, so that antigen-antibody complex can be formed. After the magnetic separation reagent is added, the complex is caughtonto the surfaces of the magnetic particles by the anti-FITC antibody connected on the surfaces of the magnetic particles. After being washed, the product is finally added with substrate and detected.The method has the advantages that (1) the magnetic particles are used for replacing the traditional enzyme-labeled plate to be taken as a solid-phase carrier, so that immunoreaction is carried out under the approximate liquid phase condition; and the reaction is more complete and rapid, and has the characteristics of high specificity and good repeatability compared with the traditional enzyme-linked immuno sorbent assay (ELISA); furthermore, (2) by adopting one-step competition law principle, the time used for detection is short.
Owner:北京倍爱康生物技术有限公司
Immune antibody for detecting pesticide organic phosphorus residus and its application
InactiveCN1598586AFast immunoassaySimple immunoassay techniqueMaterial analysisAcetic acidFiltration
The invention discloses a rudimental immune antibody which is used to detect organic phosphorus pesticide residue and its application, the character of the invention is using diethyl phosphonic acid acetic acid as hapten, produce artificial immunogen through coupling with bovine serum albumin(BSA), and produce several clone antibody through immune Zelanian giant blanc or produce mono clone antibody through chmice immune, cell amalgamation, filtration, clone planting and other steps, the several and single antibody can be used to indirect emulative ELISA, direct ELISA, or immune test paper to detect the rudimental phosphorus pesticide. The excellences of the invention including: the selected hapten has several organic phosphorus pesticides general structure, the structure reforming is not necessary, it can immune animal after coupling with protein, it can also be used to detect organic phosphorus pesticide in food and water source, this show the rapid and convenient of the immune detecting tehchnology.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Magnetic separation enzymatic chemical luminous immune detection method of human thyroglobulin antibody
InactiveCN1719256AEasy to separateAdequate and prompt responseChemiluminescene/bioluminescenceImmune complex depositionMagnetic bead
The present invention provides a human thyroglobulin antibody magnetic separation enzymatic chemiluminescent immunoassay method, belonging to the field of immunodetection and analysis technology. Said method includes the following steps: making antigen human thyroglobulin be connected with Fe3O4 mincrosphere surface as solid phase reagent, the diameter of Fe3O4 microsphere is 0.1-5.0 microns, after the self-body antibody human thyroglobulin antibody being in the sample is trapped and two antibodies are labeled by enzyme reagent, the solid phase-antigen-antibody-enzyme-labeled two-antibody sandwiched immune complex can be formed.
Owner:北京倍爱康生物技术有限公司
Adiponectin chemiluminescence immunoassay kit and its preparation method and use
InactiveCN106199011AHigh detection sensitivityImprove detection accuracyChemiluminescene/bioluminescenceImmunoglobulins against hormonesSolid phasesChemistry
The invention discloses an adiponectin chemiluminescence immunoassay kit and its preparation method and use. The adiponectin chemiluminescence immunoassay kit comprises an adiponectin monoclonal antibody-coated solid phase carrier and a chemiluminescent label-labeled adiponectin monoclonal antibody. The adiponectin chemiluminescence immunoassay kit utilizes a full-automatic chemical luminescence immunoassay instrument as a detection tool to finish adiponectin detection. An experiment proves that the adiponectin chemiluminescence immunoassay kit has detection sensitivity of 0.01ng / mL. Compared with the traditional adiponectin detection method, the adiponectin chemiluminescence immunoassay kit improves sensitivity by at least 10 times and has a high detection precision.
Owner:SHENZHEN YHLO BIOTECH
Method for determining anti-nucleosome antibody IgG (intravenous gamma globulin) and reagent device
The invention provides a method for realizing immunization detection for an anti-nucleosome antibody IgG (intravenous gamma globulin) based on the enzyme-linked immunoassay principle and a reagent device therefor; and an analytic method, the reagent device and an assorted reagent are independently, individually and disposably used for detecting the anti-nucleosome antibody IgG based on enzyme-linked immunoassay. Various reagents needed by enzyme-linked immunoassay for the anti-nucleosome antibody IgG are contained in one analytic device; and by using the method, the related immunology detection can be conveniently carried out according to the using requirements of detection items so as to provide better basis for clinical application.
Owner:SHENZHEN YHLO BIOTECH
Preparation for cadmium antimonide quantum dot immune marker and detection method for electrochemical sandwich immune
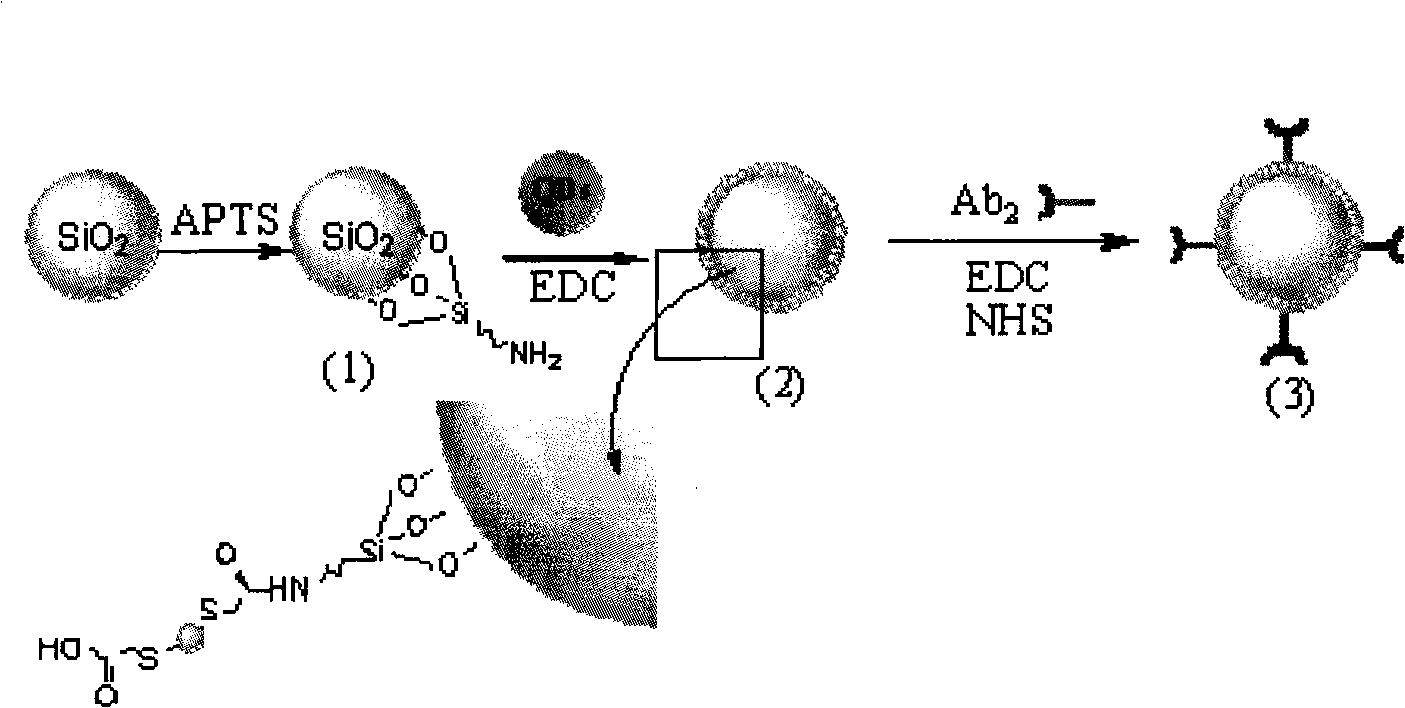
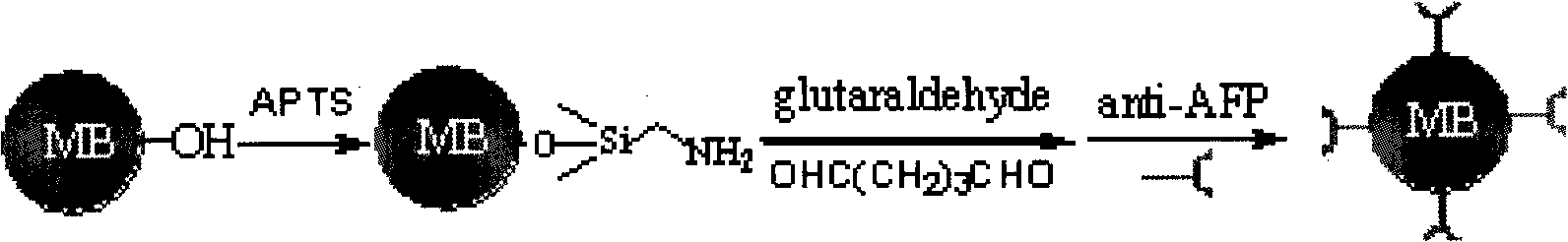
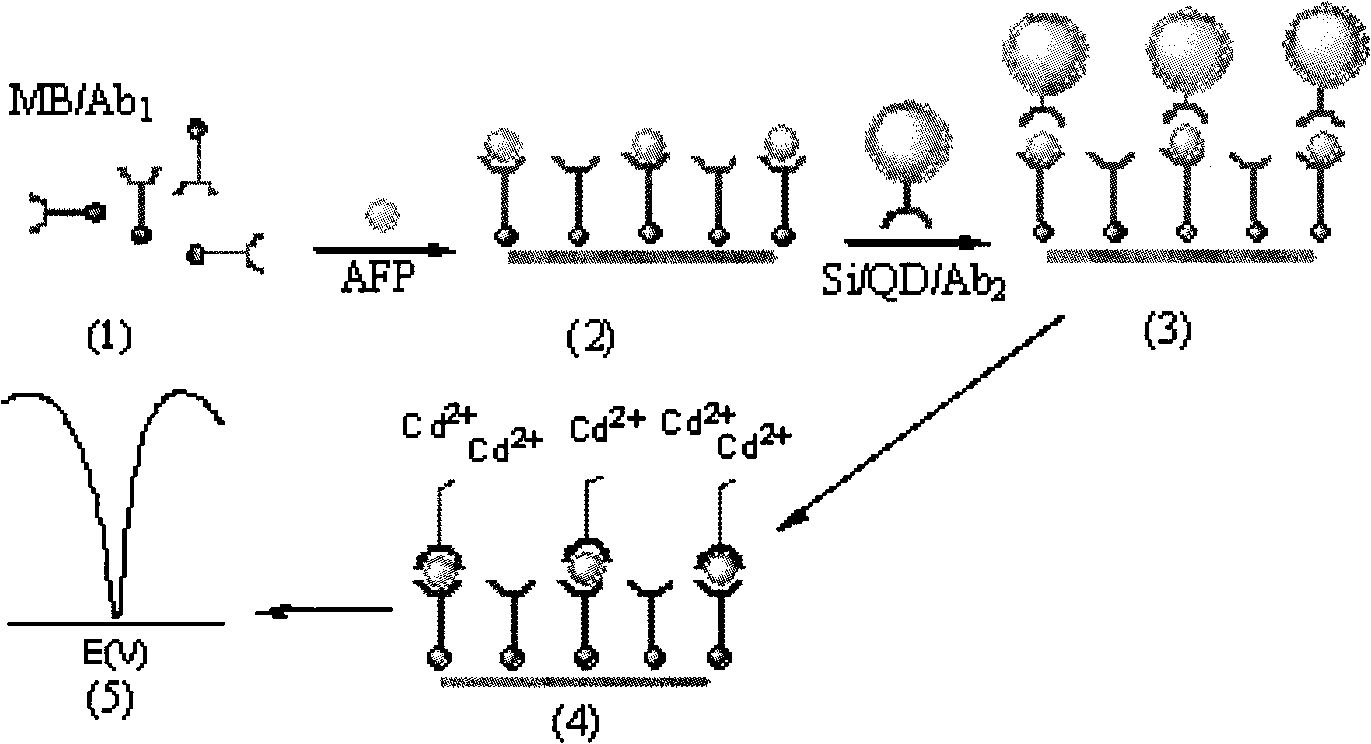
InactiveCN101526523AImprove stabilityHigh sensitivityMaterial electrochemical variablesMicrosphereSilicon dioxide
The invention relates to preparation for a cadmium antimonide quantum dot immune marker and a detection method for electrochemical sandwich immune, having the signal amplification function. Through the reaction of silicon dioxide particle and reacting ATPS, an active amido group is modified on the surface of the silicon dioxide particle under room temperature. The silicon dioxide particle is then reacted with carboxyl on the surface of a quantum dot to form a silicon dioxide microsphere with the surface modified with the quantum dot. The silicon dioxide microsphere after being modified is further modified with a second antibody on the surface of the quantum dot under the actions of EDC and NHS, and a cadmium antimonide quantum dot immune marker is obtained. The detection method for the electrochemical sandwich immune utilizes anti-AFP to modify Fe3O4 magnetic nano-microsphere, thereby Si / QD / Ab2 is modified on magnetic particles, and then electrochemical determination is carried out. Because the surface of the marker is rich in the quantum dots, the amount of the quantum dot loaded in a single sandwich immune process is greatly increased, dissolving-out voltammetry detection signals of an anode are amplified, and the sensitivity of low-concentration biomolecule detection is greatly enhanced.
Owner:SOUTHEAST UNIV
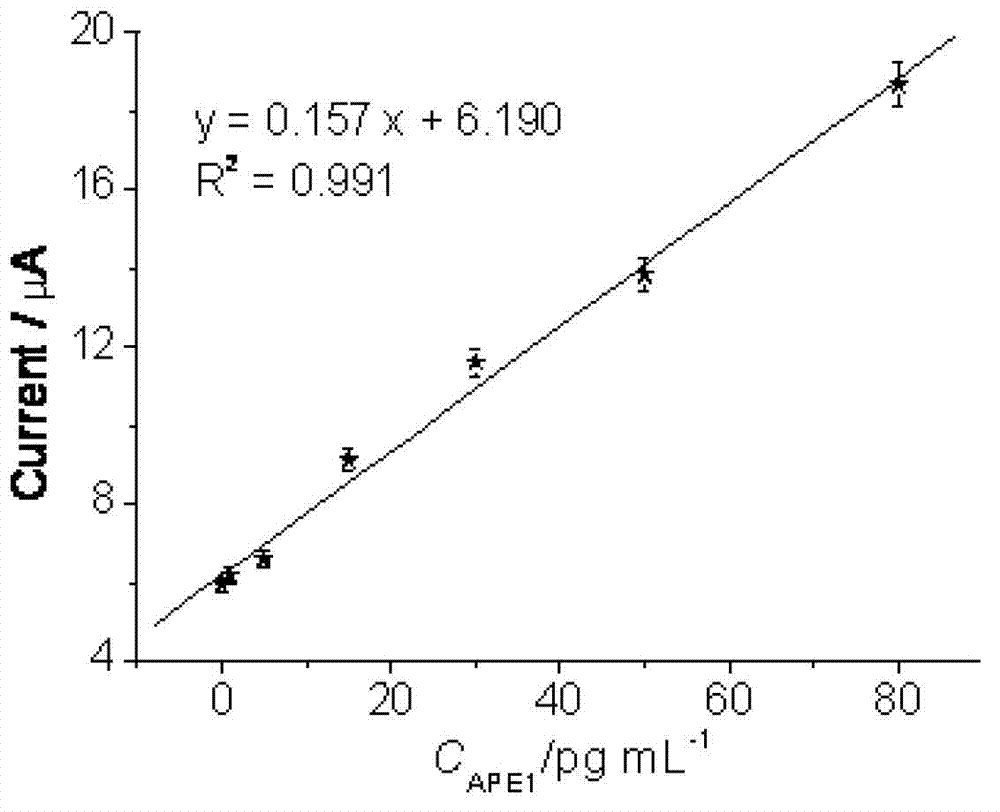
Ionic liquid-graphene nanocomposite, preparation method and electrochemical immunodetection method thereof
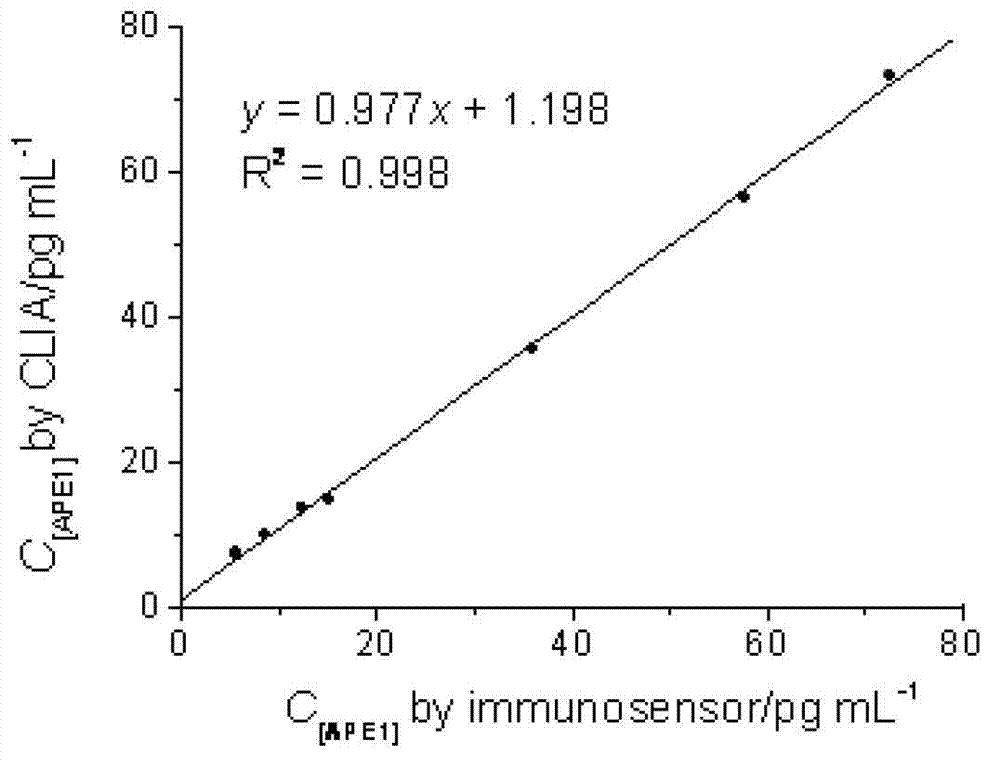
The invention relates to the field of electrochemical immunodetection, and especially relates to an ionic liquid-graphene nanocomposite, a preparation method and an electrochemical immunodetection method thereof. According to the immunodetection method, through using a double-antibody sandwich method, an apurinic / apyrimidinic endonuclease / redox factor antibody (anti-APE1) fixedly carried on the surface of an electrode carries out immunoreaction with an apurinic / apyrimidinic endonuclease / redox factor (APE1) in a sample solution, and is then combined with a room-temperature ionic liquid-graphene nanocomposite and an anti-APE1 co-coupling object marked by alkaline phosphatase (ALP) and ferrocene (Fc). Based on the electrochemical activities of the ALP-Fc-anti-APE and the room-temperature ionic liquid-graphene nanocomposite, a CV (cyclic vohammetry) catalytic current value is measured, and then the concentration of the APE1 in a detection sample is detected. The linear response range of the electrochemical immunodetection method provided by the invention is 0.1-80 pg / mL, and the lower detection limit is 0.04 pg / mL, therefore, the electrochemical immunodetection method is good in specificity and high in sensitivity.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
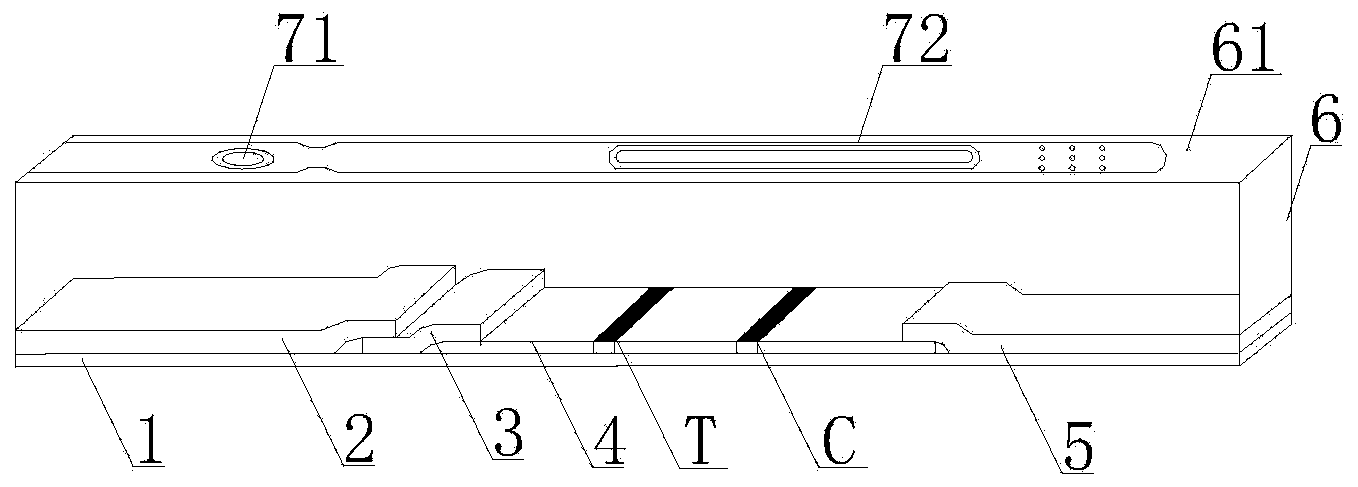
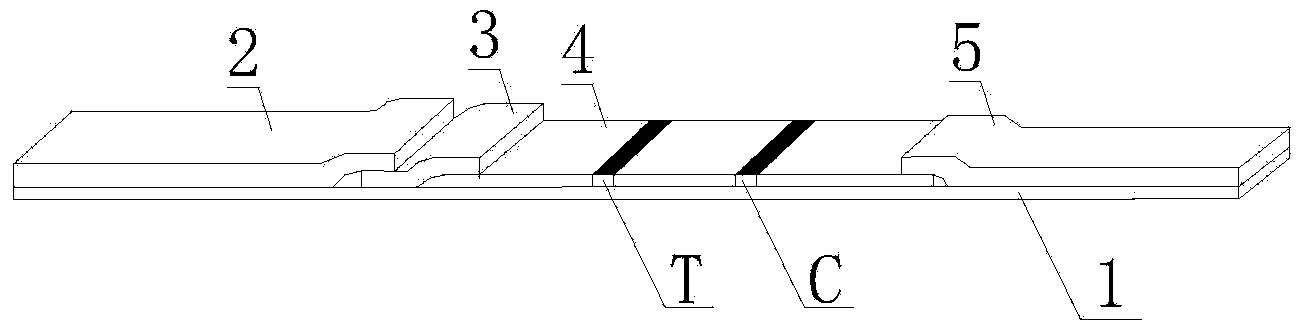
Streptococcus-A quantum dot immunochromatographic detection reagent card and preparation method thereof
ActiveCN103926403AImprove the detection rateOptimal treatment timeBiological material analysisFluorescence immunoassayFluorescence
The invention discloses a streptococcus-A quantum dot immunochromatographic detection reagent card and a preparation method of the streptococcus-A quantum dot immunochromatographic detection reagent card, and aims to provide the streptococcus-A quantum dot immunochromatographic detection reagent card which is simple, high in sensitivity and reliable in detection results. The detection reagent card comprises a box body (6), wherein a piece of detection test paper is arranged in the box body (6); the detection test paper comprises a bottom plate (1); a sample pad (2), an anti-streptococcus-A quantum dot anti-body markup pad (3), a coating film (4) and a water absorption pad (5) are connected with the bottom plate (1) in sequence; a streptococcus-A quantum antibody detection area line T and a goat anti-rabbit polyclonal anti-body quality control area line C are arranged on the coating film (4); the two lines are arranged in parallel; the streptococcus-A quantum dot immunochromatographic detection reagent card and the preparation method thereof belong to the field of fluorescence immunoassay.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
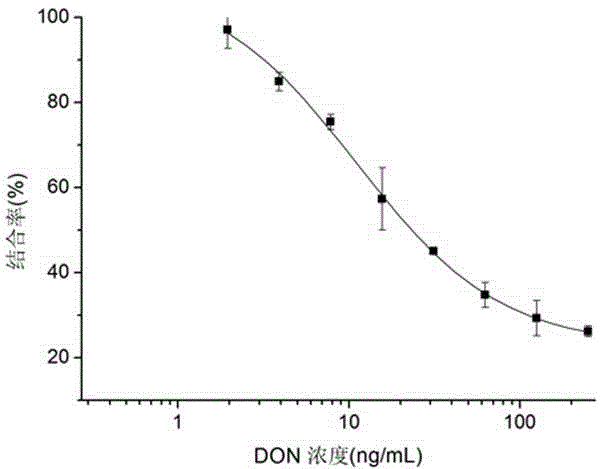
Nano antibody of anti-deoxynivalenol antibody
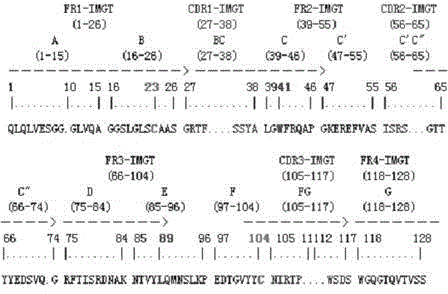
InactiveCN104592389AHave immune response propertiesGood effectImmunoglobulinsGenetic engineeringEpitopeEndurance capacity
The invention belongs to the technical field of biology and specifically relates to a preparation method and an application of a nano antibody capable of specially binding with an anti-deoxynivalenol antibody. The amino acid sequence of the nano antibody is SEQ ID No. 1. The invention also relates to a nucleotide for encoding amino acid. The nano antibody is capable of taking the place of the expensive high-toxicity DON standard substances, and can be applied to the immunological detection of the DON as a competitive antigen or a solid-phase envelope antigen; the nano antibody has immunoreaction characteristic similar to that of natural DON molecule, and is excellent in effect. Compared with the traditional antigen mimic epitopes based on polypeptides and the traditional anti-idiotype antibodies based on IgG, the nano antibody has the characteristics of more stable structure, acid-alkali resistance, high temperature resistance, high detection sensitivity and the like, and therefore, the immunodetection stability of the nano antibody is greatly improved, and meanwhile, the endurance capacity of the nano antibody to the environment is also improved.
Owner:NANCHANG UNIV
Malachite green vestigial ELISA detection kit and usage method thereof
The invention discloses an enzyme immunoassay of testing the bice green residues in animal derived food, which comprises an enzyme label plate covering bice green antigen, enzyme label bice green antibody working solution, bice green standard solution, substrate solution, substrate buffer solution, reaction termination solution, concentration washing liquid and sample dilute solution. The invention further discloses a method for using the immunoassay to test bice green residues, which comprises sample pretreatment, testing via the immunoassay, processing and analyzing result. The inventive immunoassay of bice green test uses direct competition enzyme-linked immunoassay adsorption analysis technique, with high sensitivity, high stability, simplified operation, reduced reaction time, reduced error caused by complex operation, reduced cost, wide application for testing samples and high practicality.
Owner:SOUTH CHINA AGRI UNIV
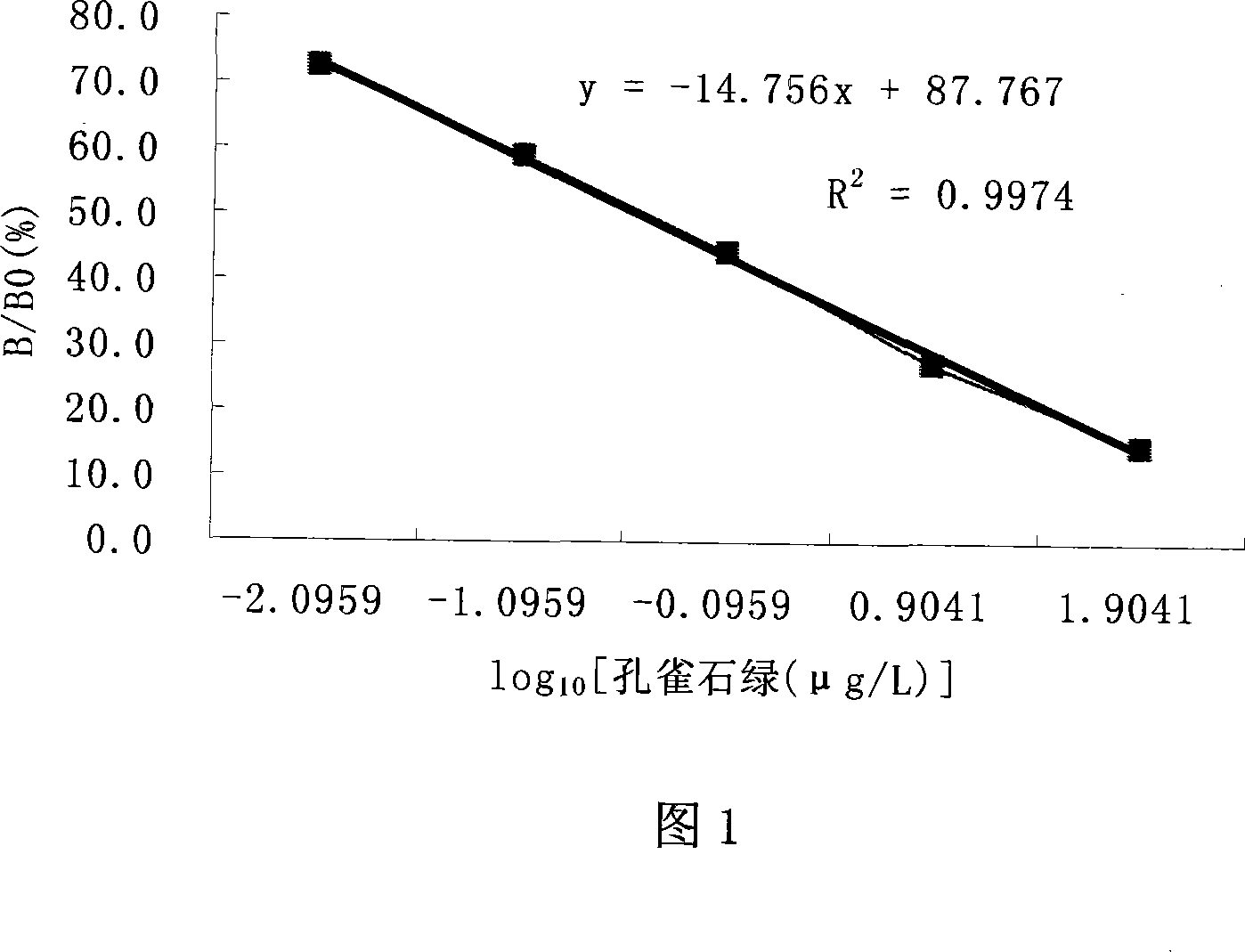
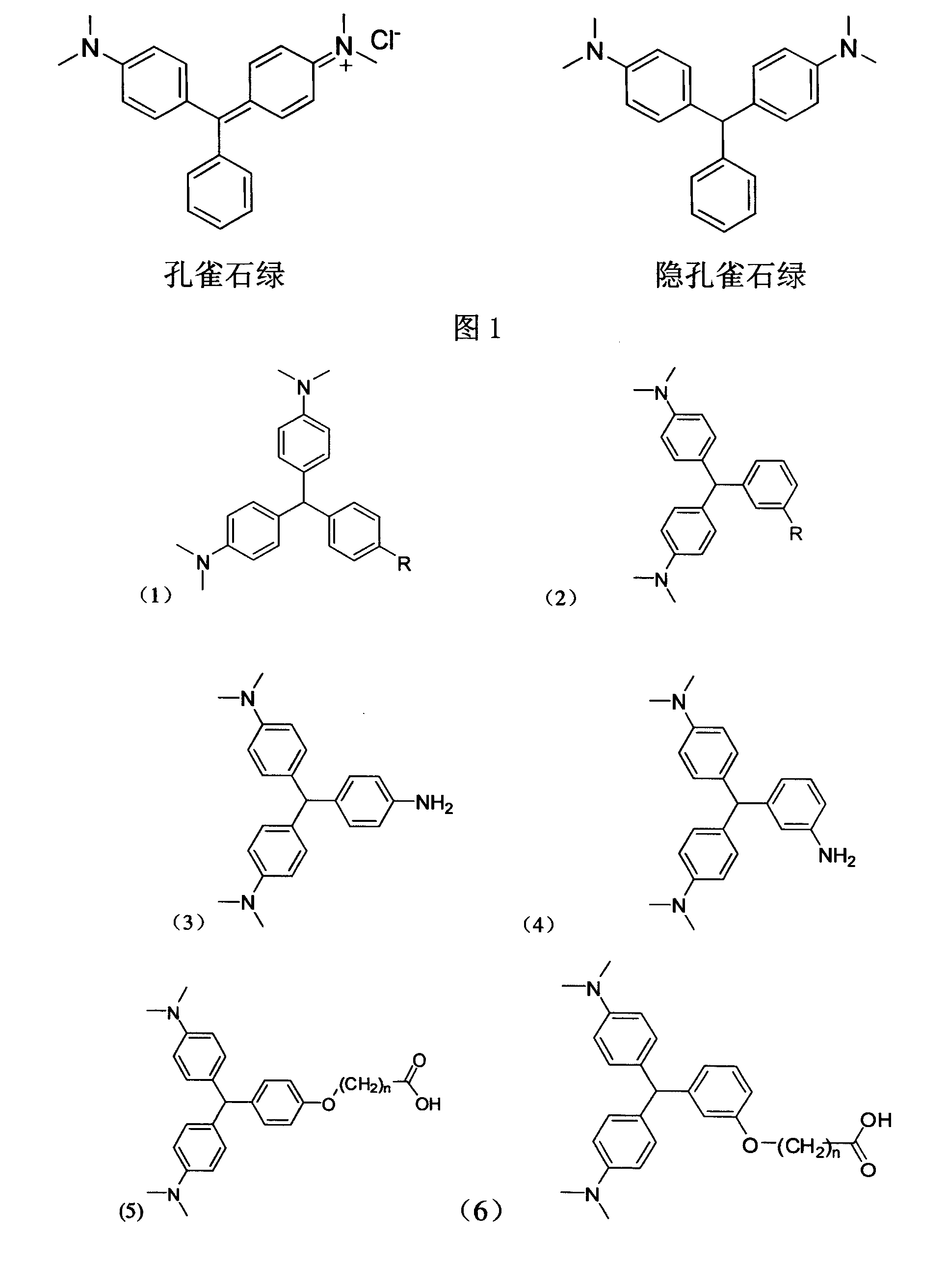
Leuco malachite green hapten, produced antibody and application of the antibody
InactiveCN101245032AHigh sensitivity and specificityImprove accuracyImmunoglobulins against animals/humansBiological testingChemistryCarbon chain
The invention discloses an implicit malachite green hapten which retains the characteristic structures of the implicit malachite green, active arms which have different electronic structural properties and different carbon chain length and is suitable for being coupled with macromolecule carriers derive from different chemical sites of the structure of the implicit malachite green, the active arm is R group, aromatic amine, terminal carboxyl group linear chain alkyl ether or amido link with the structure of linear chain terminal carboxyl group; an implicit malachite green artificial antibody is prepared and obtained from implicit malachite green hapten and when being used for detecting the residual quantity of the implicit malachite green and malachite green, the antibody has high specificity and sensitivity and high accuracy, the recovery rate can reach 80 to 110 percent, at the same time, the operation is simple and fast, no complicated pretreatment process is needed, a great amount of samples can be detected at one time, the cost is low, the operation has little requirements to operators so as to be convenient for on-site monitoring and be complementary to conventional methods, therefore, a sensitive, fast, efficient and economical implicit malachite green hapten immunity detecting method is successfully set up.
Owner:SOUTH CHINA AGRI UNIV
Multi-functional quick fluorescence immunoassay test method using functional substrate as media and using single color and multi-color quantum dots as mark
InactiveCN103149360AShort color development (fluorescence) timeStable storageFluorescence/phosphorescenceAntigenImmuno detection
The invention relates to a novel, quick, high-sensitivity, qualitative and quantitative clinical test method which uses a chemically modified functional polymorphic substrate as media and combines inorganic fluorescent semiconductor nano materials (quantum dots) and immunology means. The multi-functional quick fluorescence immunoassay test method using a functional substrate as media and using single color and multi-color quantum dots as a mark is suitable for a single or compound qualitative and quantitative test of various hormone, protein, nucleic acid and pathogens. Compared with traditional enzyme-linked immunoassay and colloidal gold test paper, the multi-functional quick fluorescence immunoassay test method has the advantages of being simple, quick, high in sensitivity, long in storage time and the like. The multi-functional quick fluorescence immunoassay test method can meet requirements of different testing conditions and samples, can conduct the single or compound qualitative and quantitative test on different antigens on the same substrate media at the same time, and has great potential on aspects of clinical applications.
Owner:HENAN UNIVERSITY
AFP nanometer antibody A83 based on AFP antigen
ActiveCN105037544AUnique molecular structureSmall molecular weightImmunoglobulins against animals/humansBiological testingAntigenDisease
The invention belongs to the technical field of biology, and relates to a camel source immunity nanometer antibody (single-domain heavy-chain antibody, VHH) specifically bound with AFP and application of the antibody. The amino acid sequence of the antibody is SEQ ID NO.:1. The invention further relates to nucleotides for encoding amino acids. The AFP nanometer antibody can be used as the antibody to be applied to immunological detection of AFP not for disease diagnosis and treatment. Compared with a Scfv antibody and a Fab antibody, the camel source immunity VHH has the higher specificity and affinity, more stable in structure, resistant to acid, base and high temperature, high in detection flexibility and the like, so that the immunity detection stability is greatly improved, and meanwhile the environment resistance is greatly improved.
Owner:NANCHANG DAJIA TECH
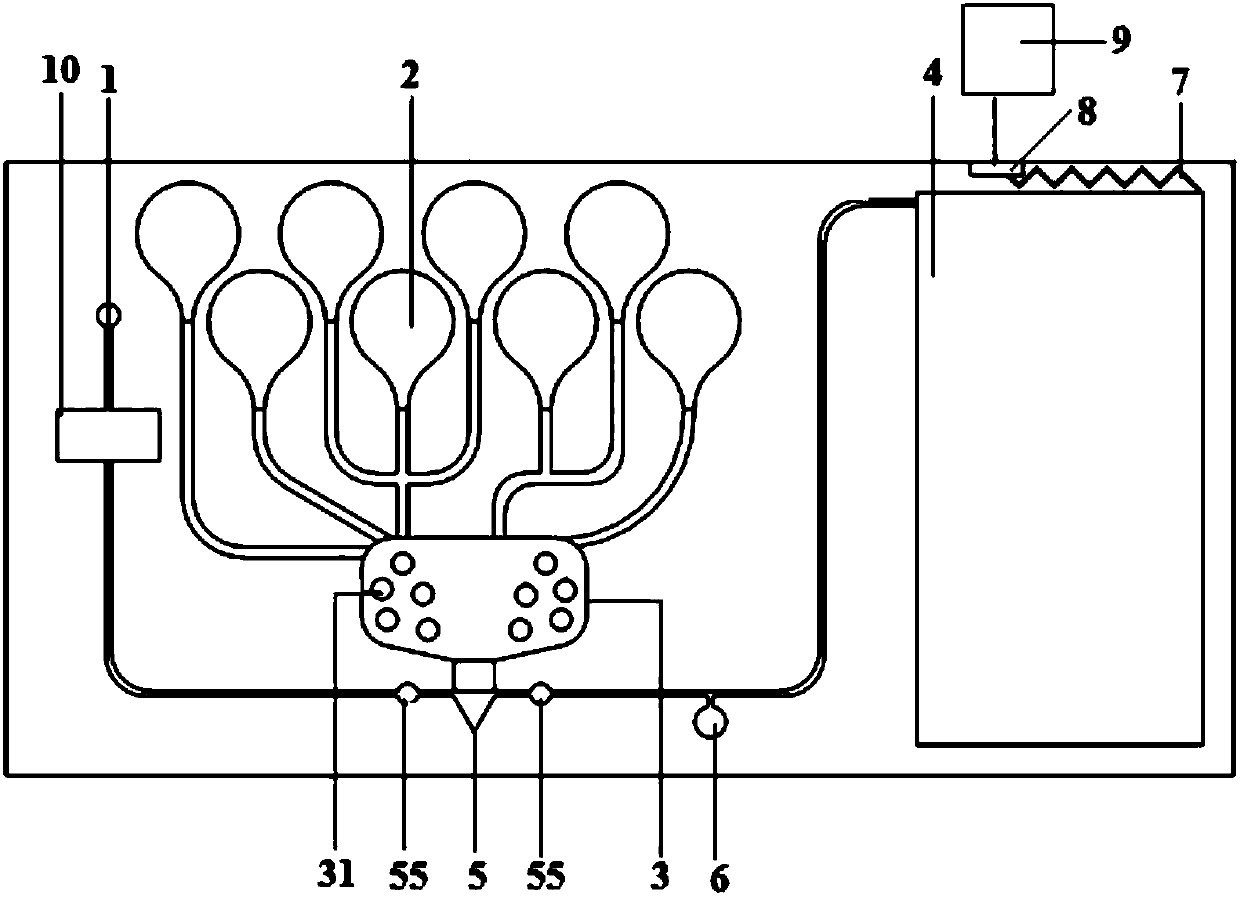
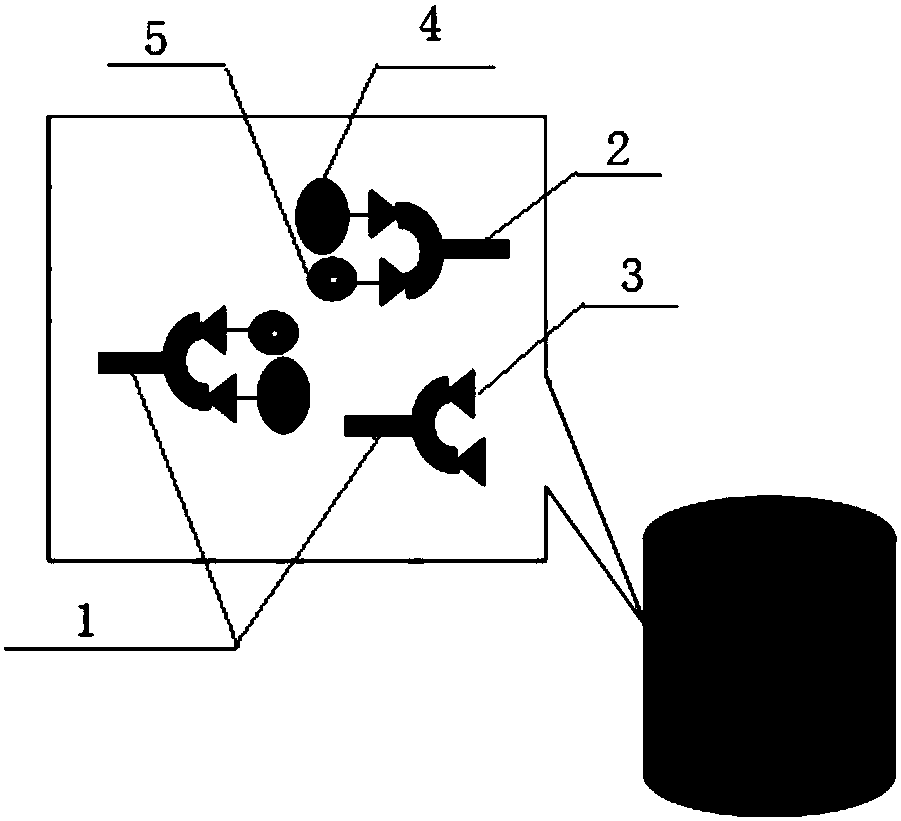
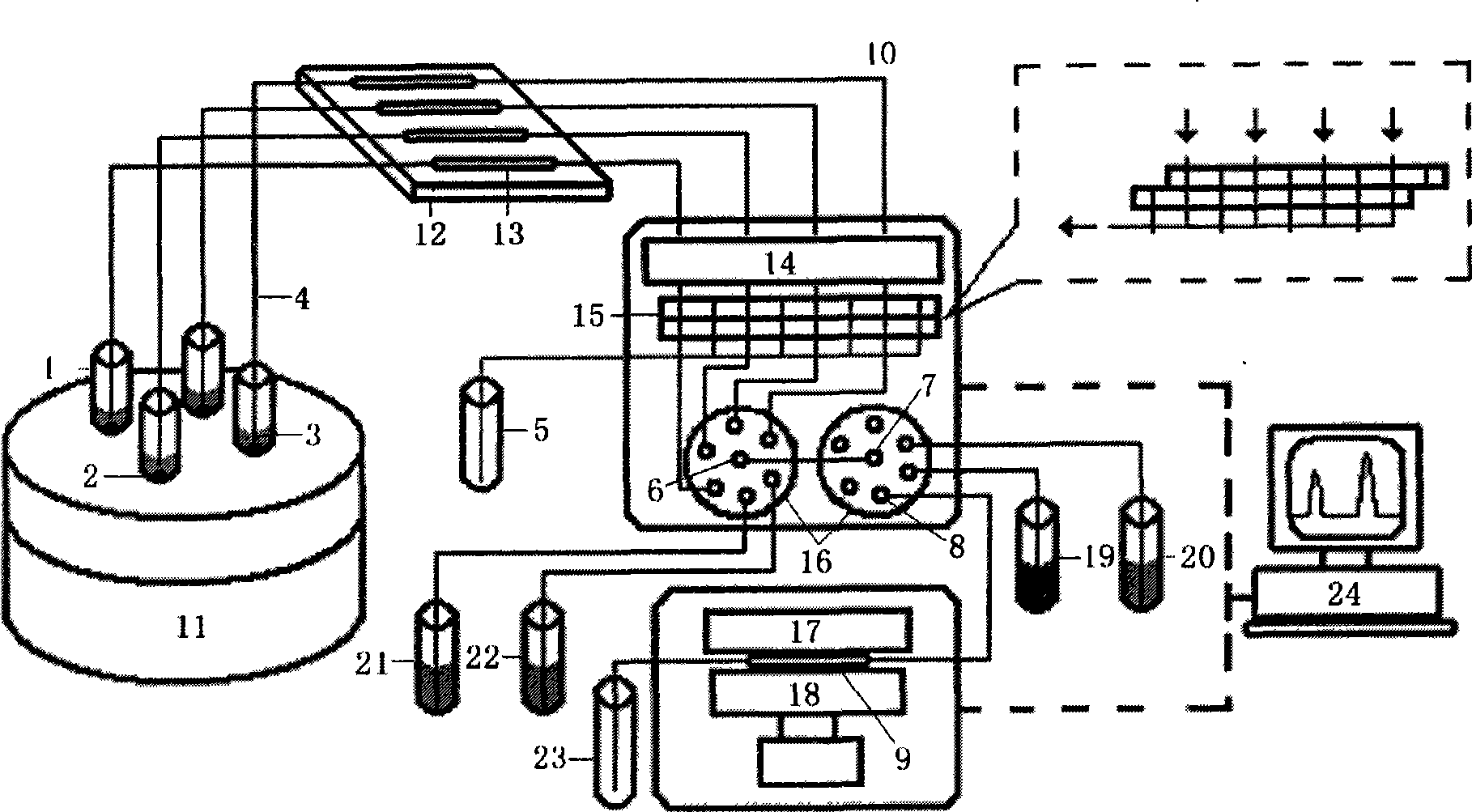
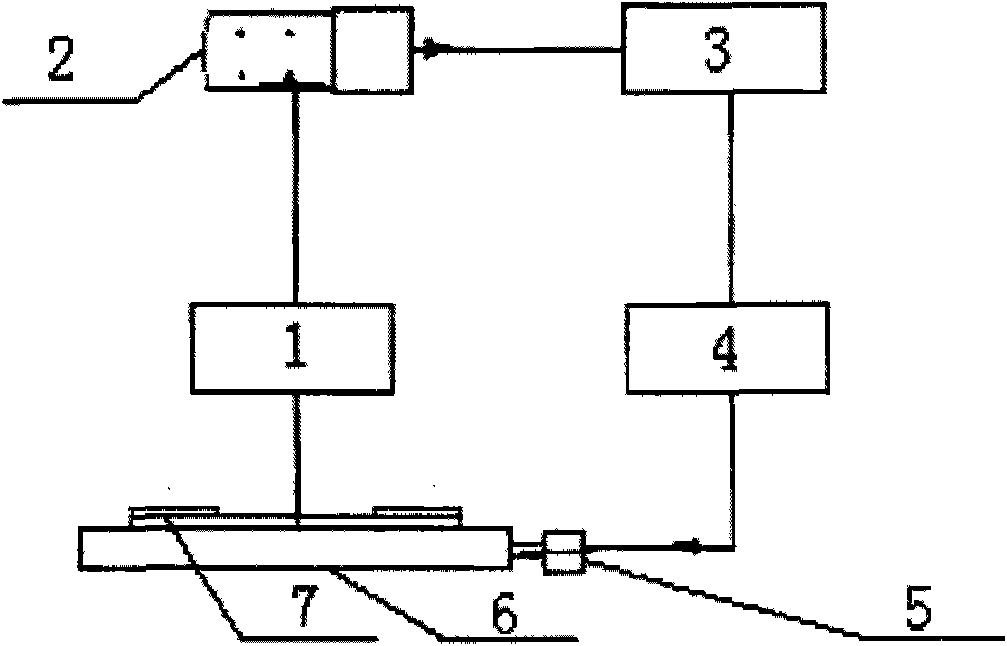
Automatic sampling distinguishing chemiluminescent multi-component immunological detection system and analysis method of same
InactiveCN101545902AEasy to operateReduce manual operationsChemiluminescene/bioluminescencePeristaltic pumpMagnetic bead
The invention relates to an automatic sampling distinguishing chemiluminescent multi-component immunological detection system and an analysis method of the same. An incubation system of the detection system consists of a test tube (1), a stir bar (2), magnetic beads (3) and a constant temperature magnetic stirring apparatus (11); a multi-channel sampling system consists of sampling channels (4), a magnet (12) and a glass tube (13); a solution conveying system consists of a peristaltic pump (14), a connecting pipe (10), a multifunctional syringe valve (15) and a multiposition valve (16); a signal acquisition system consists of a detection channel (9), a plane mirror (17) and a multiplier phototube (18); and the method is to fix various capture antibodies on the functionalized surfaces of the magnetic beads to perform specific reactions with an object to be detected and a tracing antibody so as to form a sandwiched complex, introduce chemiluminescent substrate liquid into the sandwiched complex after collecting and washing off excessive enzyme labeled antibodies by using the magnet, and inject the chemiluminescent substrate liquid into detection channels sequentially to perform detection. The method has the characteristics of simplicity, quickness, good reproducibility, high flexibility, low cost, and the like, and can be applied to the fields of clinical diagnosis, environmental monitoring, food security, and the like.
Owner:JIANGSU CANCER HOSPITAL +1
Method for qualitatively and quantitatively detecting target substance to be detected in blood serum by utilizing light initiated chemiluminescence immune assay
InactiveCN102944672AEliminate the influence of Hooks effectIncreased Quantitative Detection RangeChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexMicrosphere
The invention discloses a method for qualitatively and quantitatively detecting a target substance to be detected in blood serum by utilizing light initiated chemiluminescence immune assay, and the method comprises the following steps of placing luminous microspheres coating a substance antibody, biotin-coated target substance antibody and optical sensitization microspheres wrapped by streptavidin (SA) into a specimen to be detected to perform a primary immunity reaction and detection, and the method also comprises the step of placing anti-He agent into the reaction system to facilitate the target substance immune superimposition reaction, and carrying out the secondary optical excitation chemicalluminescence immune detection. A purpose for comprehensively correcting a hooks effect can be realized by comprehensively analyzing detection results of twice light initiated chemiluminescence assay (LiCA), and the analysis process comprises the following steps of classifying the specimen to be detected into five concentration intervals such as cathode, low anode, middle anode, high anode and ultrahigh anode according to the signal characteristics of the primary LiCA detection and the secondary LiCA detection; and quantitatively analyzing the specimen in the low anode concentration interval and the ultrahigh anode concentration interval according to the primary LiCA detection. The method has the characteristics of accuracy in result, simplicity and convenience in operation, wide application range and the like.
Owner:李方和
Monoclonal Antibodies to Progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
Up-converting phosphor bio-detector and method for detecting sampling test strips
ActiveCN101539572AReduce volumeLow costBiological testingFluorescence/phosphorescenceConvertersComputer printing
The invention provides an up-converting phosphor (UCP) bio-detector and a method for detecting sampling test strips. The UCP bio-detector consists of an optical system, a photo-detector, a control system, a driving module, a stepping motor and a scanning platform for placing the test strips, wherein, the control system comprises a micro-processor, a photo-detector interface, a signal acquisition circuit, a driving module interface, an LCD (liquid crystal display) module, a matrix keyboard, an RFID (radio frequency identification) module, a D / A (digital to analog) converter, a memorizer, an SD (secure digital) memory card and a micro-printer; and each test strip consists of test paper mounted inside the casing of the test strip and an RFID card, the test paper comprises a sample pad, a detection band and a quality control band, and the RFID card contains the information including test paper ID, substances to be detected, decision values, a parameter A and a parameter B. The control system of the invention has the advantages of simple design and convenient debugging, and the biological immunological detection can be carried out rapidly and accurately.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
Dry-type immunoassay test strip and preparation method and application thereof
InactiveCN103226143AShorten detection timeImprove featuresMaterial analysisProtein solutionCellulose
The present invention relates to a dry-type immunoassay test strip and a preparation method and application thereof. On a plastic substrate of the test strip is sequentially stuck with a sample pad, a nitrocellulose membrane anda water absorption pad from left to right, wherein the right end of nitrocellulose membrane is provided with a control line and a detection line that are parallel to each other; the left end of nitrocellulose membrane is provided with a tracer particle labeled antibody / antigen-coated line and a closed protein line I, wherein the closed protein line I is formed by coating closed protein solution I with a nitrocellulose membrane, and the antibody / antigen-coated line is formed by spraying the tracer particle labeled antibody / antigen on a closed protein line II, and the closed protein line II is formed by coating closed protein solution II with a nitrocellulose membrane. . The test strip can be applied in the immunoassay based on the double antibody sandwich method or the competitive method, and has advantages of simple operation, rapid chromatography, high specificity, accurate test results and strong stability.
Owner:GETEIN BIOTECH
Disposable multi-channel electrochemical immunosensor with high sensitivity
InactiveCN102053161AEasy to operateHigh detection throughputBiological testingElectronic transmissionCarbon nanotube
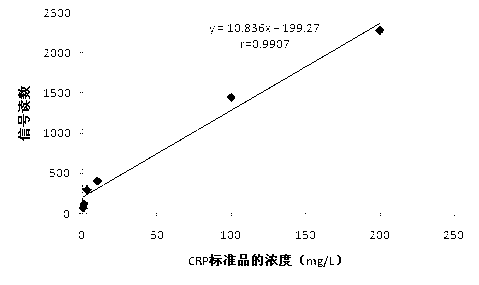
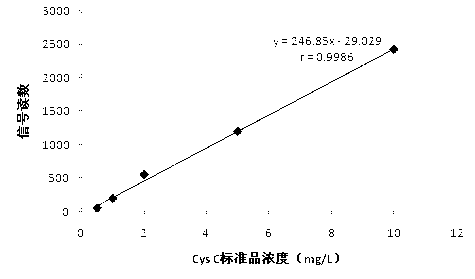
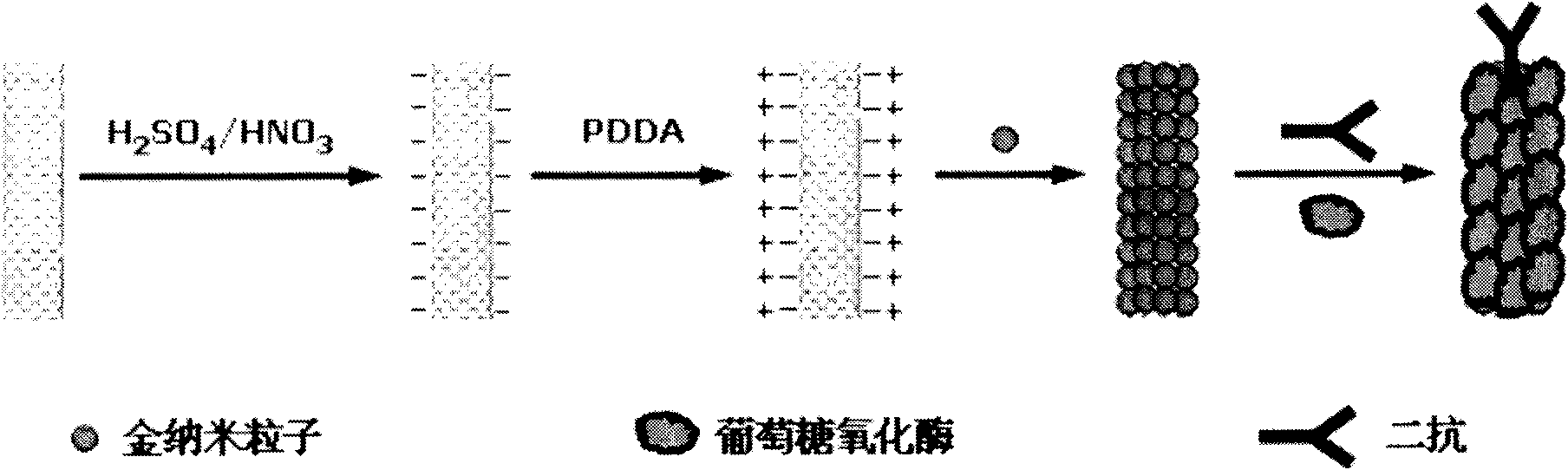
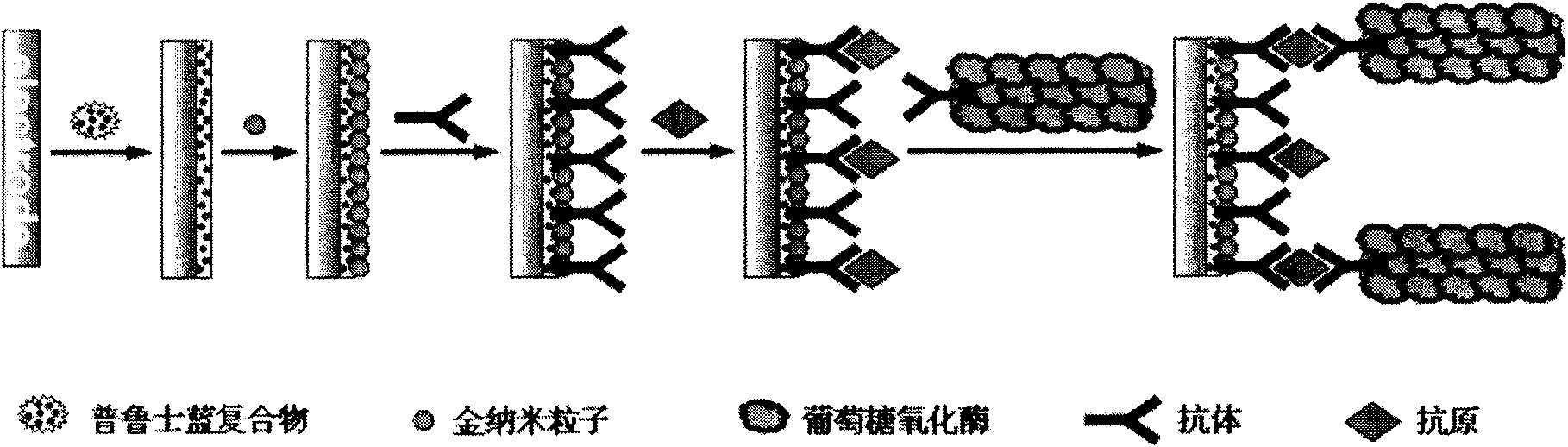
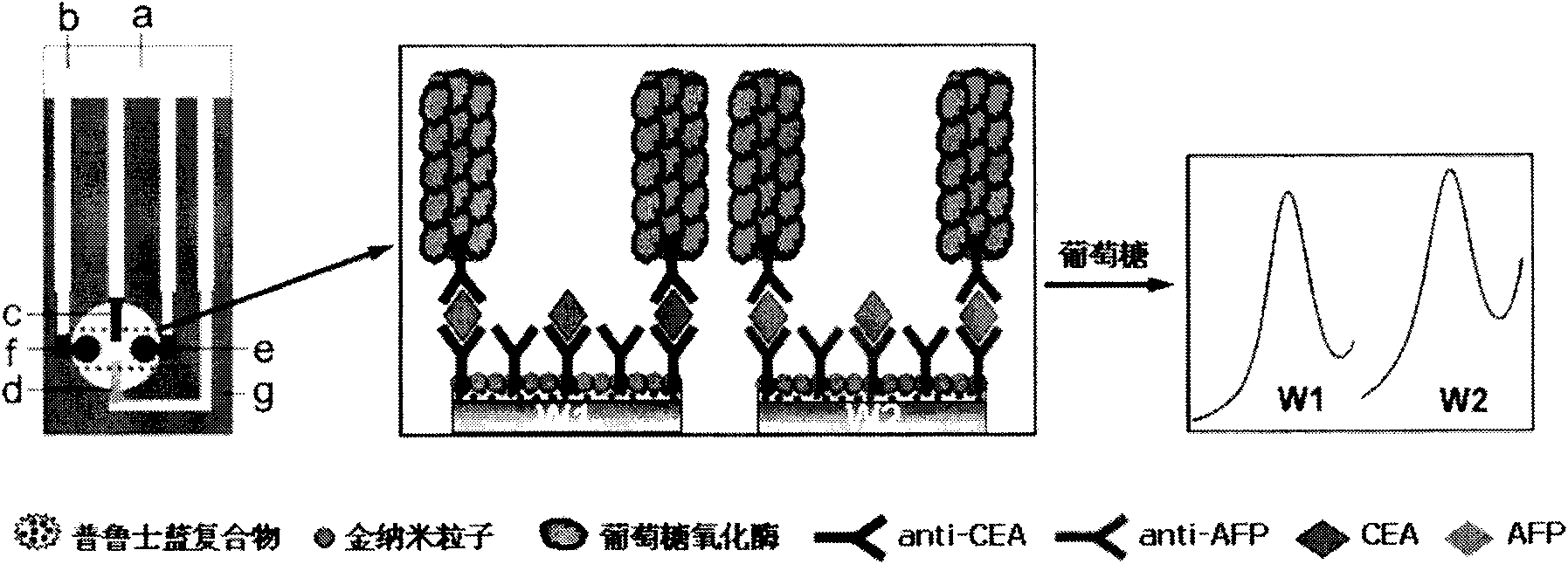
The invention relates to a disposable multi-channel electrochemical immunosensor with high sensitivity. A prussian blue composite, nanogold and a capture antibody are assembled layer by layer on a disposable printing electrode array to obtain a multi-channel immunosensor. Enzyme and secondary antibody in great proportion are assembled on a carbon nano tube loading gold nanoparticles and a novel glucose oxidase functional nano composite probe is designed for sandwich immunoassay. The nano composite probe is combined with the multi-channel immunosensor to realize double signal amplification and high sensitive immunodetection of protein. The prussian blue, as electronic transmission media, catalyzes and reduces hydrogen peroxide generated by glucose oxidation under the catalysis of glucose oxidase on oxygen so as to obtain current signal. The method avoids the interference of dissolved oxygen in the detection solution and deoxidization is not required in process of amperometric detection. The invention has the advantages of wide detection concentration range, good repeatability, accurate results and the like and has a certain clinical application value.
Owner:NANJING UNIV +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com