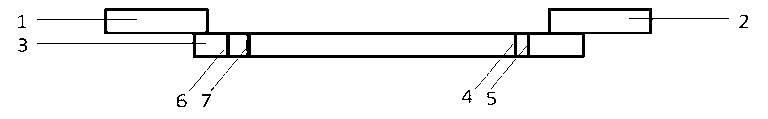Dry-type immunoassay test strip and preparation method and application thereof
A technology for immune detection and test strips, applied in the field of clinical medical diagnosis, can solve the problems of complex process and long detection time, and achieve the effects of accurate detection results, shortened detection time and cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
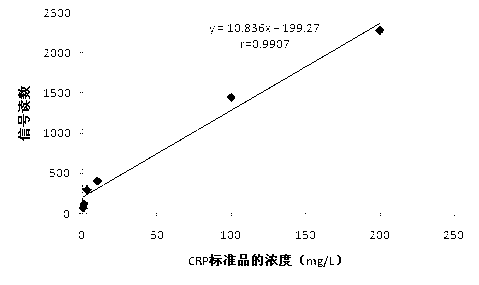
[0029] Example 1 Preparation and use of C-reactive protein colloidal gold detection test paper
[0030] 1. Preparation of C-reactive protein colloidal gold detection test paper
[0031] (1) Preparation of colloidal gold-antibody complex
[0032] a. Preparation of biotinylated C-reactive protein (CRP) monoclonal antibody complex: First, link 6-aminohexose to biotin to obtain long-arm biotin, add 80 μg of biotin per ml of mouse anti-human CRP monoclonal antibody Add and react for 1 hour to generate long-arm biotin N-hydroxysuccinamide ester, which is a biotinylated CRP monoclonal antibody complex, and dialyze through 1% BSA pH7.5 to remove free biotin;
[0033] b. Preparation of colloidal gold-labeled avidin: adjust the pH value of colloidal gold to 7.5 with 1% potassium carbonate solution, add streptavidin solution according to the amount of 10 μg streptavidin / ml colloidal gold, mix well, and place at 25°C After reacting in a water bath for 30 minutes, add 5% BSA, block for...
Embodiment 2
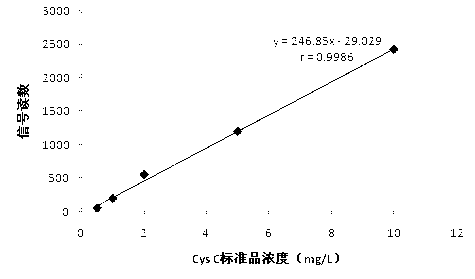
[0071] Example 2 Preparation and use of cystatin C fluorescence detection test strips
[0072] 1. Preparation of cystatin C (CysC) fluorescent test paper
[0073] (1) Cystatin C antibody labeled with fluorescent latex microspheres
[0074] a. Preparation of fluorescent latex microspheres: Dilute latex microspheres with a particle size of 400 nm to a final concentration of 30 mg / ml and a volume of 6 ml with an adsorption buffer (50 mmol / L, citrate buffer at pH 5.8) to obtain latex Microsphere suspension; add an appropriate amount of red fluorescein rhodamine-labeled streptavidin (purchased from Shanghai Enzyme Biotechnology Co., Ltd.) in the adsorption buffer, and the final volume is 6ml; add the above-mentioned latex microsphere suspension to Prepare a mixed solution in the above-mentioned adsorption buffer containing red fluorescein rhodamine-labeled streptavidin; incubate the resulting mixed solution at room temperature for 1-2 hours, and keep stirring, then centrifuge, c...
Embodiment 3
[0110] Example 3 Preparation and use of alpha-fetoprotein (AFP) fluorescent test strips
[0111] 1. Preparation of fluorescent test paper for alpha-fetoprotein detection
[0112] (1) Fluorescent latex microspheres labeled alpha-fetoprotein (AFP) antibody
[0113] a. Preparation of fluorescent latex microspheres: Dilute latex microspheres with a particle size of 200 nm to a final concentration of 30 mg / ml and a volume of 6 ml with an adsorption buffer (50 mmol / L, citrate buffer at pH 5.8) to obtain latex Microsphere suspension; add an appropriate amount of fluorescein Cy5 in the adsorption buffer, and the final volume is 6ml; add the above-mentioned latex microsphere suspension to the above-mentioned adsorption buffer containing fluorescein Cy5-labeled streptavidin to obtain Mixed solution: Incubate the resulting mixed solution at room temperature for 1-2 hours with constant stirring, then centrifuge to collect the precipitate, dissolve the precipitate in storage buffer (ads...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com