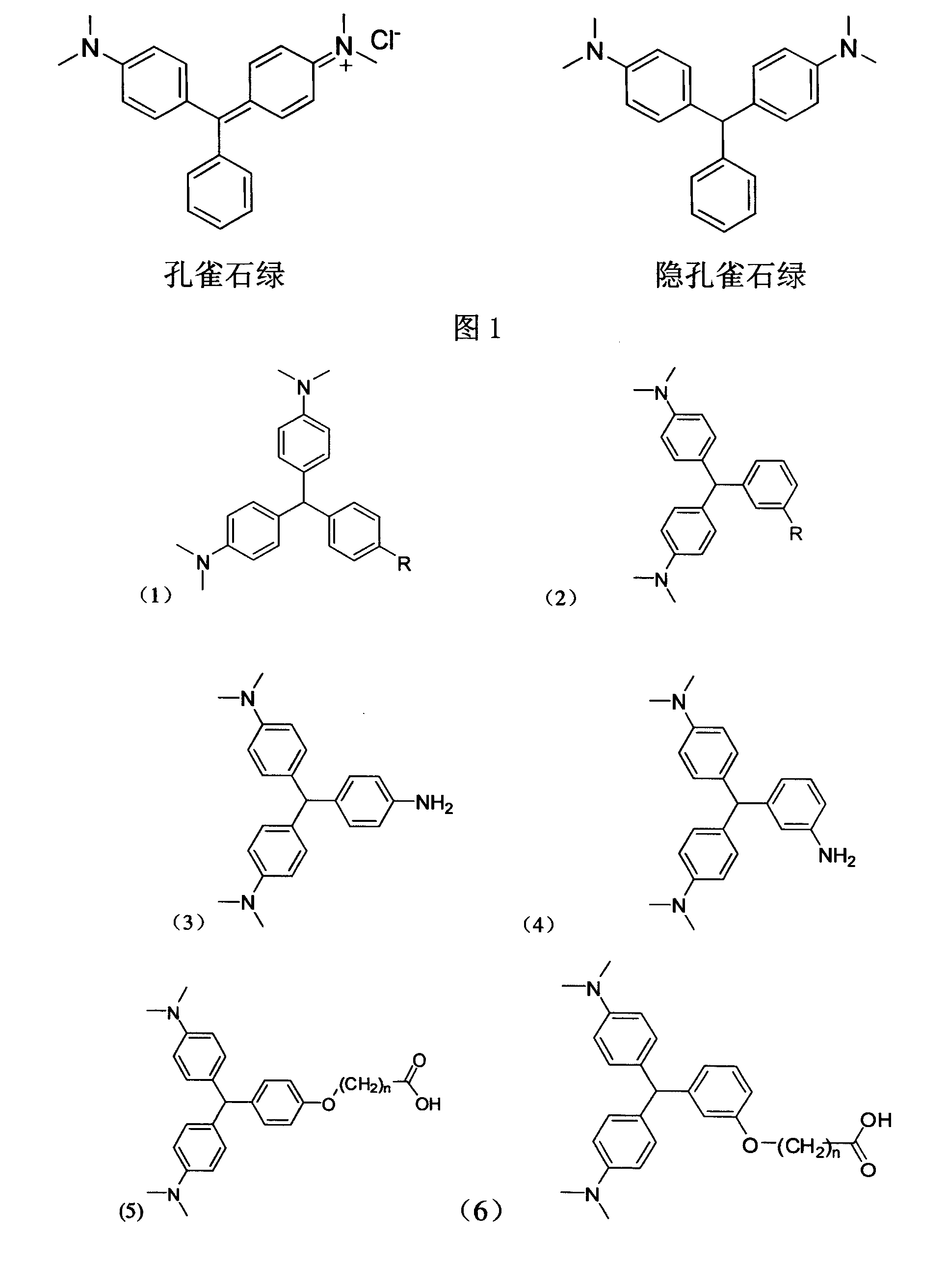
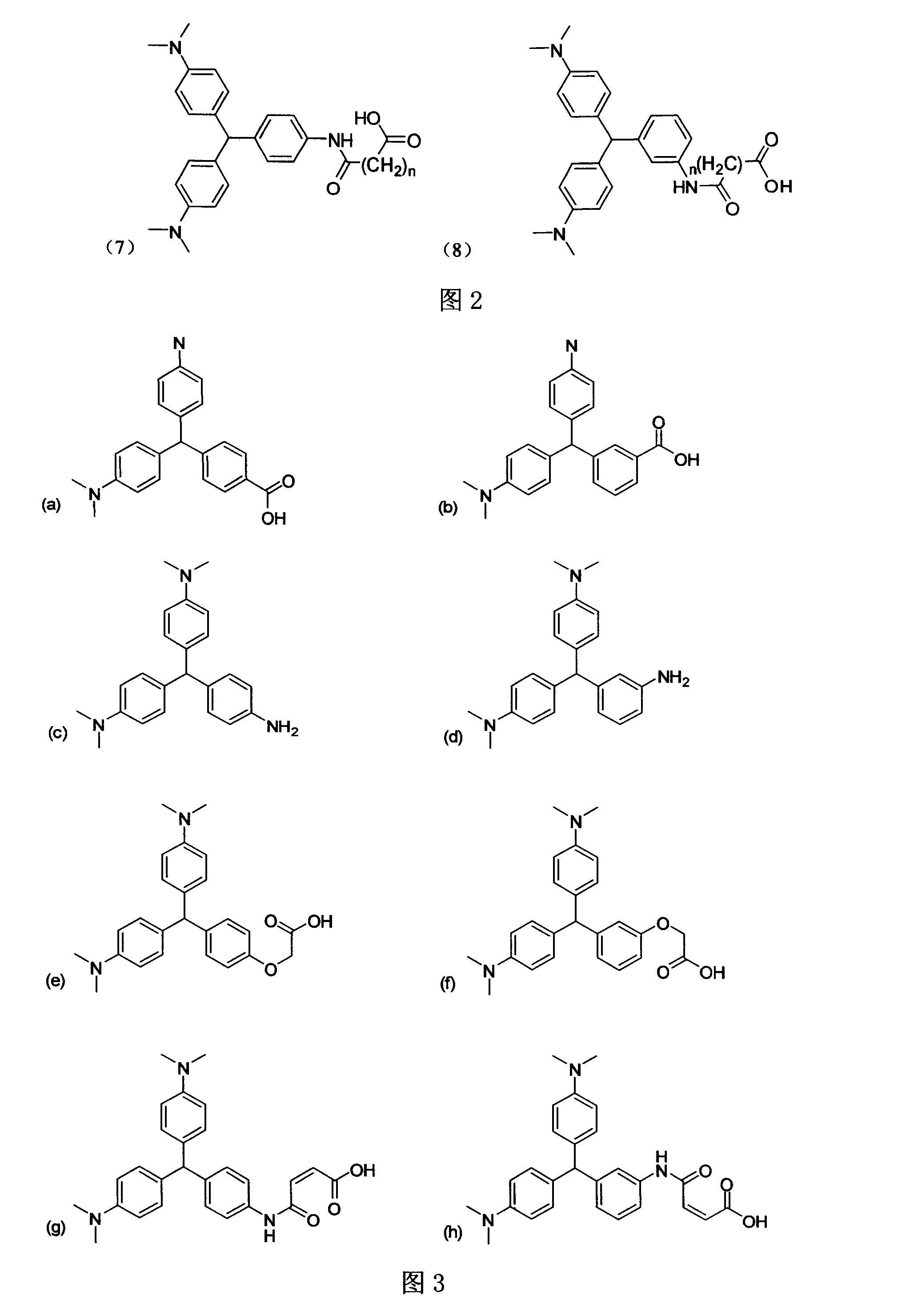
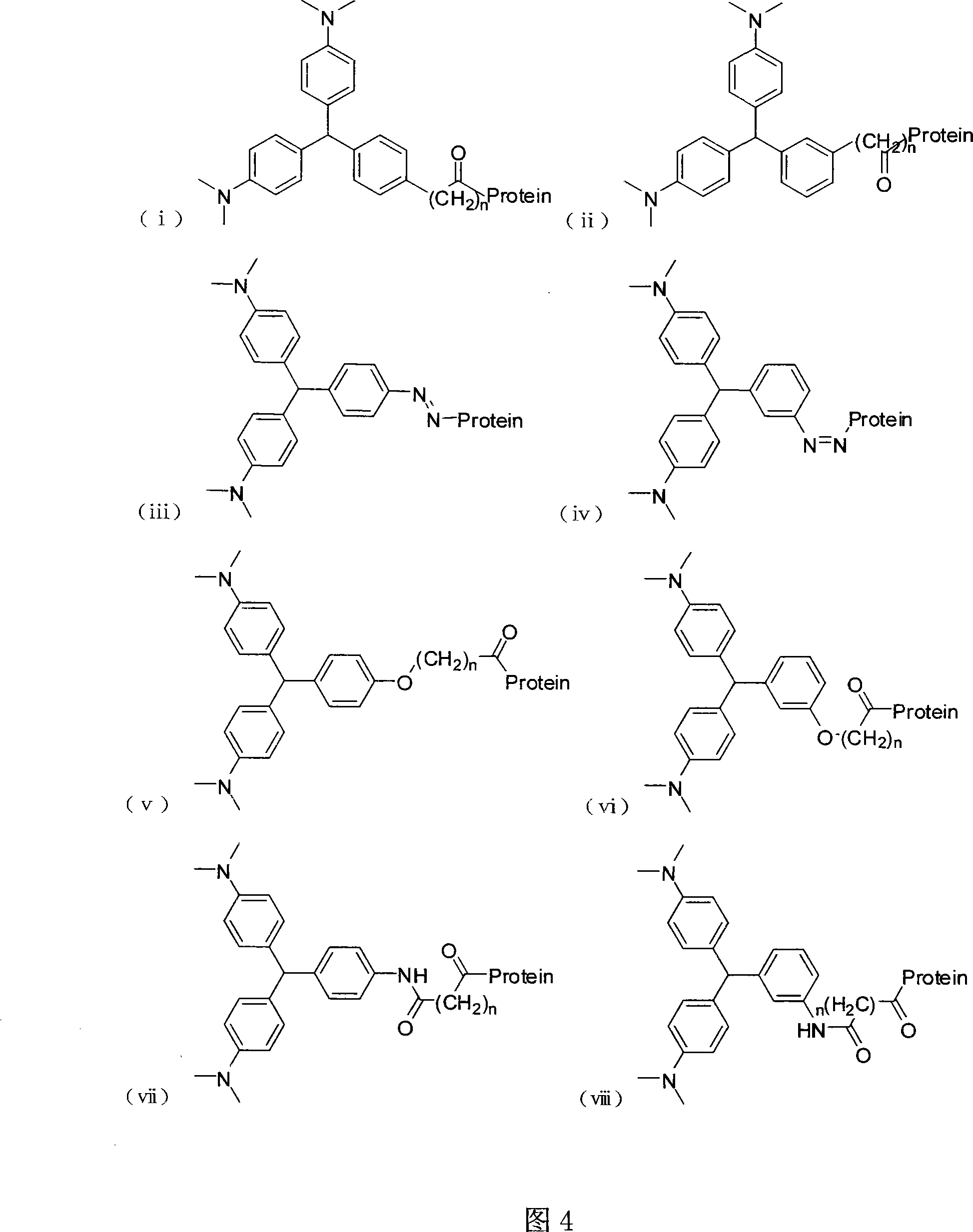
Leuco malachite green hapten, produced antibody and application of the antibody
A cryptomalachite green and hapten technology, applied in the field of immunochemistry, can solve the problems of tedious operation, expensive equipment, and inapplicability for rapid detection, etc., and achieves the effects of reducing pollution, far-reaching application value, and easy post-reaction treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 The synthetic route of the hapten is shown in Figure 5.
[0041] Take 2.50g (20.0mmol) of N,N-dimethylaniline into a 50ml two-necked flask, slowly add 5% Amberlyst 15 Resin, magnetically stir for a few minutes, the magnetic force and stirring time should be selected according to conventional technology, the time is generally 5 ~10min. Under stirring, add 1.50 g of 4-carboxybenzaldehyde or 3-carboxybenzaldehyde, and stir overnight at 80-100°C. After the completion of the reaction, the catalyst was filtered and saturated sodium bicarbonate solution was added to the reaction system to adjust the pH to pH=9.0, and extracted with dichloromethane. The aqueous phase was adjusted to pH=2 with dilute hydrochloric acid, extracted with ethyl acetate, and the organic layer was rotary evaporated under reduced pressure to obtain a crude product. The crude product was purified by reduced pressure silica gel column chromatography to a white solid substance, the structure of which...
Embodiment 2
[0043] Example 2 The synthetic route of the hapten is shown in Figure 6.
[0044] Take 2.50g (20.0mmol) of N,N-dimethylaniline in a 50ml two-necked flask, slowly add 5% Amberlyst 15 Resin, and magnetically stir for several minutes. Under stirring, add 1.53 g of 3-nitrobenzaldehyde or 4-nitrobenzaldehyde. Under nitrogen flow, stir overnight at 80-100°C. After the reaction is completed, the catalyst is filtered, and a yellow solid is obtained by rotary evaporation under reduced pressure.
[0045] Add 3.75g (about 10mmol) of the above crude product in a round bottom flask, add 10ml of 95% ethanol, stir to dissolve it, add 4g (about 70mmol) of iron powder, 20ml of distilled water, and finally add 1ml of concentrated hydrochloric acid, and reflux at 80℃. Stop the reaction at 2h. The water phase was obtained by suction filtration, the organic phase was obtained by extraction with ethyl acetate, and the light yellow crude product was obtained by evaporation under reduced pressure. The ob...
Embodiment 3
[0047] Example 3 The synthetic route of the hapten is shown in Figure 7.
[0048] Take 12.2g of 3-hydroxybenzaldehyde or 4-hydroxybenzaldehyde in a 150ml round bottom flask, dissolve it with 20ml of distilled water, add 4g of NaOH and stir to react. Then, 11.6 g of sodium chloroacetate solution was added dropwise under stirring, and the reaction was stirred at 80-100°C under reflux for 2 to 3 hours. After the reaction is over, add proper amount of concentrated hydrochloric acid to acidify, then add saturated sodium bicarbonate solution to adjust to basicity, extract with ethyl acetate, acidify the aqueous phase with dilute hydrochloric acid until precipitation is precipitated, dry and precipitate under reduced pressure to obtain the intermediate product.
[0049] The intermediate product and N,N-dimethylaniline were added to a 50ml two-necked flask at a molar ratio of 1:2, and 5% Amberlyst 15 Resin was added at the same time. Under nitrogen, the mixture was stirred at 80-100°C ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com