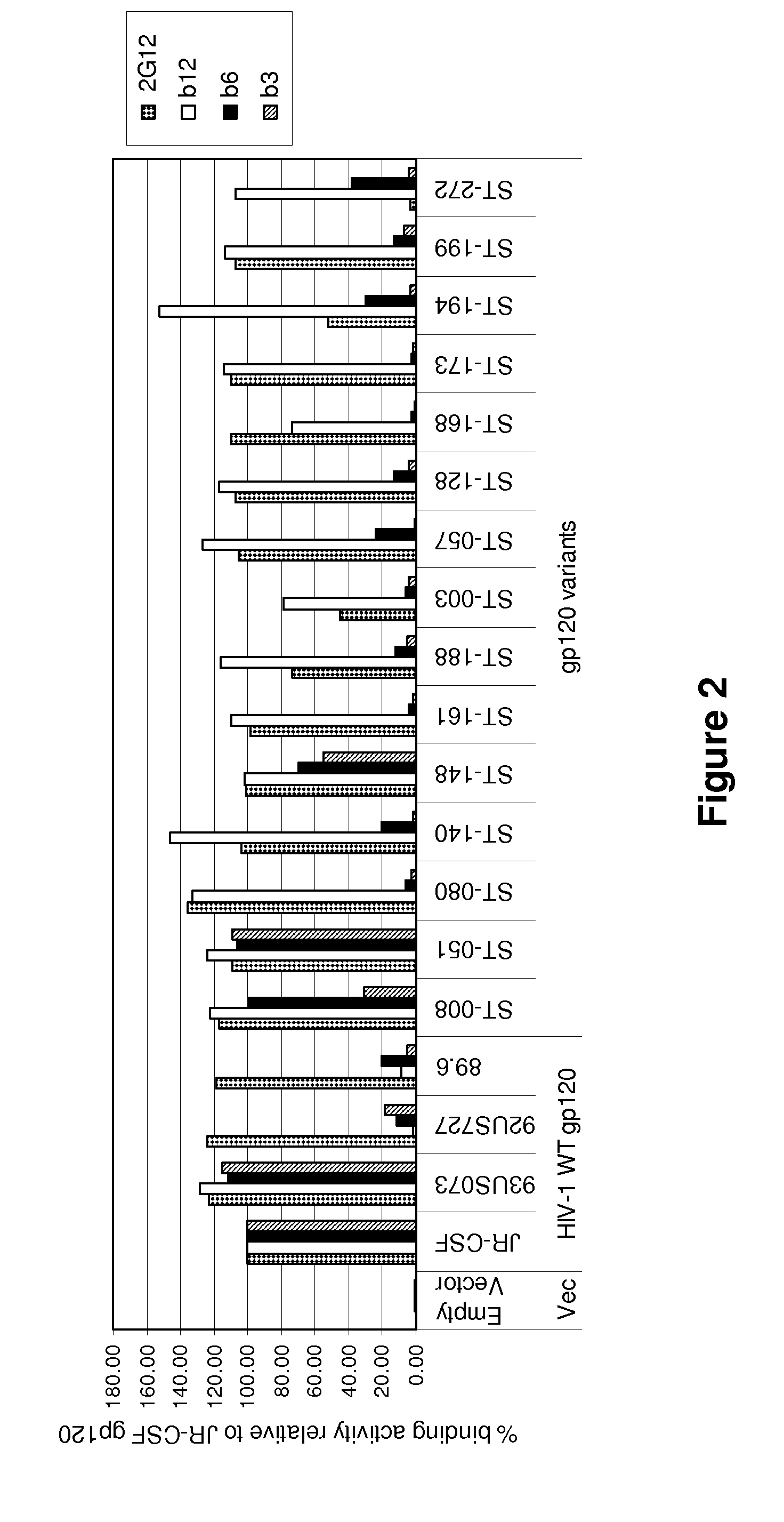
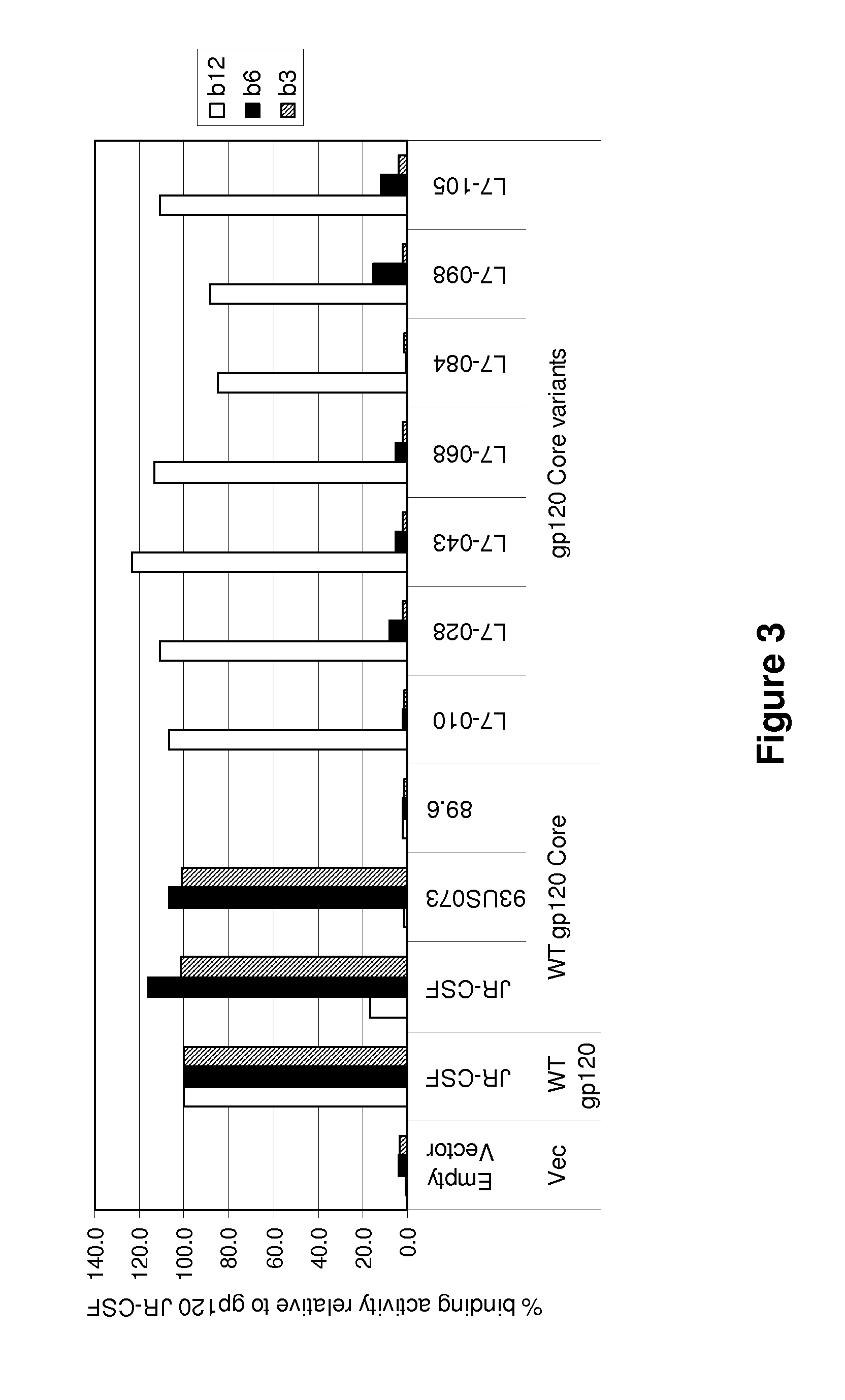
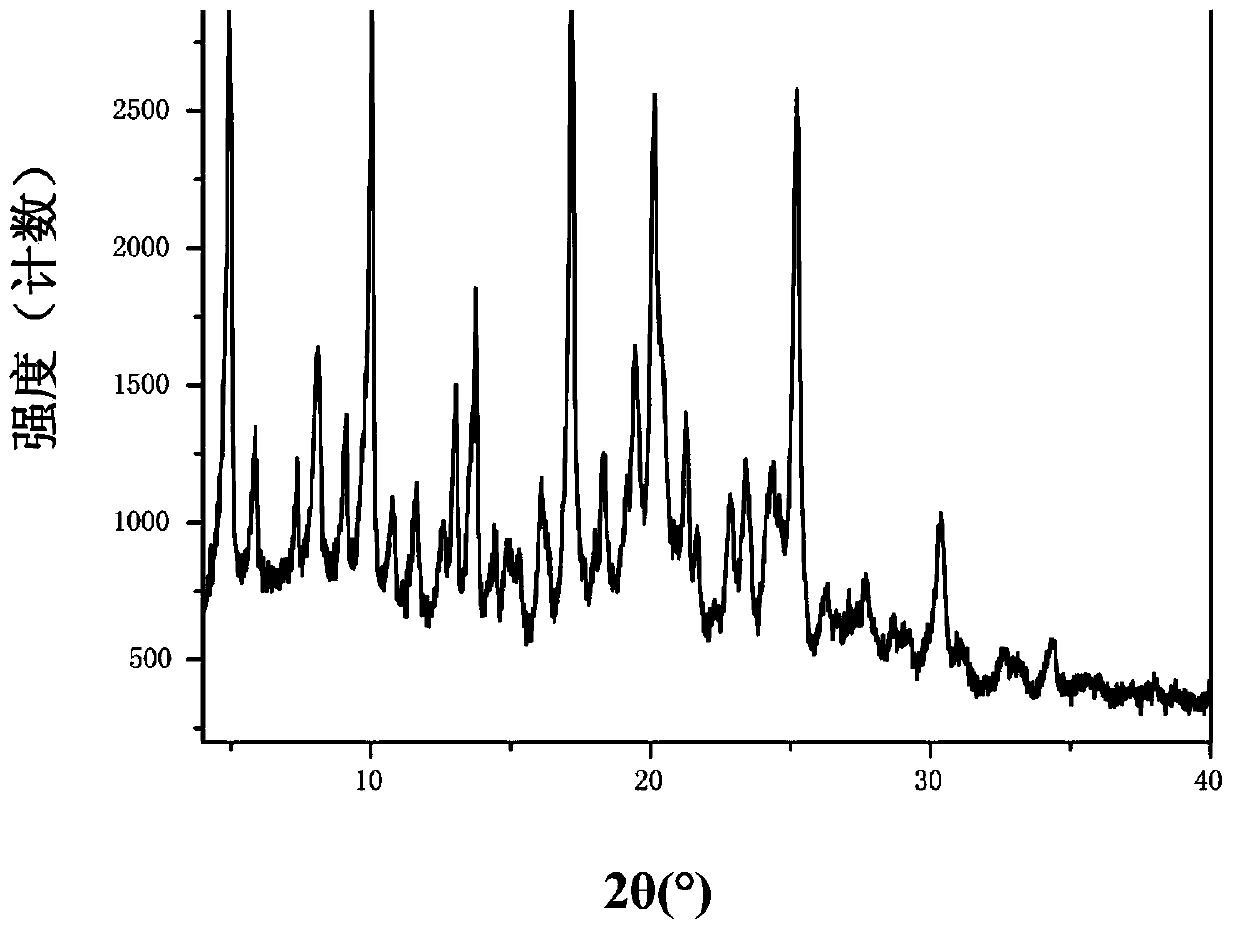
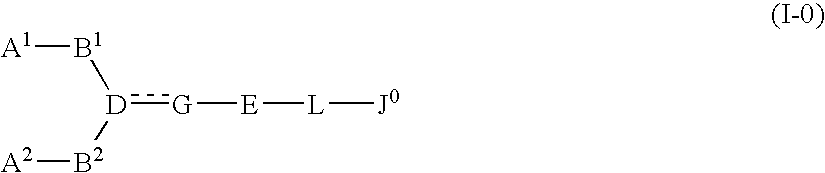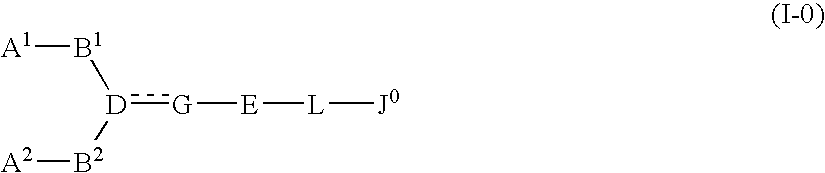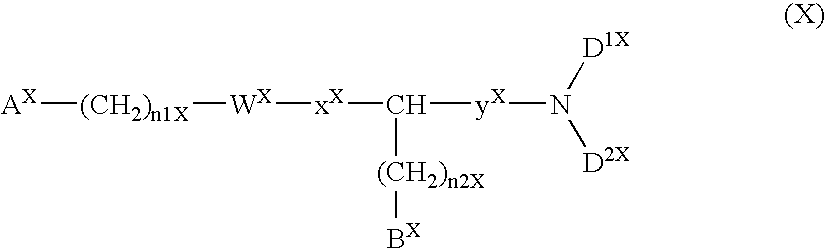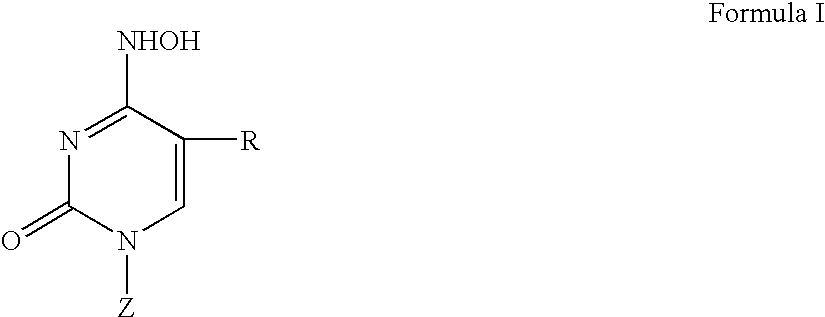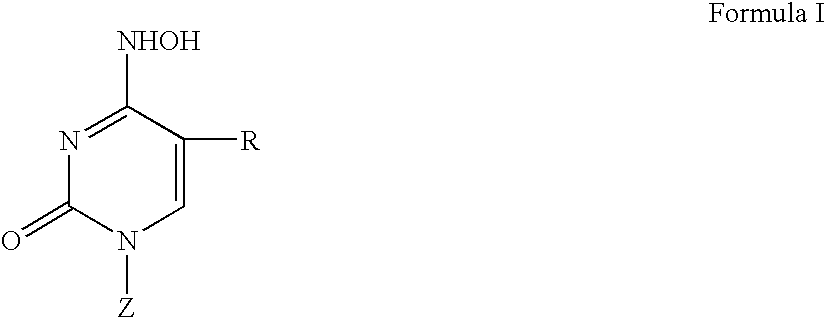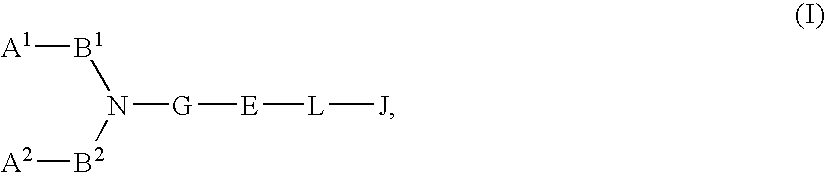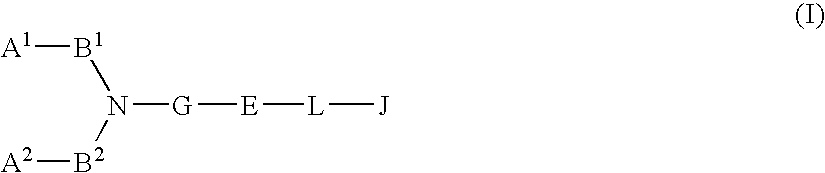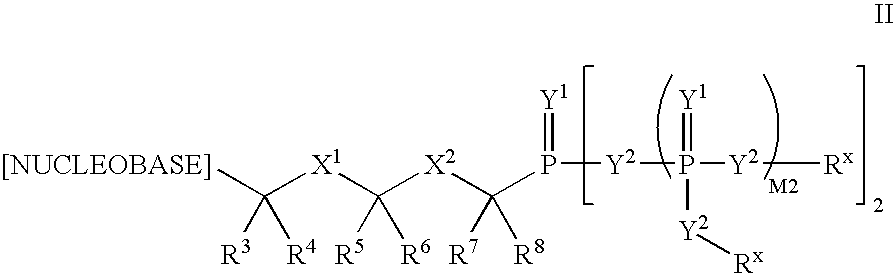Patents
Literature
302 results about "Human immuno deficiency virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The human immunodeficiency virus ( HIV) infamously causes immune deficiencies, as do a number of medications, such as the drugs used in chemotherapy. A state of immune deficiency may also be deliberately induced, classically in the case of an organ transplant, in which the patient takes drugs to suppress the immune system to reduce the risk of rejection of the donor organ.
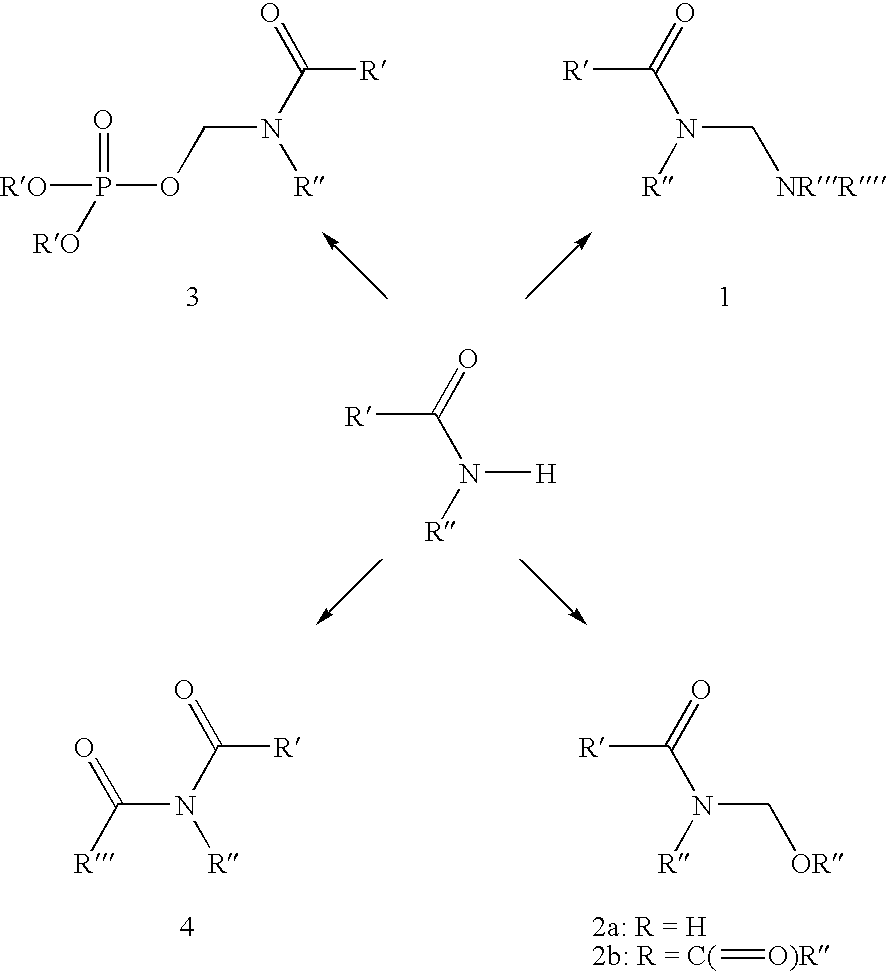
Methods and compositions for treatment of human immunodeficiency virus infection with conjugated antibodies or antibody fragments
ActiveUS20070264265A1Avoid infectionReduce eliminateOrganic active ingredientsAntiviralsDiagnostic agentBinding site
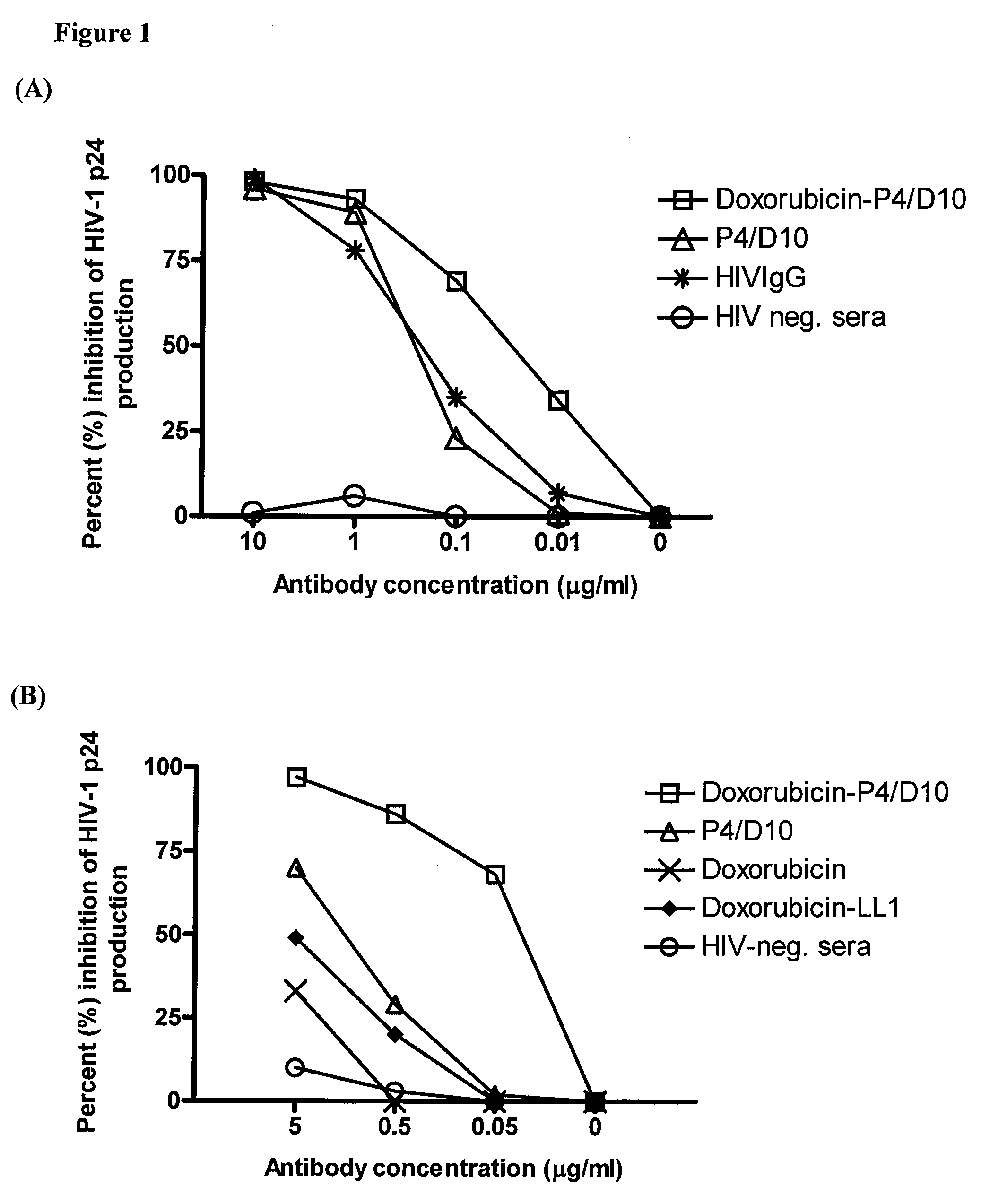
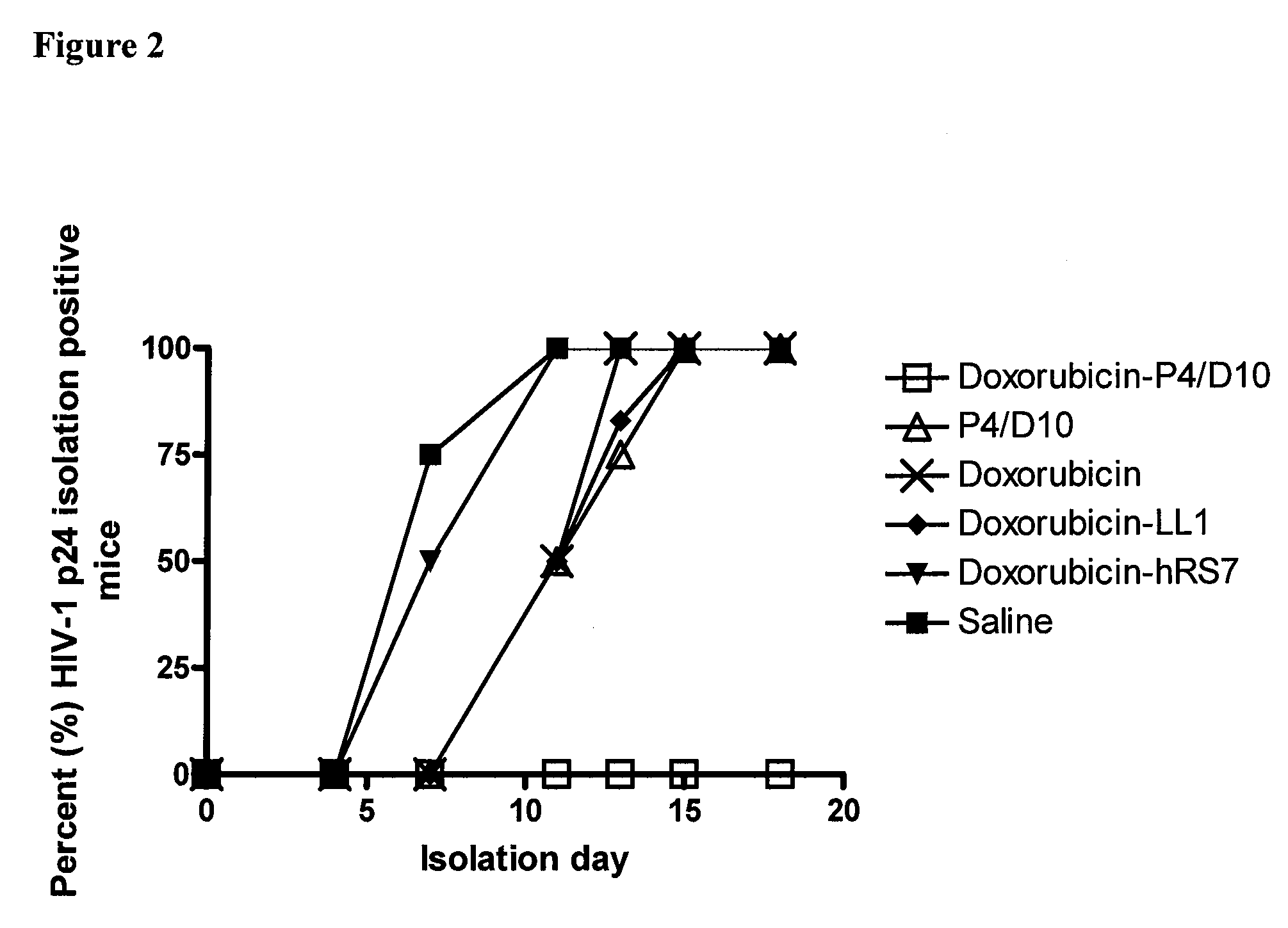
The present invention concerns methods and compositions for treatment of HIV infection in a subject. The compositions may comprise a targeting molecule against an HIV antigen, such as an anti-HIV antibody or antibody fragment. The anti-HIV antibody or fragment may be conjugated to a variety of cytotoxic agents, such as doxorubicin. In a preferred embodiment, the antibody or fragment is P4 / D10. Other embodiments may concern methods of imaging, detection or diagnosis of HIV infection in a subject using an anti-HIV antibody or fragment conjugated to a diagnostic agent. In alternative embodiments, a bispecific antibody with at least one binding site for an HIV antigen and at least one binding site for a carrier molecule may be administered, optionally followed by a clearing agent, followed by administration of a carrier molecule conjugated to a therapeutic agent.
Owner:IMMUNOMEDICS INC
Methods for measuring cellular proliferation and destruction rates in vitro and in vivo
InactiveUS6461806B1Organic active ingredientsIn-vivo radioactive preparationsStable Isotope LabelingDisease
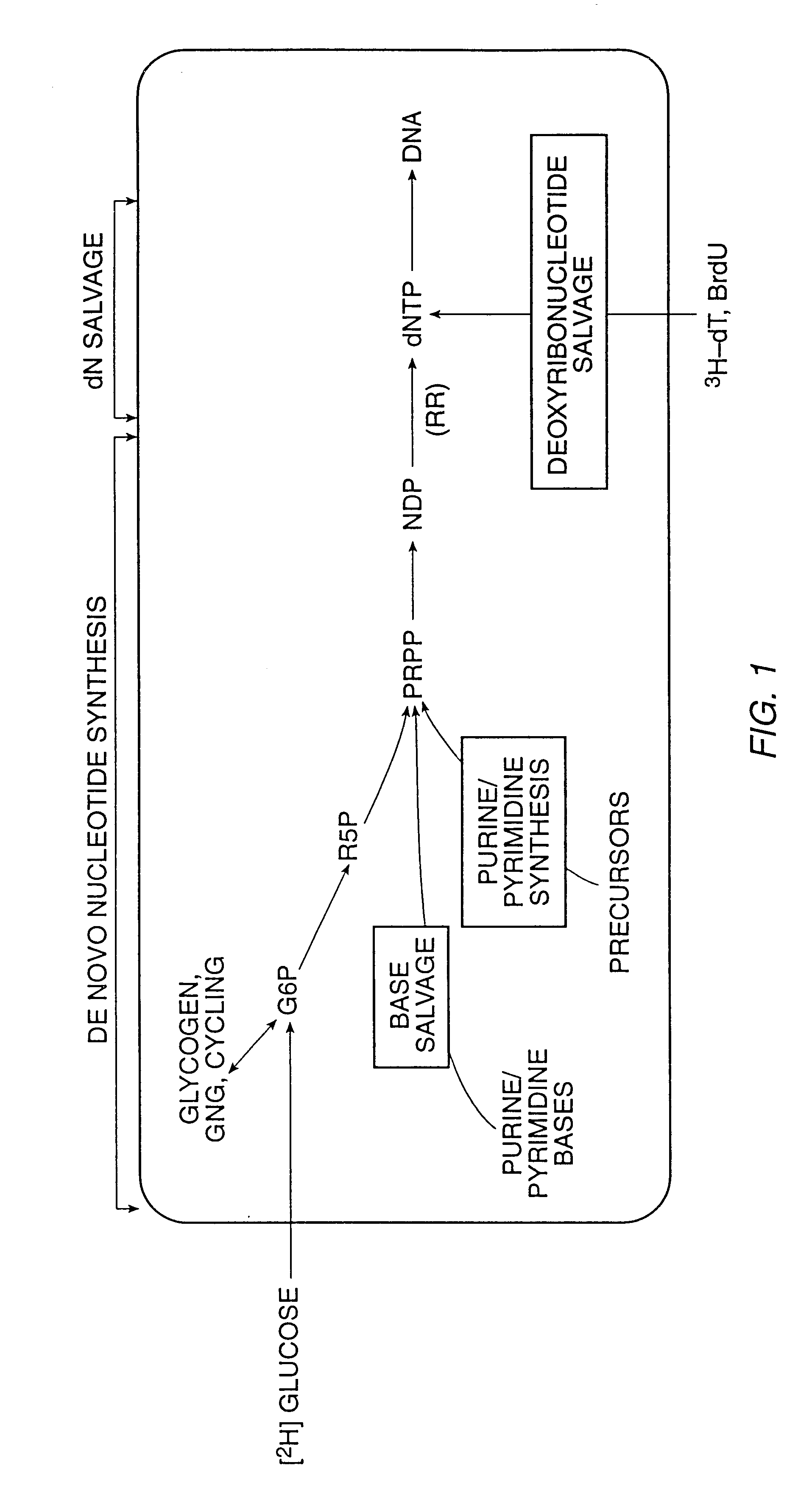
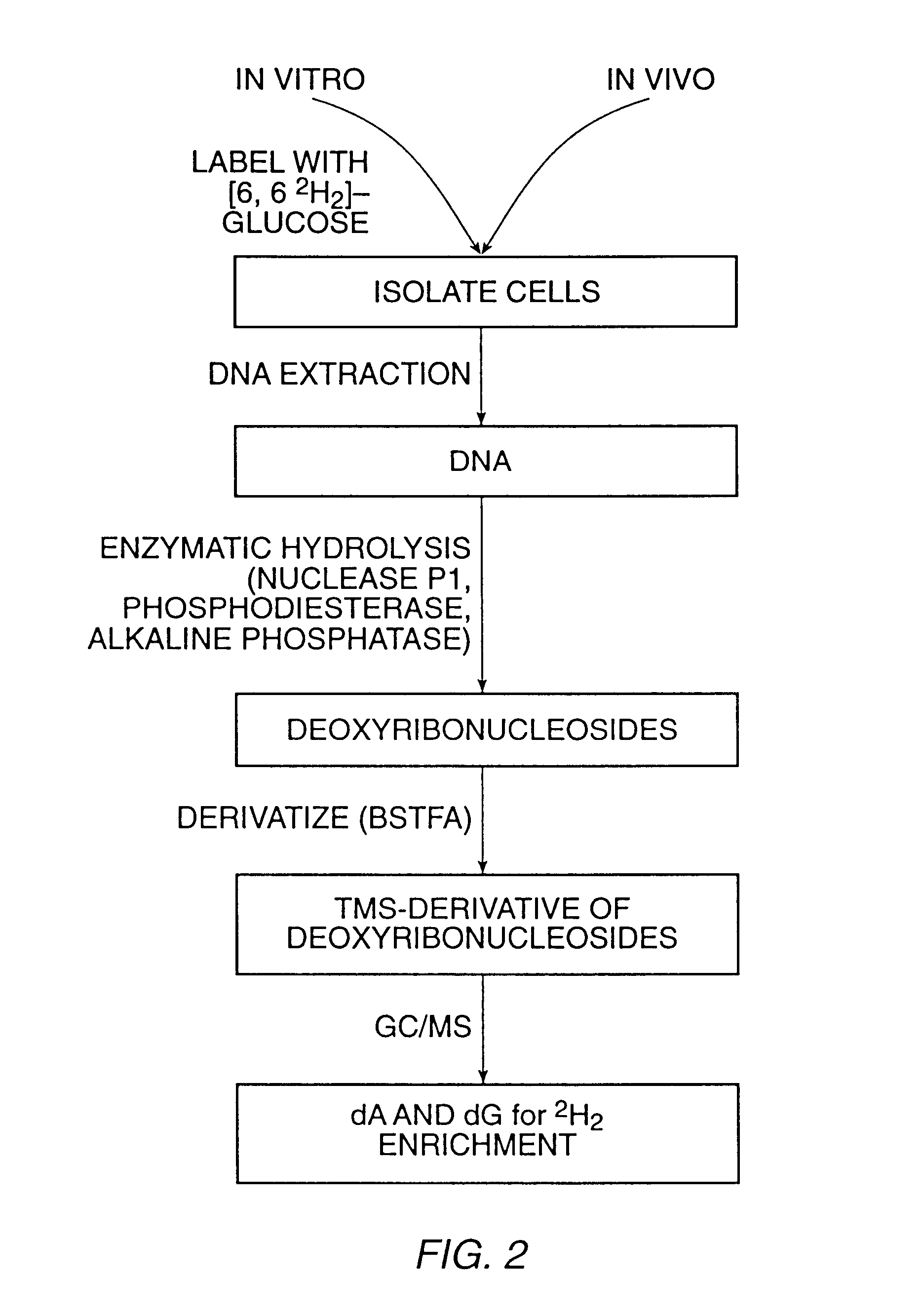
The present invention relates to methods for measuring the proliferation and destruction rates of cells by measuring deoxyribonucleic acid (DNA) synthesis and / or destruction. In particular, the methods utilize non-radioactive stable isotope labels to endogenously label DNA synthesized through the de novo nucleotide synthesis pathway in a cell. The amount of label incorporated in the DNA is measured as an indication of cellular proliferation. The decay of labeled DNA over time is measured as an indication of cellular destruction. Such methods do not involve radioactivity or potentially toxic metabolites, and are suitable for use both in vitro and in vivo. Therefore, the invention is useful for measuring cellular proliferation or cellular destruction rates in humans for the diagnosis, prevention, or management of a variety of disease conditions in which cellular proliferation or cellular destruction is involved. The invention also provides methods for measuring proliferation or destruction of T cells in a subject infected with human immunodeficiency virus (HIV) and methods of screening an agent for a capacity to induce or inhibit cellular proliferation or destruction. In addition, the invention provides methods for measuring cellular proliferation in a proliferating population which utilize both radioactive isotope labels and stable isotopes to endogenously label DNA through the de novo nucleotide synthesis pathway.
Owner:RGT UNIV OF CALIFORNIA
Hydroxyethylamino sulphonamides useful as retroviral protease inhibitors
The invention relates to sulfonamide-containing hydroxyethylamine protease inhibitor compounds, their process of making, composition and method of use for inhibiting retroviral proteases such as human immunodeficiency virus.
Owner:GD SEARLE & CO
Heterocylic antiviral compounds
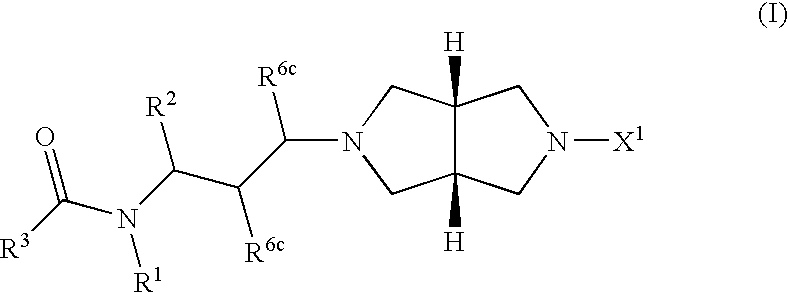
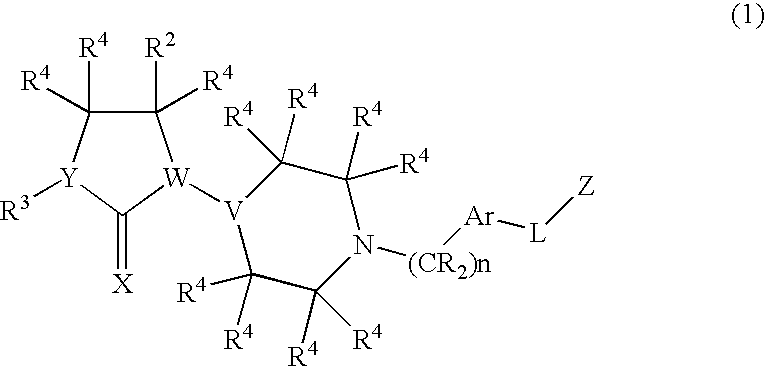
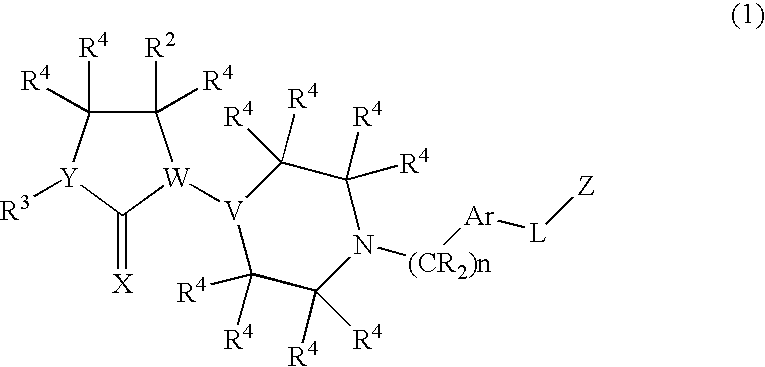
Chemokine receptor antagonists, in particular, 3,7-diazabicyclo[3.3.0]octane compounds according to formula (I) wherein R1-R3 R6c and X1 are as defined herein are antagonists of chemokine CCR5 receptors which are useful for treating or preventing an human immunodeficiency virus (HIV-1) infection, or treating AIDS or ARC. The invention further provides methods for treating diseases that are alleviated with CCR5 antagonists. The invention includes pharmaceutical compositions and methods of using the compounds for the treating diseases mediated by the CCR5 receptor.
Owner:ROCHE PALO ALTO LLC
Chemokine receptor binding compounds
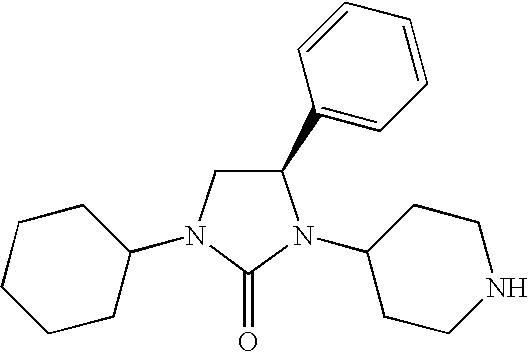
The present invention relates to chemokine receptor binding compounds, pharmaceutical compositions and their use. More specifically, the present invention relates to modulators of chemokine receptor activity, preferably modulators of CCR4 or CCR5. In one aspect, these compounds demonstrate protective effects against infection of target cells by a human immunodeficiency virus (HIV).
Owner:GENZYME CORP
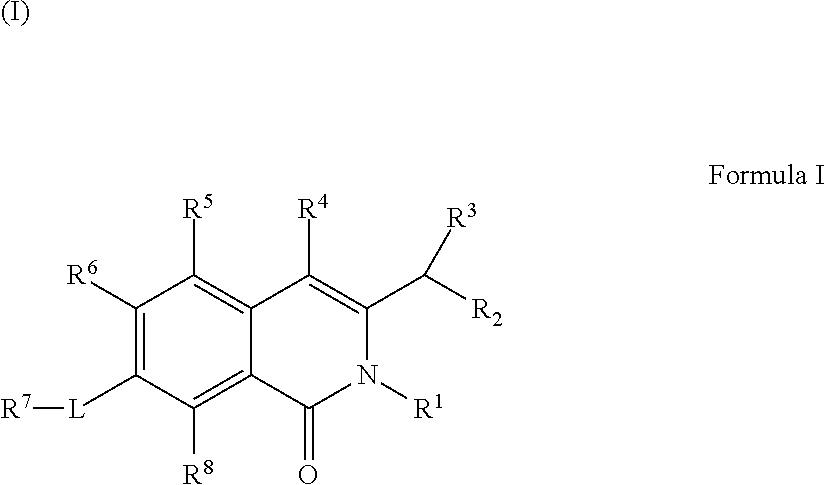
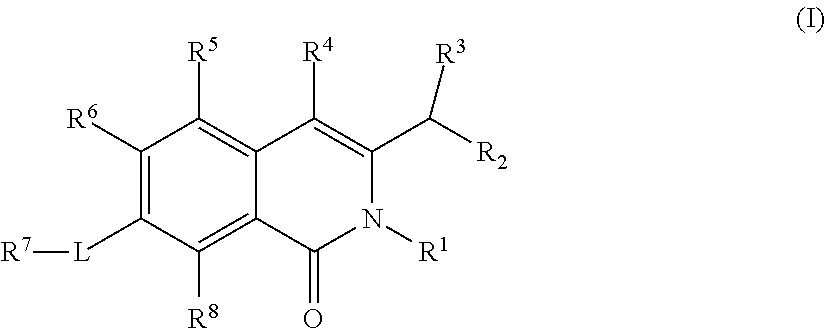
Isoquinoline Compounds And Methods For Treating HIV
Provided are compounds and pharmaceutically acceptable salts thereof, their pharmaceutical compositions, their methods of preparation, and their use for treating viral infections mediated by a member of the retrovirus family of viruses such as the Human Immunodeficiency Virus (HIV).
Owner:VIIV HEALTHCARE UK LTD
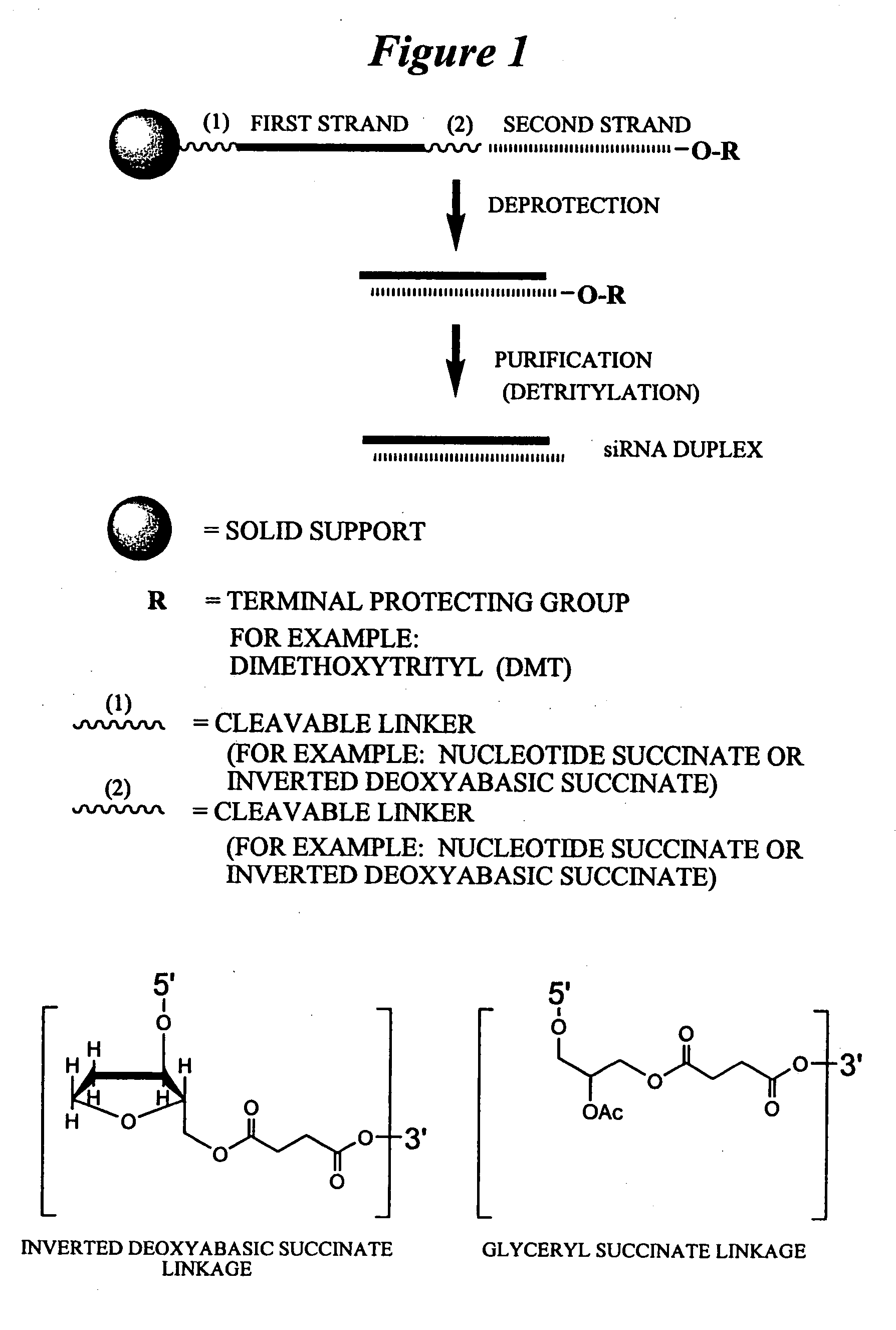
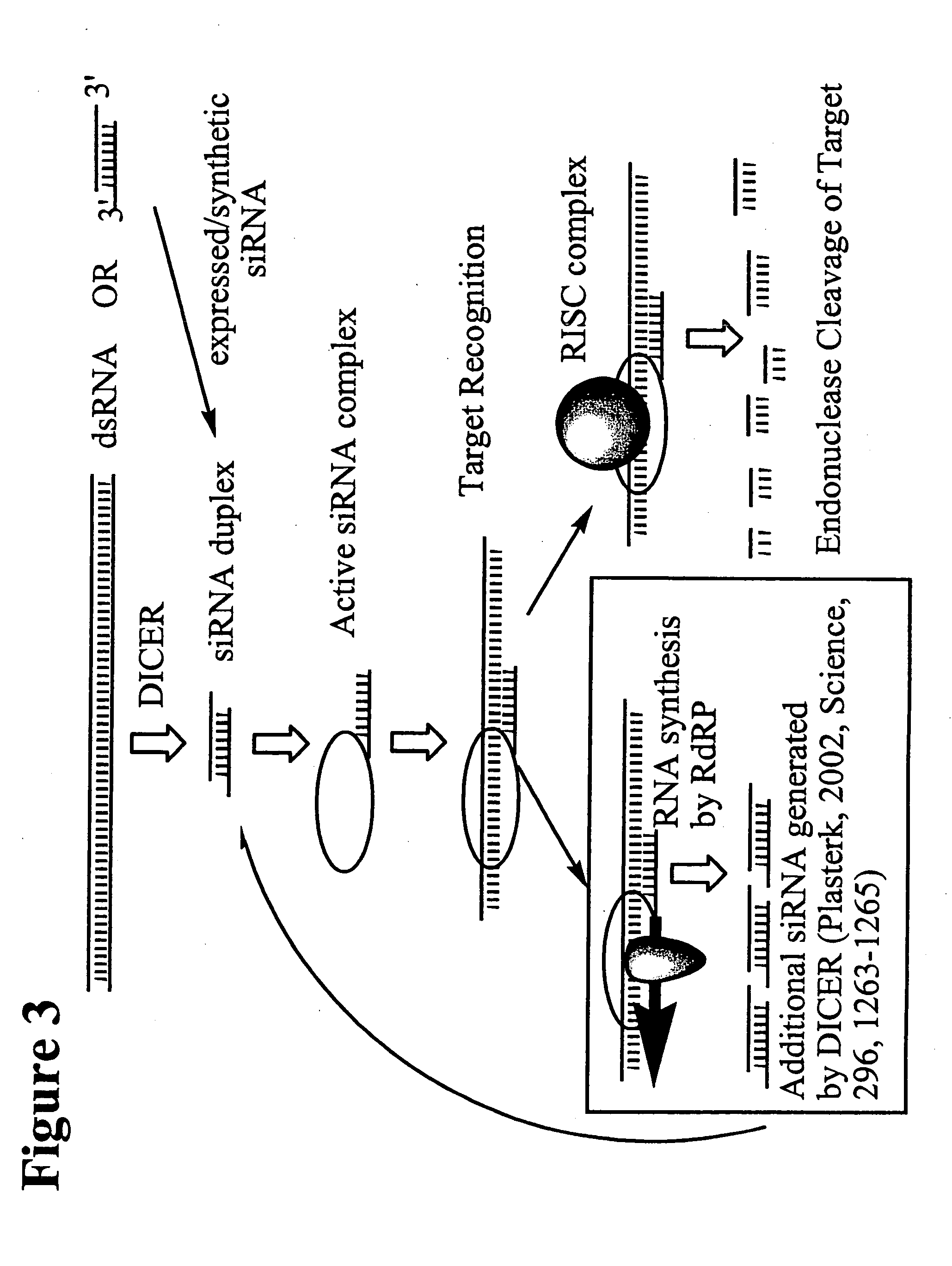
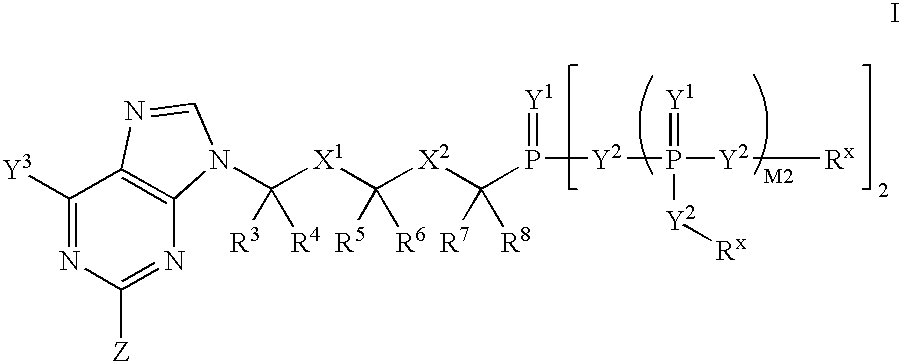
RNA interference mediated inhibition of human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050191618A1Improves various propertyImprove the immunitySugar derivativesMicrobiological testing/measurementDiseaseBiological body
This invention relates to compounds, compositions, and methods useful for modulating human immunodeficiency virus (HIV) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of human immunodeficiency virus (HIV) gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of HIV genes. The small nucleic acid molecules are useful in the treatment of HIV infection, AIDS, and / or diseaes and conditions related to HIV infection and / or AIDS in a subject or organism.
Owner:SIRNA THERAPEUTICS INC
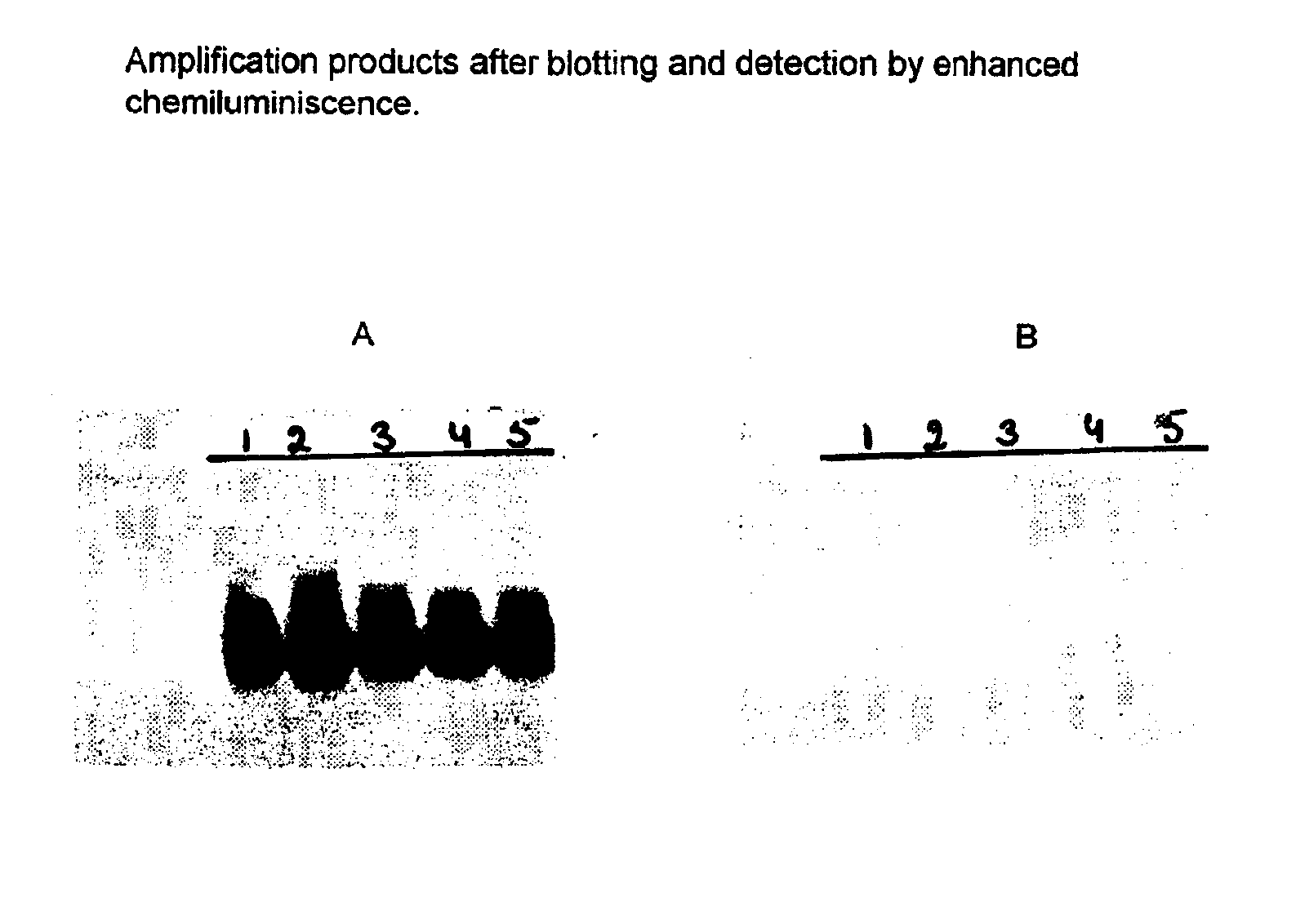
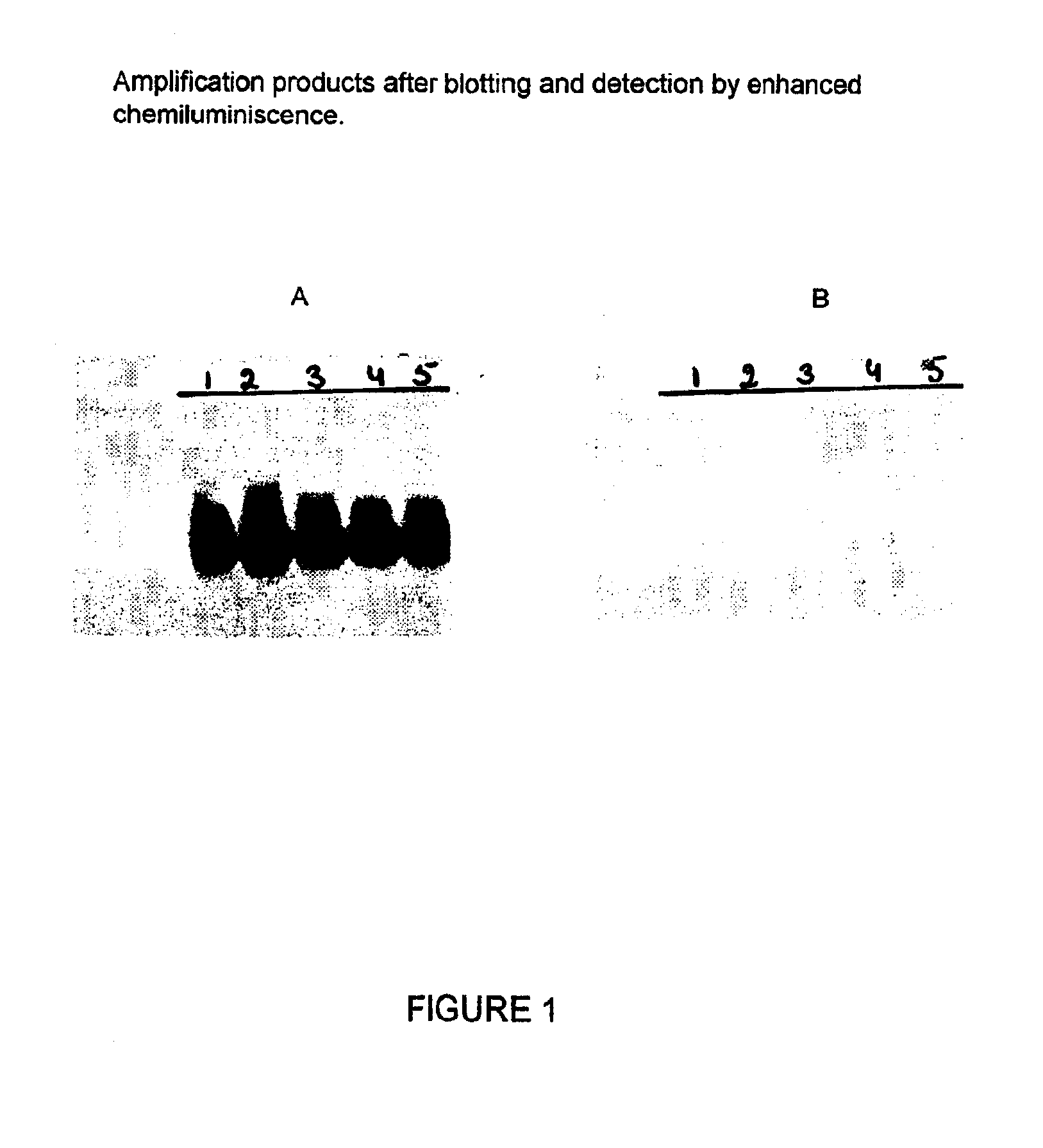
Nucleic acid sequences that can be used as primers and probes in the amplification and detection of all subtypes of HIV-1
InactiveUS6881537B1Improve accuracyHigh sensitivitySugar derivativesMicrobiological testing/measurementHybridization probeSpecific detection
The present invention is related to nucleic acid sequences that can be used in the field of virus diagnostics, more specifically the diagnosis of infections with the AIDS causing Human Immuno-deficiency Virus (HIV).With the present invention nucleotide sequences are provided that can be used as primers and probes in the amplification and detection of HIV-1 nucleic acid. The oligonucleotide sequences provided with the present invention are located in the LTR part of the HIV viral genome. It has been found that, by using the sequences of the present invention in methods for the amplification and detection of nucleic acid a sensitive and specific detection of HIV-1 can be obtained. The benefit of the sequences of the present invention primarily resides in the fact that, with the aid of primers and probes comprising the sequences according to the invention the nucleic acid of all presently known subtypes of HIV-1 can be detected with high accuracy and sensitivity. So far no primer pairs or hybridization probes have been developed that would allow the detection of such a broad range of HIV-1 variants.The oligonucleotide sequences according to the present invention are especially useful in methods for the amplification of nucleic acid.
Owner:BIOMERIEUX SA
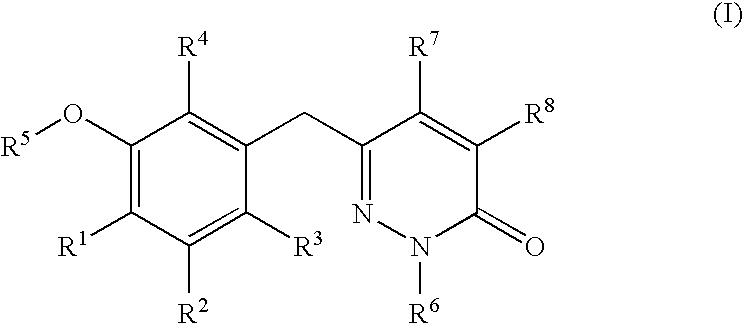
Non-nucleoside reverse transcriptase inhibitors
The present invention relates to a compounds according to formula I, methods for treating diseases mediated by human immunodeficieny virus by administration of a compound according to formula I and pharmaceutical compositions for treating diseases mediated by human immunodeficieny virus containing a compound according to formula I where R1, R2, R3, R4, R5, R6, R7 and R8 are as defined herein.
Owner:ROCHE PALO ALTO LLC
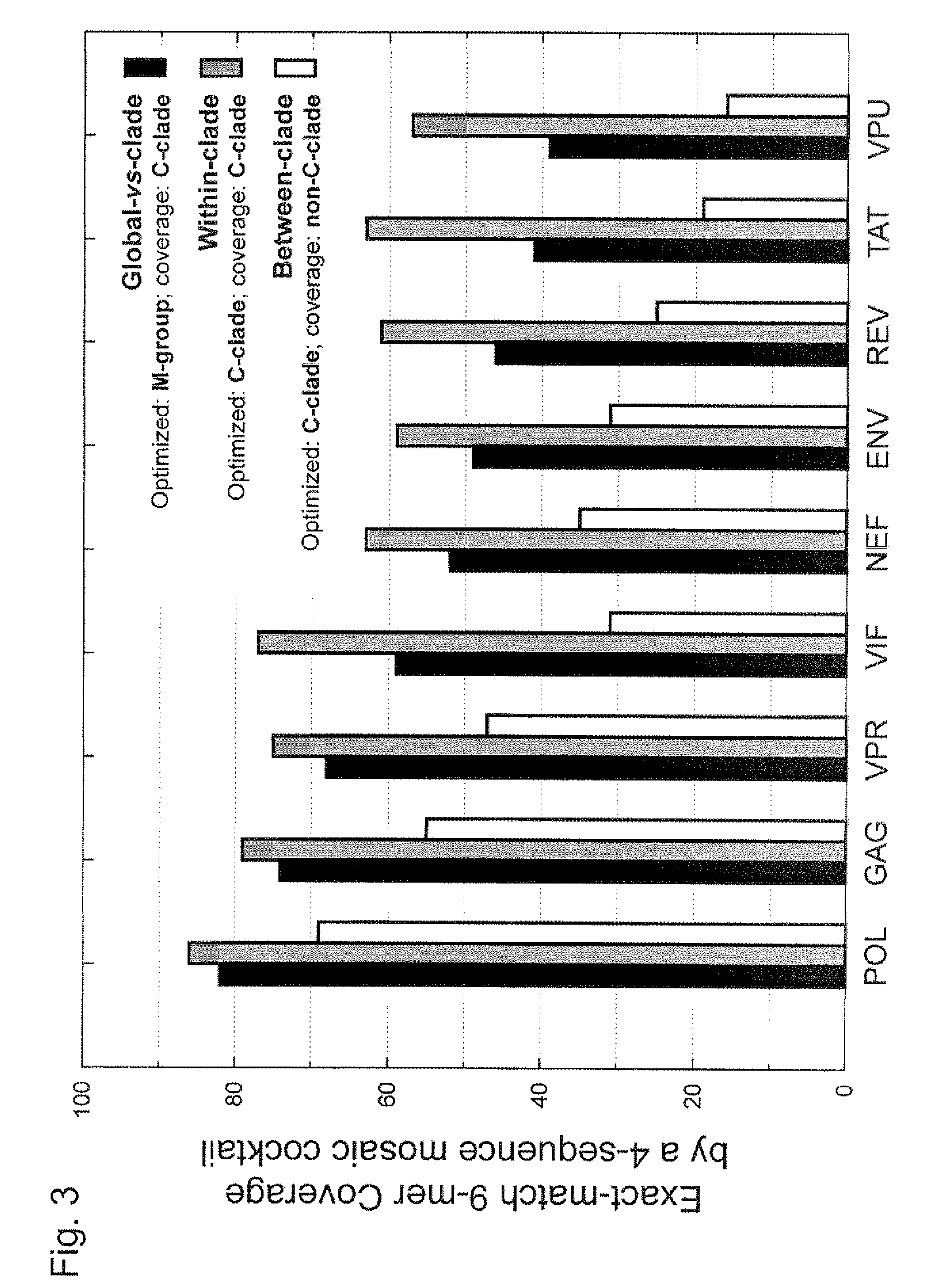
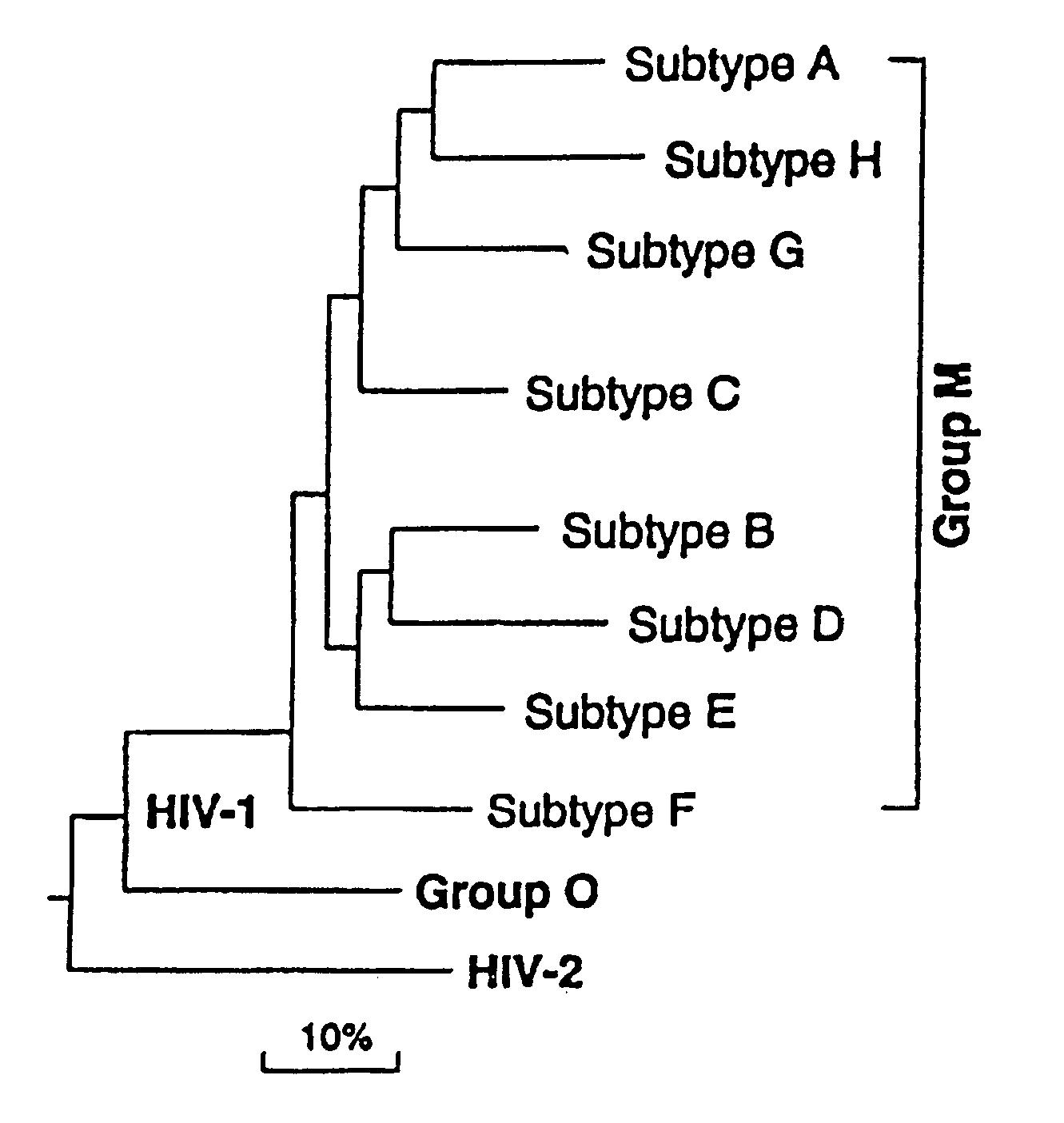
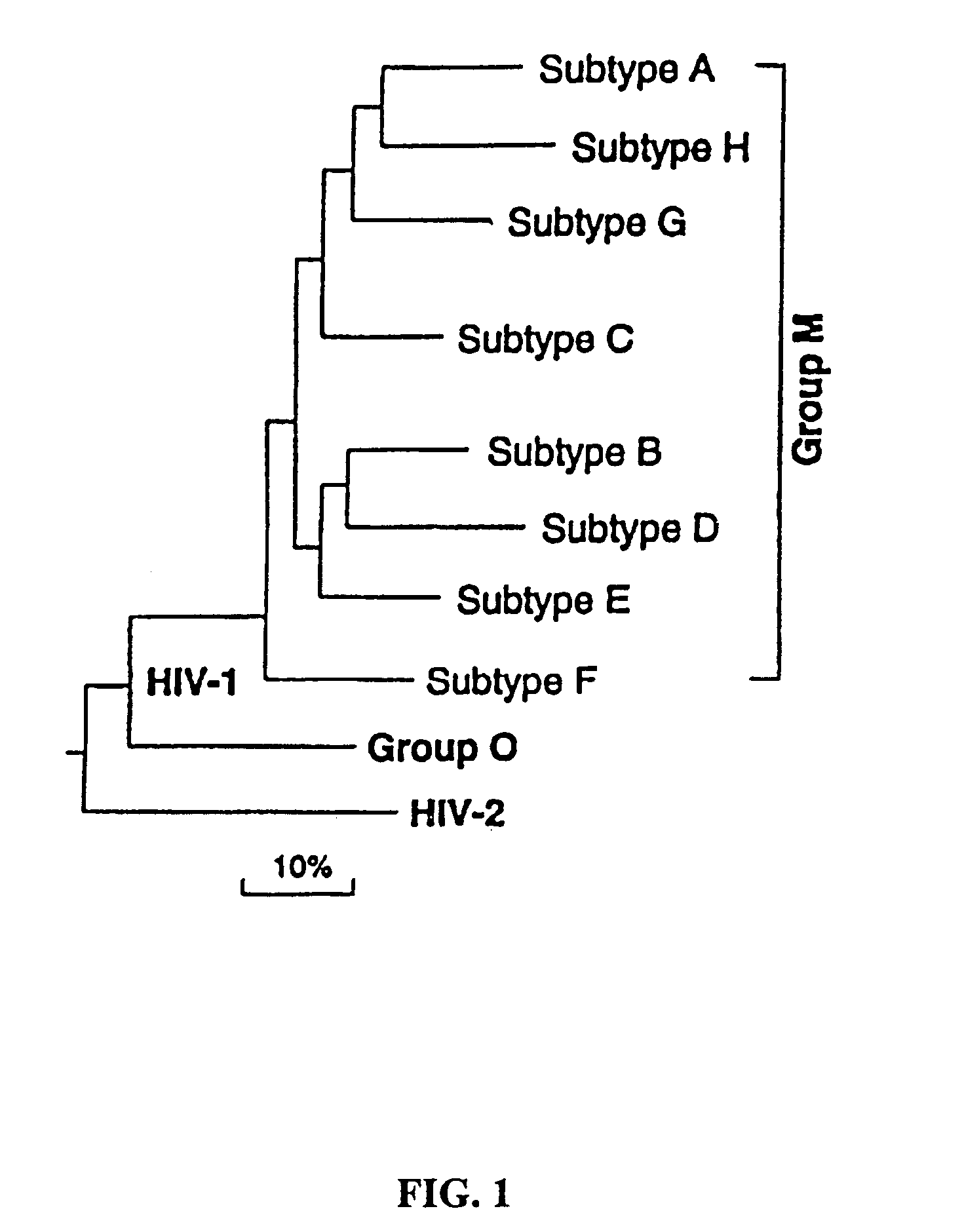
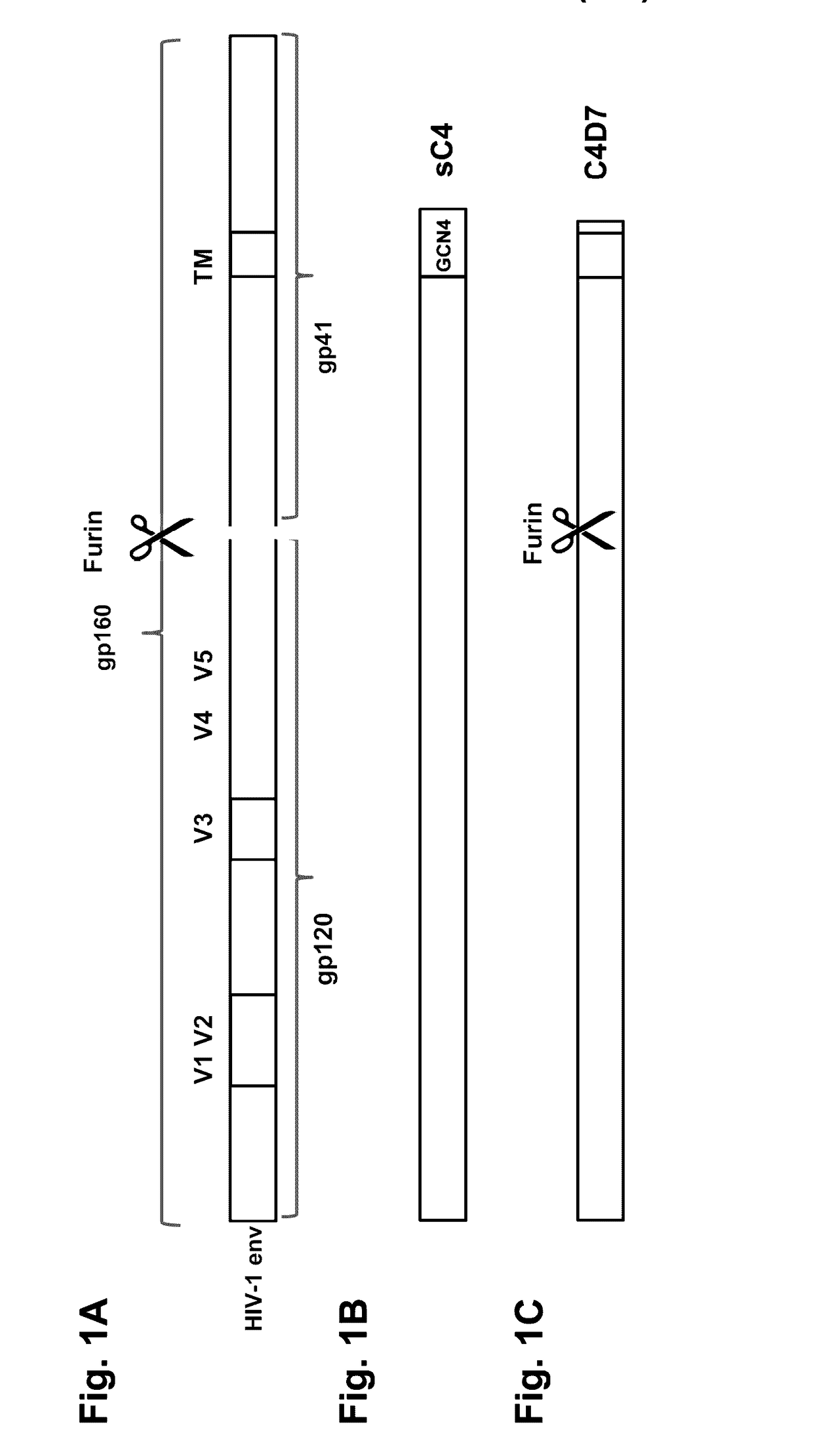
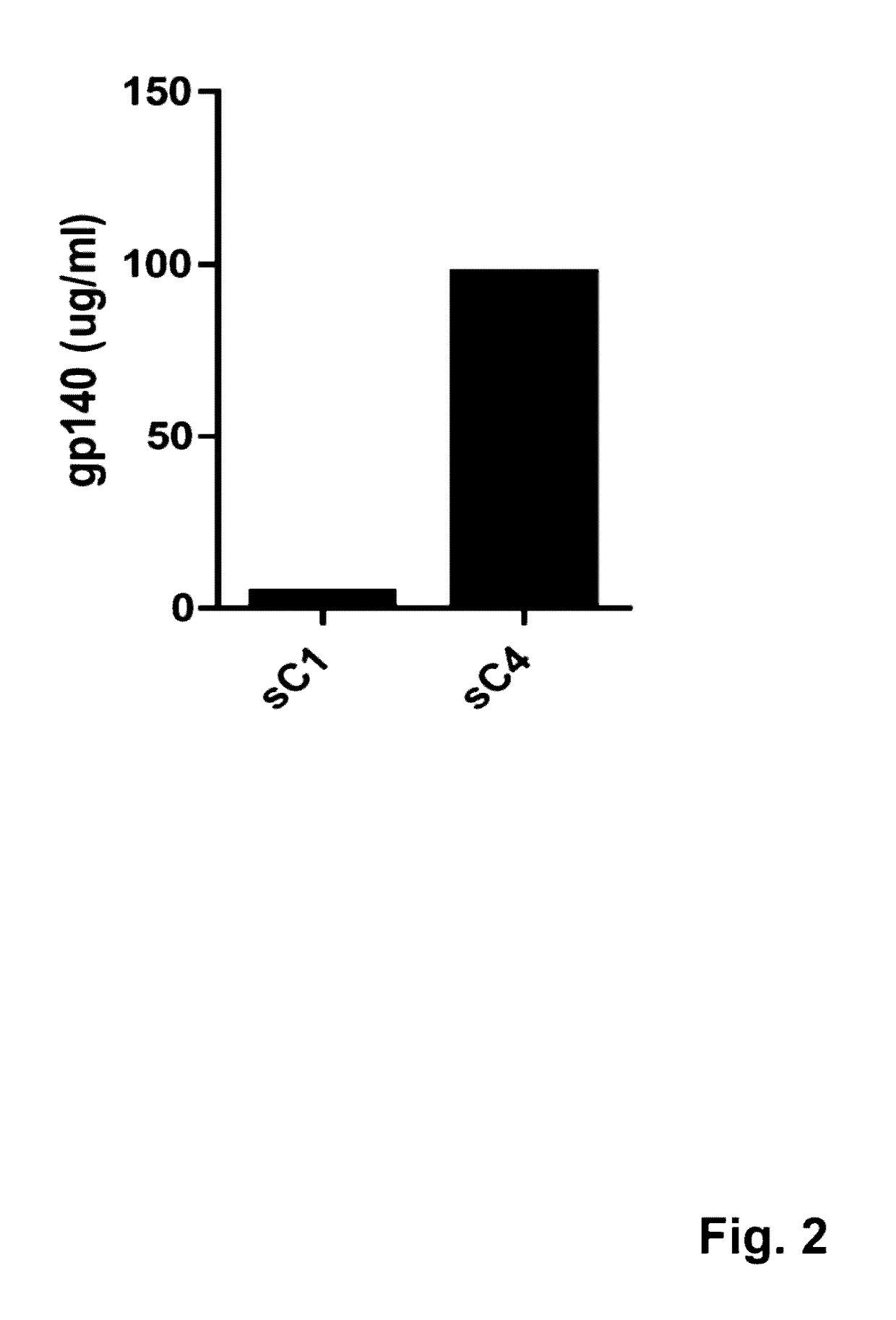
Mosaic clade M human immunodeficiency virus type 1 (HIV-1) envelope immunogens
The present invention relates to mosaic clade M HIV-1 Env polypeptides and to compositions comprising same. The polypeptides of the invention are suitable for use in inducing an immune response to HIV-1 in a human.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +3
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
ActiveUS20180064803A1Measurable immune responseMaintain viremic controlViral antigen ingredientsAntiviralsImmunodeficiency virusVaccinia
Methods for inducing an immune response against Human Immunodeficiency Virus (HIV) in HIV-infected subjects undergoing antiretroviral therapy (ART) are described. The methods include administering an adenovirus vector primer vaccine and a modified vaccinia virus (MVA) vector booster vaccine encoding mosaic HIV antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +3
Method for the development of an HIV vaccine
InactiveUS6503753B1Reduce riskSource securityAnimal cellsViral antigen ingredientsImmunodeficiency virusReverse transcriptase
Human immunodeficiency virus (HIV) comprising reverse transcriptase inactivated by photoinactivation. The inactivated virus may be more safely handled, stored, and analyzed, used in diagnostic procedures and kits, and may be used as an immunogen to evoke an immune response. The immune response may protect an individual from challenges with live virus. Alternatively, the inactivated HIV particles may be used to augment the immune response to HIV in an infected individual.
Owner:PHOTOIMMUNE BIOTECH
Compositions and methods for treating viral infections
InactiveUS6258599B1Neutralize and inactivate essential stepReduce infectivityPeptide/protein ingredientsVirus peptidesEpitopeImmunodeficiency virus
Owner:PROBE INT
Particle-bound human immunodeficiency virus envelope glycoproteins and related compositions and methods
InactiveUS20060051373A1Inhibition of growth rateSlow growth rateBiocideOrganic active ingredientsParticulate antigenPharmaceutical medicine
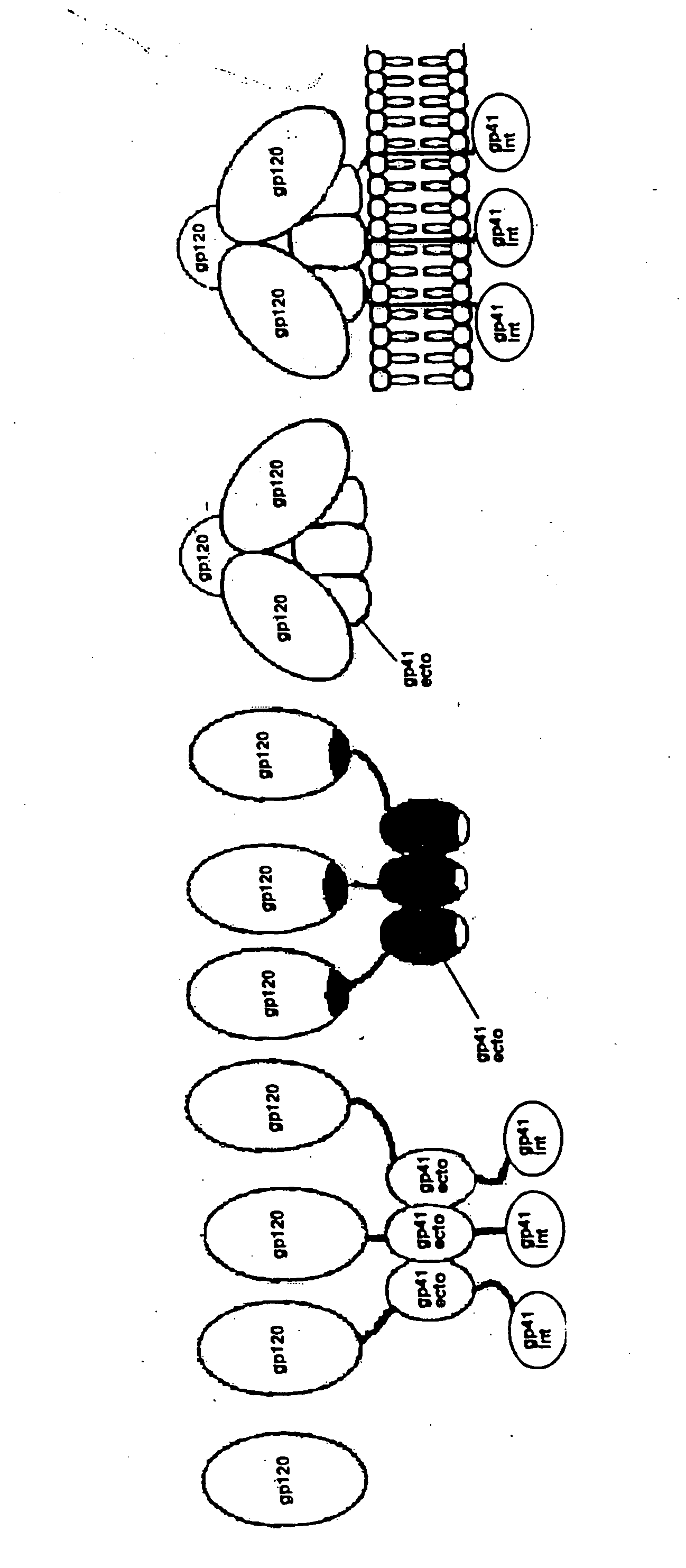
This invention provides a first composition comprising a pharmaceutically acceptable particle and a stable HIV-1 pre-fusion envelope glycoprotein trimeric complex operably affixed thereto. This invention further provides a second composition comprising (a) a pharmaceutically acceptable particle, (b) an antigen, and (c) an agent which is operably affixed to the particle and is specifically bound to the antigen, whereby the antigen is operably bound to the particle. Finally, this invention provides related nucleic acids, vectors, cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:PROGENICS PHARMA INC
Combined therapy for treatment of HIV infection
InactiveUS7094413B2Good curative effectReduce resistanceBiocideSugar derivativesImmunodeficiency virusGastrointestinal complications
The present invention relates to pharmaceutical preparations and methods for treating individuals infected with the human immunodeficiency virus (HIV). The pharmaceutical preparations comprise an immunomodulating agent and a anti-retroviral compound. The pharmaceutical preparations are used to treat HIV infected patients, particularly for gastrointestinal complications arising from viral infection. In addition, the pharmaceutical preparations of the present invention have the effect of raising the levels of CD4+ single positive and CD4+ and CD8+ double positive T cells, thus promoting restoration and normalization of the immune system following HIV infection.
Owner:SANGSTAT MEDICAL +1
Chimeric HIV Antigens
The invention provides polynucleotides and polypeptides encoded therefrom that are capable of inducing immune responses to a human immunodeficiency virus. Compositions and methods for utilizing polynucleotides and polypeptides of the invention are also provided.
Owner:DU XIAOHAN +3
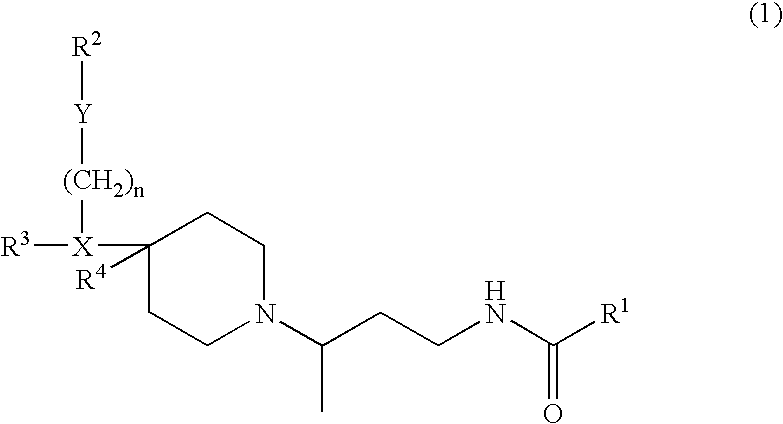
Compound Containing Basic Group and Use Thereof
InactiveUS20080009495A1Less effectPrevention and therapyBiocideSenses disorderSolventCompound (substance)
The present invention relates to a compound represented by formula (I-0): wherein symbols in formula have the same meanings as described in the present specification, a salt thereof, an N-oxide thereof or a solvate thereof or a prodrug thereof, and medical use thereof. The compound of the present invention has an antagonistic activity against CXCR4 and is therefore useful as a preventive and / or therapeutic agent for CXCR4-mediated diseases, for example, inflammatory and immune diseases (for example, rheumatoid arthritis, arthritis, retinopathy, pulmonary fibrosis, transplanted organ rejection, etc.), allergic diseases, infections (for example, human immunodeficiency virus infection, acquired immunodeficiency syndrome, etc.), psychoneurotic diseases, cerebral diseases, cardiovascular disease, metabolic diseases, and cancerous diseases (for example, cancer, cancer metastasis, etc.), or an agent for regeneration therapy.
Owner:ONO PHARMA
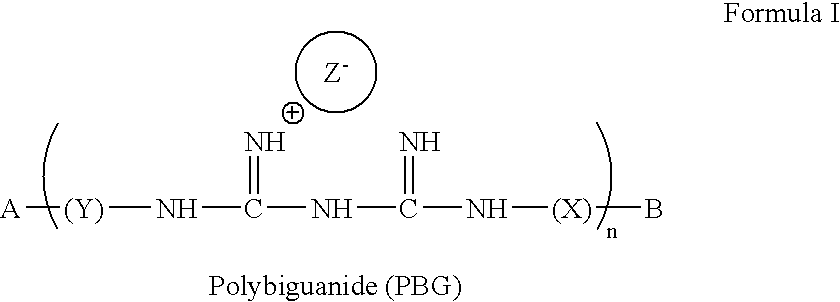
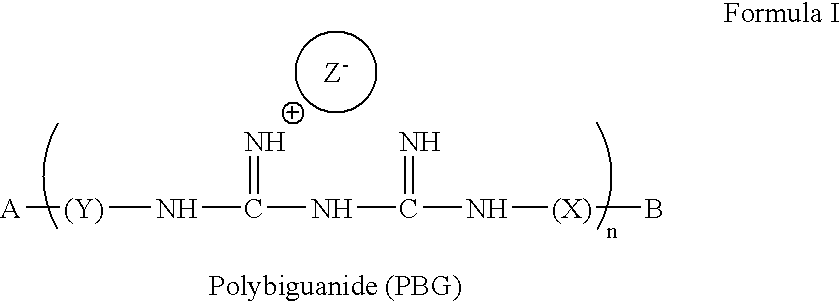
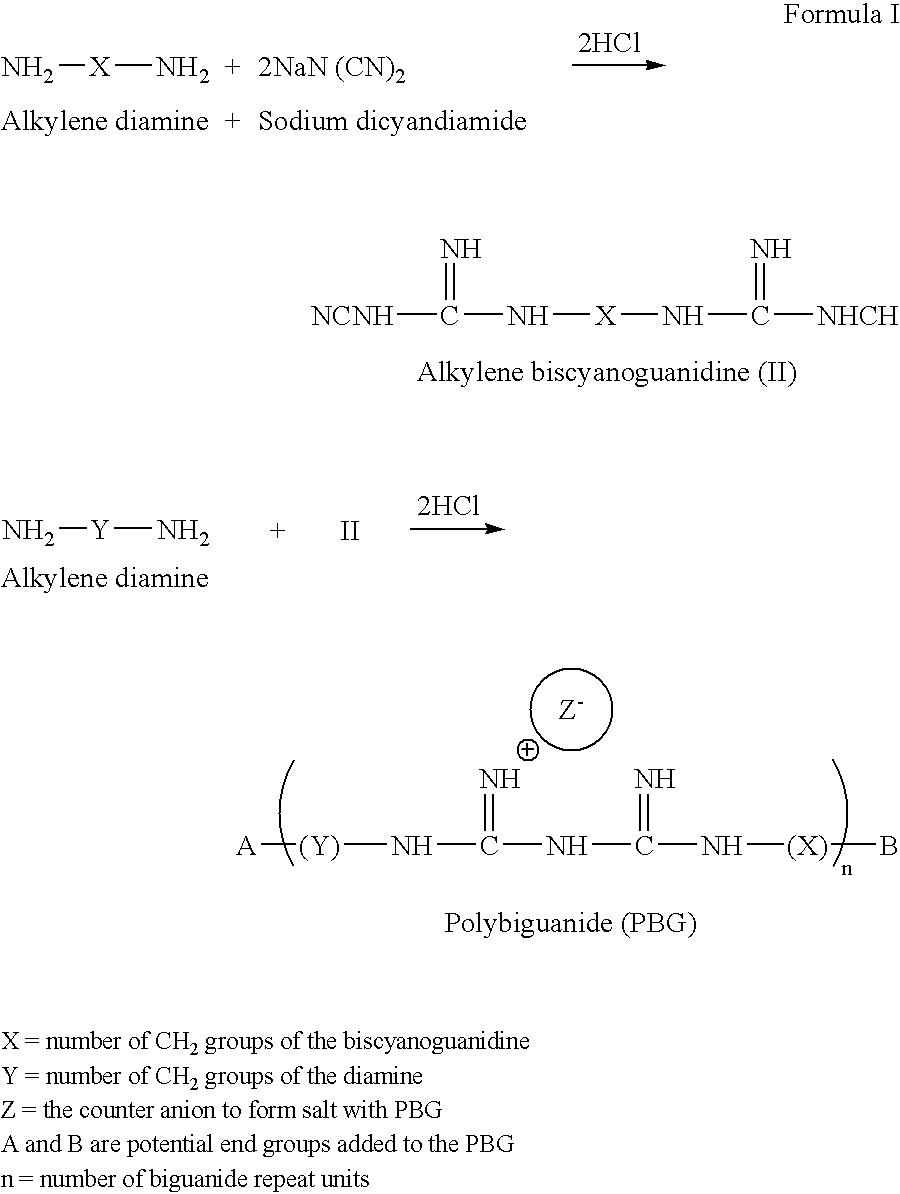
Method for the treatment or prevention of virus infection using polybiguanide-based compounds
InactiveUS20040009144A1High activityBroaden applicationAntibacterial agentsBiocideDiseasePathogenic microorganism
An inexpensive, easily available, and convenient method of treating or preventing a virus infection is provided. The present invention relates to a method for the treatment or prevention of virus infections using polybiguanide-based compounds administering a therapeutically effective amount of a compound or a pharmaceutically acceptable salt thereof. The invention relies on the unique biochemical reaction in which polybiguanide-based compounds interfere with the spread of virus within or between organisms. The compositions and formulations described in the present invention are effective means to reduce the infectivity of the human immunodeficiency virus type 1 (HIV-1), and human herpes simplex viruses, and also to kill the causative organisms of many other sexually transmitted diseases (STDs).
Owner:NOVAFLUX INC
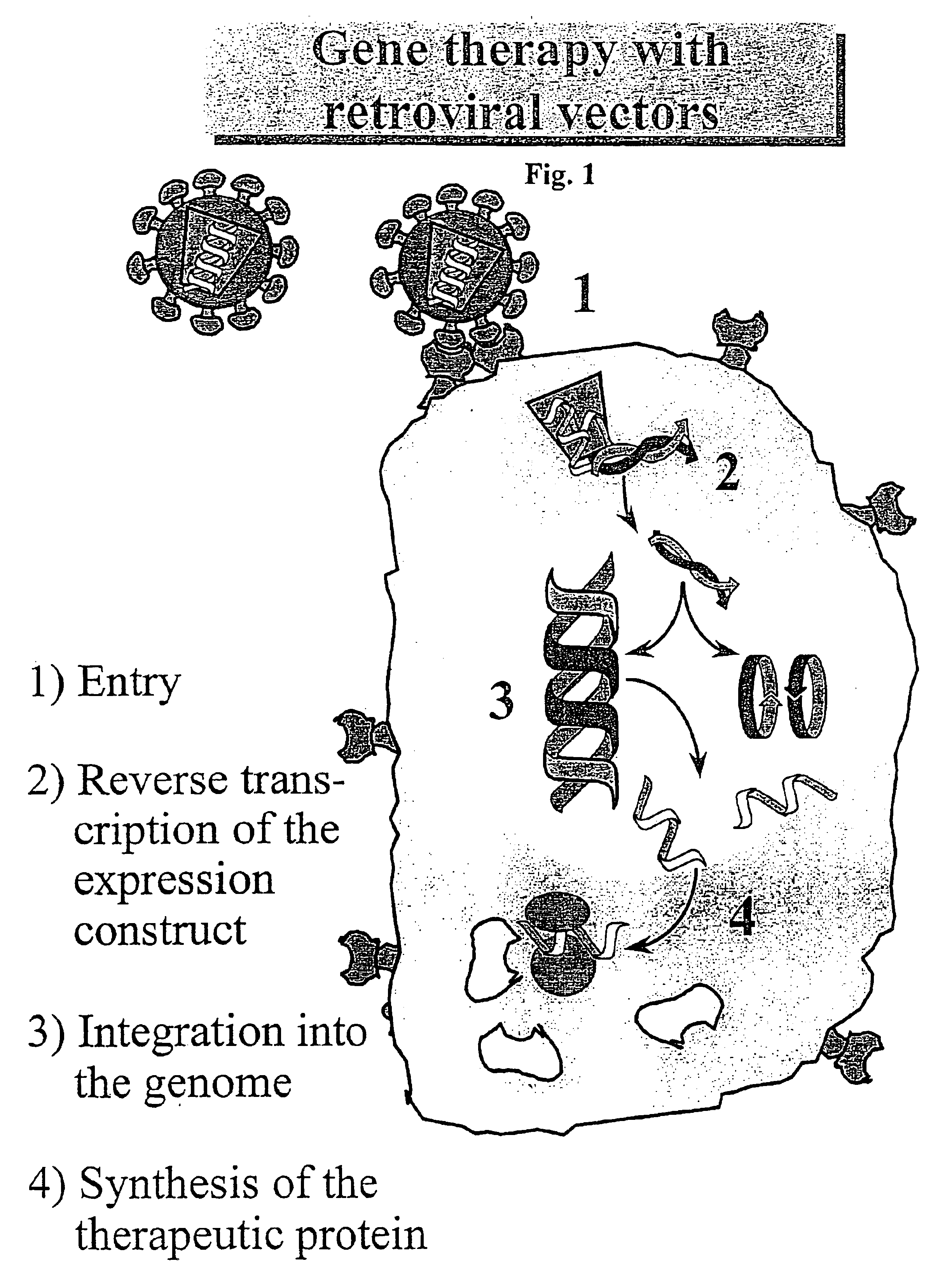
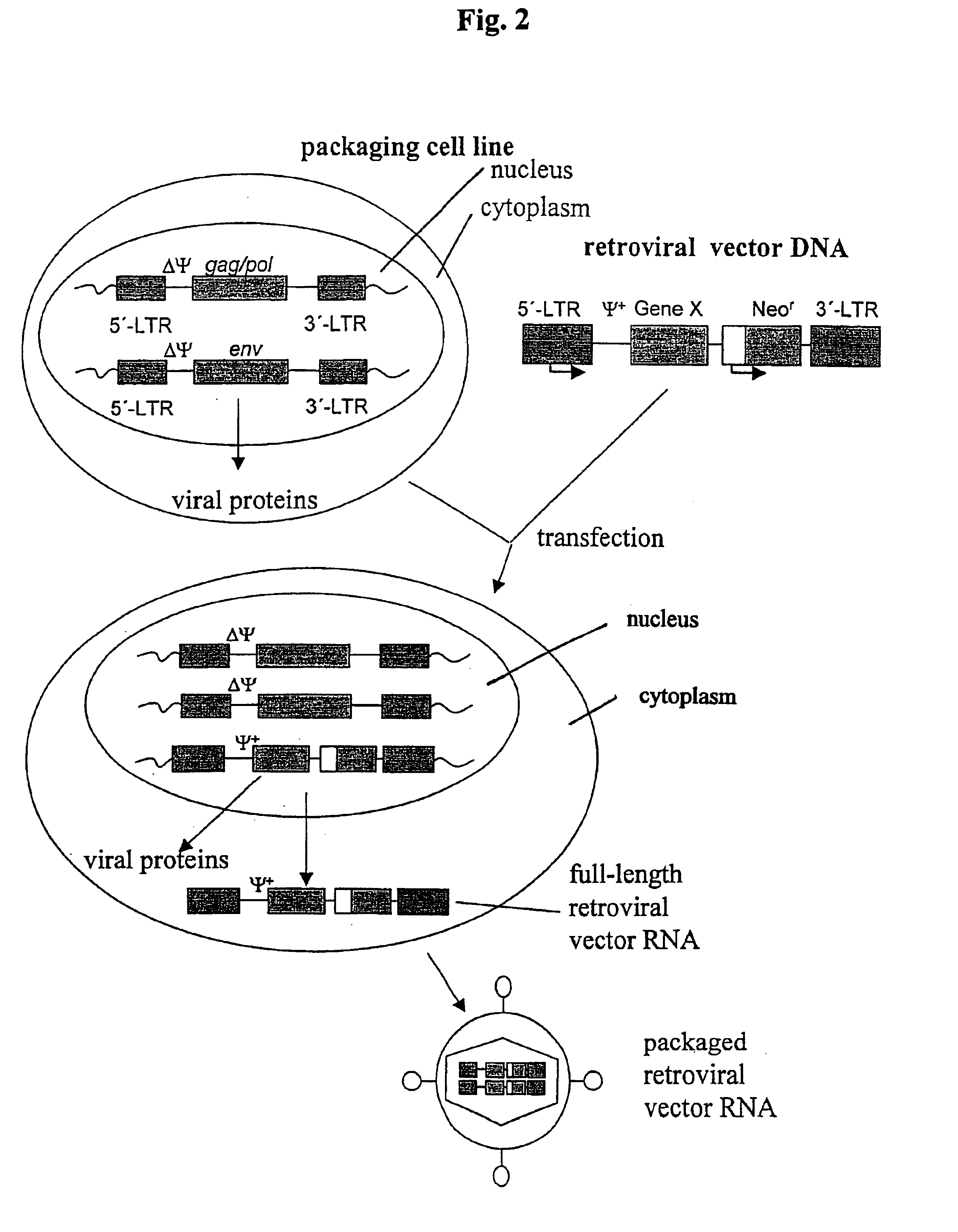
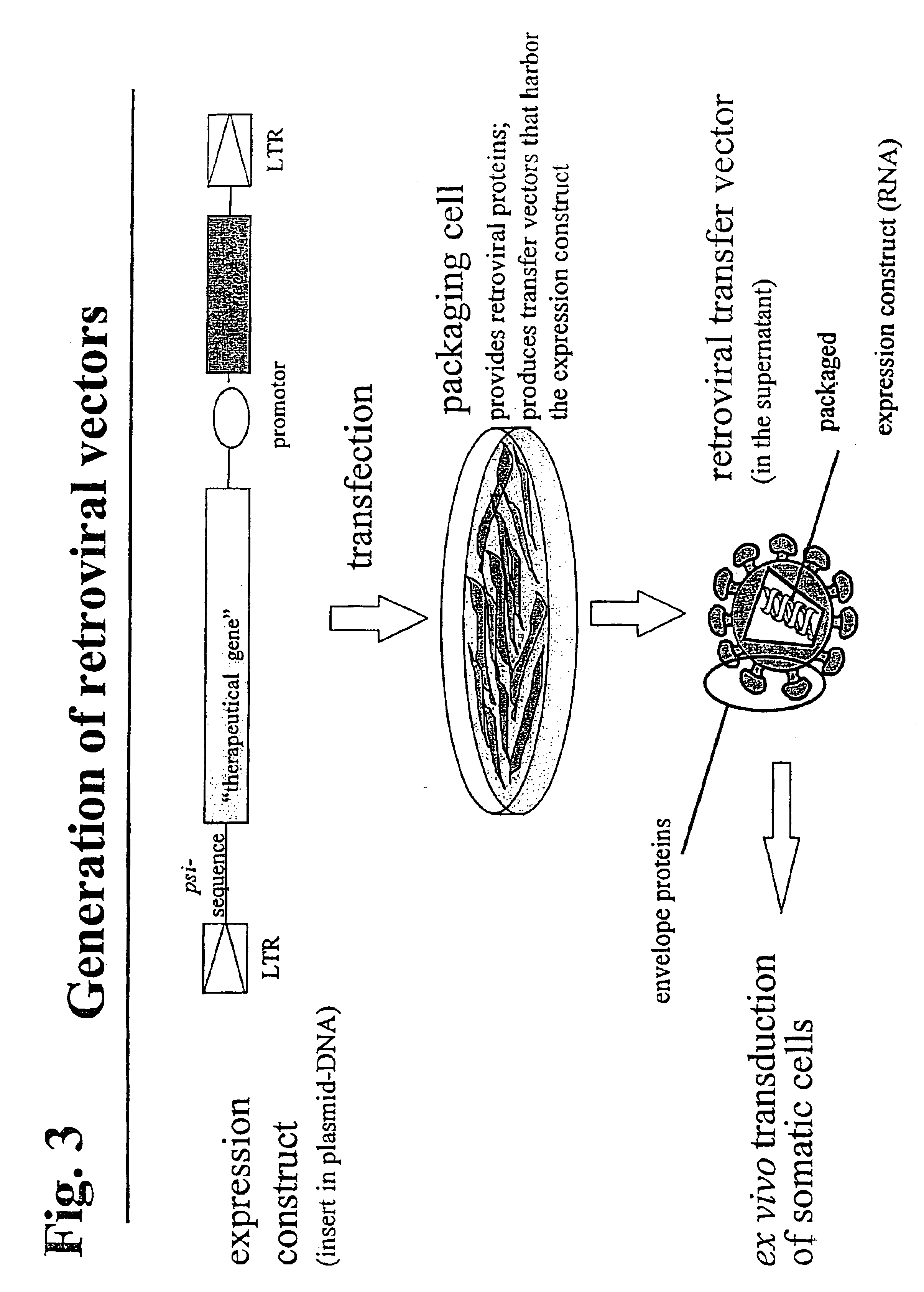
Retroviral vectors, methods for their preparation and their use for gene transfer into CD4-positive cells
InactiveUS6902929B1Improve efficiencyEasy constructionBiocideGenetic material ingredientsCell specificImmunodeficiency virus
The invention relates to the production and use of retroviral vectors for cell specific gene transfer, specially to a production method of retroviral vectors containing capsid particles of murine leukemia virus (MLV) and envelope proteins of human immunodeficiency vises (HIV) or simian immunodeficiency viruses (SIV). Said vectors can be used for gene transfer in selected cell types, specially in CD4-positive mammal cells.
Owner:BUNDESREPUBLIK DEUTLAND LETZTVERTRETEN DURCH DEN PRASIDENTEN DES PAUL EHRLICH INSTITUTS
Beta-L-N4-Hydroxycytosine Deoxynucleosides and their use as Pharmaceutical Agents in the Prophylaxis or Therapy of Viral Diseases
The invention relates to β-L-N4-hydroxycytosine nucleo-sides, pharmaceutical agents comprising same, and to the use of said β-L-N4-hydroxycytosine nucleosides and pharmaceutical agents in the prophylaxis or therapy of an infection caused by hepatitis B virus (HBV) or human immunodeficiency virus (HIV). The invention also relates to a method for the preparation of said β-L-nucleoside analogs.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
Solid of tenofovir disoproxil, and preparation method and application thereof
ActiveCN103626803AEasy to prepareCrystal form controllableOrganic active ingredientsGroup 5/15 element organic compoundsMedicineHepatitis B virus
The invention relates to a solid of tenofovir disoproxil. The solid is (1) a tenofovir disoproxil compound represented by a formula IV or (2) a tenofovir disoproxil cocrystal or salt represented by a formula V. The invention further relates to a preparation method for the solid of tenofovir disoproxil, a pharmaceutical composition containing the solid and application of the solid in preparation of drugs used for preventing and / or treating virus infection, especially hepatitis b virus (HBV) and / or human immunodeficiency virus (HIV) infection.
Owner:SICHUAN HAISCO PHARMA CO LTD
Water dispersible film
InactiveUS20050070501A1Avoid difficultyAntibacterial agentsOrganic active ingredientsDiseaseComposite film
Owner:NEW YORK BLOOD CENT
Basic group-containing compound and use thereof
A compound represented by general formula (I):a salt thereof, a solvate thereof, or a prodrug thereof wherein all symbols are as defined in the specification has an antagonistic activity against CXCR4 and is therefore useful as a preventive and / or therapeutic agent for CXCR4-mediated diseases, for example, inflammatory and immune diseases (for example, rheumatoid arthritis, arthritis, systemic erythematosus, retinopathy, macular degeneration, pulmonary fibrosis, transplanted organ rejection, etc.), allergic diseases, infections (for example, human immunodeficiency virus infection, acquired immunodeficiency syndrome, etc.), psychoneurotic diseases, cerebral diseases, cardiac / vascular disease (for example, arteriosclerosis, myocardial infarction, stenocardia, cerebral infarction, chronic arterial occlusive disease, etc.), metabolic diseases, and cancerous diseases (for example, cancer, cancer metastasis, etc.), a preventive and / or therapeutic agent for cancerous diseases or infections, or an agent for regeneration therapy.
Owner:ONO PHARMA
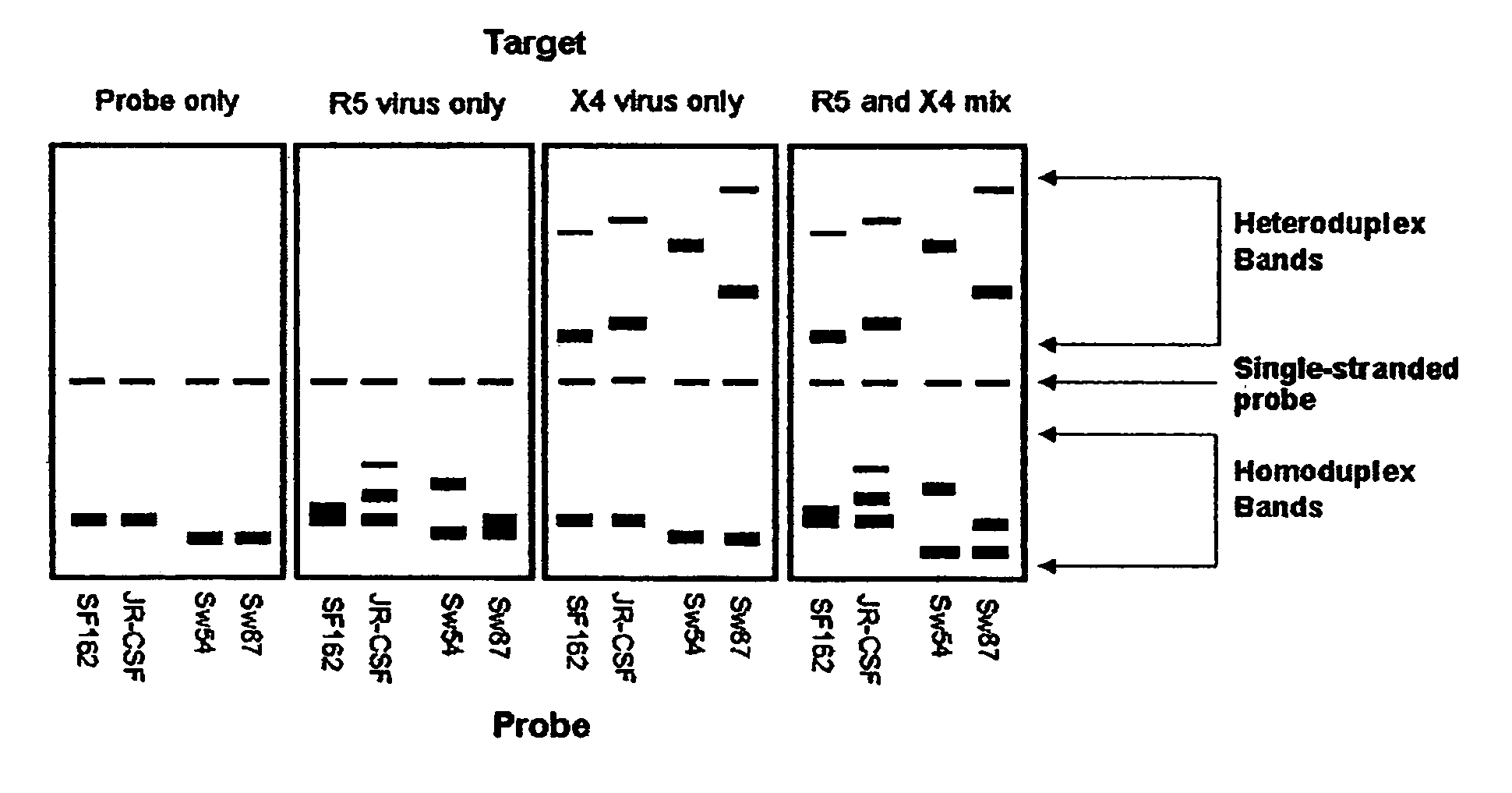
Heteroduplex tracking assay
InactiveUS20060194227A1Accurately predict disease prognosis over timeGood treatment effectMicrobiological testing/measurementVertebrate cellsHeterologousHeteroduplex
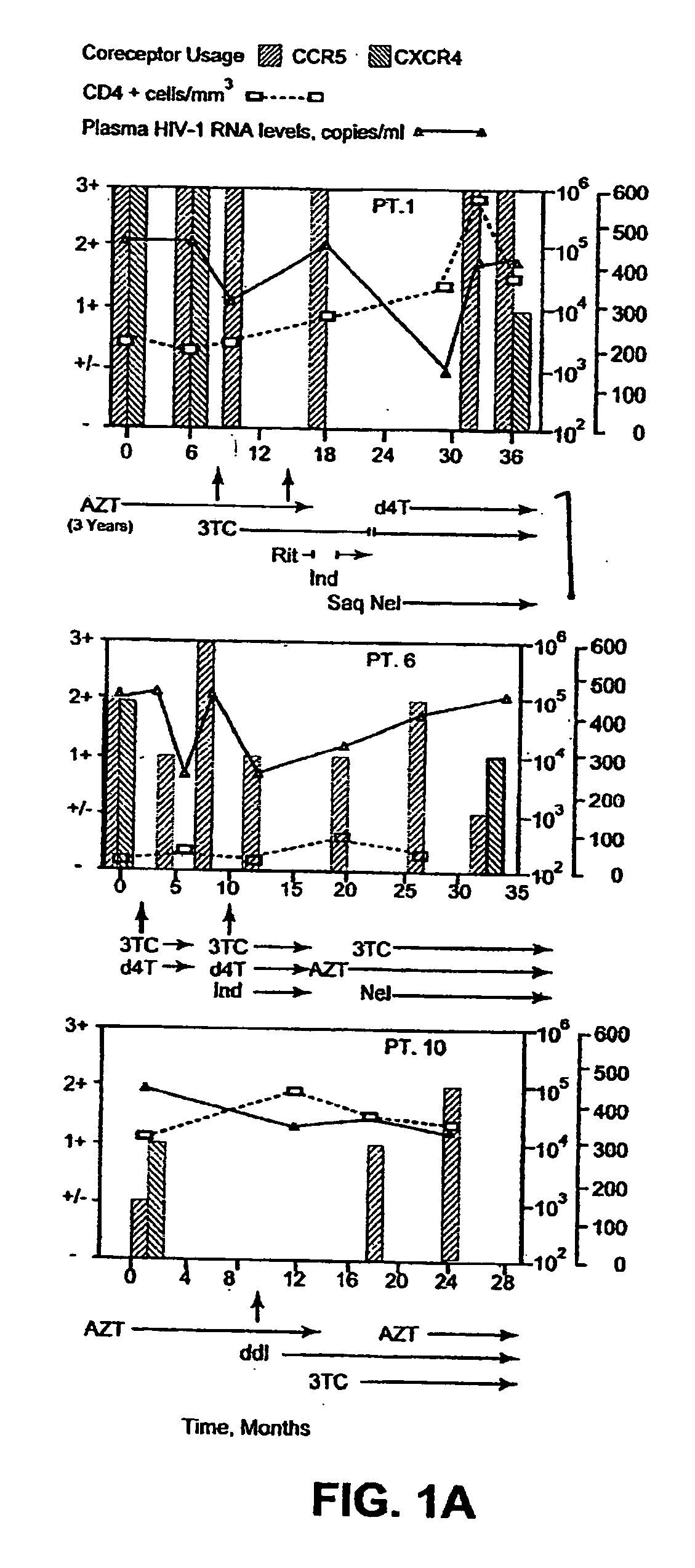
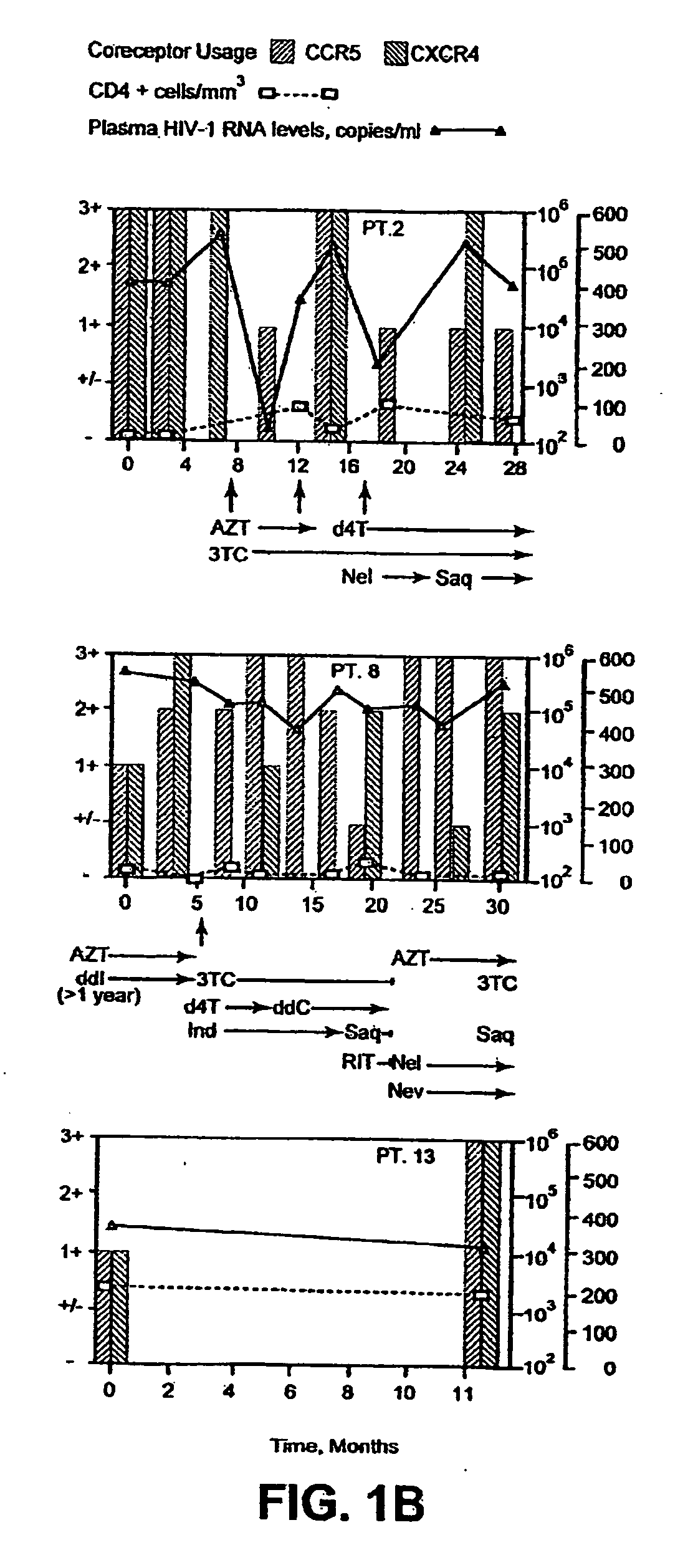
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
Human immunodeficiency virus antigens, vectors, compositions, and methods of use thereof
ActiveUS20170165355A1Improved cell surface expressionImproved expression stabilityViral antigen ingredientsVirus peptidesAntigenHiv envelope
Synthetic HIV envelope proteins, vectors and compositions thereof, and methods for inducing protective immunity against human immunodeficiency virus (HIV) infection are described. Viral expression vectors encoding the synthetic HIV envelope proteins can be used in vaccines to provide improved protective immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for the development of an HIV vaccine
InactiveUS20030104011A1Reduce riskSeriousness of riskAnimal cellsViral antigen ingredientsReverse transcriptaseHIV vaccine
Owner:PHOTOIMMUNE BIOTECH
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
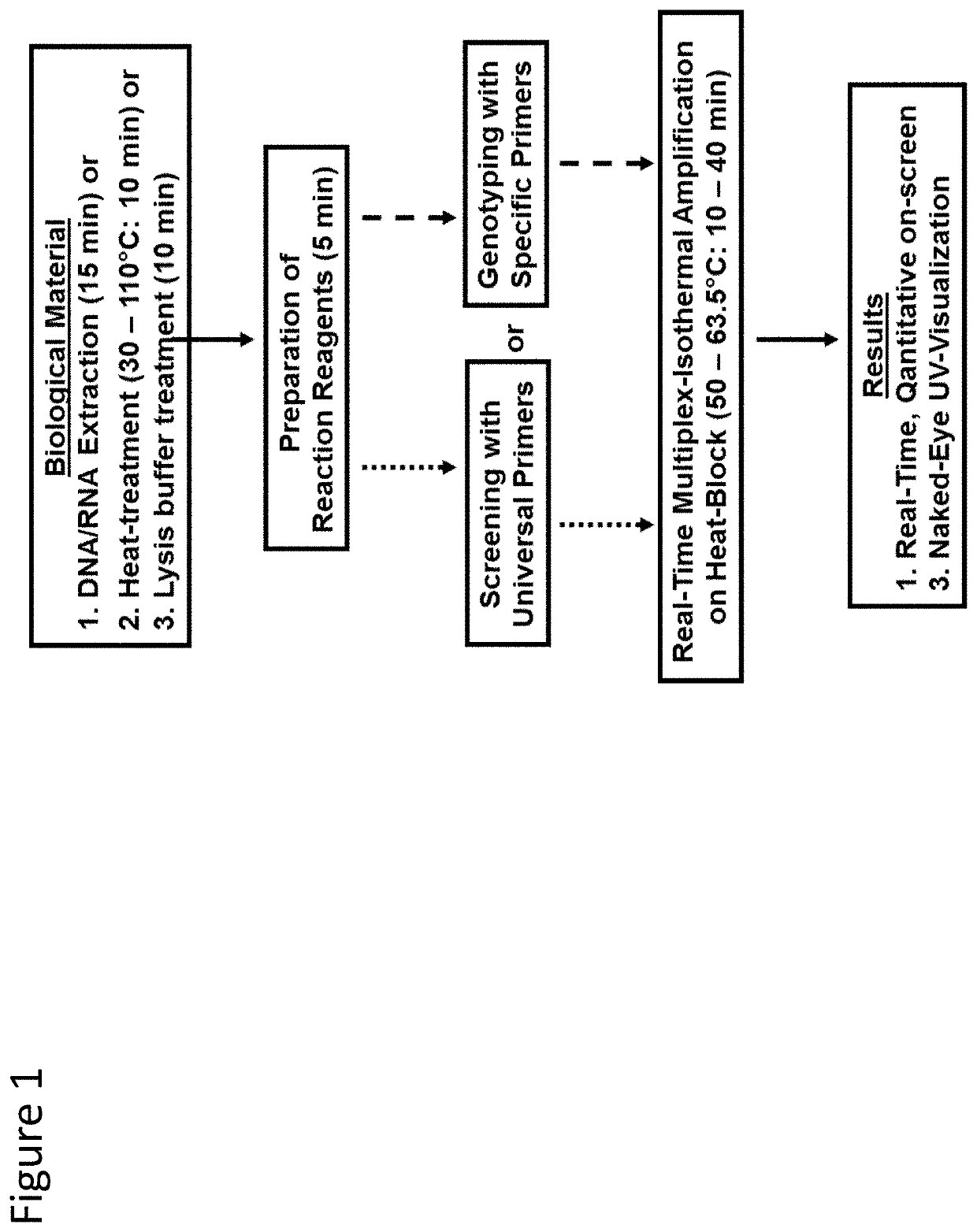
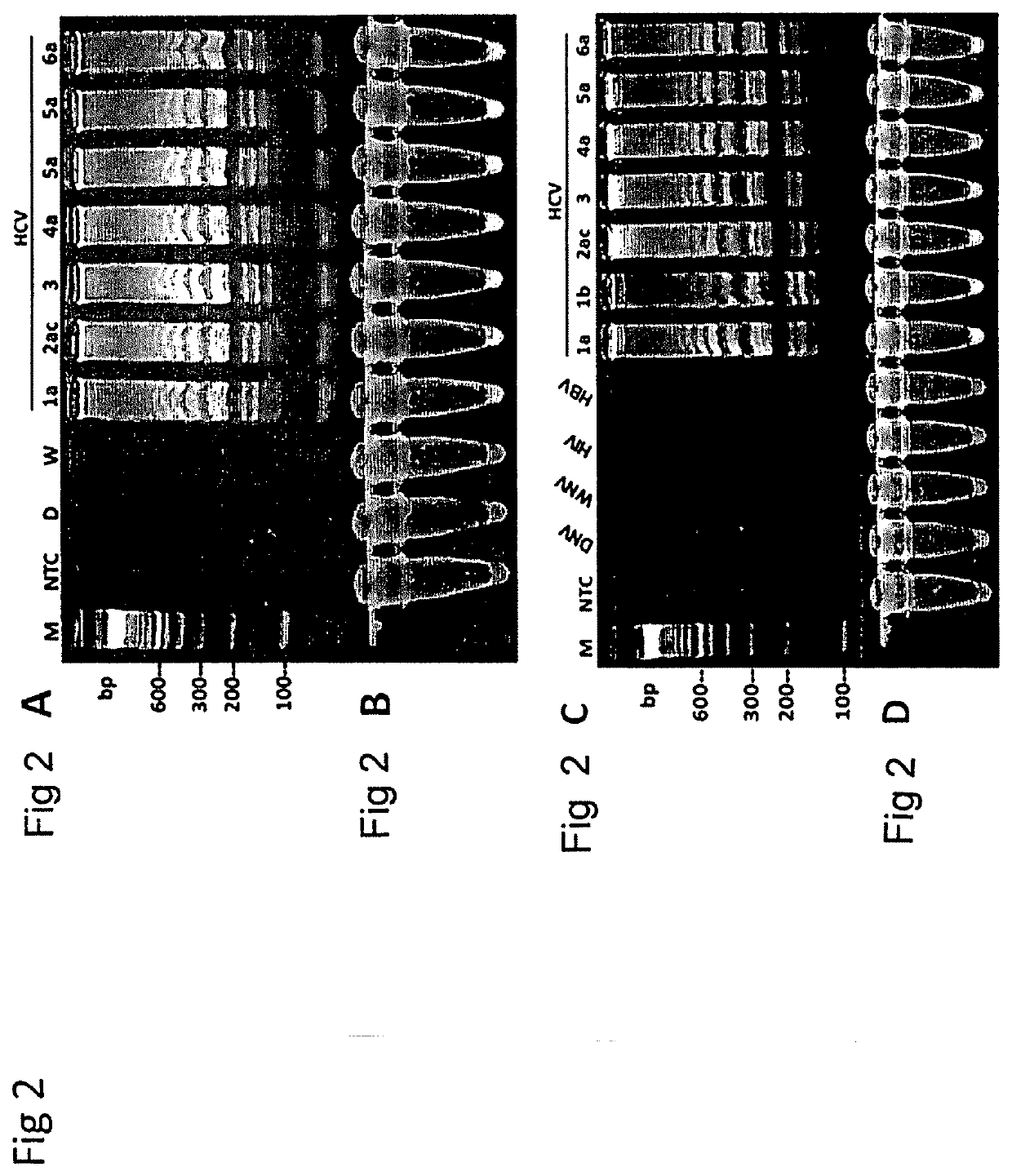
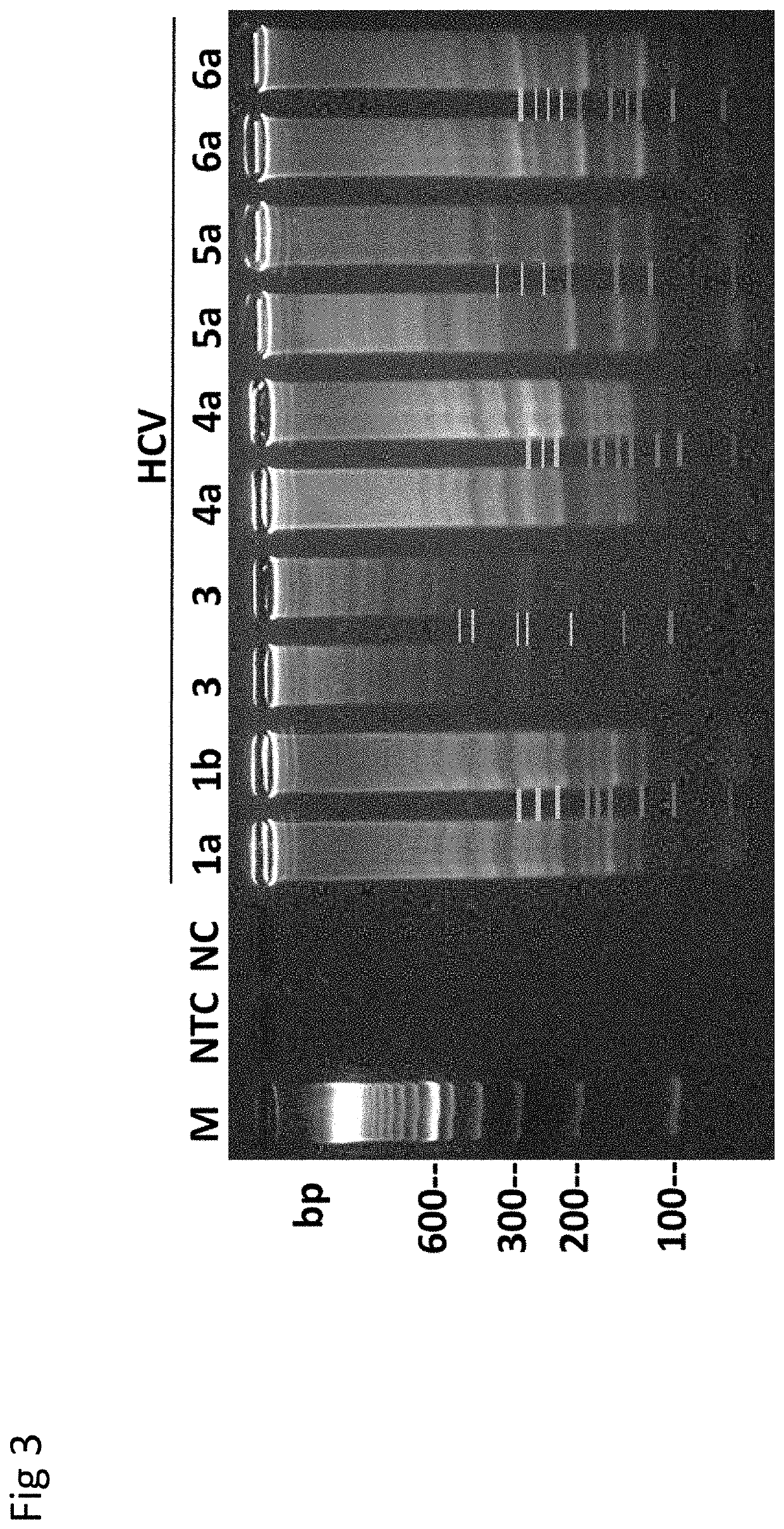
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
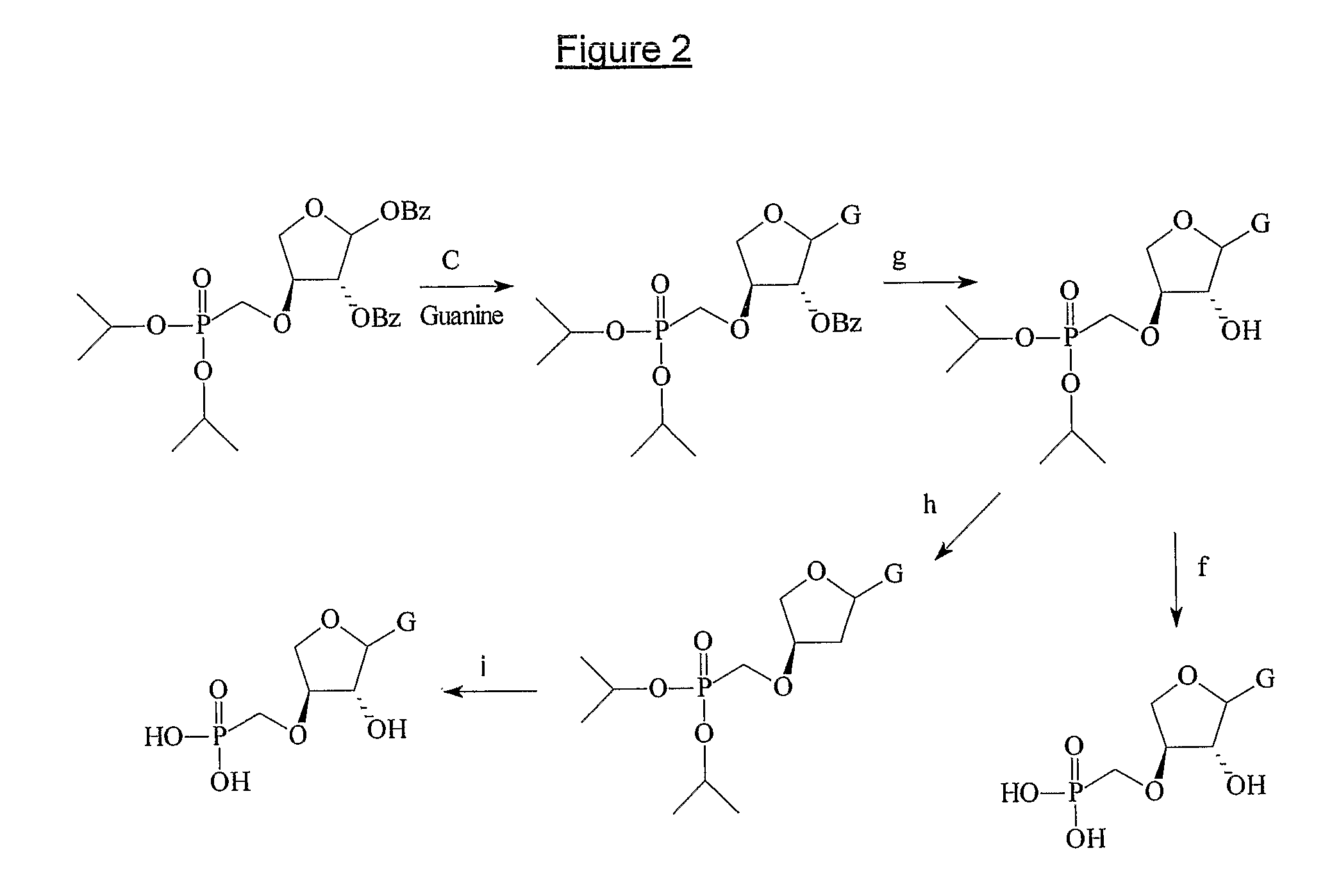
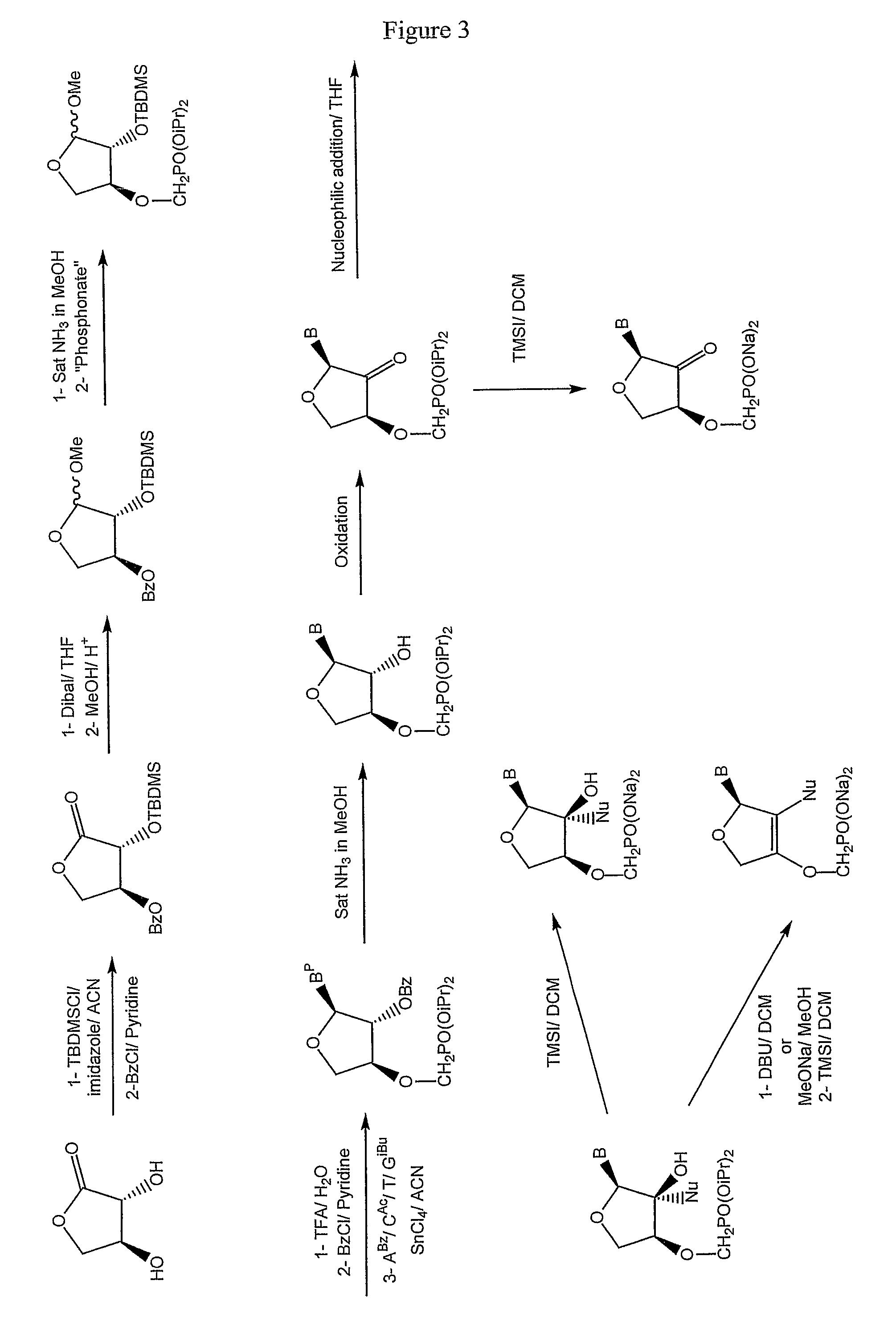
Phosphonate nucleosides useful as active ingredients in pharmaceutical compositions for the treatment of viral infections, and intermediates for their production
InactiveUS8076310B2Easy to tunePoor propertyBiocideAntibiotics chemistryNucleobaseBULK ACTIVE INGREDIENT
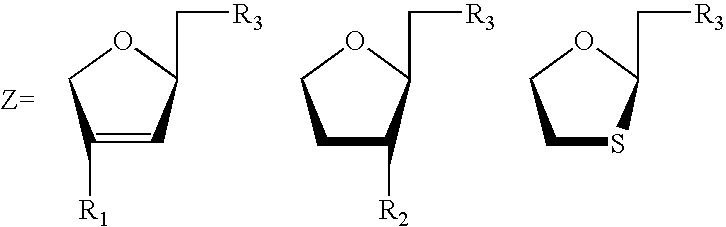
Disclosed herein are novel phosphonate nucleosides and thiophosphonate nucleosides comprising a phosphonalkoxy-substituted or phosphonothioalkyl-substituted five-membered, saturated or unsaturated, oxygen-containing or sulfur-containing ring coupled to a heterocyclic nucleobase such as a pyrimidine or purine base. The invention further relates to compounds having HIV (Human Immunodeficiency Virus) replication inhibiting properties and to compounds having antiviral activities with respect to other viruses. The invention also relates to methods for preparation of all such compounds and pharmaceutical compositions comprising them. The invention further relates to the use of said compounds as a medicine and in the manufacture of a medicament useful for the treatment of subjects suffering from HIV infection, as well as for treatment of other viral, retroviral or lentiviral infections and to the treatment of animals suffering from FIV, viral, retroviral or lentiviral infections.
Owner:K U LEUVEN RES & DEV
Chemokine receptor binding compounds
InactiveUS20050277670A1Improve the level ofInhibit bindingBiocideOrganic chemistryPharmaceutical drugVirus
The present invention relates to chemokine receptor binding compounds, pharmaceutical compositions and their use. More specifically, the present invention relates to modulators of chemokine receptor activity, preferably modulators of CCR5. These compounds demonstrate protective effects against infection of target cells by a human immunodeficiency virus (HIV).
Owner:GENZYME CORP
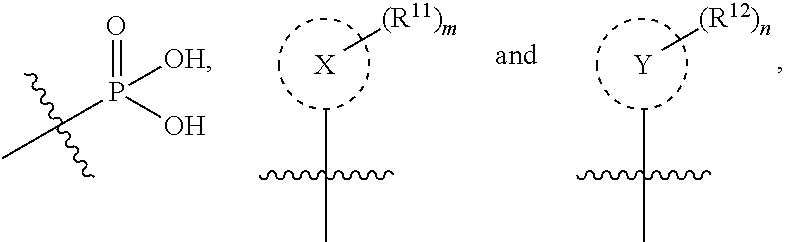
Nucleobase phosphonate analogs for antiviral treatment
ActiveUS20050059637A1Increasing cellular accumulationImprove retentionBiocideOrganic active ingredientsHigh concentrationAcquired immunodeficiency
The present invention provides novel compounds with activity against infectious viruses. The compounds of the invention may inhibit retroviral reverse transcriptases and thus inhibit the replication of the virus. They are useful for treating human patients infected with a human retrovirus, such as human immunodeficiency virus (strains of HIV-1 or HIV-2) or human T-cell leukemia viruses (HTLV-I or HTLV-II) which results in acquired immunodeficiency syndrome (AIDS) and / or related diseases. The present invention also relates generally to the accumulation or retention of therapeutic compounds inside cells. The invention is more particularly related to attaining high concentrations of active metabolite molecules in HIV infected cells. Intracellular targeting may be achieved by methods and compositions which allow accumulation or retention of biologically active agents inside cells. Such effective targeting may be applicable to a variety of therapeutic formulations and procedures.
Owner:GILEAD SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com