Patents
Literature
101 results about "HIV Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antigens associated with specific proteins of the human adult T-cell immunodeficiency virus (HIV); also called HTLV-III-associated and lymphadenopathy-associated virus (LAV) antigens.
Methods and compositions for treatment of human immunodeficiency virus infection with conjugated antibodies or antibody fragments
ActiveUS20070264265A1Avoid infectionReduce eliminateOrganic active ingredientsAntiviralsDiagnostic agentBinding site
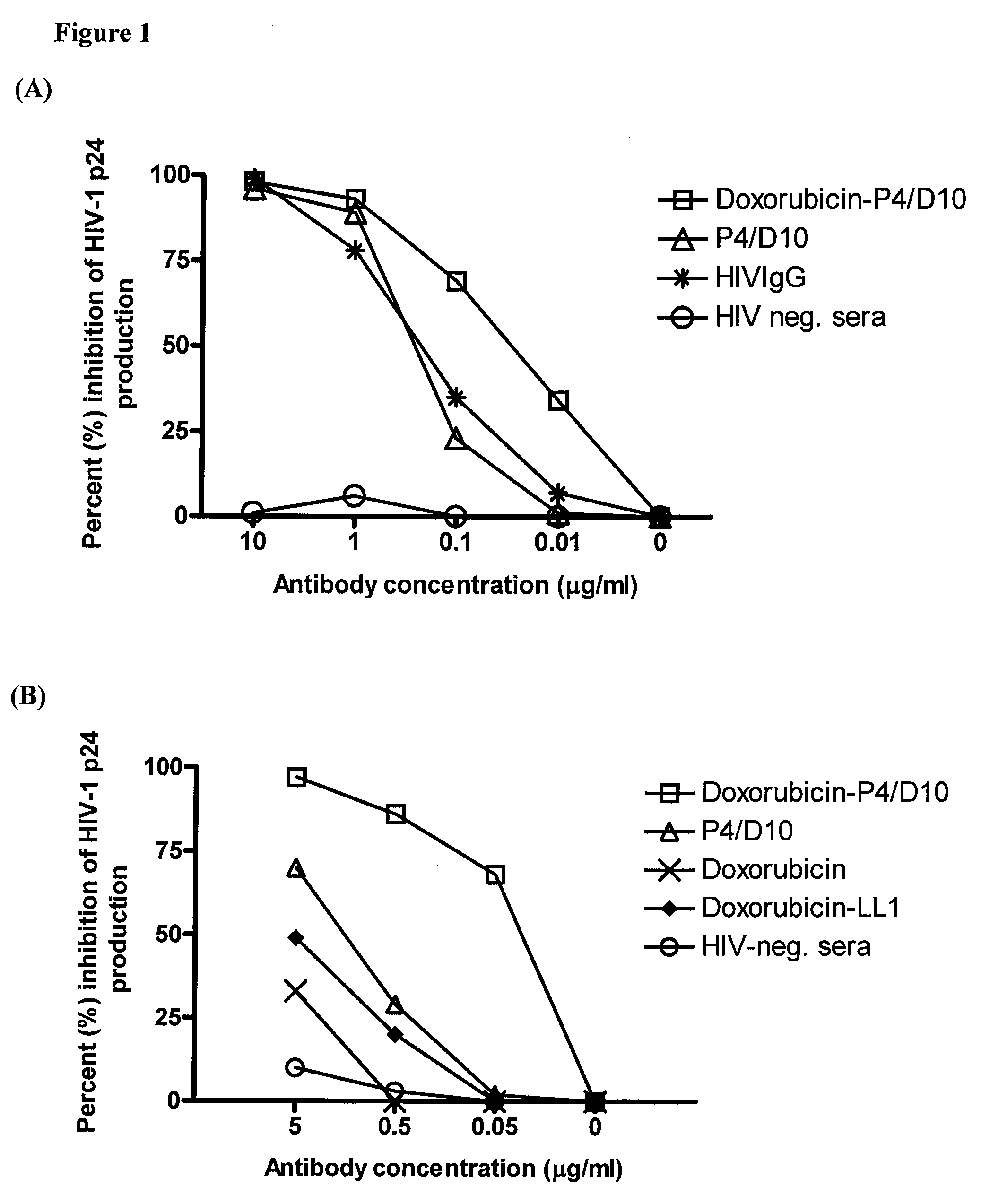
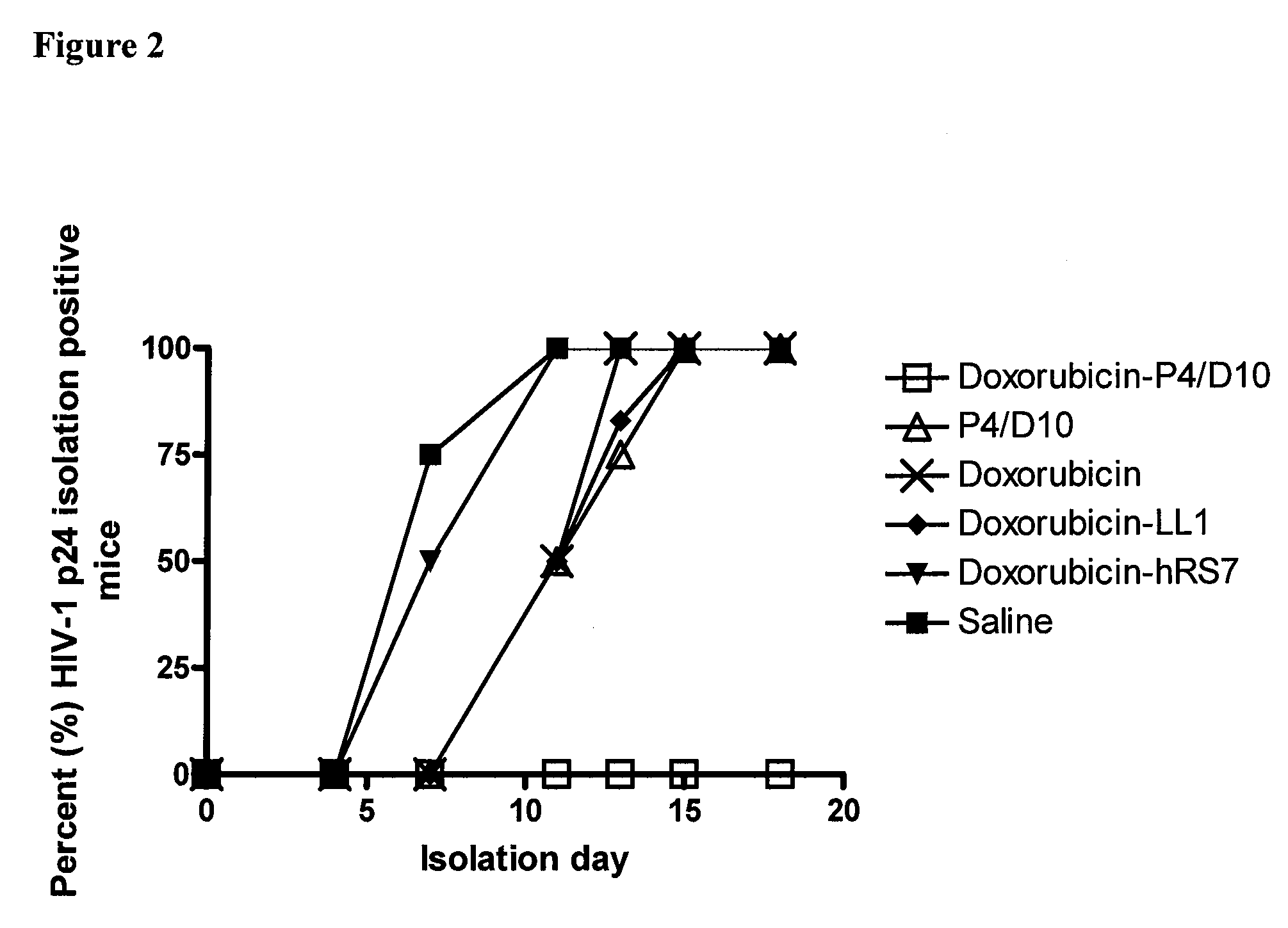
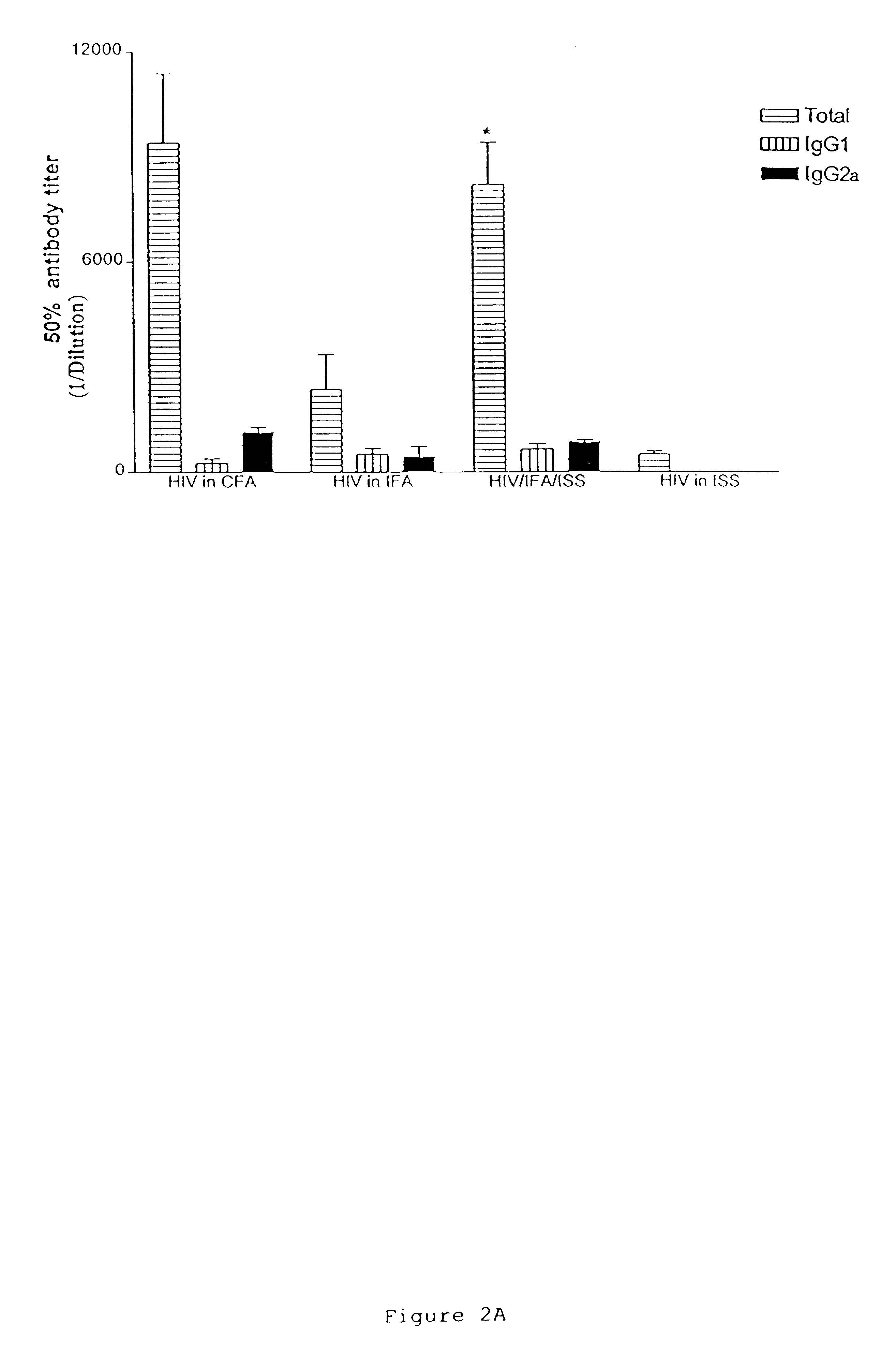
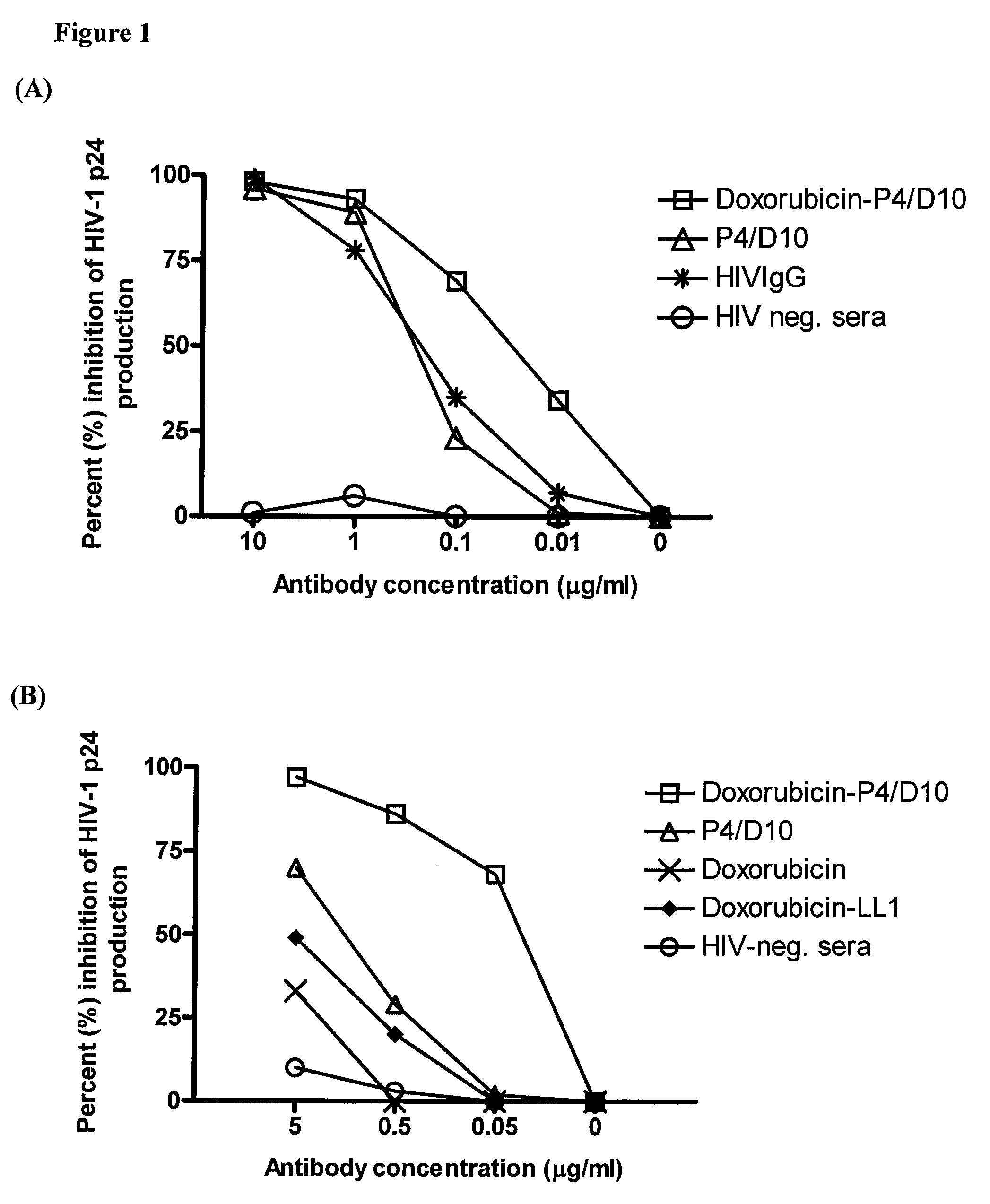
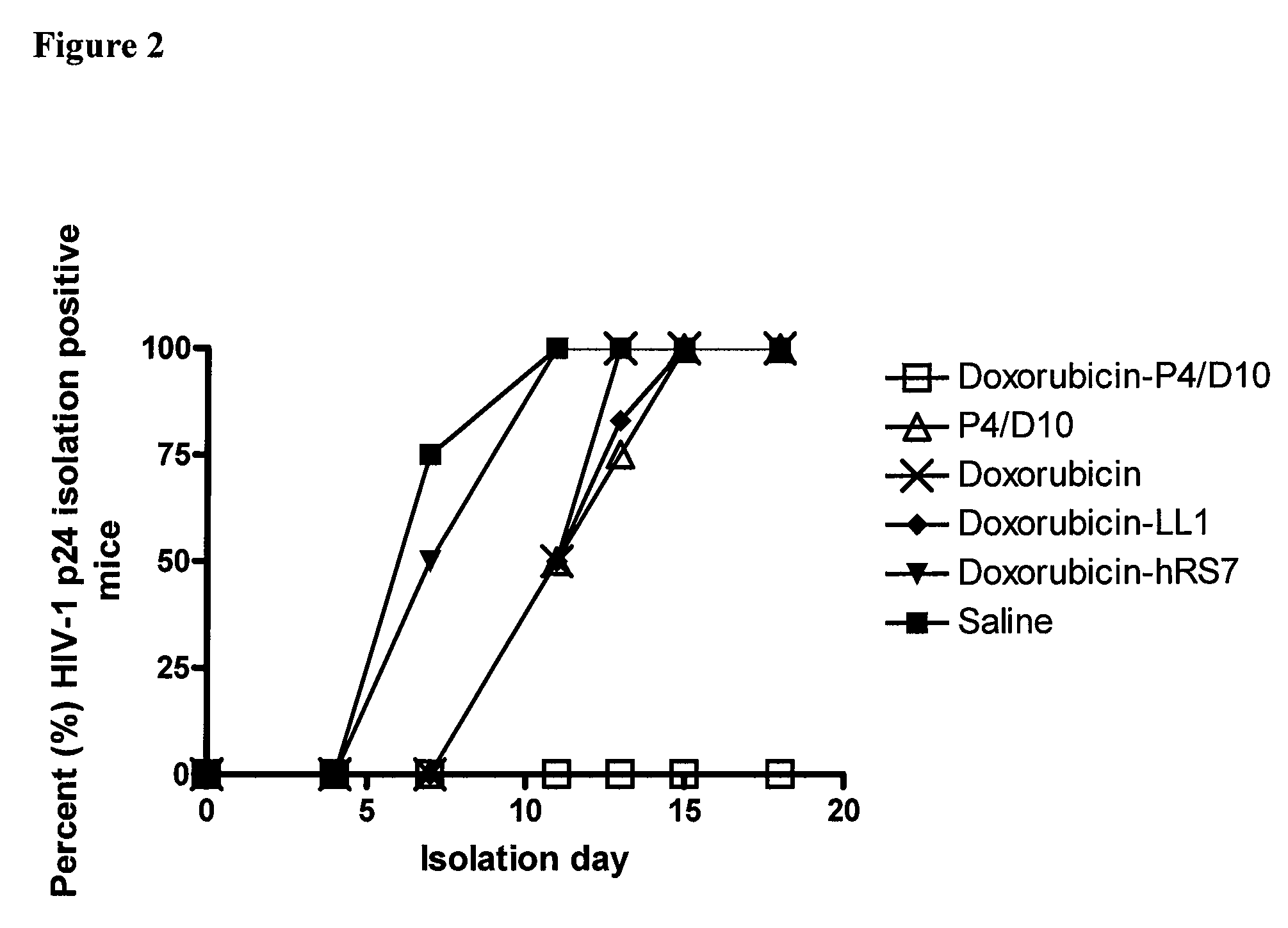
The present invention concerns methods and compositions for treatment of HIV infection in a subject. The compositions may comprise a targeting molecule against an HIV antigen, such as an anti-HIV antibody or antibody fragment. The anti-HIV antibody or fragment may be conjugated to a variety of cytotoxic agents, such as doxorubicin. In a preferred embodiment, the antibody or fragment is P4 / D10. Other embodiments may concern methods of imaging, detection or diagnosis of HIV infection in a subject using an anti-HIV antibody or fragment conjugated to a diagnostic agent. In alternative embodiments, a bispecific antibody with at least one binding site for an HIV antigen and at least one binding site for a carrier molecule may be administered, optionally followed by a clearing agent, followed by administration of a carrier molecule conjugated to a therapeutic agent.
Owner:IMMUNOMEDICS INC
HIV immunogenic compositions and methods
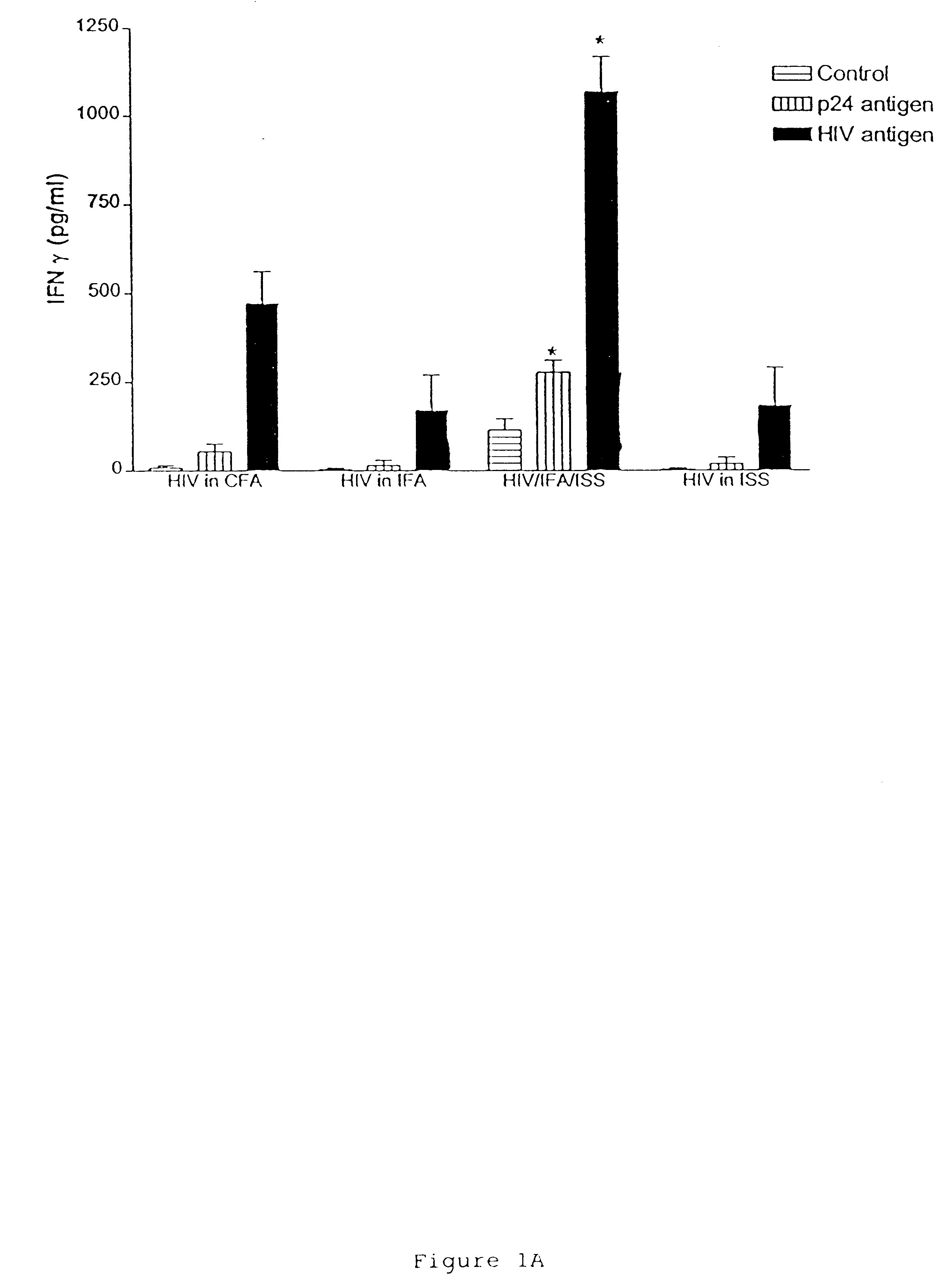
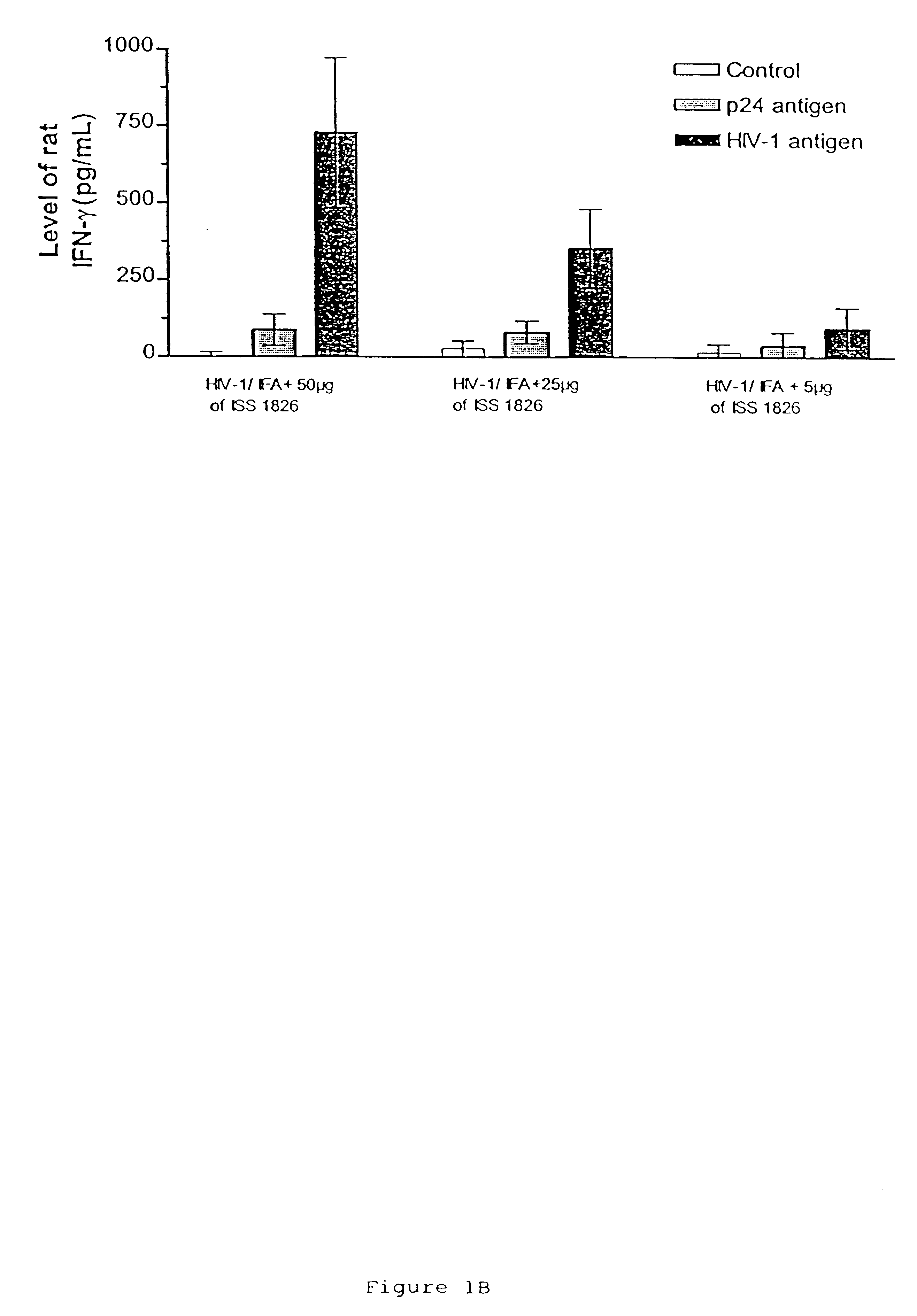
The invention provides immunogenic compositions which enhance beta-chemokine levels in a mammal. The immunogenic compositions contain an HIV antigen, an isolated nucleic acid molecule containing an immunostimulatory sequence (ISS) and an adjuvant. The HIV antigen can be a whole-killed HIV virus devoid of outer envelope protein gp120. Alternatively, the HIV antigen can be a whole-killed HIV virus, or a p24 antigen. Also provided are kits, the components of which, when combined, produce the immunogenic compositions of the invention. The invention also provides methods of making the immunogenic compositions, by combining an HIV antigen, an isolated nucleic acid molecule containing an immunostimulatory sequence (ISS) and an adjuvant. The invention further provides a method of immunizing a mammal, by enhancing beta-chemokine production in the mammal by administering to the mammal an immunogenic composition containing an HIV antigen, an isolated nucleic acid molecule containing an immunostimulatory sequence (ISS) and an adjuvant. Also provided is a method of inhibiting AIDS, by enhancing beta-chemokine production in the mammal by administering to the mammal an immunogenic composition containing an HIV antigen, an isolated nucleic acid molecule containing an immunostimulatory sequence (ISS) and an adjuvant.
Owner:THE IMMUNE RESPONSE
Methods and compositions for treatment of human immunodeficiency virus infection with conjugated antibodies or antibody fragments
ActiveUS8333971B2Avoid infectionReduce eliminateOrganic active ingredientsAntiviralsDiagnostic agentBinding site
The present invention concerns methods and compositions for treatment of HIV infection in a subject. The compositions may comprise a targeting molecule against an HIV antigen, such as an anti-HIV antibody or antibody fragment. The anti-HIV antibody or fragment may be conjugated to a variety of cytotoxic agents, such as doxorubicin. In a preferred embodiment, the antibody or fragment is P4 / D10. Other embodiments may concern methods of imaging, detection or diagnosis of HIV infection in a subject using an anti-HIV antibody or fragment conjugated to a diagnostic agent. In alternative embodiments, a bispecific antibody with at least one binding site for an HIV antigen and at least one binding site for a carrier molecule may be administered, optionally followed by a clearing agent, followed by administration of a carrier molecule conjugated to a therapeutic agent.
Owner:IMMUNOMEDICS INC
HIV antibody and antigen combined rapid detection reagent kit
ActiveCN101266246AImprove the detection rateShorten the windowMaterial analysisDiseaseDirect observation
The invention relates to biology applied technology field, especially relates to a immune chromatography assay for quickly detecting the human immunologic deficiency disease (HIV) antibody and antigen. The assay comprises two independent test papers A and B and plastic case C for storing the test paper, wherein the test paper A is used for detecting the HIV antibody and the test paper B is used for detecting the HIV p24 antigen. The test paper A and test paper B are parallely encased in the plastic case to constitute the assay. On detecting, the detected sample is added on the sample pad of the test paler and the immunoreaction result is directly observed to perform detection. The assay is used for screening or clinical diagnosis of the HIV infection and at the same time the HIV antigen and antibody are detected, the simpler antibody detection can effectively reduce the window phase for detecting the HIV, with features of quick reaction, easy operation, economy and practicality, suitable for insitu detecting.
Owner:天津中新科炬生物制药股份有限公司
Vaccine for prevention and treatment of HIV-infection
ActiveUS7612173B2Promote humoral and cellular responseAntibody mimetics/scaffoldsVirus peptidesImmunogenicityPolynucleotide
This invention relates to novel HIV polypeptide and polynucleotide fusions of Gag, Pol and Nef which are useful in immunogenic compositions and vaccines. The invention relates in particular to a polypeptide which comprises Nef or an immunogenic fragment thereof, and p17 Gag and / or p24 Gag or immunogenic fragments thereof, wherein when both p17 and p24 Gag are present there is at least one HIV antigen or immunogenic fragment between them. The polypeptide may also comprise Pol or RT or an immunogenic fragment thereof.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Chimeric HIV Antigens
The invention provides polynucleotides and polypeptides encoded therefrom that are capable of inducing immune responses to a human immunodeficiency virus. Compositions and methods for utilizing polynucleotides and polypeptides of the invention are also provided.
Owner:DU XIAOHAN +3
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
ActiveUS20180064803A1Measurable immune responseMaintain viremic controlViral antigen ingredientsAntiviralsImmunodeficiency virusVaccinia
Methods for inducing an immune response against Human Immunodeficiency Virus (HIV) in HIV-infected subjects undergoing antiretroviral therapy (ART) are described. The methods include administering an adenovirus vector primer vaccine and a modified vaccinia virus (MVA) vector booster vaccine encoding mosaic HIV antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +3
Hiv-gag codon-optimised DNA vaccines
The invention provides a nucleotide sequence that encodes an HIV-1 gag protein or fragment thereof containing a gag epitope and a second HIV antigen or a fragment encoding an epitope of said second HIV antigen, operably linked to a heterologous promoter. Preferred polynucleotide sequences further encodes nef or a fragment thereof and RT or a fragment thereof.
Owner:BEATON ANDREW +5
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS7501134B2Easy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Human immunodeficiency virus antigen/antibody chemiluminescence immune assay determination kit and method for preparing same
InactiveCN101363853AHigh sensitivityReduced detection windowChemiluminescene/bioluminescenceStreptavidinEnzyme
The invention relates to the immunization analysis medical field, and particularly provides a kit for determining the human immunodeficiency virus (HIV) antigen / antibody by the chemoluminescent immunization analysis and a preparation method thereof. The kit comprises (1) a solid carrier coated by the HIV antigens and antibodies; (2) biotin-labeled HIV antibodies; (3) enzyme-labeled HIV antigens and streptavidin; (4) a chemoluminescent primer acting with the above enzyme; and (5) a contrast. The preparation method of the kit includes the following steps of (1) coating the solid carrier with the HIV antigens and antibodies; (2) labeling the HIV antigens with a biotin; (3) labeling the HIV antigens and the streptavidin with the enzyme; (4) preparing the chemoluminescent primer, (5) preparing the contrast; (6) separately packaging; and (7) assembling into a finished product. The kit has the advantages of simpliness, rapidness, sensitiveness, stability and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
HIV vaccine formulation
ActiveUS20170362280A1Improve stabilityGood for clinical useHydroxy compound active ingredientsViral antigen ingredientsAdjuvantEngineering
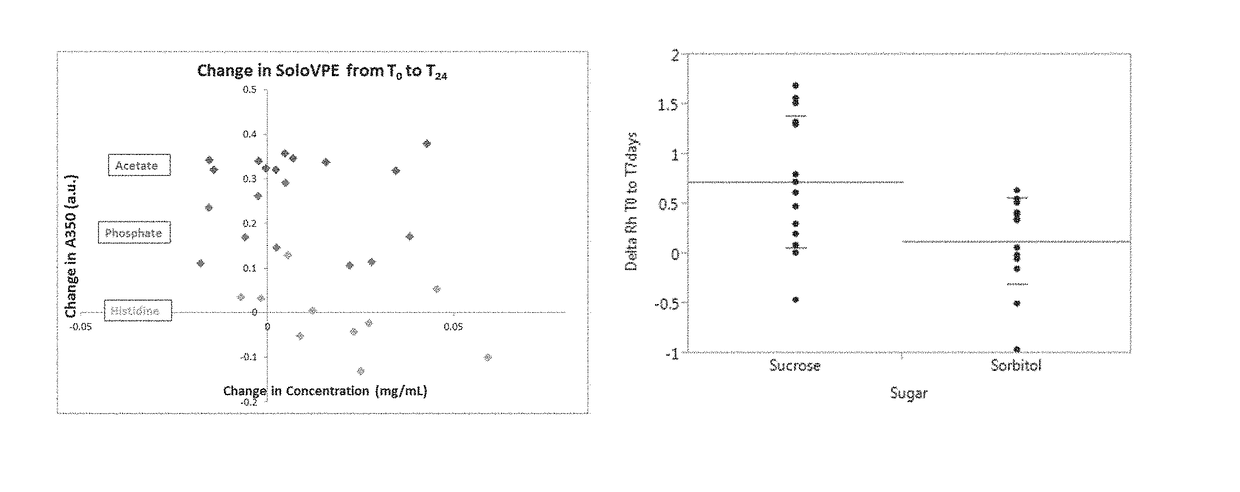
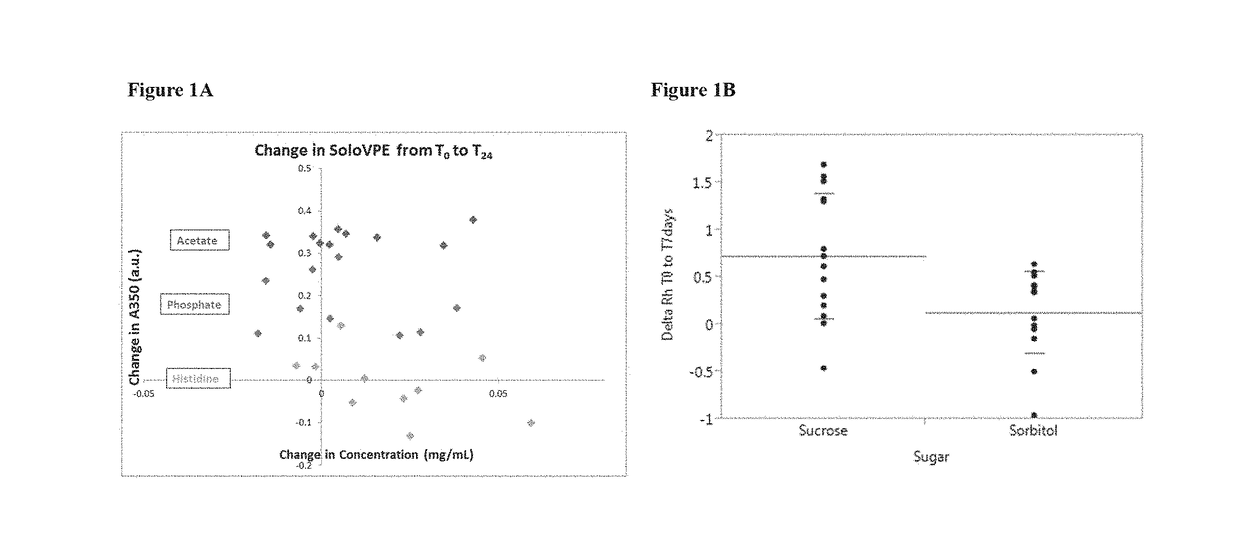
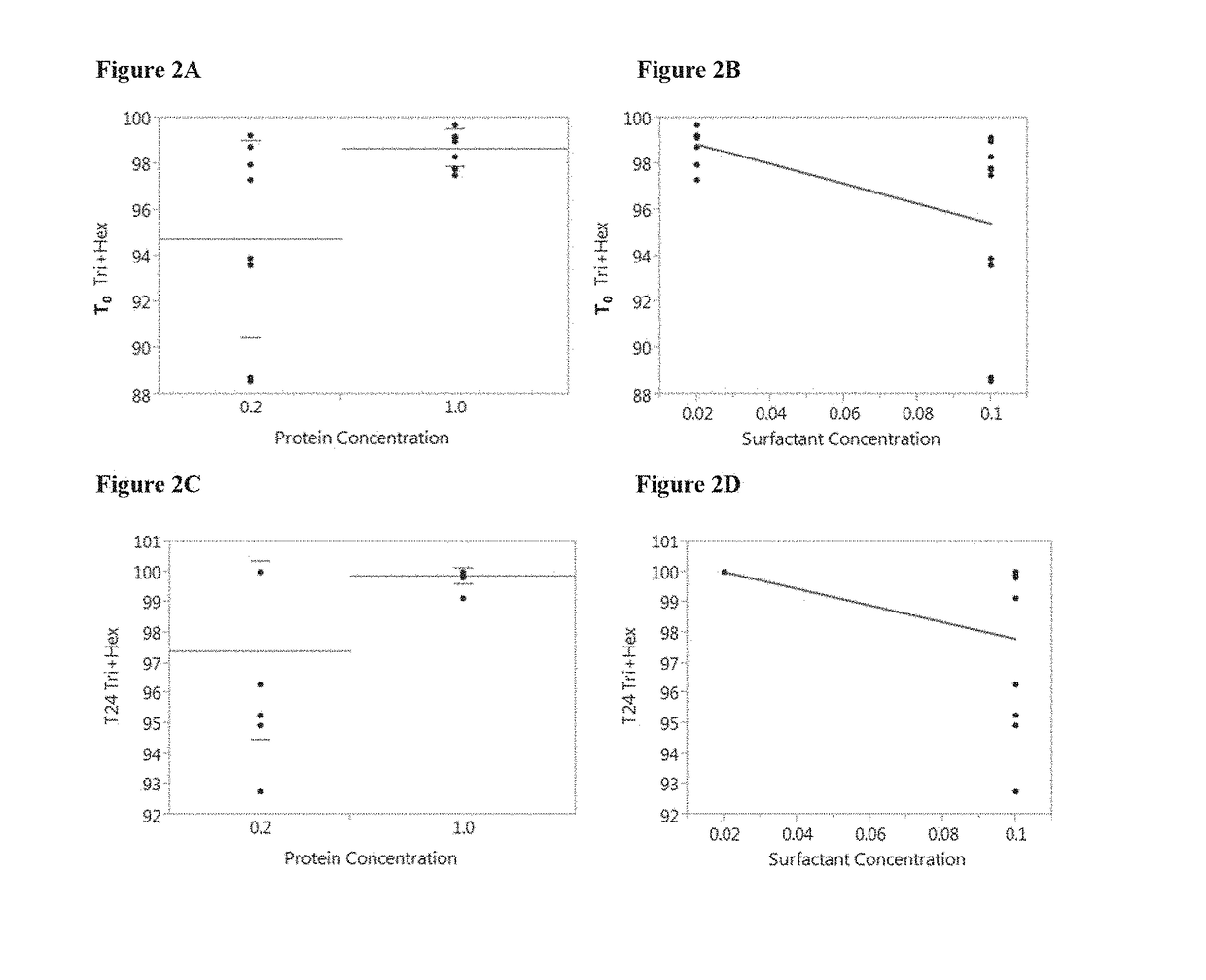
Immunogenic compositions containing a human immunodeficiency virus (HIV) gp140 protein, sorbitol, polysorbate 20, and histidine buffer are described. The described immunogenic compositions are advantageous in that they are stable at refrigerated temperature for extended periods of time, and are compatible with an adjuvant. Also described are methods of using the immunogenic compositions to induce an immune response against an HIV in a subject. The immunogenic compositions can be administered alone, or in combination with one or more additional HIV antigens, or one or more adenovirus vectors encoding the one or more additional HIV antigens.
Owner:JANSSEN VACCINES & PREVENTION BV
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS20050220883A1Without usingEasy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Microparticles with absorbed polypeptide-containing molecules formed without the use of surfactant, methods of making such microparticle compositions, and uses thereof, are disclosed. The microparticles comprise a polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, and the like. Preferred polymers are poly(D,L-lactide-co-glycolides), more preferable those having a lactide / glycolide molar ratio ranging from 40:60 to 60:40 and having a molecular weight ranging from 20,000 Daltons to 70,000 Daltons. Preferred polypeptide containing molecules are bacterial and viral antigens (including HIV antigens, meningitis B antigens, streptococcus B antigens, and Influenza A hemagglutinin antigens).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
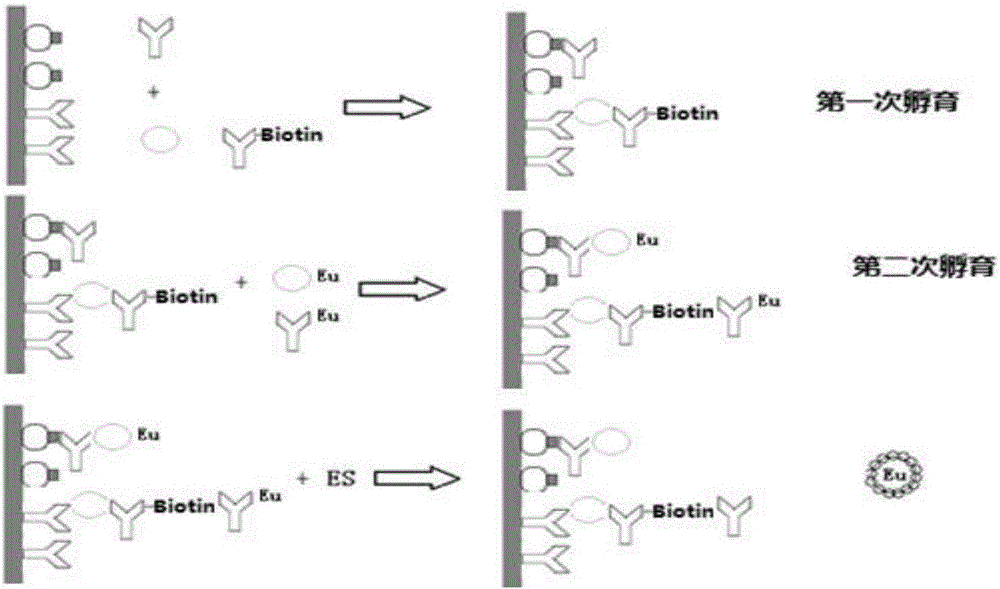
Double-labeling time-resolved fluorescence immunoassay method and kit for HIV (human immunodeficiency virus) antibody and HIV-1p24 antigen
InactiveCN103869073ARealize traceabilityEase of evaluationBiological material analysisBiotin-streptavidin complexTime resolved fluorescence immunoassay
The invention discloses a double-labeling time-resolved fluorescence immunoassay method and kit for HIV (human immunodeficiency virus) antibody-HIV-1p24 antigen. The analytical method mainly comprises the steps of preparing a solid phase carrier coated with an HIV recombinant antigen and an HIV-1p24 monoclonal antibody simultaneously; preparing biotin-labeled HIV-1p24 monoclonal antibody; preparing lanthanide 1-labeled HIV recombinant antigen; preparing lanthanide 2-labeled streptavidin; adding a calibrator containing HIV standard antibody and HIV-1p24 standard antigen or a sample to be tested into the solid phase carrier coated with the antigen and the antibody, adding the biotin-labeled HIV-1p24 antibody, incubating, washing, then adding the lanthanide 1-labeled HIV antigen and the lanthanide 2-labeled streptavidin, incubating again, washing, and adding enhancement solution for fluorescence detection. The analytical method overcomes the difficulty that the antigen and the antibody cannot be distinguished in the existing joint detection for HIV antigen and antibody, realizes simultaneous and quantitative detection of the HIV antibody and the HIV-1p24 antigen and therefore shortens the window phase of HIV detection.
Owner:GUANGZHOU FENGHUA BIOENG
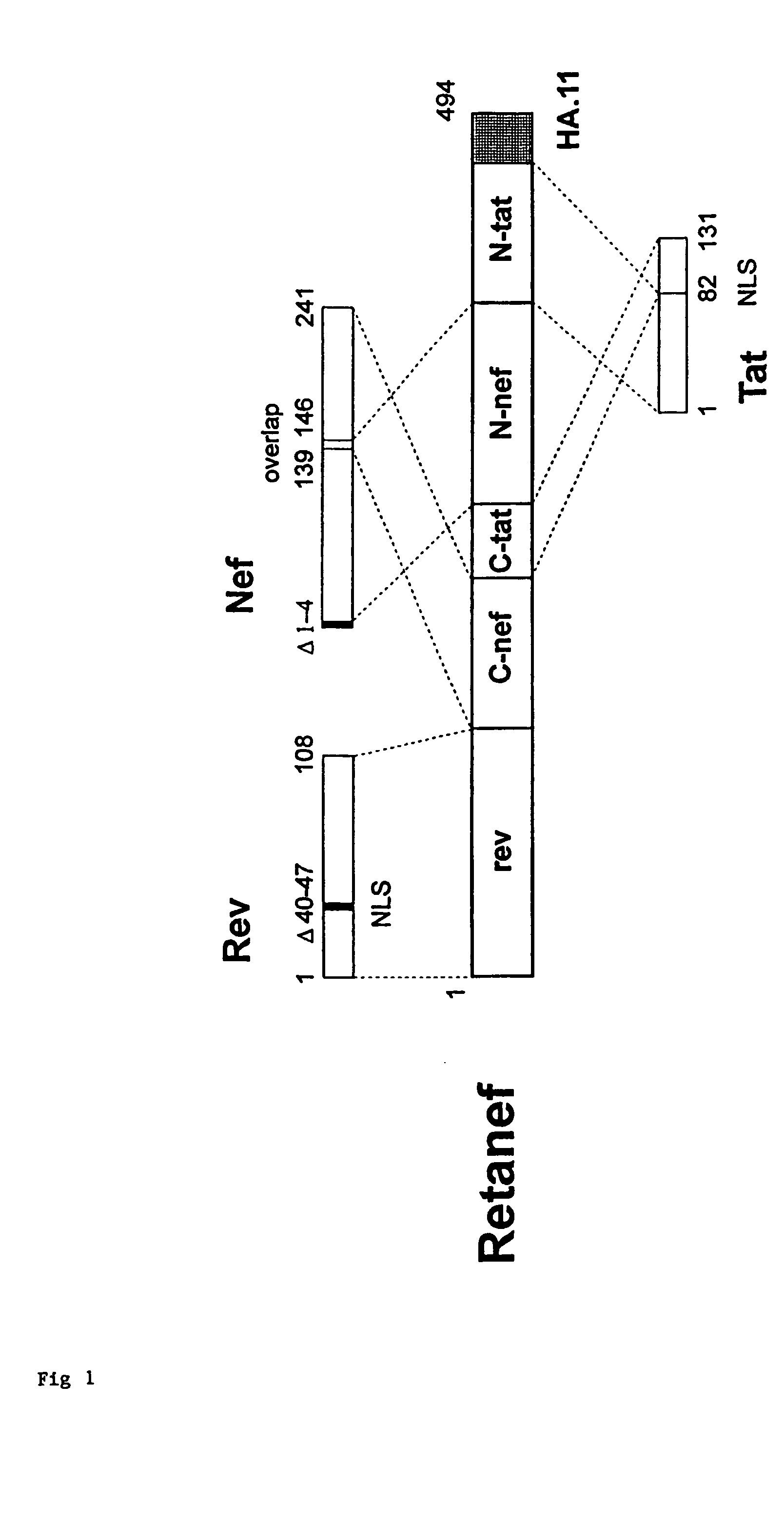
Novel chimeric rev, tat, and nef antigens
This invention provides novel HIV antigens comprising chimeric rev, tat, and nef for use in inducing an immune response. The novel antigens can be used as vaccines to prevent and / or attenuate HIV infection.
Owner:FRANCHINI GENOVEFFA +2
Live vaccine for human immunodeficiency virus
InactiveUS7189402B1Bacterial antigen ingredientsAntibody mimetics/scaffoldsSalmonella wienHIV Proteins
The present invention discloses development of a model live vaccine for HIV, using an attenuated strain of Salmonella engineered to surface express specific HIV proteins and testing of this vaccine in mice. There are provided two recombinant plasmids, containing the Lpp-OmpA genes required for surface exposure, followed by the genes for the HIV-1 proteins, Reverse Transcriptase or Transactivating protein (Tat). These plasmids are electroporated into an attenuated strain of Salmonella, and antigen expression is verified. These live vaccines are then used to orally inoculate mice and the vaccinated mice are tested for fecal IgA response and helper T cell response specific for the HIV antigens.
Owner:RES DEVMENT FOUND
Human immunodeficiency virus antibody chemiluminescence immune analyzing diagnose reagent box and method of producing the same
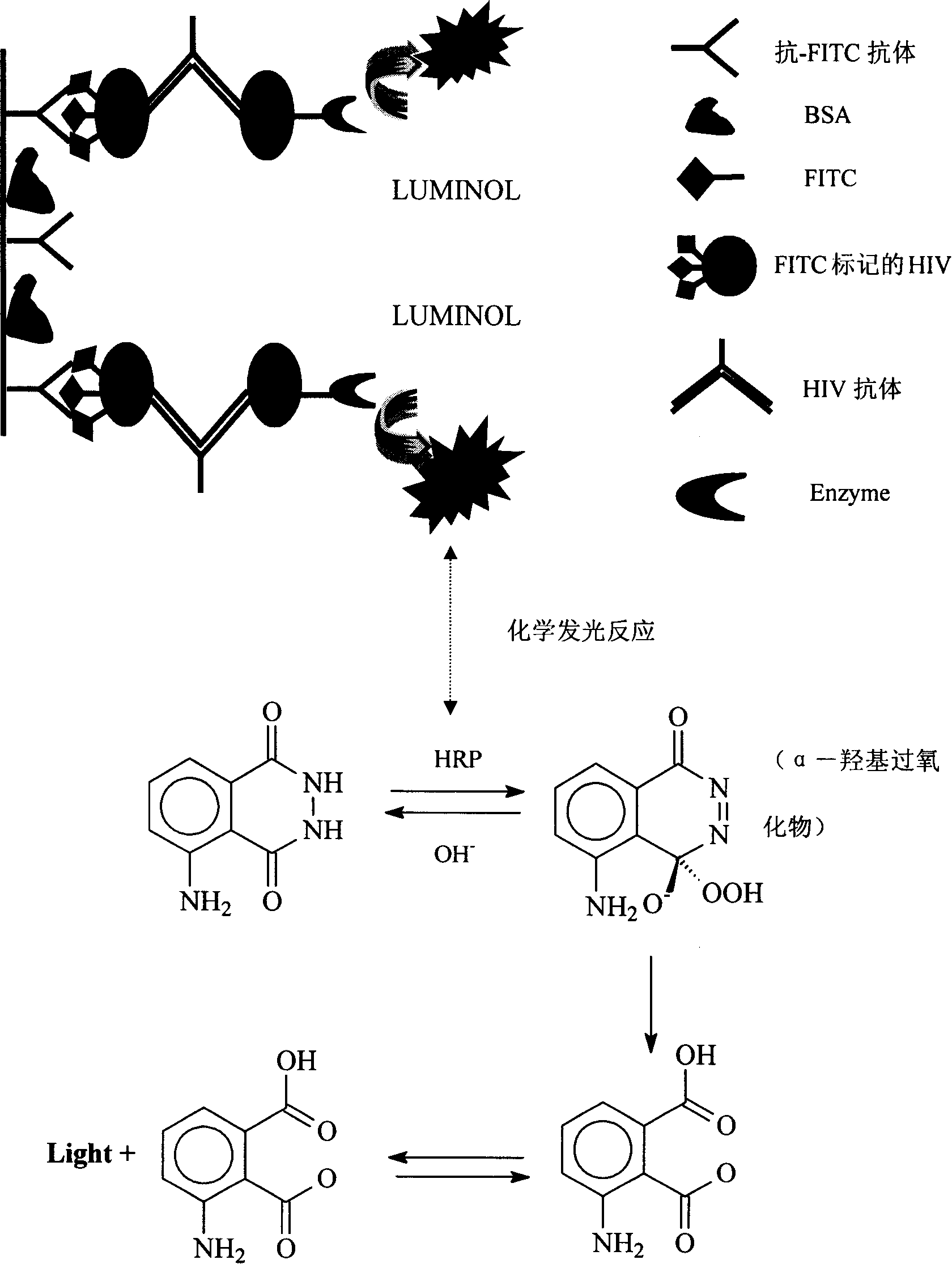
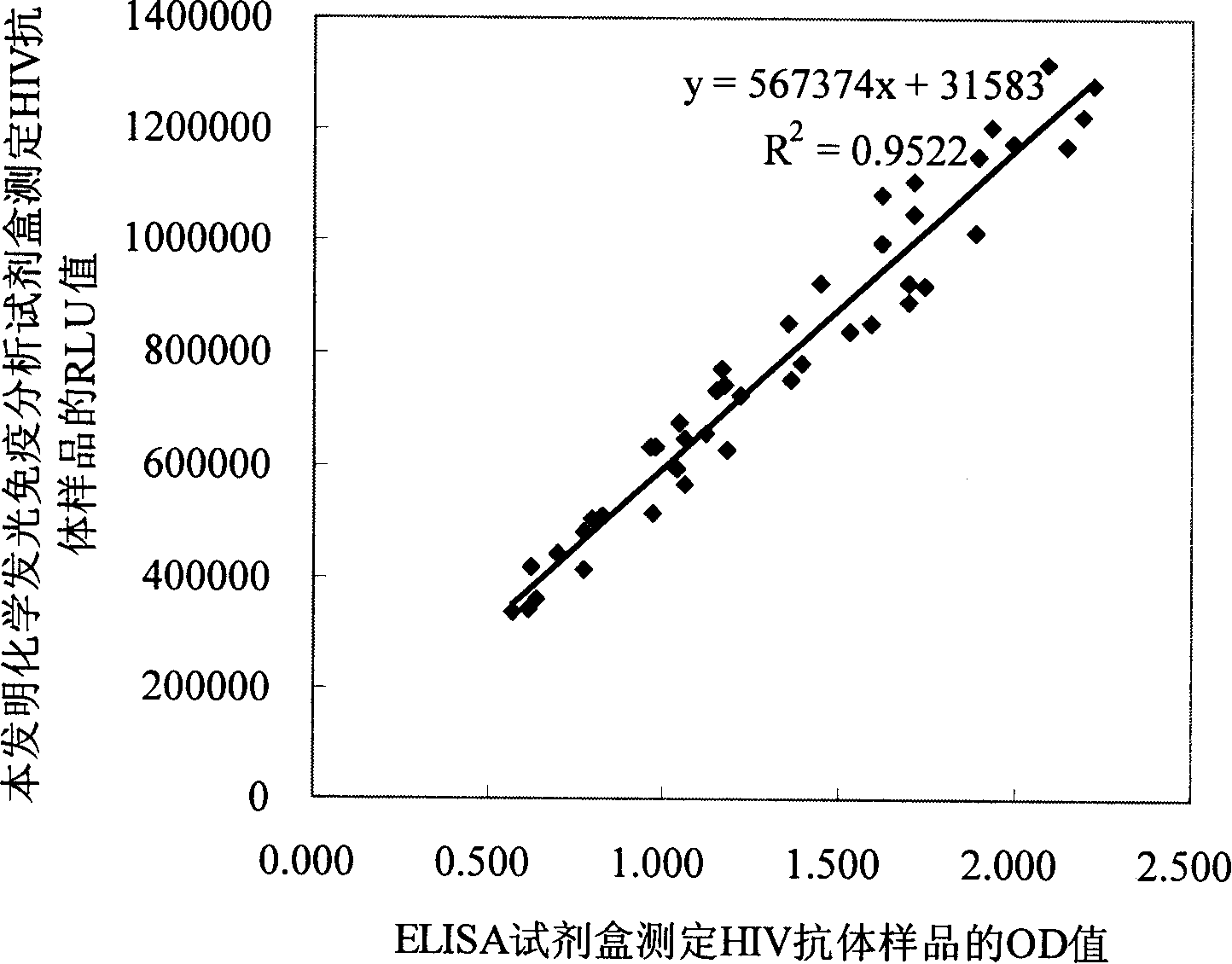
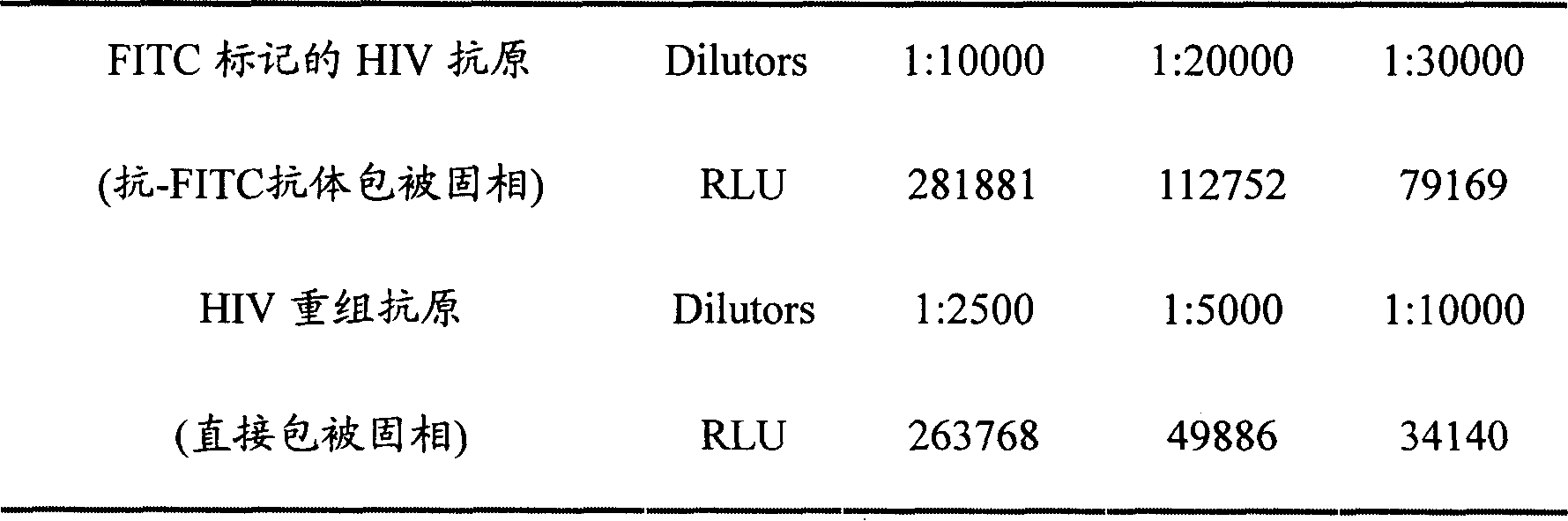
ActiveCN101178404AIncrease the amount of antigenFully exposedChemiluminescene/bioluminescenceHuman immunodeficiency virus antibodyPositive control
The invention relates to the field of immunoanalysis medicine, in particular, the invention provides a human immunodeficiency virus antibody chemiluminescence immunoassay diagnostic kit and a preparation method thereof. The kit of the invention comprises: a solid-phase carrier coated with anti-FITC antibody, FITC-labeled HIV recombinant antigen, enzyme-labeled HIV antigen, chemiluminescent substrate for the above-mentioned enzyme, and negative and positive control solutions. The method for preparing the kit includes the following steps: 1) a solid-phase carrier coated with an anti-FITC antibody; 2) labeling the HIV recombinant antigen with FITC to obtain the FITC-labeled HIV recombinant antigen; 3) labeling the HIV antigen with an enzyme to obtain Enzyme-labeled HIV antigen; 4) Subpackage and assembly. The kit of the invention has low cost and high sensitivity, and compared with the existing chemiluminescence immunoassay detection method, it can improve the analysis sensitivity and reproducibility, and reduce the amount of recombinant antigen used.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
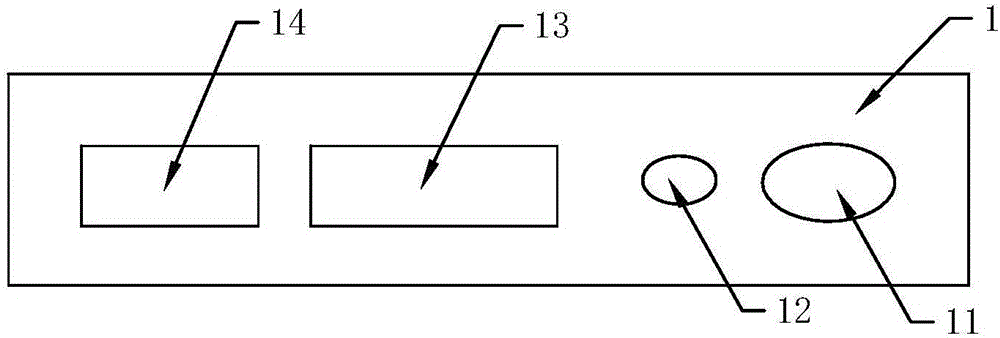
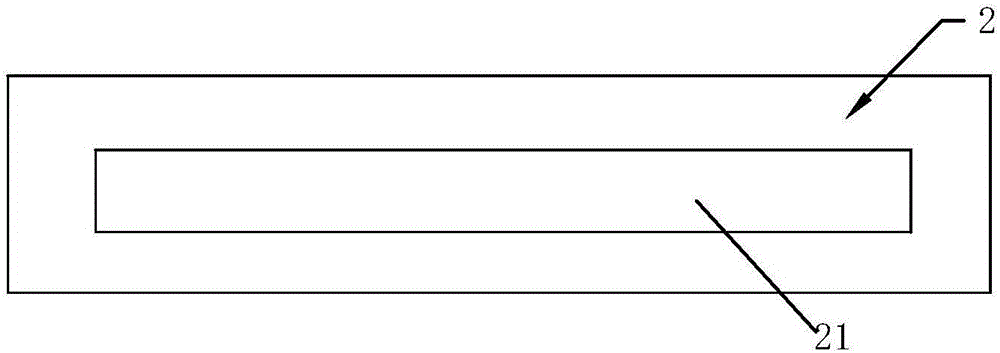
HIV (human immunodeficiency virus) antigen antibody detection card and preparation method thereof
InactiveCN106771195AHigh detection sensitivityImprove securityMaterial analysisMonoclonal antibodyBiologic DMARD
The invention belongs to the technical field of biological detection, and particularly relates to an HIV (human immunodeficiency virus) antigen antibody detection card and a preparation method thereof. The detection card comprises a card cover, a card body and a test strip, wherein the card cover is sequentially provided with a dilute solution adding hole, a sample adding hole, an observation hole and an installation marking hole; a groove for holding the test strip is arranged in the card body; and the test strip is sequentially provided with a sample pad layer, a colloidal gold layer, a nitrocellulose film and a water-absorbing layer. The preparation method comprises the following steps: preparing colloidal gold; respectively marking recombinant HIV-1 / HIV-2 antigens and mouse anti-p24 monoclonal antibodies with the colloidal gold, marking p24 antibodies with biotin, immobilizing the colloidal gold and biotin markers, coating the nitrocellulose film, preparing the test strip, and buckling the detection card. The detection card provided by the invention can detect HIV antigen antibodies, and is high in sensitivity and low in sample consumption.
Owner:山东康华生物医疗科技股份有限公司
Modifications of HIV Env, Gag, and Pol enhance immunogenicity for genetic immunization
InactiveUS20040033487A1Low cytotoxicityEnhance humoral and CTL immunityOrganic active ingredientsPeptide/protein ingredientsVaccine ImmunogenicityDNA
Modified HIV Env, Gag, Pol, or Nef DNA with improved ability to elicit antibody and CTL responses to HIV antigens have been identified as prototype immunogens for the treatment and prevention of HIV infections.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Multiple index quick detecting method for human immune defect virus antibody
InactiveCN1858594ASimultaneous diagnosis and differentiation of infectionSimple and fast operationMaterial analysis by observing effect on chemical indicatorHuman immunodeficiency virus antibodyEscherichia coli
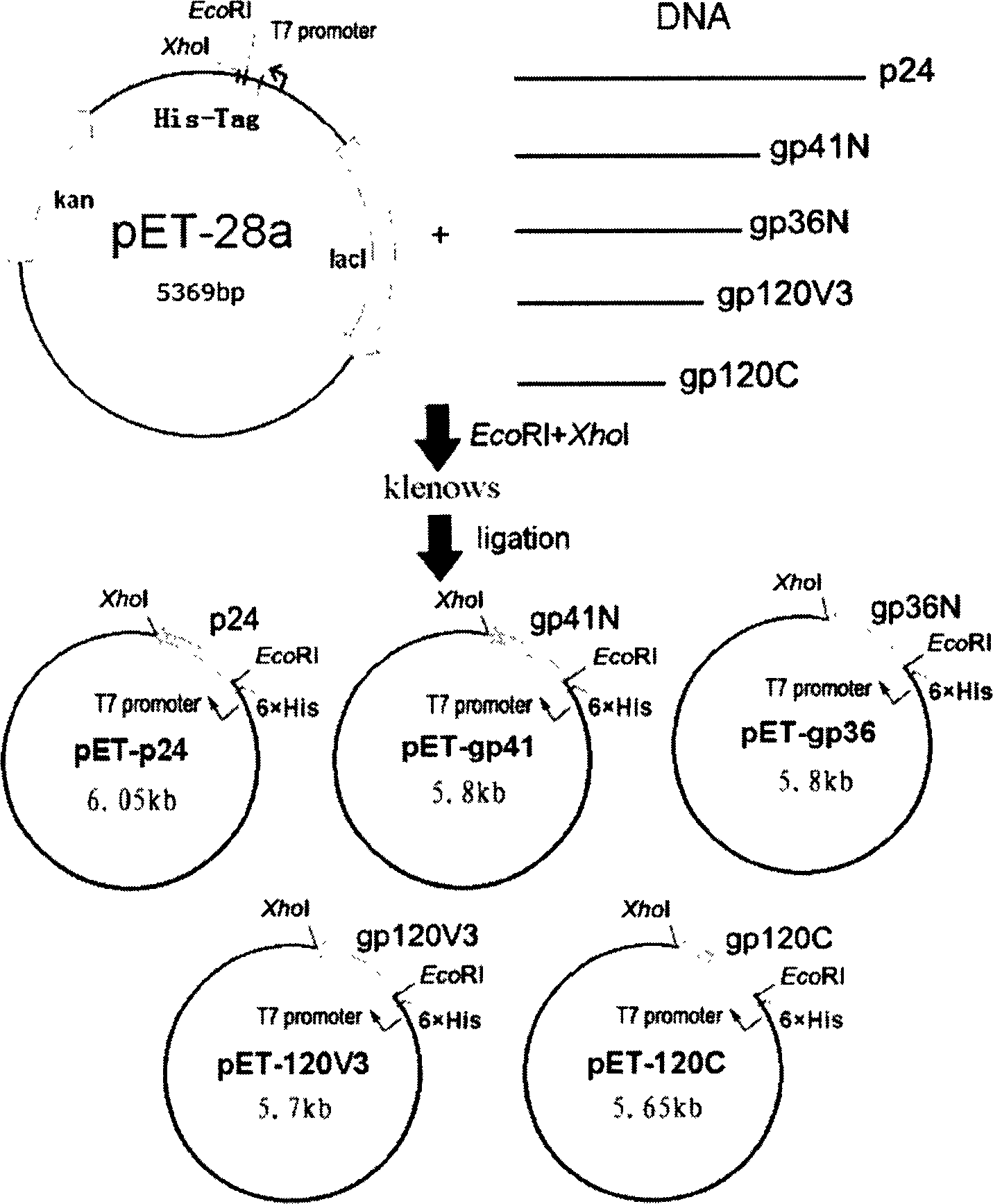
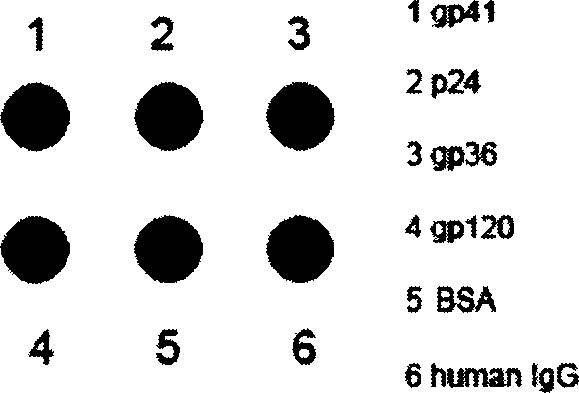
This invention discloses a multi-target quick test method for human immunodeficiency virus antibodies including: 1, expressing five kinds of HIV antigen white dribs and drabs-p24, gp41, gp36, gp120V3, gp120C in a colibacillus by a gene engineering technology, 2, fixing the five antigen whites on a pyroxylin film, 3, dripping armed serum on it and the virus antibody is combined with the antigen by a immunoreaction then adding the A(SPA) labeled by nm gold, 4, cleaning it after it penetrates the film, 5, adding SPA antibodies to increase the amplification to form red spots seen by eyes.
Owner:WUHAN UNIV
Fast urine HIV detection diagnosis test paper and method for producing the same
This invention discloses a urine test stripe for fast HIV diagnosis as well as a preparation method and a using method thereof. The test stripe consists of a base pad at the bottom and a lateral flow chromatographic pad, which is fixed at the former. Detection lines and quality control lines are arranged on the lateral flow chromatographic pad, and detection lines are positioned on upstream of quality control lines; the upstream end of the lateral flow chromatographic pad is connected to a binding pad treated by HIV antigen-colloidal gold compound or HIV antigen-colloidal selenium compound; the downstream end is connected to a blotting pad and the upstream end of the binding pad is connected to a sample pad. The test paper can correctly tell the HIV virus antibody condition of the person by testing the urine in 5-15 minutes. The invention has advantages of high testing precision, simple testing method, convenient sampling and high performance ratio, and it provides new and powerful technical support and product assurance for AIDS prevention and control in our country.
Owner:关一夫
AIDS vaccine based on replicative vaccinia virus vector
The present invention relates to replicative live AIDS carrier vaccine expressing HIV antigen and its use. The vaccine is constructed based on replicative vaccinia virus, such as vaccinia virus Tiantan strain. The replicative live AIDS carrier vaccine can induce high level HIV resisting body fluid and cellular immune response. The present invention provides the carrier for constructing the AIDS vaccine, and also relates to immunizing process with the AIDS vaccine.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Therapeutic immunization of hiv-infected individuals
InactiveUS20060216272A1Effectively maintaining low titerReceive treatment wellBiocideGenetic material ingredientsImmunodeficiency virusMedicine
The present invention provides an improved method for eliciting a therapeutic immune response in an individual infected with human immunodeficiency virus (“HIV”). The method comprises administering an adenoviral vaccine composition expressing an HIV antigen to an individual with controlled viremia. Immunization of infected individuals in this manner elicits a cellular-mediated immune response against the virus that is significant both in the level of the response and the breadth of the response. The therapeutic immune response that ensues is capable of effectively maintaining low titers of virus and, thus, offers the prospect of reducing individual dependency on antiviral therapy.
Owner:MERCK & CO INC
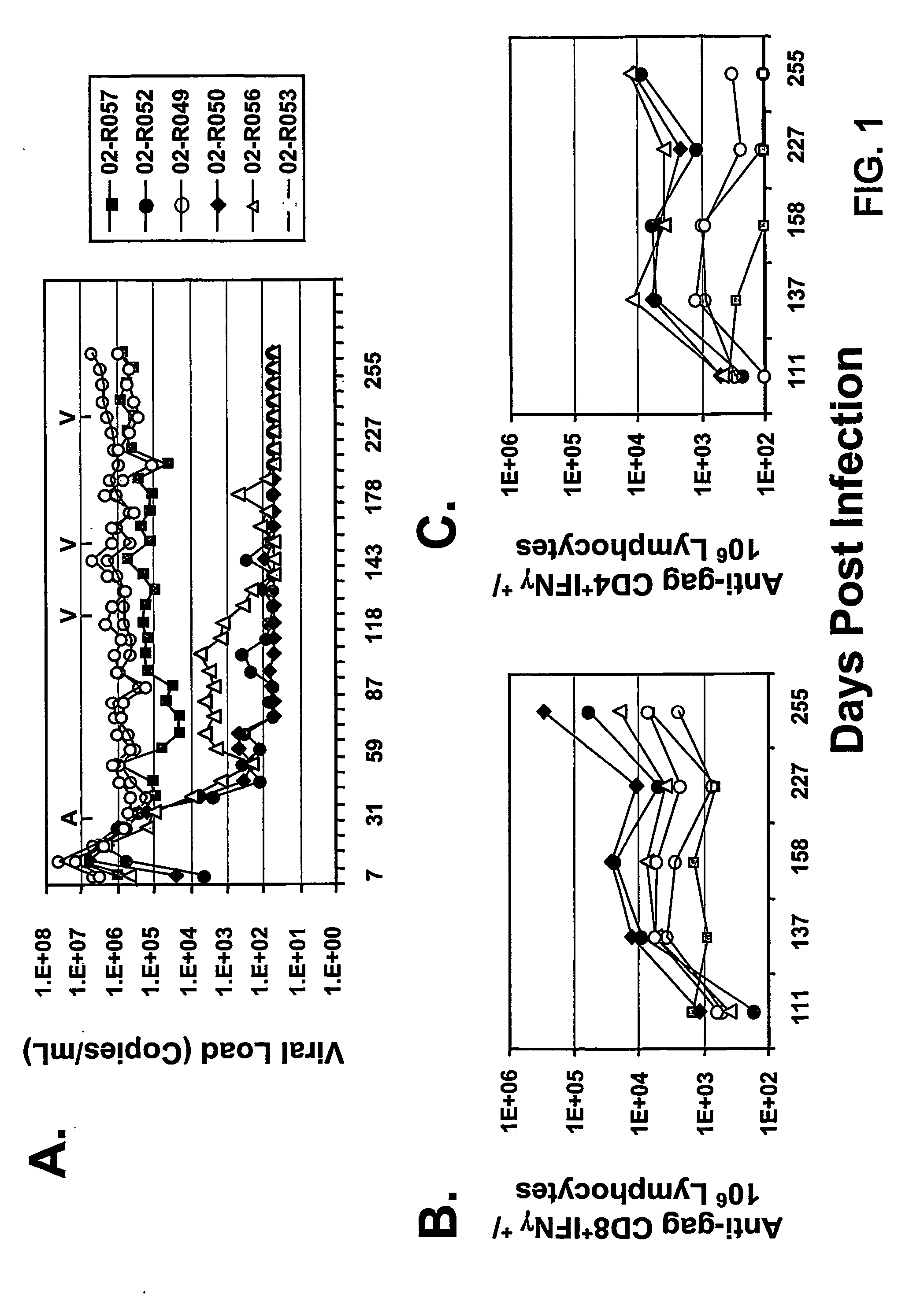
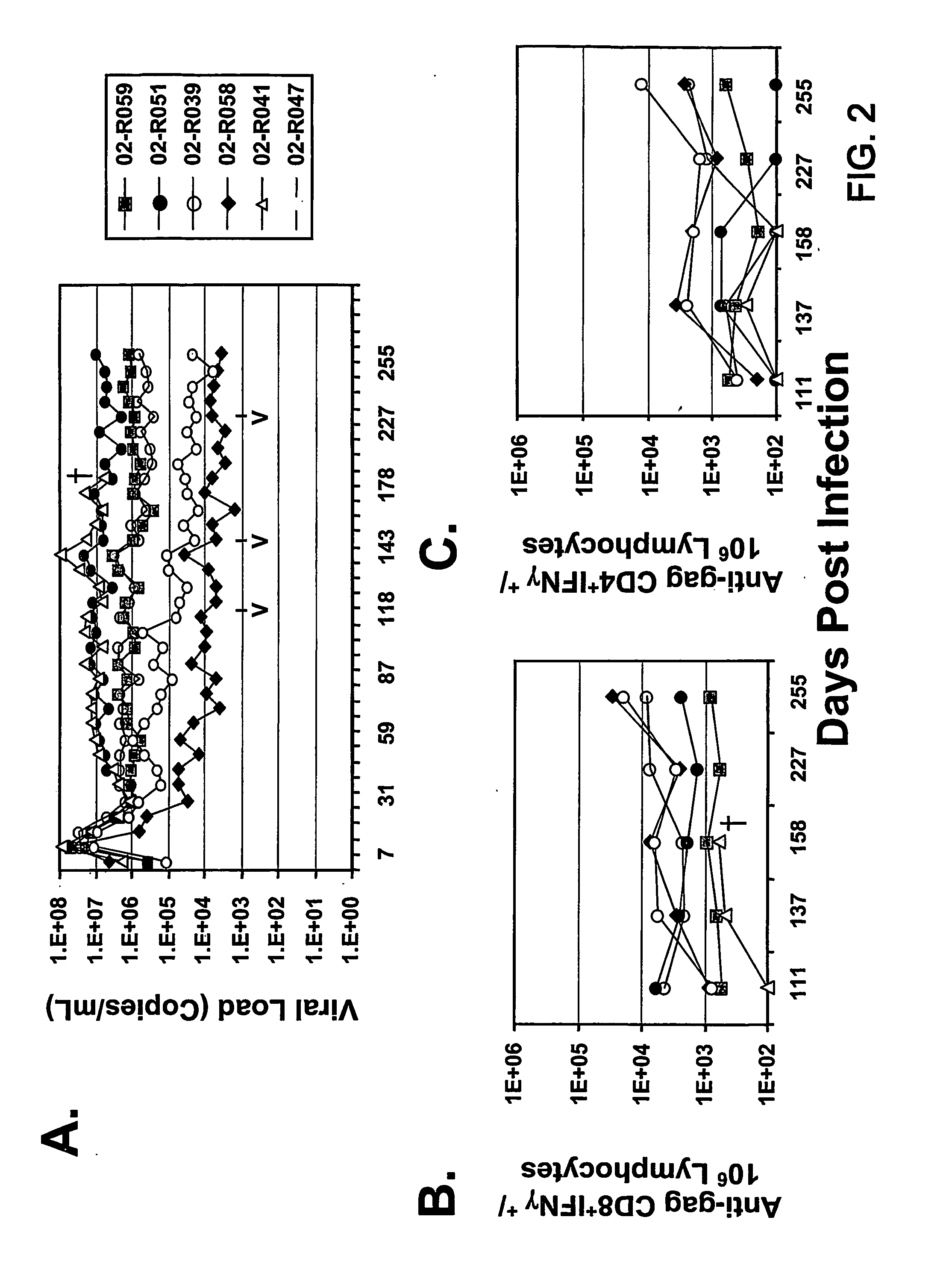
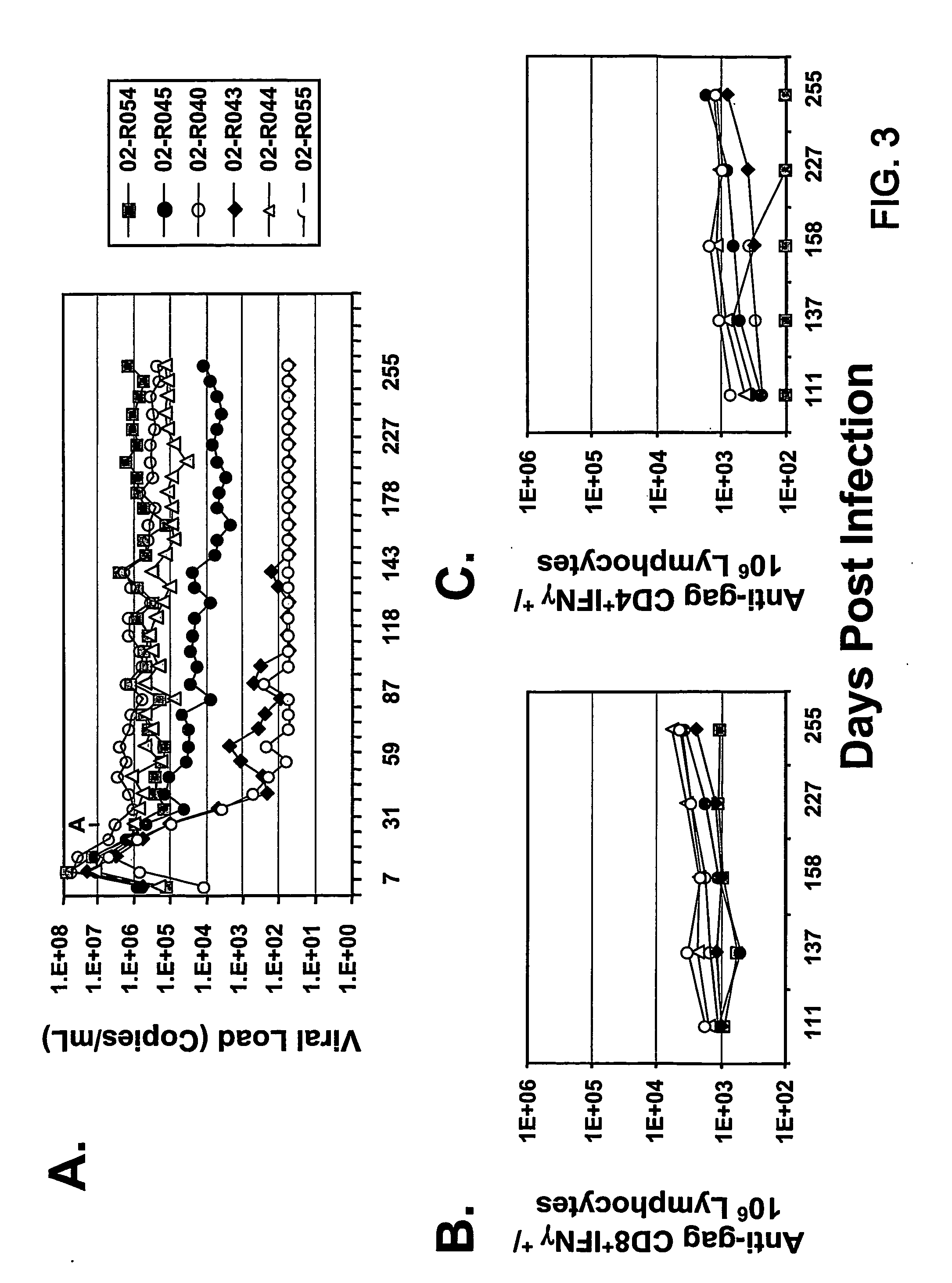
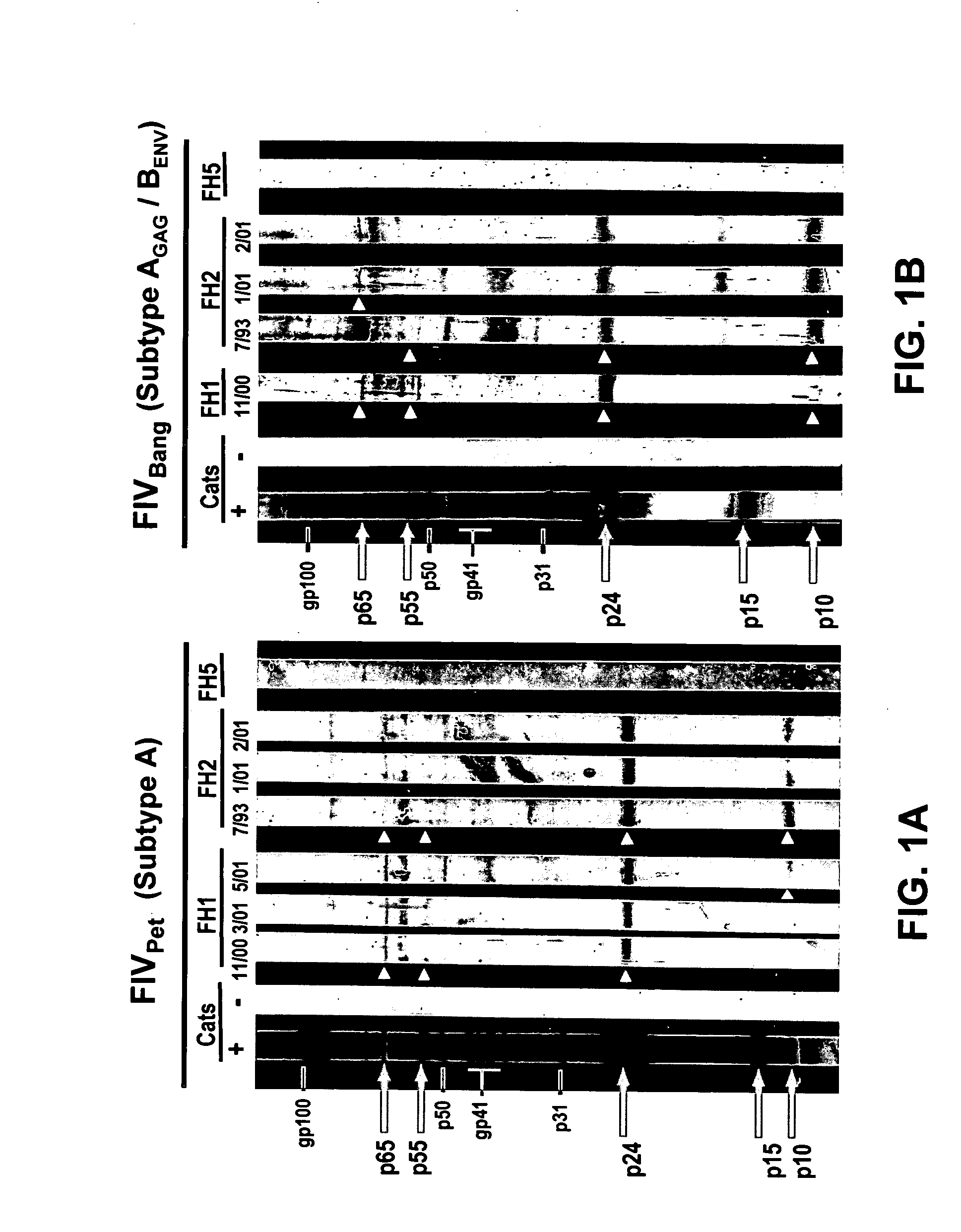
Materials and Methods for Detecting, Preventing, and Treating Retroviral Infection
InactiveUS20090274725A1Viral antigen ingredientsMicrobiological testing/measurementRetroviral infectionHuman cell
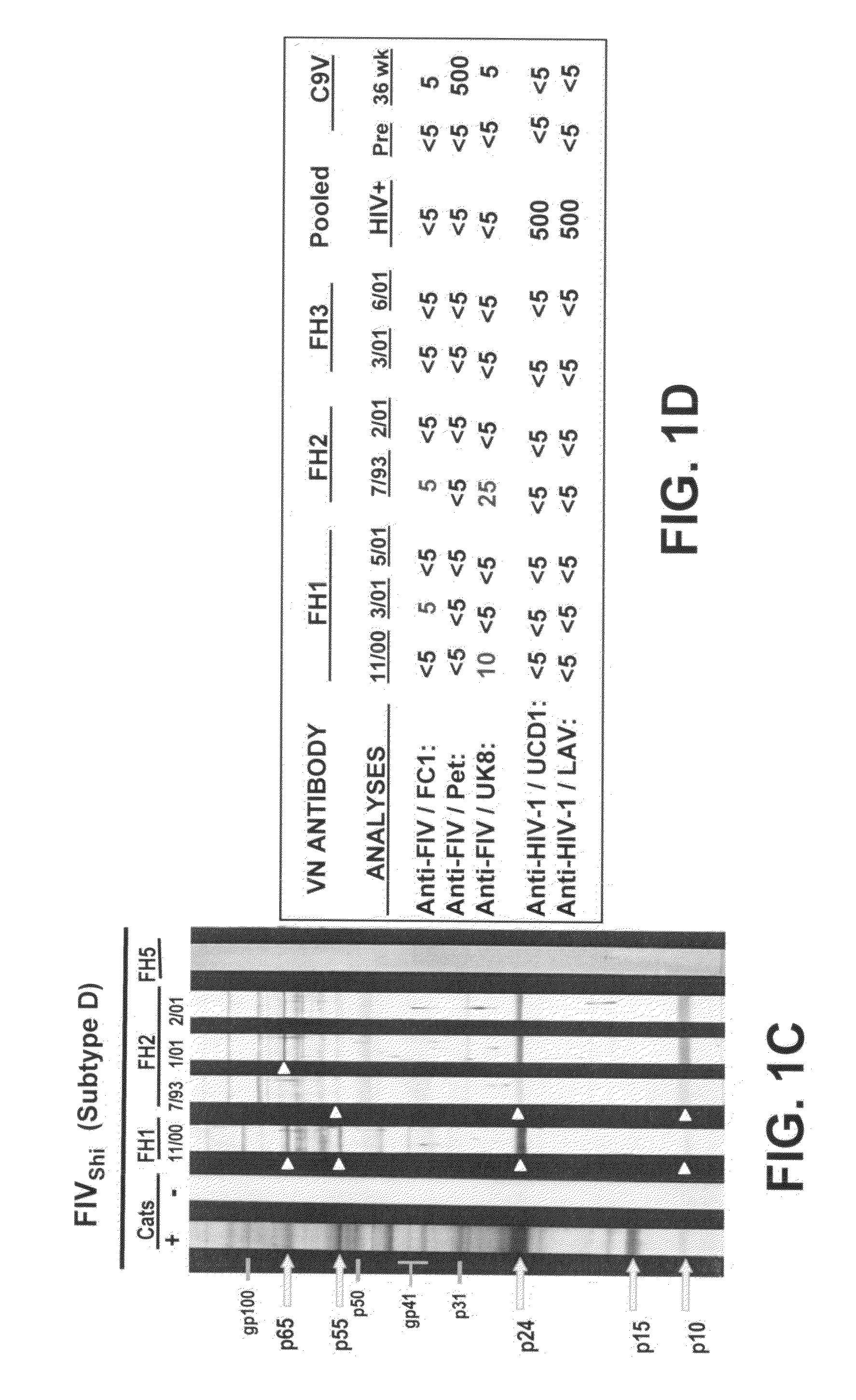
The subject invention pertains to materials and methods for detecting, preventing and treating retroviral infections in humans and other animals susceptible to infection by retrovirus. It has been discovered that FIV can be transmitted from cats to humans and that the FIV can infect human cells in vivo and that antibodies generated by the infected person cross-react with HIV antigens. Thus, the methods and compositions of the subject invention can be used to detect, prevent and treat FIV infection in humans and other non-feline animals that are susceptible to FIV infection. The methods and compositions of the invention can also be used to prevent and treat infection by HIV in humans.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
HIV viral antibody/antigen diagnostic reagent kit and preparing method thereof and detecting method
InactiveCN1673749AReduce the chance of infectionHigh sensitivityMaterial analysis by observing effect on chemical indicatorViral antibodyPhosphate
The present invention is HIV virus antibody / antigen diagnosis kit and its preparation process and detection method. The kit includes pre-coated enzyme-linked board with coated HIV antibody and P24 monoclonal antibody, rabbit anti-P24 polyclonal antibody labeled with biotin, conjugate of horseradish peroxidase labeled avidin and HIV antigen, detergent solution of phosphate buffer containing Tween, developing solution A of citrate buffer containing hydrogen peroxide, developing solution B of citrate buffer containing tetramethyl benzidine, terminating solution containing sulfuric acid solution, sample diluting solution containing phosphate buffer, normal human serum as positive contrast, HIV antibody serum as positive contrast, and P24 antigen serum as positive contrast. The present invention can detect HIV antibody and P24 antigen simultaneously in raised specificity and sensitivity and is suitable for diagnosis of human immune deficiency virus antibody.
Owner:北京科卫临床诊断试剂有限公司
Method of using adenoviral vectors to induce an immune response
InactiveUS20090286860A1Organic active ingredientsViral antigen ingredientsViral vectorBioinformatics
The invention provides a method of inducing an immune response against a human immunodeficiency virus (HIV) in a mammal. The method comprises administering to the mammal an adenoviral vector composition comprising one or more adenoviral vectors encoding two or more different HIV antigens, the production of which induces an immune response against HIV in the mammal. The invention also provides an adenoviral vector composition comprising four adenoviral vectors encoding an HIV clade A Env protein, an HIV clade B Env protein, an V clade C Env protein, and a fusion protein comprising an HIV clade B Gag protein and Pol protein, respectively.
Owner:GEN VEC INC +1
Microparticle compositions and methods for the manufacture thereof
InactiveUS7846479B2Convenient introductionImprove efficiencyPowder deliveryAntiviralsHemagglutininAdjuvant
Microparticles with adsorbed complexes of macromolecule and detergent, methods of making such microparticles, and uses thereof, are disclosed. The microparticles comprise a polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, and the like, and are formed using cationic, anionic, or nonionic detergents. The surfaces of the microparticles have adsorbed thereon a complex of biologically active macromolecules, such as nucleic acids, polypeptides, antigens, and adjuvants, and a detergent. Preferred polymers are poly(D,L-lactide-co-glycolides), more preferably those having a lactide / glycolide molar ratio ranging from 40:60 to 60:40 and having a molecular weight ranging from 30,000 Daltons to 70,000 Daltons. Preferred macromolecules are bacterial and viral antigens (such as HIV antigens, meningitis B antigens, streptococcus B antigens, and Influenza A hemagglutinin antigens) as well as polynucleotides that encode for such antigens.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Hiv-gag codon-optimised dna vaccines
InactiveUS20070015721A1Slow and controlled releaseAntibody mimetics/scaffoldsGenetic material ingredientsHeterologousEpitope
The invention provides a nucleotide sequence that encodes an HIV-1 gag protein or fragment thereof containing a gag epitope and a second HIV antigen or a fragment encoding an epitope of said second HIV antigen, operably linked to a heterologous promoter. Preferred polynucleotide sequences further encodes nef or a fragment thereof and RT or a fragment thereof.
Owner:GLAXO GROUP LTD
HIV positive serum surrogate
Substitute of HIV positive blood serum is cross-linking object obtained from cross-linking reaction between unhuman anti HIV antibody or its treating fluid and normal person immunoglobulin or its treating fluid. Essence of the invention is to combine unhuman anti HIV antibody activity (i.e. activity reacting to HIV antigen) with antigen activity of normal person immunoglobulin (i.e. activity reacting to the conjugate of anti abzyme of human immunoglobulin) so as to eliminate risk of containing pathogenesis factor possibly in blood serum (or blood plasma), which is positive in human anti antibody of pathogenesis factor, but possess reactivity on blood serum, which is positive in anti antibody of pathogenesis factor. Since the substitute is from unhuman anti HIV antibody and normal person immunoglobulin, thus it is essential to prevent risk infected by positive serum potentially.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST
Method of Using Adenoviral Vectors to Induce an Immune Response
The invention provides a method of inducing an immune response against a human immunodeficiency virus (HIV) in a mammal. The method comprises administering to the mammal an adenoviral vector composition comprising one or more adenoviral vectors encoding two or more different HIV antigens, the production of which induces an immune response against HIV in the mammal. The invention also provides an adenoviral vector composition comprising four adenoviral vectors encoding an HIV clade A Env protein, an HIV clade B Env protein, an HIV clade C Env protein, and a fusion protein comprising an HIV clade B Gag protein and Pol protein, respectively.
Owner:UNITED STATES OF AMERICA +1
Diagnostic kit for jointly detecting HIV antigen and HIV antibody and preparation method of diagnostic kit
InactiveCN105929157AStrong specificityThe interference of specific fluorescence greatly improves the sensitivityFluorescence/phosphorescenceTime resolved fluorescence immunoassayImmunofluorescence
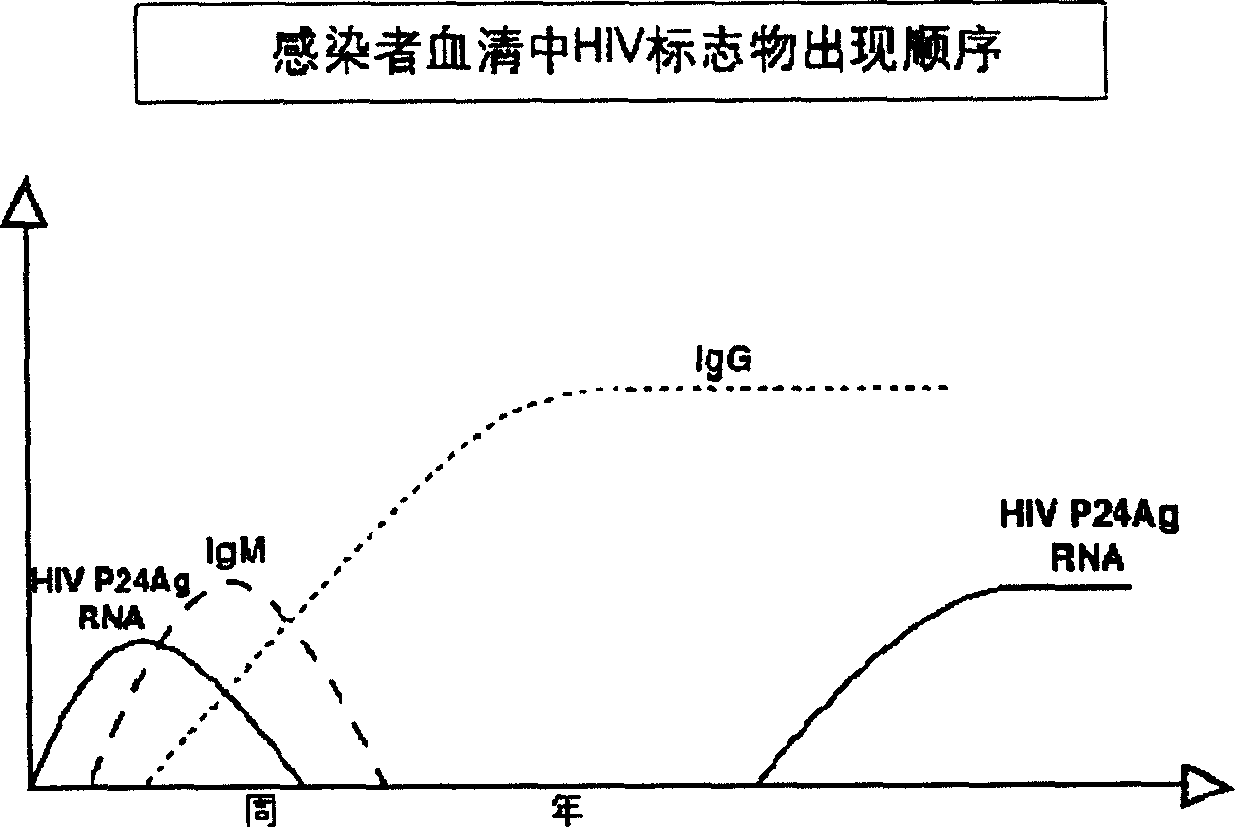
The invention provides a diagnostic kit for jointly detecting an HIV antigen and an HIV antibody. The HIV-1type p24 antigen and the HIV-1 / 2 type antibody are qualitatively detected by adopting the immunoadsorption test principle of a double antibody sandwich method and a double antigen sandwich method and combining a time-resolved fluorescence immunoassay technique; the window period of existing HIV detection can be shortened to be 2-3 weeks, and the diagnostic kit is beneficial for early diagnosis of an HIV and also can serve as a basis for prognostically judging and evaluating the antiviral therapy effect; meanwhile, the kit further has the advantages of being high in specificity and sensitivity, good in repeatability, excellent in stability, wide in measurable range, high in detection automation degree, free of environmental pollution and the like.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com