HIV antibody and antigen combined rapid detection reagent kit
A technology for human immunodeficiency and detection kits, applied in measurement devices, instruments, scientific instruments, etc., can solve problems such as incompatibility with rapid diagnostic reagents, and achieve the effects of shortening the window period, improving the detection rate, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1HI
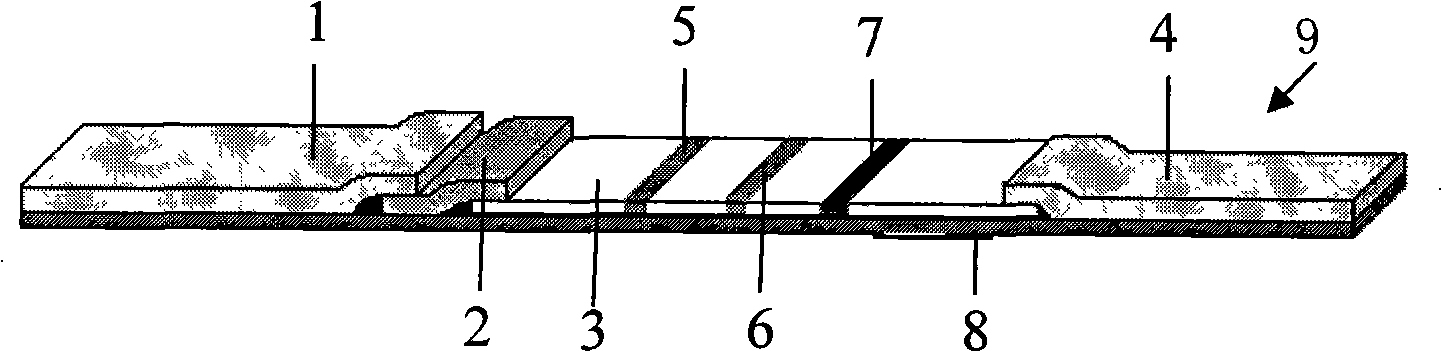
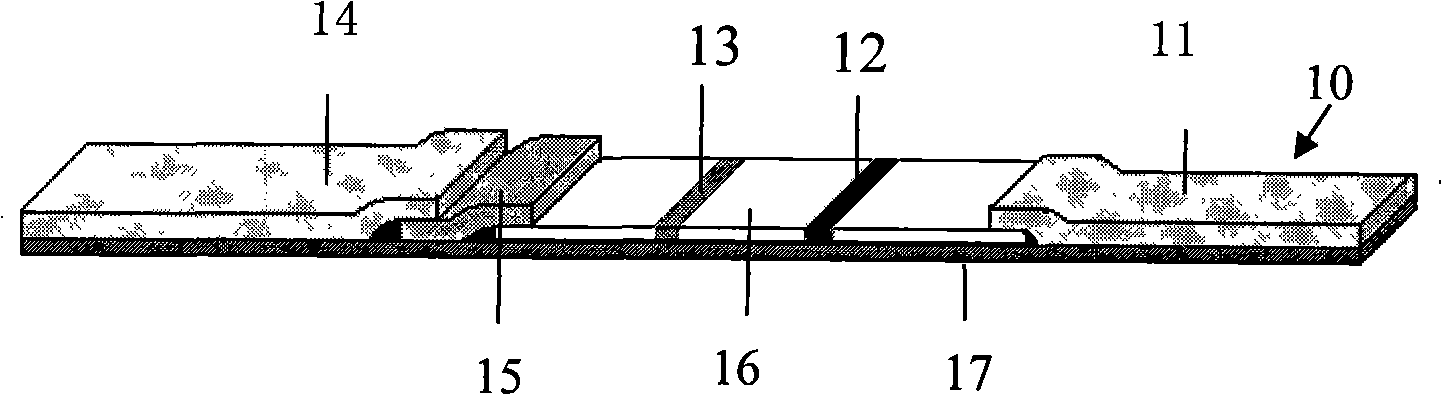
[0047] The preparation of embodiment 1 HIV antibody and antigen combined rapid detection detection test paper A9, test paper BI0:
[0048] 1 main material
[0049] 1.1 Recombinant antigens: HIV gp41, gp36: American Biotech Atlantic Inc (BAI.) products, used for coating and labeling of test paper A; anti-HIV P24 antibody pairing: products of BAI Company, used for coating and labeling of test paper B; Auric acid: product of Sigma company, NC membrane: product of Millipore company; BSA, PEG 20000, hydrolyzed casein: product of Sigma company. Other commonly used reagents are analytical reagents.
[0050] 1.2 HIV antibody national reference product (colloidal gold): developed by China Institute for the Control of Pharmaceutical and Biological Products. Including 20 positive sera, 20 negative sera, 3 sensitivity sera, and 1 precision sera. National reference materials for HIV1 P24 antigen, including 10 positive sera, 20 negative sera, 10 sensitivity sera, and 2 precision sera.
...
Embodiment 2
[0059] Example 2: Performance Analysis of HIV Antibody Antigen Combined Rapid Diagnostic Kit
[0060] 1 main material
[0061] 1.1 HIV Antibody Antigen Combined Rapid Diagnostic Kit: See Example 1 for the preparation method;
[0062] 1.2 National reference materials for HIV antibody and antigen: inspected and developed by China National Institute for the Control of Pharmaceutical and Biological Products, see Example 1 for description;
[0063] 1.3 Serum containing interfering substances: including 10 serums of common interfering substances hyperlipidemia, hemolysis, and jaundice, 10 serums each containing positive antibodies to related infectious diseases HAV, HBV, HIV, TP, and HP, and containing different anticoagulants heparin, EDTA, and 10 copies of sodium citrate plasma were collected, verified and stored by our company in relevant hospitals in Tianjin. The above serum or plasma were tested by more than two ELISAs and all were HIV antibody and antigen negative serum.
[...
Embodiment 3
[0082] Example 3: Preparation of HIV Antibody and Antigen Combined Rapid Kit 18:
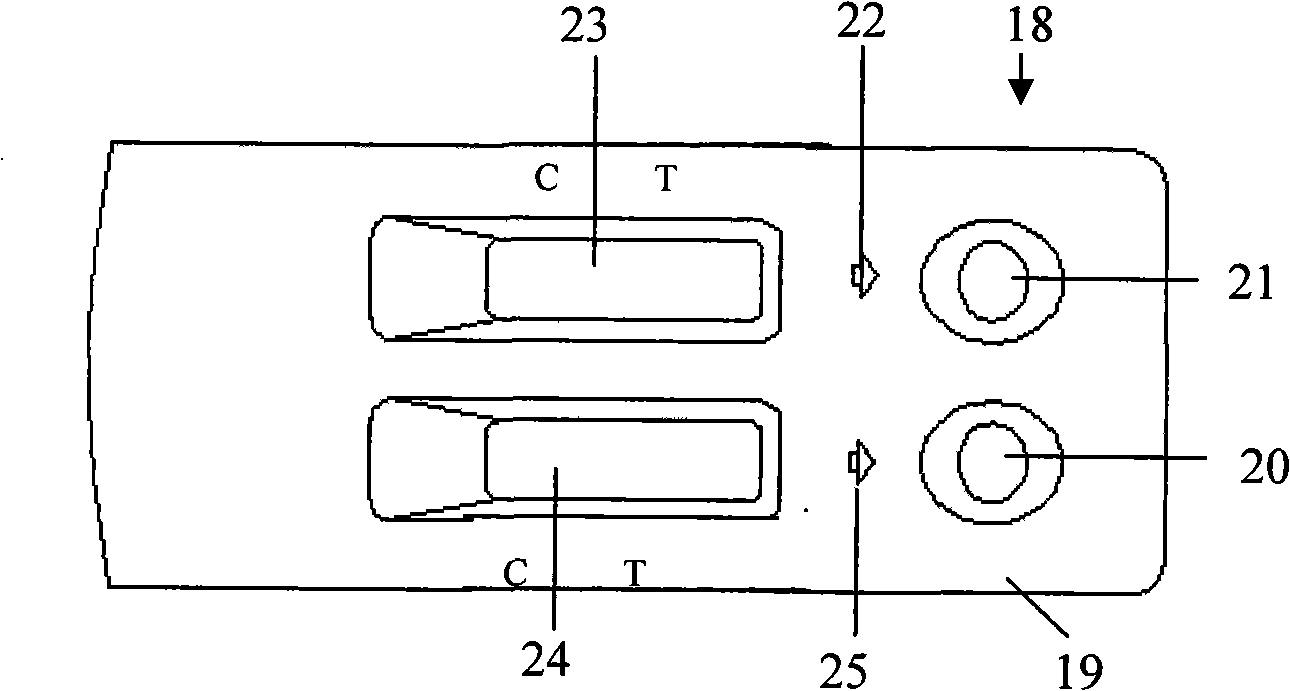
[0083] image 3 Front view of the cover of HIV Antibody and Antigen Combined Rapid Test Kit. Figure 4 Rear view of the lid of the HIV Antibody and Antigen Combined Rapid Test Kit. Figure 5 Bottom view of HIV Antibody and Antigen Combined Rapid Detection Kit. Such as image 3 , Figure 4 , Figure 5 Shown, human immunodeficiency virus (HIV) antibody and antigen combined rapid kit 18 are made of box cover 19, box bottom 31, box cover 19, box bottom 31 all are to adopt plastic injection molding to form integrally, box cover 19, box bottom The bottom 31 is two rectangular box-shaped bodies.
[0084] The length, width and height of the lid 19 are set with the length, width and height of the test paper A9 and the test paper B10, and two rectangular holes of the same size are set on the front left side of the lid 19 as the B reaction zone hole 23 and the A reaction zone Hole 24, on the right s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com