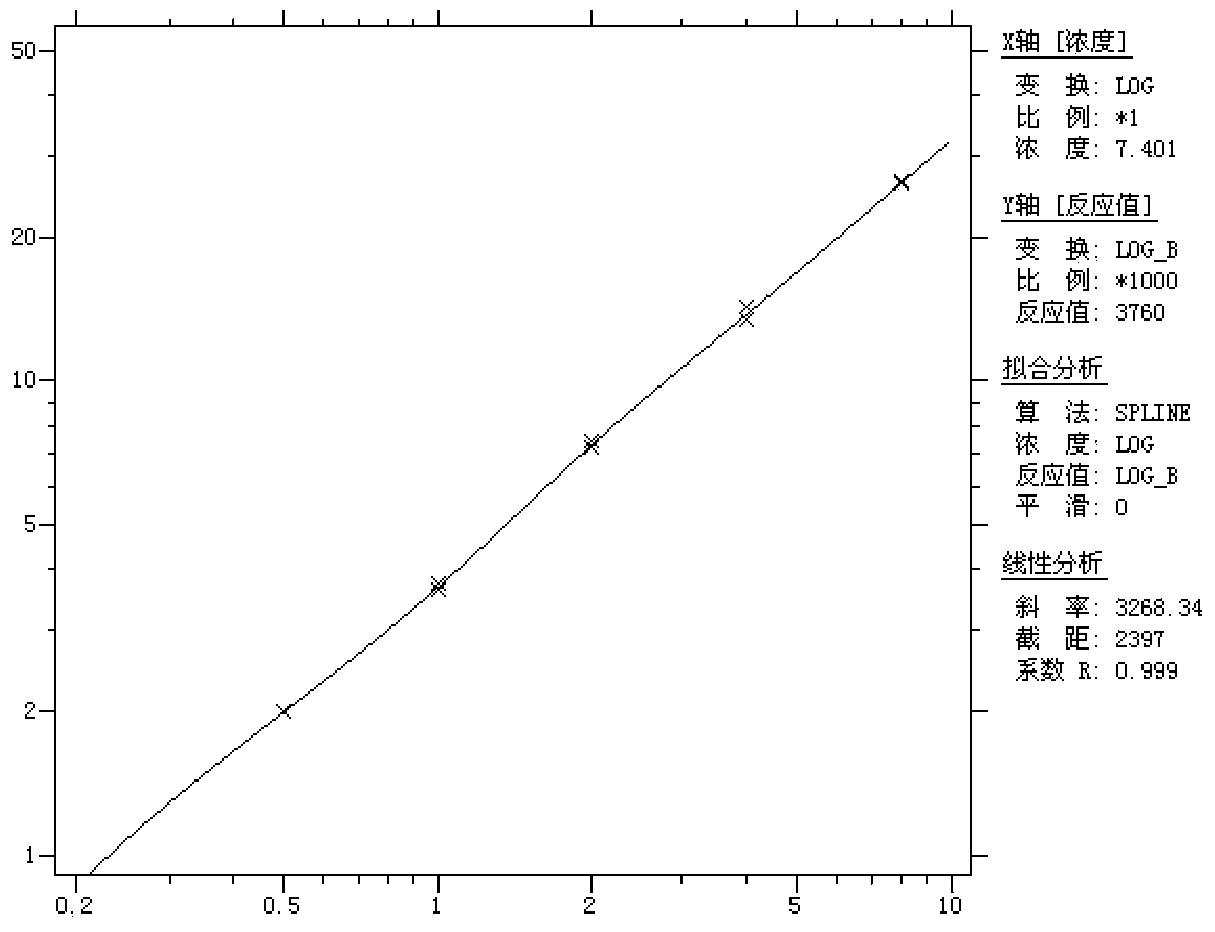
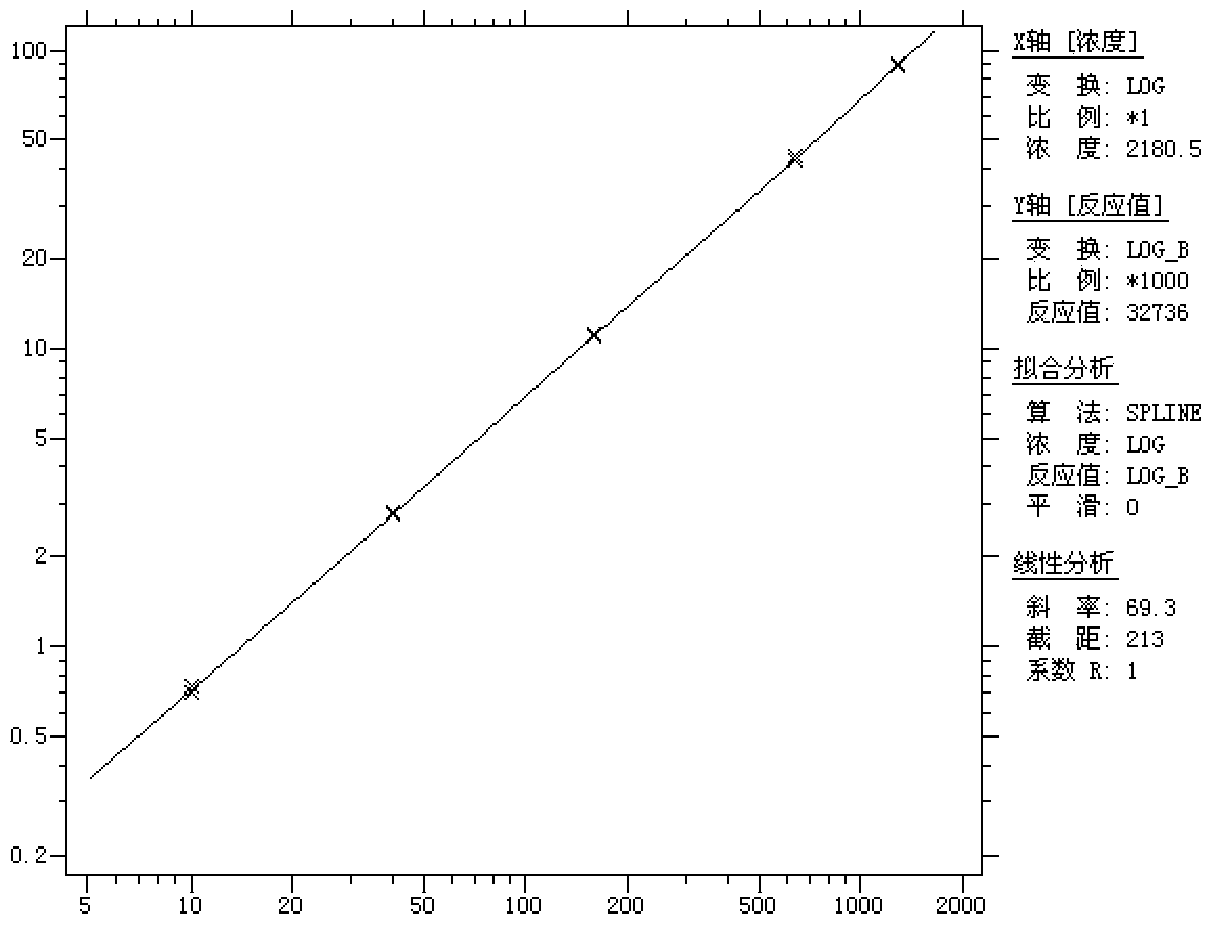
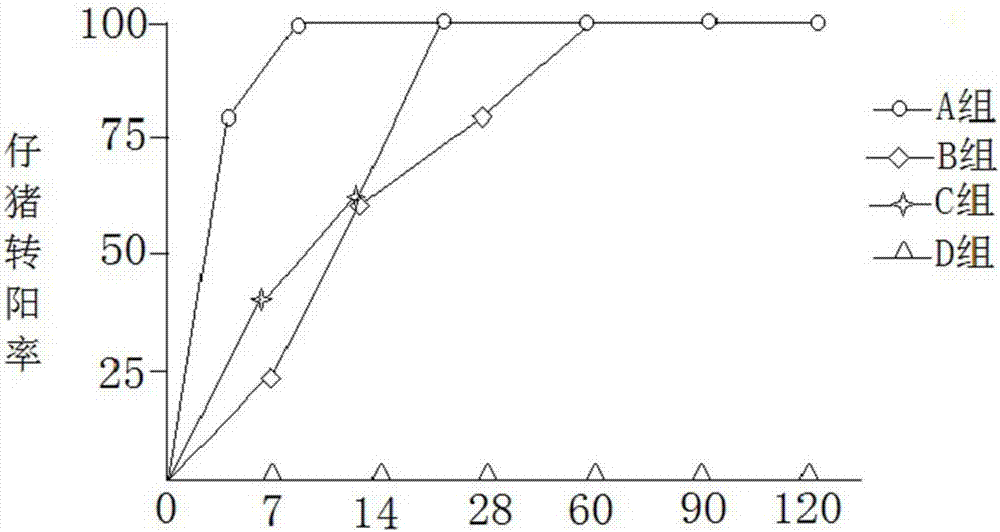
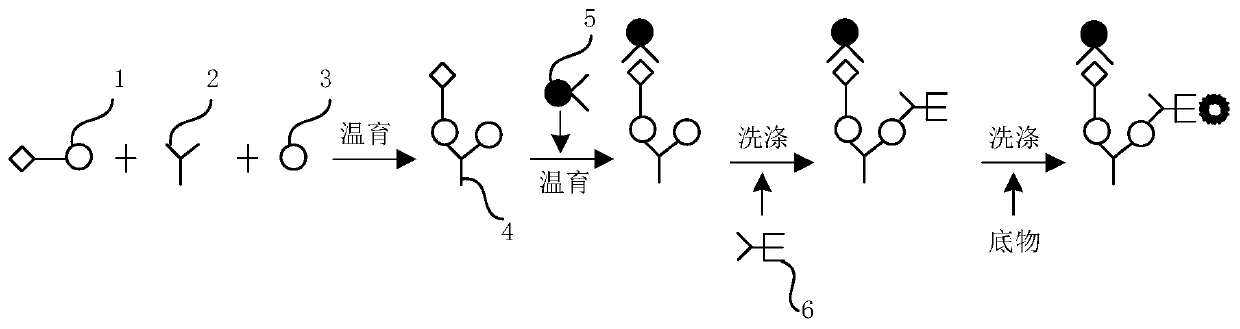
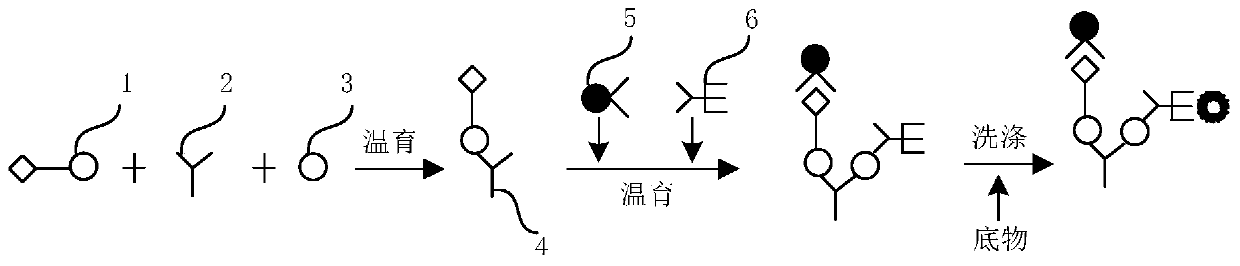
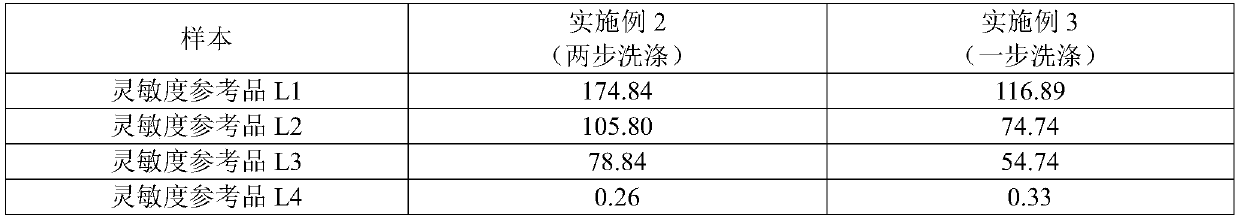
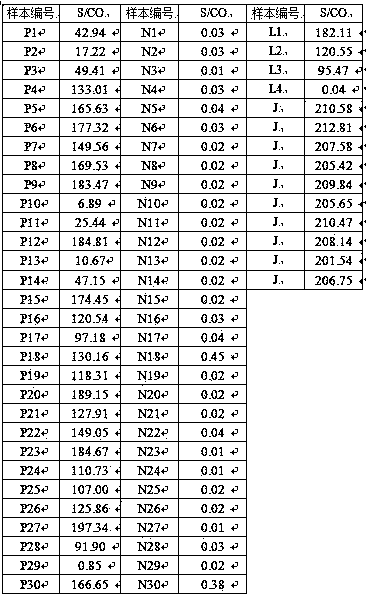
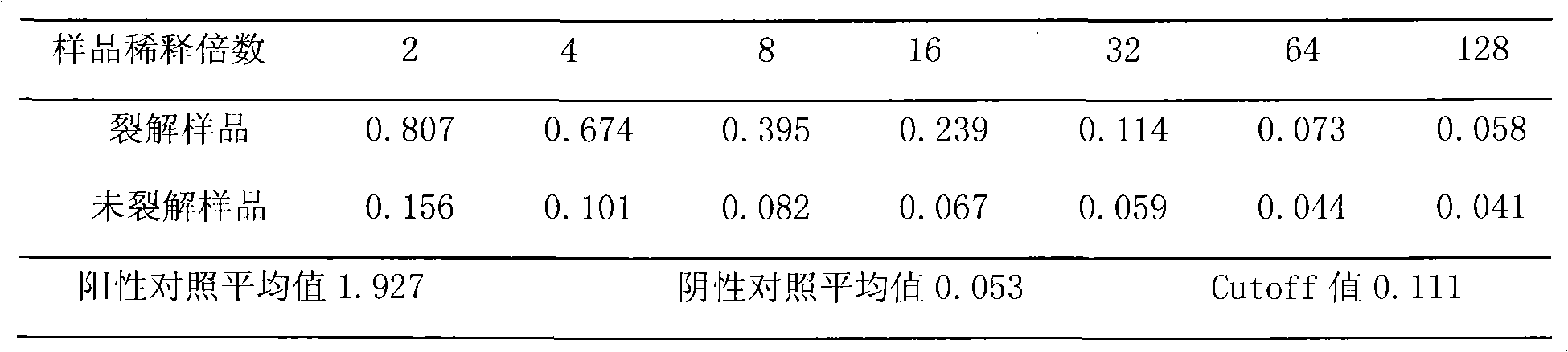
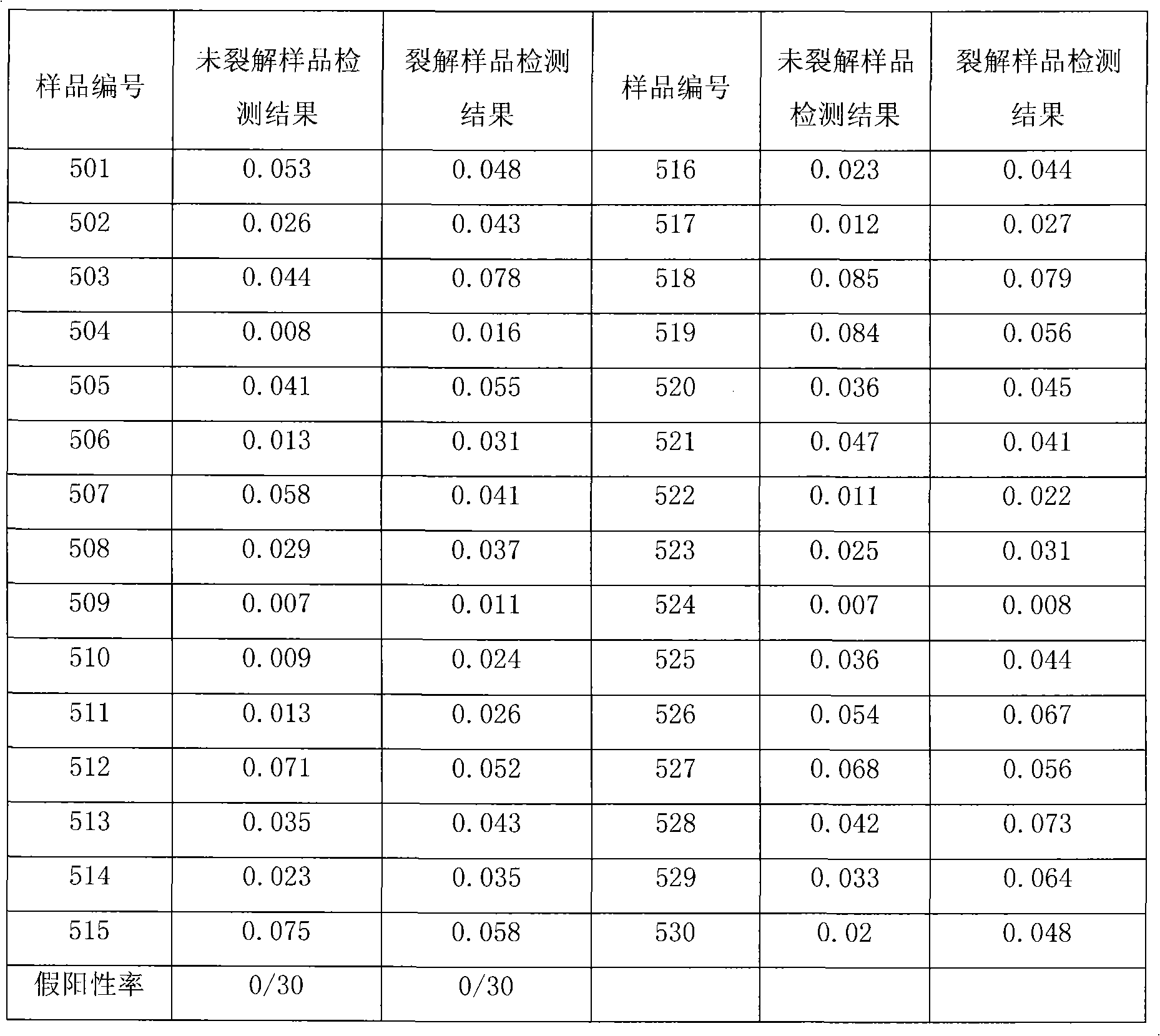
Patents
Literature
53results about How to "Shorten the window" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
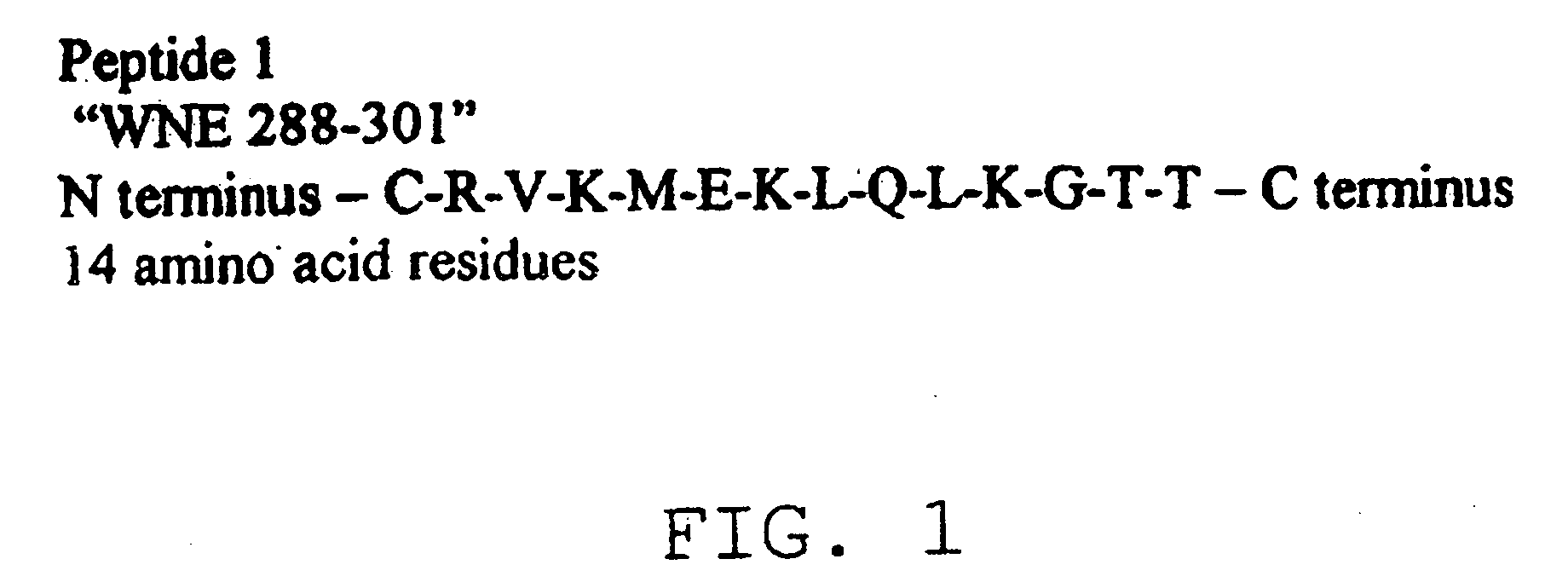
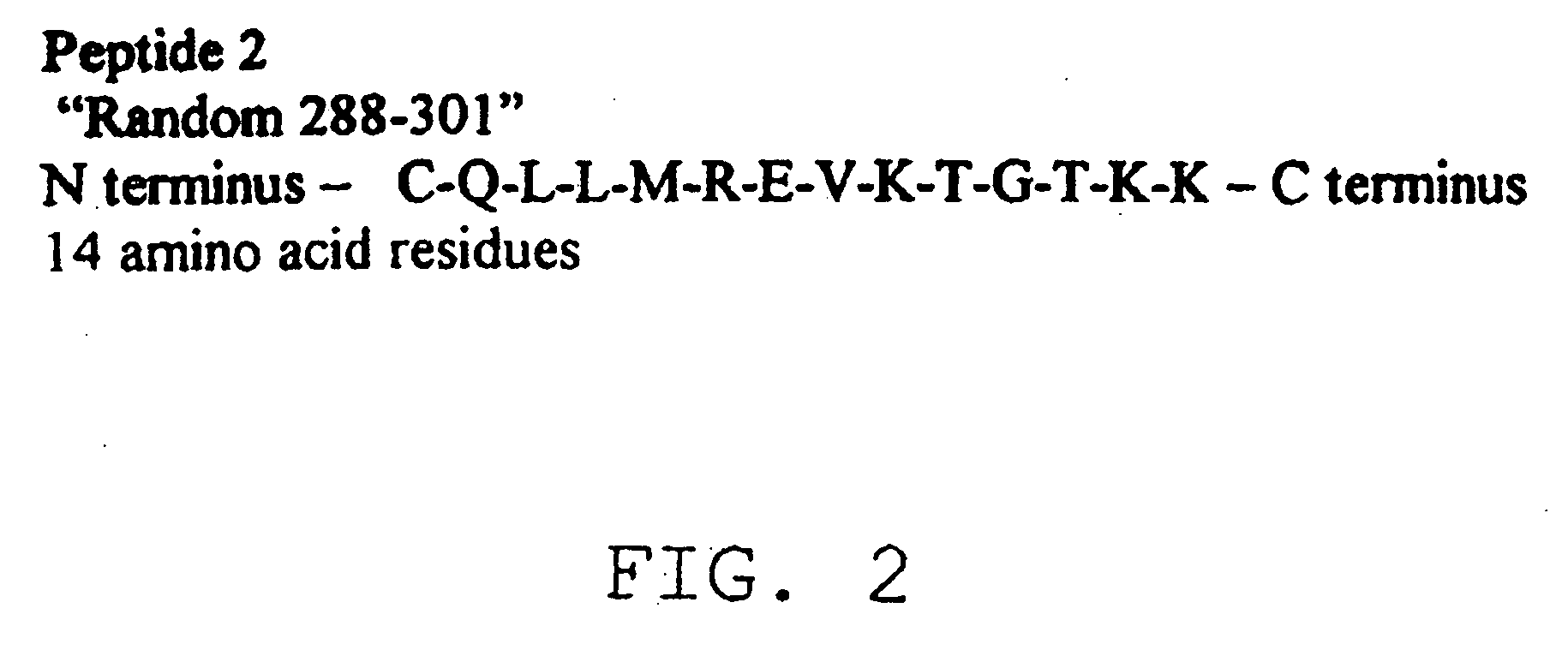
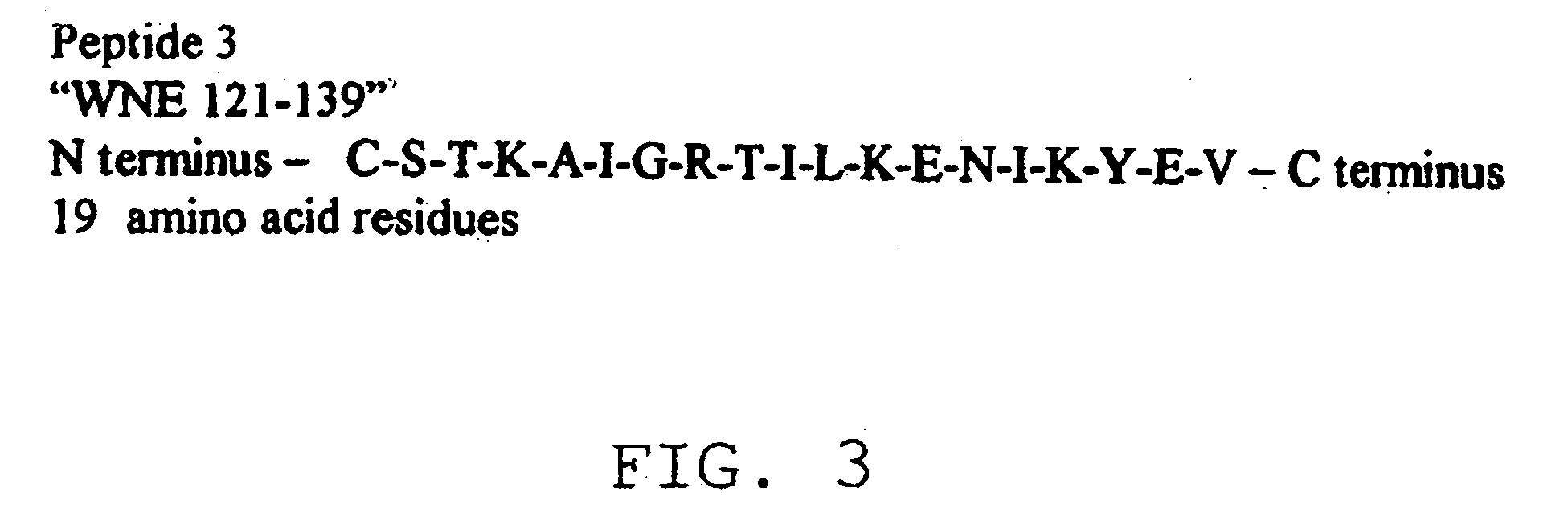
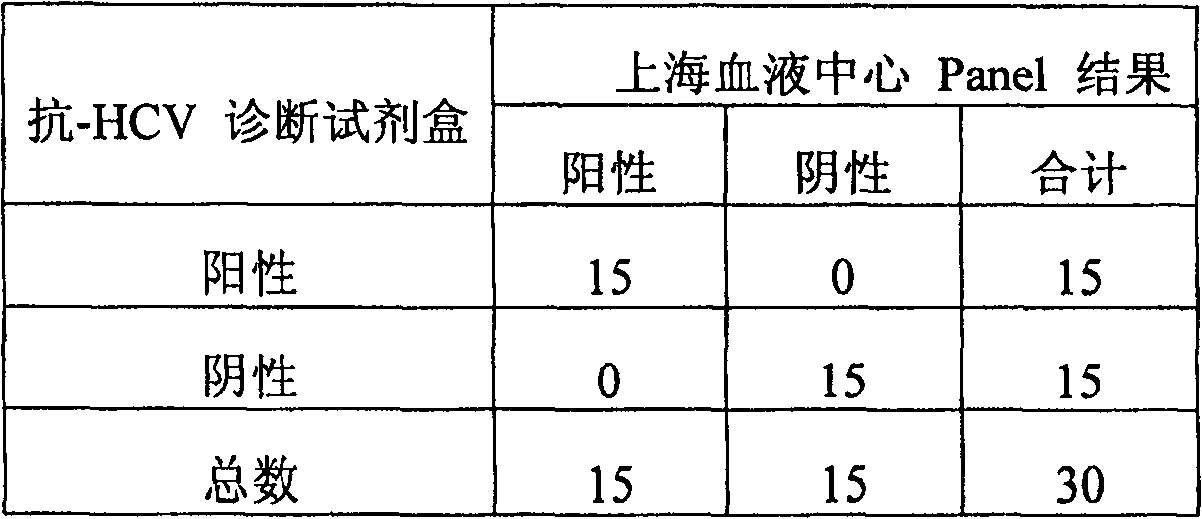
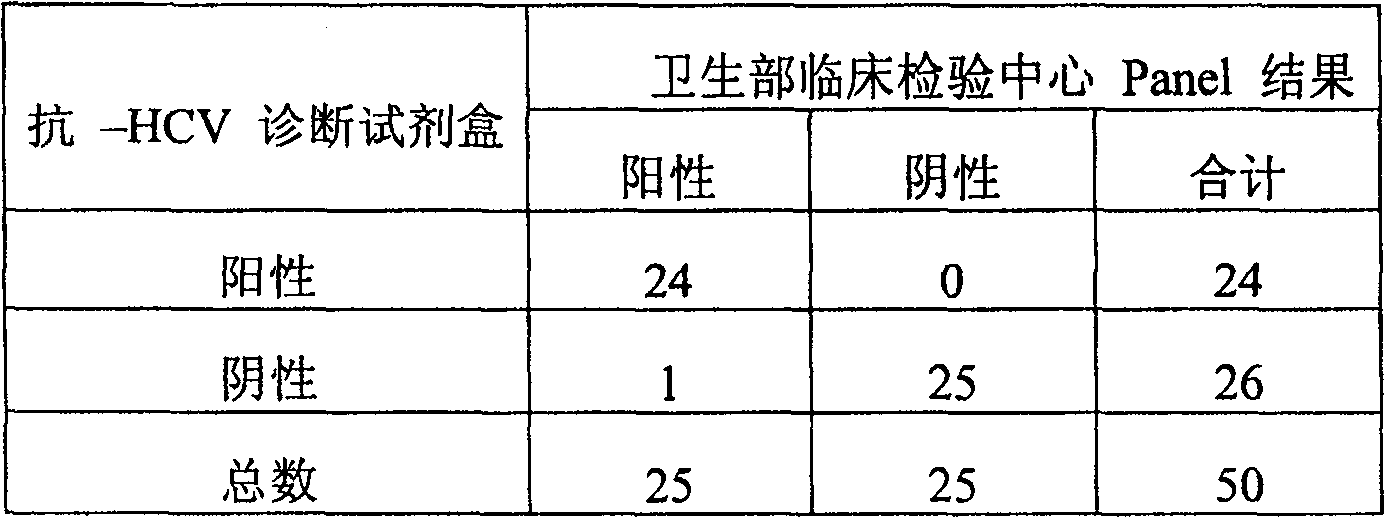
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
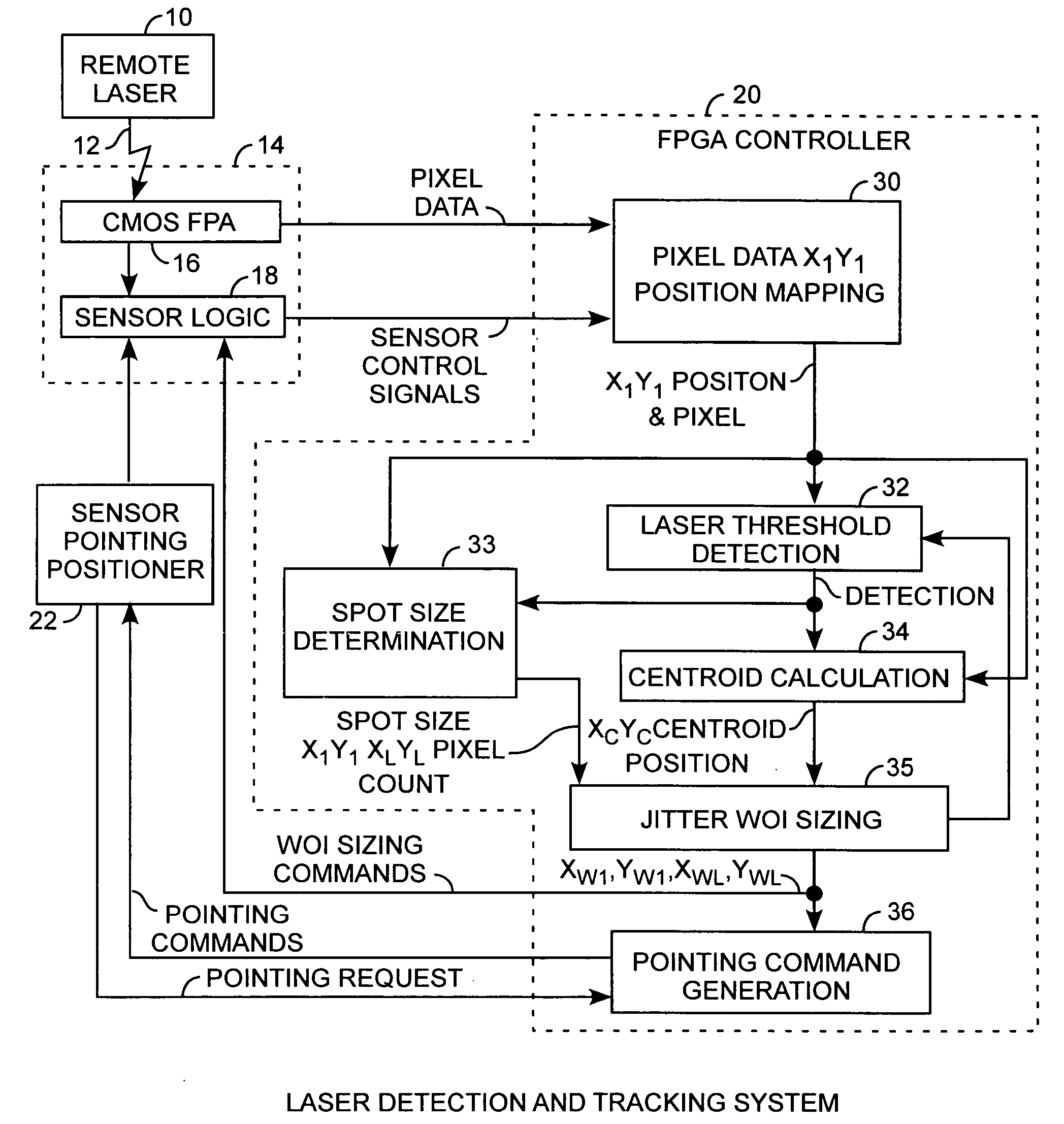
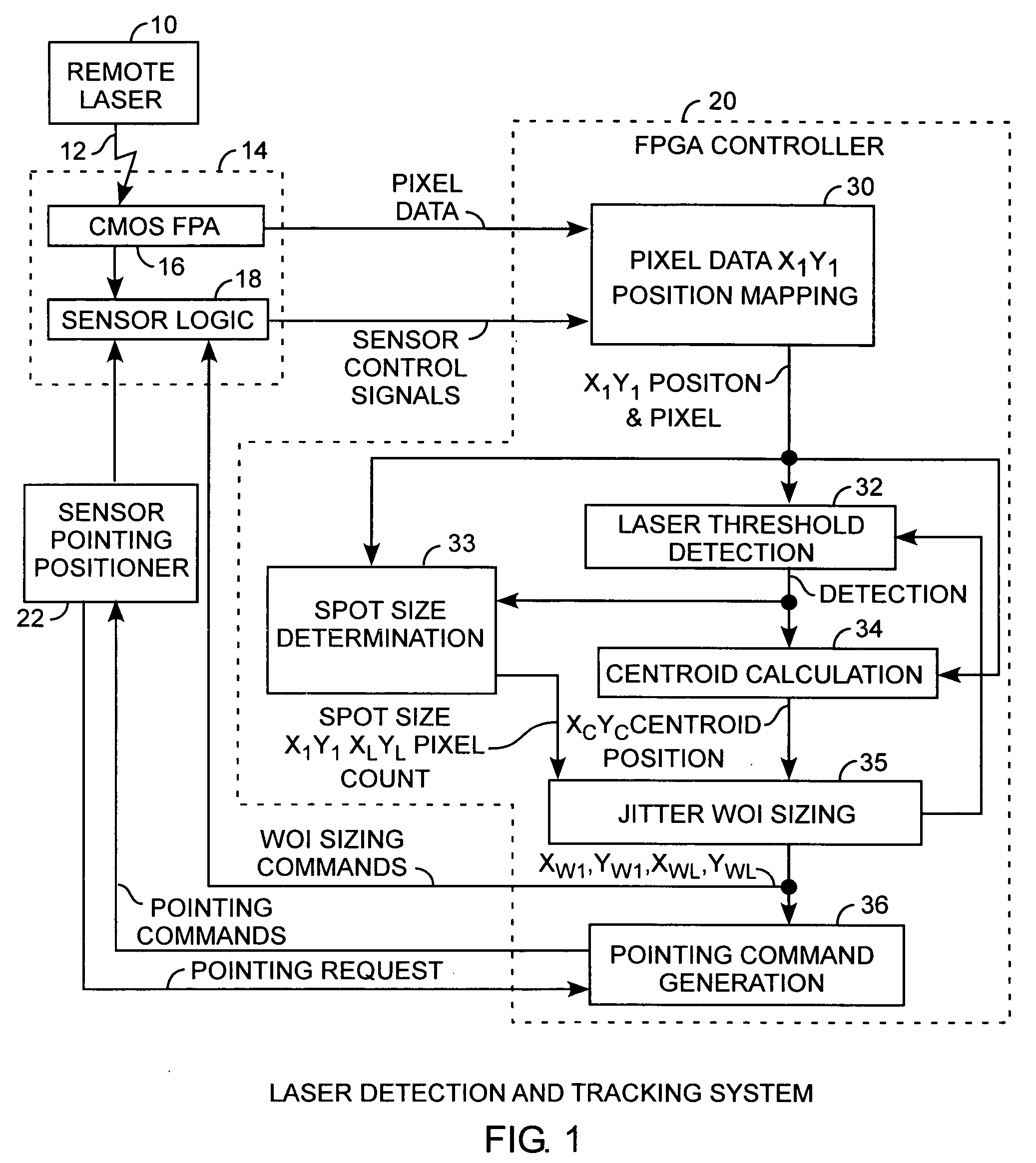
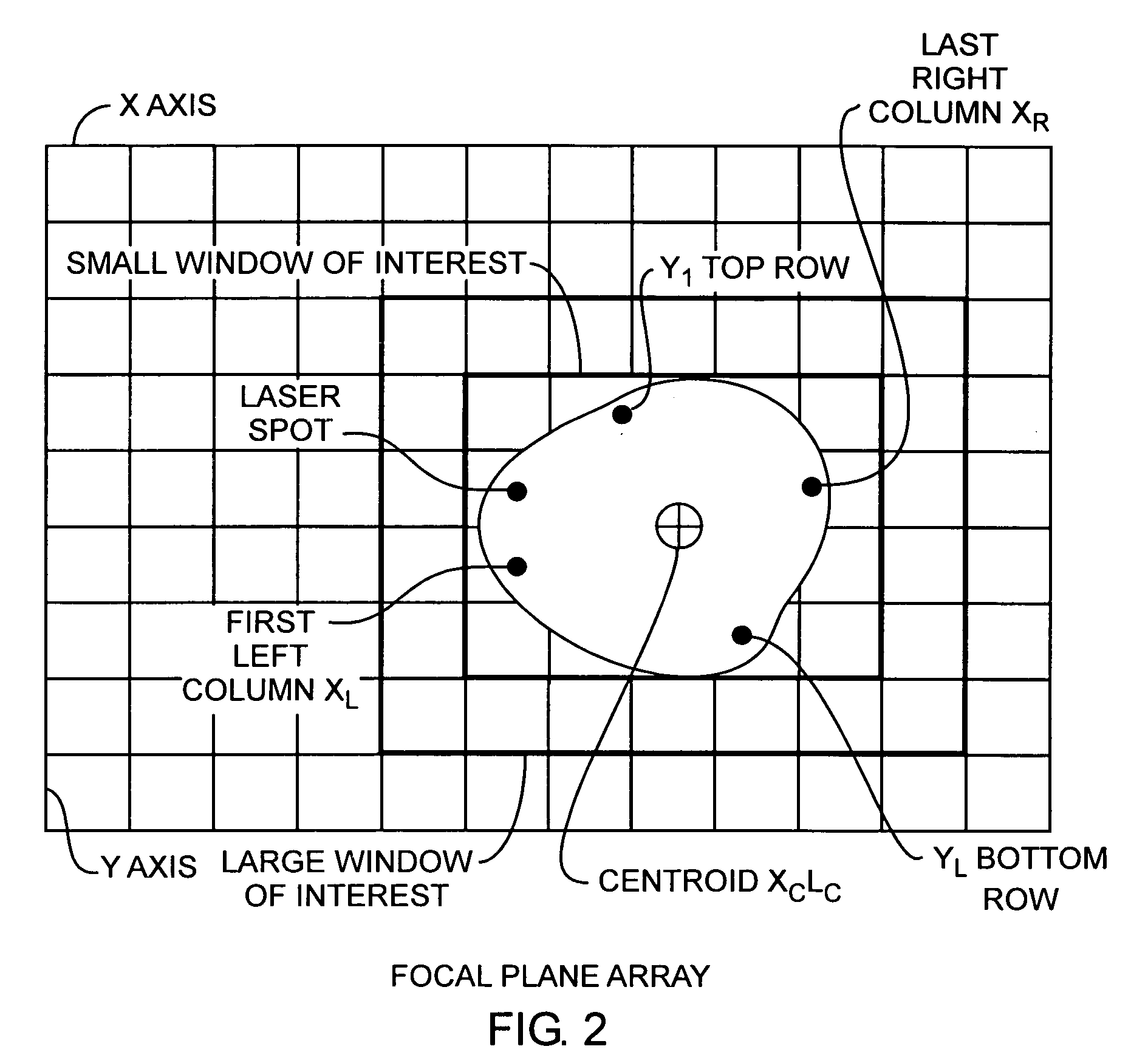
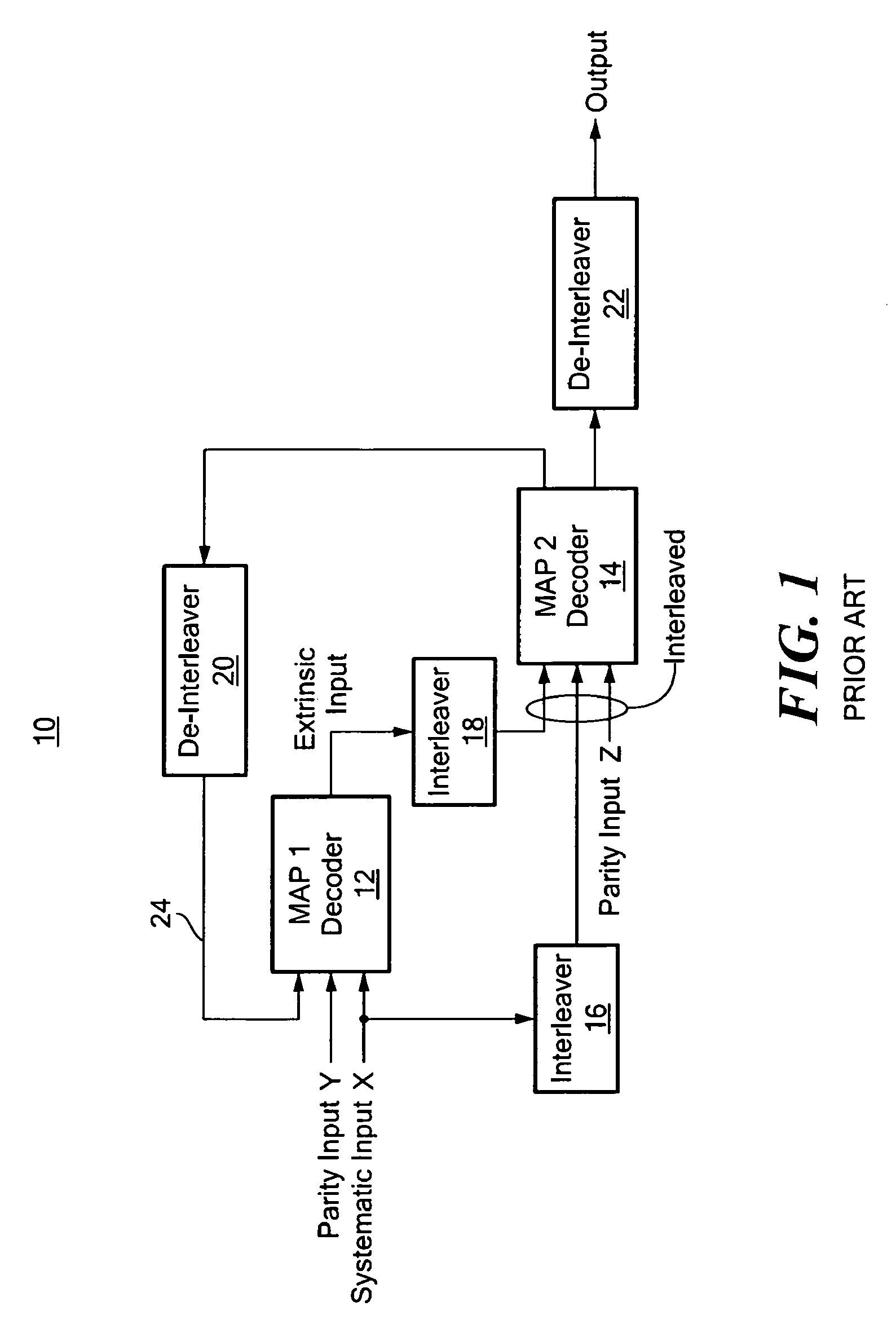
Electro-optical focal plane array digital sensor system
ActiveUS20070007436A1Increase frame rate pixel dataReduce jitterTelevision system detailsTelevision system scanning detailsLaser beamsField-programmable gate array
A laser beam detection and tracking system includes a focal plane array sensor providing pixel data and a field programmable gate array controller receiving the pixel data for determining a centroid of the illuminating spot of the impinging laser beam and for determining variably sized windows of interest disposed around the spot for selecting a subset of pixels of the array for communicating a subset of the pixel of a window frame of data from the array to the controller for increasing the array frame rate for reducing laser jitter during tracking of the beam for improved beam tracking performance.
Owner:THE AEROSPACE CORPORATION
Fluorescence microballoon immunochromatography testing card for testing HIV and preparation method
InactiveCN101576562AQuick checkSimple and fast operationBiological testingMicrosphereHIV p24 Antigen
The invention discloses a fluorescence microballoon immunochromatography testing card for quantitatively testing HIV and preparation method. The testing card comprises A and B test paper, a sample cushion, a glass fibrous membrane, a nitrocellulose membrane and drinking paper, wherein, the nitrocellulose membrane is thereon fixed with a test line and a quality control line for realizing the simultaneous test of HIV antibody and HIV p24 antigen. The invention takes nucleocapsid dual-structural light-emitting nano-particles compounded by silicon dioxide and fluorescent substance as marks and adopts immunochromatographic technique to realize quantitative immunoassay of HIV. In the testing process, fluorescence microballoon excitation light source is adopted to carry out excitation, after the emitted fluorescence passes through an optical filter device, all emission spectra are collected, aggregated and multiplied by CCD scanning technique or optical fiber technique, and are then converted into numerical signals, the concentration of the substance to be measured is automatically calculated by using the built-in analysis software in a fluorescence analyzer. The invention has the advantages of high sensitivity, precise quota, fast detection, convenient operation, and economy and practicality.
Owner:WUXI ZODOLABS BIOTECH
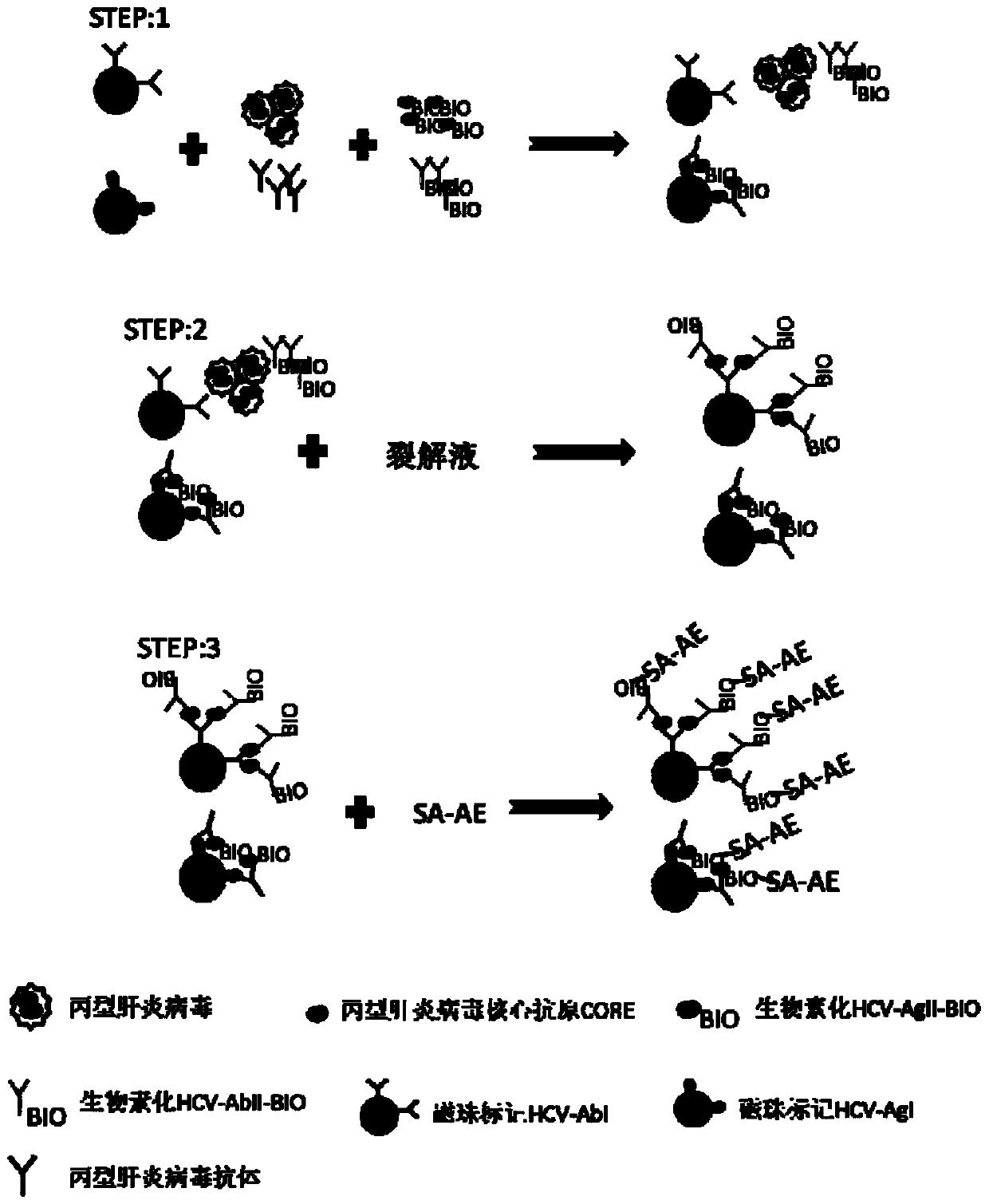
Micro-molecule indirectly labeled dual-antigen sandwich method for determining hepatitis C virus total antibody
The invention relates to a method for testing the total antibody of hepatitis C virus (HCV), based on dual-antibody interlayer method of small-molecule indirect mark, wherein said method is characterized in that: it uses small molecule matter as mark; uses dual-antigen interlayer method to test the total antibody of sample; and said method comprises: using small molecule material to mark HCV antigen; using HCV antigen to pack solid material; using single generate material to mark small molecule antibody or other material that combined with small molecule; the HCV antibody of sample can be reacted with HCV antigen of solid material surface and small molecule mark, to form dual-antigen interlayer composite; then reacting the small molecule with single report molecule, to make the composite with detected signal generate material. The invention can keep activity of antigen, with signal amplifying function and better specificity of dual-antigen interlayer mode, while it can detect variable kinds of antibodies, to improve the detecting sensitivity and specificity.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Gene detection reagent kit for SARS virus and its detection method
InactiveCN1468965AFacilitate early diagnosis"The shortened windowMicrobiological testing/measurementVenous blood specimenRNA extraction
The detection process of SARS virus of the gene detection reagent kit with rationing standard specific SARS virus nucleic acid as reference includes RNA extraction, PCR amplification and fluorescent detection. The venous blood sample, gargled liquid or respiratory tract secretion of the patient is used as the analysis sample directly, RNA of the sample is extracted as the nucleic acid template, and the template is used in fluorescent PCR amplification. The used fluorescent amplification detecting reagents includes one-step process RT-PCR buffering liquid, deoxynucleoside triphosphate (dNTP) mixture, specific amplification primer and specific probe. The method of the present invention is fast and convenient in detecting SARS virus.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE +1
Human immunodeficiency virus antigen and antibody combined detection kit, use and detection method thereof
InactiveCN106546732AShorten the windowImprove early detection rateBiological material analysisAntigenAntibody
The invention discloses a human immunodeficiency virus antigen and antibody combined detection kit, use and a detection method thereof. The detection kit comprises the following components: (a) magnetic particle coating matter, which includes an HIV-1 / M specific antigen, an HIV-1 / O specific antigen, an HIV-2gp36 antigen, and an HIV p24 antibody that coat the magnetic particles and exist in a magnetic particle diluent; (b) an enzyme marker, which inlcudes marker enzyme marked HIV-1 / M specific antigen, HIV-1 / O specific antigen, HIV-2gp36 antigen, and HIV p24 antibody in an enzyme marker diluent; (c) a sample treatment solution, which contains a nonionic surfactant; and (d) a test diluent, which is a buffer solution for diluting a to-be-tested sample. The kit can realize combined detection of human immunodeficiency virus antigens and antibodies, and has good specificity and high sensitivity.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
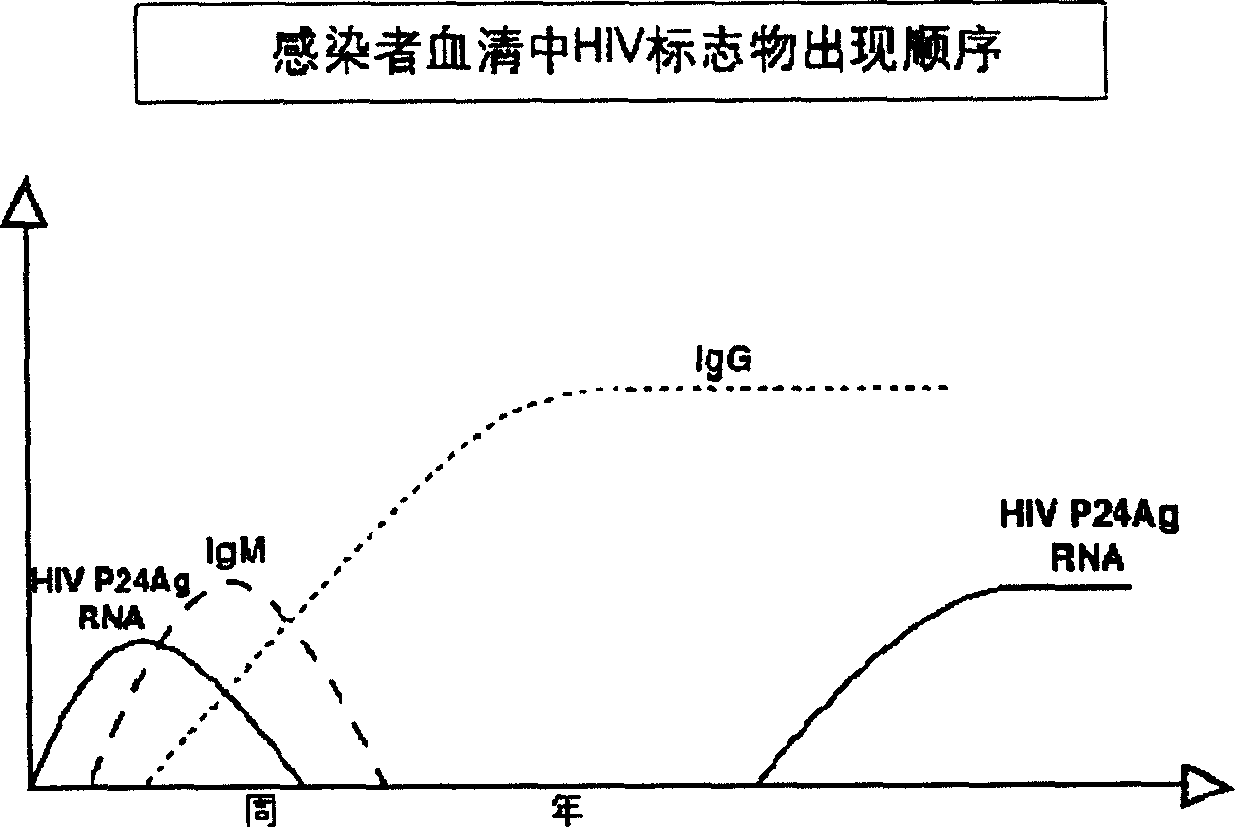
Double-labeling time-resolved fluorescence immunoassay method and kit for HIV (human immunodeficiency virus) antibody and HIV-1p24 antigen
InactiveCN103869073ARealize traceabilityEase of evaluationBiological material analysisBiotin-streptavidin complexTime resolved fluorescence immunoassay
The invention discloses a double-labeling time-resolved fluorescence immunoassay method and kit for HIV (human immunodeficiency virus) antibody-HIV-1p24 antigen. The analytical method mainly comprises the steps of preparing a solid phase carrier coated with an HIV recombinant antigen and an HIV-1p24 monoclonal antibody simultaneously; preparing biotin-labeled HIV-1p24 monoclonal antibody; preparing lanthanide 1-labeled HIV recombinant antigen; preparing lanthanide 2-labeled streptavidin; adding a calibrator containing HIV standard antibody and HIV-1p24 standard antigen or a sample to be tested into the solid phase carrier coated with the antigen and the antibody, adding the biotin-labeled HIV-1p24 antibody, incubating, washing, then adding the lanthanide 1-labeled HIV antigen and the lanthanide 2-labeled streptavidin, incubating again, washing, and adding enhancement solution for fluorescence detection. The analytical method overcomes the difficulty that the antigen and the antibody cannot be distinguished in the existing joint detection for HIV antigen and antibody, realizes simultaneous and quantitative detection of the HIV antibody and the HIV-1p24 antigen and therefore shortens the window phase of HIV detection.
Owner:GUANGZHOU FENGHUA BIOENG
Kit for detecting RNA of hepatitis E virus
InactiveCN101538619AEfficient detectionShorten the windowMicrobiological testing/measurementMicroorganism based processesBlood serumRNA
The invention relates to a kit for detecting RNA of a hepatitis E virus (HEV). The kit can carry out qualitative detection to an HEV RNA in blood serum sample and a fecal sample of man and animals by adopting fluorescent PCR technology, wherein the HEV nucleic acid is separated by adopting extracted nucleic acid in a silicone gel membrane method, and the detection of the HEV RNA is realized by adopting the fluorescent PCR technology.
Owner:BEIJING KINGHAWK PHARMA
Kit and process for PCR amplification detecting type 2 pig streptococcus virulence gene
InactiveCN101020929AEasy diagnosisConvenient researchMicrobiological testing/measurementDNA/RNA fragmentationVirulent characteristicsStreptococcus infection
The (Streptococcus suis Serotype 2 (SS2) virulence gene PCR amplification kit includes a SS2DNA contrast template, a PCR amplification detecting reagent, an agarose gel electrophoresis reagent and a DNA gradient standard. The PCR amplification detecting process includes the measurement of 7 important SS2 genes, diagnosing Streptococcus infection and SS2 infection based on whether to amplify specific Streptococcus gene, specific SS2 gene and 5 relevant important SS2 virulence genes, and predicting the virulence, invasiveness trend and disease prognosis fast and early. The present invention is favorable to the clinical diagnosis of borderline case and early warning, and is hopeful to be applied in emergency detection of human SS2 infection, etc.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
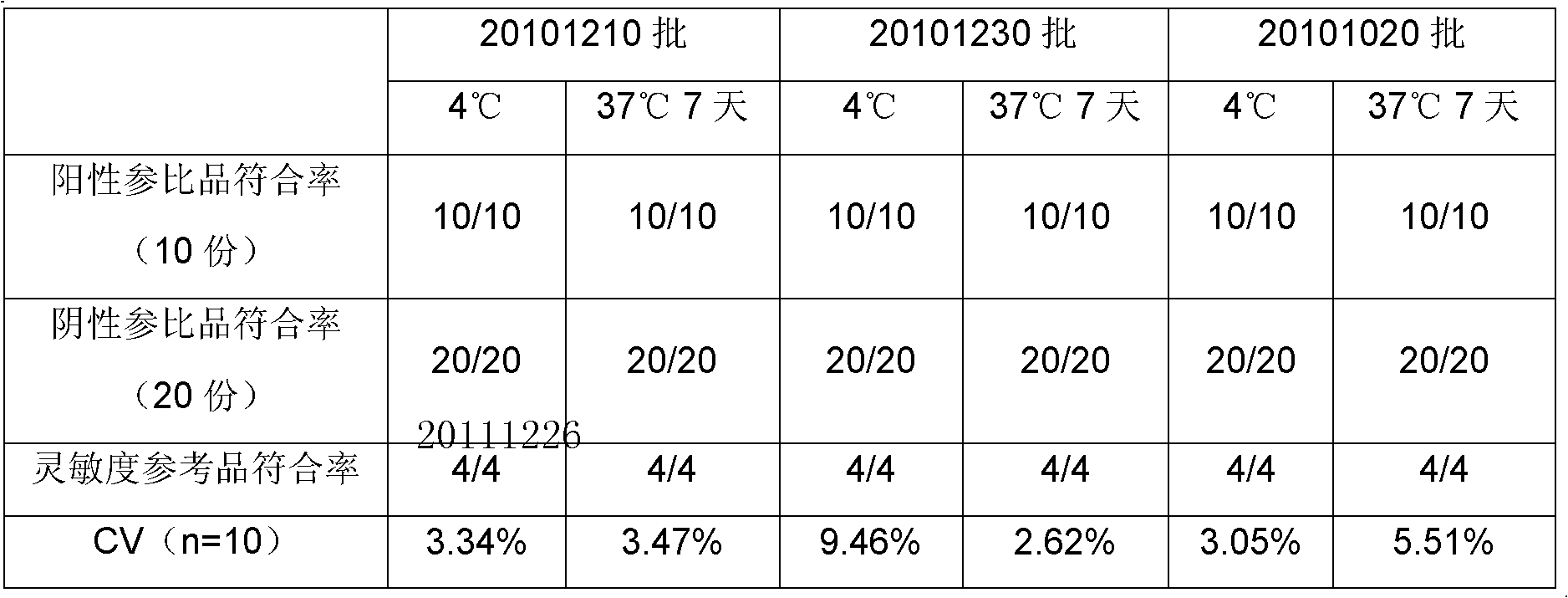
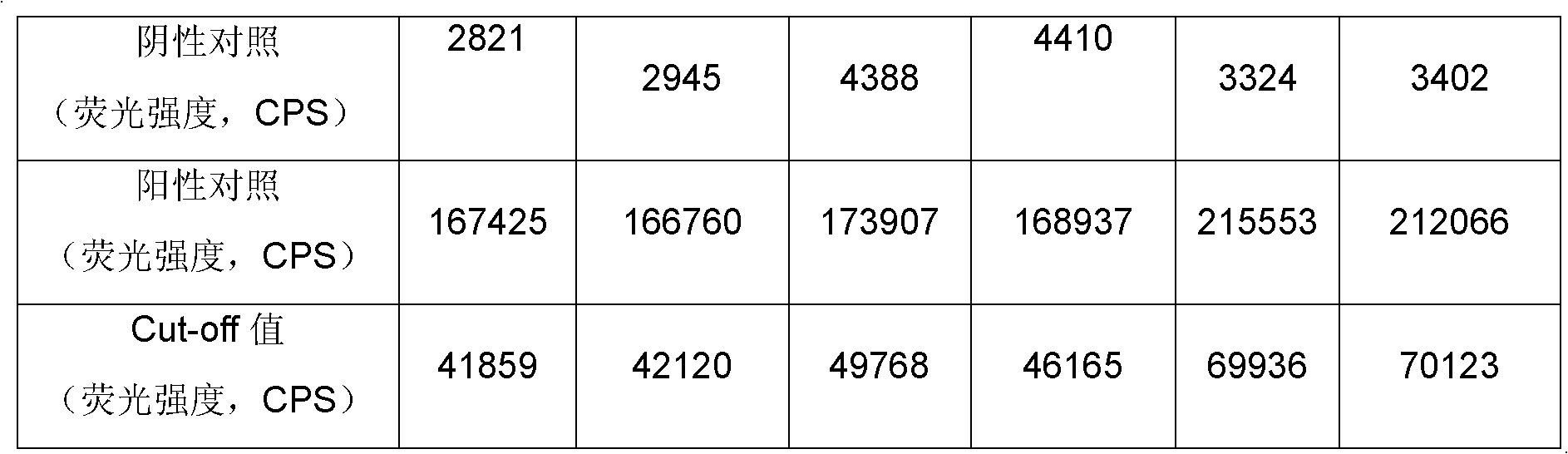
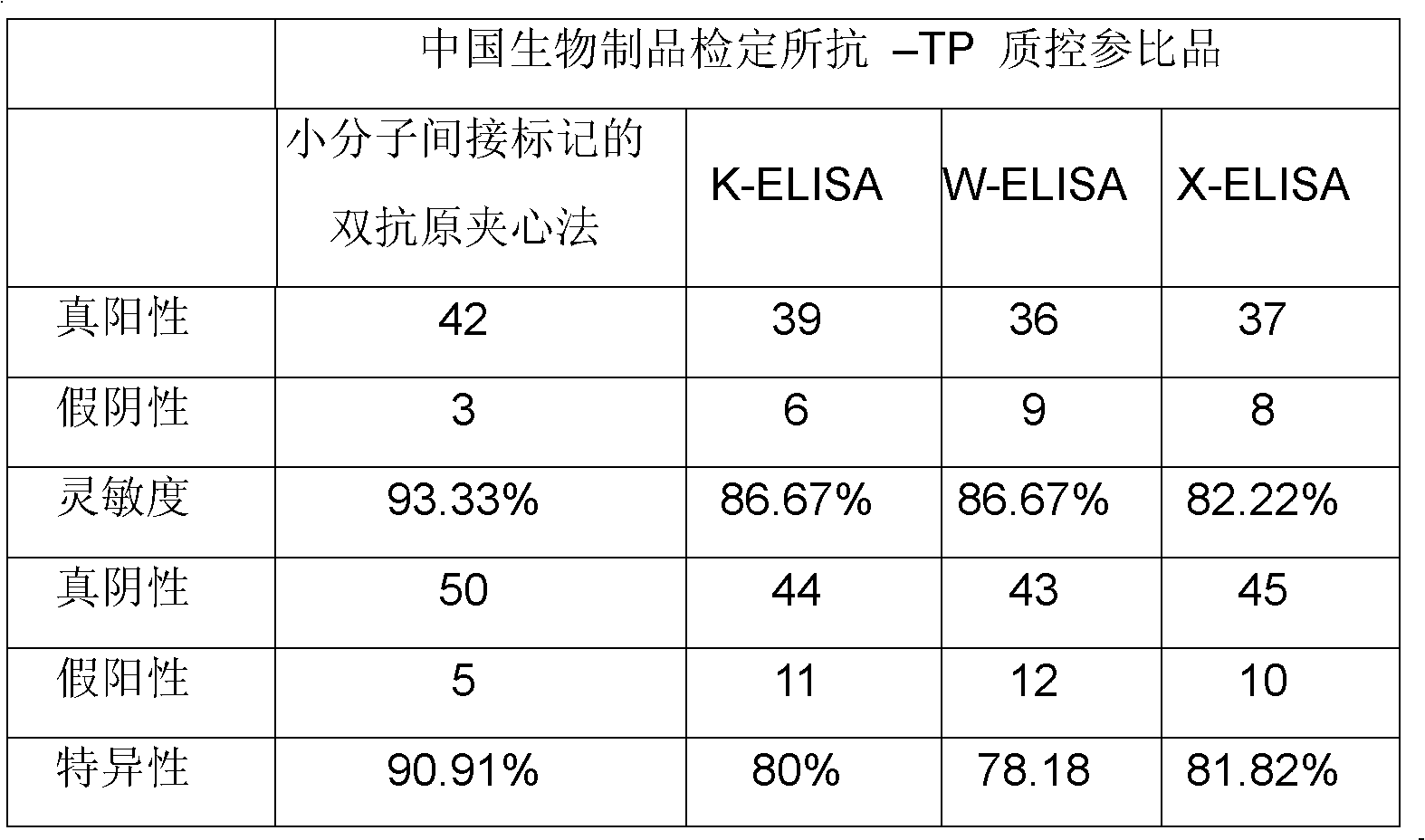
Treponema (TP) antibody detection method and detection kit thereof
The invention discloses a treponema (TP) antibody detection method and a detection kit of the TP antibody detection method. The detection method comprises the following steps of: (a) marking a first TP antigen by a small molecular substance; (b) coating a solid phase material by a second TP antigen; (c) marking an anti-small-molecular substance antibody or other substances capable of being combined with a small molecule by a signal generating substance, and then preparing a signal reporter molecule; (d) carrying out immune reaction between the second TP antigen on the surface of the solid phase material, the first TP antigen marked by the small molecular substance and the TP antibody in a sample, and then carrying out conjugation reaction between the signal reporter molecule and the small molecular substance, thus forming a detachable signal generation substance for detecting the content of the TP antibodies in the sample. The detection kit comprises (a) the first TP antigen marked by the small molecular substance, (b) the solid phase material coated with the second TP antigen, and (c) the signal reporter molecule. By adopting the TP antibody detection method and the detection kit of the TP antibody detection method, the sensitivity and specificity in the detection of the TP antibody can be effectively improved.
Owner:PERKINELMER MEDICAL DIAGNOSTICS PROD SHANGHAI
HIV viral antibody/antigen diagnostic reagent kit and preparing method thereof and detecting method
InactiveCN1673749AReduce the chance of infectionHigh sensitivityMaterial analysis by observing effect on chemical indicatorViral antibodyPhosphate
The present invention is HIV virus antibody / antigen diagnosis kit and its preparation process and detection method. The kit includes pre-coated enzyme-linked board with coated HIV antibody and P24 monoclonal antibody, rabbit anti-P24 polyclonal antibody labeled with biotin, conjugate of horseradish peroxidase labeled avidin and HIV antigen, detergent solution of phosphate buffer containing Tween, developing solution A of citrate buffer containing hydrogen peroxide, developing solution B of citrate buffer containing tetramethyl benzidine, terminating solution containing sulfuric acid solution, sample diluting solution containing phosphate buffer, normal human serum as positive contrast, HIV antibody serum as positive contrast, and P24 antigen serum as positive contrast. The present invention can detect HIV antibody and P24 antigen simultaneously in raised specificity and sensitivity and is suitable for diagnosis of human immune deficiency virus antibody.
Owner:北京科卫临床诊断试剂有限公司
Friendly fire avoidance/self-defense system
InactiveUS7055420B1No harmPrevent accidental engagementAircraft componentsDefence devicesCommunications systemEngineering
The invention is a defense system whereby all primary delivery weapons systems with some types of communications systems will be able to identify friends from foes and with the ability to take appropriate actions afterward so that no harm will come to friendly assets.
Owner:LOIS WILLIAM
Treponema pallidum IgG antibody biotin avidin enzyme-linked immune detection kit and preparation method thereof
ActiveCN104155450AAccurate detectionEfficient technical meansMaterial analysisProteomics methodsPositive control
The invention relates to a treponema pallidum IgG antibody biotin avidin enzyme-linked immune detection kit and a preparation method thereof, which relates to the syphilis detection. The kit comprises a concentrated washing liquid bottle, a negative and positive control bottle, a biotin mark antihuman IgG specific fragment gamma chain monoclonal antibody bottle, a horseradish peroxidase mark avidin bottle, a TMB coloured solution bottle A, a coloured solution bottle B, a reaction stopping solution bottle, a micropore plate coated with recombinant antigen. The treponema pallidum has infectious standard strain Nichol strain and treponema pallidum non-infectious standard strain Reiter strain, by using a proteomics method, the treponema pallidum proteomics can be separated through dimensional electrophoresis, protein with strong immunogenicity can be identified by different patient or infectious animal serum, and then an antigen marker can be searched. The antigen marker is used for syphilis confirmation test, so that diagnosis sensitivity and specificity can be increased, a window stage is shortened. The treponema pallidum antibody can be sensitively detected and enables quantitative measurement, and has the advantages of cheap price, simple operation and accurate result.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
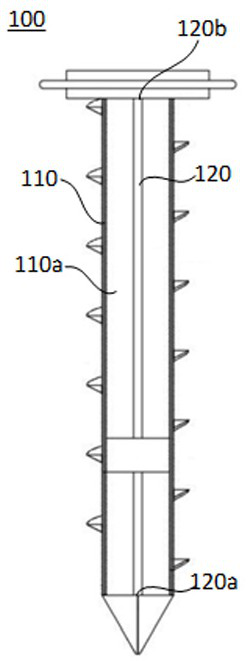
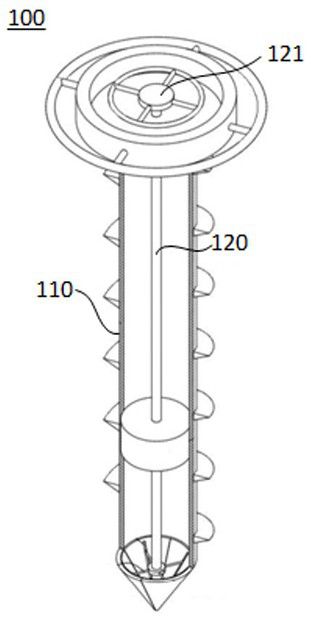
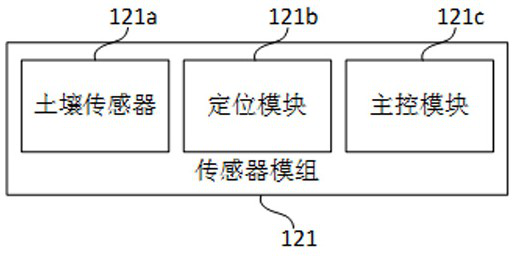
Sampling drill bit for soil remediation, soil information system and information management method
PendingCN112798333AGuaranteed traceabilityHigh data reliabilityWithdrawing sample devicesEarth material testingSoil scienceSoil remediation
The embodiment of the invention discloses a sampling drill bit for soil remediation. The sampling drill bit comprises a drill bit body and a sampler, wherein the drill bit body comprises a hollow part, and the sampler is arranged in the hollow part, so that sampling is completed from the first end of the sampler when the drill bit body performs tunneling; a sensor module is arranged at the second end of the sampler; the sensor module comprises a soil sensor, a positioning module and a main control module. The soil sensor is arranged in the sampler of the sampling drill bit, and the main control module serves as an independent node of the block chain to be connected into the block chain. Based on the sampling drill bit, the embodiment of the invention further provides a soil information system and an information management method for soil remediation, the data transparency and investigation data credibility in the soil sampling investigation process can be effectively improved, and timely and credible soil parameters can be provided for related parties of soil sampling investigation.
Owner:JIANGXI ACAD OF ECO-ENVIRONMENTAL SCI & PLANNING
Micro-molecule indirectly labeled dual-antigen sandwich method for determining hepatitis C virus total antibody
The invention relates to a method for testing the total antibody of hepatitis C virus (HCV), based on dual-antibody interlayer method of small-molecule indirect mark, wherein said method is characterized in that: it uses small molecule matter as mark; uses dual-antigen interlayer method to test the total antibody of sample; and said method comprises: using small molecule material to mark HCV antigen; using HCV antigen to pack solid material; using single generate material to mark small molecule antibody or other material that combined with small molecule; the HCV antibody of sample can be reacted with HCV antigen of solid material surface and small molecule mark, to form dual-antigen interlayer composite; then reacting the small molecule with single report molecule, to make the composite with detected signal generate material. The invention can keep activity of antigen, with signal amplifying function and better specificity of dual-antigen interlayer mode, while it can detect variable kinds of antibodies, to improve the detecting sensitivity and specificity.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Assembly type high-pile bearing platform for offshore wind power
PendingCN113047330AShorten the construction periodReduce volumeWind motor supports/mountsFoundation engineeringOffshore wind powerSteel tube
The invention discloses an assembly type high-pile bearing platform for offshore wind power. The assembly type high-pile bearing platform comprises a plurality of steel pipe piles embedded into bed rock, a lower bearing platform erected at the upper ends of the steel pipe piles, a plurality of pieces of lower-layer profile steel used for connecting every two adjacent steel pipe piles, a plurality of pieces of upper-layer profile steel correspondingly arranged at the tops of the steel pipe piles, an upper bearing platform formed by pouring concrete materials on the lower bearing platform and hardening the concrete materials, and a bolt assembly; pile holes corresponding to the steel pipe piles in a one-to-one manner are formed in the bottom of the lower bearing platform; the bottoms of the lower bearing platform are supported through the lower-layer profile steel; each piece of upper-layer profile steel is arranged in the radial direction of the corresponding steel pipe pile; the bolt assembly comprises a plurality of bolts in the axial direction; and the bolt assembly and the lower bearing platform are poured together. According to the assembly type high-pile bearing platform, the construction efficiency of the high-pile bearing platform structure can be improved, the window period time needed by construction is shortened, the influences of the offshore environment conditions on construction of the high-pile bearing platform are reduced, and the construction quality of the high-pile bearing platform can be better controlled.
Owner:CCCC THIRD HARBOR ENG
Hepatitis c virus detection kit
ActiveCN110261616AAvoid cross-reactionLow costBiological testingImmunoassaysRapid detectionHepacivirus
The invention relates to a hepatitis c virus detection kit. The hepatitis c virus detection kit comprises a first antibody and a second antibody for detecting a hepatitis c virus core antigen. The first antibody combines 95th-117th amino acid sequence of the hepatitis c virus core antigen aiming at an epitope or specificity in the 95th-117th amino acid sequence of the hepatitis c virus core antigen. The second antibody combines 55th-72th amino acid sequence of the hepatitis c virus core antigen aiming at the epitope or the specificity in the 55th-72th amino acid sequence of the hepatitis c virus core antigen. The kit has high sensitivity, good stability and simple operation, and can be used for rapid detection of early acute hepatitis c.
Owner:GUANGDONG FAPON BIOTECH CO LTD
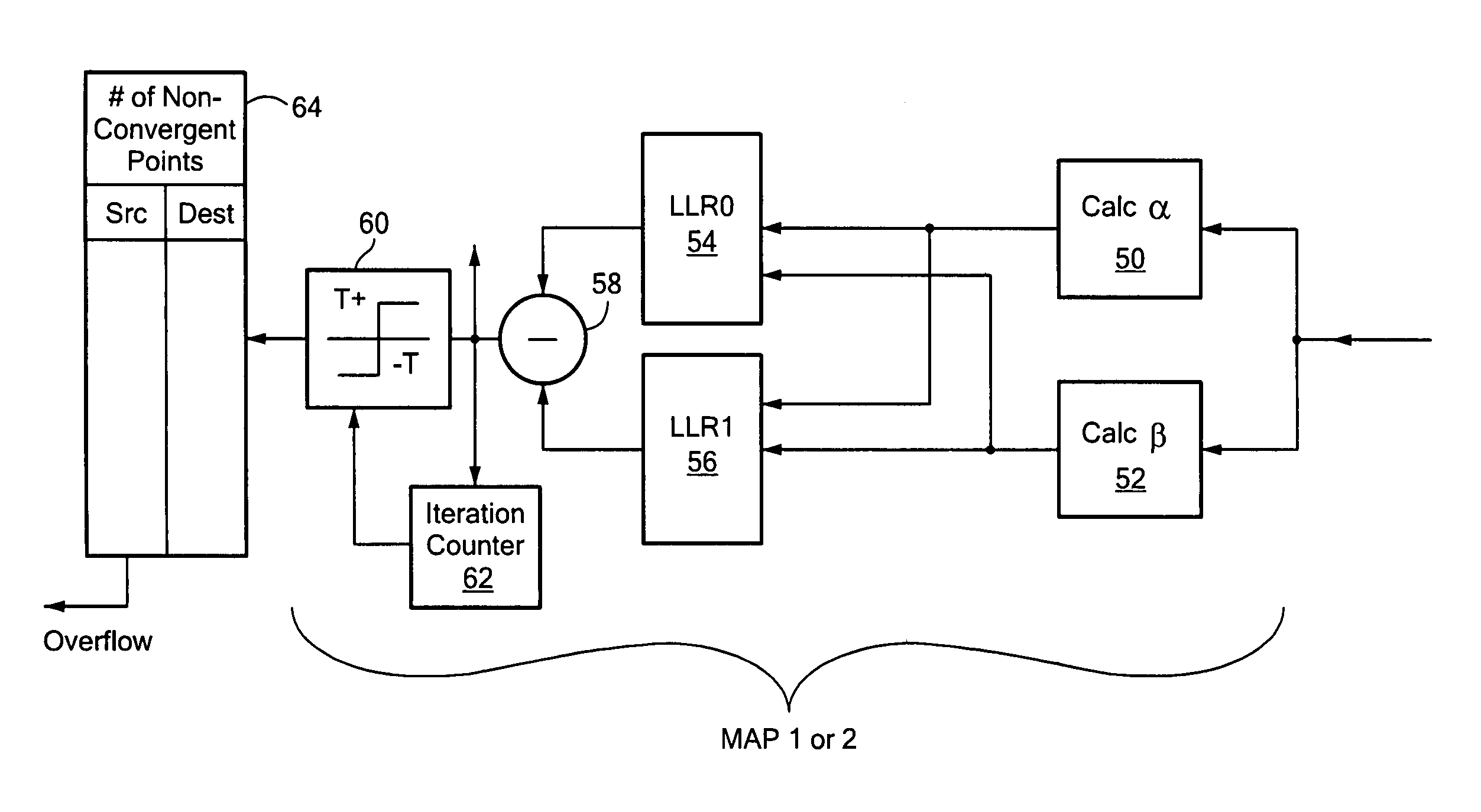
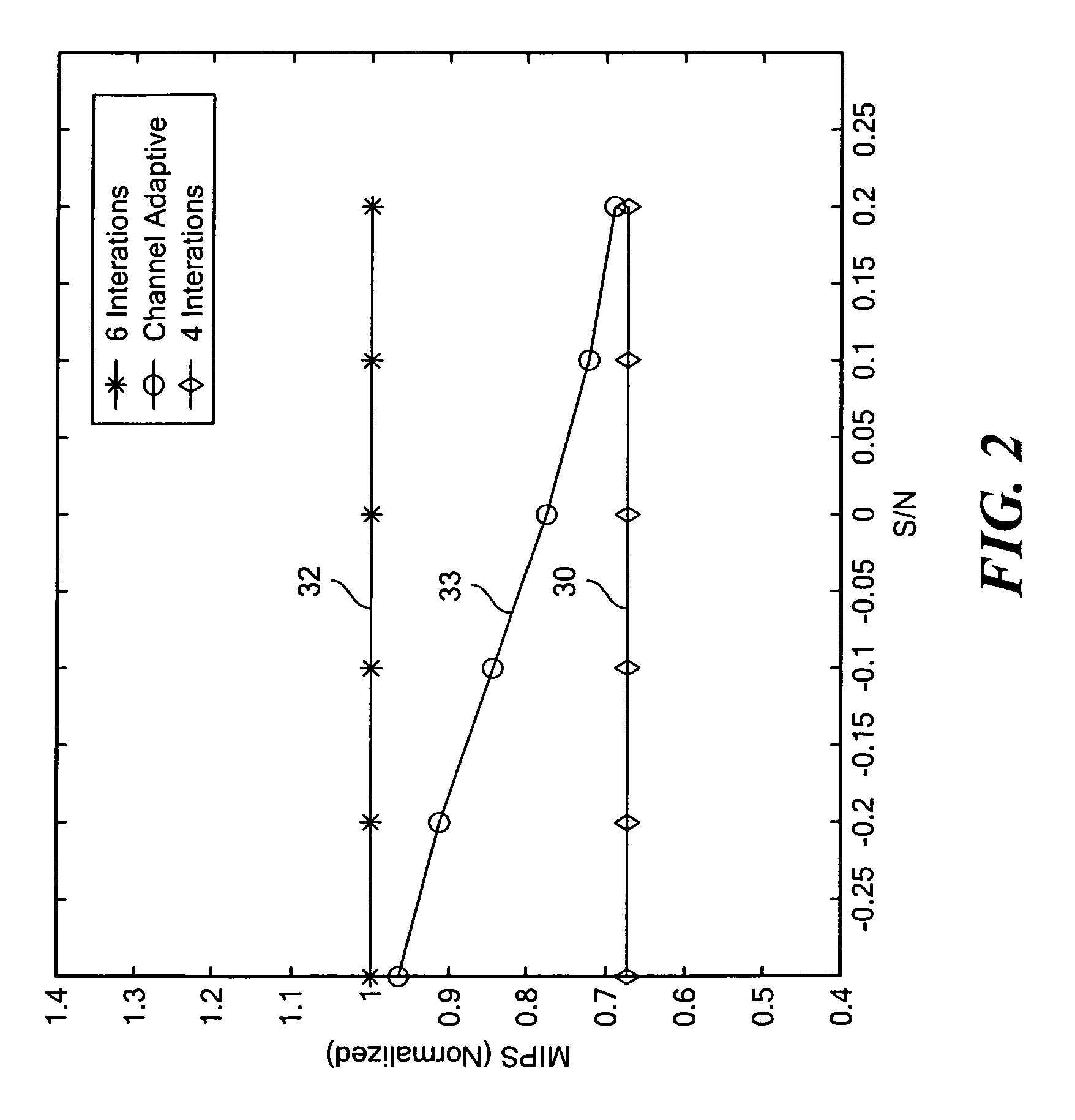
Channel adaptive iterative turbo decoder system and method
ActiveUS8321744B2Less powerImprove powerData representation error detection/correctionCode conversionLog likelihoodComputer science
A channel adaptive iterative turbo decoder for computing with MAP decoders a set of branch metrics for a window of received data, computing the forward and reverse recursive path state metrics and computing from the forward and reverse recursive path state metrics the log likelihood ratio for 1 and 0 and interleaving the decision bits; and identifying those MAP decoder decision bits which are non-convergent, computing a set of branch metrics for the received data, computing from the forward and reverse recursive path state metrics the log likelihood ratio (LLR) for 1 and 0 for each non-converged decision bit and interleaving the non-convergent decision bits.
Owner:ANALOG DEVICES INC
Immunopotentiator for swine atrophic rhinitis inactivated vaccine and preparation method of immunopotentiator
ActiveCN107261134ASuppress latent infectionAvoid latent infectionAntibacterial agentsBacterial antigen ingredientsPig farmsVitamin C
The invention discloses an immunopotentiator for a swine atrophic rhinitis inactivated vaccine. The immunopotentiator comprises a liquid containing dry powder immunity particles, and a solvent, wherein the liquid containing the dry powder immunity particles mainly comprises an aluminium hydroxide aqueous solution, ginsenoside, cholesterol, a vitamin C, diethylaminoethyl glucan, potassium iodide and polyethylene glycol according to an appropriate ratio by compatibility. Compared with the prior art, the prepared immunopotentiator for the swine atrophic rhinitis inactivated vaccine can effectively inhibit establishment and reactivation of latent infection of bordetella bronchiseptica and pasteurella multocida, can shorten a window phase of antibody production, improves an immunity effect of the swine atrophic rhinitis vaccine, prolongs the immunity duration, effectively avoids latent infection of swine atrophic rhinitis, provides guarantee for prevention and elimination of the swine atrophic rhinitis, can be widely applied to a pig farm for prevention and treatment of the swine atrophic rhinitis, and increases an economic benefit of the pig farm.
Owner:SHANGHAI CHUANG HONG BIOTECH
Hepatitis C virus antibody detection kit, preparation method and detection method
PendingCN111579781AFully combinedHigh detection sensitivityChemiluminescene/bioluminescenceAntibody combining siteBinding site
The invention relates to the technical field of in-vitro diagnosis and detection, in particular to a hepatitis C virus antibody kit, a preparation method and a detection method. The hepatitis C virusantibody kit comprises a reagent R1, a reagent R2 and a reagent R3, and the reagent R1 comprises a streptavidin magnetic particle solution; the R2 reagent comprises a mixed reagent of a biotinylated hepatitis C virus antigen and a hepatitis C virus recombinant antigen; and the R3 reagent comprises an alkaline phosphatase labeled hepatitis C virus monoclonal antibody solution. According to the invention, the hepatitis C virus antibody, the biotinylated hepatitis C virus antigen and the hepatitis C virus recombinant antigen form a 'sandwich' sandwich compound, so that the blocking of antigen andantibody binding sites due to the addition of magnetic particles and enzyme markers is avoided, and the sensitivity and specificity of the detection of the hepatitis C virus antibody are remarkably improved.
Owner:深圳市爱康试剂有限公司
Method and device for detecting HIV-1 P24 antigen based on silicon nitride (SiNx) solid nanopore
The invention discloses a method and a device for detecting an HIV-1P24 antigen based on a silicon nitride (SiNx) solid nanopore, and the method comprises the following steps: S1, a pretreatment step: carrying out pretreatment on a SiNx film chip with a window before use; s2, preparing a nanopore, namely preparing the required nanopore in the SiNx thin film chip by using a multistage current pulse breakdown method; s3, detecting an ion blocking current pulse signal generated by the nanopore in the sample; and S4, analyzing, detecting and calculating the detection limit of the sample. According to the present invention, the HIV-1P24 antigen molecule can be well detected, the spatial structure change of the sample during the translocation can be inferred, and the HIV-1P24 antigen molecule at the low concentration can be detected. The method and the device have the advantages of rapidness, detection timeliness, high sensitivity, high signal-to-noise ratio and high flux, further shorten the window period of HIV detection, and strive for earlier diagnosis opportunities for infected persons.
Owner:CHONGQING INST OF GREEN & INTELLIGENT TECH CHINESE ACADEMY OF SCI +1
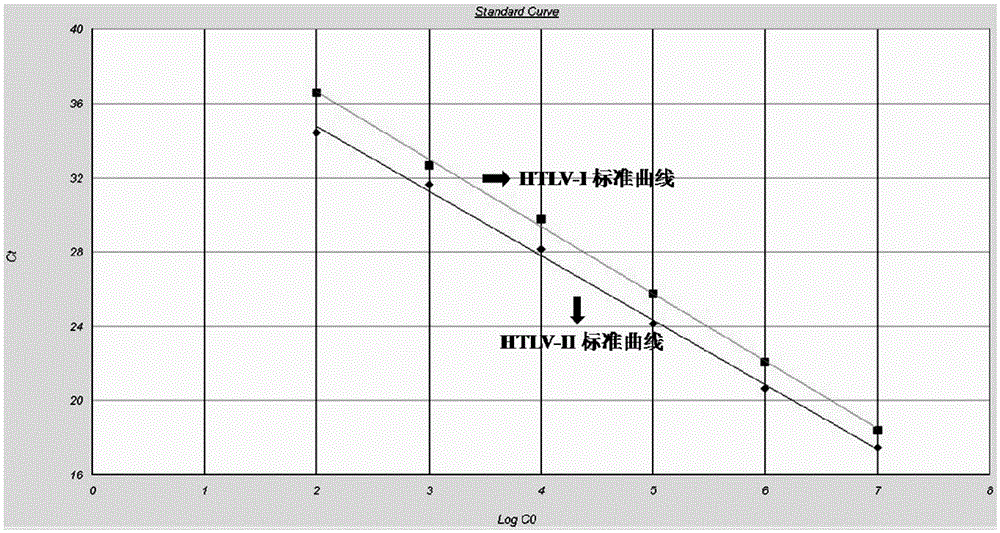
A kind of primer and method for detecting htlv-i and htlv-ii provirus in one tube
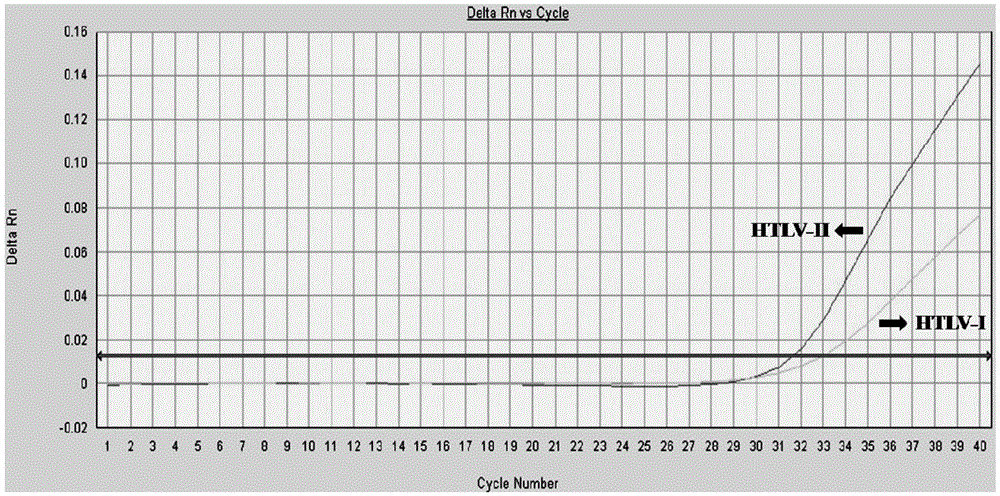
ActiveCN103898239BHelp monitorLimit transmissionMicrobiological testing/measurementDNA/RNA fragmentationConcentration ratioBiology
The invention provides a primer and a method for detecting HTLV (human T-lymphotropic virus)-I and HTLV-II proviruses. The primer comprises a specific primer and a probe for detecting an HTLV-I provirus and a specific primer and a probe for detecting an HTLV-II provirus, the two primers and the two probes are added in the same PCR (polymerase chain reaction) pipe according to a reasonable concentration ratio, and a sample is detected by means of optimized Real-time PCR reaction conditions. The detection method can be used for detecting whether HTLV infection exists before serological changes, thereby greatly shortening the window phase; meanwhile, compared with a conventional serological detection method, the detection method has the advantages of short detection period, high specificity, high accuracy, high sensitivity, little dependence on the conditions, low pollution risk and the like. The detection result is beneficial to monitor the change of the carrying capacity of the HTLV provirus of a patient and limit the propagation of the HTLV virus.
Owner:SHANGHAI ADICON CLINICAL LAB LNC
Method and device for detecting viral antigen of C type hepatitis
The invention discloses a method and device for detecting virus antigen of type-C hepatitis. The invention designs a compound antigen for type-C hepatitis virus made up of ten specificity antigens in epi-position, the reagent for detecting the type-C hepatitis virus antigen constructed by the inducer and by using the antigens, it can identifies the type-C hepatitis antigen in serum and plasma infected by the type-C hepatitis virus through the detection. The reagent box has a high specificity and low false negative rate and false positive rate.
Owner:KUNMING KELAISEN BIOLOGICAL TECH
Receptor reagent for detecting novel coronavirus and application thereof
PendingCN113391065AReduce pollutionShorten the windowChemiluminescene/bioluminescenceColor/spectral properties measurementsReceptorCoronavirus antibody
The invention relates to a receptor reagent for detecting novel coronavirus and application thereof. The receptor comprises receptor microspheres capable of reacting with active oxygen to generate detectable chemiluminescence signals; the receptor microsphere is filled with a chemiluminescence agent, and the surface of the receptor microsphere is connected with a novel coronavirus antibody 1. The kit containing the receptor reagent is used for detecting the novel coronavirus by using a double-antibody sandwich method, the window period can be effectively shortened, the detection result is quick, and the result is reported within 30 minutes; the detection flux is large, can reach 200-500 test / hour, is suitable for large-scale sample detection, and can reduce the pollution of high-risk viruses at the same time.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Genetic detection kit for communicating hydrocephalus
InactiveCN104561324AFacilitate early diagnosis"The shortened windowMicrobiological testing/measurementDiagnosis earlyVenous blood specimen
The invention discloses a genetic detection kit for communicating hydrocephalus. The kit comprises a RT-PCR buffer, a deoxy nucleoside triphosphate mixture, specific amplification primers and probes. The kit is convenient to sample, and venous blood samples of a to-be-detected patient can be sampled; the fluorescence amplification detection kit has the advantages of high sensitivity and strong specificity and can be used for early diagnosis; and the kit is of great significance for giving the early warning of patients with communicating hydrocephalus and taking effective treatment means.
Owner:申爱华
Hepatitis C virus (HCV) antigen and antibody combined detection kit
The invention discloses a hepatitis C virus (HCV) antigen and antibody combined detection kit. The kit comprises magnetic particles, a sample diluent, a negative control, an antigen positive control,an antibody positive control and an enzyme conjugate, the magnetic particles are coated with recombinant HCV virus antigens and mouse anti-human HCV core antigen monoclonal antibodies; the antibody positive control contains HCV antibody positive human plasma; and the antigen positive control contains HCV recombinant core antigens. According to the kit, the chemiluminescence and magnetic particle separation technologies are combined, and the HCV recombinant core antigens and the HCV antibodies in serum are detected at the same time, by matching with a full-automatic chemiluminiscence instrumentof the Zhengzhou Antu Bio-Engineering Co.,Ltd, and can be used as a primary screening test for clinical detection, so that the window period is further shortened, and the HCV propagation is effectively controlled.
Owner:AUTOBIO DIAGNOSTICS CO LTD
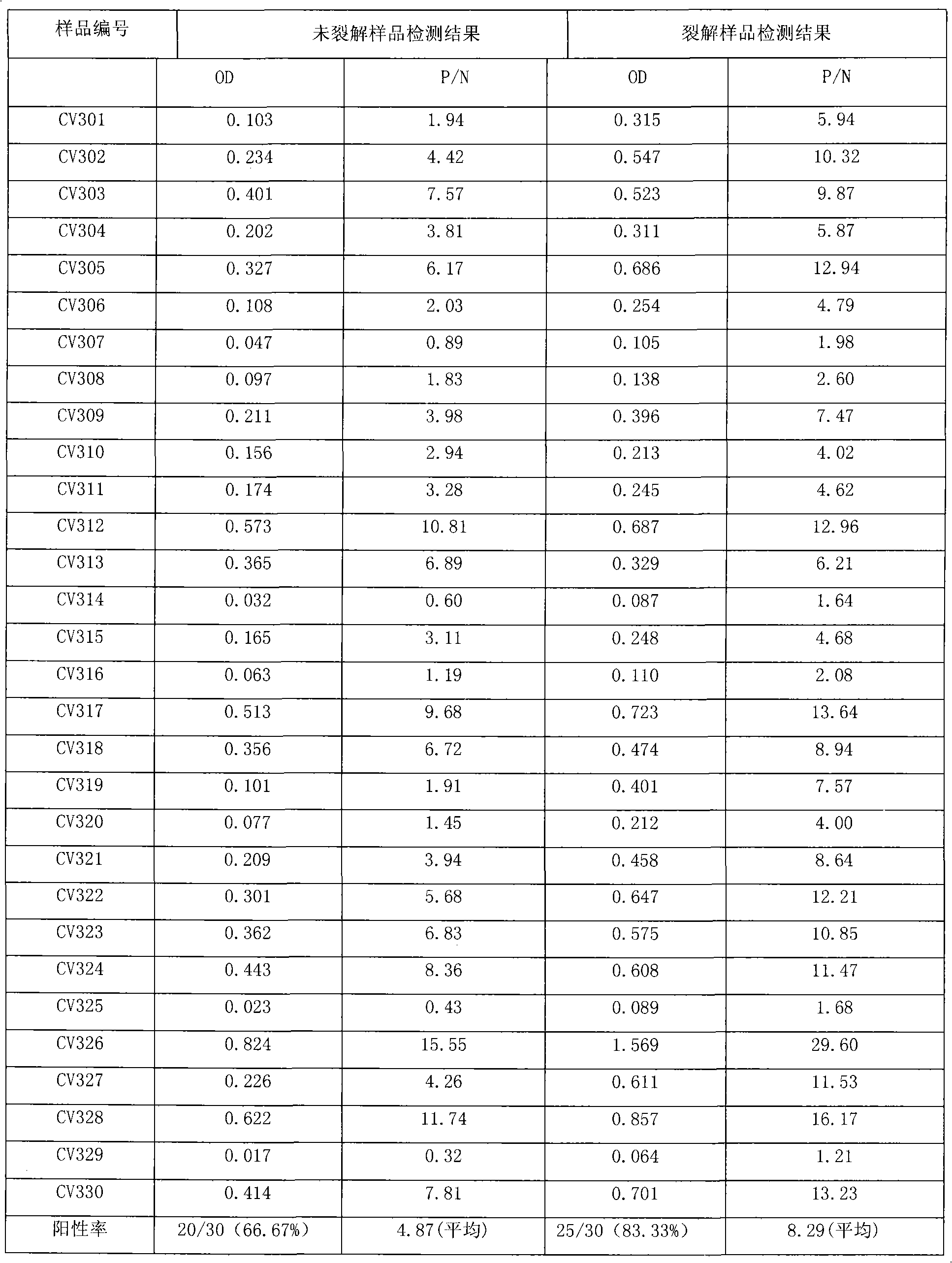
Virus disaggregating agent and method for disaggregating virus antigen-antibody complex and detecting HCV (Hepatitis C Virus) antigen
ActiveCN101975857AHigh sensitivityShorten the windowSerum immunoglobulinsImmunoglobulins against virusesSolventRNA
The invention provides a virus disaggregating agent and a method for disaggregating virus antigen-antibody complex and detecting HCV (Hepatitis C Virus) antigen. The virus disaggregating agent is characterized in that each 100ml of disaggregating agent comprises 0.1-1ml of detergent, 0-20g of protein denaturant, 0.01-1ml of reducing agent, 0.1-1ml of fat solvent, and base liquid as remainder amount. The antigen-antibody complex obtained by the neutralization reaction of testing blood sample or antiserum and virus antigen is disaggregated into free components, or virus core antigen is fully exposed and the antigen reactivity of the virus core antigen is remained, thereby remarkably improving the sensitivity by comparing with the prior HCV core antigen detection method; in addition, the detection window period of the antigen is 49 days shorter than that of the anti-HCV antibody averagely, the situation appears within 1-2d after HCV-RNA appears, thereby efficiently shortening the window period of the blood serum before transformation, which is of important signification on improving the detection rate of affected person in the window period and the safety of blood transfusion and blood products. In the invention, the virus disaggregating agent is suitable for the disaggregation of common virus antigen-antibody complex including HCV and HAV (Hepatitis A Virus).
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Gene detection method for communicating hydrocephalus
InactiveCN104561325AFacilitate early diagnosis"The shortened windowMicrobiological testing/measurementVeinVenous blood specimen
The invention discloses a gene detection method for communicating hydrocephalus. According to the method, a venous blood sample of a to-be-tested patient is directly used as an analysis sample. The method comprises the following steps: (1) extracting RNA; (2) performing fluorescent PCR (polymerase chain reaction) amplification; (3) performing fluorescent amplification; and (4) performing result analysis. The detection method disclosed by the invention is simple to sample, is high in sensitivity and strong in specificity, can be used for early diagnosis, and is of great significance for carrying out early warning on communicating hydrocephalus patients and taking effective treatment means.
Owner:唐延辉
Detection kit for monkey pox virus antigen and preparation method thereof
PendingCN114755418AShorten the windowReduce the risk of invisible transmissionMaterial analysisPox virusIncubation period
The invention relates to a monkey pox virus antigen detection kit and a preparation method thereof, and belongs to the technical field of in vitro diagnostics, the monkey pox virus antigen detection kit comprises a detection card and a sample extracting solution, the detection card comprises an upper cover, a lower cover and a monkey pox virus antigen detection test strip, the monkey pox virus antigen detection test strip comprises a sample pad, a silicon core gold shell combination pad, a solid-phase antibody reaction membrane, absorbent paper and a PVC plate, a sample adding hole and an observation window are formed in the upper cover, and the preparation method of the monkey pox virus antigen detection kit comprises a preparation method of the silicon core gold shell combination pad, a preparation method of the sample pad and a preparation method of the solid-phase antibody reaction membrane. The kit can detect various samples including oropharynx swabs, body fluid, blood and skin focus tissues (including vesicular fluid, pustule fluid and scab), is high in sensitivity, and can effectively shorten the window period of virus diagnosis so as to reduce the hidden transmission risk caused by the incubation period and effectively control the large-scale transmission of epidemic situations.
Owner:山东康华生物医疗科技股份有限公司
Optical control system of two-photon fluorescence immunoassay analyzer
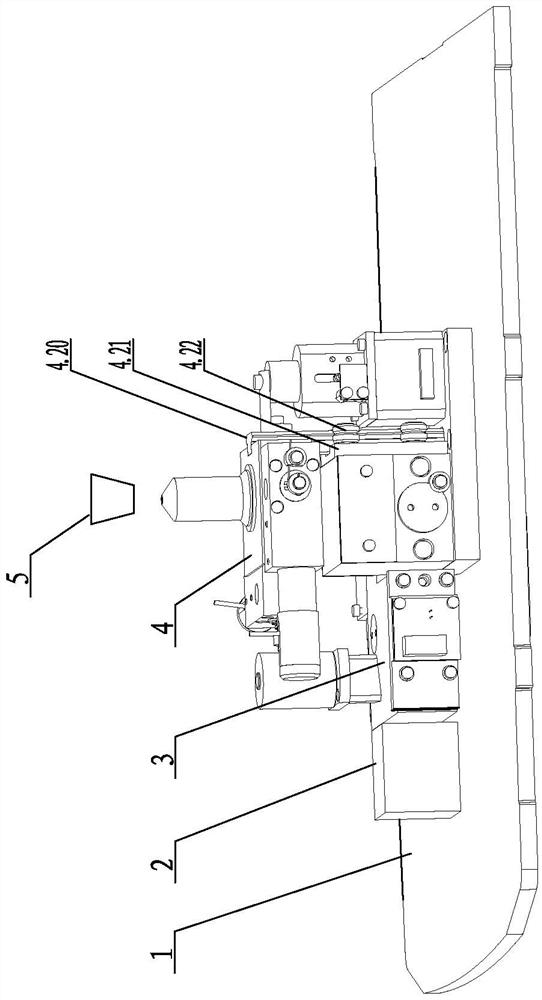
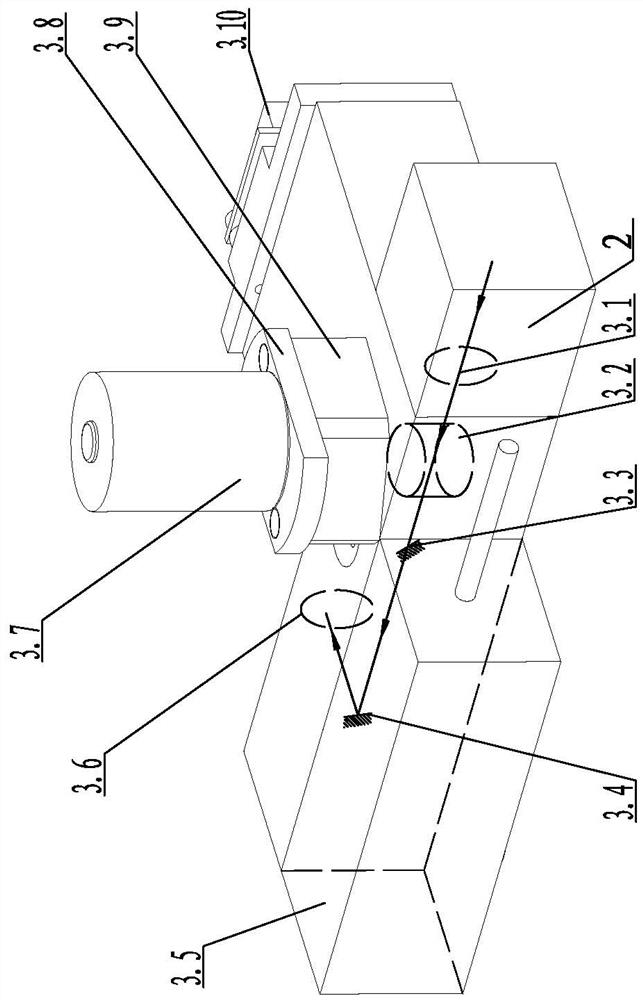
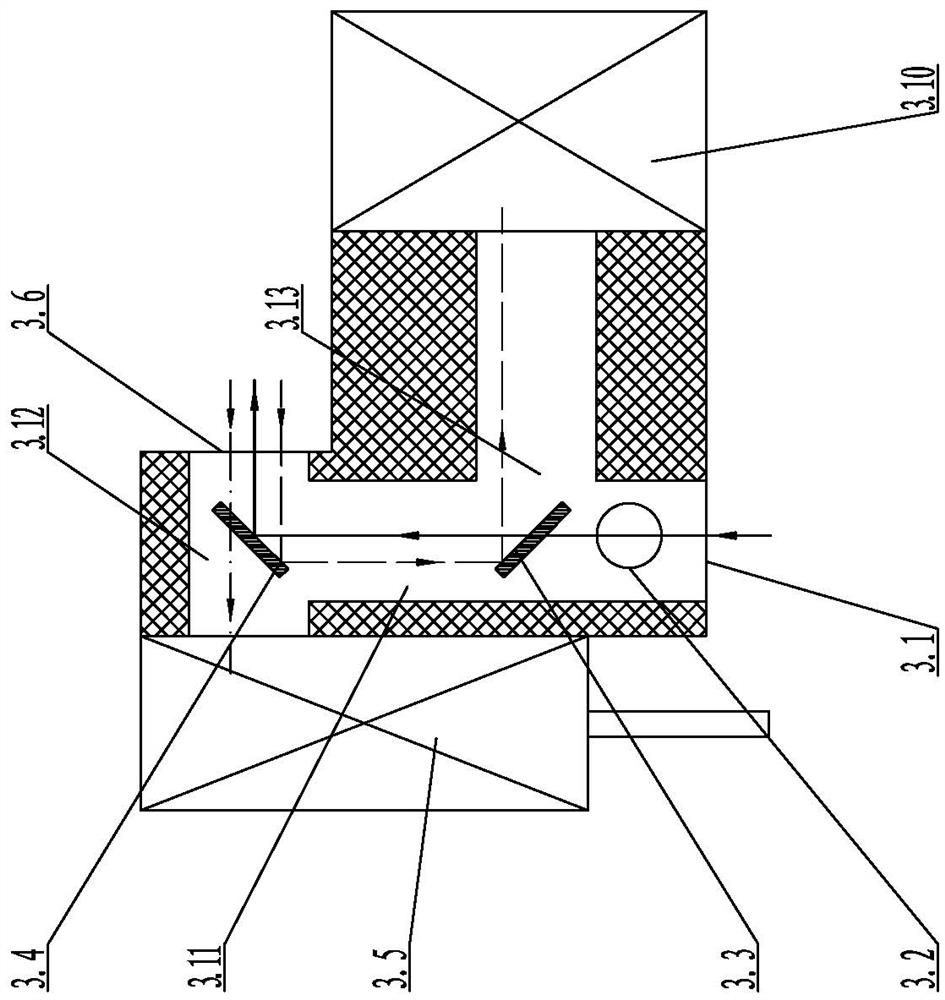
InactiveCN111812073AScattering effect is smallStrong penetrating powerBiological testingFluorescence/phosphorescenceImmune profilingLight excitation
The invention relates to the technical field of optical control systems, in particular to an optical control system of a two-photon fluorescence immunoassay analyzer. The system is characterized in that a two-photon laser generation module, a detection control module and an adjustment transmission module are mounted on a mounting bottom plate; a photomultiplier is arranged in the detection controlmodule; two-photon laser emitted by the two-photon laser generation module can be focused into a reaction cup through the detection control module and the adjustment transmission module; and the two-photon laser excites a detection substance in the reaction cup to generate fluorescence, and the fluorescence can return to the photomultiplier of the detection control module through the adjustment transmission module to be measured. The two-photon laser generation module is used for generating two photons, and due to the fact that the wavelength of the two photons is long, compared with short-wavelength light, the long-wavelength light is less affected by scattering and easily penetrates through a specimen, background interference fluorescence is further reduced, and the accuracy of equipment detection and analysis is improved.
Owner:山东新华普阳生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com