Immunopotentiator for swine atrophic rhinitis inactivated vaccine and preparation method of immunopotentiator
An immune enhancer and technology for atrophic rhinitis, which is applied in the field of immune enhancer for porcine atrophic rhinitis inactivated vaccine and its preparation, can solve the problems of long duration of immunity, expensive raw materials, high cost, etc., and achieve the extension of duration of immunity , avoid latent infection, inhibit the effect of latent infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
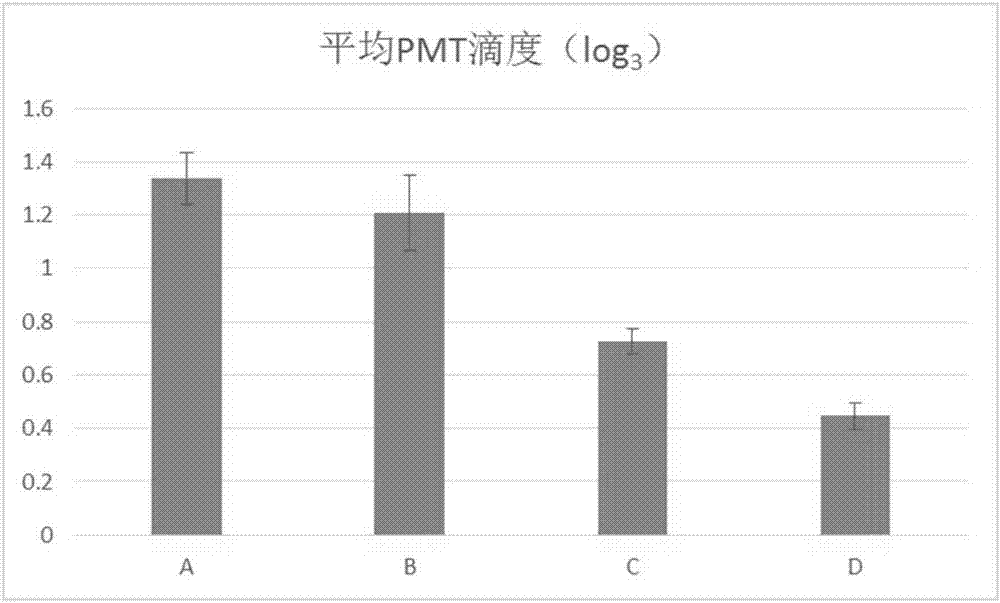
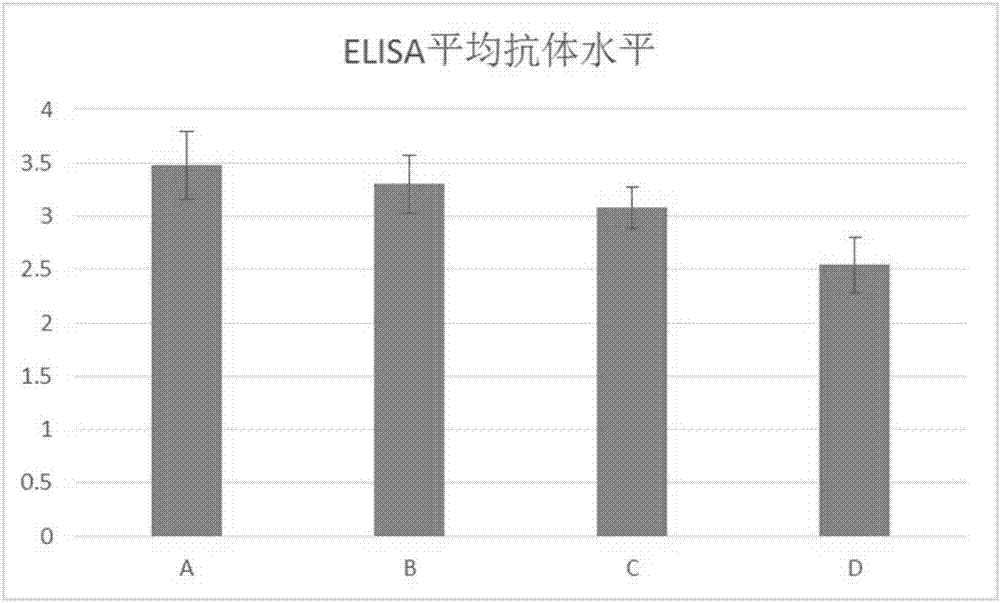
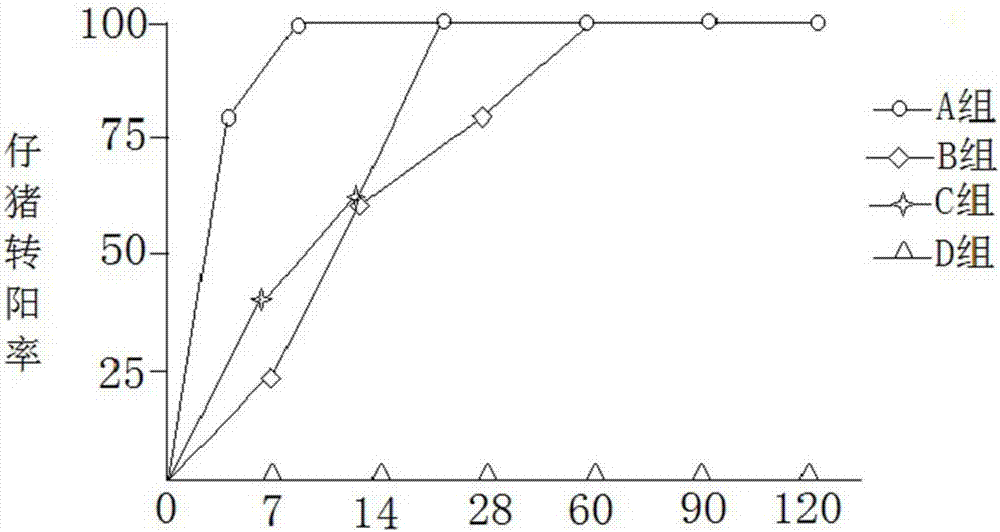
Image
Examples
Embodiment 1
[0022] a. Liquid preparation containing dry powder immune particles: take 30ml of an aqueous solution with a concentration of 100ug / ml of aluminum hydroxide, add 4ug of ginsenoside, 4ug of cholesterol, and 4ug of diethylaminoethyl dextran under slow stirring, and continue stirring Add polyethylene glycol after 5 minutes, the total moles of vitamin C and potassium iodide / moles of aluminum hydroxide in the liquid containing dry powder immune particles=4, and the mass ratio of aluminum hydroxide to polyethylene glycol is 1:1 After continuing to stir for 20 minutes, a golden liquid containing dry immune particles was obtained. Observing with an atomic microscope, the obtained dry powder immune particles had a diameter of 50 nm. The liquid containing dry powder immune particles was packaged in a brown collection bottle and filled with liquid nitrogen. Do aseptic storage, the shelf life is 2 years;
[0023] b. Preparation of immune enhancer: take a suitable amount of liquid containi...
Embodiment 2
[0025] a. Liquid preparation containing dry powder immune particles: Take 30ml of an aqueous solution with a concentration of 100ug / ml of aluminum hydroxide, add 80ug of ginsenoside, 80ug of cholesterol, and 40ug of diethylaminoethyl dextran under slow stirring, and continue stirring Add polyethylene glycol after 3 minutes, the total moles of vitamin C and potassium iodide in the liquid containing dry immune particles / moles of aluminum hydroxide = 1, and the mass ratio of aluminum hydroxide to polyethylene glycol is 1:0.1 , and continue to stir for 10 minutes to obtain a golden liquid containing dry immune particles. Observing with an atomic microscope, the diameter of the obtained dry immune particles is 120nm. The liquid containing dry immune particles is encapsulated in a brown collection bottle and filled with liquid nitrogen. Do aseptic storage, the shelf life is 2 years;
[0026] b. Preparation of immune enhancer: take an appropriate amount of liquid containing dry powde...
Embodiment 3
[0028] a. Liquid preparation containing dry powder immune particles: take 30ml of an aqueous solution with a concentration of 100ug / ml of aluminum hydroxide, add 40ug of ginsenoside, 40ug of cholesterol and 20ug of diethylaminoethyl dextran under slow stirring, and continue stirring for 4min After adding polyethylene glycol, the total moles of vitamin C and potassium iodide in the liquid containing dry powder immune particles / moles of aluminum hydroxide = 2, and the mass ratio of aluminum hydroxide to polyethylene glycol is 1:0.5, After continuing to stir for 15 minutes, golden yellow liquid containing dry immune particles was obtained. Observing with an atomic microscope, the obtained dry powder immune particles had a diameter of 100nm. The liquid containing dry powder immune particles was encapsulated in a brown collection bottle, filled with liquid nitrogen to make Aseptically stored, the shelf life is 2 years;
[0029] b. Preparation of immune enhancer: take an appropriate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com