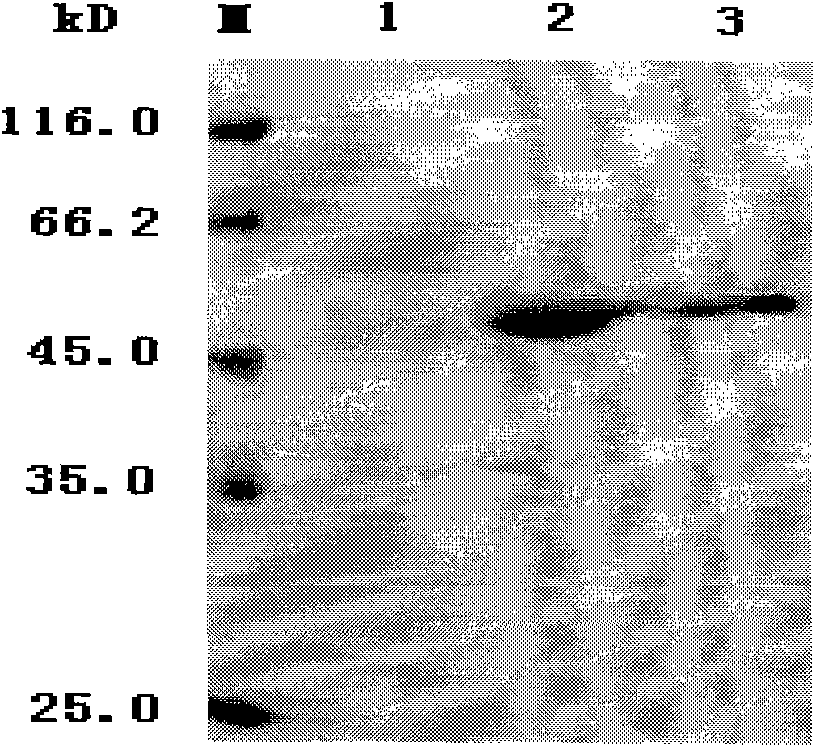
Construction and application of mycobacterium tuberculosis fusion protein
A Mycobacterium tuberculosis and fusion protein technology, which is applied in the field of fusion protein construction, can solve the problems of low antigen expression, no immune protection of Mycobacterium tuberculosis, and inability to produce immune responses in dormant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Fusion protein AMH (Ag85B-Mpt64 190-198 - Construction of HspX)
[0024] 1. Materials and methods:
[0025] 1.1 Main reagent materials:
[0026] 1.1.1 Main materials: Mycobacterium tuberculosis virulence standard strain H37Rv was provided by Shanghai Pulmonary Hospital; DDA was purchased from Sigma-Aldrich; recombinant plasmids pET28a-AMM and pET28a-Ag85B were constructed by the Institute of Genetics, Fudan University. MPT64 190-198 The polypeptide fragments were synthesized by Jill Biochemical (Shanghai) Co., Ltd. with a purity of 90.45%; Ni-NTA His·Bind Resins were purchased from Novagen (EMD Chemicals Inc); the rest of the reagents were domestic or imported analytically pure.
[0027] Plasmid extraction kits and PCR product purification kits were purchased from Huashun Company, endonucleases and ligases were purchased from Biolab Company; IFN-γ ELISPOT kits were products of U-Cytech Company (Netherlands).
[0028]1.1.2 Main instruments and equipment: SC...
Embodiment 2
[0048] The preparation of embodiment 2 subunit vaccine:
[0049] BCG-PSN was dissolved to 0.6 mg / ml with physiological saline, and the fusion protein AMH was diluted to 0.5 mg / ml with PBS. DDA was prepared with water for injection to 2.5 mg / ml, placed in a water bath at 80°C for 10 minutes, and cooled to room temperature. Take 50 μl of BCG-PSN solution and mix well with an equal amount of AMH protein solution, and let stand at room temperature for 1 min. Add 100 μl of DDA dropwise to the mixed solution, and then fully emulsify it so that the vaccine is in the form of a uniform cream.
Embodiment 3
[0050] Example 3 Animal Immunization Test
[0051] 1. Grouping of experimental animals (six groups in total)
[0052] A. AMH (20ug / time / piece) + DDA (250ug / time / piece) + BCG polysaccharide nucleic acid (BCG-PSN, 30ug / time / piece)
[0053] B.Ag85B (20ug / time / piece)+DDA (250ug / time / piece)+BCG polysaccharide nucleic acid (30ug / time / piece)
[0054] C.DDA (250ug / time / piece) + BCG polysaccharide nucleic acid (30ug / time / piece)
[0055] D.AMM (Ag85B-Mpt64 190-198 -Mtb8.4, 20ug)+DDA(250ug / time / piece)+BCG-PSN(30ug / time / piece)
[0056] E. Physiological saline (NS, 200μl / time / only)
[0057] F.BCG (5×10 6 CFU / only)
[0058] 2. Immunization method:
[0059] In the 1st, 4th, and 7th weeks, the animals were subcutaneously immunized with the prepared protein vaccine in the groin (200 μl / only), and BCG 5×10 6 Animals were immunized with CFU subcutaneously in the groin once in the first week. Blood was collected 5 weeks after the last protein vaccine immunization to detect humoral immune...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com