Hepatitis C virus (HCV) antigen and antibody combined detection kit
A hepatitis C virus, antigen antibody technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inconvenient promotion and application of primary screening tests, high requirements for the experimental environment, and low degree of full automation, so as to achieve reliable detection results , improved sensitivity, and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1 Preparation of hepatitis C virus antigen antibody combined detection kit
[0012] 1. Preparation of magnetic particle coating
[0013] The process of connecting specific antigens and antibodies to magnetic beads by covalent coupling is called magnetic particle coating; the prepared magnetic particle antigen and magnetic particle antibody combination is called magnetic particle coating.
[0014] The basic principle of coating is: the amino groups on the surface of the antigen or antibody react with the chemical groups on the surface of the magnetic beads under the action of the chemical cross-linking agent to form a covalent combination of antigen / antibody-magnetic particles. The conjugate is washed and blocked to remove unreacted antigen or antibody, and block non-specific binding sites, and finally prepare the kit magnetic bead coating. The normal operation process is as follows:
[0015] 1. Take a solution of paramagnetic nanoparticles containing a certain function...
Embodiment 2
[0035] Example 2 Detection method of the kit of the present invention
[0036] The hepatitis C virus antigen antibody combined detection kit prepared in Example 1 is used in conjunction with the automatic chemiluminescence analyzer A2000 and A2000 Plus, and the process is as follows:
[0037] In the first step, add the sample, sample diluent and magnetic particle coating working solution to the reaction cup, and incubate at 37°C for 15 minutes. The HCV antibody in the sample and the HCV recombinant antigen coated with magnetic particles and the biotin in the sample diluent Antigen binding, the HCV core antigen in the sample is combined with the HCV core antigen monoclonal antibody coated with magnetic particles. After the incubation is completed, the solid phase is placed in a magnetic field and is attracted, and the substances bound to the solid phase are retained, while other Unbound material is removed by washing. The sample diluent and the magnetic particle coating working sol...
Embodiment 3
[0041] Example 3 Performance test of the kit of the present invention
[0042] HCV antibody national reference materials and WHO HCV core antigen standard materials were used to test the performance of antibody and antigen detection systems.
[0043] 1. Performance test of antibody detection system
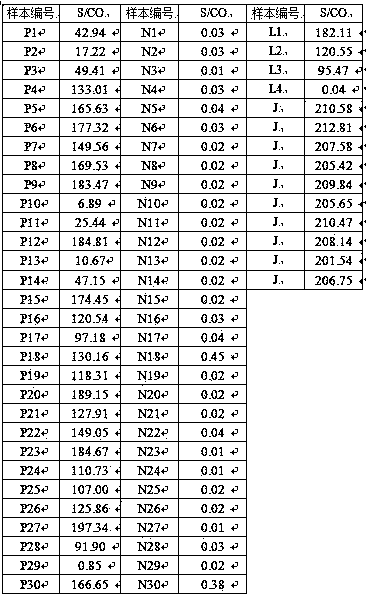
[0044] The HCV antibody detection reagents were tested with the sixth set of national reference products. The positive coincidence rate was 29 / 30, and the negative coincidence rate was 30 / 30. The minimum detection limit reference samples L1-L3 were positive, L4 was negative, and the precision was 1.6%. Meet the requirements of national standards: positive compliance rate ≥29 / 30; negative compliance rate ≥29 / 30; the lowest detection limit reference product L1-L2 is positive, L4 is negative, L3 is positive or negative; precision reference product CV≤15 %. The test results are shown in Table 1:
[0045] Table 1: HCV antibody national panel test results
[0046]
[0047] 2. Performance test ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com