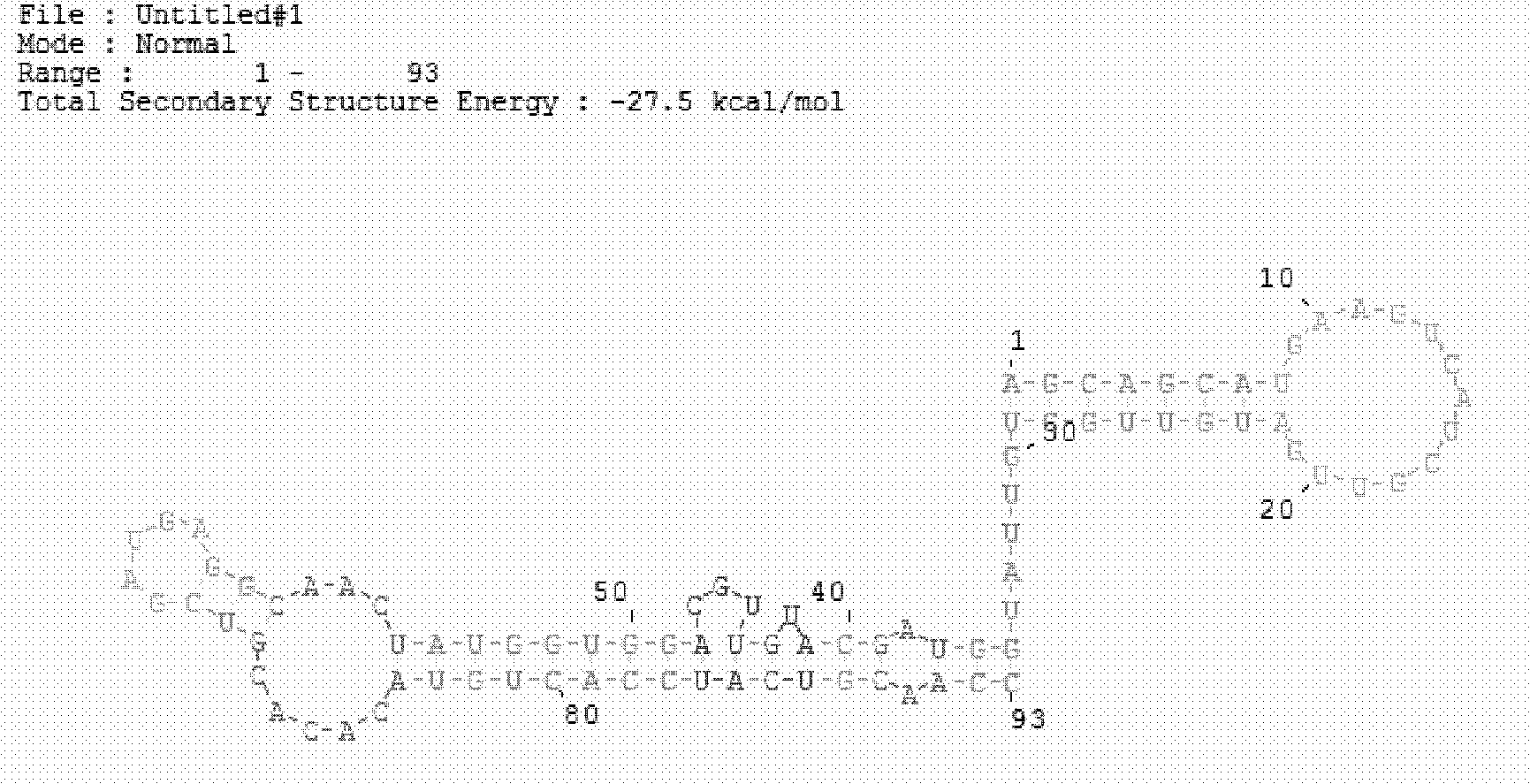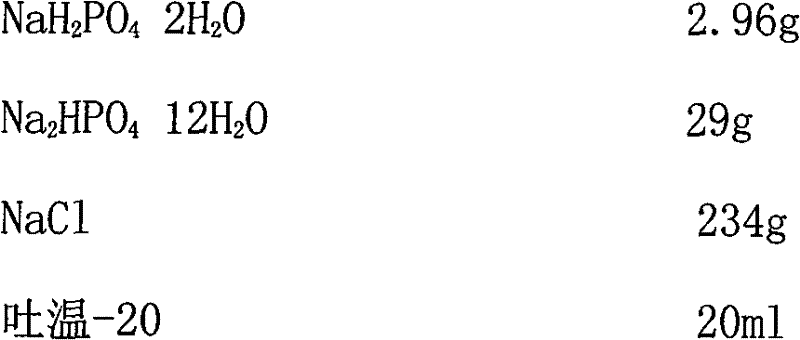Patents
Literature
33 results about "Hcv core antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
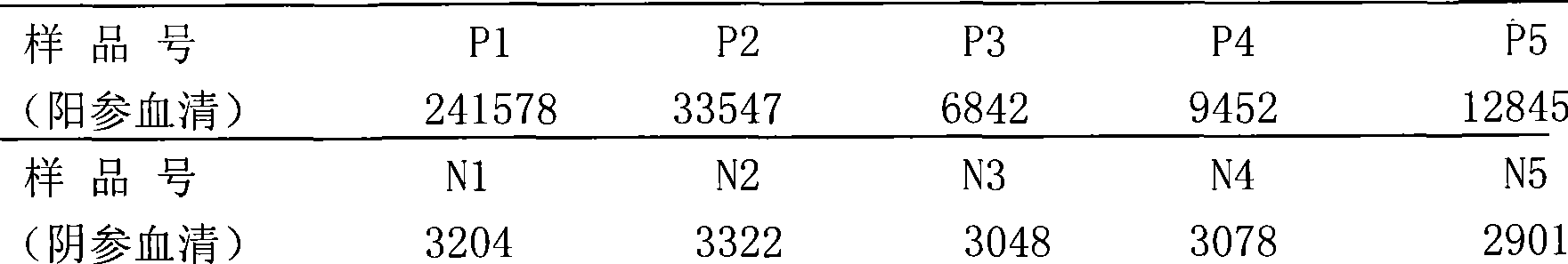
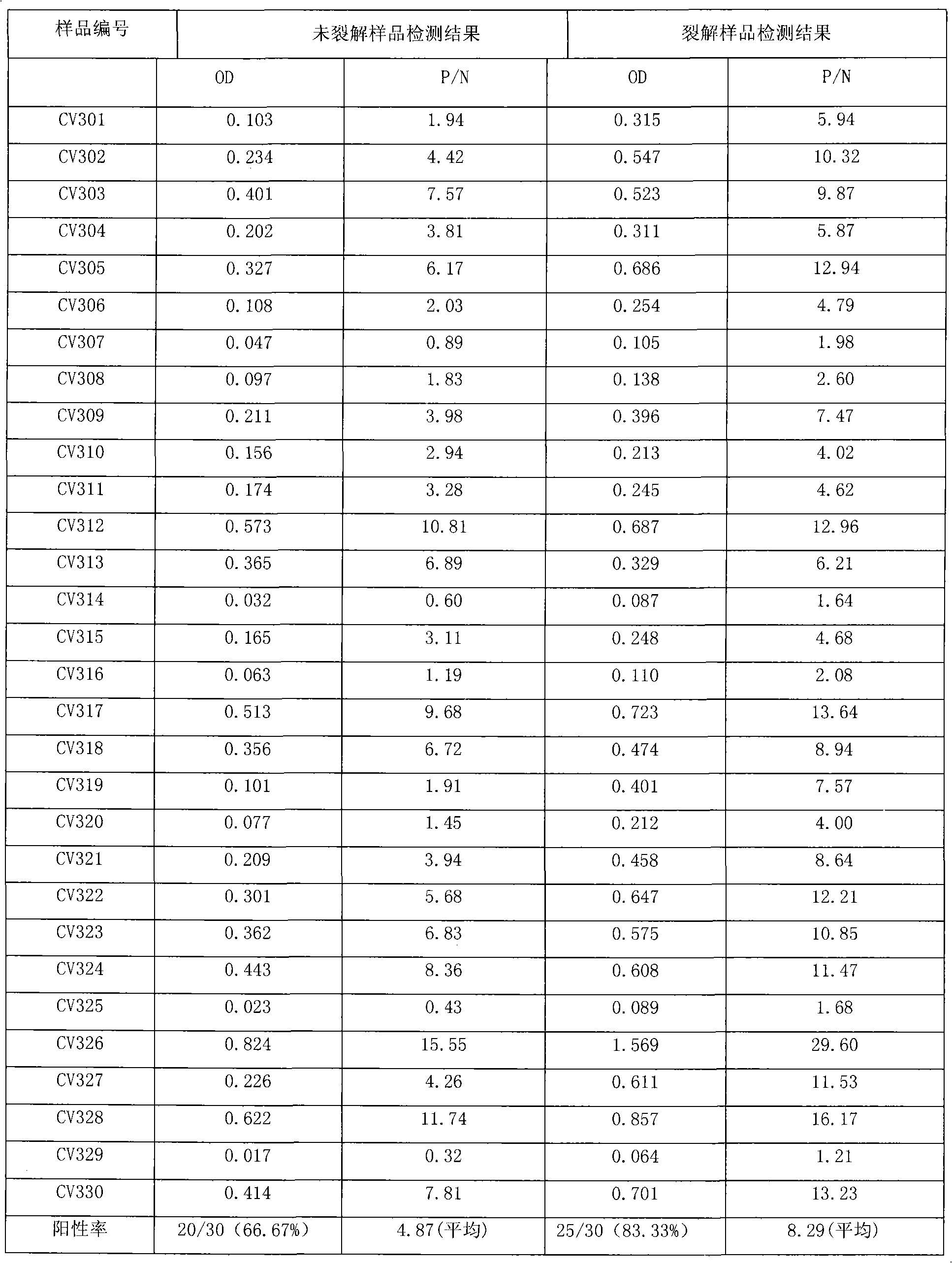
Since the core antigen is part of hepatitis C virus, it can usually be found in the bloodstream two weeks after infection. Since HCV core antigen testing is simpler and less expensive than viral-load testing, some experts suggest using it in resource-limited settings.
Hepatitis C virus antigen-antibody combined detection method
The invention discloses a method for jointly detecting hepatitis C virus (HCV) antigen-antibody. A monoclonal antibody of an anti-HCV core antigen and a chimeric antigen are coated with enzyme-linked plate to be detected simultaneously, so that the method can shorten the 'window period' of HCV virus detection. Moreover, HCV antibodies in serum and the compatibility with the coated monoclonal antibody can be detected as possible so as to avoid the combination of the chimeric antigen and the monoclonal antibody by modifying HCV overall length core antigen, telescoping non-structural areas and realizing the expression of the chimeric antigen, and the positive detection rate is close to the PCR positive detection rate. The HCV detection method also has the advantages of simple operation, low price, so the method is suitable for promotion and application.
Owner:湖南景达生物工程有限公司
Reagents For HCV Antigen-Antibody Combination Assays
The present invention is directed to combination immunoassays, reagents and kits for simultaneous detection of HCV antigens and anti-HCV antibodies in a sample. The combination immunoassays of the present invention employ a non-ionic detergent that effectively exposes or releases the HCV core antigen from virions in a sample without interfering with the performance of other reagents such as the capture of anti-HCV antibodies by recombinant HCV antigens.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Microfluidic paper base chip for detecting antibody to hepatitis C virus as well as preparation method of chip
The invention relates to a microfluidic paper base chip for detecting an antibody to hepatitis C virus as well as a preparation method of the chip. The microfluidic paper base chip provided by the invention comprises a plurality of micro detection channels, wherein each channel comprises an HCV core antigen, an HCV NS5 antigen, an HCV NS4 antigen, an HCVNS3 antigen as well as the combination thereof. Owing to the multi-channel detection capability, the microfluidic paper base chip can detect a plurality of immune projects synchronously; miniaturized screening and confirming testing are integrated on the microfluidic paper base chip, so that the complex HCV diagnosis process is simplified greatly, and the diagnosis efficiency is improved greatly.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Hepatitis C virus core antigen chemiluminescence ELISA detection kit
ActiveCN101419238ARealize full detectionImprove featuresMaterial analysisAntigenOperating instruction
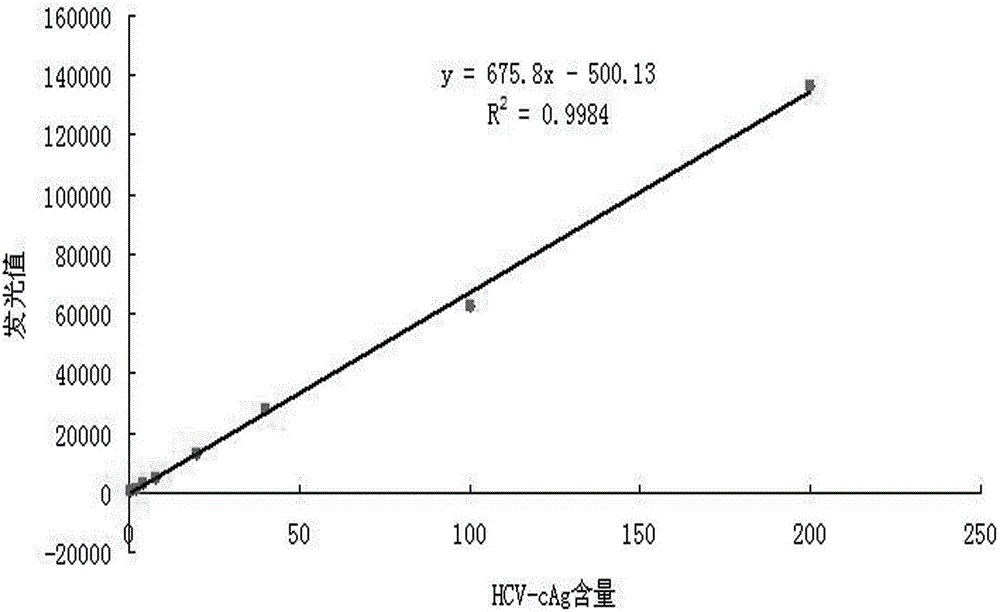
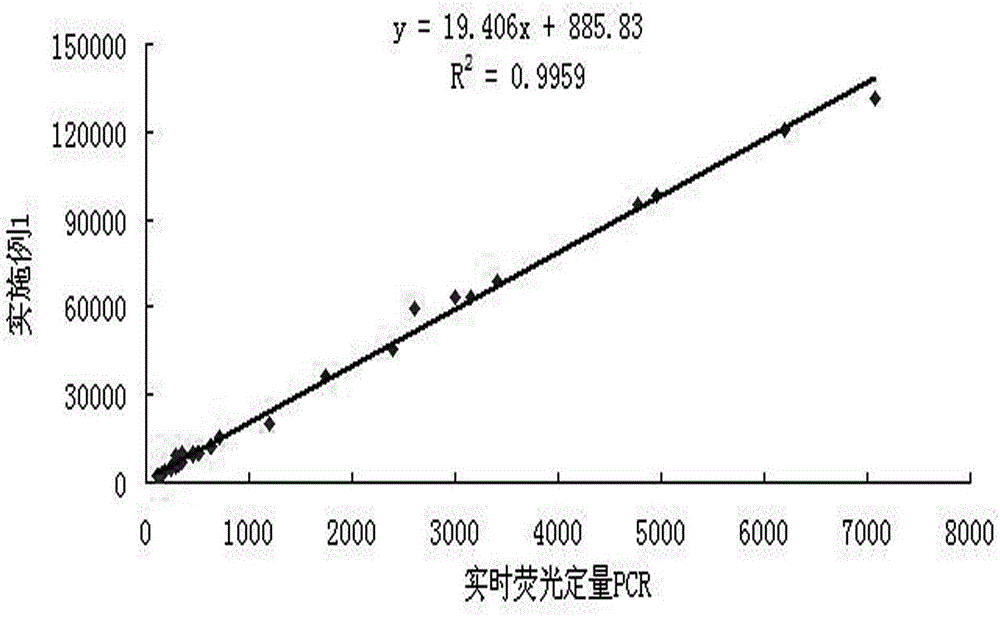
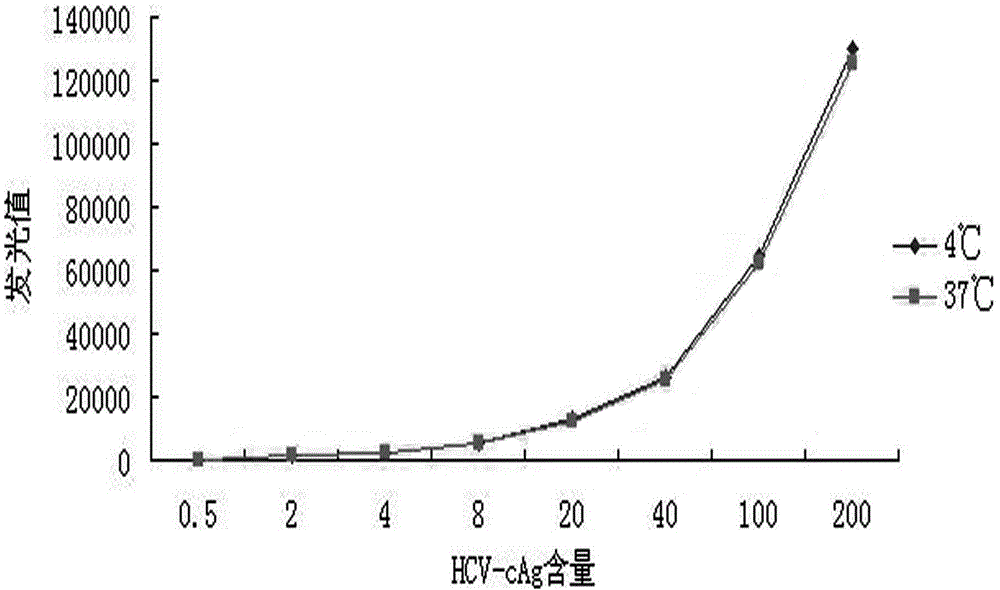
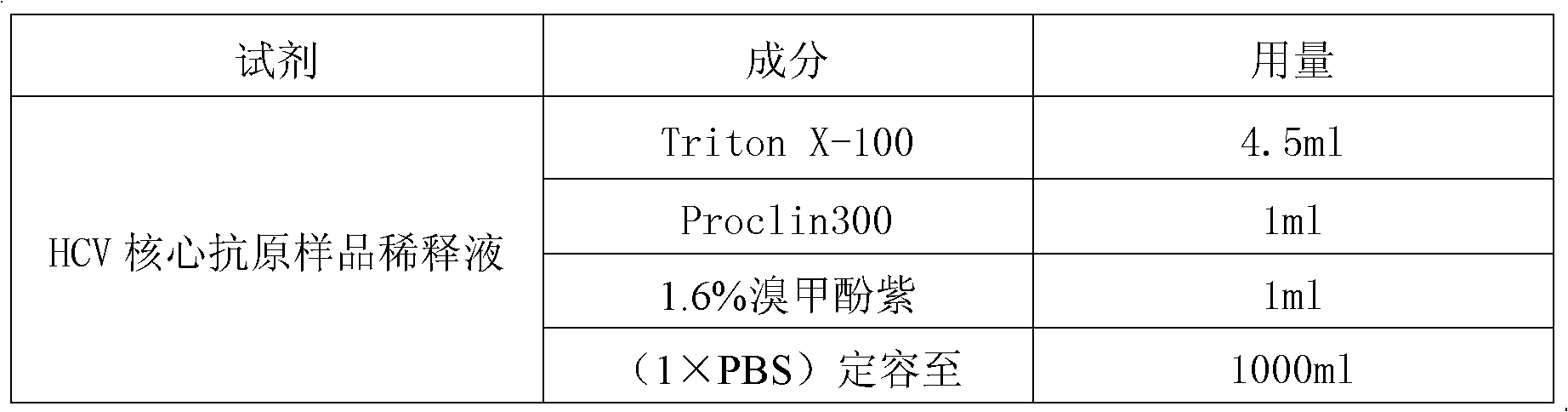
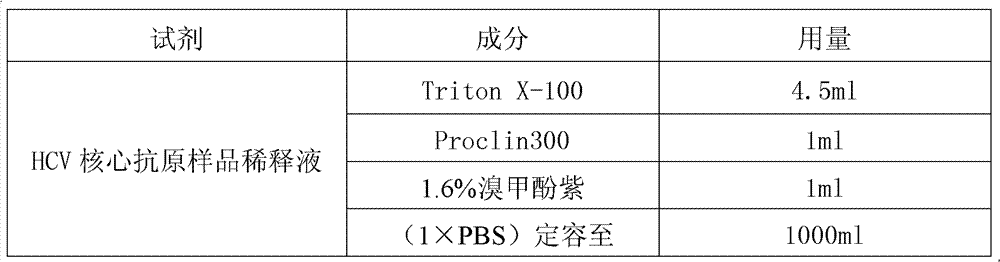
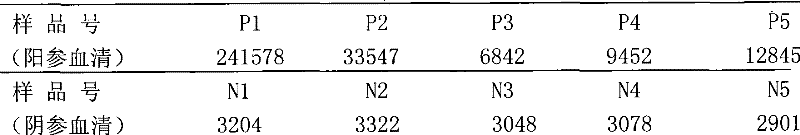
The invention discloses a reagent kit for carrying out chemiluminescent enzyme-linked immunodetection on a hepatitis C virus core antigen. The reagent kit comprises a kit body, and an enzyme label plate, a reagent, a non-drying glue sealing strip and an operating instruction manual which are arranged inside the kit body, wherein the enzyme label plate is a chemiluminescent enzyme label plate; each hole of the plate is coated with coated antibodies Mab1and Mab2 with concentration of 5ug / ml; and the reagent comprises a HCV-cAg antienzyme composition, a gradient solution of a HCV core antigen standard product, positive control serum, negative control serum, a pretreatment solution, a diluent of a HCV-cAg sample, a chemiluminescent zymolyte solution A, a chemiluminescent zymolyte solution B and a 20 * concentrated cleaning solution. The positive detection rate of the reagent kit is close to that of PCR; and simultaneously, the reagent kit has the advantages of simple operation, high sensitivity and high specificity, and is suitable for popularization and application in clinic.
Owner:山东莱博生物科技有限公司
Enzyme-linked immunologic diagnosis kit for core antigen of C type hepatitis virus and method for preparing same
Disclosed are a hepatitis C virus antigen enzyme-linked immunoassay reagent box and a method for making the same. The invention obtains the cell strain of excretive anti HCV core antigen by analyzing the core antigen array of the hepatitis C virus different type and cloning the core antigen gene of HCV, purifying out the high activity monoclonal antibody with four core aa expression sites of HCV, wherein the Cab1 and Cabs are used as coating antibodies, the Cab3 and Cab4 are used as enzyme labeled antibodies; employing double antibodies sandwich technology to prepare HCV-cAg ELISA diagnosing reagent box.
Owner:湖南景达基因有限公司
Combined detection kit for hepatitis c virus (HCV) antigen-antibody
InactiveCN106093402AImprove specific recognition abilityReduce non-specific bindingMaterial analysisHCV AntibodyHcv core antigen
The invention discloses a combined detection kit for HCV antigen-antibody. The kit comprises a first monoclonal antibody against HCV core, a first HCV recombinant chimeric antigen, a first diluted sample solution, a second diluted sample solution, a first enzyme-labeled second monoclonal antibody against HCV core antigen, a first ligand-labeled second HCV recombinant chimeric antigen, a second enzyme-labeled second ligand, a developer and a stopping solution, wherein the first diluted sample solution comprises a reducing agent, a first surfactant and a first denaturant; and the second diluted sample solution comprises a second surfactant and a second denaturant. The combined detection kit for the HCV antigen-antibody opens mismatched disulfide bonds in coating antigen and in an antigen-antibody complex of a sample in virtue of the first diluted sample solution, and destroys HCV core antigen and HCV antibody compounds in virtue of the second diluted sample solution, so more antigen is released; and thus, detection sensitivity is improved.
Owner:HUNAN KANGRUN PHARMA
Method for measurement of hepatitis C virus
InactiveUS7316905B1Reduce secondary infectionPeptide/protein ingredientsMicrobiological testing/measurementAntigenHcv core antigen
A method for measurement of the hepatitis C virus (HCV) characterized by measuring HCV core antigen and HCV core antibody by their binding with probes in the presence of an anionic surfactant or a non-ionic surfactant, or both.
Owner:ADVANCED LIFE SCI INST
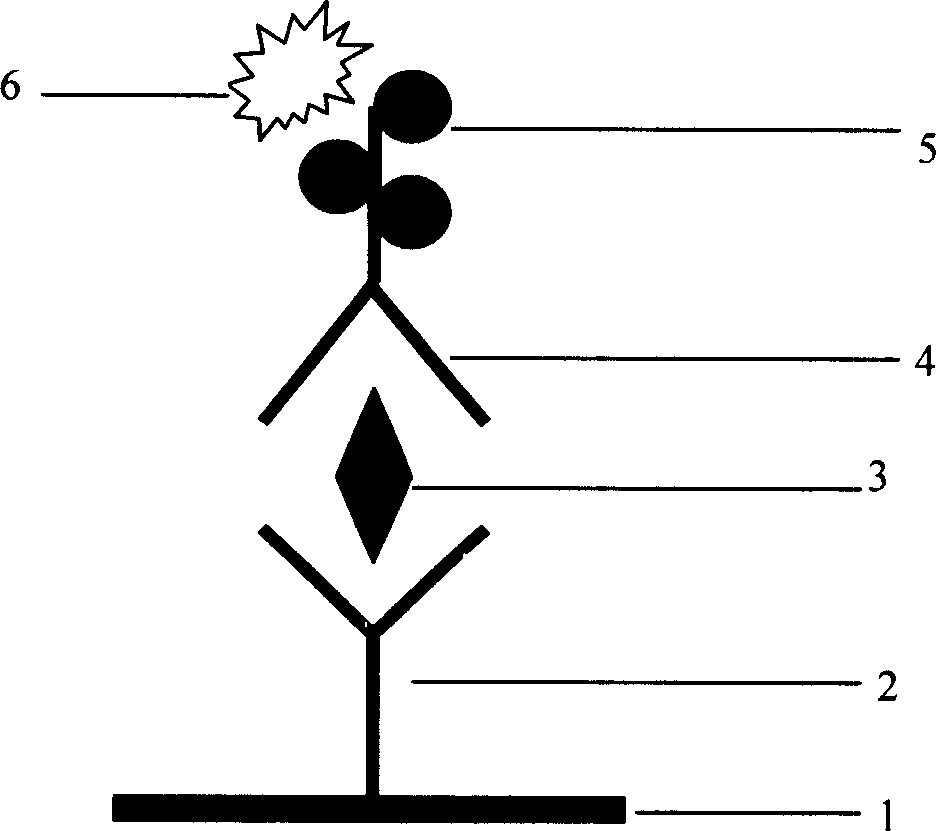
HCV (hepatitis C virus) core antigen and antibody thereof as well as hybridoma cell lines secreting antibody
ActiveCN102329378AGood antigenicityHigh purityVirus peptidesImmunoglobulins against virusesAntigenNucleotide
Owner:SHANDONG UNIV QILU HOSPITAL
Application of kaempferol in preparation of anti-HCV (hepatitis c virus) infective medicaments
InactiveCN104306368ASmall side effectsThe purification process is matureOrganic active ingredientsAntiviralsAntigenInflammatory factors
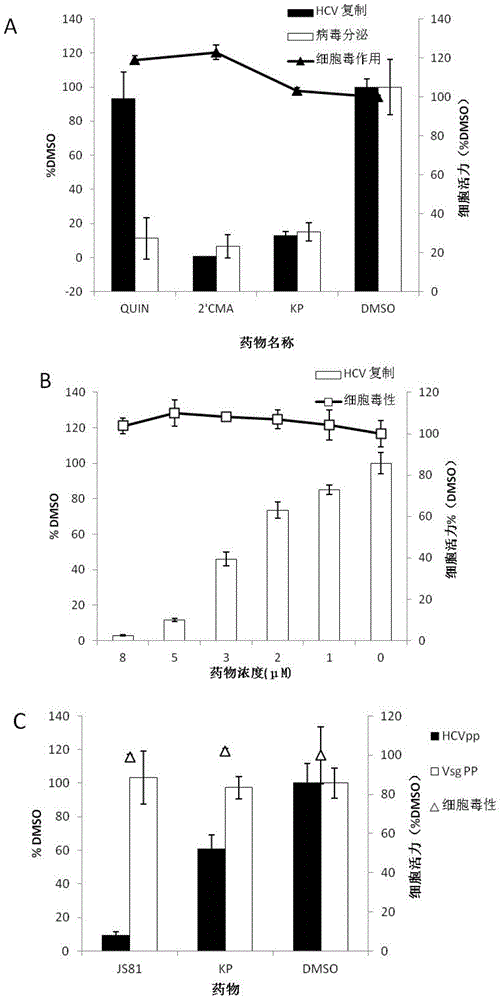
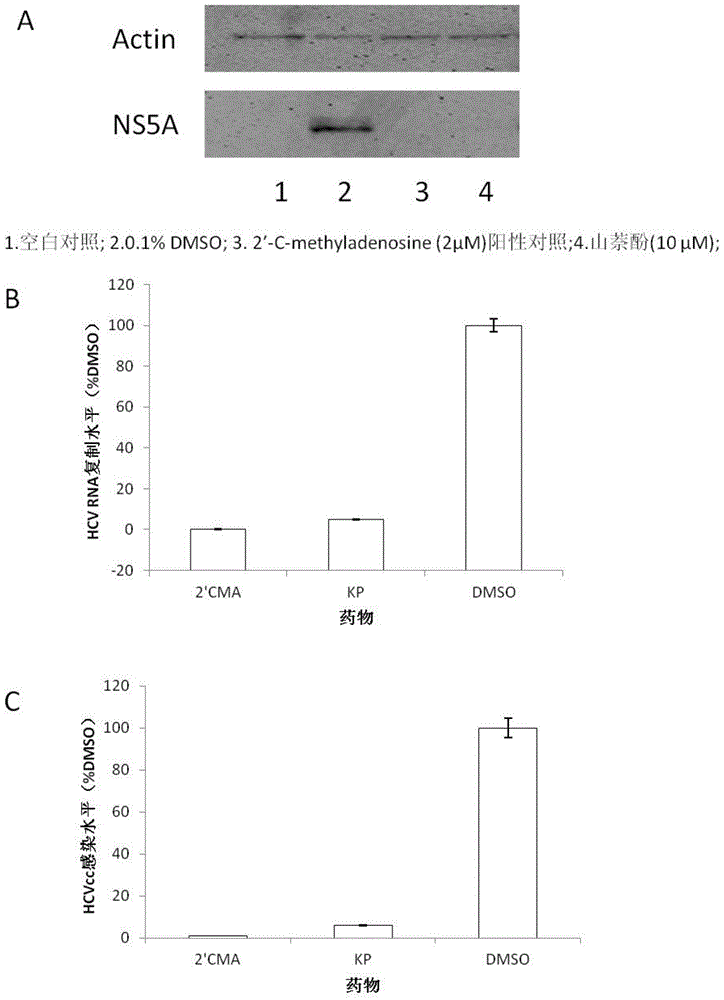
The invention relates to the technical field of medicine and provides the application of kaempferol in preparation of anti-HCV (hepatitis c virus) infective medicaments. Kaempferol is called KP for short, is a flavonoids compound, and widely exists in various fruits and traditional Chinese medicines such as gingko, propolis, tilia amurensis and Chinese scholartree. The experiment verifies that kaempferol can inhibit HCV replication in terms of protein level and RNA level, is dose-dependent, can inhibit replication of HCV of different gene types, has a synergistic effect with IFN, can inhibit acute and chronic HCV infection, and meanwhile can obviously inhibit inflammatory factor RNA expression induced by an HCV core antigen and an NS5A antigen, especially the epoxidase 2 gene expression.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
HCV core antigen detection kit based on magnetic micro-particle chemiluminescence method
InactiveCN104360063AExpand the scope of detectionShorten the windowChemiluminescene/bioluminescenceSerum igeSerum samples
The invention relates to an HCV core antigen detection kit based on a magnetic micro-particle chemiluminescence method, which belongs to the technical field of clinical in-vitro detection. Total or free HCV core antigens in a serum sample are qualitatively or quantitatively detected by virtue of a double-antibody sandwich method. The HCV core antigen detection kit mainly comprises a reagent R1, a reagent R2, a bottle of positive reference, a bottle of negative reference, a luminous substrate A, a luminous substrate B and a solid washing solution. The kit is high in sensitivity and stability, is simple, convenient and quick to operate, is easy to automatically operate, and can well meet domestic clinical using requirements.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
HCV Nucleic acid aptamer and application thereof in preparing HCV-cAg detection kit
ActiveCN102433339AAvoid defectsHigh sensitivityMaterial analysisDNA/RNA fragmentationAntigenNucleotide
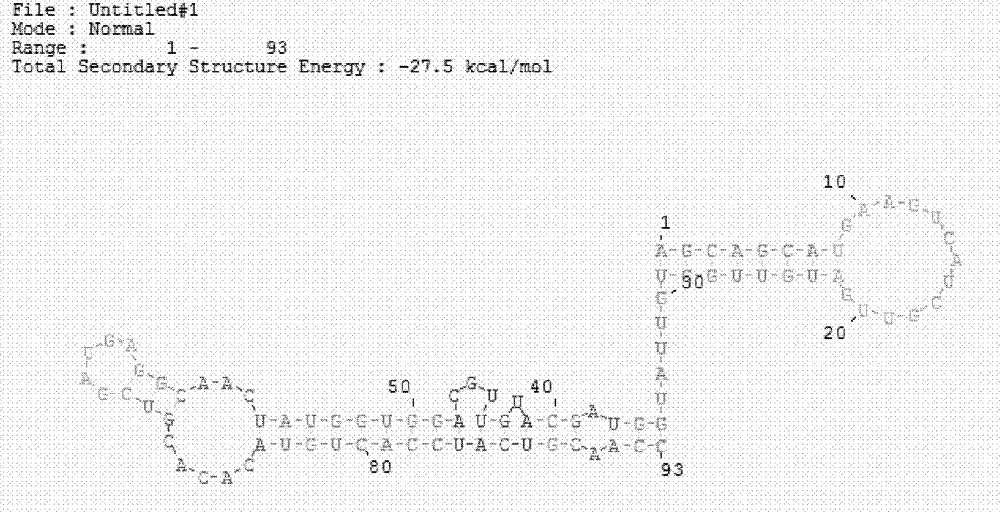
The invention discloses a hepatitis C virus core antigen nucleic acid aptamer, wherein the nucleotide sequence of the aptamer is as indicated in SEQ ID No.1. The invention also discloses the application of the hepatitis C virus core antigen nucleic acid aptamer in preparing a hepatitis C virus (HCV) core antigen detection kit. The aptamer and the HCV core antigen detection kit prepared therefrom in the invention are capable of detecting the core antigen at pg / ml level; the sensitivity of the hepatitis C virus core antigen is greatly improved; therefore, the HCV nucleic acid aptamer and the HCV-cAg detection kit has important clinic diagnosis meaning and wide application prospect for early diagnosis of window-phase infected patients.
Owner:山东莱博生物科技有限公司
Reagents For HCV Antigen-Antibody Combination Assays
The present invention is directed to combination immunoassays, reagents and kits for simultaneous detection of HCV antigens and anti-HCV antibodies in a sample. The combination immunoassays of the present invention employ a non-ionic detergent that effectively exposes or releases the HCV core antigen from virions in a sample without interfering with the performance of other reagents such as the capture of anti-HCV antibodies by recombinant HCV antigens.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Multi-epitope fusion protein of an hcv antigen and uses thereof
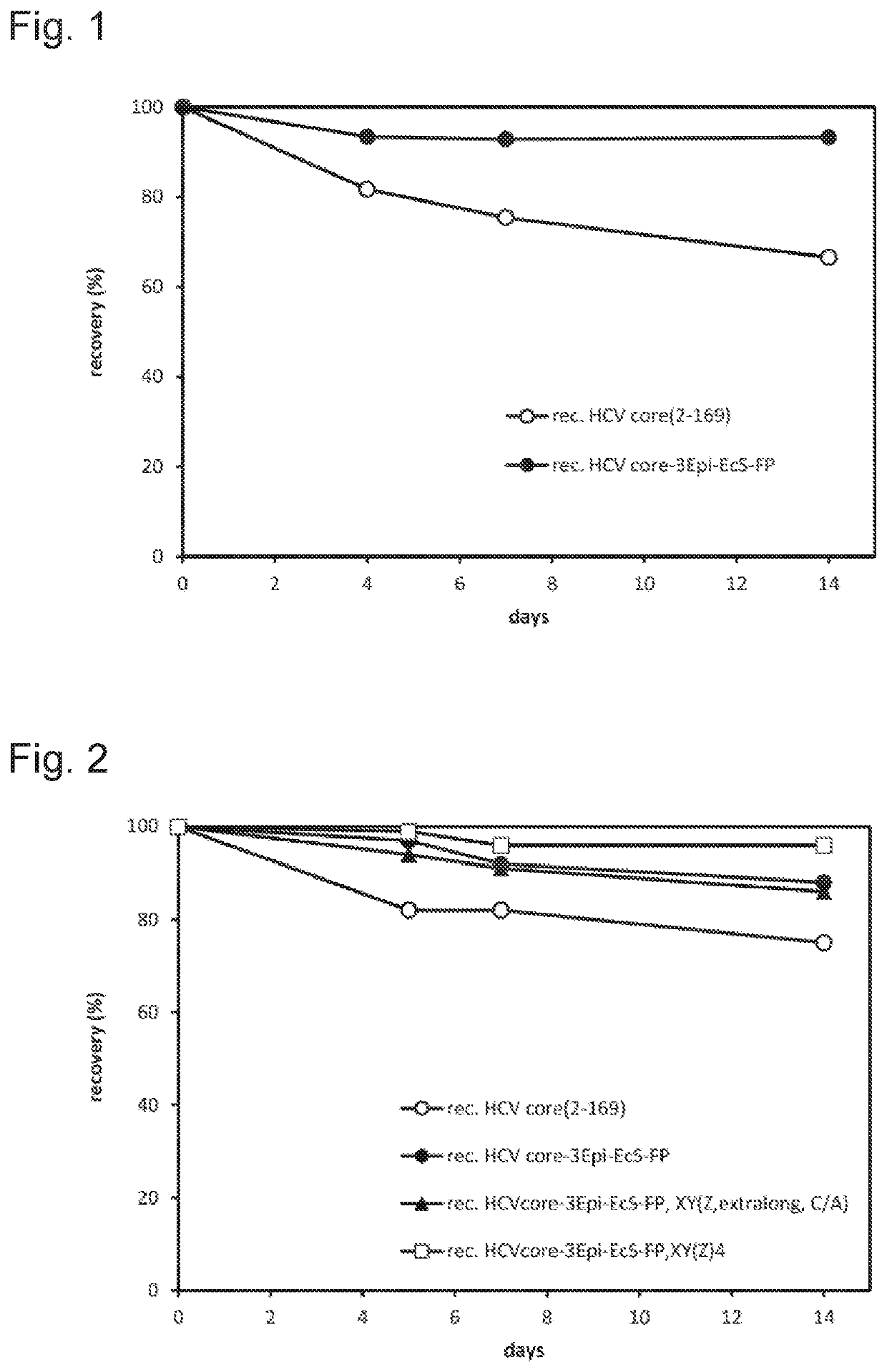
ActiveUS20200148726A1SsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenHepatitis C virus core
The disclosure relates to a multi-epitope fusion protein as well as to its use as calibrator and / or control in an in vitro diagnostics immunoassay for detecting HCV core antigen. The multi-epitope fusion protein has two to six different non-overlapping linear peptides present in the amino acid sequence of hepatitis C virus (HCV) core protein, wherein each of the peptides is separated from the other peptides by a spacer consisting of a non-HCV amino acid sequence and having a chaperone amino acid sequence. No further HCV specific amino acid sequences are present in the polypeptide. A further aspect relates to a reagent kit for detecting HCV core antigen containing said multi-epitope fusion protein as calibrator or control or both.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
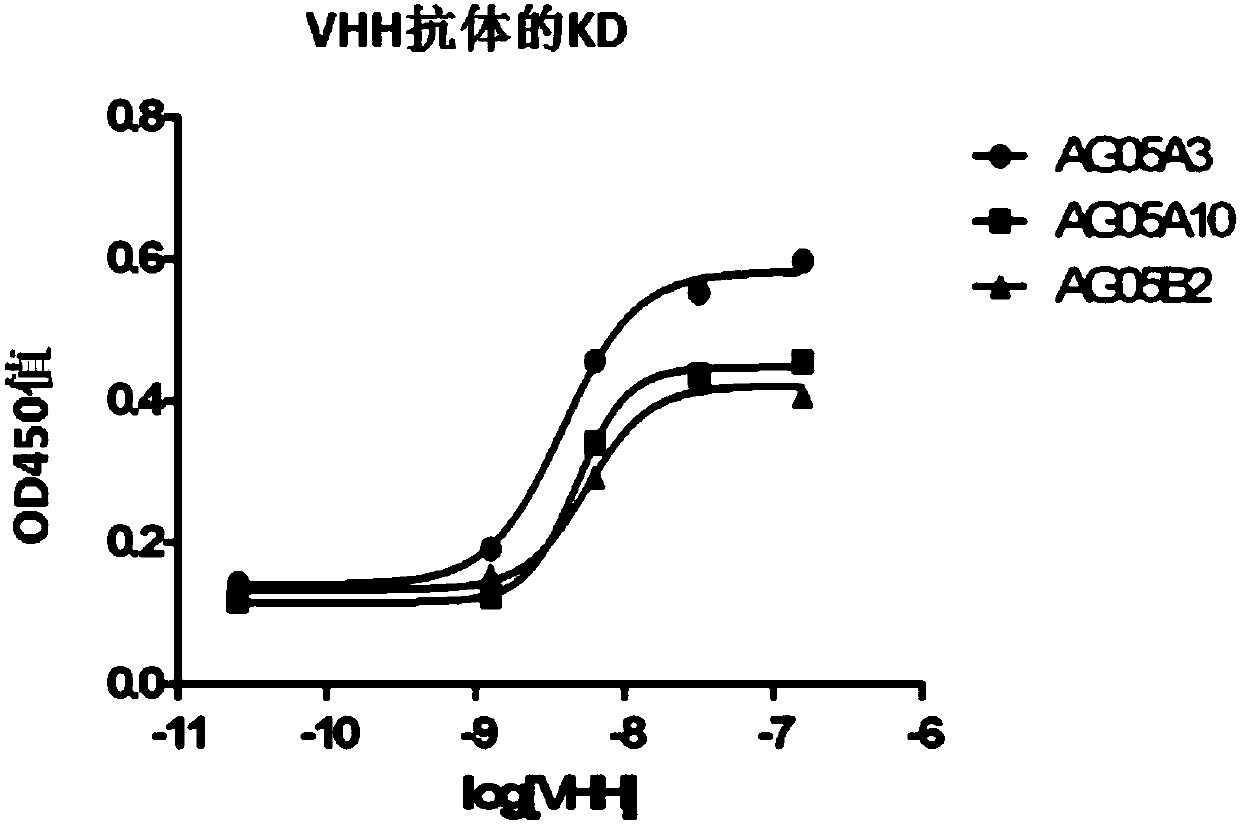
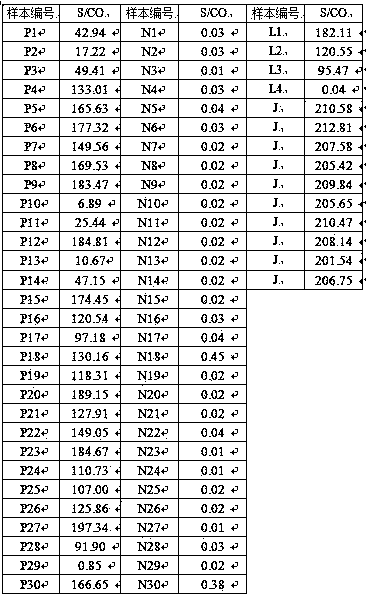
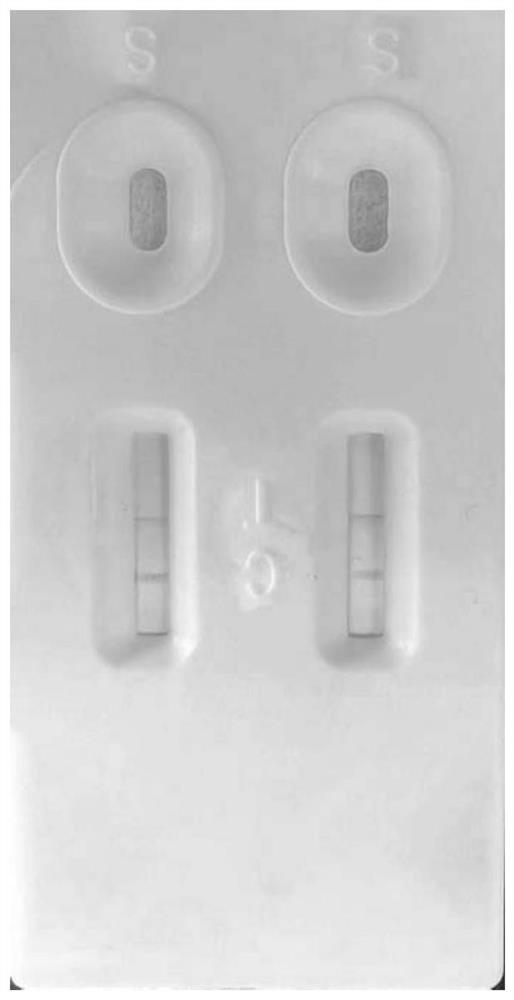
Camelid single-domain hcv antibodies and methods of use
InactiveCN107921123ASsRNA viruses positive-sensePolypeptide with affinity tagAnalyteAntibody fragments
The present disclosure in some aspects relates to HCV core antigen polypeptides. In some aspects, the present disclosure further relates to HCV antibodies, including camelid antibodies that specifically bind to HCV core antigen, and antibody fragments. The disclosure further relates to methods of detecting an analyte in a sample using a camelid antibody, such as a camelid VHH antibody or fragmentsthereof, hi one aspect, provided herein is a technology platform for isolating highly specific antibodies and applying these antibodies in an immunoassay, such as a lateral flow immunoassay (LFIA). In some aspects, this technology is used to develop HCV core antigen specific antibodies and to produce LFIA devices for rapid and early diagnosis of HCV. In other aspects, a rapid test is provided forscreening and detection of hepatitis C virus infection to improve the diagnosis rate and effectively prevent HCV infection transmission.
Owner:QOOLABS INC
Hepatitis C virus (HCV) antigen and antibody combined detection kit
The invention discloses a hepatitis C virus (HCV) antigen and antibody combined detection kit. The kit comprises magnetic particles, a sample diluent, a negative control, an antigen positive control,an antibody positive control and an enzyme conjugate, the magnetic particles are coated with recombinant HCV virus antigens and mouse anti-human HCV core antigen monoclonal antibodies; the antibody positive control contains HCV antibody positive human plasma; and the antigen positive control contains HCV recombinant core antigens. According to the kit, the chemiluminescence and magnetic particle separation technologies are combined, and the HCV recombinant core antigens and the HCV antibodies in serum are detected at the same time, by matching with a full-automatic chemiluminiscence instrumentof the Zhengzhou Antu Bio-Engineering Co.,Ltd, and can be used as a primary screening test for clinical detection, so that the window period is further shortened, and the HCV propagation is effectively controlled.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Hepatitis C virus antigen-antibody joint detection method and kit
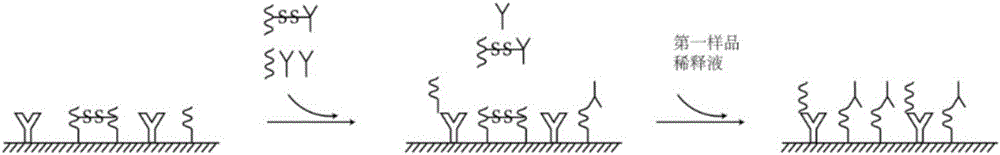
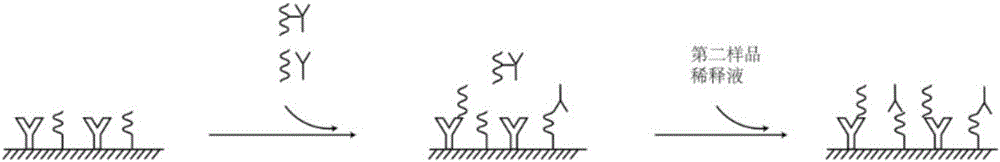
The invention relates to a hepatitis C virus antigen-antibody joint detection method in the technical field of immunodetection. The kit comprises at least two detection areas, whether a first compoundformed by a donor-hepatitis C virus antibody-receptor exists or not is detected in one detection area, and whether a second compound formed by a donor-hepatitis C virus antigen-receptor exists or notis detected in the other detection area, a donor can generate active oxygen in an excited state, and a receptor can react with the active oxygen to generate a detectable chemiluminescence signal. Themethod is advantaged in that the HCV core antigen and the antibody are jointly detected, so detection accuracy is improved, detection cost is low, moreover, a treating agent is added into a core antigen detection hole, so interference of a low-affinity antibody in an early body is reduced, the antigen detection sensitivity in a conversion period is improved, and the HCV detection sensitivity is further improved.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
HCV (hepatitis C virus) core antigen and antibody thereof as well as hybridoma cell lines secreting antibody
ActiveCN102329378BHigh affinitySensitive detection methodVirus peptidesMicroorganism based processesAntigenNucleotide
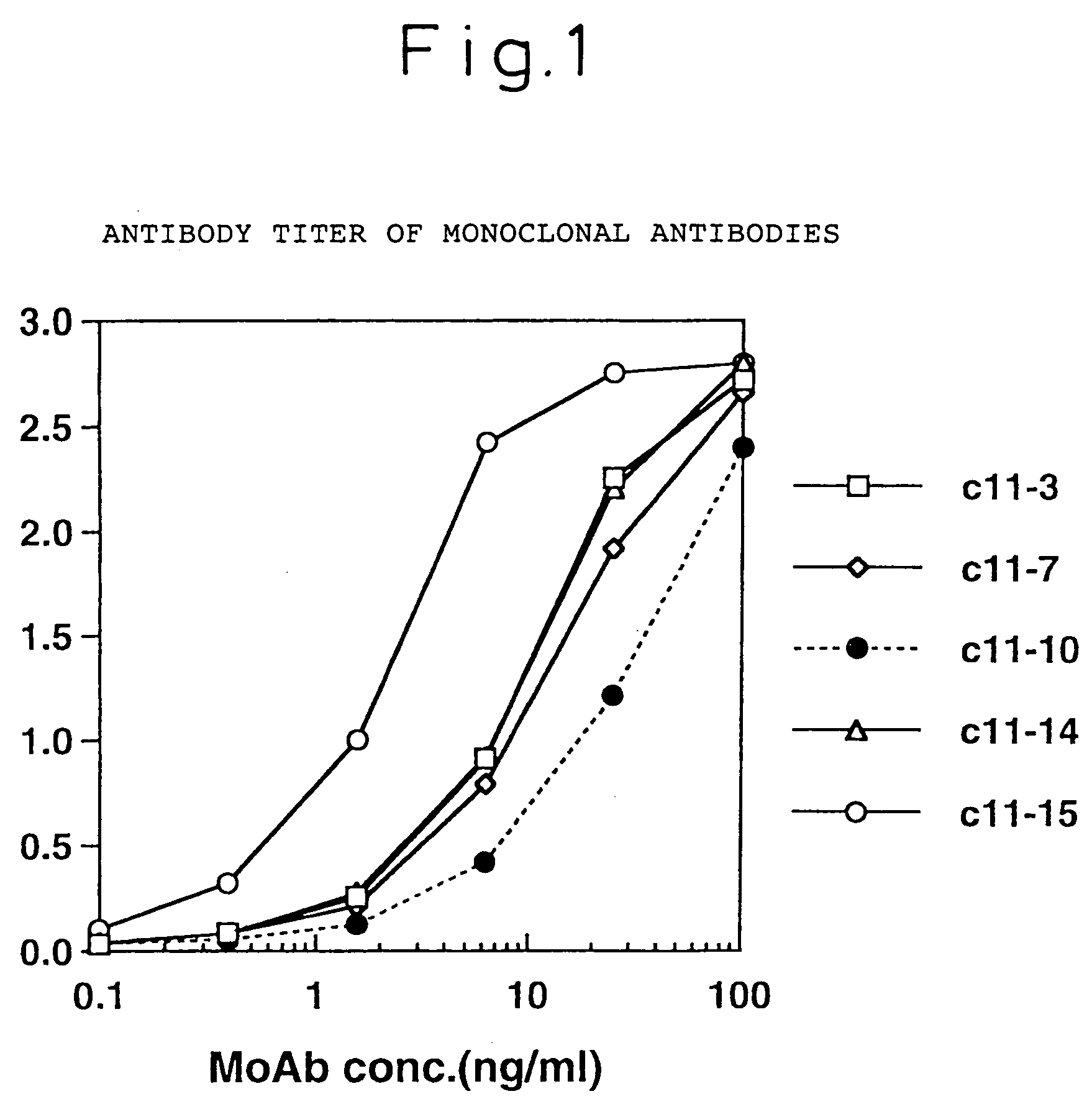
The invention discloses an HCV (hepatitis C virus) core antigen, which is a polypeptide encoded by a DNA (deoxyribonucleic acid) of which the amino acid sequence is shown in SEQ ID NO.1 and the nucleotide sequence is shown in SEQ ID NO.2. The invention also discloses a hybridoma cell line secreting monoclonal antibodies, the hybridoma cell line is named as SC16-H6 and has been preserved in the China General Microbiological Culture Collection Center on July 14th, 2011, and the preservation number is CGMCC No. 5074. The invention also discloses another hybridoma cell line secreting monoclonal antibodies, the hybridoma cell line is named as SC23-G5 and has been preserved in the China General Microbiological Culture Collection Center on July 14th, 2011, and the preservation number is CGMCC No. 5075. The invention also discloses a monoclonal antibody of an anti-HCV core antigen, which is secreted by the hybridoma cell line (preservation number: CGMCC No. 5074) or the hybridoma cell line (preservation number: CGMCC No. 5075), and has a specificity to the polypeptide shown in SEQ ID No.1.
Owner:SHANDONG UNIV QILU HOSPITAL
Multi-epitope fusion protein of an HCV antigen and uses thereof
ActiveUS11078241B2SsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenHepatitis C virus core
The disclosure relates to a multi-epitope fusion protein as well as to its use as calibrator and / or control in an in vitro diagnostics immunoassay for detecting HCV core antigen. The multi-epitope fusion protein has two to six different non-overlapping linear peptides present in the amino acid sequence of hepatitis C virus (HCV) core protein, wherein each of the peptides is separated from the other peptides by a spacer consisting of a non-HCV amino acid sequence and having a chaperone amino acid sequence. No further HCV specific amino acid sequences are present in the polypeptide. A further aspect relates to a reagent kit for detecting HCV core antigen containing said multi-epitope fusion protein as calibrator or control or both.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Hepatitis C virus antigen-antibody joint inspection kit and application thereof
PendingCN111521780AReduced detection windowReduce distractionsMaterial analysisReceptorAntigen testing
The invention relates to a hepatitis C virus antigen-antibody joint detection kit and an application thereof, belongs to the technical field of immunodetection. The kit comprises the following components, a receptor bound with a first antigen, a second antigen, a receptor binding to the first antibody, and a second antibody, wherein the first antigen and the second antigen can be specifically combined with a variable region of a hepatitis C virus antibody, the first antibody and the second antibody can be specifically combined with different epitopes of a hepatitis C virus antigen. The kit isadvantaged in that the HCV antigen-antibody joint detection kit can be used for joint detection of HCV core antigen and antibody, so a detection window period is shortened, detection cost is low, moreover, the kit further comprises a treating agent, and the treating agent can reduce the interference of a low-affinity antibody in an early body and improve the antigen detection sensitivity in a conversion period, so the HCV detection sensitivity is improved, and the HCV detection window period is further shortened.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
A highly efficient hepatitis C virus core antigen ELISA kit
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Preparation and application of recombinant hepatitis C antigen
InactiveCN108530521AOvercome the defects of low detection sensitivity and prone to false negative resultsLow detection sensitivitySsRNA viruses positive-senseViral antigen ingredientsAntigenNucleotide
The invention discloses preparation and application of recombinant hepatitis C antigen. The amino acid sequence of the recombinant hepatitis C antigen is shown in SEQ ID No. 1, and the protein structure of the recombinant hepatitis C antigen is soluble protein. The nucleotide sequence of amino acid for encoding the recombinant hepatitis C antigen is shown in SEQ ID No. 2. A preparation method of the recombinant hepatitis C antigen comprises the steps of culturing host cells having the nucleotide sequence shown in SEQ ID No. 2, isolating and purifying for culturing to obtain polypeptide or protein molecule antigen so as to obtain the recombinant hepatitis C antigen. The recombinant hepatitis C antigen is beneficial to the normalization and standardization of hepatitis C virus (HCV) core antigen detection, can be used for preparing a kit for detecting HCV, and is beneficial to making up for the defect that the existing kit is insufficient in sensitivity when being used for detecting theHCV.
Owner:南京京达生物技术有限公司
Virus disaggregating agent and method for disaggregating virus antigen-antibody complex and detecting HCV (Hepatitis C Virus) antigen
ActiveCN101975857BImprove securityPreserve antigen reactivitySerum immunoglobulinsImmunoglobulins against virusesWhole blood productHepatitis A viruses
The invention provides a virus disaggregating agent and a method for disaggregating virus antigen-antibody complex and detecting HCV (Hepatitis C Virus) antigen. The virus disaggregating agent is characterized in that each 100ml of disaggregating agent comprises 0.1-1ml of detergent, 0-20g of protein denaturant, 0.01-1ml of reducing agent, 0.1-1ml of fat solvent, and base liquid as remainder amount. The antigen-antibody complex obtained by the neutralization reaction of testing blood sample or antiserum and virus antigen is disaggregated into free components, or virus core antigen is fully exposed and the antigen reactivity of the virus core antigen is remained, thereby remarkably improving the sensitivity by comparing with the prior HCV core antigen detection method; in addition, the detection window period of the antigen is 49 days shorter than that of the anti-HCV antibody averagely, the situation appears within 1-2d after HCV-RNA appears, thereby efficiently shortening the window period of the blood serum before transformation, which is of important signification on improving the detection rate of affected person in the window period and the safety of blood transfusion and blood products. In the invention, the virus disaggregating agent is suitable for the disaggregation of common virus antigen-antibody complex including HCV and HAV (Hepatitis A Virus).
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Reagents for HCV antigen-antibody combination assays
The present invention is directed to combination immunoassays, reagents and kits for simultaneous detection of HCV antigens and anti-HCV antibodies in a sample. The combination immunoassays of the present invention employ a non-ionic detergent that effectively exposes or releases the HCV core antigen from virions in a sample without interfering with the performance of other reagents such as the capture of anti-HCV antibodies by recombinant HCV antigens.
Owner:ORTHO-CLINICAL DIAGNOSTICS
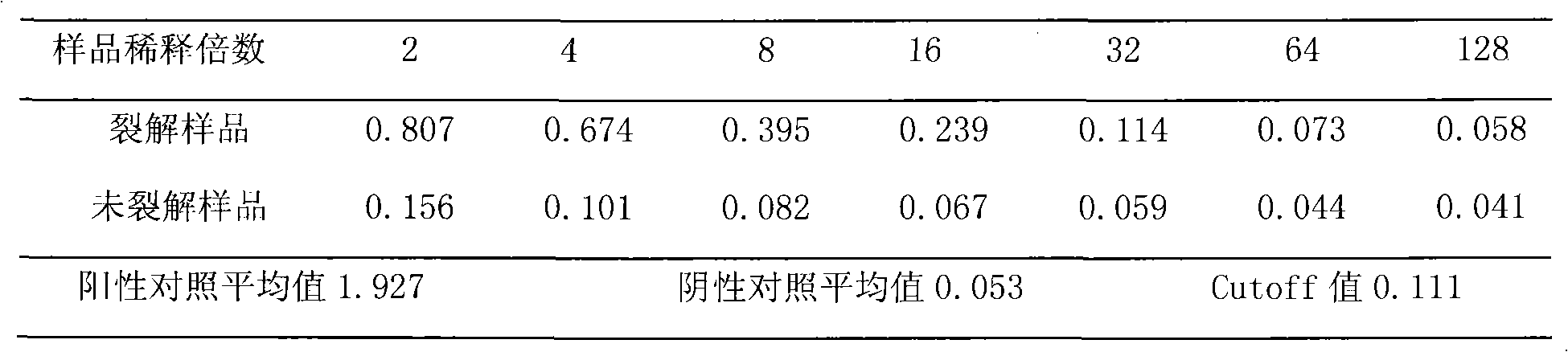
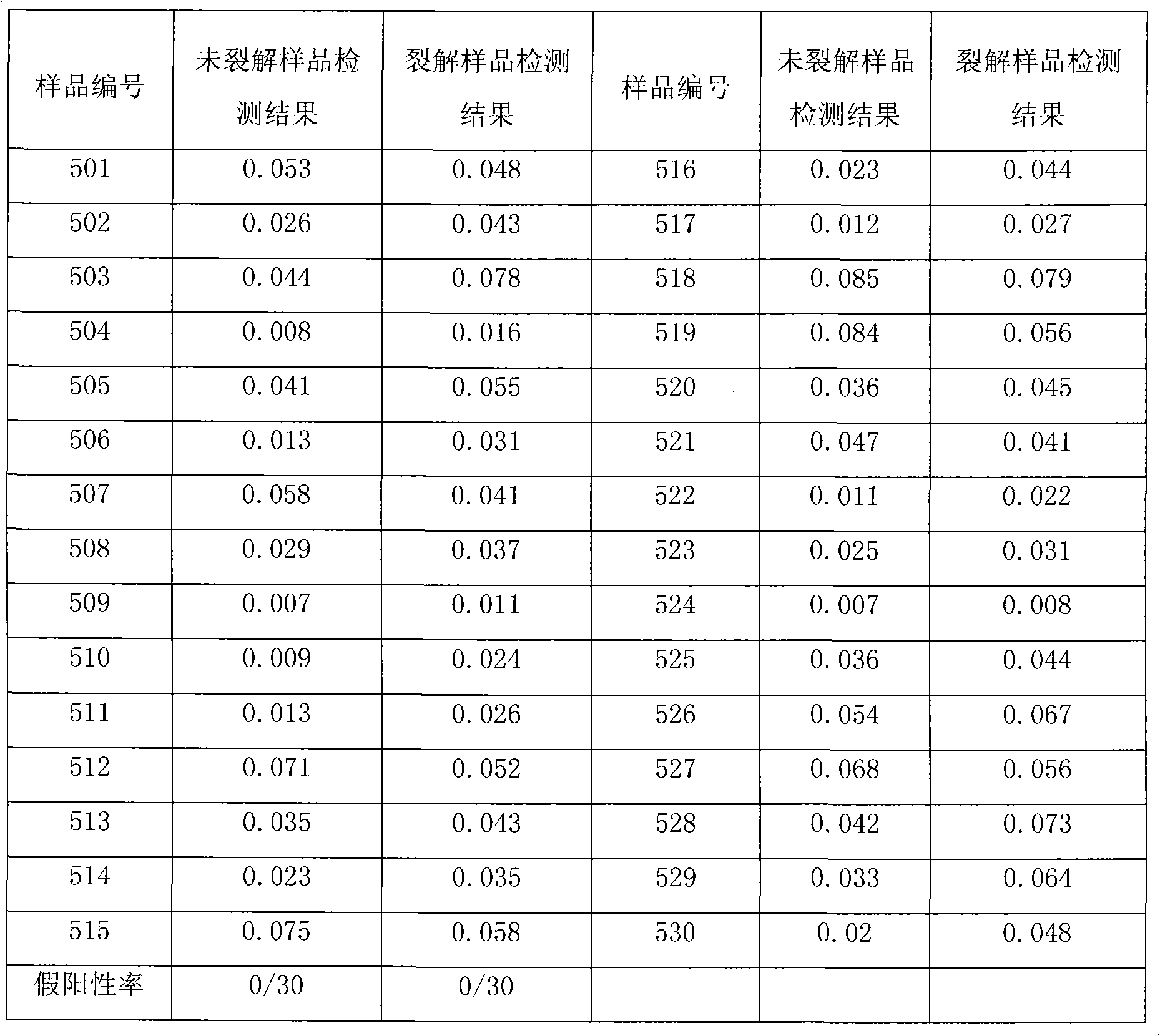
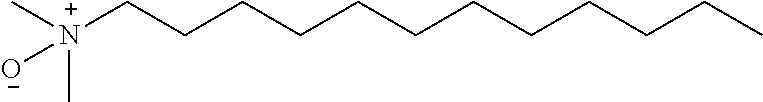
Pretreatment method for rapid detection of HCV core antigen
The present disclosure relates to a method for detecting a core polypeptide of a hepatitis C virus (HCV) in a sample from a subject with the steps of (a) contacting the sample with a surfactant comprising a cationic detergent; (b) contacting the sample with a binding compound; and (c) detecting a core polypeptide of the HCV in the sample; wherein step a) is immediately followed by step b). The present disclosure further relates to a method for pre-processing a sample from a subject for detection of an HCV core polypeptide, involving (a) contacting the sample with a surfactant comprising a cationic detergent and, optionally, with an agent inducing a pH shift, immediately followed by (b) contacting the sample with a binding compound. Moreover, the present disclosure further relates to uses, devices, and analytical systems related to aforesaid methods.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Hepatitis C virus detection related peptide and visible time-resolved fluorescent microsphere test strip thereof
ActiveCN113912677AShort detection timeHigh detection sensitivitySsRNA viruses positive-senseVirus peptidesViral testHepatovirus
The invention provides a quality control peptide, an HCV hapten and an HCV antigen for HCV detection, and a test strip based on the quality control peptide, the HCV hapten and the HCV antigen. The quality control peptide, the HCV hapten and the HCV antigen can be used for simultaneously detecting an HCV core antigen and an HCV antibody in a fluorescent or naked-eye manner.
Owner:苏州华益美生物科技有限公司
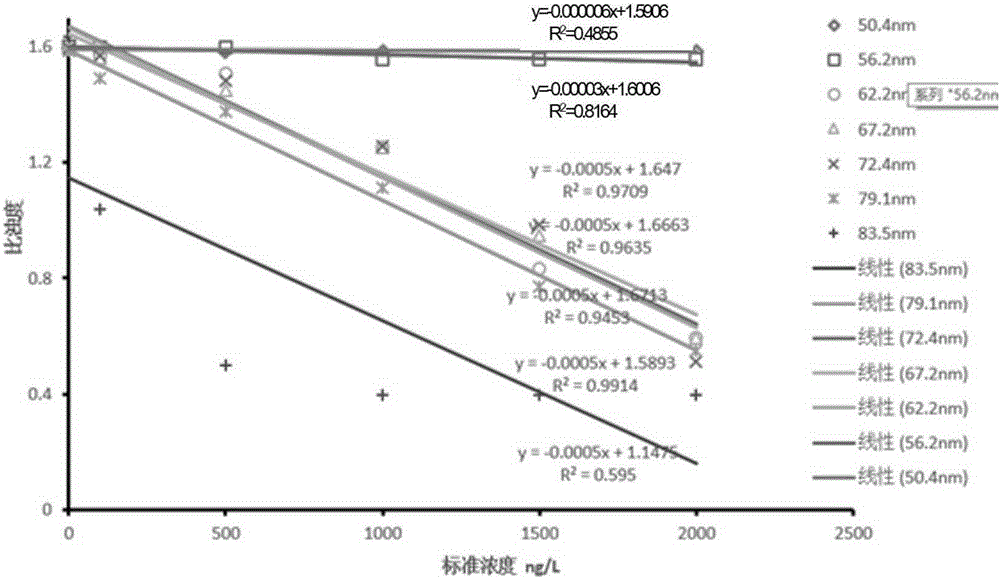
Hepatitis C virus core antigen detection kit and its preparation
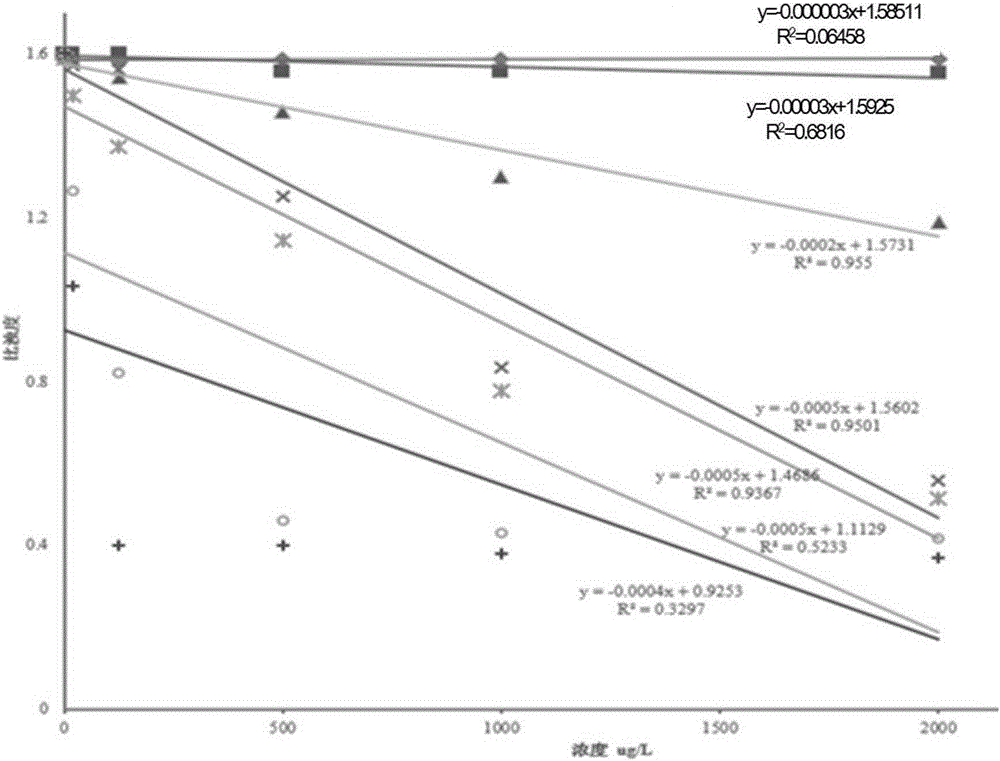
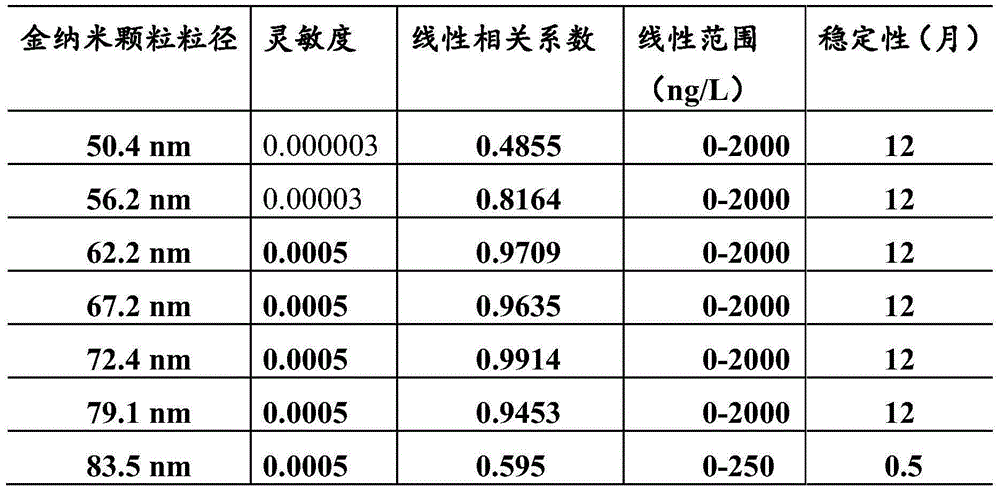
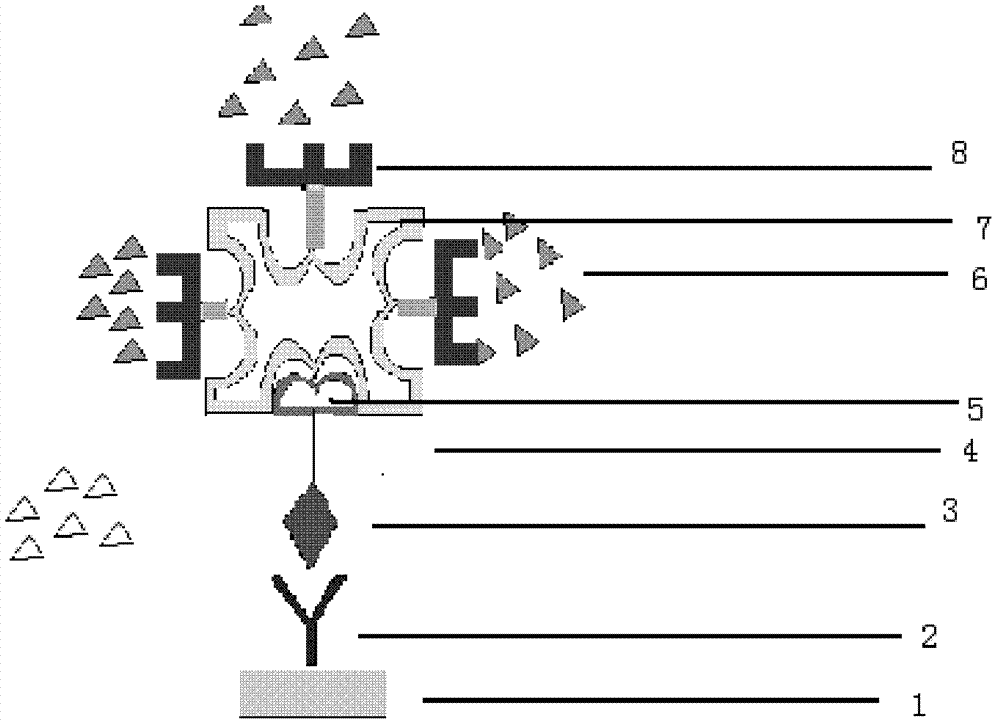
The invention provides a hepatitis C virus (HCV) core antigen detection kit. Based on colloidal gold immunoturbidimetry, the kit contains a reagent R2 which is a solution containing gold nanoparticles labeled with an HCV core antigen antibody. The kit is characterized in that particle size of the gold nanoparticles is 62.2nm-79.1nm; and the mass ratio of the gold nanoparticles to the antibody is 50:20-60. The invention also provides a preparation method of the kit. The kit provided by the invention has characteristics of high sensitivity, high specificity, rapid reaction and good stability. No precipitate is generated after a reaction. It is convenient to clean a biochemical analyzer, and service life of the biochemical analyzer is prolonged.
Owner:BEIJING JIUJIAYI TECH
HCV Nucleic acid aptamer and application thereof in preparing HCV-cAg detection kit
ActiveCN102433339BEasy to getShort screening cycleMaterial analysisDNA/RNA fragmentationAntigenNucleotide
The invention discloses a hepatitis C virus core antigen nucleic acid aptamer, wherein the nucleotide sequence of the aptamer is as indicated in SEQ ID No.1. The invention also discloses the application of the hepatitis C virus core antigen nucleic acid aptamer in preparing a hepatitis C virus (HCV) core antigen detection kit. The aptamer and the HCV core antigen detection kit prepared therefrom in the invention are capable of detecting the core antigen at pg / ml level; the sensitivity of the hepatitis C virus core antigen is greatly improved; therefore, the HCV nucleic acid aptamer and the HCV-cAg detection kit has important clinic diagnosis meaning and wide application prospect for early diagnosis of window-phase infected patients.
Owner:山东莱博生物科技有限公司
Hepatitis C virus core antigen chemiluminescence ELISA detection kit
ActiveCN101419238BRealize full detectionImprove featuresMaterial analysisOperating instructionAntigen
The invention discloses a reagent kit for carrying out chemiluminescent enzyme-linked immunodetection on a hepatitis C virus core antigen. The reagent kit comprises a kit body, and an enzyme label plate, a reagent, a non-drying glue sealing strip and an operating instruction manual which are arranged inside the kit body, wherein the enzyme label plate is a chemiluminescent enzyme label plate; each hole of the plate is coated with coated antibodies Mab1and Mab2 with concentration of 5ug / ml; and the reagent comprises a HCV-cAg antienzyme composition, a gradient solution of a HCV core antigen standard product, positive control serum, negative control serum, a pretreatment solution, a diluent of a HCV-cAg sample, a chemiluminescent zymolyte solution A, a chemiluminescent zymolyte solution B and a 20 * concentrated cleaning solution. The positive detection rate of the reagent kit is close to that of PCR; and simultaneously, the reagent kit has the advantages of simple operation, high sensitivity and high specificity, and is suitable for popularization and application in clinic.
Owner:山东莱博生物科技有限公司
Treating agent and application thereof
PendingCN111521804AReduced detection windowHigh detection sensitivityMaterial analysisAntiendomysial antibodiesActive agent
The invention relates to a treating agent and an application thereof in the technical field of immunodetection. The treating agent is prepared from the following components in percentage by weight of5wt%-15wt% of urea, 1 v%-5 v% of n-butyl alcohol, 0.5 wt%-2wt% of ionic surface active agent, 0.1 v%-2 v% of non-ionic surface active agent, and 5 wt%-15 wt% of metal salt. The treating agent disclosed by the invention can be used for cracking HCV viruses and denaturing core antigens, so HCV core epitopes are exposed, detection sensitivity of the HCV core antigens is improved,an HCV detection window period is further shortened; in addition, the treating agent disclosed by the invention can dissociate the low-affinity antibody, so interference of the early-stage in-vivo low-affinity antibody isreduced.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
A microfluidic paper-based chip for detecting hepatitis C virus antibody and its preparation method
The invention relates to a microfluidic paper base chip for detecting an antibody to hepatitis C virus as well as a preparation method of the chip. The microfluidic paper base chip provided by the invention comprises a plurality of micro detection channels, wherein each channel comprises an HCV core antigen, an HCV NS5 antigen, an HCV NS4 antigen, an HCVNS3 antigen as well as the combination thereof. Owing to the multi-channel detection capability, the microfluidic paper base chip can detect a plurality of immune projects synchronously; miniaturized screening and confirming testing are integrated on the microfluidic paper base chip, so that the complex HCV diagnosis process is simplified greatly, and the diagnosis efficiency is improved greatly.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com