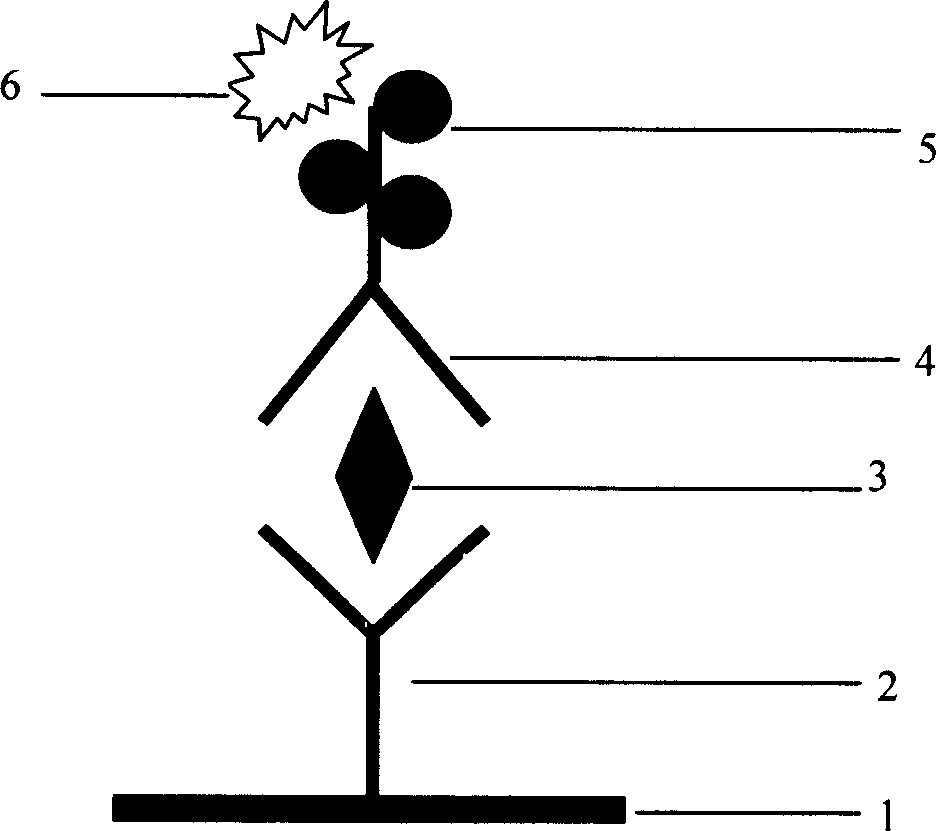
Enzyme-linked immunologic diagnosis kit for core antigen of C type hepatitis virus and method for preparing same
A technology of hepatitis C virus and diagnostic kits, applied in biological testing, material inspection products, measuring devices, etc., can solve the problems of not applying for invention patents in China, high prices, promotion and popularization, etc., achieve good application prospects, shorten Window period, effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] 1. Preparation of HCV-core recombinant antigen
[0030] 1.1 Recombinant antigen cloning and expression: call out the full sequence gene of HCV from the gene bank, and analyze the sequences of the core antigens of different subtypes of HCV:
[0031]
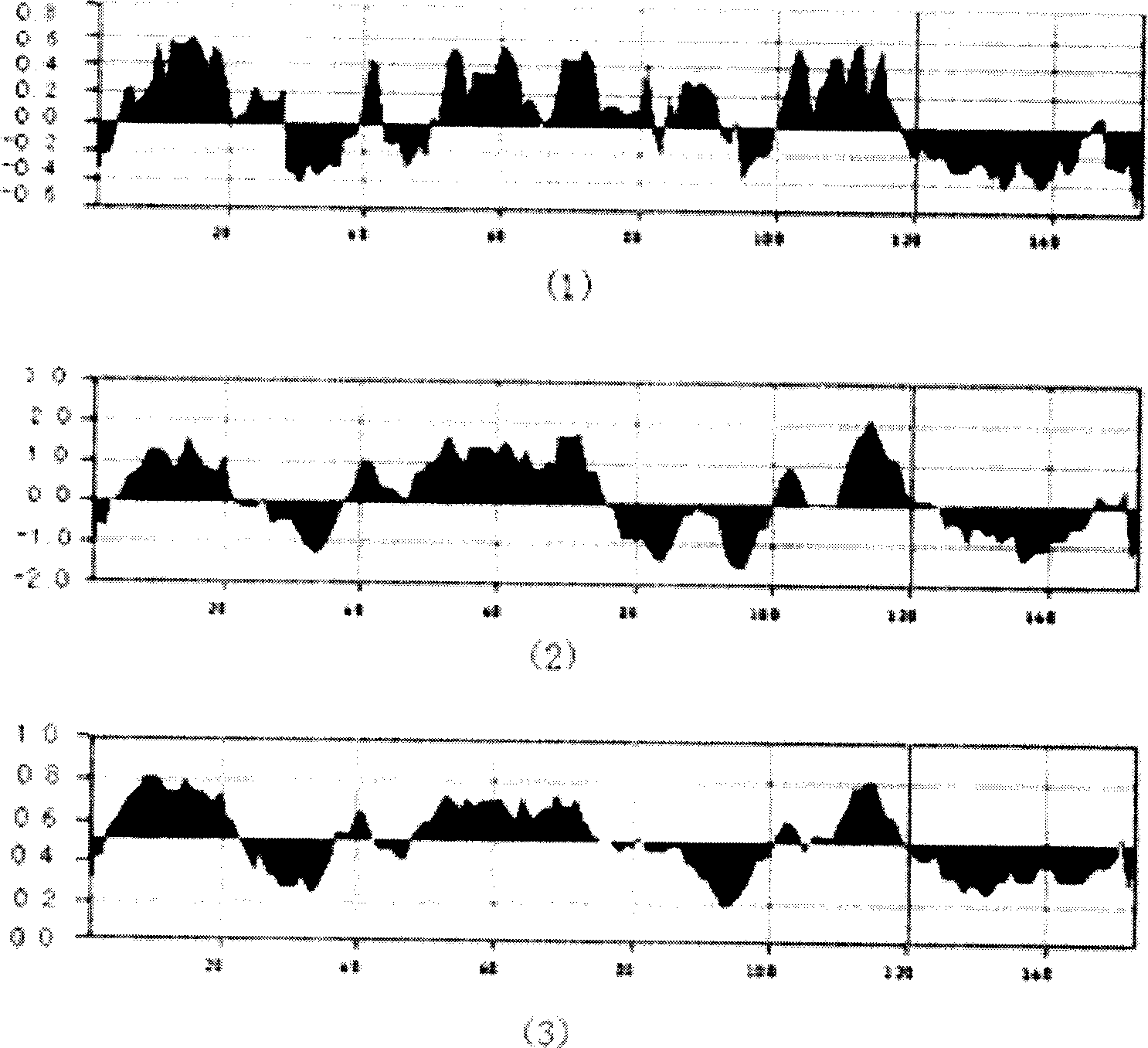
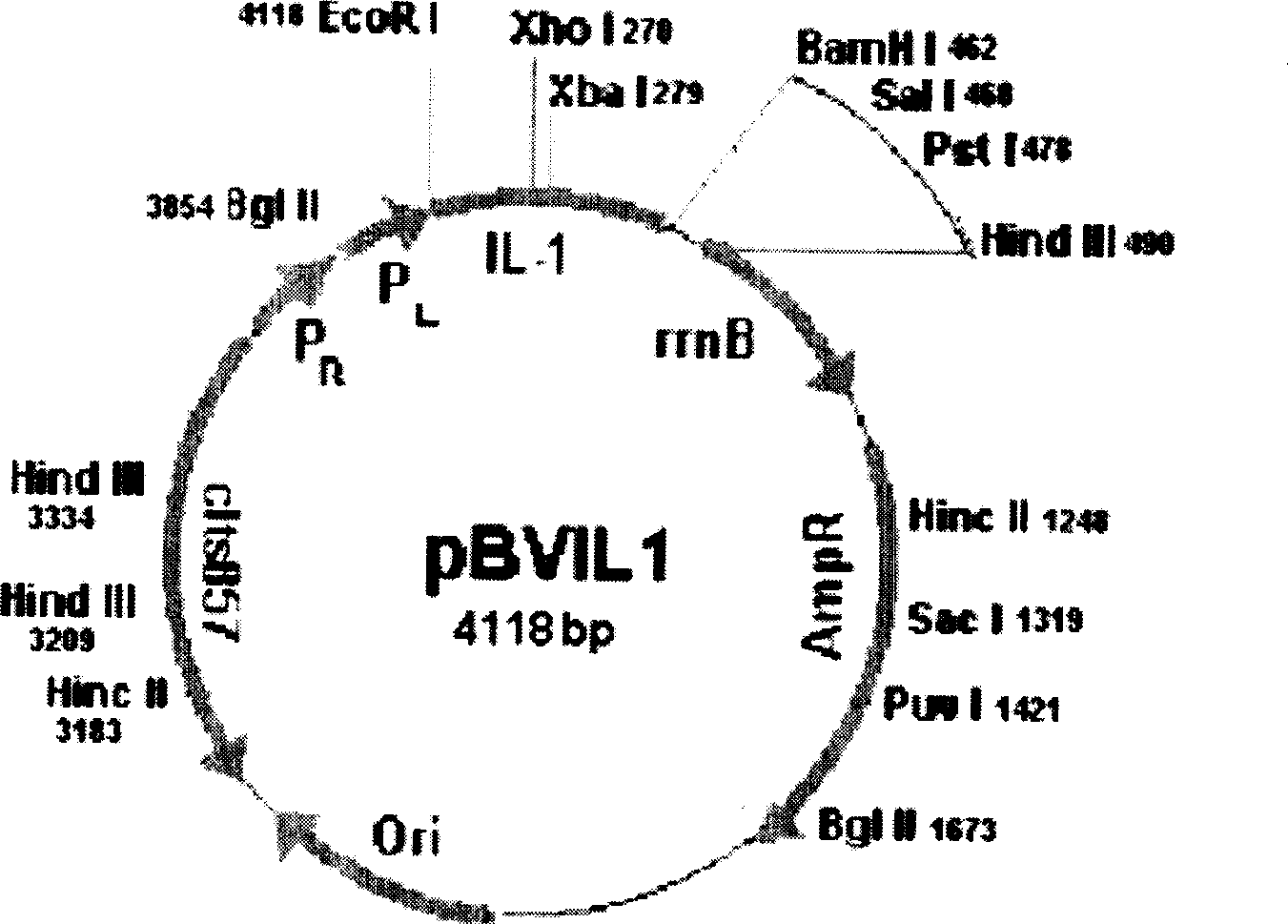
[0032] At the same time, the Macvector program was used to analyze the antigenicity, hydrophilicity and antigenic epitope of the hepatitis C core antigen 1-160 amino acid sequence ( figure 2 ). Among them, there are 4 antigenic epitopes with conserved sequences and strong antigenicity, which are located at amino acid residues 20-35, 35-50 and 100-115, 115-130, respectively. The 2-160 amino acid sequence was selected and inserted into the expression vector pBVIL1 for recombinant cloning and expression ( image 3 ).
[0033] 1.2 Purification and activity identification of recombinant HCV-cAg: The recombinant HCV-C antigen extracted from engineering bacteria was eluted by S-Sepharose column step gradient, and the elutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com