Camelid single-domain hcv antibodies and methods of use
An antibody, camel technology, applied in the direction of antibodies, chemical instruments and methods, botanical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
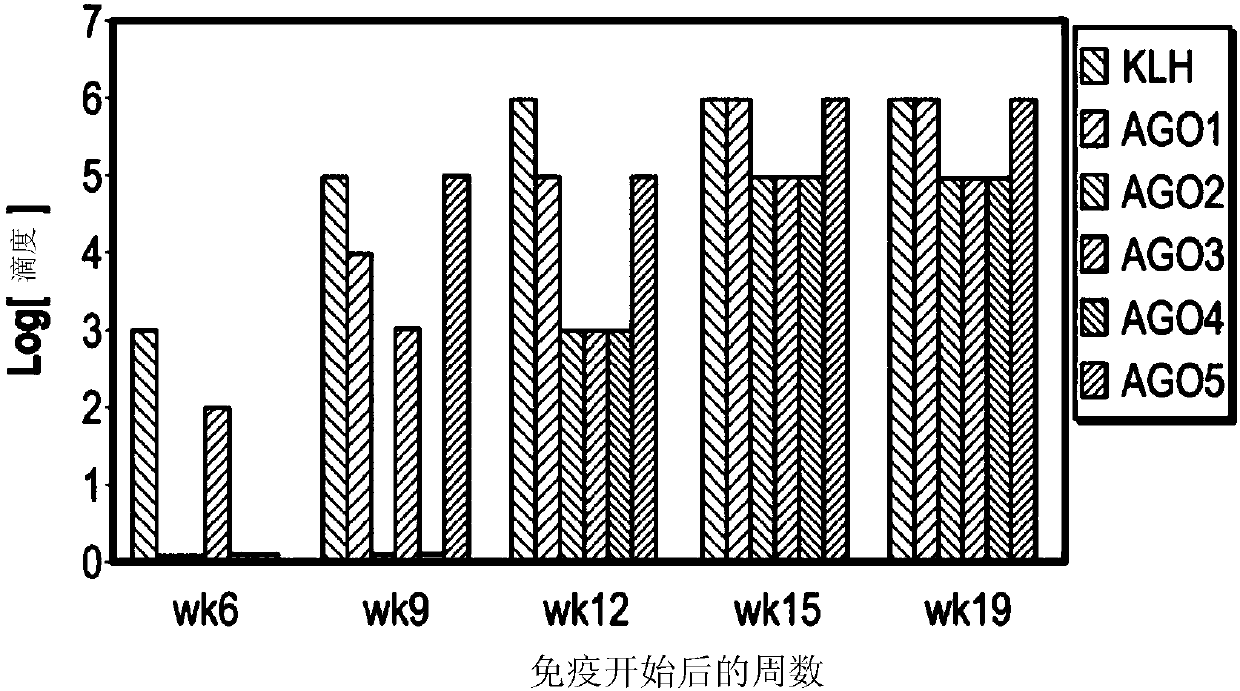
[0381] Example 1: Llama immunity
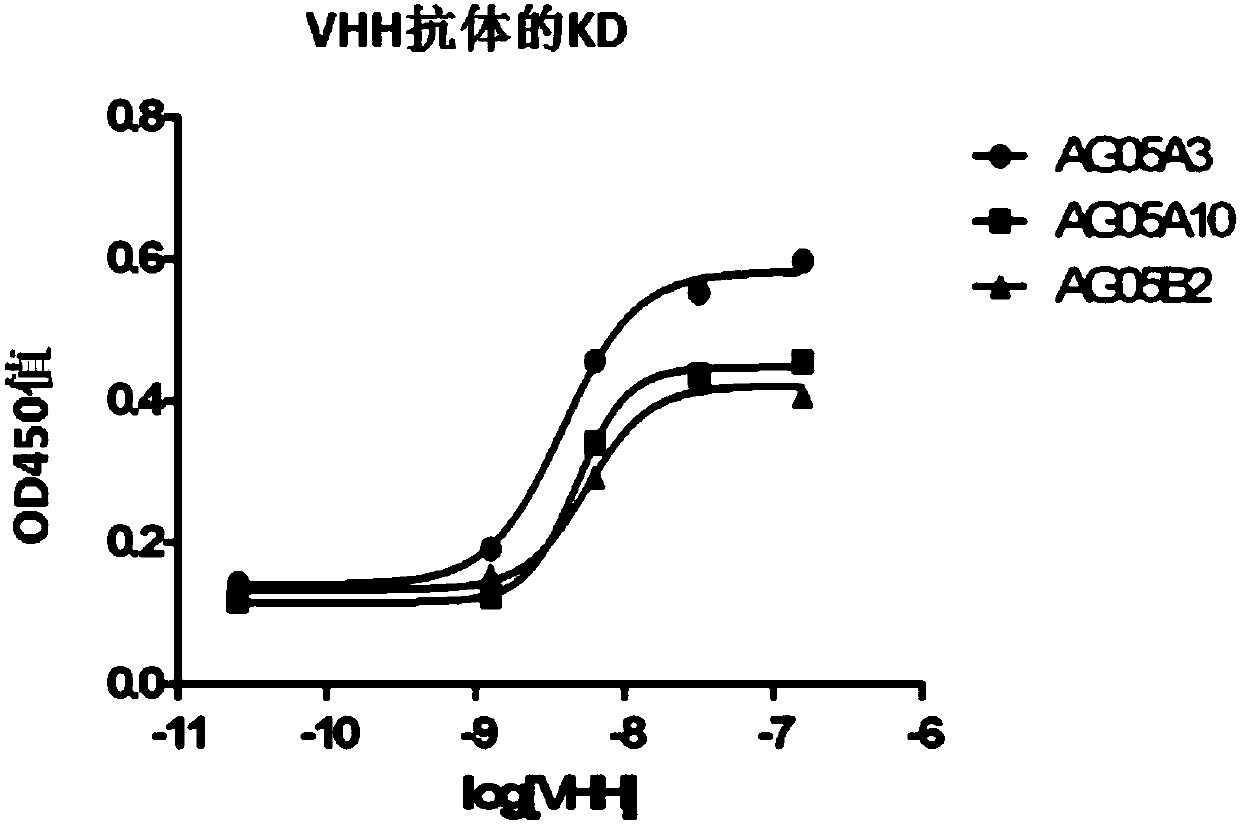
[0382] This example provides a method for isolating high affinity VHH antibodies from immunized llamas by in vitro screening. Using this method, multiple VHH antibodies to small molecule haptens were isolated, one of which was a high affinity antibody with a kD of 60pM. Many of these VHH antibodies had kD in the ~100 pM range as determined by ELISA.
[0383]In one experiment, five small molecule compounds were routinely synthesized / modified by Annova Chem (San Diego, CA) to generate a carboxyl group for attachment. The compounds are named AG01, AG02, AG03, AG04, and AG05, and their molecular structures are shown in Table 2.
[0384] Table 2 List of small molecule compounds used for llama immunization
[0385]
[0386]
[0387] Each compound was dissolved in DMSO at a concentration of 50 mg / ml and diluted to 20 mg / ml with MES buffer (0.1 M, pH 6.4). KLH was diluted to 20 mg / ml before use. Equimolar ratios of NHS and EDC were disso...
example 2
[0394] Example 2: Application of VHH Antibody in Rapid Detection Platform
[0395] Fusion proteins of VHH antibodies were prepared using rabbit Fc domains (VHH-rFc). The antibodies are expressed / purified and can be detected using widely available secondary antibodies against rabbit IgG. These antibodies were successfully used to produce LFIA devices ( Figure 4 ). Without any optimization, the detection limit of AG01 using LFIA is 250 ng / ml. These data demonstrate that VHH antibodies can be successfully used to prepare highly sensitive LFIA devices targeting small molecules.
[0396] Figure 4 Competitive lateral flow immunoassay for detection of AG01 using VHH-rFc fusion antibody is shown. The quality control line is printed with goat anti-rabbit antibody. The test line is printed with the AG01-BSA conjugate. A VHH-rFc fusion antibody against AG01 was labeled with colloidal gold and printed on the conjugate pad. Samples containing serially diluted AG01 were applied ...
example 3
[0401] Example 3: Quantitative LFIA of quantum dot (QD) technology
[0402] Traditional lateral flow assays use colloidal gold or latex microsphere-conjugated antibodies for colorimetric detection and are primarily qualitative. Fluorescent and luminescent labels are used to increase sensitivity. Semiconductor nanocrystals, also known as quantum dots, are a class of light-emitting materials whose electrical properties are closely related to the size and shape of individual crystals. By simply changing the crystal size, quantum dots can emit light or colors in a wide range of wavelengths not easily covered by organic dyes. A single light source can excite quantum dots of many colors so that multiple targets can be marked and detected simultaneously. In addition to this composite function, quantum dots have bright colors and long-term photostability, so they are brighter and retain their luminescence longer than organic dyes. For these reasons, quantum dots are the best choi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com