HCV core antigen detection kit based on magnetic micro-particle chemiluminescence method
A chemiluminescence method and a detection kit technology, applied in chemiluminescence/bioluminescence, analysis through chemical reactions of materials, measurement devices, etc., can solve the problems of long window period, difficult promotion, low sensitivity, etc., and achieve easy The effects of automated operation, expanded detection range, and shortened window period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The composition and preparation method of the kit
[0029] 1. Reagent R1: Add 0.1 g of magnetic beads coated with anti-HCV-cAg antibody purchased from Dynal to 100 ml of 0.1M PBS buffer with pH=7.6 and mix well.
[0030] 2. The preparation method of HRP-labeled anti-HCV-cAg antibody in reagent R2 is as follows:
[0031] (1) Weigh 25 mg of HRP and dissolve it in 1.25% glutaraldehyde solution, and let it stand overnight at room temperature.
[0032] (2) The reacted enzyme solution was eluted with a Sephadex G-25 chromatographic column with physiological saline. The flow rate was controlled at 1ml / min, and the brown effluent was collected. If the volume is greater than 5ml, concentrate to 5ml with PEG. Place in a 25ml small beaker and stir slowly.
[0033] (3) Dilute 12.5mg of the antibody to be labeled to 5ml with physiological saline, and add it dropwise to the enzyme solution while stirring.
[0034] (4) Add 0.25ml of 1M pH9.5 carbonic acid buffer and continue to s...
Embodiment 2
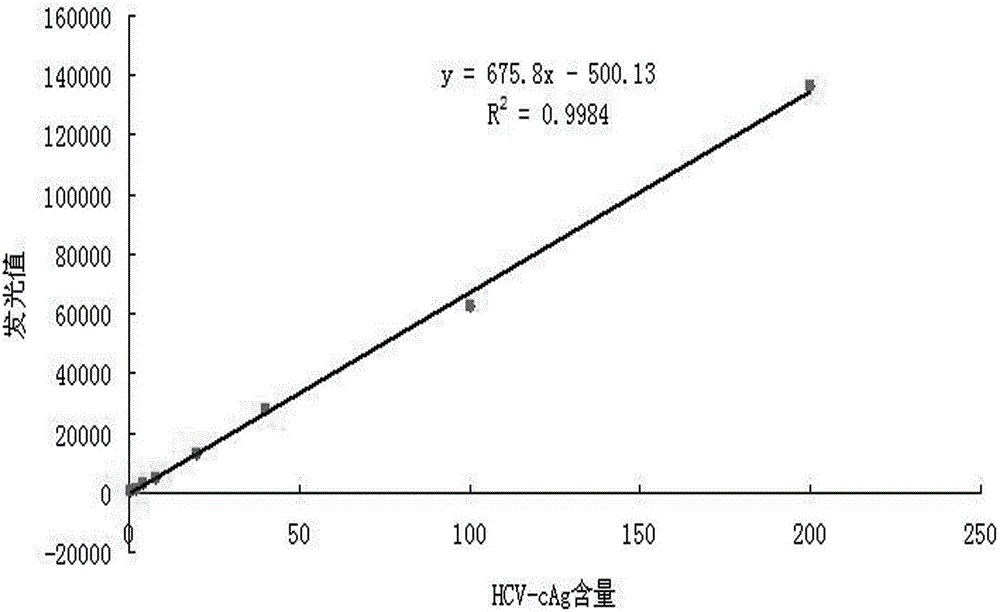
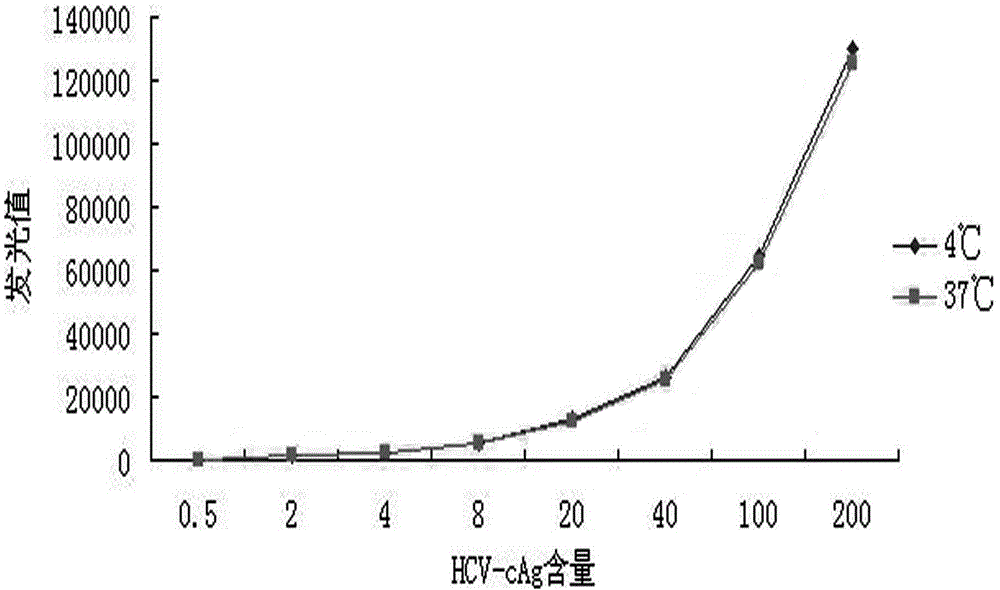
[0046] Sensitivity and linear range experiments
[0047] Through experimental detection and analysis, the determination of the cut off value of the reagent: the average RLU value of the negative reference product detection × 2.1 (if the average RLU value of the negative reference product detection is ≤ 60, calculate as 60; when RLU > 60, calculate according to the actual Calculate the average RLU value of the negative control × 2.1). The low-value samples were detected, and the luminescence values of the negative reference product and normal human serum were compared at the same time, as shown in Table 1. By comparison, when the low-value sample concentration is 0.5ng / mL, it is still detected as positive, indicating that the sensitivity of the reagent in Example 1 can reach 0.5ng / mL, which has good sensitivity.
[0048] Table 1 Sensitivity determination
[0049] Test items Luminescence value (RLU) Result judgment HCV-cAg 0.5ng / mL 331 + 20 normal h...
Embodiment 3
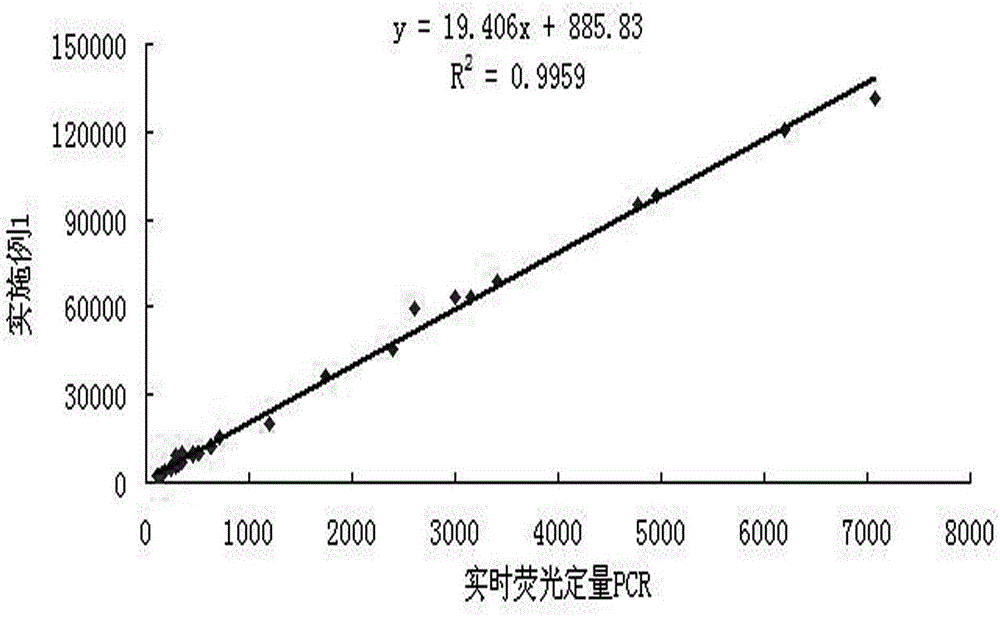
[0052]The detection kit for hepatitis C core antigen by magnetic particle chemiluminescence method of the present invention has a good correlation with the result obtained by real-time fluorescent quantitative PCR.
[0053] The specific method is as follows: 40 cases of clinical samples are detected simultaneously with the kit obtained in Example 1 and the hepatitis C virus nucleic acid detection kit (PCR-fluorescent probe method) produced by Sun Yat-sen University Daan Gene Co., Ltd., and the results are very good. good correlation, specific results such as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com