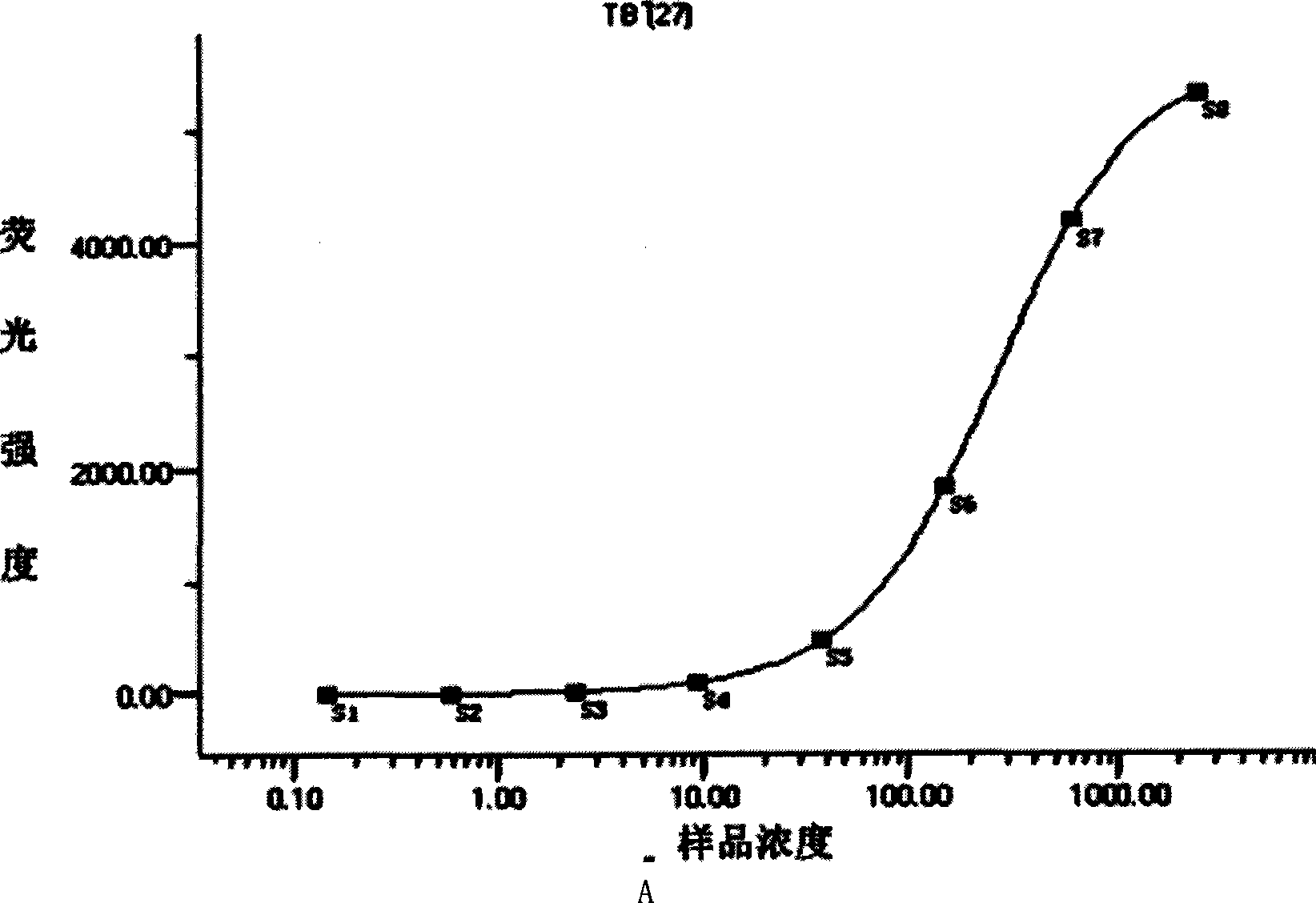
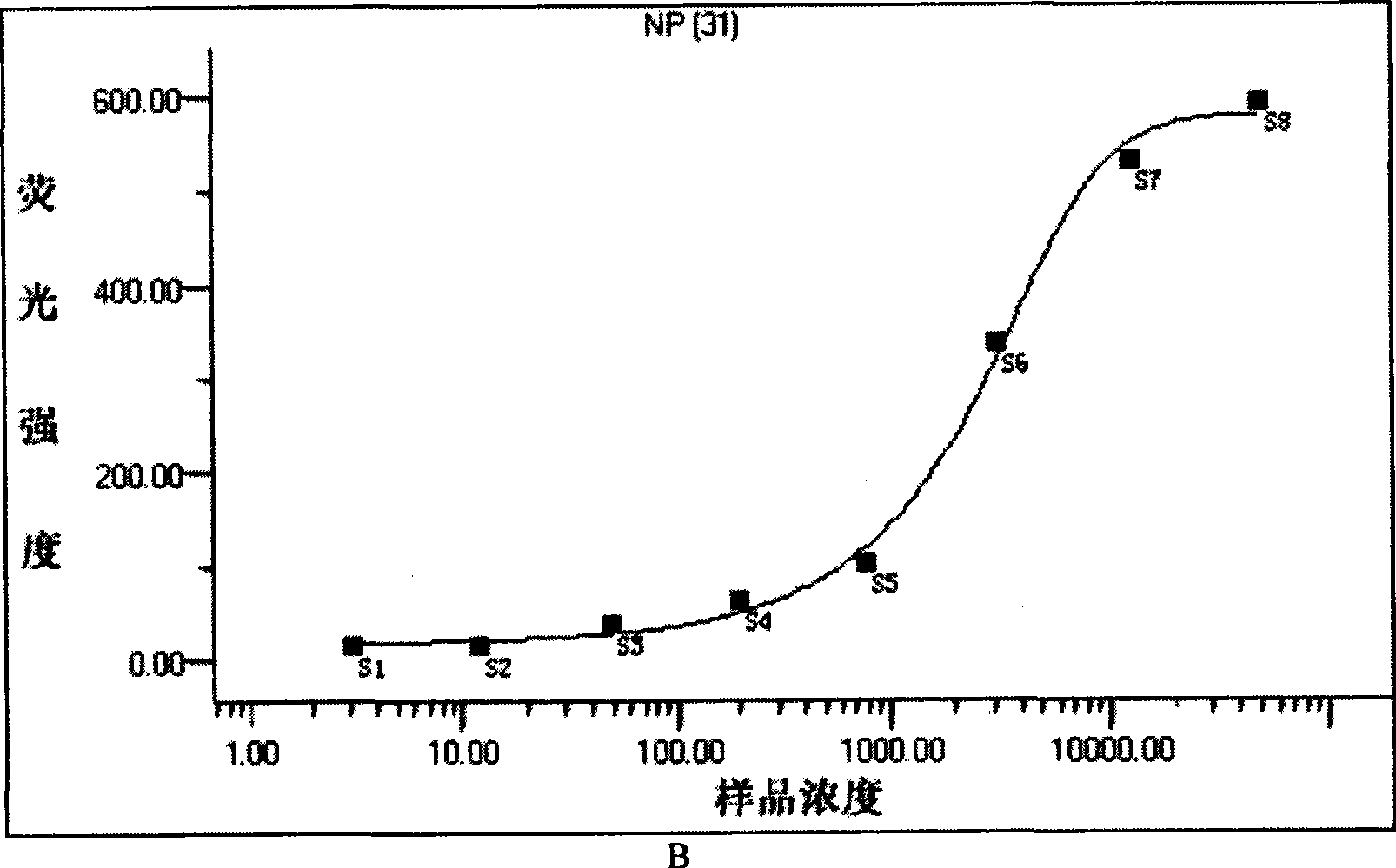
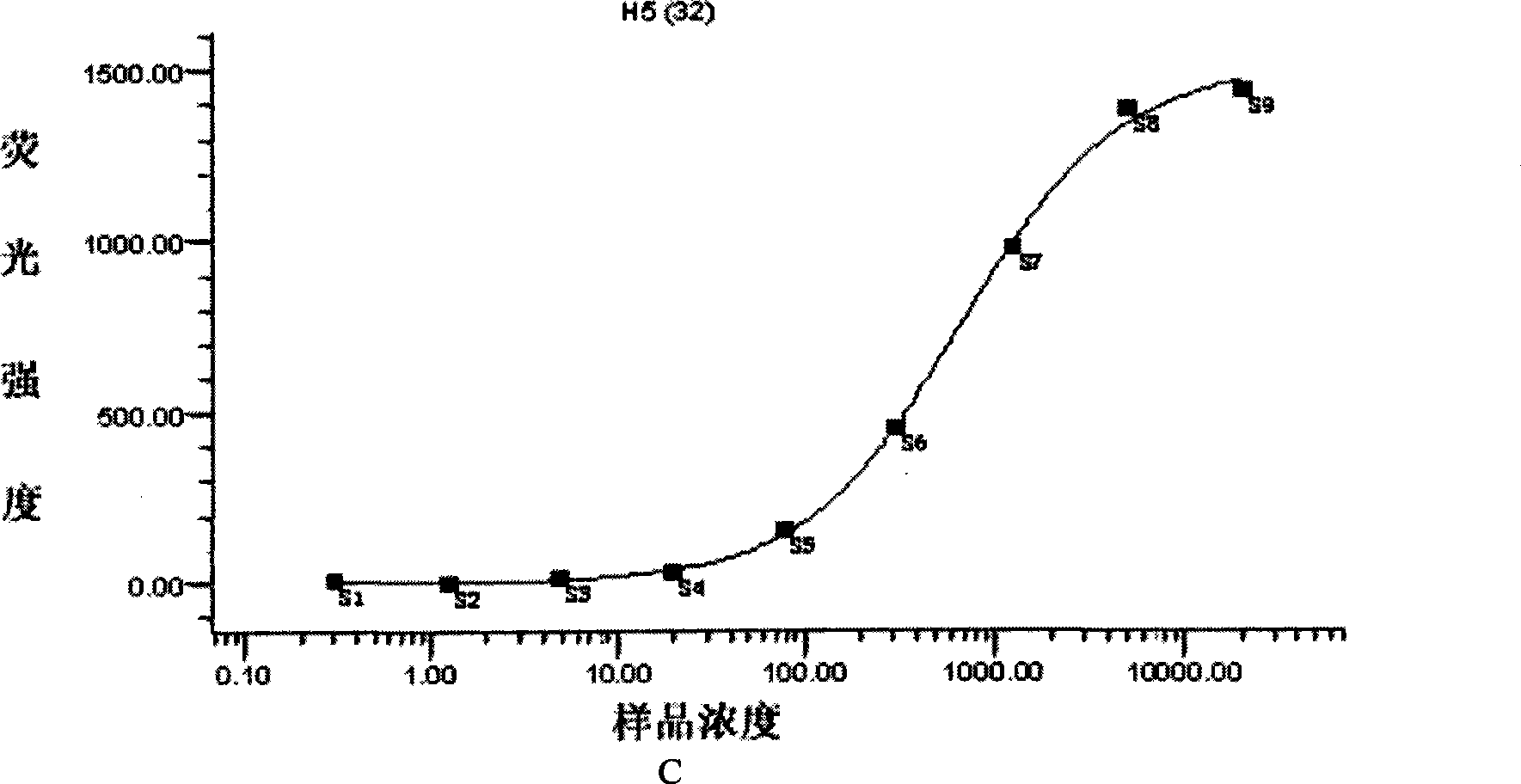
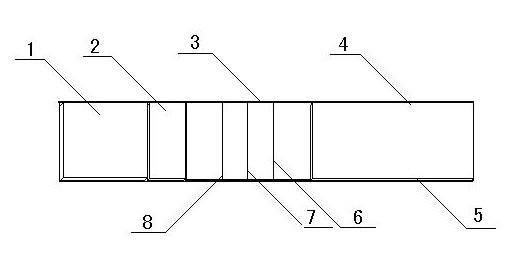
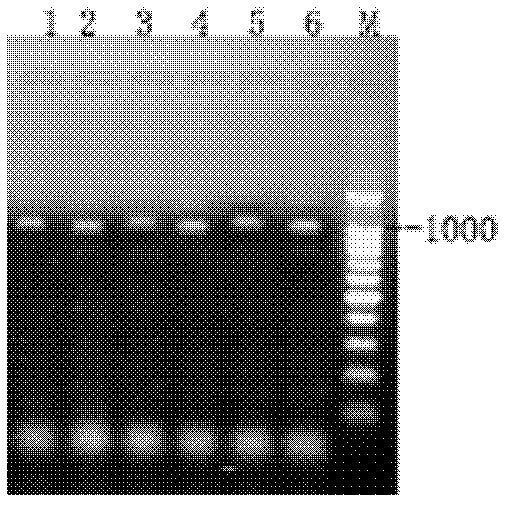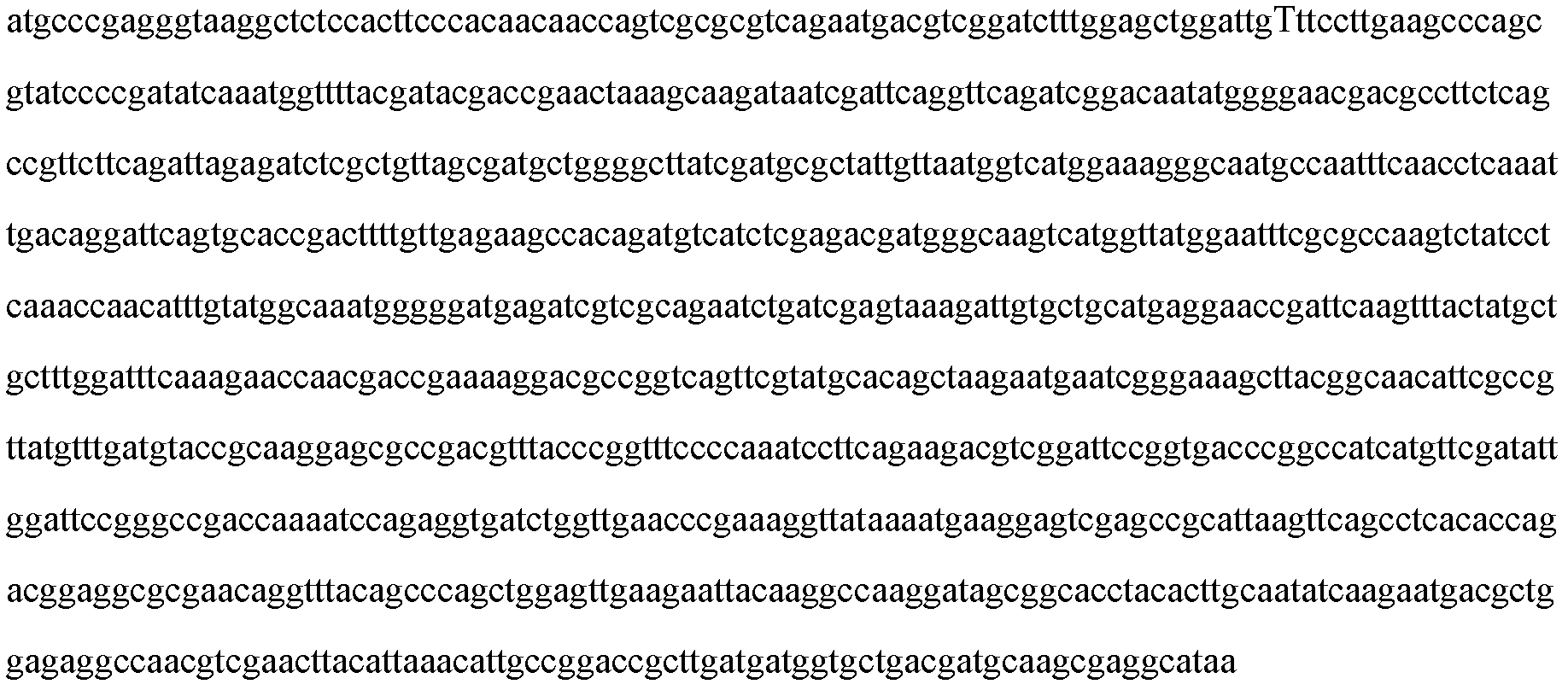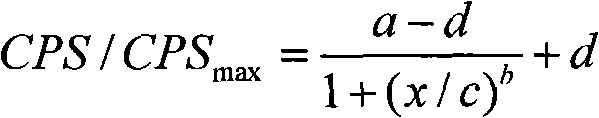Patents
Literature
224 results about "Antigen testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antigen detection is a test usually done to detect or identify what organisms are causing a disease in a patient. Antigens are foreign substances or organisms, like parasites, bacteria, fungi or viruses, which enter the human body and stimulate the immune system to produce specific antibodies.
Diagnostic panel of cancer antibodies and methods for use
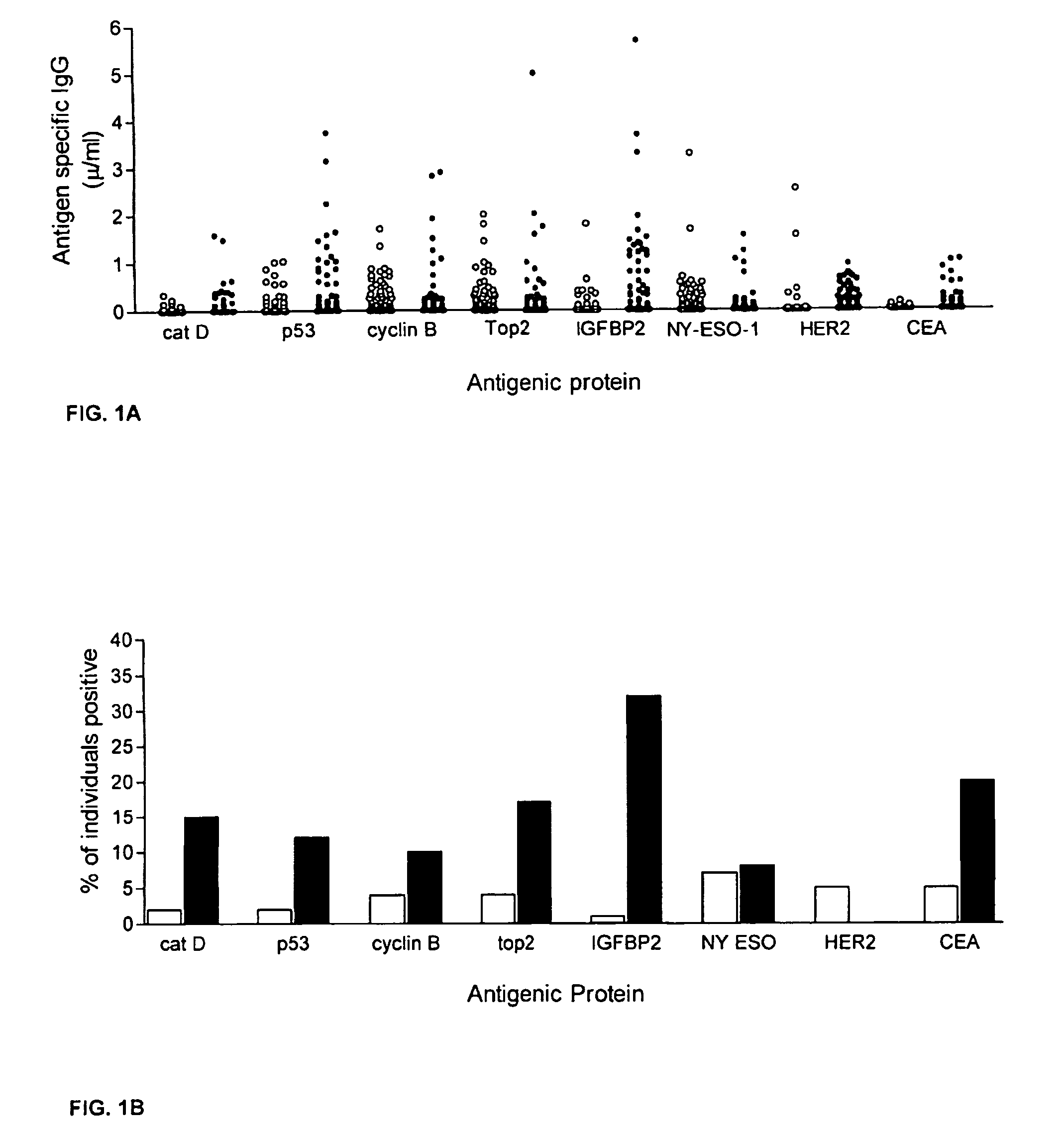
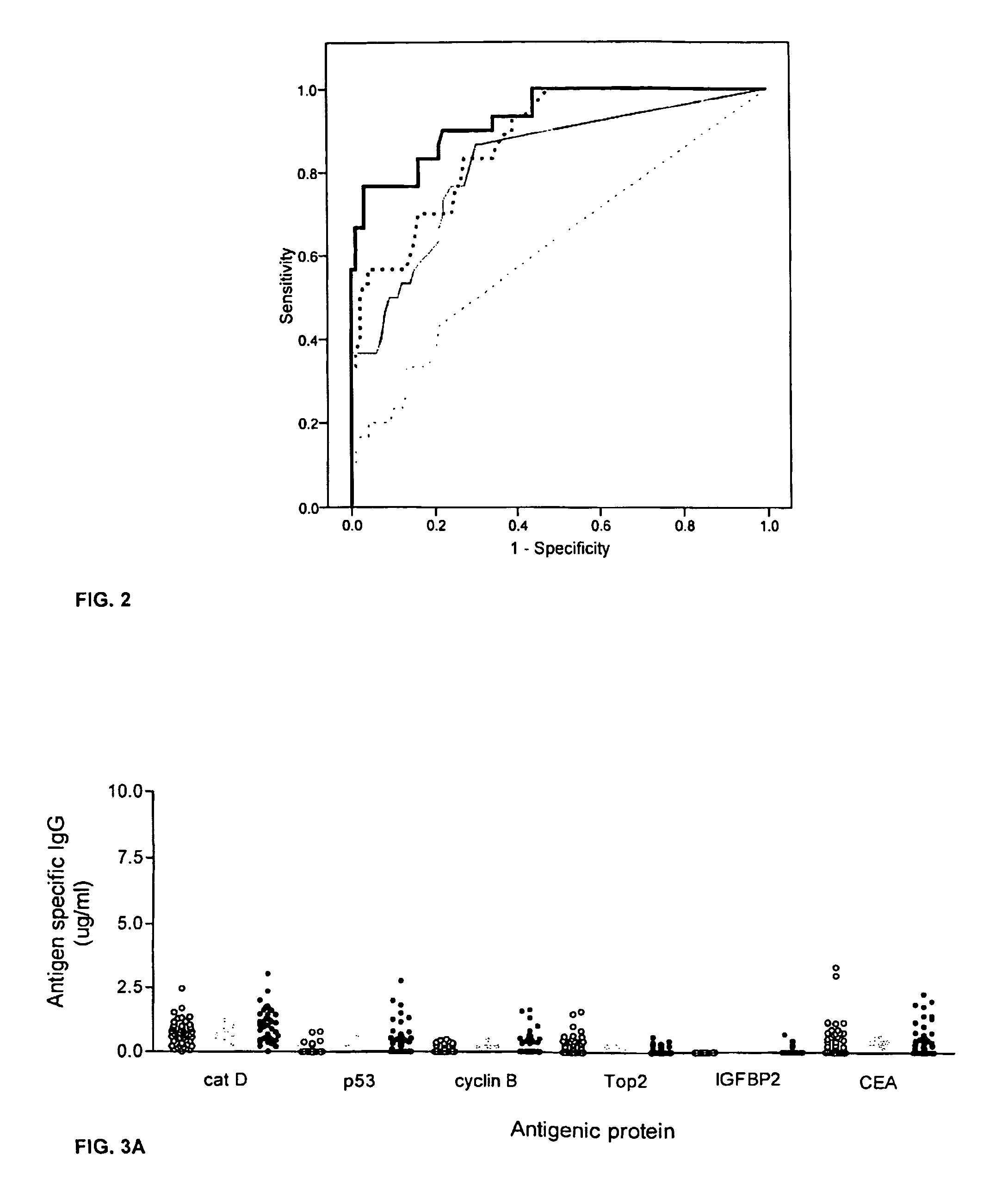
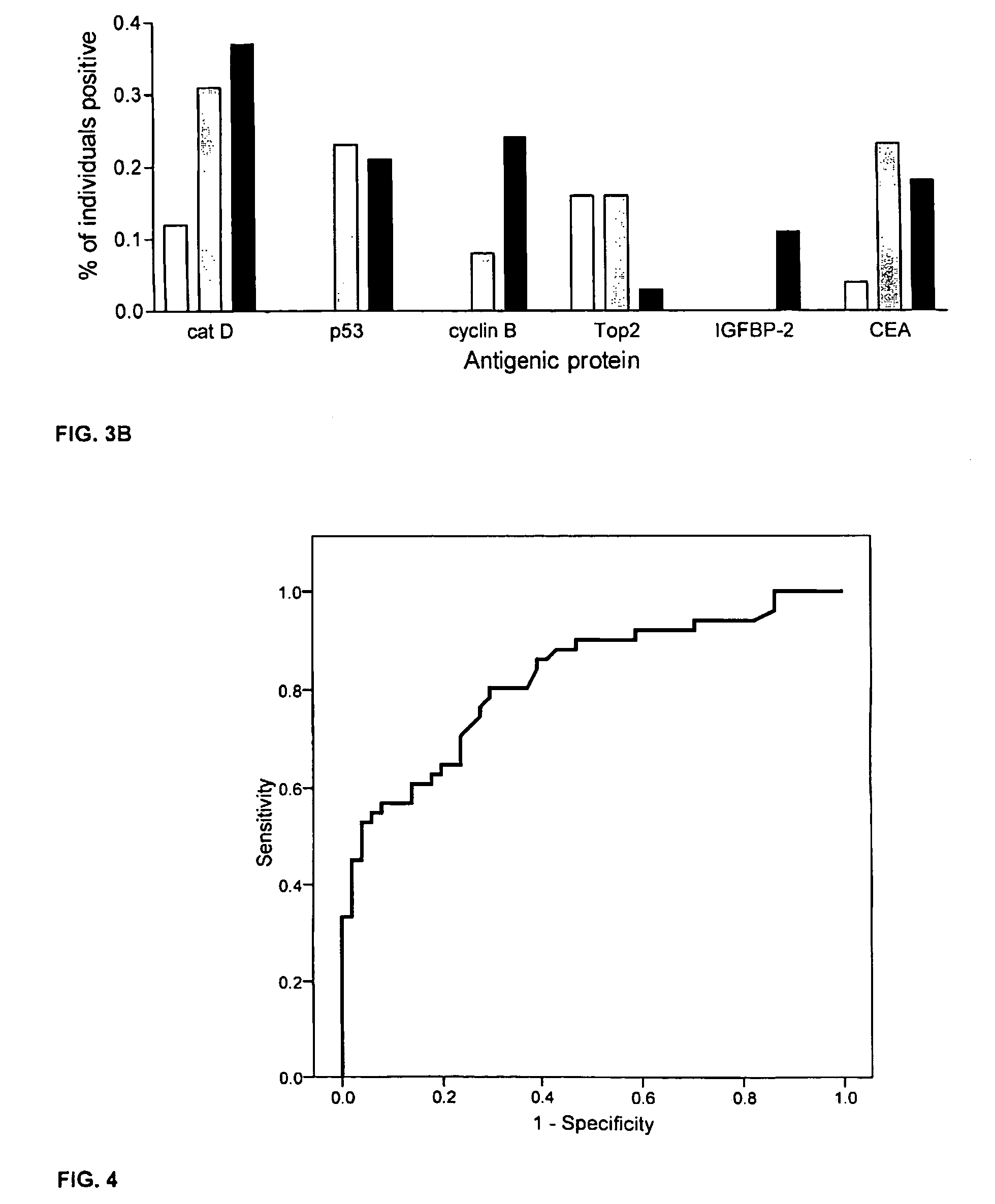
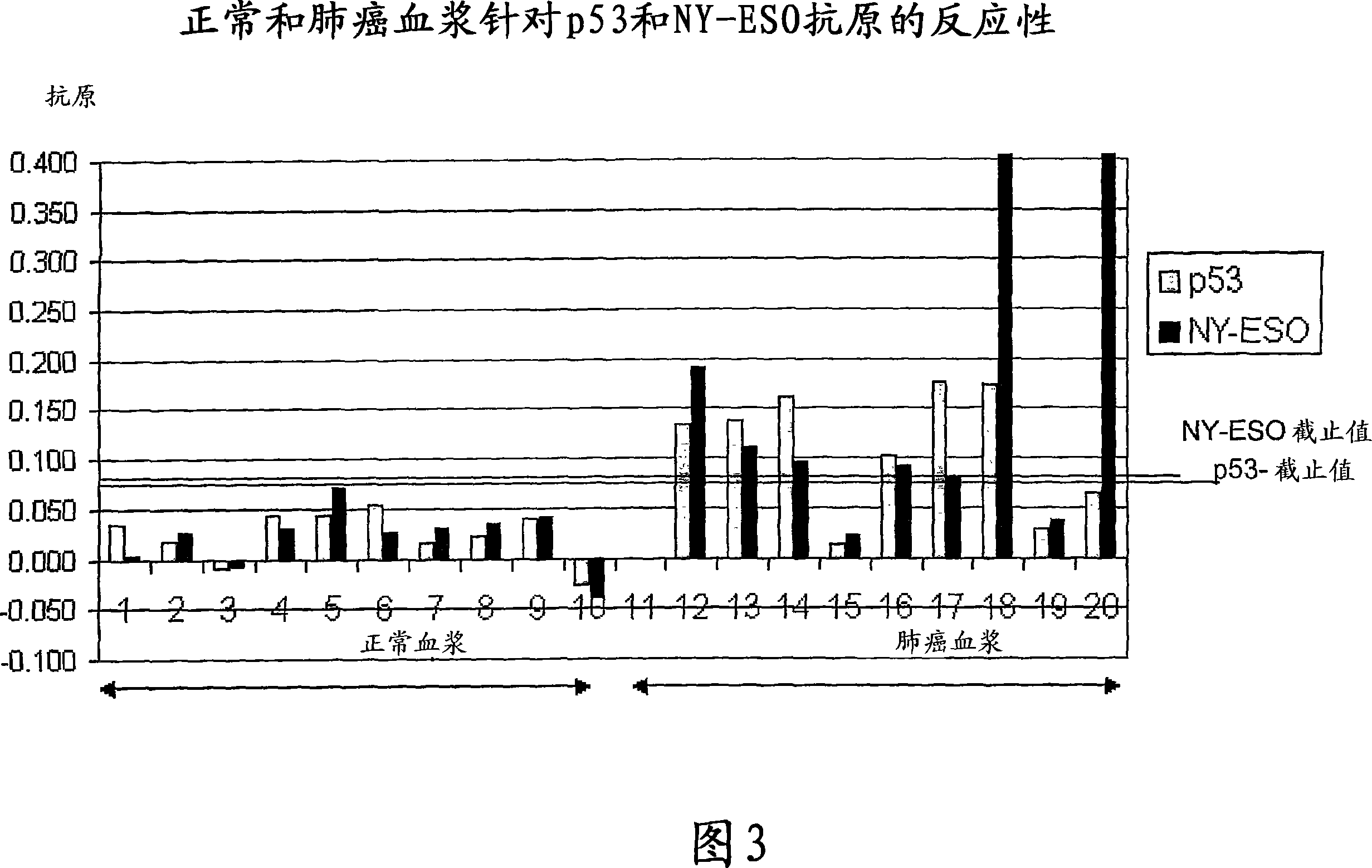
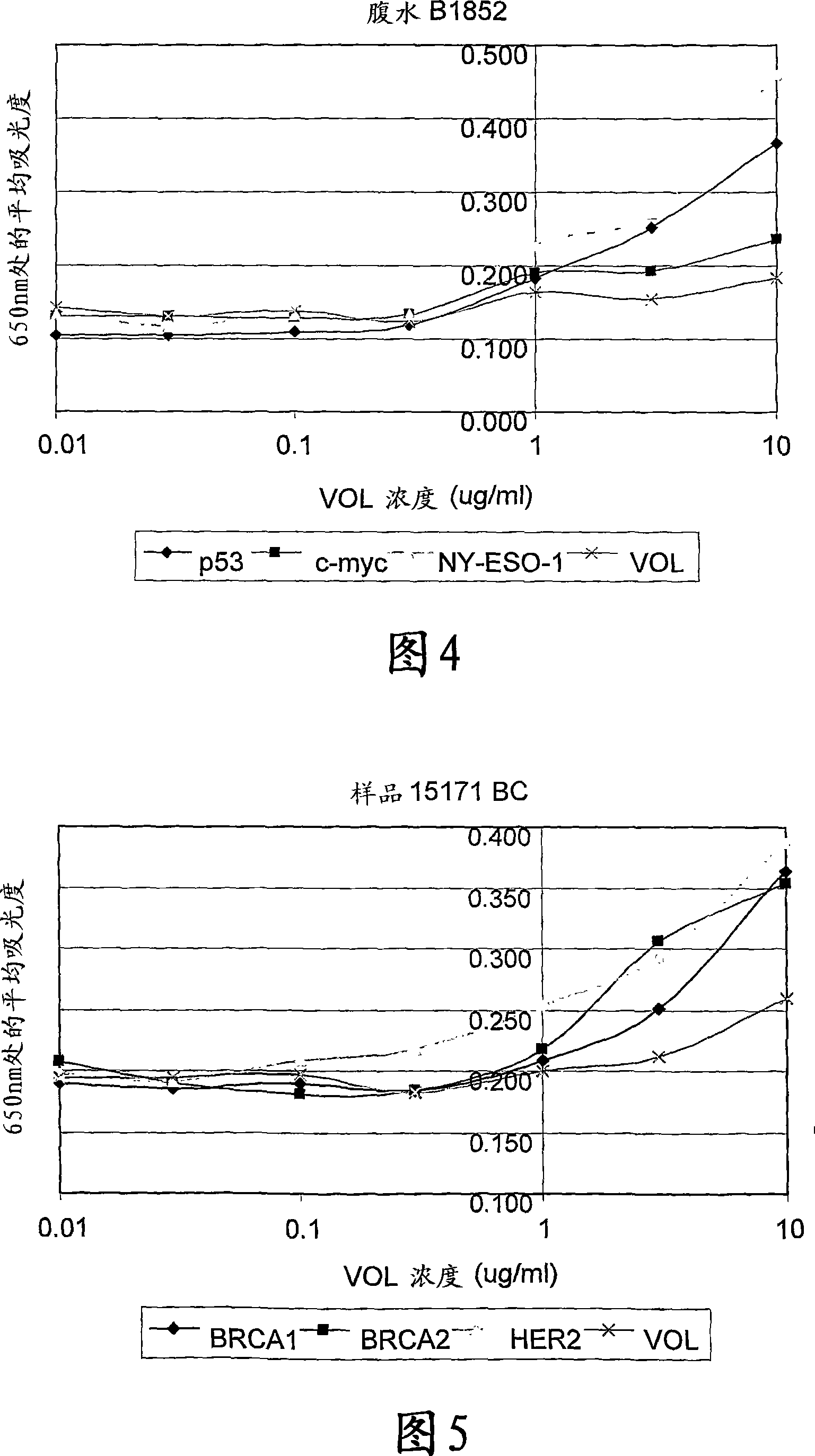
The invention provides a method for detection of a malignancy in a specimen of bodily fluid. The method comprises contacting the specimen with at least two antigens selected from the group consisting of p53, IGFBP2, Topo2α, cathepsin D, cyclin B, cyclin D1, MUC1, HER-2 / neu and CEA. The method further comprises incubating the specimen and the antigen for a duration and under conditions that are sufficient for the formation of immunocomplexes; and detecting the presence or absence of immunocomplex formation between the antigens and antibodies specific for the antigens in the specimen, thereby determining the presence or absence of the malignancy. Also provided is a method for monitoring the effectiveness of cancer therapy related to a malignancy in a warm-blooded animal, a method for distinguishing between Stage I and Stage II colorectal cancer in a specimen of bodily fluid.
Owner:UNIV OF WASHINGTON
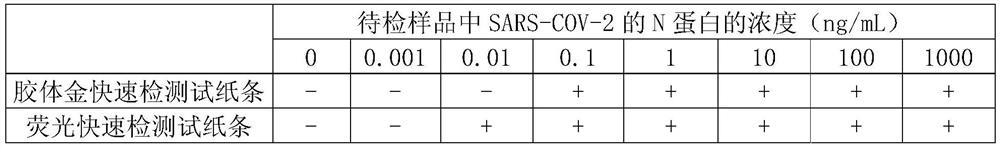
Detection kit for antigens of novel coronavirus (SARS-CoV-2)
InactiveCN111303254AThe test result is accurateHigh sensitivitySsRNA viruses positive-senseVirus peptidesAntigen testingColloidal au
The invention relates to the technical field of biology, and specifically provides a detection kit for a detection kit for antigens of a novel coronavirus (SARS-CoV-2). According to the detection kitprovided by the invention, the antigens of the SARS-CoV-2 are detected by virtue of a colloidal gold double antibody sandwich method, and two monoclonal antibodies and colloidal gold are mixed for labeling, so that a detection result of the antigens of the SARS-CoV-2 is accurate and high in sensitivity, and meanwhile, the detection rate can be remarkably increased. According to the detection kit,a blank in immunological detection of the SARS-CoV-2 is filled up, and field detection can be realized without a detection instrument, so that the time and the labor are saved, and operation is flexible.
Owner:北京新创生物工程有限公司
Rapid vaccinia antibody detection device, method and test kit
InactiveUS20040002063A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeTest sample
The invention relates to a rapid vaccinia antibody detection device, method and test kit for the detection of vaccinia antibody in a serum, plasma or whole blood test sample utilizing a purified vaccinia cell lysate as the capture antigen. The detection device operates on the basis of a 2-step flow-through format in use with a push buffer.
Owner:MEDMIRA
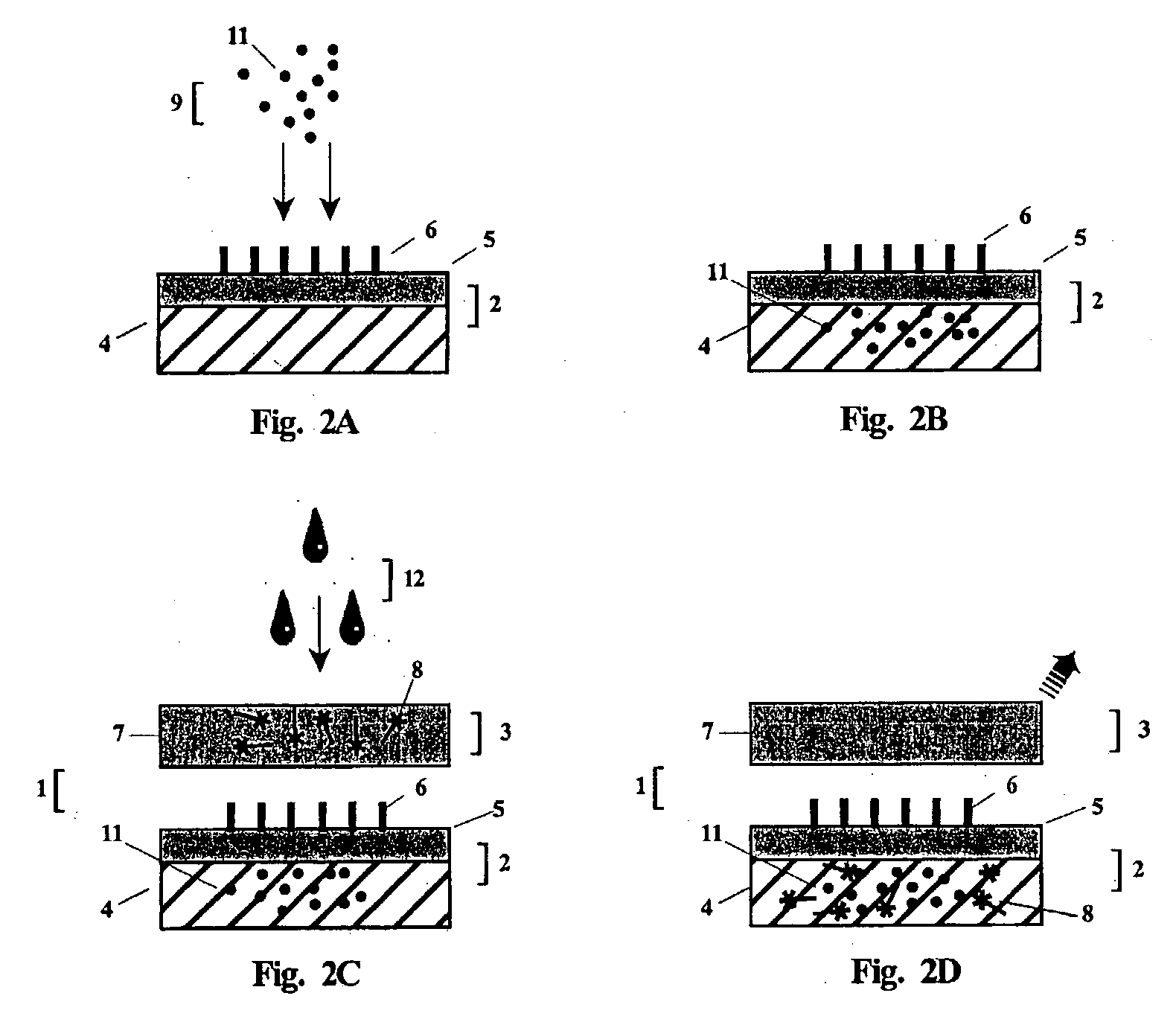
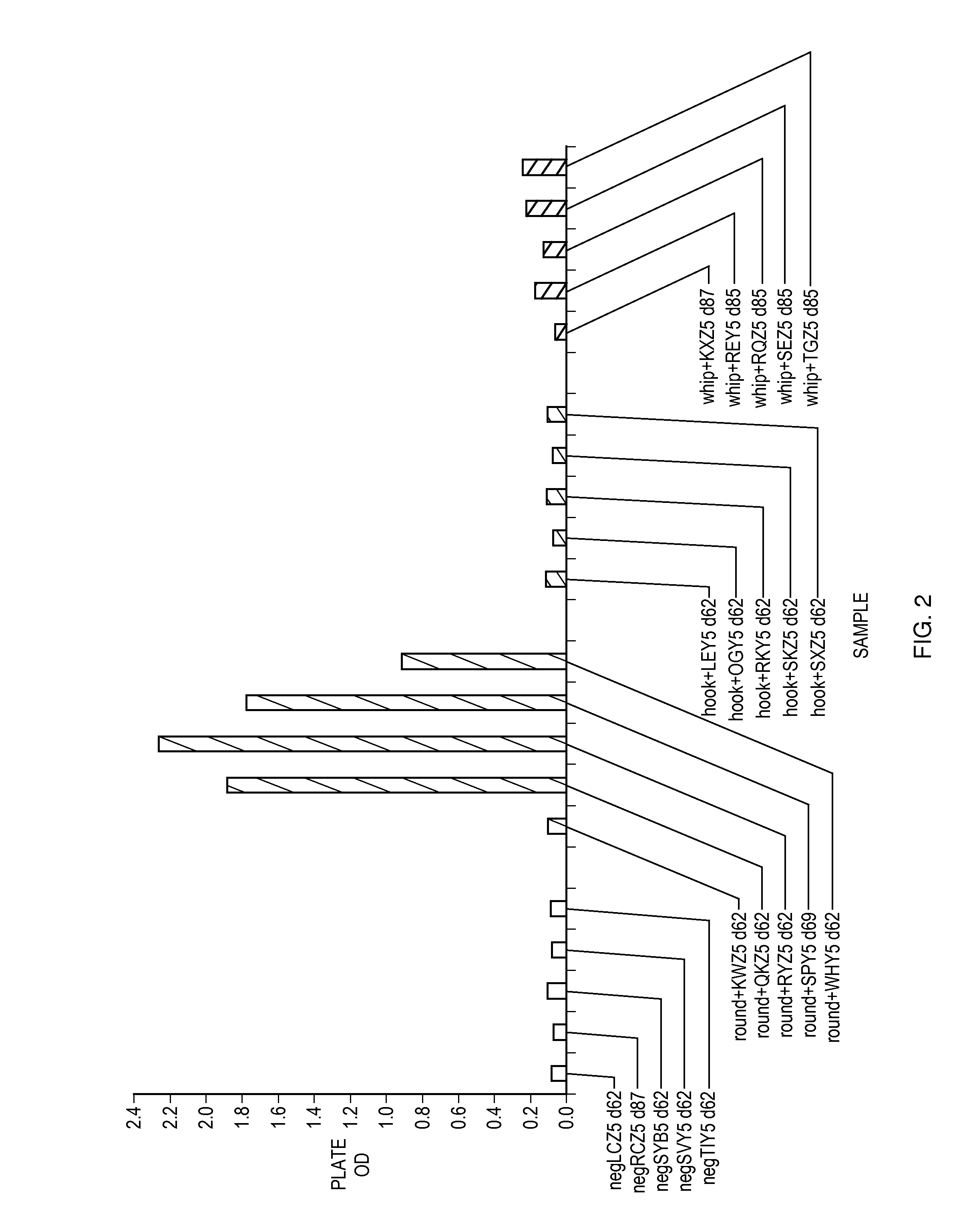
Roundworm coproantigen detection
ActiveUS20080311600A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAntigenFeces
A composition, device, kit and method for detecting the presence or absence of roundworm in a fecal sample. The composition, device, kit and method of the present invention may be used to confirm the presence or absence of roundworm in a fecal sample from a mammal that may also be infected with one or more of hookworm, whipworm, and heartworm.
Owner:IDEXX LABORATORIES
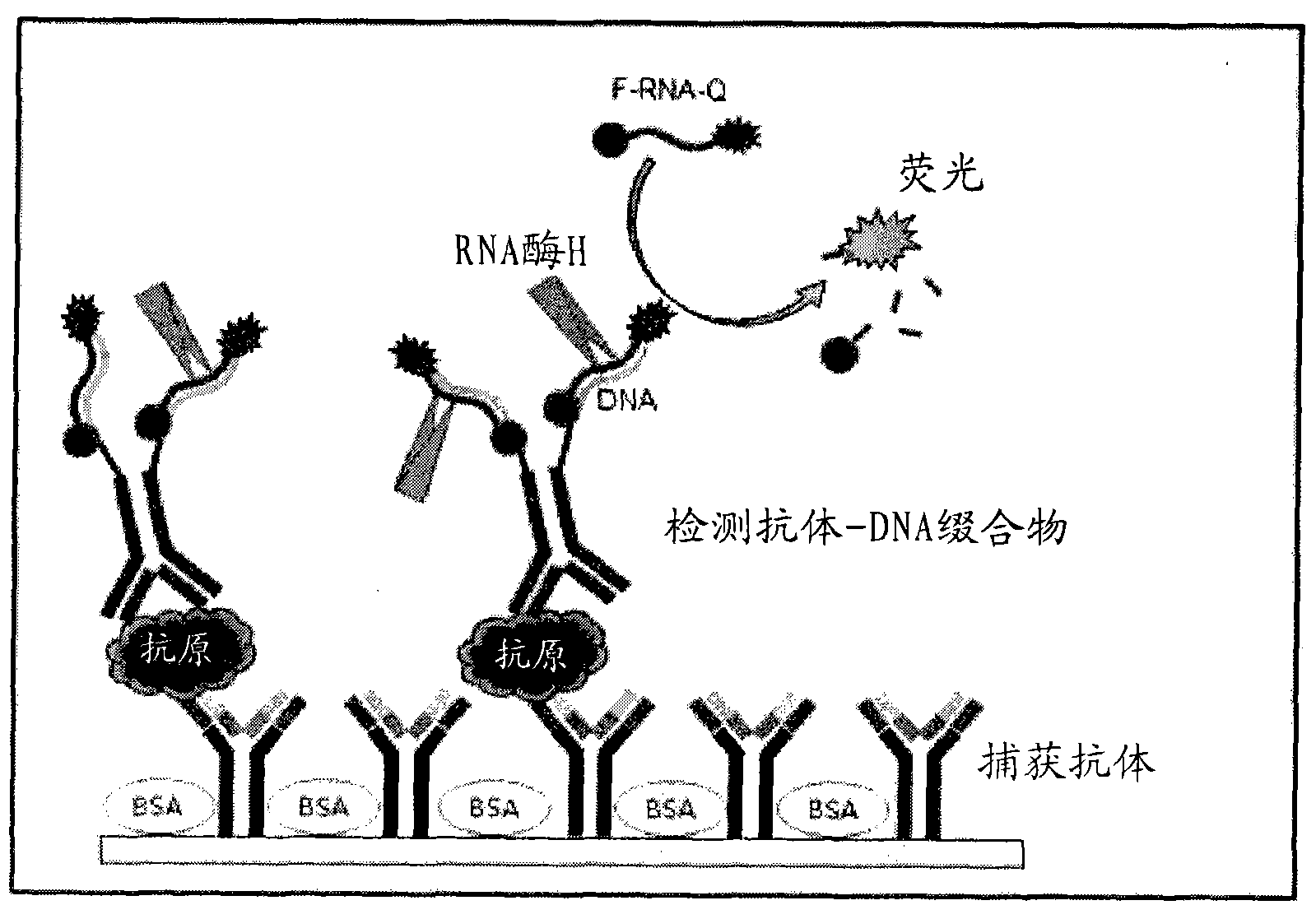
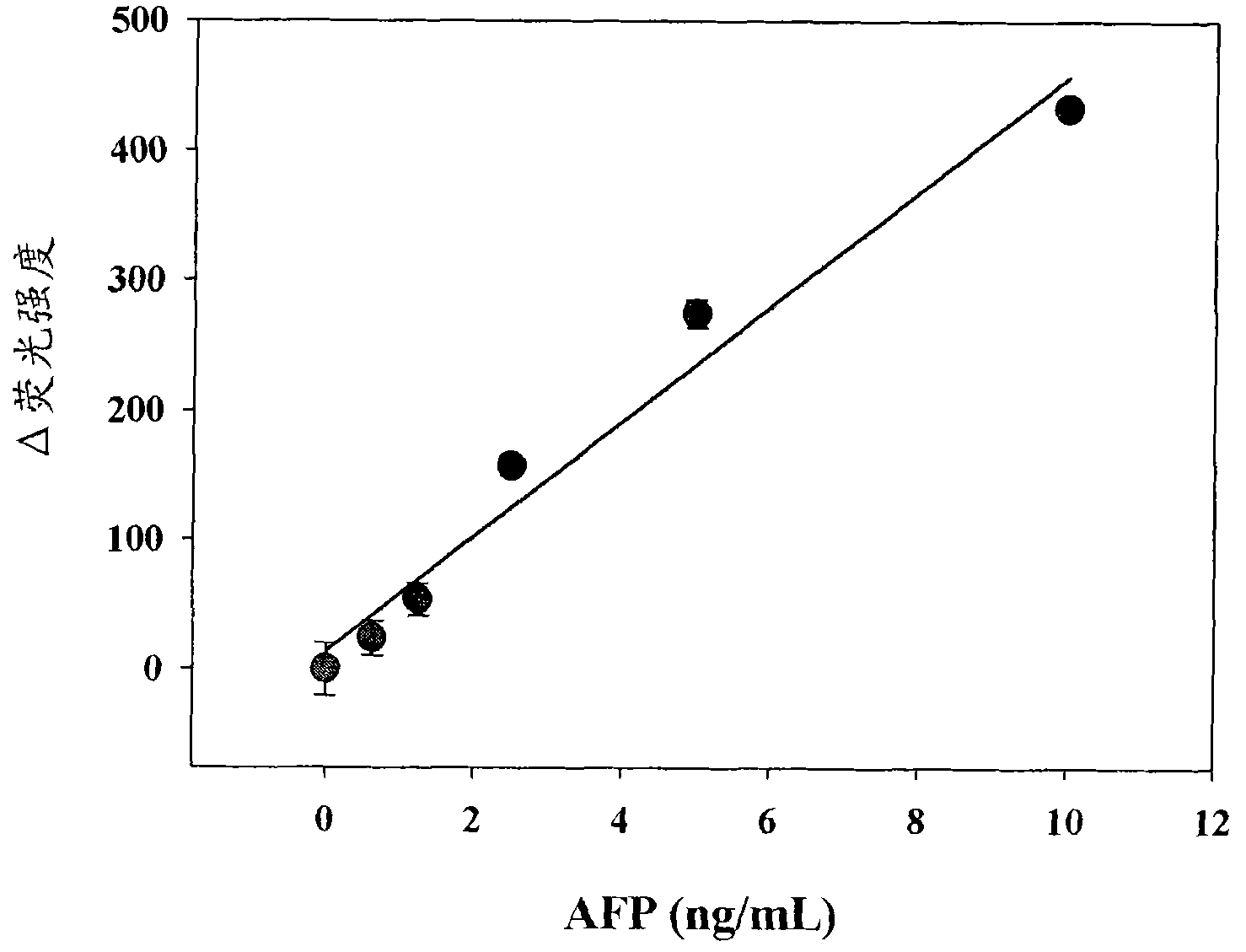
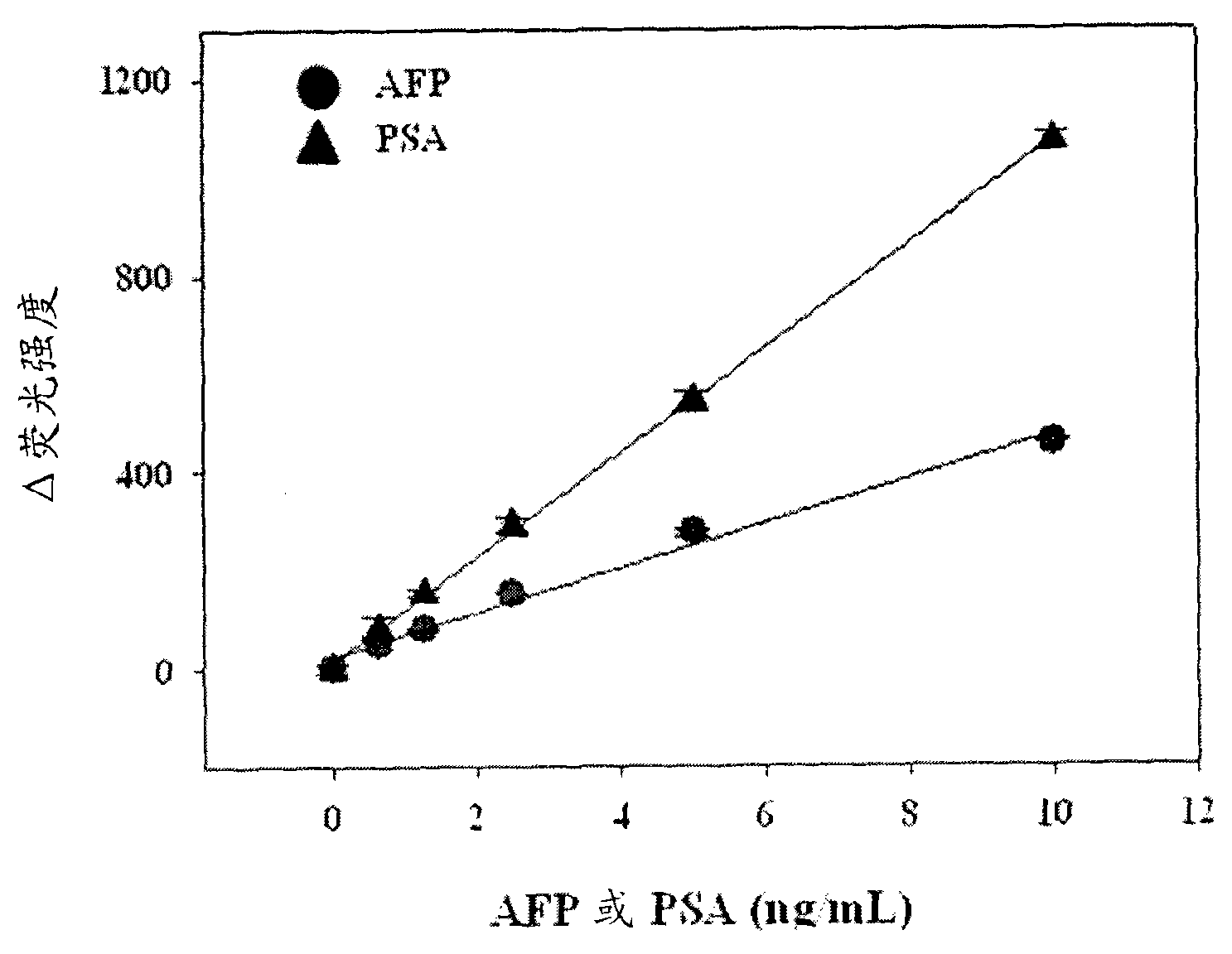
Antigen detection kit and method
InactiveCN101988920AMicrobiological testing/measurementBiological testingSingle-Stranded RNACapture antibody
An antigen detection kit and an antigen detection method using the same are provided. The antigen detection kit comprises a capture antibody, a detection antibody bound to a single stranded DNA oligonucleotide, a single stranded RNA oligonucleotide complementary sequence to the DNA oligonucleotide, and an RNase.
Owner:KOREA INST OF SCI & TECH
ELISA reagent box for determining antibody and antigen of swine circular virus II
InactiveCN1584597AEasy to operateSuit one's needsColor/spectral properties measurementsBiological testingPositive controlAntigen testing
Owner:ZHEJIANG UNIV
Hybridoma cell capable of secreting an anti-novel coronavirus N protein monoclonal antibody, onoclonal antibody and application
ActiveCN111733141AQuick monitoringIncreased sensitivityBiological material analysisImmunoglobulins against virusesProtein.monoclonalAntigen testing
The invention discloses a hybridoma cell capable of secreting an anti-novel coronavirus (SARS-COV-2) N protein monoclonal antibody, a monoclonal antibody and anapplication. The invention provides a hybridoma cell strain N-3G3, wherein the preservation number of strain is CCTCC NO: C202075. The invention also protects the monoclonal antibody secreted by the hybridoma cell strain N-3G3. The antibodywith high sensitivity and high specificity is the key to the development and implementation of an antigen detection technology. The specific antibody of the N protein of the SARS-CoV-2 is obtained onthe basis of a monoclonal antibody technology, and an SARS-COV-2 detection test strip is prepared from the N protein of the SARS-CoV-2. When the test strip provided by the invention is used for detecting the SARS-CoV-2, the operation is simple, the sensitivity is high, the specificity is strong, and the rapid monitoring and prevention of the SARS-COV-2 can be realized.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Antibody pointed at SARS coronavirus N protein antigen and its use in detecting SARS coronavirus or its antigen
InactiveCN1590409AImmunoglobulins against virusesMaterial analysisSARS coronavirus IgGAntigen testing
An antibody against the nucleocapsidprotein of SARS coronavirus, the method for using said antibody to detect the SARS coronavirus or its antigen, and the reagent kit and relative products for said method are disclosed.
Owner:马杰
Improved immunoassay methods
ActiveCN101203756AImprove featuresHigh sensitivityDisease diagnosisBiological testingTest sampleAntigen testing
The invention relates to a method of detecting a disease state or disease susceptibility in a mammalian subject which comprises detecting an antibody in a test sample comprising a bodily fluid from said mammalian subject wherein said antibody is a biological marker of a disease state or disease susceptibility, the method comprising: (a) contacting said test sample with a plurality of different amounts of an antigen specific for said antibody, (b) detecting the amount of specific binding between said antibody and said antigen, (c) plotting or calculating a curve of the amount of said specific binding versus the amount of antigen for each amount of antigen used in step (a) and (d) determining the presence or absence of said disease state or disease susceptibility based upon the amount of specific binding between said antibody and said antigen at each different antigen concentration used.
Owner:福瑞姆有限公司
Immunofluorescence kit for detecting PD-L1 and CD8 antigens and application method
PendingCN110632292AAccurately reflectEasy to get materialsMaterial analysisLymphatic SpreadAntigen testing
The present invention relates to an immunofluorescence kit for detecting PD-L1 and CD8 antigens and a detection method using the kit. The kit comprises the following reagents: buffer solutions CYP1, CYP2 and CYPP, a blocking solution, a specific antibody for detecting PD-L1, a corresponding fluorescent second antibody, a fluorescently labeled CD45 antibody, a fluorescently labeled CD8 antibody, anantibody dilution solution and a nuclear staining solution. The invention also provides a method for carrying out antigen detection by using the kit. A detection result of the kit can guide actual drug use, and not only can reflect the whole tumor comprising carcinoma in situ and possibly existent invisible micro-metastasis load, but also can reflect the expression condition of the PD-L1 in CTC;a reference can be provided for precise selection of therapeutic drugs according to the proportion of PD-L1 and CD8 positive cells in PBMC; and the kit is more convenient than a detection technology based on tumor tissues in material acquisition.
Owner:CYTTEL BIOSCI BEIJING
Reagent box for enzyme linked immunosorbent assay of EB virus protease and its preparation
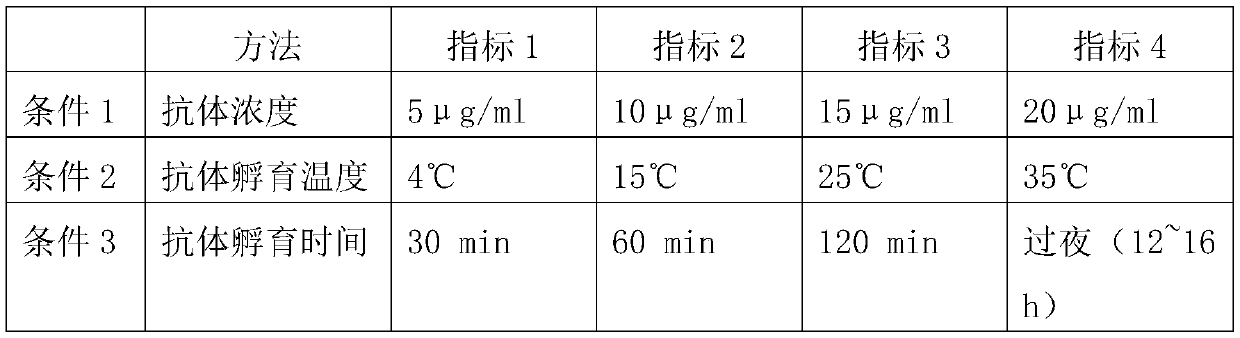
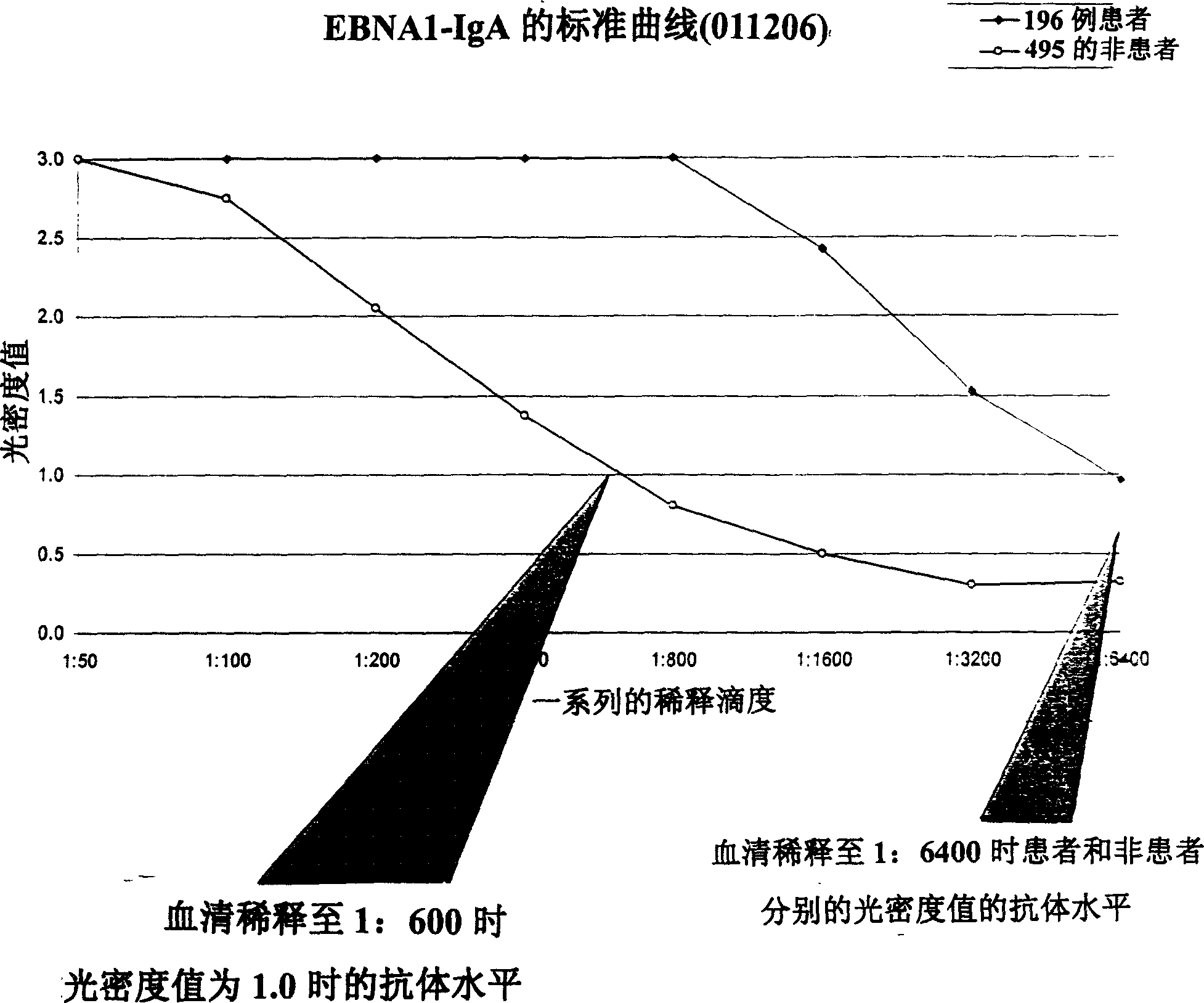
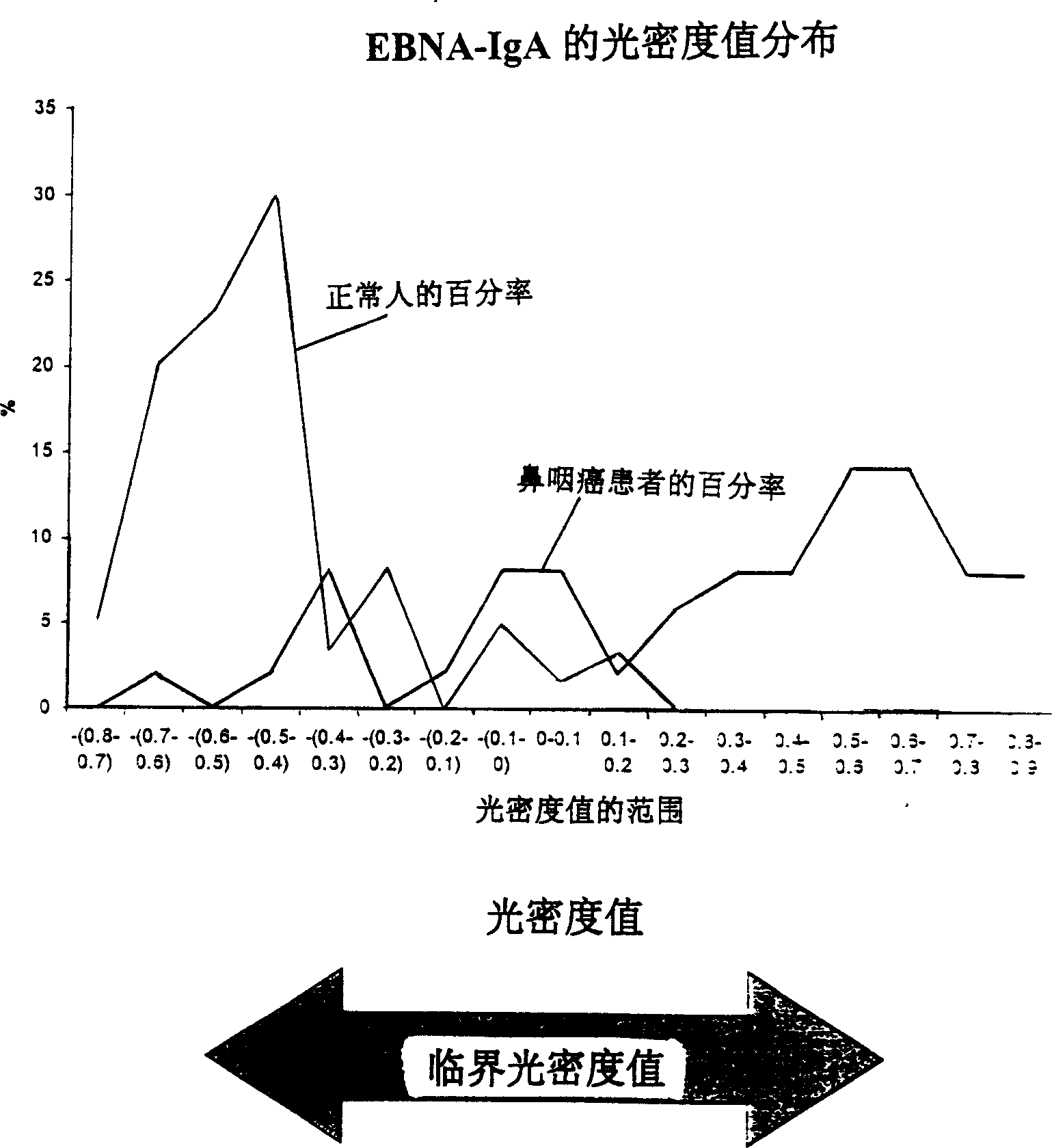
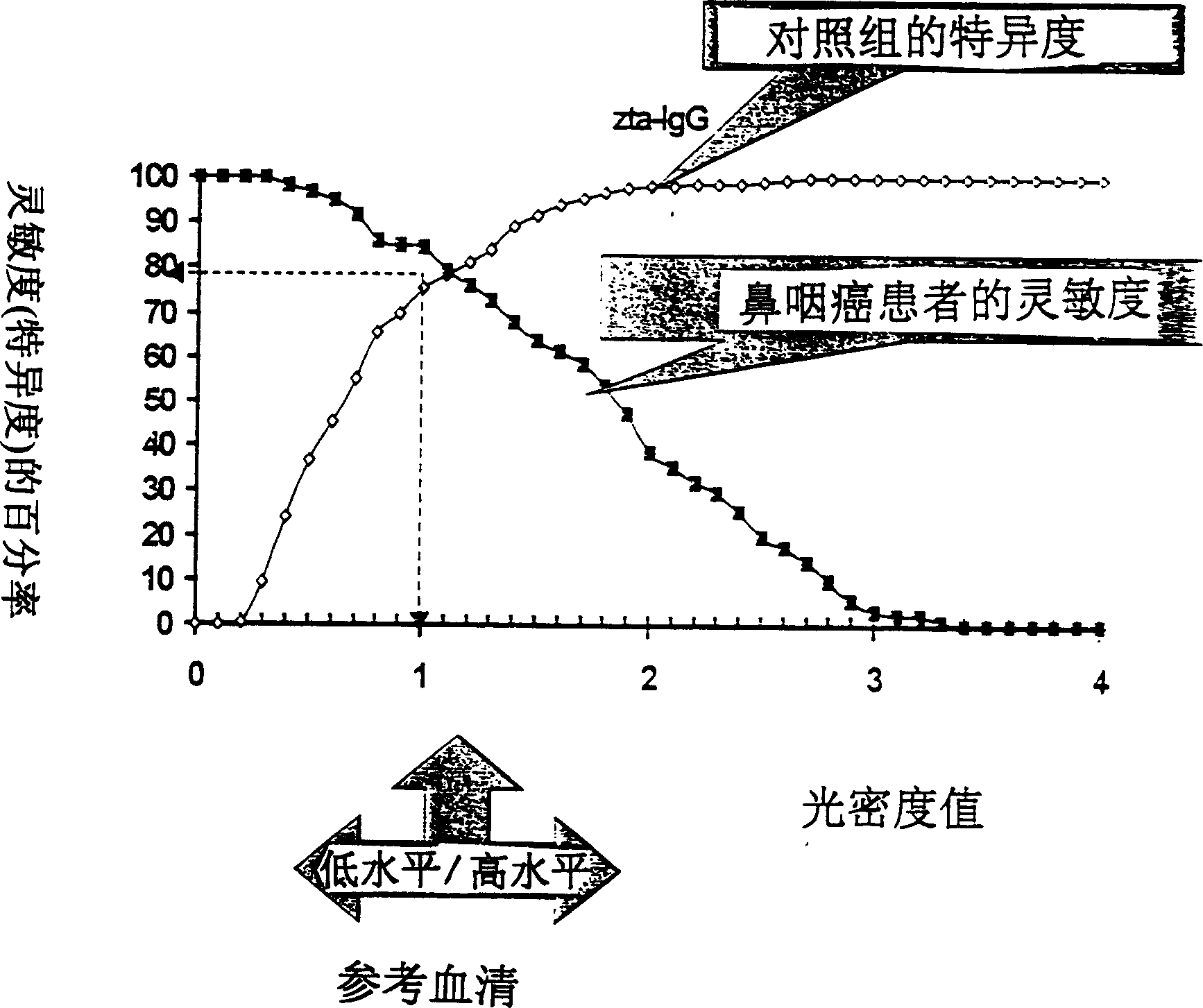
A method for preparing EB viral protein enzyme-linked immunosorbent diagnostic kit includes selectng EBNAl (BKRF1) prote, Zta (BZLF1) protein and VCA-p18 protin in EB viral protein for diagnosing nasopharyngeal carcinoma of serodiagnosis as target antigen for detecting antibody level in blood serum, using glutathione-transferase gene fusion system to carry on clone, presentation and purification of EB viral protein for creating diagnostic kit.
Owner:SINOCLONE LTD
Important heating pathogen fast screening system
The invention discloses a method for detecting a system for quickly screening a fever pathogen, and mainly relates to detection of a tuberculosis antibody, a flu antibody, a bird flu antibody, a plague antibody and an SARS antibody in blood serum. The chip mainly comprises coding microspheres, a coating antigen, a detection antibody, a biotinylated antibody and a streptavidin-phycoerythrin. The method comprises the following steps that: the coating antigen and the coding microshperes are coupled; the detection antibody is respectively coupled with the corresponding microspheres in separate specificity; the red laser excites the classified fluorescence on the spherical substrate; the type is determined according to different colors of the spherical substrates, wherein the biotinylated antibody is combined with the detection antibody; and the streptavidin-phycoerythrin is combined with the biotin of the detection antibody captured by the microshperes; the green laser excites the phycoerythrin, the number of report fluorescence molecules combined on the spherical substrate is measured, and the number is used for indirectly determining the content of the detection antibody combined onthe spherical substrate, so that whether the pathogen infection exists is determined, and the aim of quickly screening the fever pathogen is achieved.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Magnetic immune chromatography method capable of synchronously detecting Tm and Pa allergens
InactiveCN102156191ARealize synchronous detectionImprove stabilityMaterial analysisMagnetic beadAntigen testing
The invention discloses a magnetic immune chromatography method capable of synchronously detecting Tm and Pa allergens. The method is characterized by comprising the following steps of: sequentially sticking a sample pad, a combination pad combining anti-Tm and anti-Pa immune magnetic beads, a chromatography film and a water absorption pad to a bottom plate crossly at intervals of about 2 millimeters, and then covering a transparent plastic sealing film on the upper layer to construct a magnetic immune chromatography test strip capable of synchronously detecting the Tm and Pa allergens. A Pa antigen detection line T1, a Tm antigen detection line T2 and a goat anti-mouse IgG quality control line C are pre-coated on the chromatography film, the immune magnetic beads can be captured by chromatography, and quick qualitative detection of the Tm and Pa allergens is realized according to 1 to 3 macroscopic color development strips formed after the chromatography of a sample; or quantitative detection of single allergen or synchronous quantitative detection of two allergens is realized by detecting the constructed magnetic immune test strips through a magnetic analyzer according to the magnetic signal detection values of the detection lines and the control line formed after the chromatography of the sample.
Owner:SHANGHAI OCEAN UNIV
EV71 virus neutralization epitope detection kit or reagent and preparation method thereof
The invention relates to an EV71 virus neutralization epitope detection kit or a reagent and a preparation method thereof. Particularly, the invention performs quantitative detection on EV71 virus antigen by adopting an HD6 monoclonal antibody with spectral activity. The kit or the reagent has the advantages of easy operation, high sensitivity and the like.
Owner:SINOVAC BIOTECH
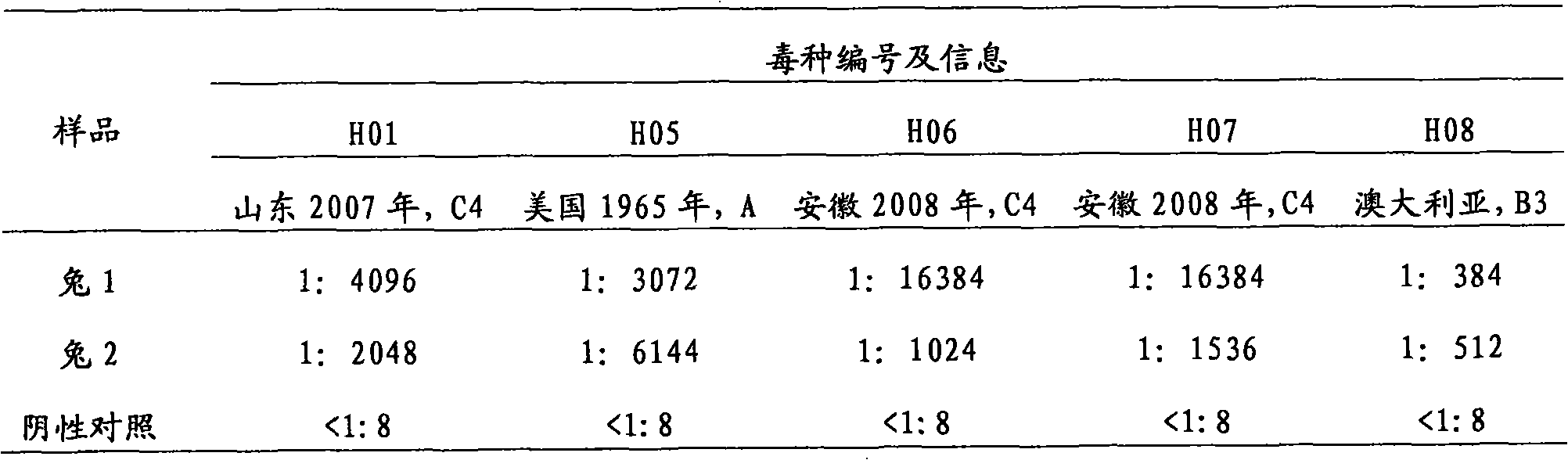
Tuberculosis antibody multi-antigen ELISA detecting kit and making method
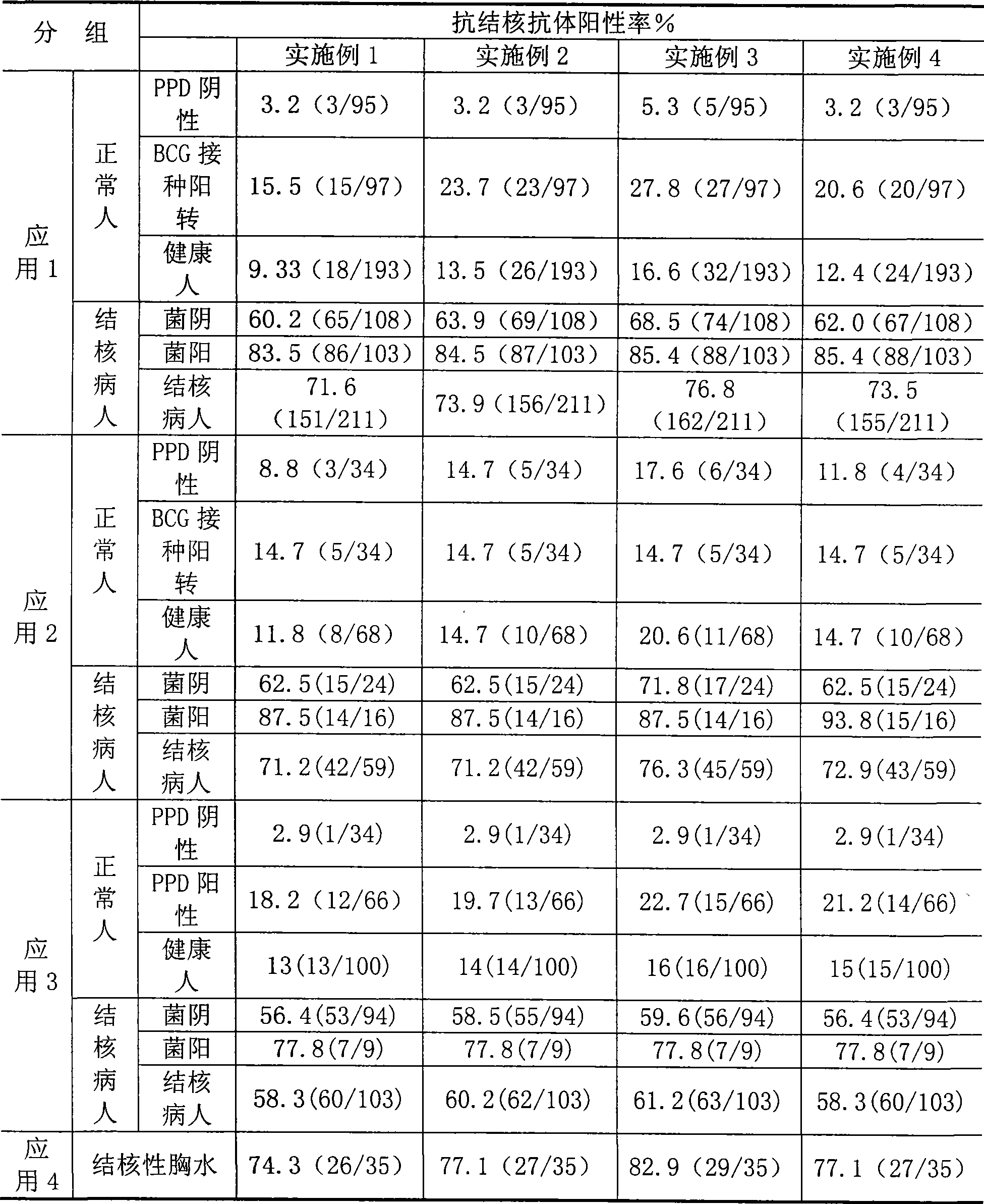
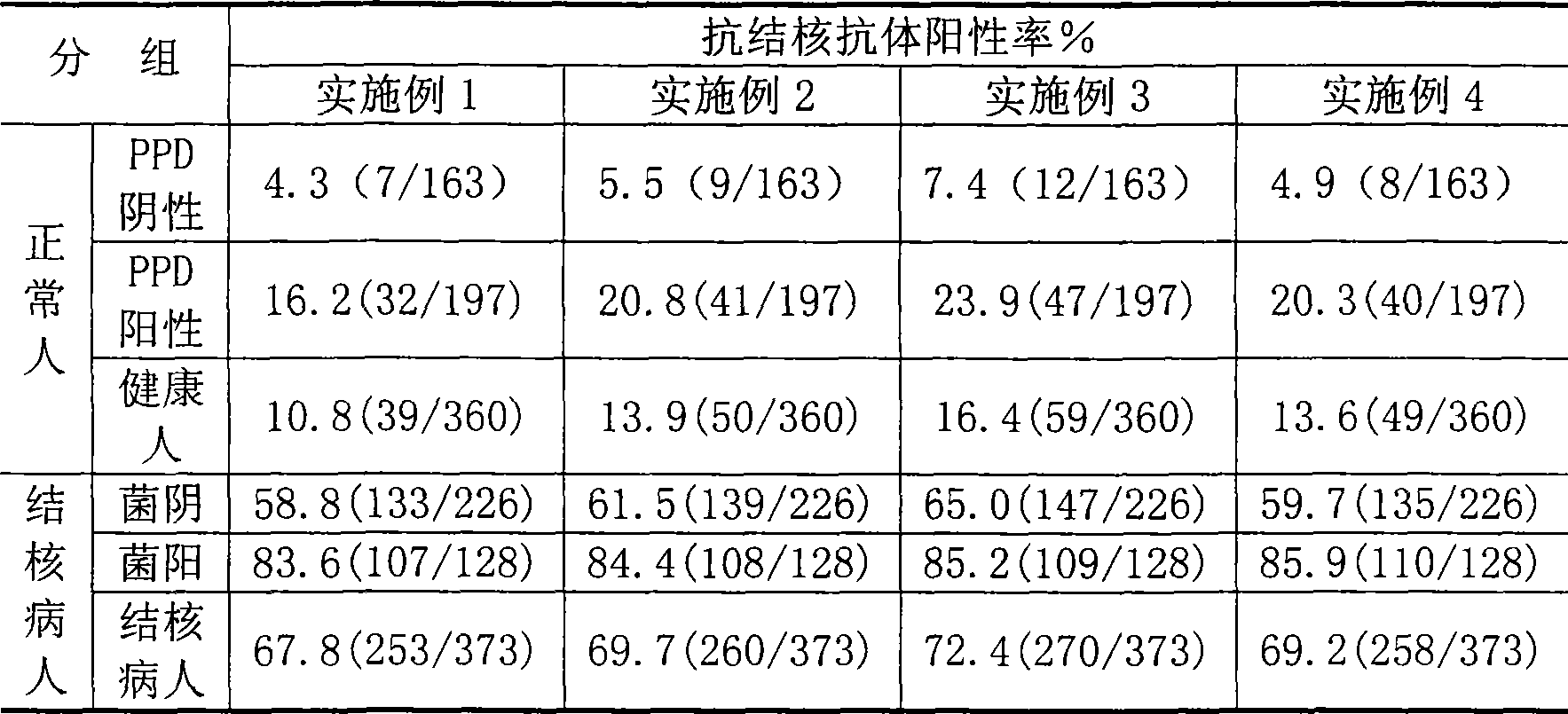
The invention relates to a tuberculosis antibody multiple antigen ELISA detection kit and a preparation method thereof, which pertains to the field of tuberculosis medical immunology diagnostic techniques and mainly uses detection antigen, enzyme-linked antihuman IgG antibodies, substrates, positive control serum of tuberculosis patients, control serum of normal person, calf serum and polystyrene microplates to form the kit, wherein, the detection antigen adopts the mycobacterium tuberculosis complex strains of lipid Arabian mannose (LAM), 38kD and 16kD to be combined with arbitrary one or more than one mycobacterium tuberculosis recombinant proteins in the recombinant proteins of MPT63, MTB48 and CFP10-ESAT6. The mycobacterium tuberculosis has high sensitivity, strong specificity and complementarity, can be used for detecting specific antitubercular antibodies in such body fluid samples as serum, hydrothorax and the like, and assisting the diagnosis and differential diagnosis of tuberculosis.
Owner:中国人民解放军总医院第二附属医院
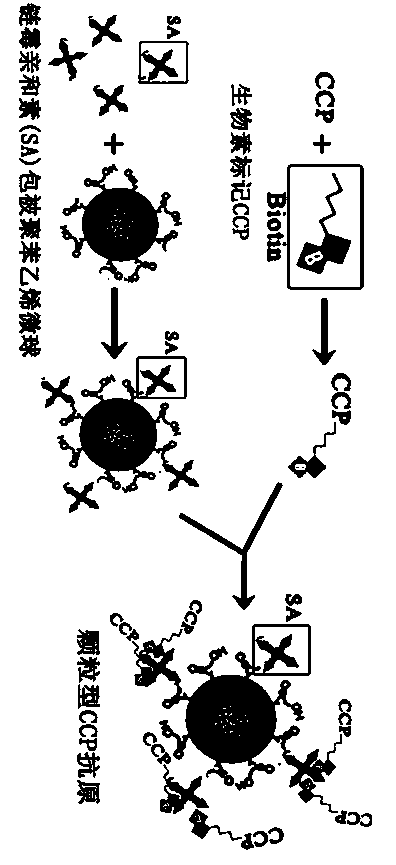
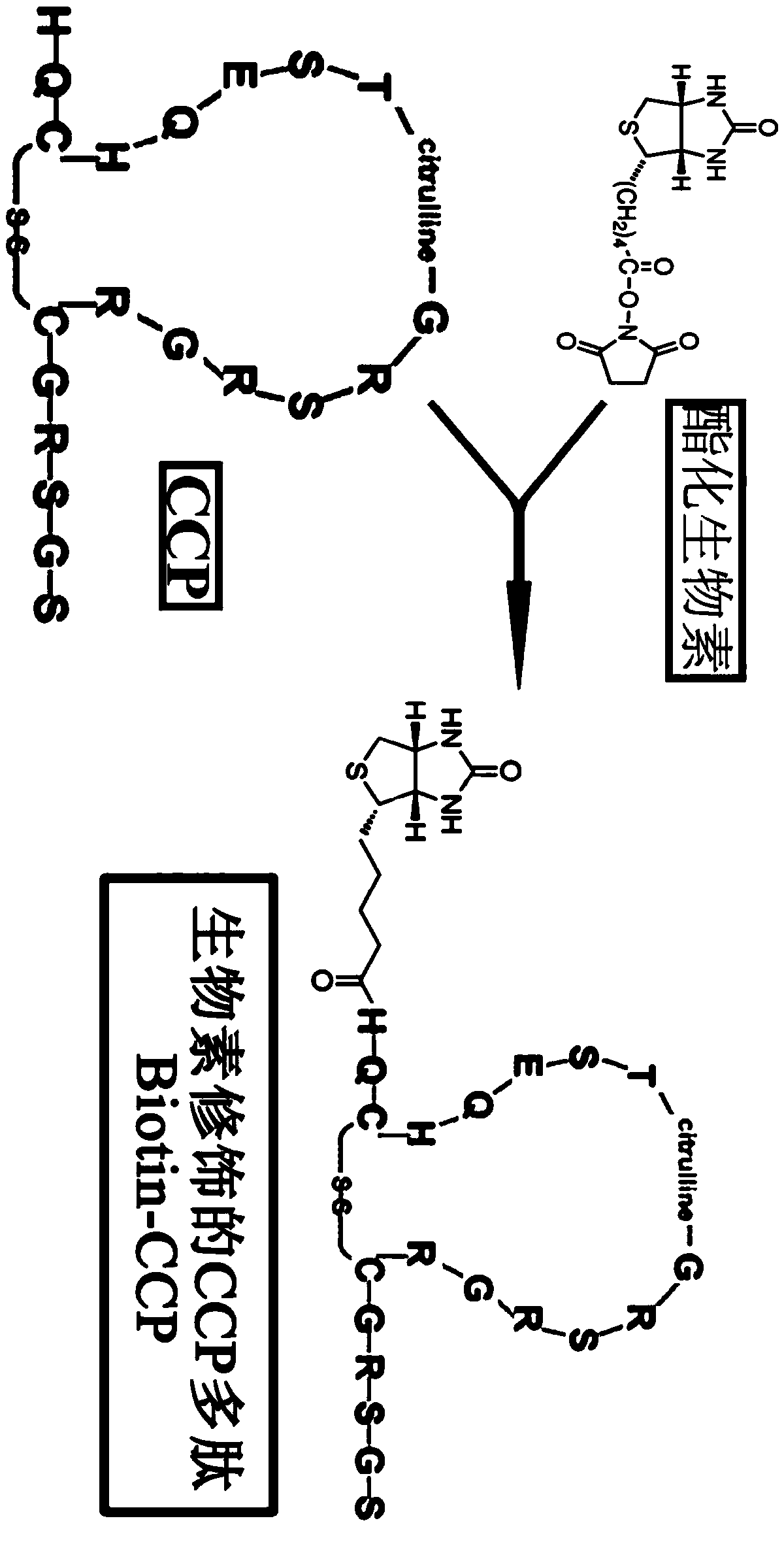
Preparation of CCP polypeptide series and its use
InactiveCN1491959AImprove early diagnosis rateEasy to operateDepsipeptidesBiological testingChemical synthesisDiagnostic Specificity
The present invention relates to CCP polypeptide and its application. CCP polypeptide is prepared through the following steps: calculating the hydrophilicity value, water treatment index and higher structure emergence frequency of amino acid, calculating the site with maximum antigenicity based on protein structure and thus obtaining synthetic peptide chain sequence SHQESTXGRSRGRSGRSGS; synthesizing in Fmoc chemical synthesis process with the said peptide chain the destination peptide of sequence HQCGQESTXGRSRGRSGRSGS. The enzyme-linked immunological method with the CCP peptide as antigen to detect rheumatoid arthritis is provided. The rheumatoid arthritis diagnosing kit has high diagnosis specificity, up to 80 %. The present invention has high early diagnosis rate on rheumatoid arthritis.
Owner:昆明广博生物技术有限公司
Rapid combined detection device for novel coronavirus antigen and antibody in saliva and preparation method thereof
PendingCN111912980AEasy to acceptReduce the risk of infectionBiological material analysisAntigen testingWindow period
Owner:江苏维尔生物科技有限公司
Method for detecting valence of antibody
InactiveCN103439515AImprove linearityEasy accessCarrier-bound/immobilised peptidesBiological testingAntiendomysial antibodiesMicrosphere
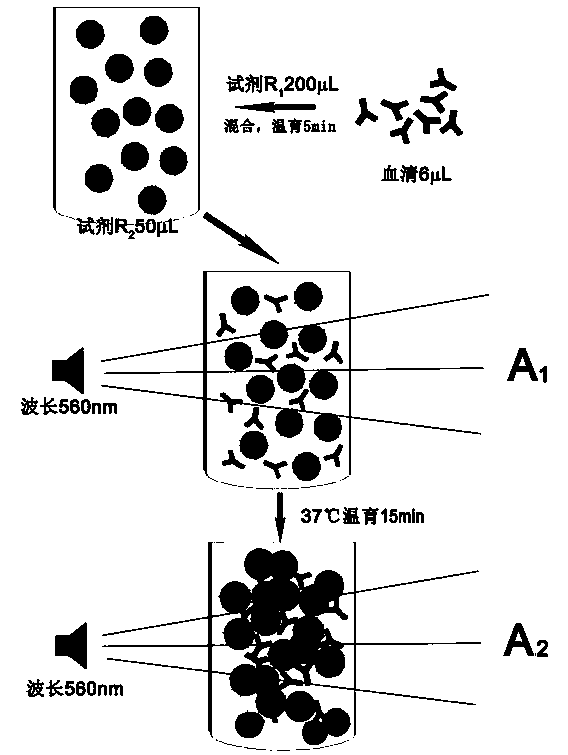
The invention discloses a method for detecting the valence of an antibody. The method comprises the following steps: 1), coupling an antigen on a microsphere to obtain a modified microsphere; 2), mixing and reacting the modified microsphere with an antibody solution; 3), measuring the turbidity of the reaction liquid, so as to determine the valence of the antibody. According to the method provided by the invention, CCP is modified on the surface of a polystyrene microsphere by adopting a vitamin H-streptavidin system, so as to prepare a granular CCP antigen successfully; in case that the granular CCP antigen prepared by adopting the method is used for detecting the valence of anti-CCP antibody in blood serum, the linearity of the detecting result is good, and the detecting process can be completed by 20 minutes, so that the method can be used for acquiring the detecting result more quickly as compared with the conventional ELISA detecting method consuming 2-3 hours.
Owner:SOUTHERN MEDICAL UNIVERSITY
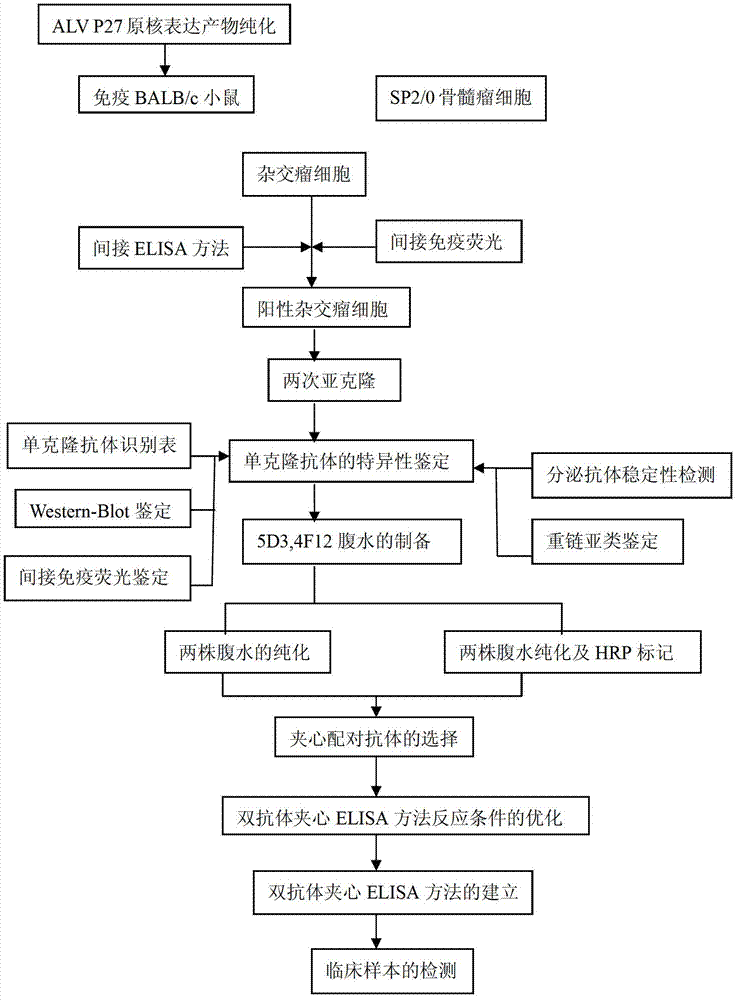
Avian leukosis double-antibody sandwich enzyme-linked immuno sorbent assay (ELISA) antigen detection kit
The invention provides an avian leukosis double-antibody sandwich enzyme-linked immuno sorbent assay (ELISA) antigen detection kit. The kit comprises ELISA plates which are coated on anti-avian leukosis virus (ALV) p27 monoclonal antibodies, enzyme labeling anti-ALVp27 monoclonal antibodies and the like. Antibody capturing and antibody detecting are respectively conducted aiming at different antigenic determinants of p27 protein; anti-ALVp27 monoclonal antibodies coated by the ELISA plates are obtained through secretion of hybridoma cell strain ALVP27-5D3, and the enzyme labeling anti-ALVp27 monoclonal antibodies are obtained through secretion of hybridoma cell strain ALVP27-4F12. The avian leukosis double-antibody sandwich ELISA antigen detection kit is easy, convenient and fast to operate, can be used in detecting of all subgroup virus of ALV, and is suitable for all levels of veterinarian departments in the basic level and rapid and mass screening detecting of the avian leukosis of leaving and entering the country. The avian leukosis double-antibody sandwich ELISA antigen detection kit has the advantages of being low in cost, notable in economical benefit, and wide in application prospect.
Owner:YANGZHOU UNIV
Immunoassay and kit for an early and simultaneous detection of biochemical markers in a patient's sample
The present invention comprises an immunochemical assay for determination of at least two antigens in a sample. The immunochemical assay comprises contacting a sample from a patient with a carrier molecule that contains at least two capture antibodies, each of which specifically binds to a binding moiety of an antigen in the sample. The assay further contains a detection antibody that specifically binds the same antigens on different binding moieties than binding moieties used by the capture antibodies. The detection agent is further attached to one or more detection probes to facilitate the detection of antigens in the sample. The immunochemical assay of the present invention is specifically designed to detect biochemical markers that are released at different time intervals in a patient's sample.
Owner:DIAGENICS INT CORP
High-affinity anti-carcinoembryonic antigen nanobody and application thereof
ActiveCN108659130ASpecific recognition abilitySpecific bindingHydrolasesImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenCarcinoembryonic antigen
The invention discloses a high-affinity anti-carcinoembryonic antigen nanobody. The nanobody has three unique complementary determining regions CDR1, CDR2 and CDR3. The invention further provides an expression vector containing a nanobody variable region coding sequence and a host cell containing the expression vector, and further provides fusion protein of a nanobody variable region and alkalinephosphatase, the application of the nanobody to preparation of a carcinoembryonic antigen detection kit, a method for performing immunodetection on a carcinoembryonic antigen by applying the nanobody,and the corresponding detection kit. The anti-carcinoembryonic antigen (CEA) nanobody provided by the invention has a specific CEA recognizing and bonding ability, has the affinity which can reach 4.791E-10, and has unique antigen-determining cluster recognition sites; an excellent detection result can be obtained in CEA immunodetection, especially in a double-antibody sandwich method.
Owner:长春力太生物技术有限公司
Mycoplasma pneumonia mosaic antigen, antigen detection reagent, and preparation method of both
InactiveCN107573417AStrong specificityEasy to culture and purifyBiological testingHybrid peptidesAntigenMycoplasma pneumonia
The invention provides a mycoplasma pneumonia mosaic antigen amino acid sequence containing an amino acid sequence as shown in SEQ ID NO:1 as well as a full-gene synthesized mycoplasma pneumonia mosaic antigen full-gene sequence containing an amino acid sequence as shown in SEQ ID NO:2. The invention also provides a method for constructing the two gene sequences. The invention also provides a preparation method of the mycoplasma pneumonia mosaic antigen containing full-gene synthesis, a mycoplasma pneumonia detection kit and a preparation method thereof. Mp recombinant mosaic antigen is selected as a mark material and is applied to a gold immunochromatography system, and the detection system is directly marked and captured, so the sensitivity is greatly improved, the specificity of the antigen is high, the antigen is easy in cultivation and purification, and cost is reduced; and a new method for detecting mycoplasma pneumoniae IgG rapidly and accurately is provided for clinical use, and a good market prospect is achieved.
Owner:HANGZHOU CLONGENE BIOTECH
Multi-item respiratory tract antigen detection card and kit
ActiveCN112362869AAdequate responseHigh sensitivityBiological testingImmunoassaysAntibody conjugateAntigen testing
The invention relates to a multi-item respiratory tract antigen detection card which comprises an influenza A virus antigen test strip, an influenza B virus antigen test strip, a respiratory tract adenovirus antigen test strip, a respiratory tract syncytial virus antigen test strip and a mycoplasma pneumoniae antigen test strip, wherein each of the influenza A virus antigen test strip, the influenza B virus antigen test strip, the respiratory tract adenovirus antigen test strip, the respiratory tract syncytial virus antigen test strip and the mycoplasma pneumoniae antigen test strip comprisesa colloidal gold conjugate pad, and each colloidal gold conjugate pad comprises a streptavidin conjugate pad and a double-nanoparticle double-labeled antibody conjugate pad; the test strip in the detection card enables biological raw materials to react fully, improves the sensitivity of antigen detection, effectively reduces missing detection, and meanwhile, the blocking agent is added into the sample pad to improve the specificity, so that influenza A virus, influenza B virus, respiratory adenovirus, respiratory syncytial virus and mycoplasma pneumoniae antigen can be detected at the same time.
Owner:山东康华生物医疗科技股份有限公司
Hepatitis B virus surface antigen detection particles, preparation thereof and use thereof
InactiveCN101666801AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingSerum igeAntigen testing
The invention relates to a reagent for the diagnosis of hepatitis B and discloses hepatitis B surface antigen detection particles, which are luminous particles coated by a hepatitis B surface antigen.The invention also discloses the preparation and use of the hepatitis B surface antigen detection particles. In addition, the invention also discloses an in-vitro diagnostic kit for determining the hepatitis B surface antigen in a sample and also discloses the using method of the kit. The kit of the invention can be used in combination with other serums and clinic information to diagnose the acute or chronic hepatitis B infection condition of an individual and can also be used for screening hepatitis B in perinatal females to judge the hepatitis B infection risk of the newborns.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Visible protein chip for detecting poultry disease serum antibody, its preparation method and application
The invention discloses a visual protein chip for detecting serum antibody of new-castle disease virus of chickens, infectious bronchitis virus of chickens, avian influenza virus and infectious bursal disease virus of chickens , which is prepared by the following steps: purifying and diluting whole proteins of the four virus respectively; pointing samples of the positive control serum, the negative control serum and the four virus proteins onto a chip carrier respectively; drying, fixing, sealing and washing the samples to obtain the visual protein chip. The visual protein chip uses the purified whole proteins as capturing antigens to detect the virus-specific antibodies in chicken serum so as to simplify the preparation technology and reduce the production cost, and the visual protein chip has better specificity but no cross, has high reliability of results and has the advantages of quickness, simplicity and convenience, high sensitivity, good specificity and the like. When the serum is diluted by 6,400 times, the visual protein chip still can detect the antibodies, the sensitivity is 400 times of that of the prior AGP detection method. According to the detection to serum samples in-place, the detection rate of the visual protein chip is higher than the proir AGP method remarkably.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Indirect ELISA (Enzyme Linked Immunosorbent Assay) method for detecting anti-roundworm antibody by recombined roundworm ALAg protein antigen
InactiveCN102279271AIncreased sensitivityBiological testingFermentationReverse transcriptaseAntigen testing
The invention discloses an indirect ELISA (Enzyme Linked Immunosorbent Assay) method for detecting anti-roundworm antibody by a recombined roundworm ALAg protein antigen. In the method, RNA (Ribonucleic Acid) of a roundworm adult body or infective egg serves as a template; an ALAg gene is amplified by RT-PCR (Reverse Transcriptase-Polymerase chain Reaction); an ALAg expression carrier is built, and expression bacteria are transformed for inducible expression; recombined protein is purified; and the indirect ELISA method is built by taking the purified protein as a coated antigen. A stable and specific indirect ELISA method for an anti-roundworm body IgG is built, can be used for the clinical monitoring and diagnosis for roundworm infection and is suitable to basically and clinically widely popularize.
Owner:GUIYANG COLLEGE OF TRADITIONAL CHINESE MEDICINE
Novel coronavirus (COVID-19) antigen detection kit and detection method thereof
PendingCN112129937AStrong specificityThe detection process is fastBiological testingImmunoassaysProtein.monoclonalEpidemic spread
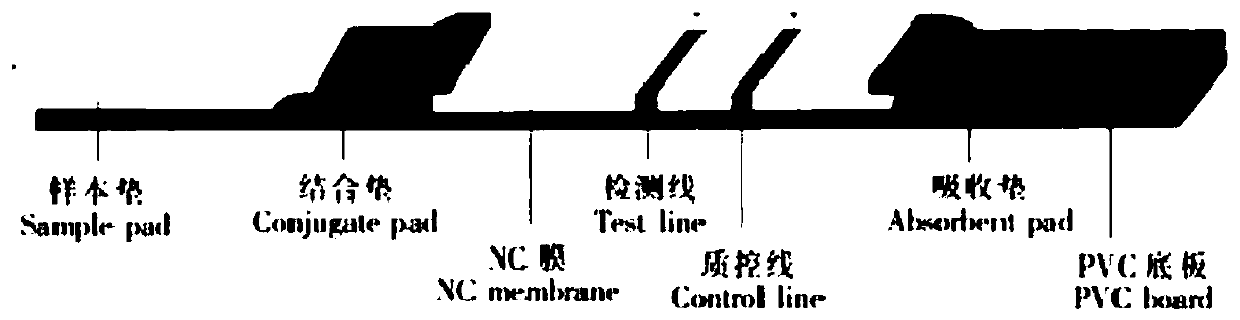
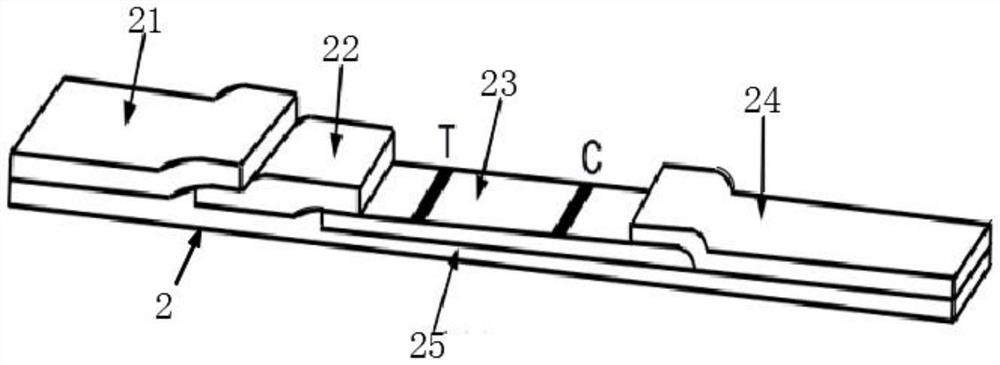
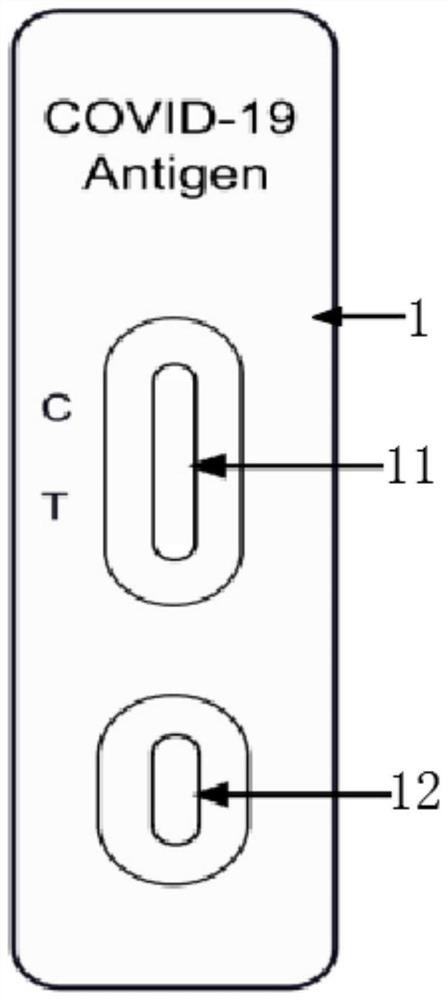
The invention relates to the technical field of novel coronavirus detection, and discloses a novel coronavirus (COVID-19) antigen detection kit. The novel coronavirus (COVID-19) antigen detection kitcomprises a cuboid paper box package, wherein a detection card, a sterile swab, a sample extraction solution, a sample extraction tube, a suction head and a specification are arranged in the cuboid paper box package; the detection card comprises a card shell and a test strip; the test strip comprises a sample pad, a marking pad, an NC film, absorbent paper and a PVC bottom plate; and the marking pad is provided with a colloidal gold-marked murine N protein monoclonal antibody I. The detection kit disclosed by the invention is high in specificity, high in detection speed and simple and convenient to operate, does not need special equipment or professional operation, can be applied to preliminary screening of various places such as communities, primary hospitals, airports, customs and even families, can judge results within several minutes, and provides a simpler, more convenient and faster field detection means for suspected patient investigation and asymptomatic infected person screening, thereby preventing epidemic spread as soon as possible.
Owner:深圳容金科技有限公司
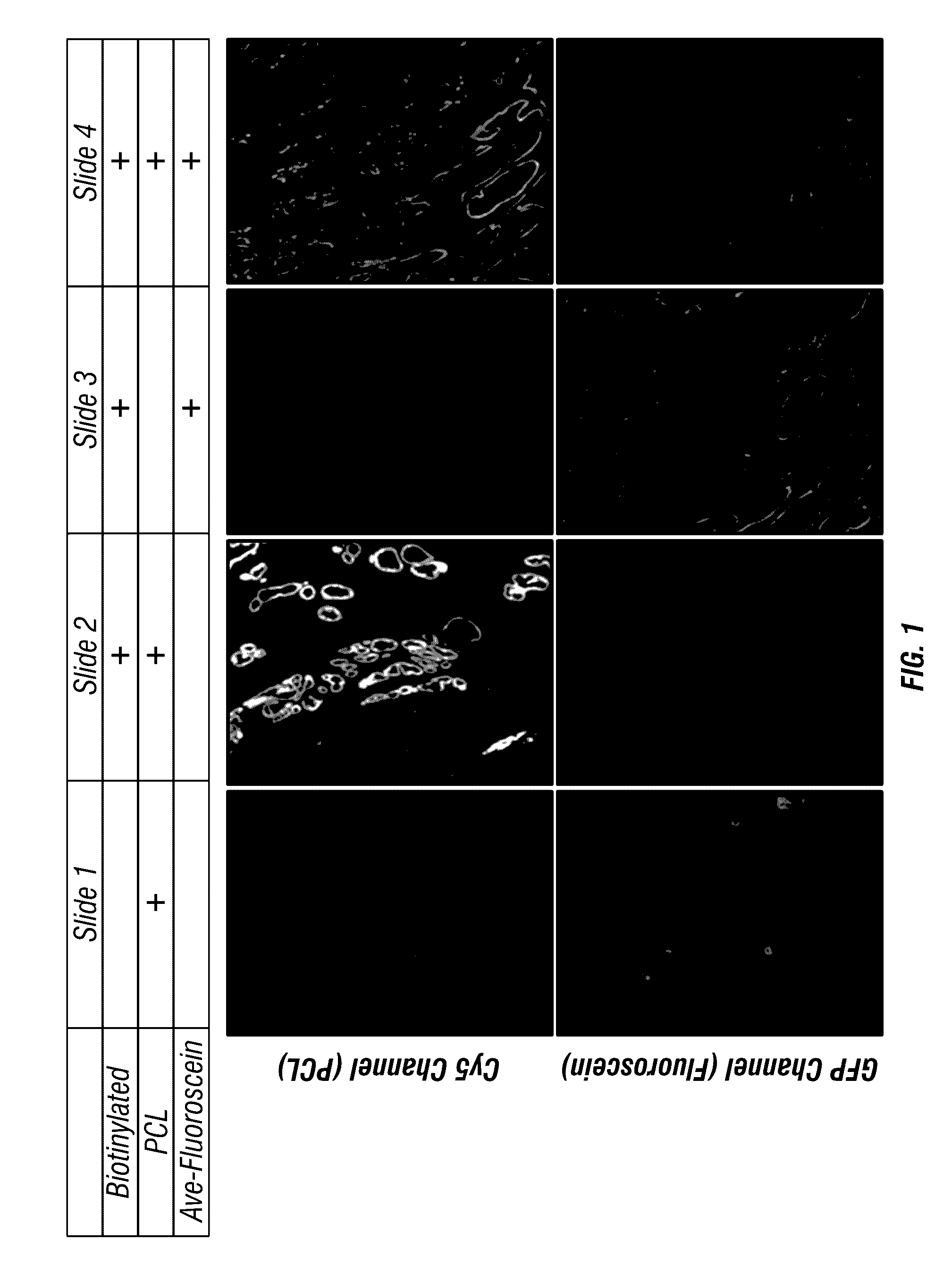
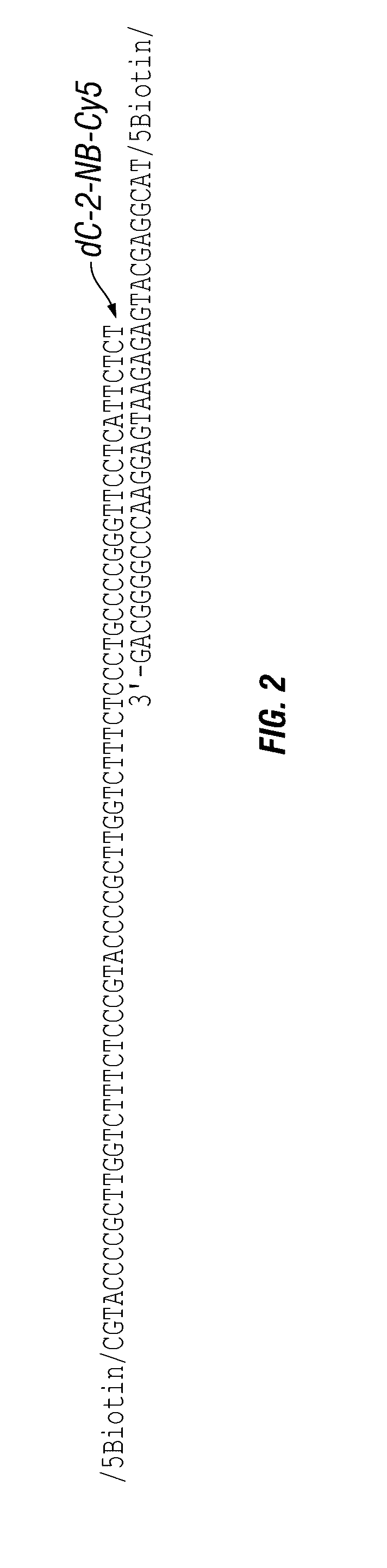
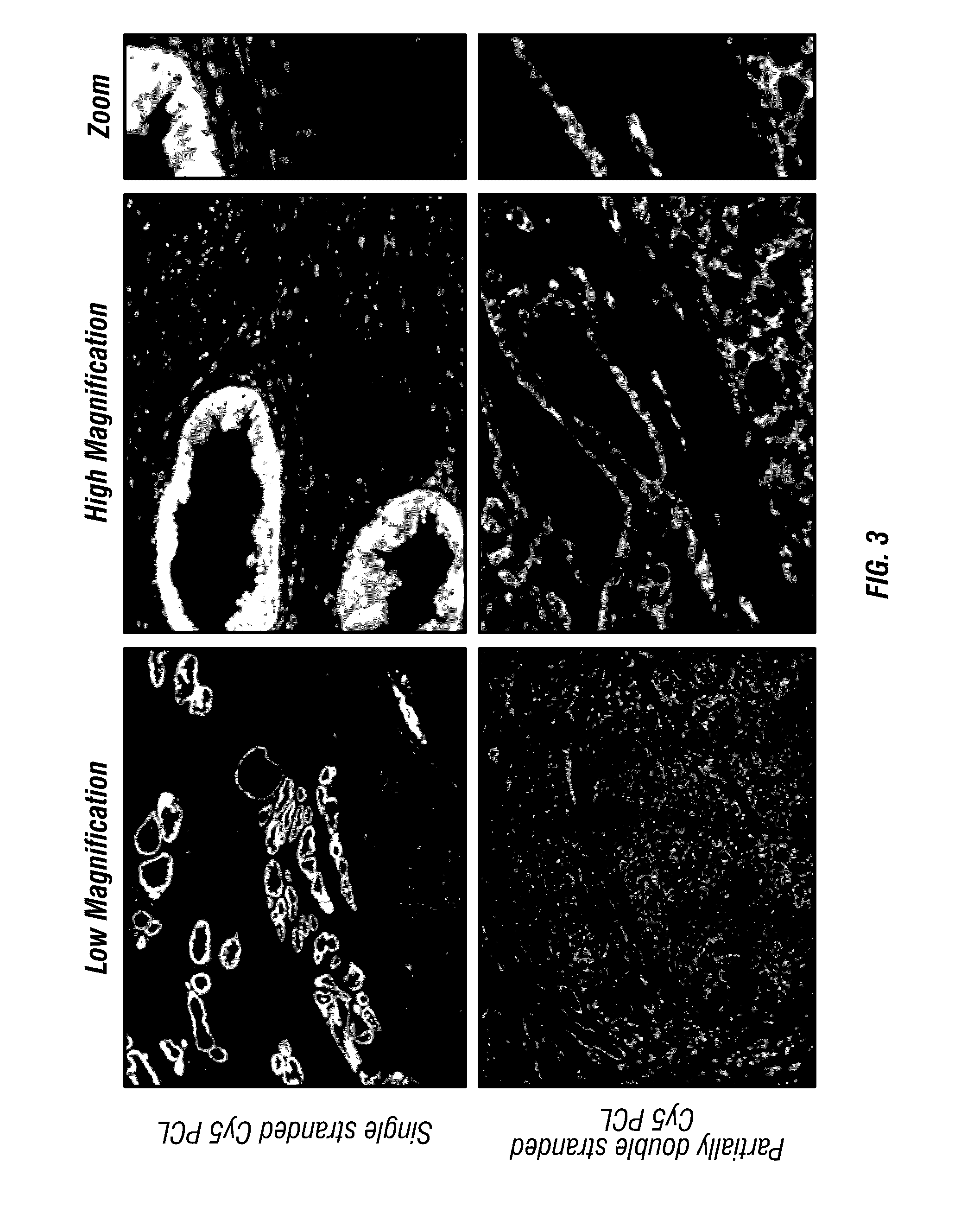
Antigen detection using photocleavable labels
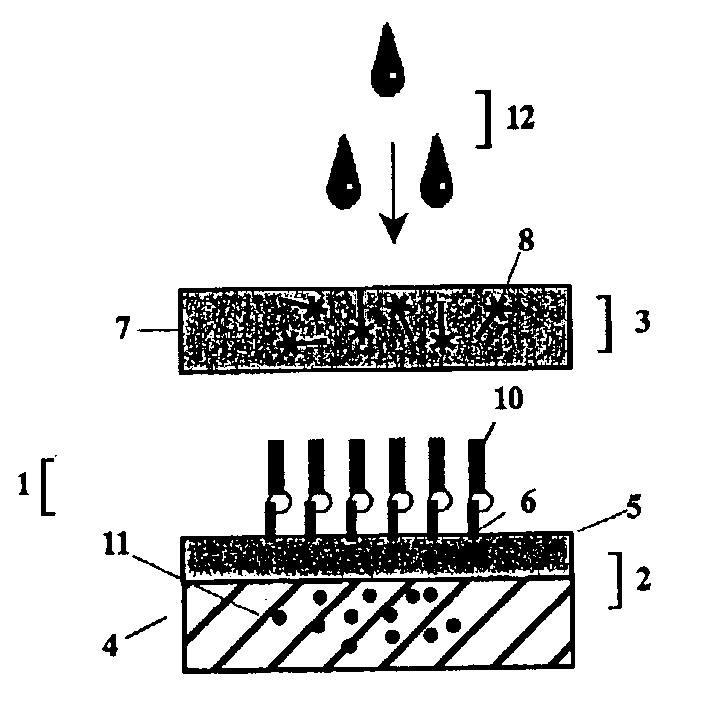
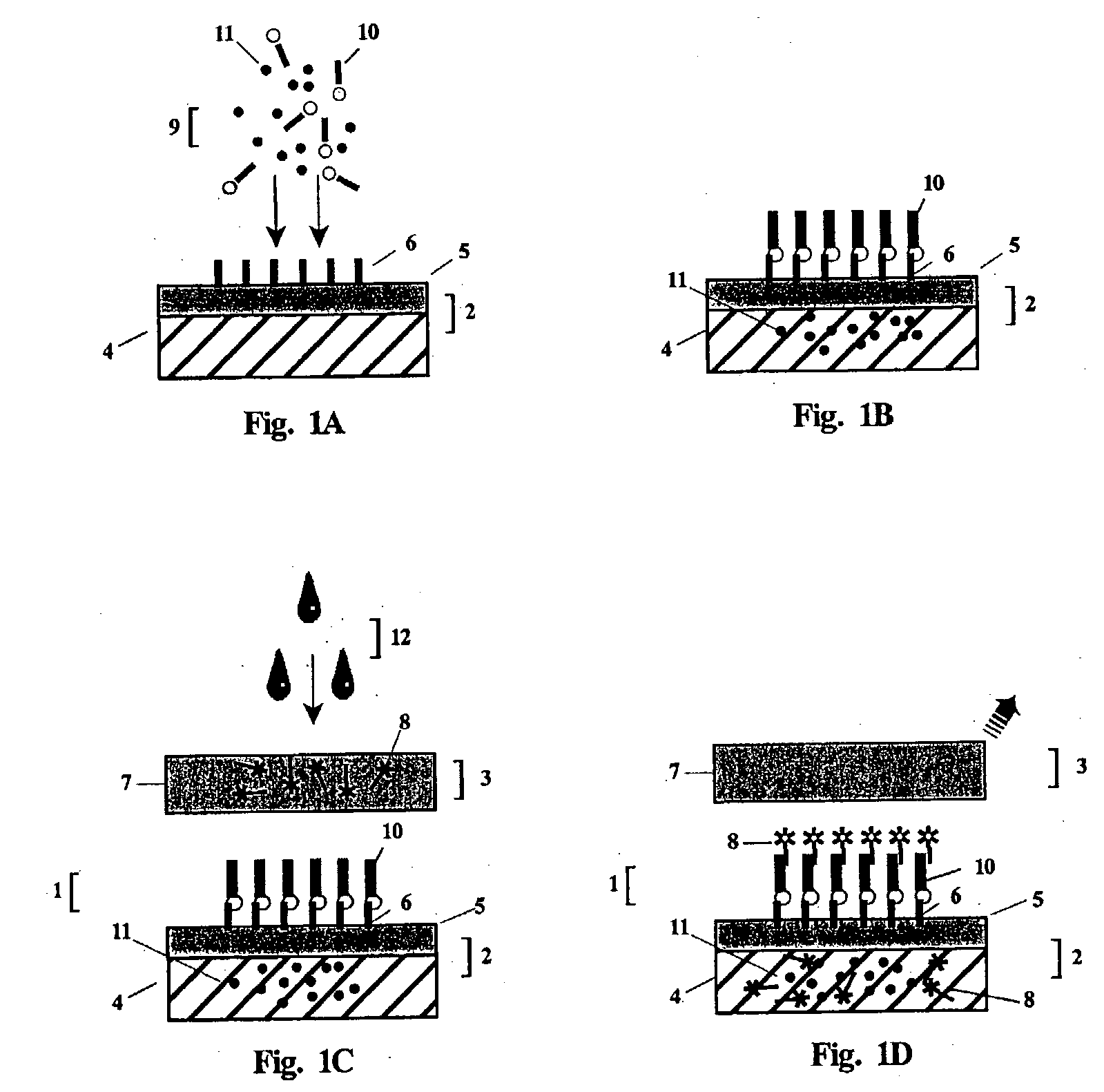
Provided herein are methods of using photocleavable labels for multiplex and serial antigen detection. The methods comprise detecting the presence of photocleavable labels, which are conjugated through functional linkers to antigen-binding complexes, which in turn non-covalently bind to antigens. The presence of a photocleavable label is indicative of the presence of an antigen specifically or selectively bound by an antigen-binding complex. Also provided are apparatuses for using photocleavable labels for multiplex and serial antigen detection.
Owner:AGILENT TECH INC
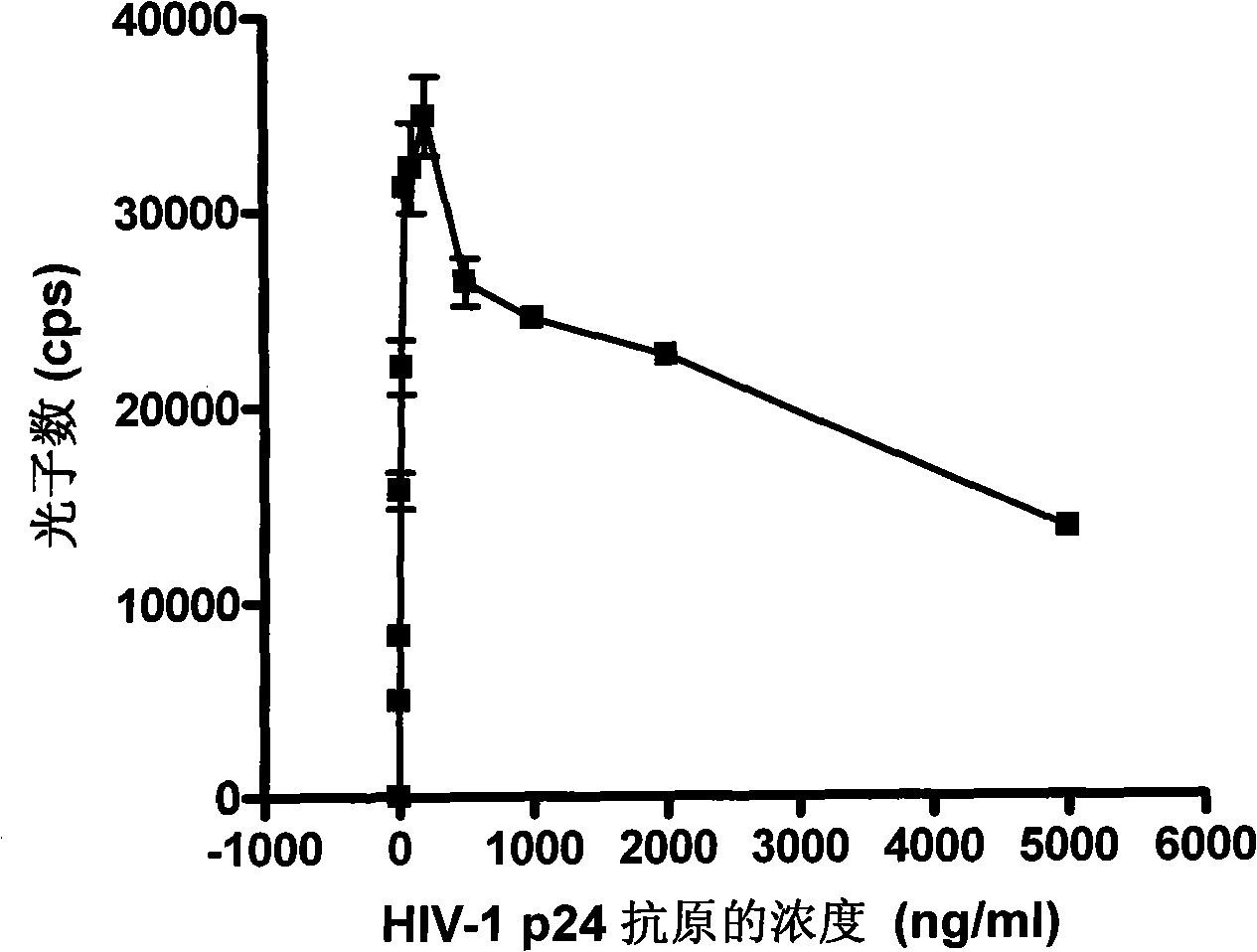
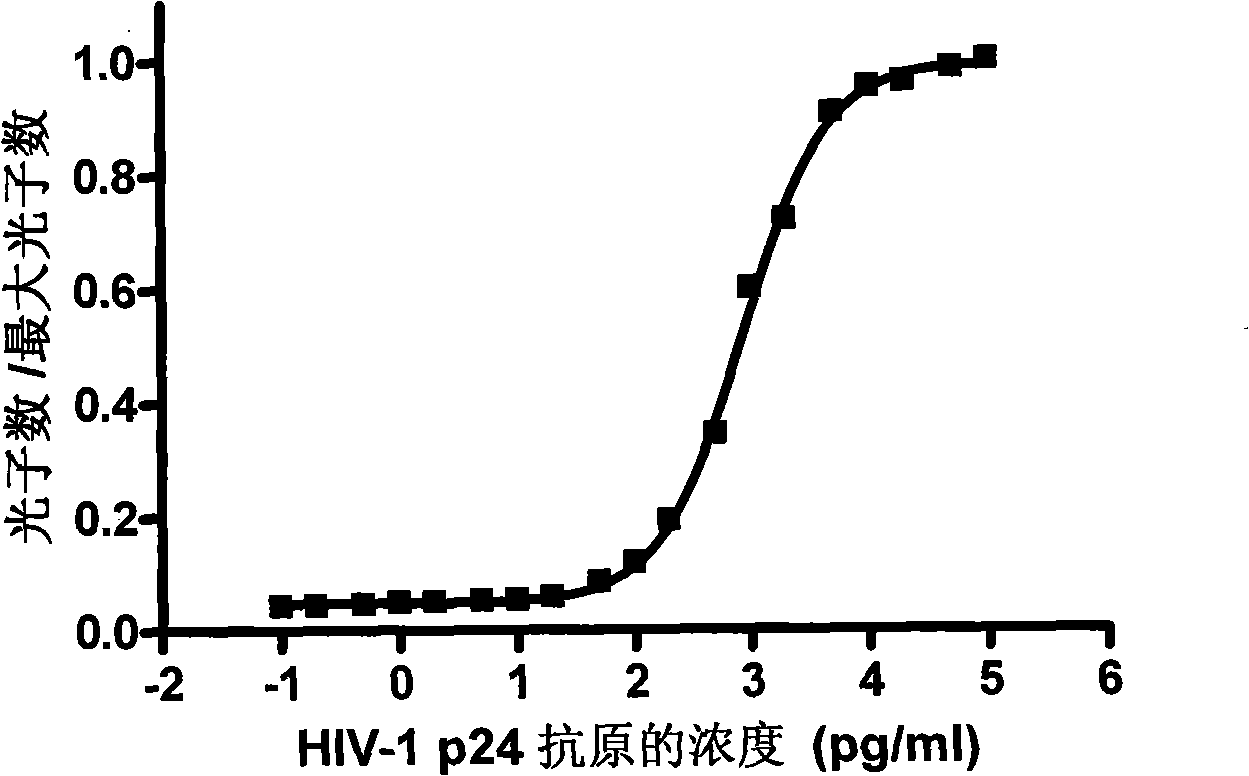
HIV-1 p24 antigen acridine ester chemiluminescence immune analyse detecting method
InactiveCN101281196AShorten the timeReduce stepsChemiluminescene/bioluminescenceAntigen testingSodium hydroxide
HIV-1 p24 antigen acridine ester chemiluminescence immunity analyzing testing method pertains to the clinical blood testing analysis method technique field. The prior HIV-1 p24 antigen testing method has problems of low sensitivity, rigmarole operations and the like. The invention uses acridine ester series compounds to mark the HIV-1 p24 antibody, adopts a double antibody / antigen sandwich method to execute immunity combination between the p24 antigen-antibody; uses excitant hydrogen peroxide, nitric acid, Triton X-100, and sodium hydrate to excite acridine ester series compounds to execute chemiluminescence reaction; and tests the number of photon to execute a qualitative and / or quantitative analysis. The invention has good correlativity with the general ELISA testing method, which has a correlation coefficient of 0.951 at the instance of P no more than 0.01; has high sensitivity, wherein, the testing limit is 0.5pg / ml; has wide detection range in 5-6 magnitude order; and the invention also has advantages of short reaction time, easier operation and the like.
Owner:BEIJING UNIV OF TECH
HPV16E7 monoclonal antibody and preparation method and application thereof
The invention discloses an HPV16E7 monoclonal antibody and a preparation method and application thereof.A correct sequence of an HPV16E7 gene is obtained from a clinical specimen through cloning, the gene effectively expresses HPV16E7 fusion protein in the form of soluble protein in BL21(DE3) escherichia coli under the induction of IPTG after being correctly inserted in a pET28a(+) prokaryotic expression plasmid, the HPV16E7 monoclonal antibody is successfully prepared through a protein immunized BALB / C mouse, the antibody can recognize a prokaryotic and eukaryotic expression HPV16E7 antigen, quite high application value is achieved for detection of the HPV16E7 antigen, and a basis is provided for research of protein and related diseases and establishment of a detection method of the HPV16 antigen.
Owner:SHANGHAI PUTUO DISTRICT CENT HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com