Detection kit for antigens of novel coronavirus (SARS-CoV-2)
A sars-cov-2, antigen technology, applied in the direction of viruses, viral peptides, viruses/phages, etc., can solve the problems of time-consuming, cumbersome and time-consuming, difficult to guarantee specificity and sensitivity, and achieve accurate detection results and improve detection. Output rate, flexible operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
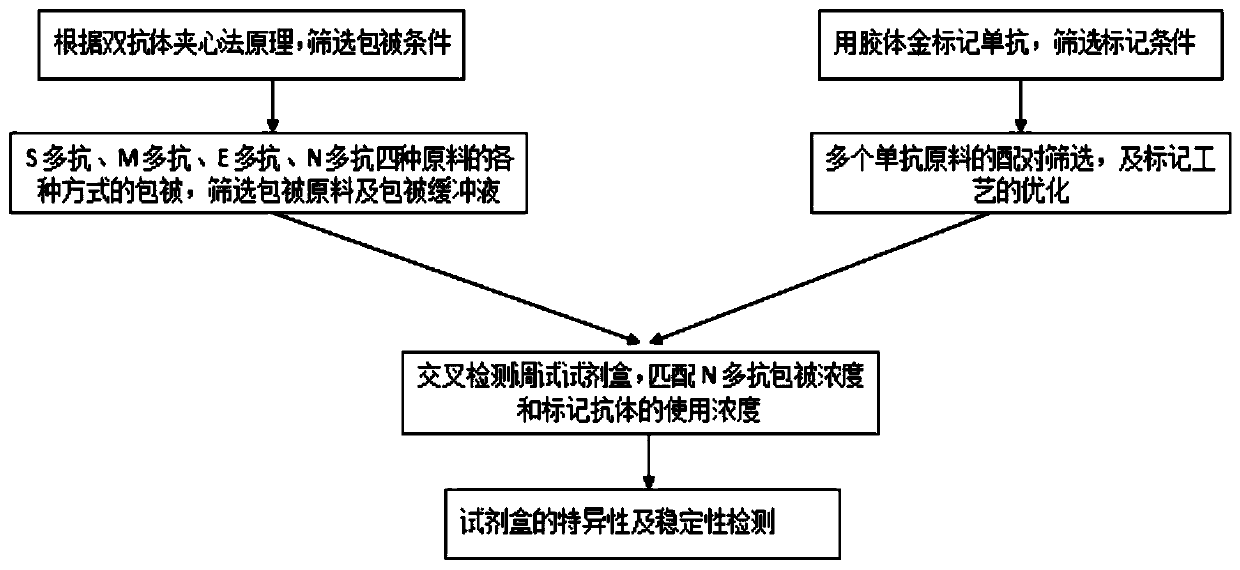
[0060] The present invention also protects the preparation method of the above-mentioned antibody, which uses a protein composed of any one of the amino acid sequences shown in SEQ ID NO.2-SEQ ID NO.11 as an antigen, prepares an immunogen with an adjuvant, and obtains an antibody after immunizing animals. Further, for example, a protein composed of any one of the amino acid sequences shown in SEQ ID NO.2-SEQ ID NO.11 is used as an antigen, and an adjuvant is used to prepare an immunogen, and after the animal is immunized, the titer of the antibody meets the 10-fold dilution activity After the level above L6, splenocytes were immunized again to perform cell fusion with myeloma cells, positive clones were screened, amplified and purified to obtain antibodies. The inventor determined the preparation scheme provided by the present invention by exploring the conditions of animal immunization, cell fusion, screening of specific hybridoma cells, and mass production of monoclonal antib...
Embodiment 1
[0075] The preparation process of embodiment 1 monoclonal antibody
[0076] animal immunity
[0077] ① Antigen preparation.
[0078] Prepare the immunization antigen of monoclonal antibody, the screening antigen of present embodiment is as follows 10 kinds: N1 antigen (SEQ ID NO.2), N2 antigen (SEQ ID NO.4), N3 antigen (SEQ ID NO.3), N4 antigen ( SEQ ID NO.5), S1 antigen (SEQ ID NO.6), S2 antigen (SEQ ID NO.7), S3 antigen (SEQ ID NO.8), M1 antigen (SEQ ID NO.9), M2 antigen (SEQ ID NO.9) ID NO.10), E antigen (SEQ ID NO.11).
[0079] ② Selection of immune animals.
[0080] Mice can be selected as immunized animals according to the myeloma cells used. Because the mouse myeloma cell lines used in hybridoma technology are all derived from BALB / c mice. Mice should be 8-12 weeks old for the first immunization, and female mice are easier to operate.
[0081] ③ Determination of the immunization program.
[0082] Choose the appropriate immunization scheme according to the specifi...
Embodiment 2
[0130] The preparation of embodiment 2 polyclonal antibody
[0131] immunity
[0132] Antigens were selected from N protein (SEQ ID NO.1), S protein (SEQ ID NO.12), M protein (SEQ ID NO.13) and E protein (SEQ ID NO.14), and polyclonal antibodies were prepared respectively.
[0133] For the first immunization, the antigen was mixed with Freund's complete adjuvant in a volume ratio of 1:1, stirred by a vortex mixer to prepare a water-in-oil adjuvant, and each guinea pig was subcutaneously immunized with multiple injections of 2ml. 0.5 mg antigen per guinea pig.
[0134] For the second immunization, 3 weeks after the first immunization, the antigen was mixed with Freund’s incomplete adjuvant in a volume ratio of 1:1, stirred by a vortex mixer to prepare a water-in-oil adjuvant, and each guinea pig was subcutaneously immunized at multiple points Inject 2ml, 0.25mg antigen per guinea pig.
[0135] For the third immunization, 3 weeks after the second immunization, take the antige...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com