Patents
Literature
298 results about "Guinea pig" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The guinea pig or domestic guinea pig (Cavia porcellus), also known as cavy or domestic cavy, is a species of rodent belonging to the family Caviidae and the genus Cavia. Despite their common name, guinea pigs are not native to Guinea, nor are they biologically related to pigs, and the origin of the name is still unclear. They originated in the Andes of South America, and studies based on biochemistry and hybridization suggest they are domesticated descendants of a closely related species of cavy such as C. tschudii, and therefore do not exist naturally in the wild. They were originally domesticated as livestock, as a source of food, and continue to be.
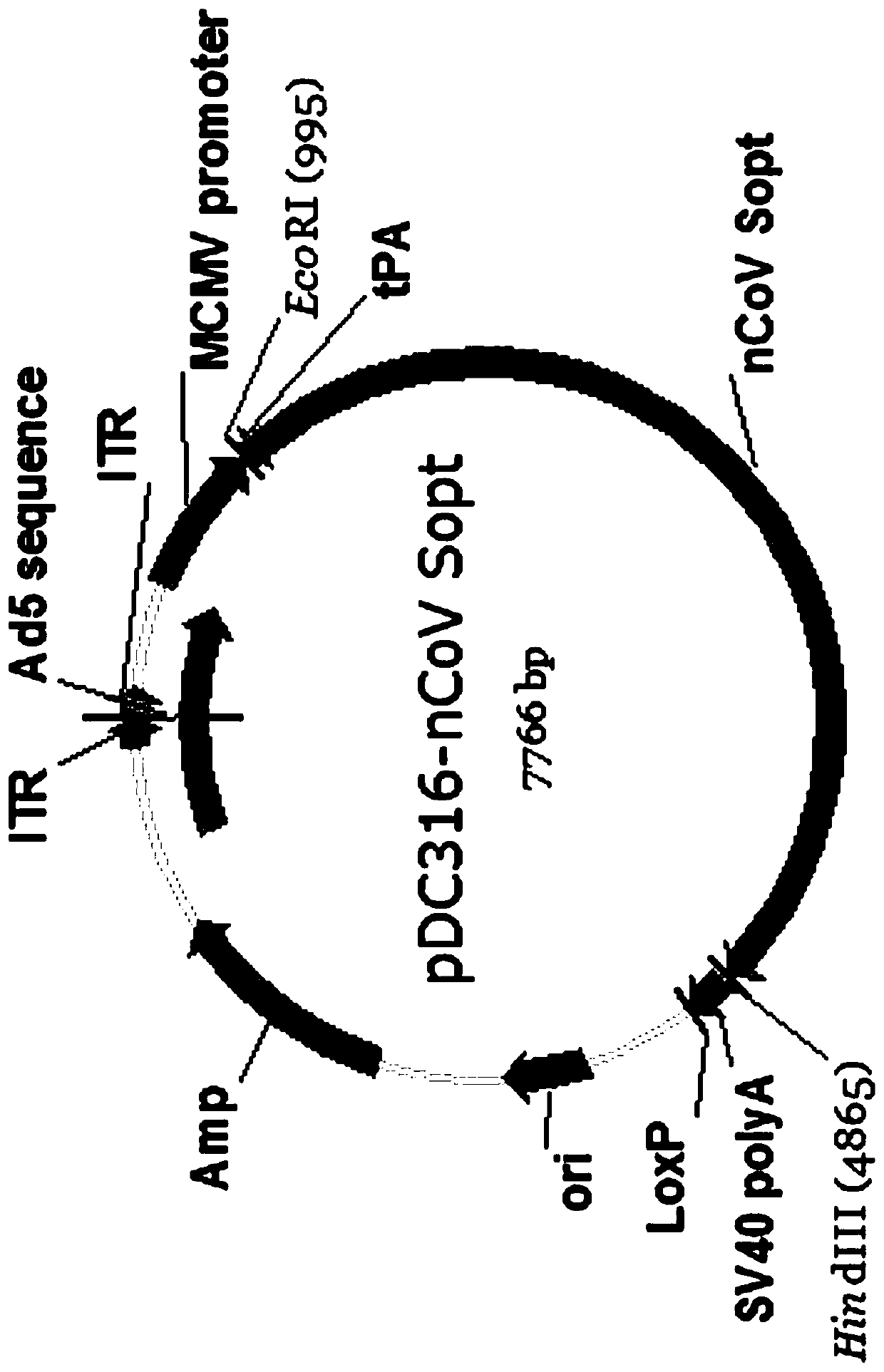
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
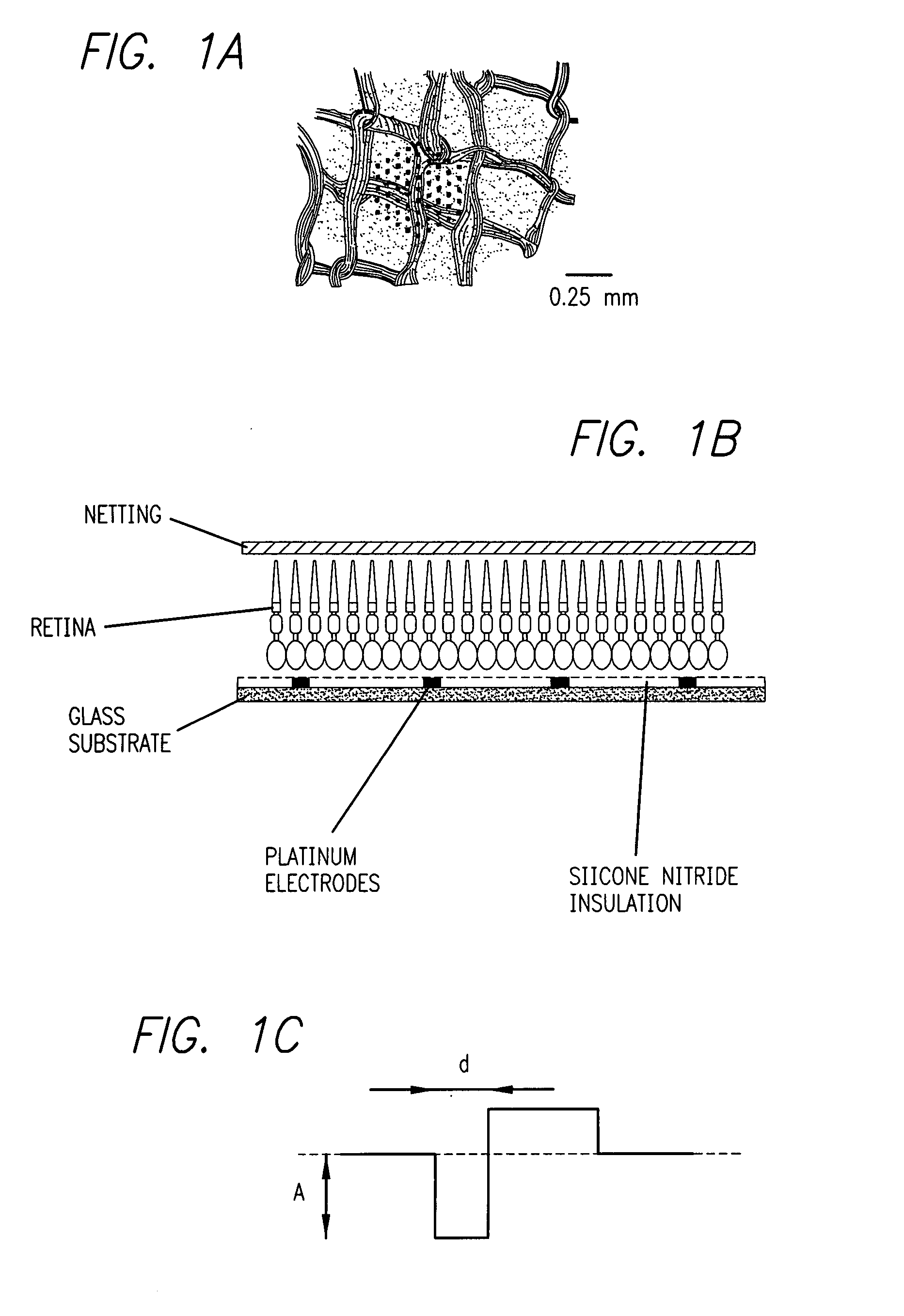
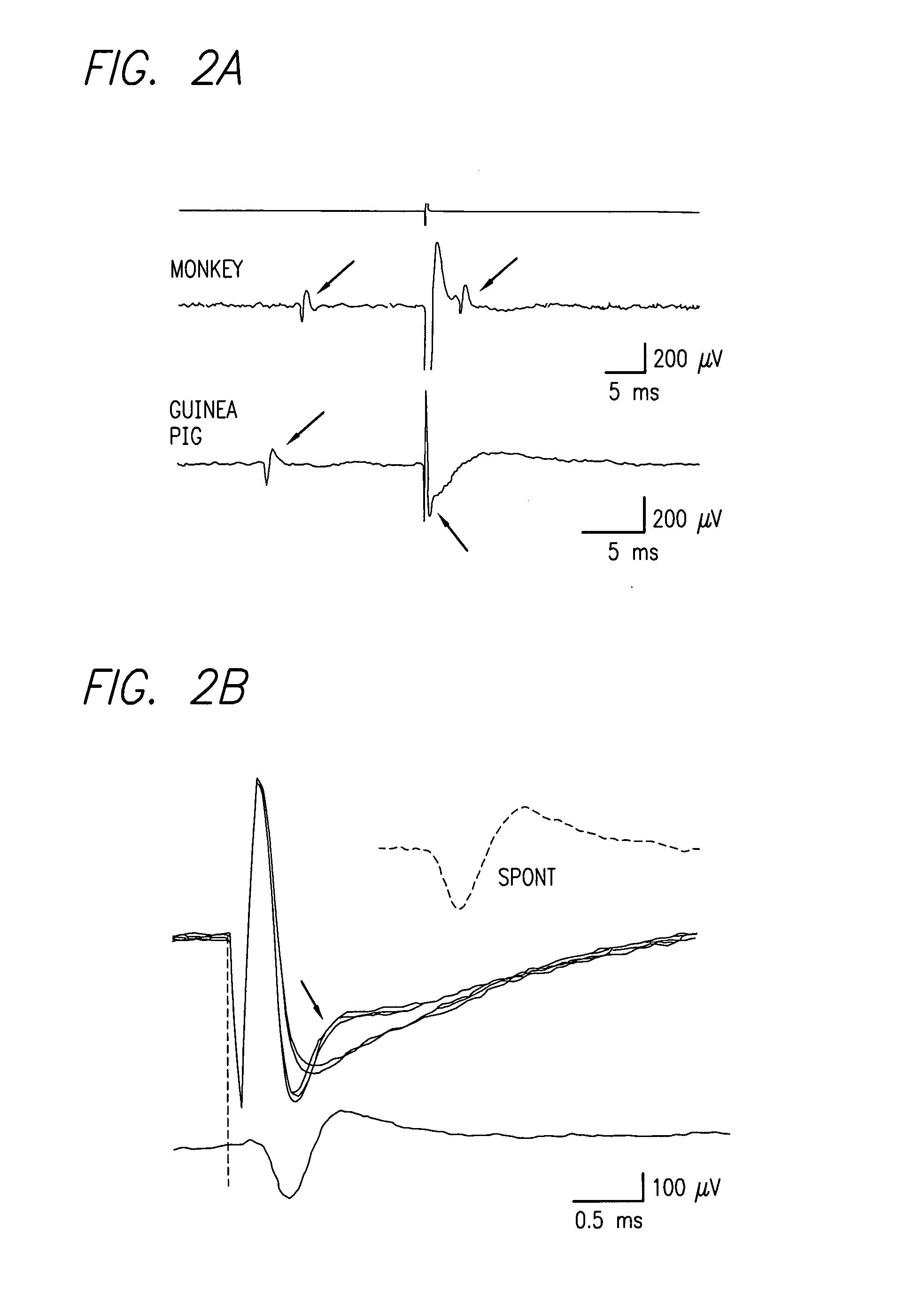
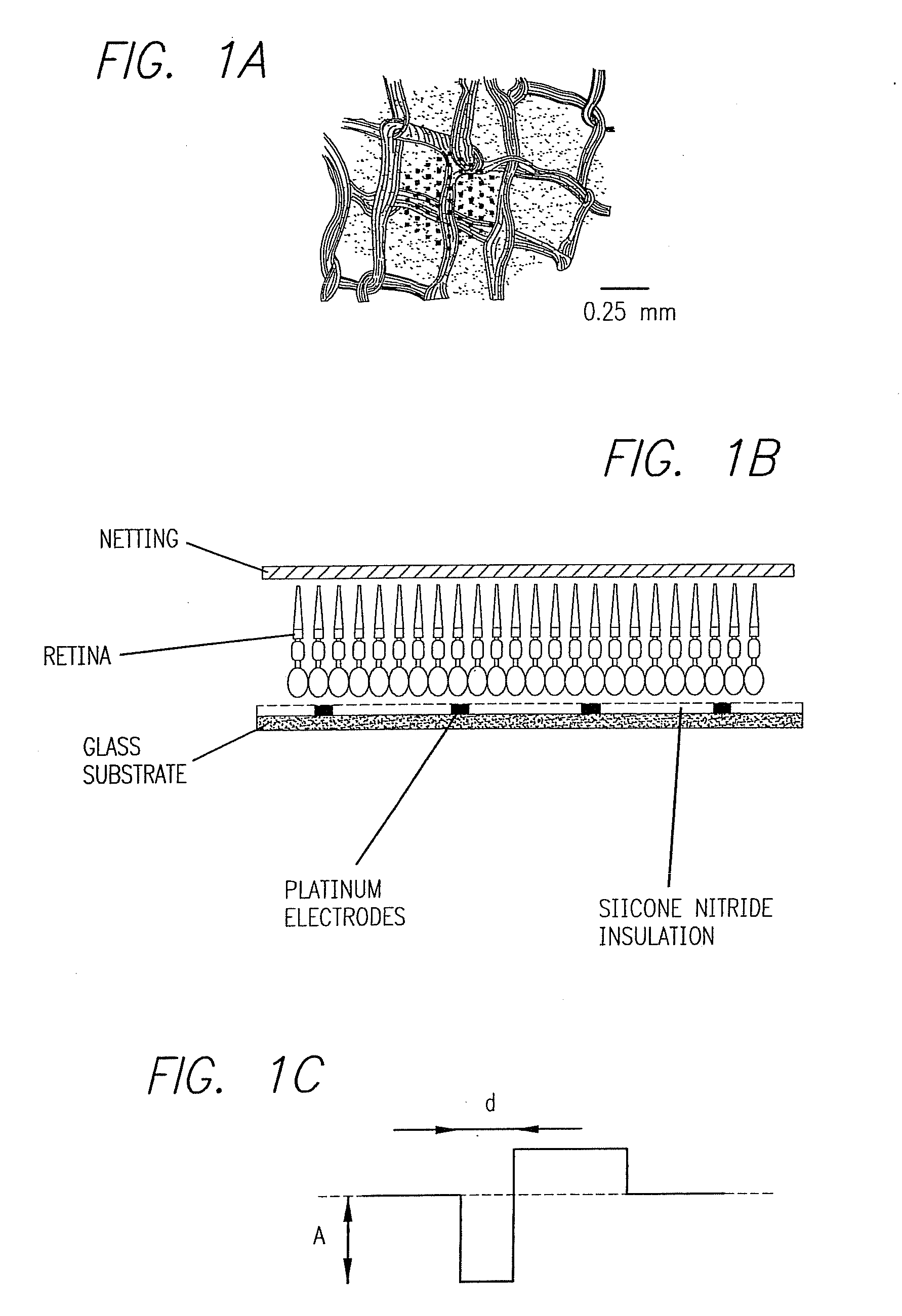
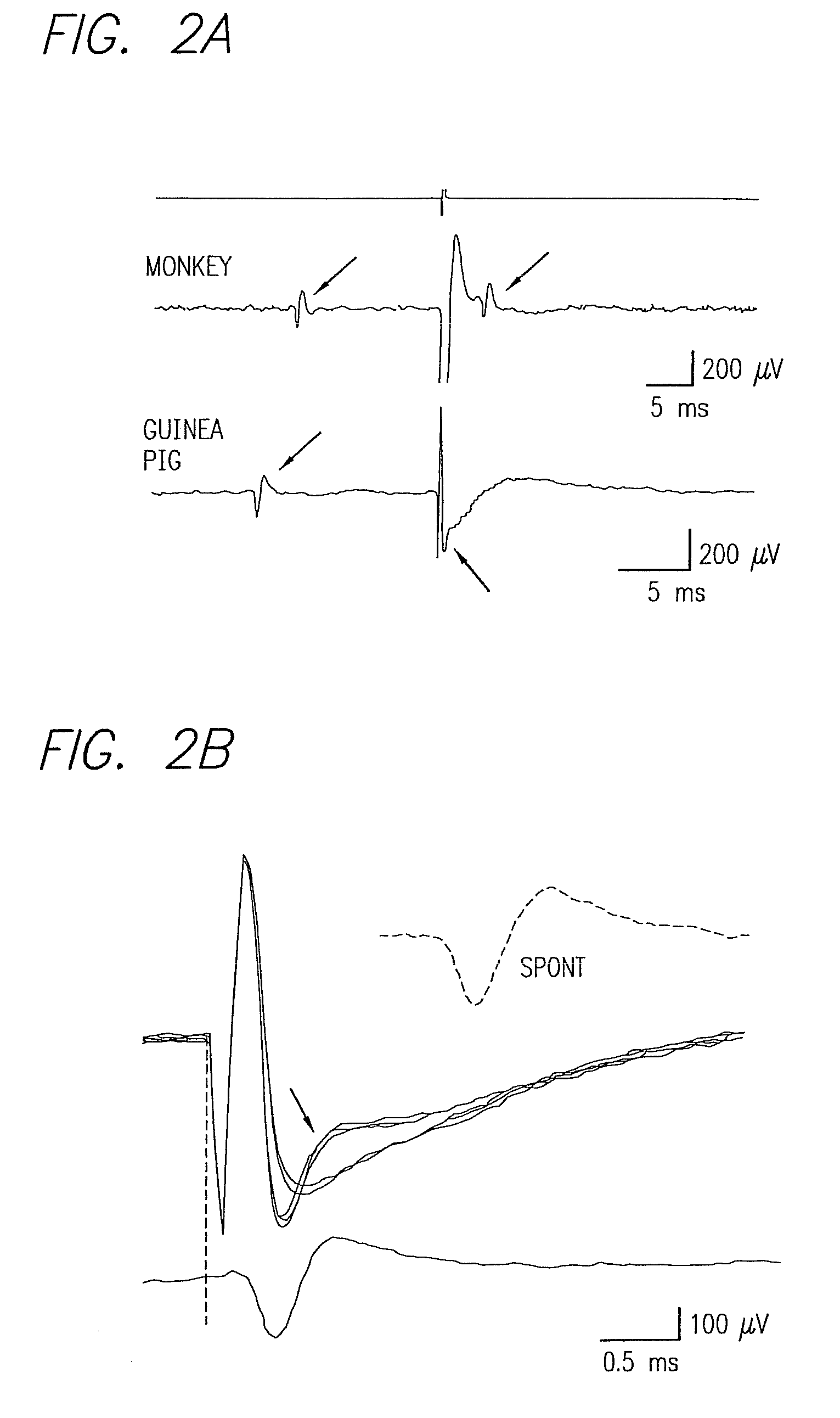
Method and apparatus for visual neural stimulation
Existing epiretinal implants for the blind are designed to electrically stimulate large groups of surviving retinal neurons using a small number of electrodes with diameters of several hundred μm. To increase the spatial resolution of artificial sight, electrodes much smaller than those currently in use are desirable. In this study we stimulated and recorded ganglion cells in isolated pieces of rat, guinea pig, and monkey retina. We utilized micro-fabricated hexagonal arrays of 61 platinum disk electrodes with diameters between 6 and 25 μm, spaced 60 μm apart. Charge-balanced current pulses evoked one or two spikes at latencies as short as 0.2 ms, and typically only one or a few recorded ganglion cells were stimulated. Application of several synaptic blockers did not abolish the evoked responses, implying direct activation of ganglion cells. Threshold charge densities were typically below 0.1 mC / cm2 for a pulse duration of 100 μs, corresponding to charge thresholds of less than 100 pC. Stimulation remained effective after several hours and at high frequencies. To demonstrate that closely spaced electrodes can elicit independent ganglion cell responses, we utilized the multi-electrode array to stimulate several nearby ganglion cells simultaneously. From these data we conclude that electrical stimulation of mammalian retina with small-diameter electrode arrays is achievable and can provide high temporal and spatial precision at low charge densities. We review previous epiretinal stimulation studies and discuss our results in the context of 32 other publications, comparing threshold parameters and safety limits.
Owner:SALK INST FOR BIOLOGICAL STUDIES +1
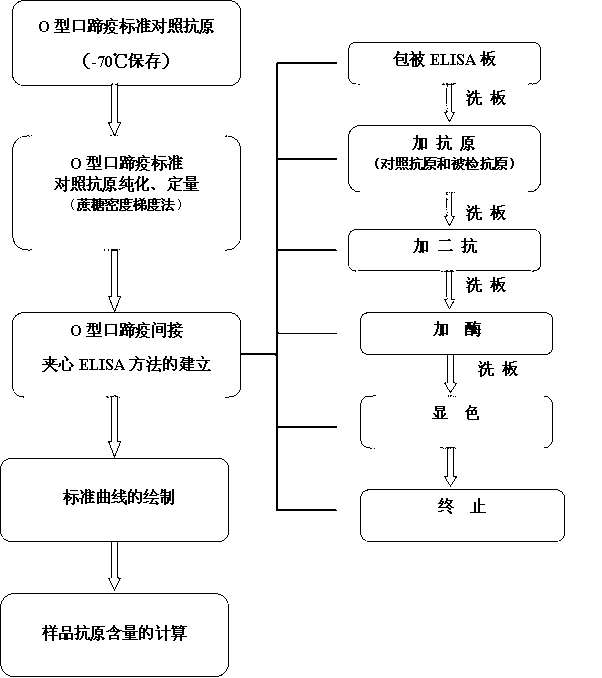
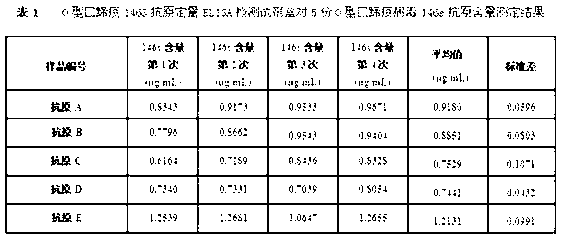
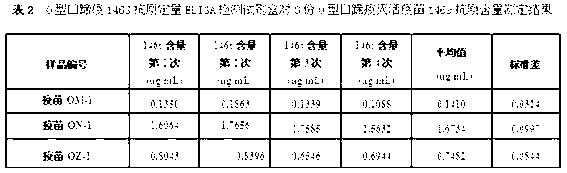
O type foot-and-mouth disease 146S antigen quantitative ELISA detection kit and method for using same
ActiveCN103076451ASolve efficiency problemsSolving the power test substitution problemMaterial analysisDiseaseVaccine Potency
The invention discloses an O type foot-and-mouth disease 146S antigen quantitative ELISA (enzyme-linked immuno sorbent assay) detection kit and a method for using the same. The kit comprises an ELISA plate, an O type foot-and-mouth disease standard reference antigen, a demulsifier, an O type foot-and-mouth disease rabbit antiserum, an O type foot-and-mouth disease guinea pig antiserum, a rabbit anti-guinea pig-horse radish peroxidase conjugate, a guinea pig antiserum dilute solution, a 25-fold PBST (phosphate buffer solution tween) concentrated solution, a carbonate buffer solution capsule, a citric acid-phosphate buffer solution tablet, an OPD (o-phenylenediamine) tablet, a stop solution, a plate sealing membrane, a moving liquid tank and a 96-mesh U-shaped dilution plate. The kit is an organic combination of a sucrose density gradient centrifugation method and an indirect sandwich ELISA method, integrates the advantages of the sucrose density gradient centrifugation method and the indirect sandwich ELISA method, is simple to operate and good in stability, is suitable for batch detection, can be used for distinguishing serum types, and is an ideal substitution method for antigen quantitative and vaccine efficacy detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Kit for detecting guinea pig aeromonas by utilizing Loop-mediated isothermal amplification technique
InactiveCN101235411AAccurate identificationRapid identificationMicrobiological testing/measurementAeromonas caviaeLoop-mediated isothermal amplification
The invention relates to a reagent kit and a detecting method for using loop-mediated isothermal amplification (LAMP) technique to detect aeromonas caviae, which belongs to the pathogen diagnosis field. The main technical scheme of the invention comprises: utilizing the LAMP technique, designing four pairs of high specificity primers aiming at six zones of 16s and 23s ribosome RNA gene transcription spacer region, and achieving the purpose for detecting the aeromonas caviae with specificity. Compared with a traditional method, equipment and operational steps which are needed in the method are simple, the method has the advantages of rapidness and high specificity, and the detecting cost is lower, which is suitable for common clinics and field detections.
Owner:NANKAI UNIV
Method and Apparatus for Visual Neural Stimulation
Existing epiretinal implants for the blind are designed to electrically stimulate large groups of surviving retinal neurons using a small number of electrodes with diameters of several hundred μm. To increase the spatial resolution of artificial sight, electrodes much smaller than those currently in use are desirable. In this study we stimulated and recorded ganglion cells in isolated pieces of rat, guinea pig, and monkey retina. We utilized micro-fabricated hexagonal arrays of 61 platinum disk electrodes with diameters between 6 and 25 μm, spaced 60 μm apart. Charge-balanced current pulses evoked one or two spikes at latencies as short as 0.2 ms, and typically only one or a few recorded ganglion cells were stimulated. Application of several synaptic blockers did not abolish the evoked responses, implying direct activation of ganglion cells. Threshold charge densities were typically below 0.1 mC / cm2 for a pulse duration of 100 μs, corresponding to charge thresholds of less than 100 pC. Stimulation remained effective after several hours and at high frequencies. To demonstrate that closely spaced electrodes can elicit independent ganglion cell responses, we utilized the multi-electrode array to stimulate several nearby ganglion cells simultaneously. From these data we conclude that electrical stimulation of mammalian retina with small-diameter electrode arrays is achievable and can provide high temporal and spatial precision at low charge densities. We review previous epiretinal stimulation studies and discuss our results in the context of 32 other publications, comparing threshold parameters and safety limits.
Owner:SECOND SIGHT MEDICAL PRODS +1
Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and preparation method thereof
ActiveCN101775399AImproving immunogenicityFacilitate presentationGenetic material ingredientsVirus peptidesAdjuvantRecombinant vaccines
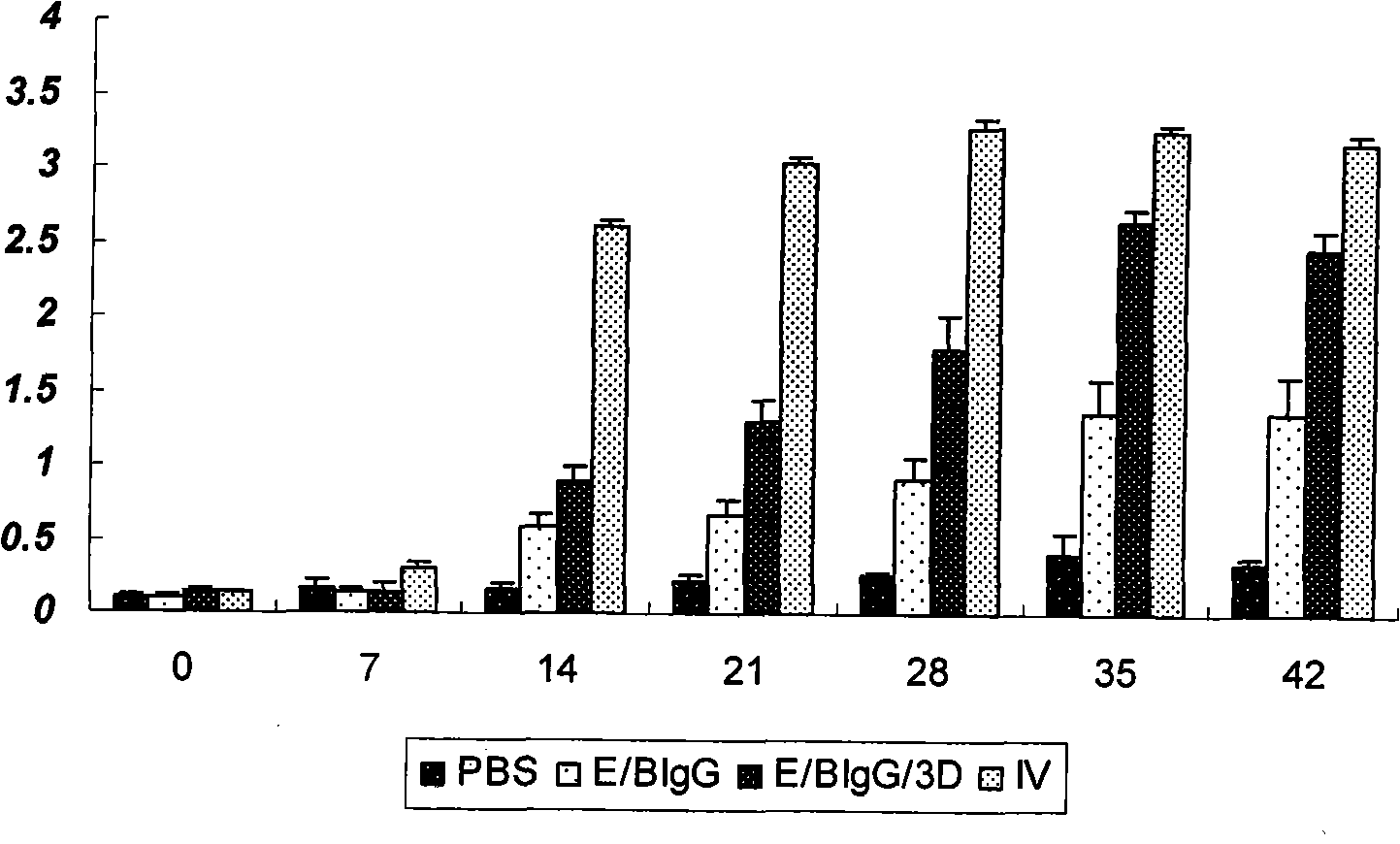
The invention discloses an Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and a preparation method thereof. The recombinant vaccine comprises the following components: proteins coded by foot-and-mouth disease virus multi-epitope genes and the fusion genes of carrier proteins, and foot-and-mouth disease virus 3D proteins. The preparation method comprises the following steps: diluting the proteins expressed by the foot-and-mouth disease virus multi-epitope genes and the fusion genes of the carrier proteins and the foot-and-mouth disease virus 3D proteins, mixing the diluted proteins uniformly, adding an adjuvant into the mixture to emulsify the mixture. Animal models and animal immune effect experiments show that the bovine Asia1 epitope recombinant vaccine can make comprehensive immune protective response, can induce injected and immunized bovine and guinea pigs to generate high level neutralizing antibodies, and can also induce cell immune response, so the recombinant vaccine can effectively protect animals against the virulent attack of the foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Polypeptide vaccine of anti Asiatic I virus of foot-and-mouth disease and its preparing method
The invention is a kind of polypeptide vaccine and the manufacturing method which can resist Asia one foot and mouth disease virus. The vaccine is prepared by that DNA array of B , T cell form location amino acid on VP1 and VP4 contact with coding form location albumen alone, or link with macromolecule of the carrier coding schedule location merged protein. The invention uses chemical method to synthesize the peptide gene of B cell and T cell form location on coding Asia1 type VP1 and VP4, and they are contacted, the gene is inserted with particle carrier alone or connecting with the carrier molecular, and transferred into bacteria express, and yeasted and gets the product.
Owner:FUDAN UNIV
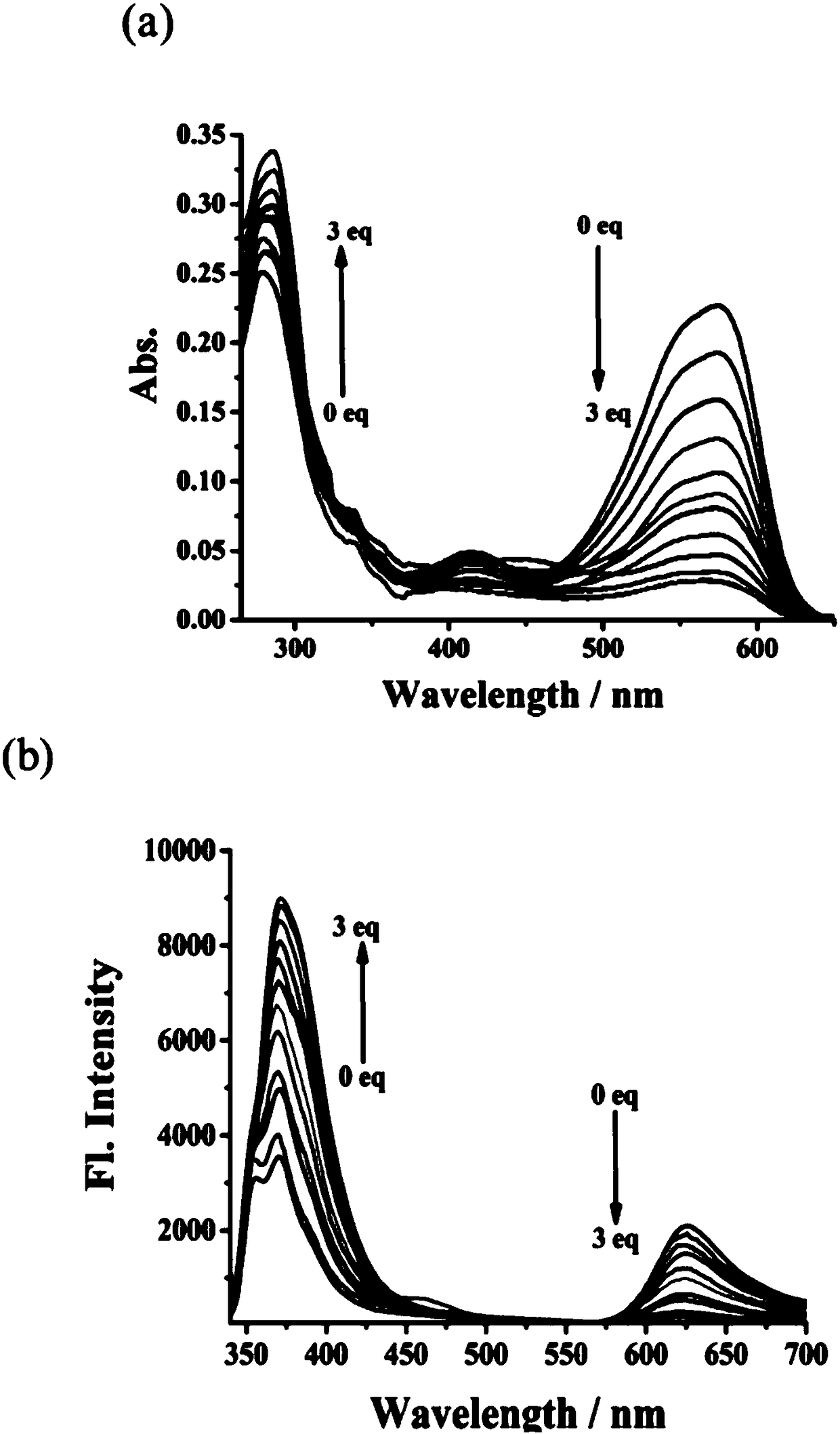
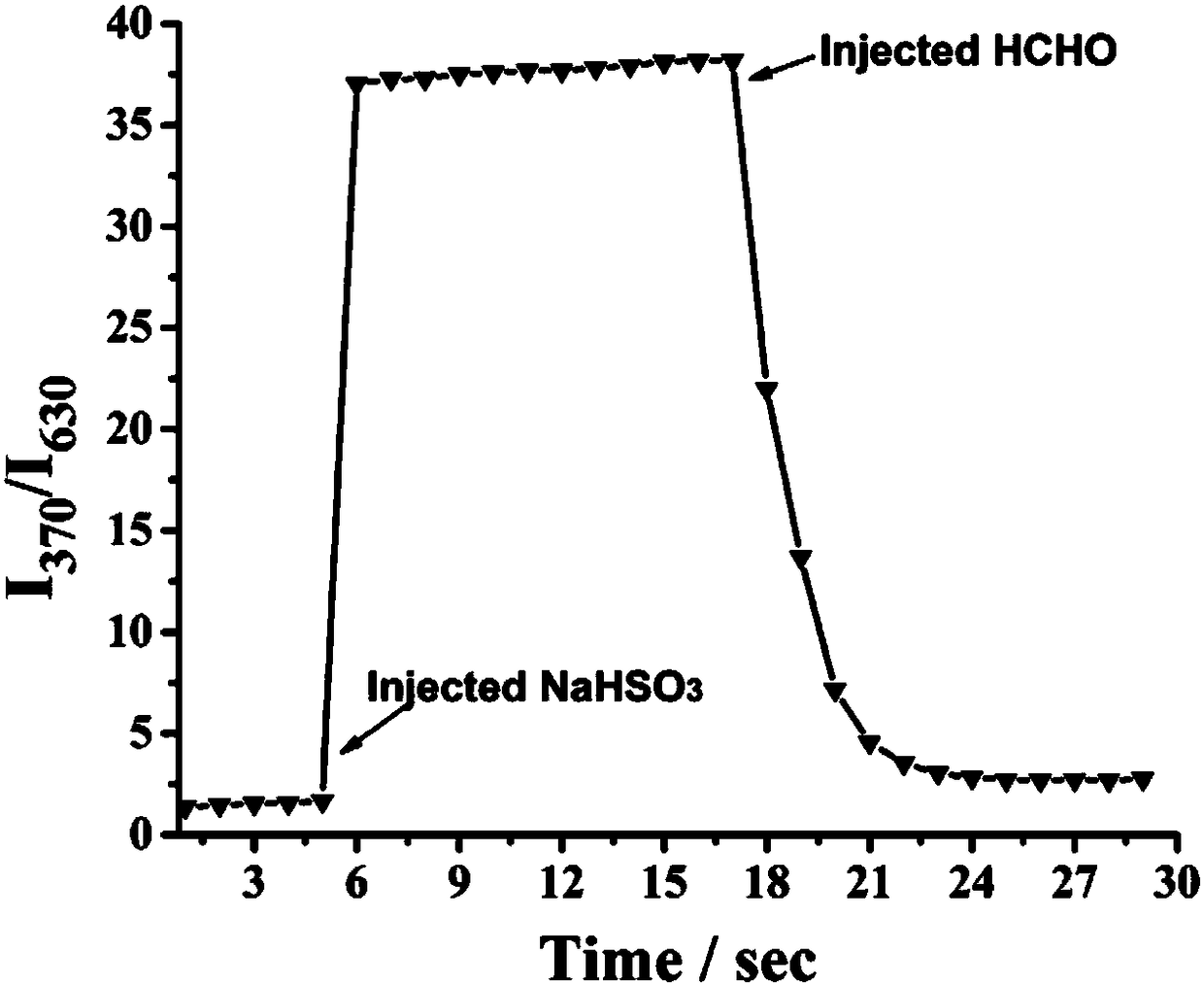
Fluorescent probe of reversible sulfur dioxide/sulphurous acid (hydrogen) salt
InactiveCN108117544AThe synthesis steps are simpleRaw materials are easy to getOrganic chemistryFluorescence/phosphorescenceHydrogenOxonium ion
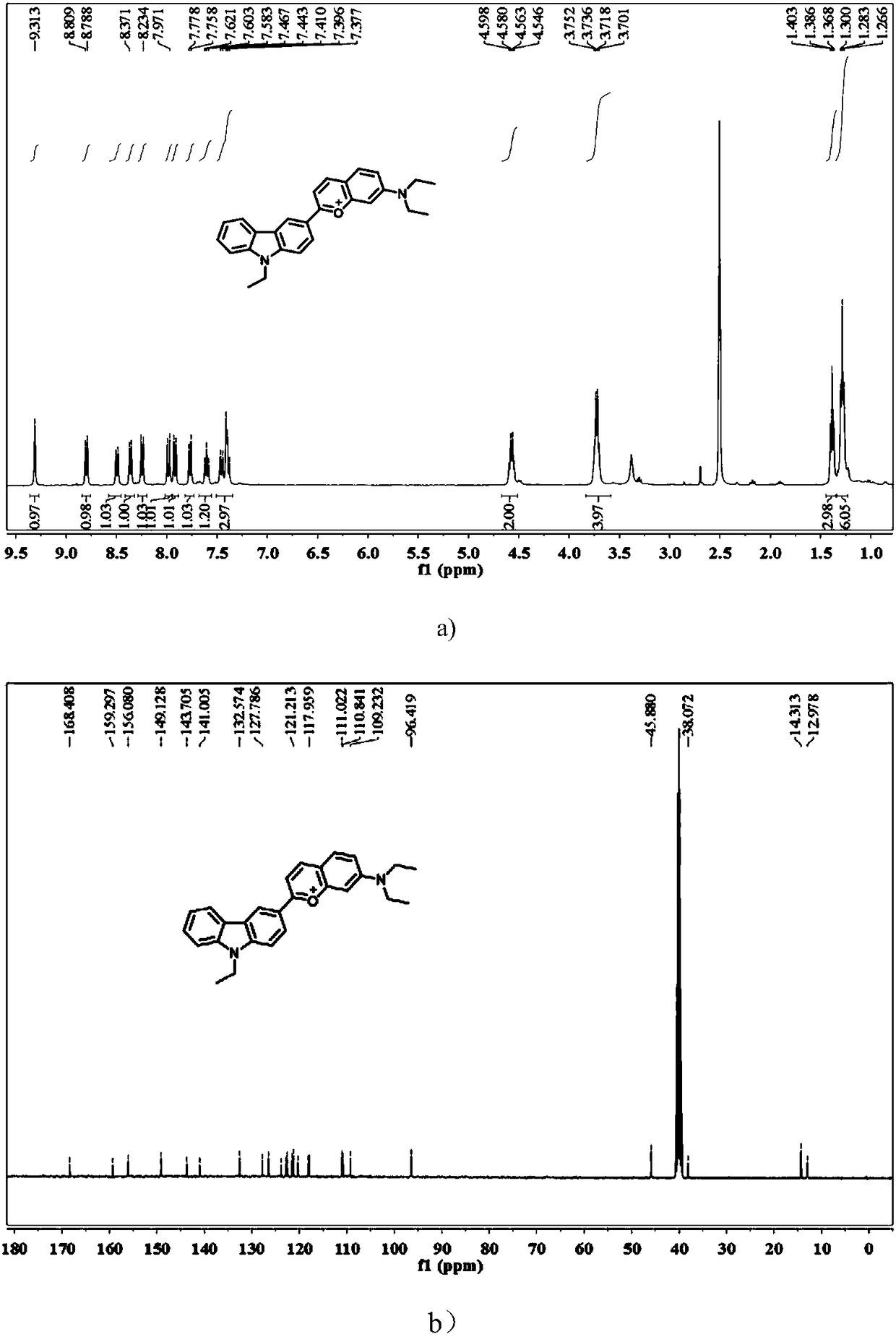
The invention provides a fluorescent probe of reversible sulfur dioxide / sulphurous acid (hydrogen) salt, with the chemical name of 7-diethylin-2-(9-ethyl-9H-carbazole-3) benzo iso-pyran oxonium ion. The fluorescent probe can detect sulfur dioxide / sulphurous acid (hydrogen) salt in a solution, cells, tissues or a living body, wherein the living body includes fish, mice, rat, guinea pig and rabbit;and reversibility is realized by formaldehyde. The fluorescent probe is simple in synthesis steps, easily available in material, high in yield and suitable for industrial application.
Owner:UNIV OF JINAN
ELISA reagent kit for testing Asial type foot-and-mouth disease virus
The invention discloses an ELISA agent box to test Asial foot-and-mouth disease virus and making method of the agent box, which comprises the following parts: solid support to connect the detected sample, mono-clone antibody to clad Asial foot-and-mouth disease virus on the solid support connected with the detected sample, multi-clone antibody of guinea pig as dianti-Asial foot-and-mouth disease virus, rabbit anti-guinea pig lgG marked by enzyme, standard serum, colorless substrate, terminal liquid.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Compound angelica sinensis preparations and preparation and application
ActiveCN101234168ASignificant anti-inflammatory and cough-relieving effectSignificant anti-asthma, cough-relieving and phlegm-reducing effectsAntipyreticAerosol deliveryDiseaseCaladium
The invention provides a compound angelica preparation, the dosage of the raw medicinal materials of which is calculated according to the weight, namely 10-30g of angelica, 5-15g of caladium and 2-8g of sophora Flavescens Ait. The preparation includes oral liquid, spray, and aerosol, dropping pill, capsule or tablet. Compared to the prior art, the preparation of the invention has remarkable effect on curing asthma, cough and expectoration, while the animal experiment also shows that the preparation has remarkable anti-inflammation and cough relieving actions on mice and has better asthma curing action on Guinea pig. Moreover, the preparation only contains three Chinese traditional medicines and is of refined formula, advanced preparation process and can be prepared to be various formulations like dropping pill, aerosol, spray, capsule and tablet and so on with easy administration. The beneficial effects of the preparation is further specified combining with the embodiment and the clinical experiment as below; the preparation can be applied in preparing drugs with actions of anti-inflammation, cough relieving and asthma curing, can also be applied in preparing drugs for treating acute and chronic bronchitis, chronic obstructive pulmonary diseases and upper respiratory tract infection respiratory diseases.
Owner:ZHEJIANG UNIV
System for Small Animal Aerosol Inhalation Chamber
InactiveUS20090211534A1Excellent decontaminationAnimal housingVeterinary instrumentsSmall animalRODENT EXPOSURE
This patent application is for an apparatus to safely expose guinea pigs and other small animals to biohazardous aerosol. It is composed of three main chambers housed in an outer box which fits within a conventionally sized biosafety cabinet. The animal chamber contains a removable housing unit for four or eight guinea pigs. The aerosol chamber is separate to minimize fur contamination. The nebulizer chamber is also sealed to reduce risks from leakages. This apparatus is easily decontaminated by immersion in disinfectant. The first version of the prototype, tentatively named the AXS1, has been tested for safety, ergonomics, efficiency of rodent exposure to bacteria, airflow, access points, seal mechanisms, and size.
Owner:ALAN ROWE SCHENKEL
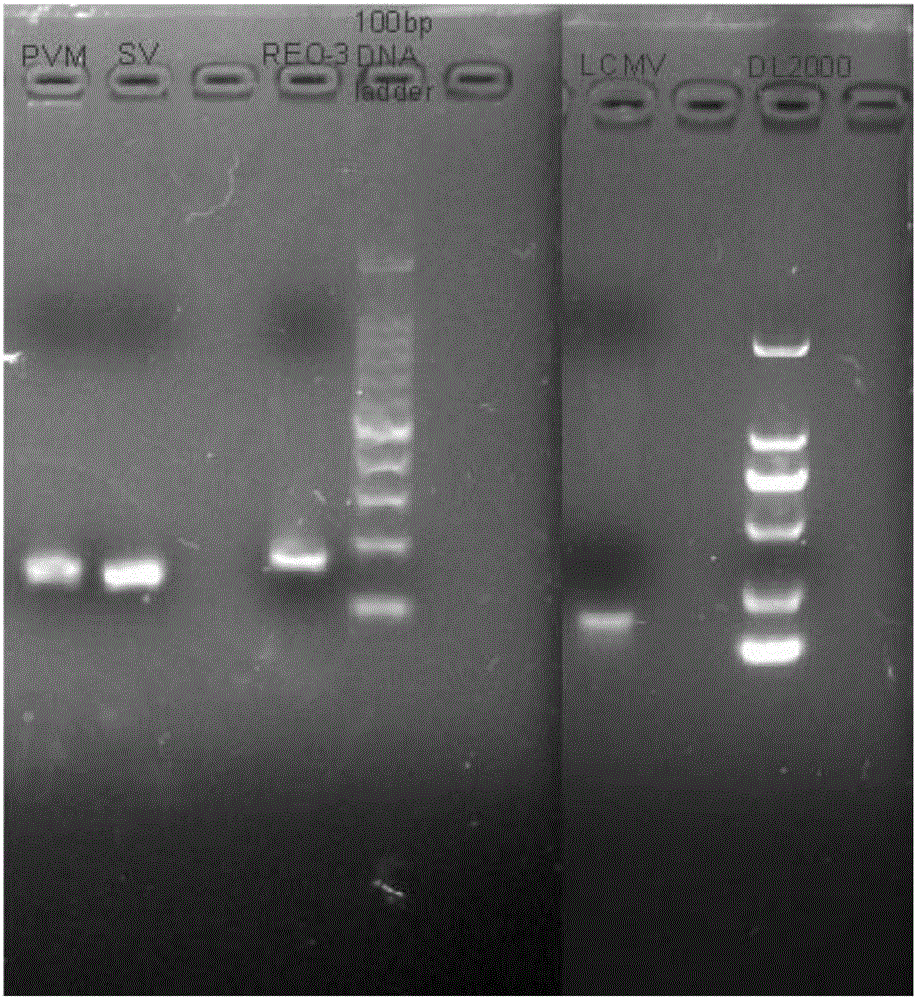
Multiple liquid phase gene chip method and reagent for rapidly detecting guinea pig LCMV, SV, PVM and Reo-3 viruses
ActiveCN106191311AAccurate detectionReduce dosageMicrobiological testing/measurementMicroorganism based processesBiotin-streptavidin complexFluorescence
The invention discloses a multiple liquid phase gene chip method and a reagent for rapidly detecting guinea pig LCMV, SV, PVM and Reo-3 viruses. The operation is simple. A target amplified fragment is acquired through PCR, and then the amplified product, fluorescent coding micro-balloon and streptavidin-phycoerythrin are hybridized, MFI value is read through a tester and different viruses are distinguished. According to the method provided by the invention, the rat lymphocyte choriomeningitis virus, sendai virus, rat pneumonia virus and Reo-3 virus can be accurately detected at the same time; the specificity is strong, the sensitivity is high and the repeatability is excellent; compared with the traditional detection method, the method has the advantages that different target molecules in a same sample can be detected, the detection flux is high, the sample dosage is less, the operation is simple and quick, the detection cost is greatly reduced and the detection efficiency is increased.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
Pet dog feed
InactiveCN102217738AHigh nutritional valueEasy to makeAnimal feeding stuffAccessory food factorsAnimal scienceArginine
The invention discloses pet dog feed, which comprises the following components in parts by weight: 400-600 parts of wheat gluten, 400-600 parts of bone powder, 400-600 parts of fish powder, 200-300 parts of corn powder, 100-200 parts of potato powder, 1-5 parts of tryptophane, 1-5 parts of phenylalanine, 1-5 parts of valine, 1-5 parts of arginine, 1-5 parts of lysine, 1-5 parts of glutamic acid, 1-5 parts of threonine and 1-5 parts of sodium chloride. The pet feed is suitable for feeding pet dogs; under proper situations, the pet feed can also be used for feeding pet cats, guinea pigs and other pets. In the formula, the wheat gluten plays a role in increasing the flexibility and the plasticity of the feed, the fish powder, the bone powder and the potato powder have abundant nutrition and contain abundant amino acids, vitamins and other essential nutrition, and the sodium chloride can supplement the content of sodium ions in pet bodies. The feed is high in nutritional value and does not contain any additives; furthermore, the preparation is simple, the cost is low and the pet dog feed is suitable for purchase and use by ordinary families.
Owner:陈晓蕾
Method for extracting plastocyte of mouse
The invention relates to an extraction method of mouse platelet. Blood drawn from orbita vein of mouse is placed into an anticoagulation centrifugal tube, centrifugated at the speed of 800rmp for 10min after standing for 30min, and the extracted supernatant fluid is plasma rich in platelet. The supernatant fluid is placed in a clean test tube and centrifugated at the speed of 3500rpm for 10min. After filtering the supernatant fluid, the sediments at the bottom of the tube are the platelets. 50ul to 100ul of ammonium oxalate solution with a mass concentration of 1 percent is added into the test tube and slightly stirred with a glass rod, and then 2ml to 3ml of ammonium oxalate solution with a mass concentration of 1 percent is added, and stood for 5min, to make sure the hematid is dissolved; the hematid is centrifugated at the speed of 3500rpm for 10min, after the supernatant fluid is abandoned, the test tube is added with a few platelet scrub solution and blown and beaten to turn the platelet into a platelet suspension. The suspension is centrifugated at the speed of 3300rpm for 10min, removed the supernatant in the suspension, the test tube is added with a few platelet scrub solution and blown and beaten to turn the platelet into a platelet suspension. The platelet is used as an antigen to immunify guinea pigs, and the serum of such guines pigs is injected into the mouse to prepare allergic purpura. The preparation method has simple operation, can obtain high concentration platelet, and can comparatively better solve the problem of impure platelet.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Metabonomics analysis method base on acute anaphylactic reaction
ActiveCN103235073AOvercome subjectivityOvercoming sensitivityComponent separationHypersensitive responseMetabolome
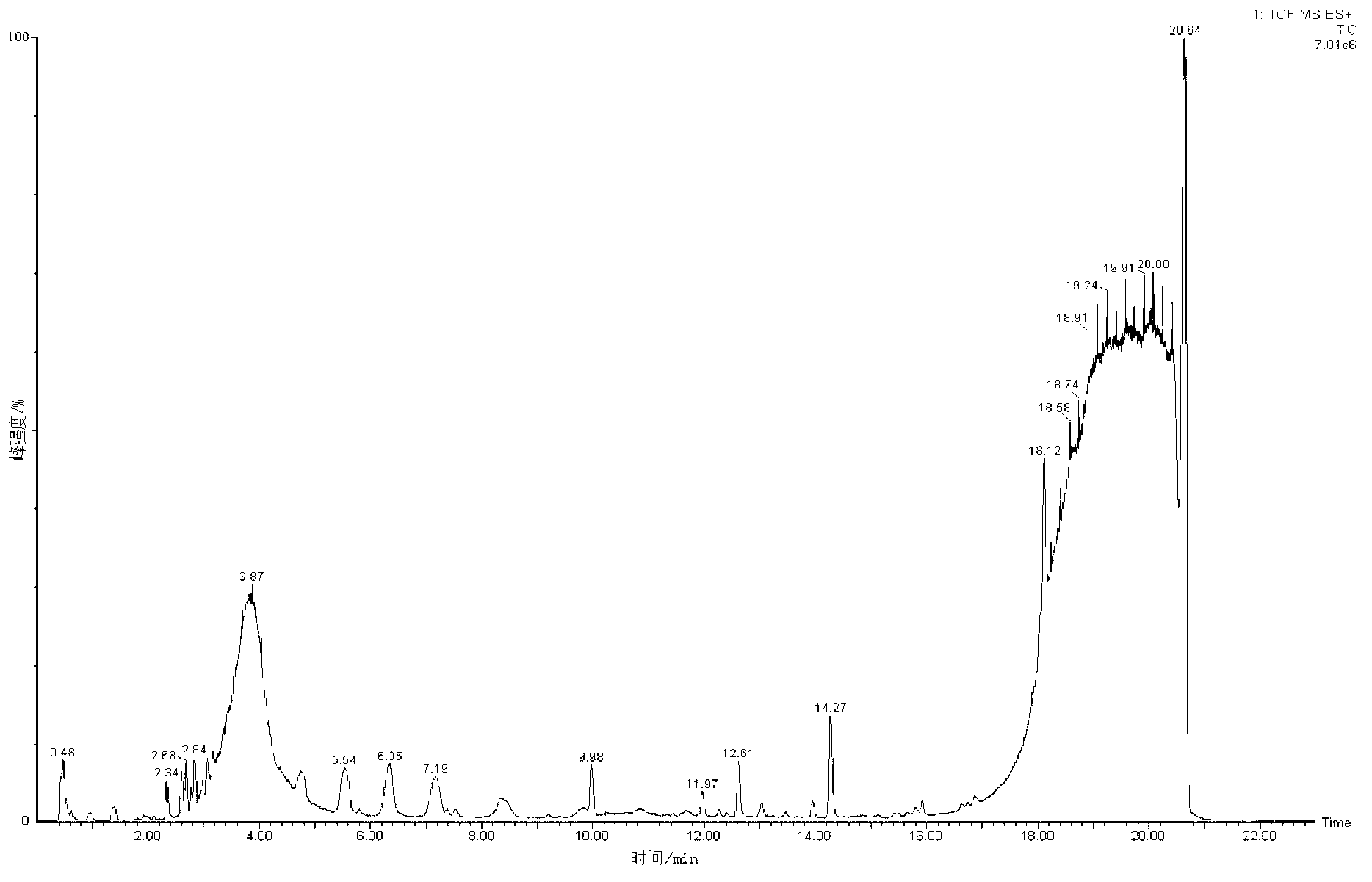
The invention discloses a metabonomics analysis method base on acute anaphylactic reaction. The invention mainly starts from the systems biology, and applies a UPLC-QTof / MS technology platform for UPLC and SPE-RRLC-Q-TOF combination, and establishes an acute anaphylactic reaction guinea pig serum metabonomics evaluation model, and provides a basis for further research of medicament acute anaphylactic reaction. During analysis, the UPLC and SPE-RRLC-Q-TOF combination technology is used for detecting serum samples of normal guinea pig and experiment guinea pig with acute anaphylactic reaction generated by induction, and a metabolome spectrogram is obtained by an acquisition method after optimization, and a mode identification method suitable for evaluating animal acute anaphylactic reaction is established for analyzing the metabolome data, which establishes a base for subsequently and furtherly screening biological tag with characteristics and developing a new animal acute anaphylactic reaction detection method.
Owner:HUNAN INST FOR FOOD & DRUG CONTROL
Double antibody sandwich ELISA kit used for Seneca viral antigen detection and application thereof
InactiveCN107741495AAvoid cross reactionShorten detection timeBiological material analysisElisa kitSenecavirus
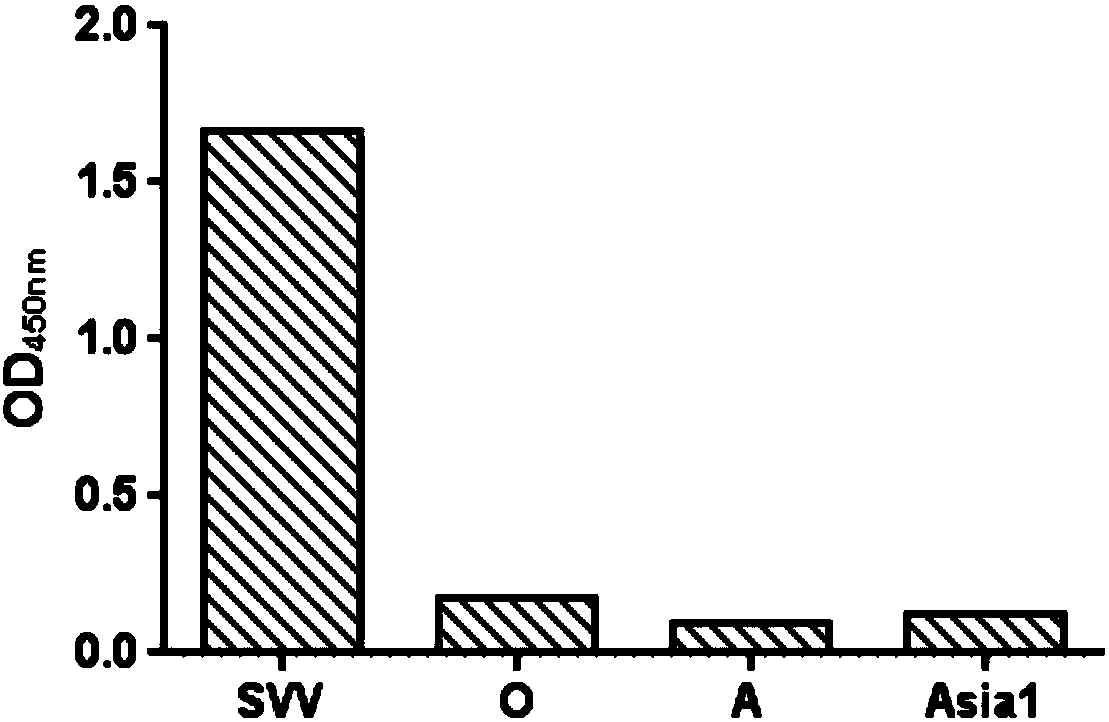
The invention discloses a double antibody sandwich ELISA kit used for Seneca viral antigen detection and application thereof. The kit comprises a Seneca viral guinea pig anti-IgG enveloped elisa plateand an HRP marked Seneca viral rabbit anti-IgG. The HRP marked rabbit anti-IgG is used for replacing primary antibodies and secondary antibodies in traditional ELISA, so that the operating steps aresimplified. Simultaneously, the SVV guinea pig anti-IgG is enveloped to the surface of a solid-phase carrier by changing an enveloping stabilization technology, the preparation technology of an enzyme-labeled antibody diluent is changed, an enzyme-labeled antibody working solution can be stored stably without changing the activity and valence, and a double antibody sandwich ELISA kit for SVV antigen specific detection and a detection method thereof are established. The vacancy of making up SVV ELISA antigen detection is proposed, the problem that existing virus separation and detection for SVVantigens are low in repeatability and sensitivity and complex in operational program is overcome, and effective technical means is provided for SVV antigen detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Application of maidenhair volatile oil in rhinitis resistance medicine
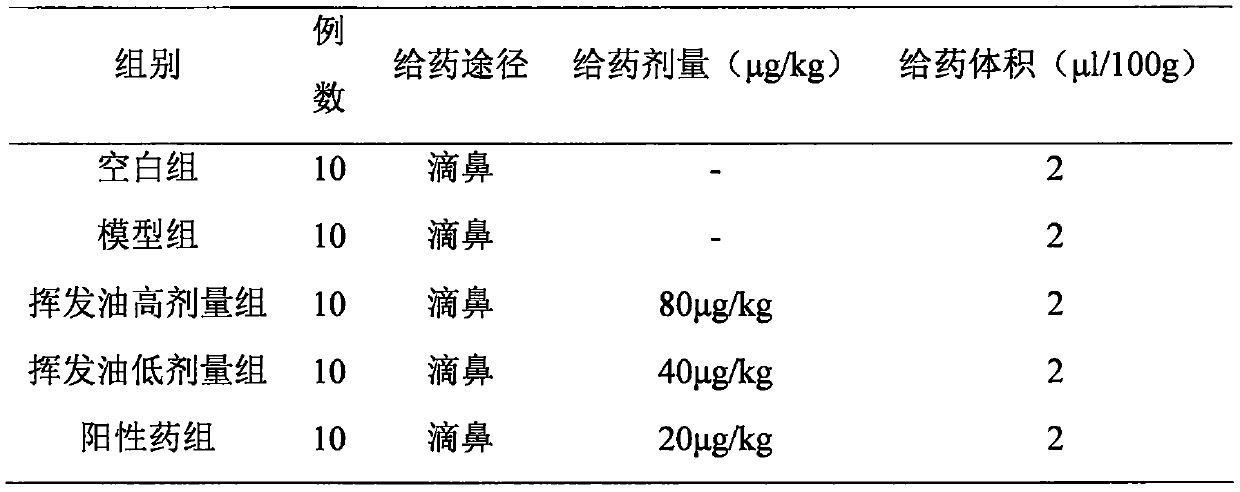
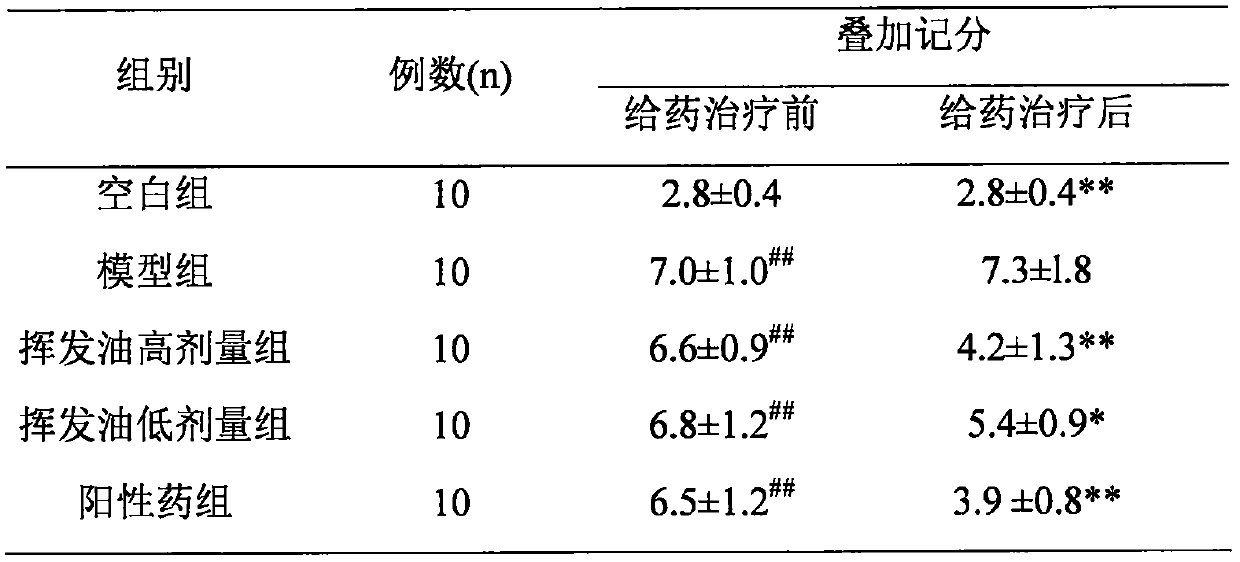
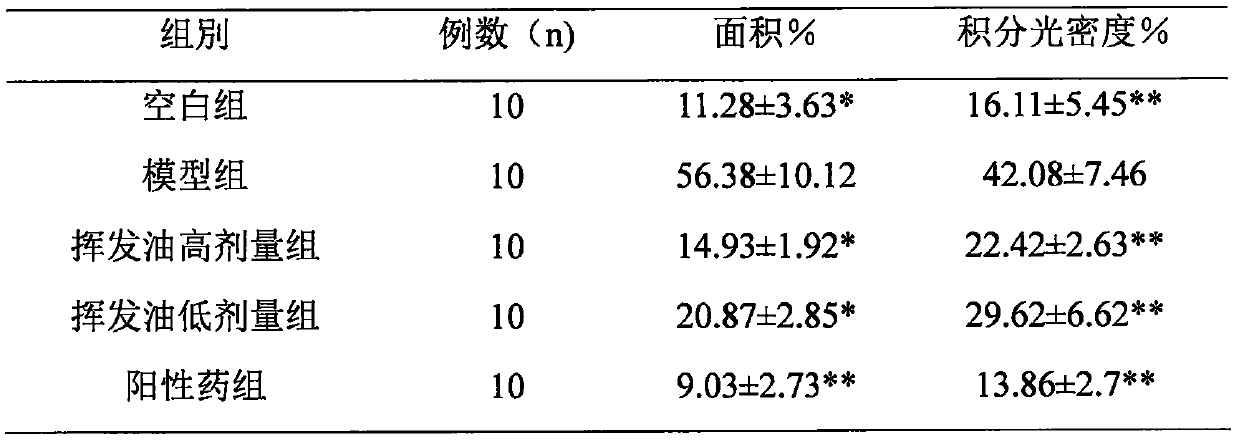
The invention relates to application of maidenhair volatile oil in a medicine for treating rhinitis. A method comprises the step of grinding a whole herb of maidenhair and then extracting the maidenhair by a steam distillation method or a carbon dioxide supercritical fluid extraction method to form the volatile oil. No toxic reagent is used by the two extraction methods, and the extracted volatile oil has the characteristics of no toxicity, no residue, simple extraction technology and high extraction efficiency. A medicinal auxiliary material can be added into the volatile oil to prepare a nasal drop or a spray as the medicine for treating the rhinitis. Pharmacological experiments show that the volatile oil has a better pharmacological action on an allergic rhinitis model of a guinea pig, can obviously alleviate symptoms of a nose of the allergic rhinitis model of the guinea pig, reduces an ethological accumulative integral, and significantly relieves edema of a nasal mucosa of the allergic rhinitis model of the guinea pig.
Owner:蔡德成 +2
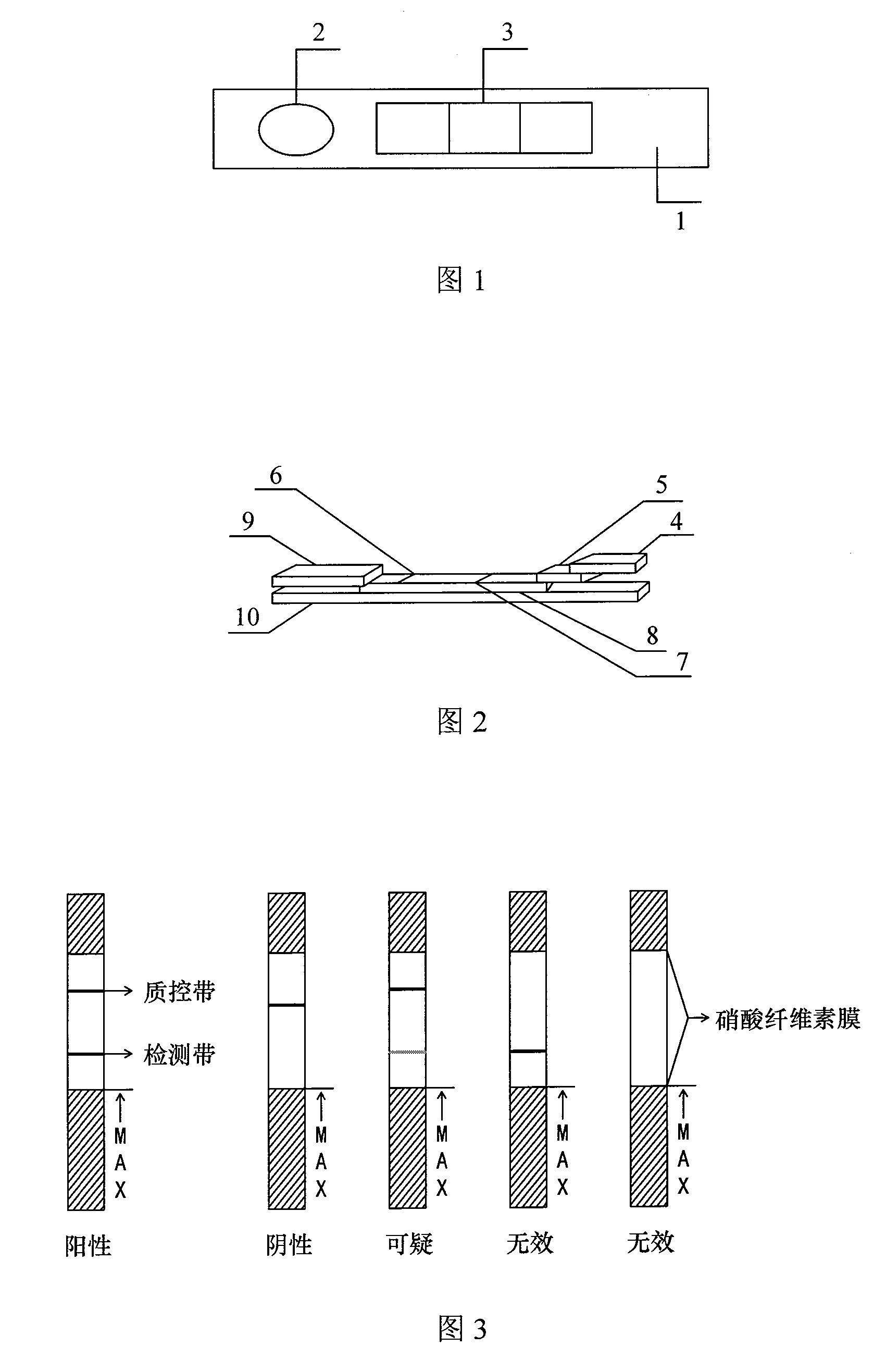
O -type foot-and-mouth disease antibody horizontal detection test paper and preparation method
ActiveCN101236204ADetermining Antibody TitersDetermine the protectiveBiological testingSpecific detectionMedicine
The invention relates to a colloidal gold test paper strip for carrying out the quick detection of the O-shaped foot and mouth disease antibody level of artiodactyls, the preparing method of the test paper strip and a specific detection method. A sample application absorption cushion made of porous fiber material, an immune gold label layer which is arranged on the tail end of the sample application absorption cushion and is connected with the sample application absorption cushion, a detection section made of nitrocellulose film, and an absorption section which is made of water absorption material and is arranged on the tail end of the detection section and is connected with the detection section are fixed on the base plate of the test paper strip. A quality control line is an O-shaped foot and mouth disease resistant rabbit or guineapig IgG antibody, a detection line is coated with O-shaped foot and mouth disease virus, and the O-shaped foot and mouth disease virus of the detection line of the detection section and the colloidal gold label attached to the immune gold label layer is natural virus, or DNA recombination expression foot and mouth disease virus, or the decomposed antigen of the foot and mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Aseptic cultivating technology for cavy and feed formula
InactiveCN1748493AComprehensive and balanced nutritionImprove palatabilityAnimal feeding stuffAccessory food factorsMicroorganismGerm free isolator
The present invention discloses the cultivating technology of aseptic cavy and the feed formula. The cultivating technology includes abdominal incision to take out cavy embryo, raising and propagating in aseptic isolating apparatus, and providing delicious feed comprising protein, carbohydrate, fat, vitamins and minerals and Co-60 ray irradiated. The cavy thus raised has high survival rate and no microbe and parasite infection, and makes it possible to obtain correct and reliable experiment result. The present invention may be used widely in various research and production fields.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Systems and methods for electrically grounding animals
Systems and methods are effective in electrically grounding animals that are kept indoors, such as pets (dogs, cats, hamsters, iguanas, fish, birds, etc.), lab caged animals used for clinical trials (guinea pigs, rats, etc.). The inventive apparatus and concepts are also applicable to fish and other creatures in aquariums and animals that are kept indoors at night or during harsh weather, including livestock (cattle, sheep, pigs, lamb, etc.) and horse stables.
Owner:MANKOVITZ ROY J
Varicella-zoster virus gB-gE-gH-gL fusion protein, genetic engineering subunit vaccine and preparation methods
ActiveCN105906721AImprove immunityStrong immune memoryAntibody mimetics/scaffoldsViral antigen ingredientsEscherichia coliAdjuvant
The invention relates to the technical field of biomedicine, and particularly provides varicella-zoster virus gB-gE-gH-gL fusion protein, a genetic engineering subunit vaccine and preparation methods. The fusion protein comprises a VZV gB extracellular region, a gE extracellular region, a gH truncated fragment and a gL truncated fragment. The amino acid sequence of encoded protein of the fusion protein is SEQ ID NO:1, and one amino acid sequence is SEQ ID NO:2. A prokaryotic expression vector is utilized for constructing escherichia coli BL21 (DE3) host bacteria capable of expressing the VZV gB-gE-gH-gL fusion protein. The fusion protein is purified and mixed with a medicinal adjuvant to be prepared into the genetic engineering subunit vaccine. Compared with a currently-used VZV attenuated live vaccine, the vaccine can induce immune mice to generate higher specific humoral immunity and cell-mediated immunity and can prevent dormant infection that VZV is spread to dorsal root ganglions and intestinal ganglions through blood flow, the safety of the VZV vaccine is effectively improved, and therefore the genetic engineering subunit vaccine is an alternative vaccine with potential clinical application value.
Owner:ANHUI MEDICAL UNIV
Chinese medicinal composition for treating irritable bowel syndrome and its preparation method
ActiveCN1654063AAnthropod material medical ingredientsDigestive systemIntestinal structureCyclodextrin
The Chinese medicine composition for treating intestinal irritable syndrome is prepared with gallnut, nutmeg, red ginseng, deglued antler powder, dark plum and myrobalan fruit as material and through the technological process including water extraction, extracting volatile oil, inclusion with cyclodextrin, grinding into fine powder and other steps. The pharmacological experiments show that the medicine of the present invention can inhibit the motion of small intestine, resist the sthenic motion of small intestine caused by Neostigmine, resist diarrhea caused by rhubarb, promote the absorption of water inside intestine caused by mannitol, etc. and has relatively high anti-diarrhea effect and high safety.
Owner:GUIZHOU LIANSHENG PHARMA
Cordyceps militaris sporocarp heteropolysaccharide and application thereof
ActiveCN105585639AReduce savingsAnti-atherosclerotic effectMetabolism disorderCardiovascular disorderArcus aortaeFreeze-drying
The invention discloses a preparation method and application of cordyceps militaris sporocarp heteropolysaccharide. The preparation method mainly comprises the following steps that cordyceps militaris sporocarp is subjected to drying, pulverization, ethanol degreasing and hot water ultrasonic extraction, and an extracting solution is subjected to concentration, alcohol precipitation, deproteinization, column chromatography, dialysis and freeze-drying, so that the cordyceps militaris sporocarp heteropolysaccharide TY258 is obtained. The cordyceps militaris polysaccharide has the efficacy of lowering lipid and resisting to atherosclerosis and can remarkably lower the cholesterol level, the triglyceride level and the low density lipoprotein level of high-fat induced atherosclerosis model mice (mice with apolipoprotein and low density lipoprotein receptors knocked out) and lipid accumulation of the livers and arcus aortae at the concentration of 25 mg / kg, lift the high density lipoprotein cholesterol level of the mice and improve activity of superoxide dismutase and lipoprotein esterolysis enzyme. The cordyceps militaris sporocarp polysaccharide can also remarkably lower the contents of plasma triglyceride, cholesterol, malonaldehyde and oxidized low-density lipoprotein of high-fat induced guinea pigs and rats.
Owner:TAISHAN MEDICAL UNIV
Method of preparing porcine parvovirus virus-like particle subunit vaccine by using Escherichia coli expression system and application of method
InactiveCN106148358AImprove solubilityGood specific immune responseBacteriaViral antigen ingredientsEscherichia coliEukaryotic plasmids
The invention discloses an encoding gene of porcine parvovirus VP2 protein, a method of prokaryotically expressing VP2 protein virus-like particles, and application of the method in vaccine preparation. Sequences are optimized, VP2 gene is artificially synthesized, the synthesized gene is inserted into pET28a vector, the gene and chaperone protein plasmids are co-transferred to BL21(DE3) host bacteria, the VP2 protein and chaperone protein are co-expressed to promote correct folding of the VP2 protein. Experiments prove that recombinant bacteria expressed VP2 protein can be self-assembled in vitro and has good immunogenicity; by immunizing mice and guinea pigs with the virus-like particle subunit vaccine prepared with the VP2 protein expressed herein, it is possible to induce the production of a high level of hemagglutination inhibition antibodies and neutralizing antibodies, and the vaccine can prevent guinea pigs from being affected by strong porcine parvovirus. The recombinant bacteria according to the invention can be utilized to efficiently prepare porcine parvovirus virus-like particles, the production cost is low, operation is simple, and biosafety is better.
Owner:HENAN ACAD OF AGRI SCI +1
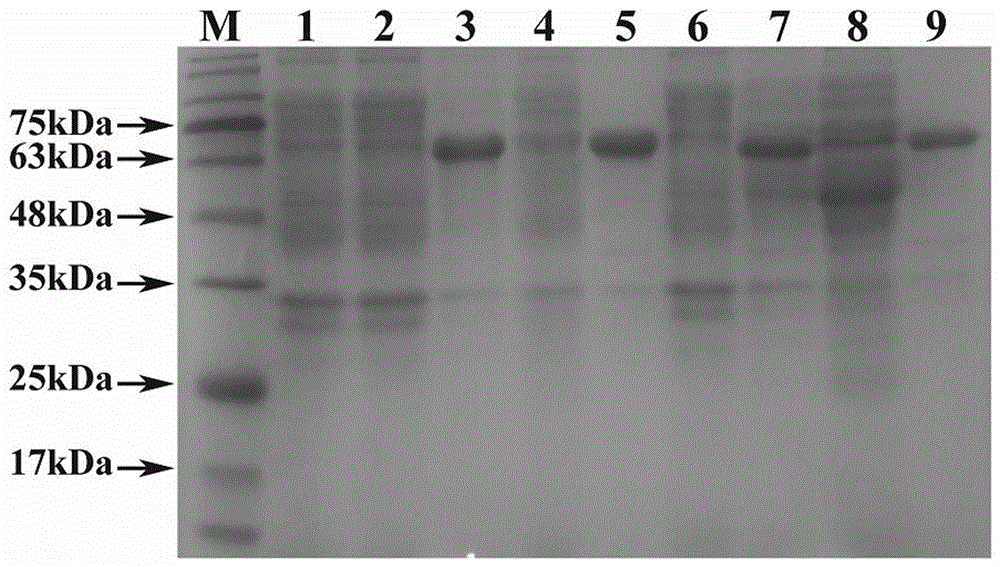
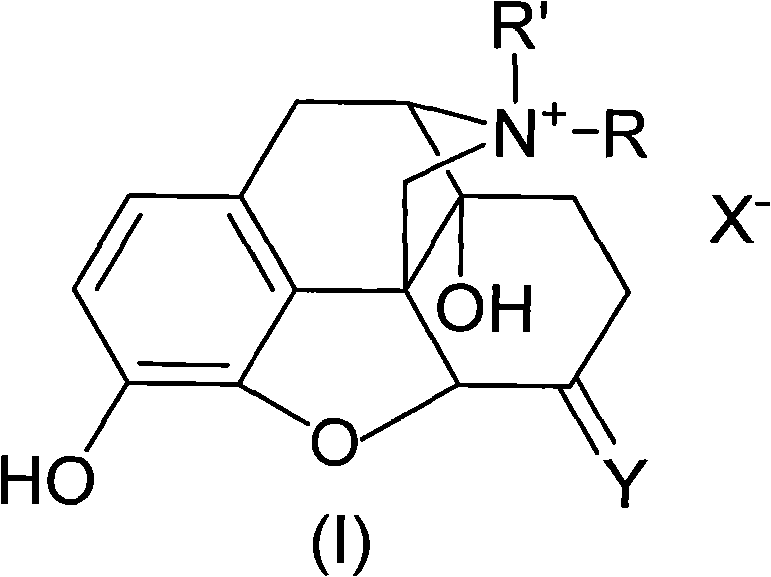
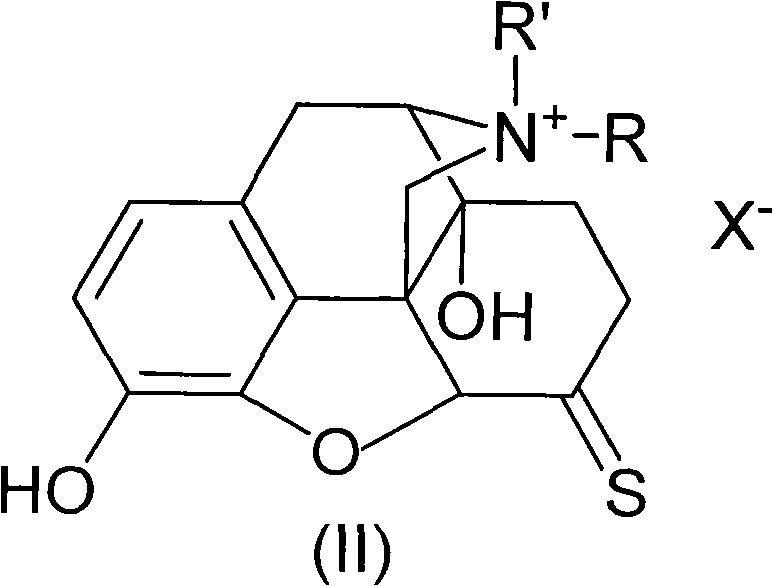
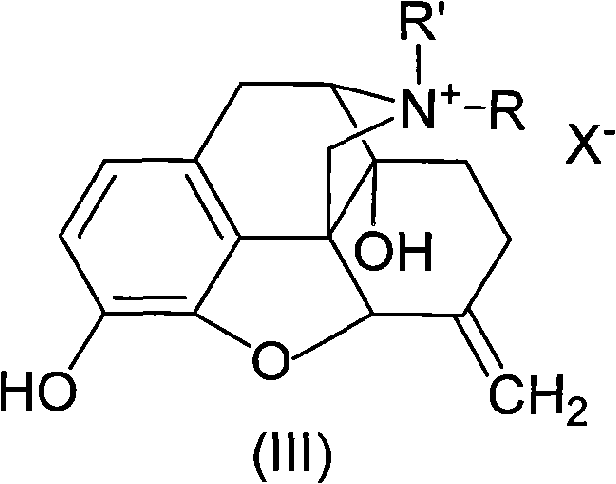
Morphinone quaternary ammonium salt derivatives and preparation method thereof
ActiveCN101570539AEasy to operateFew reaction stepsOrganic active ingredientsNervous disorderIntestinal ObstructionsIntestinal peristalsis
The invention provides morphinone quaternary ammonium salt derivatives which are prepared through the following steps: obtaining morphinone derivatives from morphine as the starting material through demethylation, oxidation, reduction, condensation and other chemical reactions; and obtaining the morphinone quaternary ammonium salt derivatives from the morphinone derivative through the reaction with halocarbon. The preparation method of the invention has the characteristics of few technological steps, low production cost, simple operation, stable quality and the like. The compounds provided by the invention have obvious contraction effect on the morphine-dependent guinea pig ilea, thereby promoting the intestinal peristalsis, the compounds are applicable to the preparation of drugs for curing constipation and intestinal obstruction; and the compounds provided by the invention further have obvious inhibiting effect on the conditioned place preference (CPP) of morphine-induced rats to the drug-paired compartment, therefore, the compounds can be used for preparing anti-relapsing drugs and effectively reducing the relapse rate, and the invention is favorable for the all-round promotion of rehabilitation and anti-relapsing work.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications
InactiveUS20090053261A1Many of characteristicSsRNA viruses positive-senseAntibody mimetics/scaffoldsVaccinationADAMTS Proteins
Display of peptides or proteins in an ordered, repetitive array, such as on the surface of a virus-like particle, is known to induce an enhanced immune response relative to vaccination with the “free” protein antigen. The 2100 coat proteins comprising the rod-shaped capsid of Tobacco mosaic virus (TMV) can accommodate short peptide insertions into the primary sequence, but the display of larger protein moieties on the virion surface by genetic fusions to the capsid protein has not been possible. Since TMV lacks surface exposed residues compatible with commonly available linker chemistries, we employed a randomized library approach to introduce a reactive lysine at the externally located at the amino-terminus of the coat protein. We found that we could easily control the extent of virion conjugation and demonstrated stoichiometric biotinylation of the introduced lysine. To characterize this modular platform for the display of heterologous proteins, we bound a model antigen (streptavidin (SA)-green fluorescent protein (GFP), expressed and purified from plants) to the surface of TMV, creating a GFP-SA decorated virus particle. Rapid and quantitative determination of the level of TMV capsid decoration was accomplished by subjecting the complex to amino acid analysis and solving the family of linear equations relating the pmoles of each residue to the known amino acid composition of the complex components. We obtained a GFP-SA tetramer loading of 26%, which corresponds to display of approximately 2200 GFP moieties per intact virion. We evaluated the immunogenicity of GFP decorated virions in both mice and guinea pigs, and found augmented humoral IgG titers in both species, relative to unbound GFP-SA tetramer. In mice, we observed a detectable humoral immune response after only a single immunization with the TMV-protein complex. By demonstrating the presentation of whole proteins, this study expands the utility of TMV as a vaccine scaffold beyond that which is possible by genetic manipulation.
Owner:KENTUCKY BIOPROCESSING
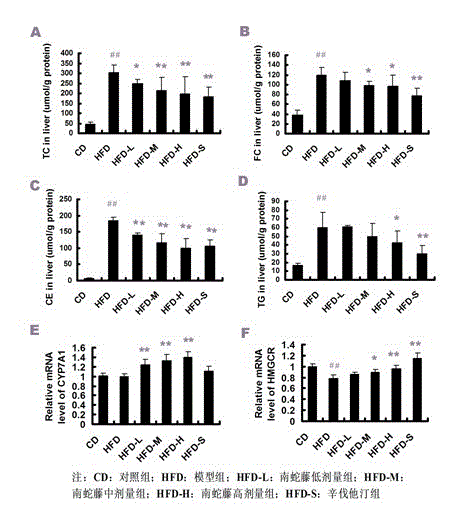
Application of celastrus orbiculatus alcohol extract in preparing medicine for treating non-alcoholic fatty liver disease (NAFLD)
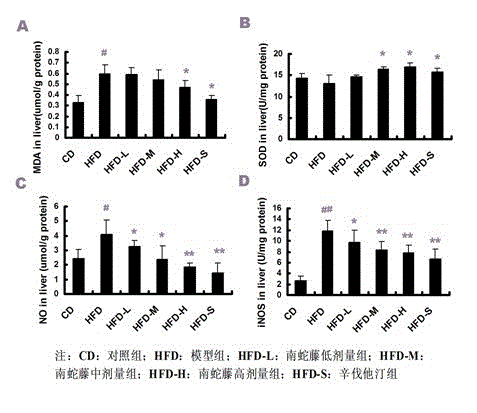
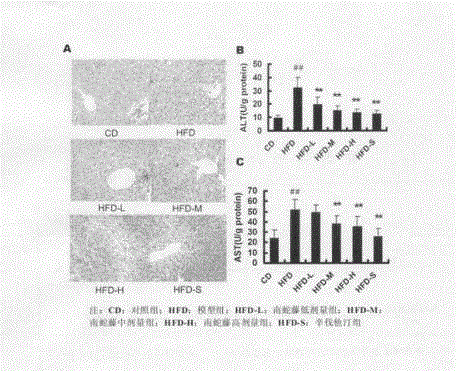
InactiveCN103142679AHas antioxidant activityReduce sensitivityDigestive systemPlant ingredientsCelastrus orbiculatusPositive control
The invention discloses an application of a celastrus orbiculatus alcohol extract in preparing a medicine for treating non-alcoholic fatty liver disease (NAFLD). By adopting a high-fat diet induced guinea pig NAFLD model and taking simvastatin as a positive control medicine, the celastrus orbiculatus alcohol extract is orally taken for 8 weeks, then influence of the celastrus orbiculatus alcohol extract on NAFLD is observed, and a corresponding mechanism is explored; and the result indicates that the celastrus orbiculatus alcohol extract can be used for obviously improving the high-fat induced pathological alteration of liver tissue, easing the damage of liver cells, reducing the lipid accumulation in liver and suppressing oxidative stress reaction in liver. According to the invention, a new application of the celastrus orbiculatus alcohol extract is developed, and a new means of treating NAFLD is provided.
Owner:TAISHAN MEDICAL UNIV
Novel porcine reproductive and respiratory syndrome virus variant GP5 recombinant protein and preparation method and application thereof
ActiveCN104974231ASolve the problem that it is difficult to express the complete GP5 proteinGuaranteed biological functionSsRNA viruses positive-sensePeptide/protein ingredientsComplete proteinRespiratory syndrome virus
The invention discloses a novel porcine reproductive and respiratory syndrome virus variant GP5 recombinant protein and a preparation method and application thereof. The novel porcine reproductive and respiratory syndrome virus variant GP5 recombinant protein is a soluble complete protein expressing PRRSV GP5 or soluble protein expressing PRRSV GP5 fused with TAT. A pCold 1F DNA is employed for efficient soluble expression of the PRRSV mutant GP5 protein and TAT-GP5 fusion protein, so as to ensure the biological function of the recombinant protein and solve the problem that the existing technology is difficult to express the full GP5 protein; and a guinea pig immunization inoculation test shows that TAT-GP5 fusion protein can significantly improve the level of humoral immunity and cellular immunity of the body.
Owner:QINGDAO AGRI UNIV
Bovini Asia 1/O type foot-and-mouth disease bivalent multi-epitope vaccine and preparation method and application thereof
ActiveCN103897065ARealize prevention and controlOvercome limitationsGenetic material ingredientsAntiviralsIgG.heavy chainImmunogenicity
The invention discloses a bovini Asia 1 / O type foot-and-mouth disease bivalent multi-epitope vaccine and a preparation method and an application thereof and belongs to the field of veterinary biological vaccines. The bovini Asia 1 / O type foot and mouth disease virus compound multi-epitope recombinant antigen is obtained by coupling dominant epitope of epidemic bovini O and Asia 1 type foot-and-mouth disease virus representative strains connected in series in China with a bovini IgG heavy chain constant region. Immune efficacy experiments verify that the bovini Asia 1 / O type foot-and-mouth disease bivalent multi-epitope vaccine prepared by the invention has good immunogenicity, and high level protective antibodies can be generated by the body induced by immune guinea pig or immune bovine. The inoculated vaccine is safe and harmless to immune animals. The vaccine is a novel vaccine which is abroad in prospect, supports matter storage and technical support to prevention and control of bovini Asia 1 / O type foot-and-mouth disease in China, and is of great importance.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
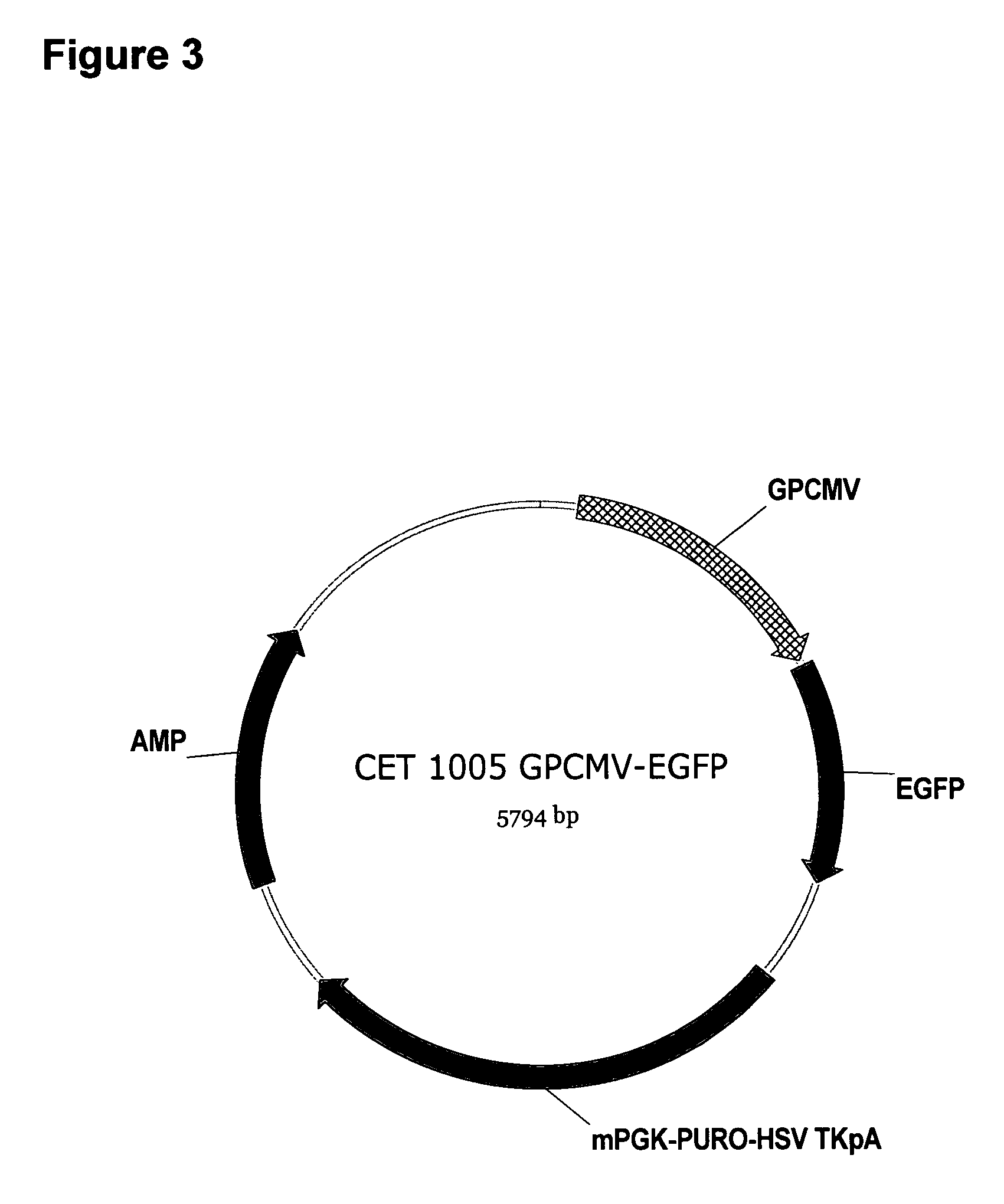
Vectors comprising guinea pig CMV regulatory elements
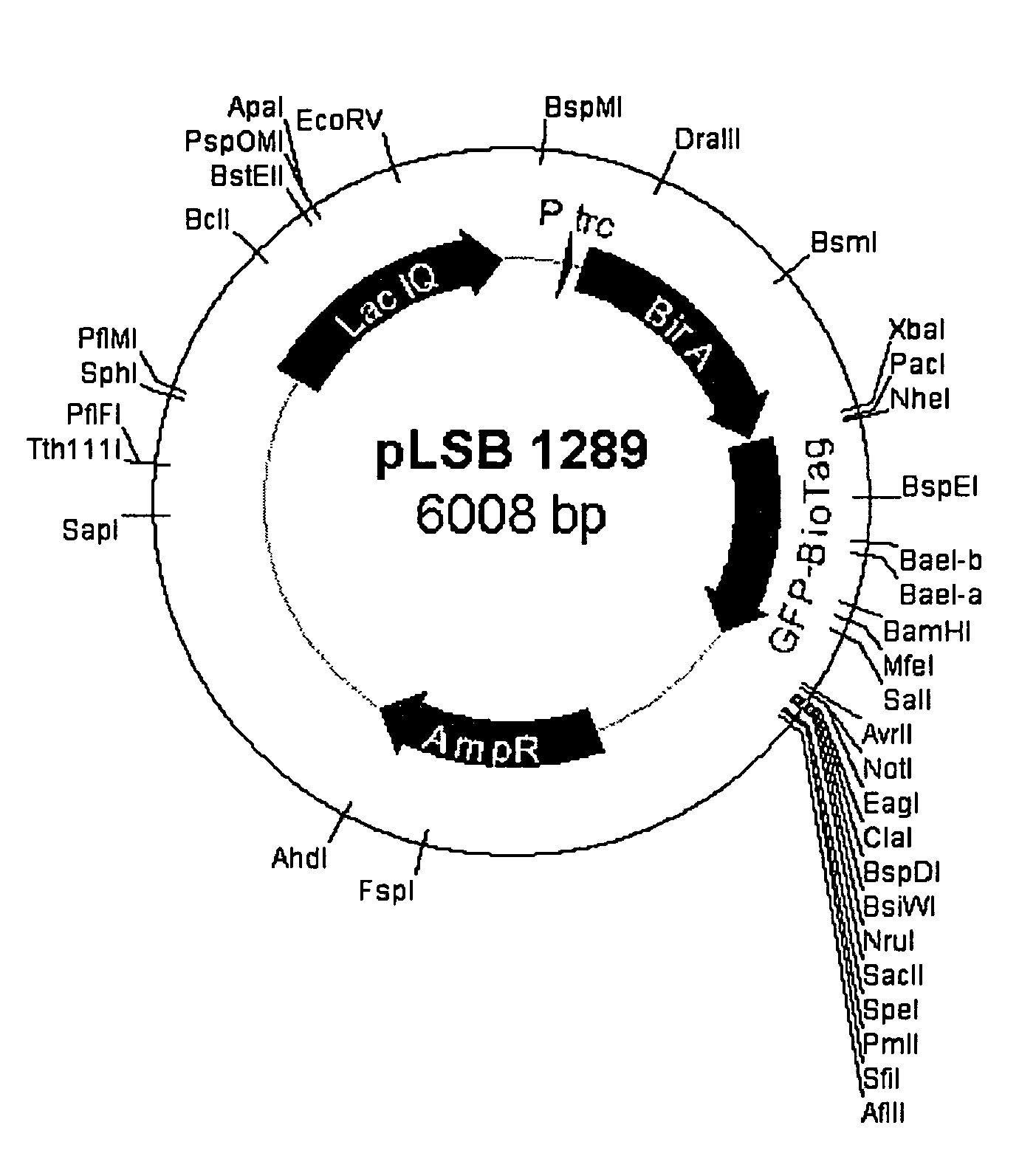
ActiveUS7501129B2High expressionIncrease transcriptionVirus peptidesImmunoglobulinsNucleic acid sequencingGenetic element
The invention comprises novel polynucleotides, and related vectors, host cells, methods, and compositions, containing transcriptional enhancers providing very high levels of expression of operably-linked expressible nucleic acid sequences in eukaryotic cells. Advantageously the enhancers may be used in combination with their naturally-associated promoters and / or other genetic elements that increase transcription. The invention comprises eukaryotic expression vectors that are capable of providing increased levels of expression in many cell types over that obtainable from human or murine CMV IE enhancer / promoter elements.
Owner:MILLIPORE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com