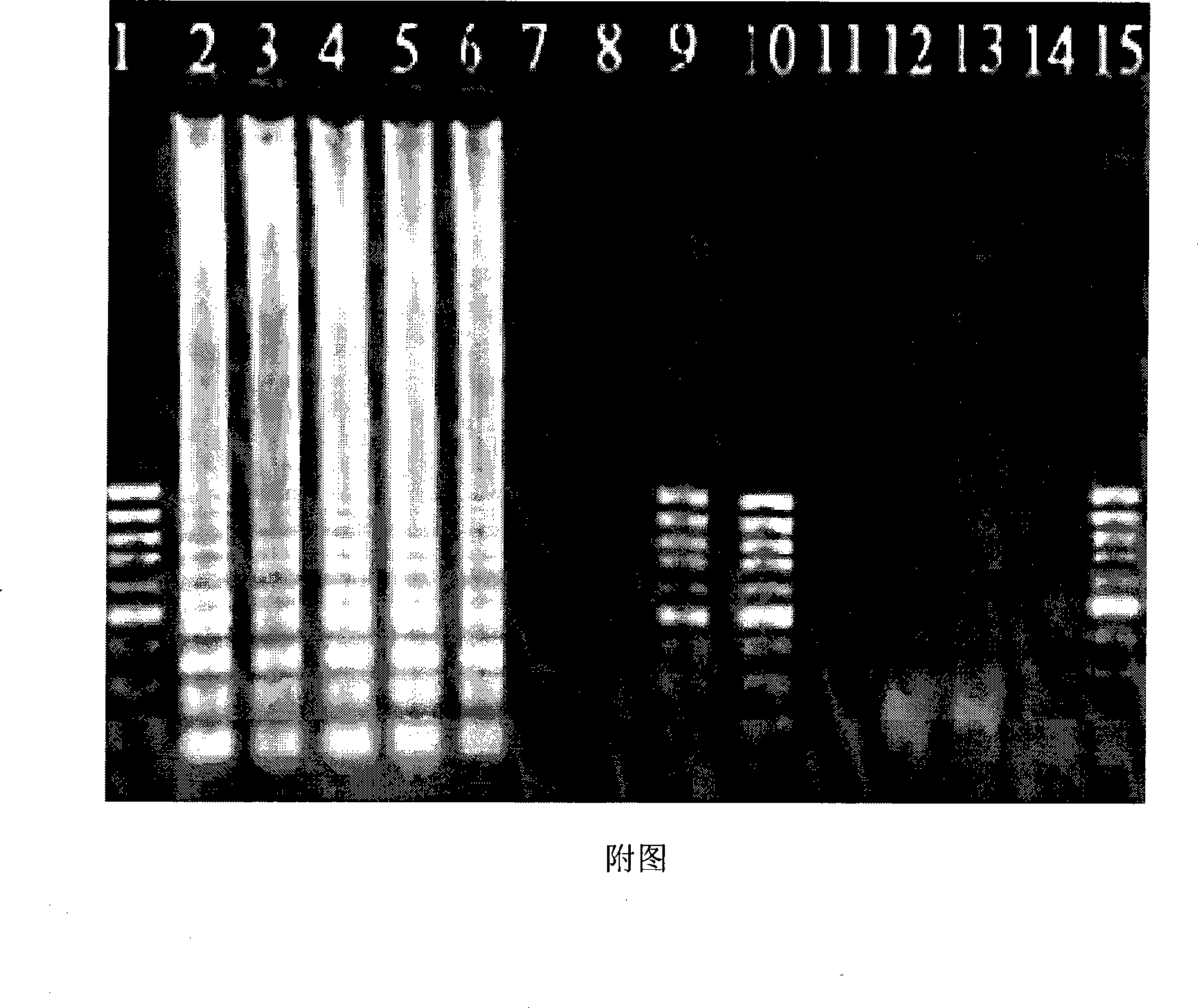
Kit for detecting guinea pig aeromonas by utilizing Loop-mediated isothermal amplification technique
A ring-mediated isothermal technology for Aeromonas caviae, applied in the field of pathogenic bacteria diagnosis, can solve the problems of lack of rapid and accurate detection methods for pathogenic bacteria, and achieve the effects of convenient operation, strong specificity, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Patient Sample 1
[0041] 1. DNA extraction
[0042] Pick suspected colonies and add them to 0.4mLTE buffer (pH8.0) for shaking and mixing. Add 10 μL of proteinase K solution and incubate at 55°C for 1-2 hours. Add the same volume of Tris-saturated phenol (pH 8.0), shake vigorously, centrifuge at 8000 rpm for 3 minutes, take the supernatant, and repeat the phenol extraction. Take the supernatant, add 0.1 times the volume of sodium acetate (3mol / L), mix well, add an equal volume of ice ethanol, mix well, let stand at low temperature for 30 minutes, centrifuge at 12000 rpm for 5 minutes, discard the supernatant, Wash once with 70% cold ethanol, centrifuge at 12,000 rpm for 5 minutes at room temperature, discard the supernatant, add 50 μL TE solution, and store at -20°C.
[0043] 2) Loop-mediated isothermal amplification (LAMP)
[0044] Add the following reagents to the PCR reagent tube to make a total volume of 25 μL:
[0045] Composition Final concentration...
Embodiment 2
[0070] Example 2: Patient Sample 2
[0071] 1. DNA extraction
[0072] Pick suspected colonies and add them to 0.4mLTE buffer (pH8.0) for shaking and mixing. Add 10 μL of proteinase K solution and incubate at 55°C for 1-2 hours. Add the same volume of Tris-saturated phenol (pH 8.0), shake vigorously, centrifuge at 8000 rpm for 3 minutes, take the supernatant, and repeat the phenol extraction. Take the supernatant, add 0.1 times the volume of sodium acetate (3mol / L), mix well, add an equal volume of ice ethanol, mix well, let stand at low temperature for 30 minutes, centrifuge at 12000 rpm for 5 minutes, discard the supernatant, Wash once with 70% cold ethanol, centrifuge at 12,000 rpm for 5 minutes at room temperature, discard the supernatant, add 50 μL LTE solution, and store at -20°C.
[0073] 2) Loop-mediated isothermal amplification (LAMP)
[0074] Add the following reagents to the PCR reagent tube to make a total volume of 25 μL:
[0075] 10× Reaction Buffer (2.5 μL)...
Embodiment 3
[0088] Example 3: Patient Sample 3
[0089] 1. DNA extraction
[0090] Pick suspected colonies and add them to 0.4mLTE buffer (pH8.0) for shaking and mixing. Add 10 μL of proteinase K solution and incubate at 55°C for 1-2 hours. Add the same volume of Tris-saturated phenol (pH 8.0), shake vigorously, centrifuge at 8000 rpm for 3 minutes, take the supernatant, and repeat the phenol extraction. Take the supernatant, add 0.1 times the volume of sodium acetate (3mol / L), mix well, add an equal volume of ice ethanol, mix well, let stand at low temperature for 30 minutes, centrifuge at 12000 rpm for 5 minutes, discard the supernatant, Wash once with 70% cold ethanol, centrifuge at 12,000 rpm for 5 minutes at room temperature, discard the supernatant, add 50 μL LTE solution, and store at -20°C.
[0091] 2) Loop-mediated isothermal amplification (LAMP)
[0092] Add the following reagents to the PCR reagent tube to make a total volume of 25 μL:
[0093] 10× Reaction Buffer (2.5 μL)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com