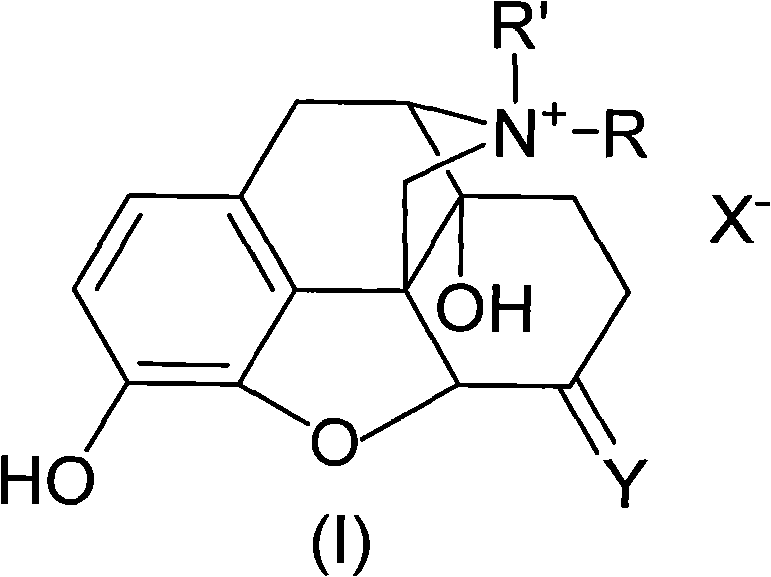
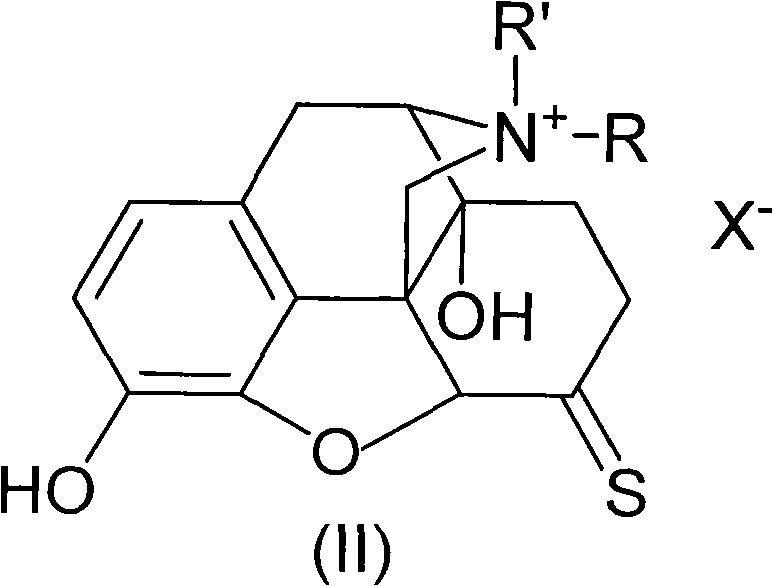
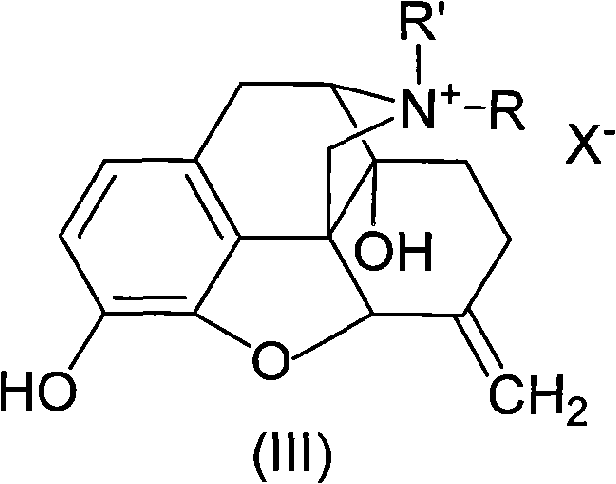
Morphinone quaternary ammonium salt derivatives and preparation method thereof
The technology of a derivative and morphone, which is applied in the field of quaternary ammonium salt derivatives of morphone and the preparation thereof, can solve the problems of cumbersome process route, high price, limited sources and the like, and achieves a technology with few process steps, low production cost and stable quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of 3,1-bis(2,2,2-trichloroethyl carboxylate)normorphine (compound b1)
[0034]
[0035] At room temperature, morphine (28.53g, 0.1mol), trichloroethyl chloroformate (42.37g, 0.2mol) and a certain amount of pyridine were added to the reaction flask, and the mixture was reacted and stirred overnight. After the reaction was complete, water was added to precipitate out. After washing and drying, 3,17-bis(2,2,2-trichloroethyl formate) normorphine was obtained.
[0036] The compound H NMR spectrum (D6-DMSO+D2O): δ1.88, 1.63 (CH 2 , m, 2H), 2.88 (CH, m, H), 3.19, 2.94 (CH 2 , m, 2H), 3.39, 3.29 (CH 2 , m, 2H), 3.73 (CH, m, H), 4.19 (CH, s, H), 4.30 (CH, s, H), 4.84 (CH 2 , s, 2H), 4.92 (CH 2 , s, 2H), 5.59 (2CH, m, 2H), 6.54 (CH, d, H), 6.63 (CH, d, H) ppm.
Embodiment 2
[0038] Synthesis of 3,17-bis(2,2,2-trichloroethyl formate) normorphone (compound c1)
[0039]
[0040] 3,17-bis(2,2,2-trichloroethyl formate) normorphine (14.3 g, 0.023 mol) was dissolved in 60 ml of trichlorethylene, and 28 ml of water was added. The pH was adjusted to 5 with sulfuric acid, the mixture was heated to reflux, and then Jones reagent (7.5g sodium dichromate dihydrate dissolved in 22ml water and 6ml sulfuric acid) was slowly added for 1 hour, and the oxidation reaction was carried out under reflux for 1.5 hours, Excess oxidizing agent was destroyed by adding 6 ml of 2-propanol. The layers were separated, and the organic layer was washed with 10% aqueous sodium bicarbonate solution and water, and dried over anhydrous sodium sulfate. Concentrate to dryness, and dissolve the residue in ethanol to obtain 3,17-bis(2,2,2-trichloroethyl formate)normorphone.
[0041] The compound H NMR spectrum (D6-DMSO+D2O): δ1.88, 1.63 (CH 2, m, 2H), 2.88 (CH, m, H), 3.19, 2.94 (C...
Embodiment 3
[0043] Synthesis of 14-hydroxy-3,17-bis(2,2,2-trichloroethyl formate)normorphone (compound d1)
[0044]
[0045] Dissolve 3,17-bis(2,2,2-trichloroethyl formate) normorphone (13.02g, 0.021mol) in 135ml of ethanol, heat to 60°C, add 2.6g (0.0147 mol) cobalt acetate and 0.5g (0.006mol) sodium acetate and pass into air, TLC traces until the reaction is complete, and the reaction solution is treated with activated carbon (0.3g) and filtered. Concentrate to volume (8.91 g 14-hydroxy-3,17-bis(2,2,2-trichloroethyl carboxylate)normorphone in 50 ml ethanol) and proceed directly to the next step.
[0046] The compound H NMR spectrum (D6-DMSO+D2O): δ1.88, 1.63 (CH 2 , m, 2H), 3.19, 2.94 (CH 2 , m, 2H), 3.39, 3.29 (CH 2 , m, 2H), 3.81(CH, t, H), 4.84(CH 2 , s, 2H), 4.92(CH, s, H), 4.92(CH 2 , s, 2H), 6.34 (CH, d, H), 6.54 (CH, d, H), 6.63 (CH, d, H), 7.01 (CH, d, H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com