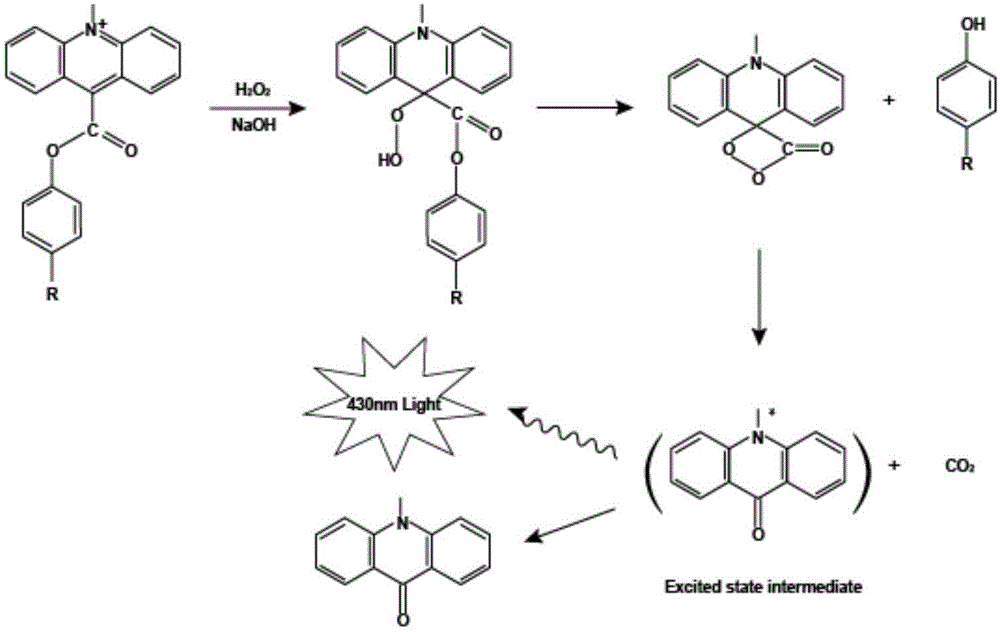
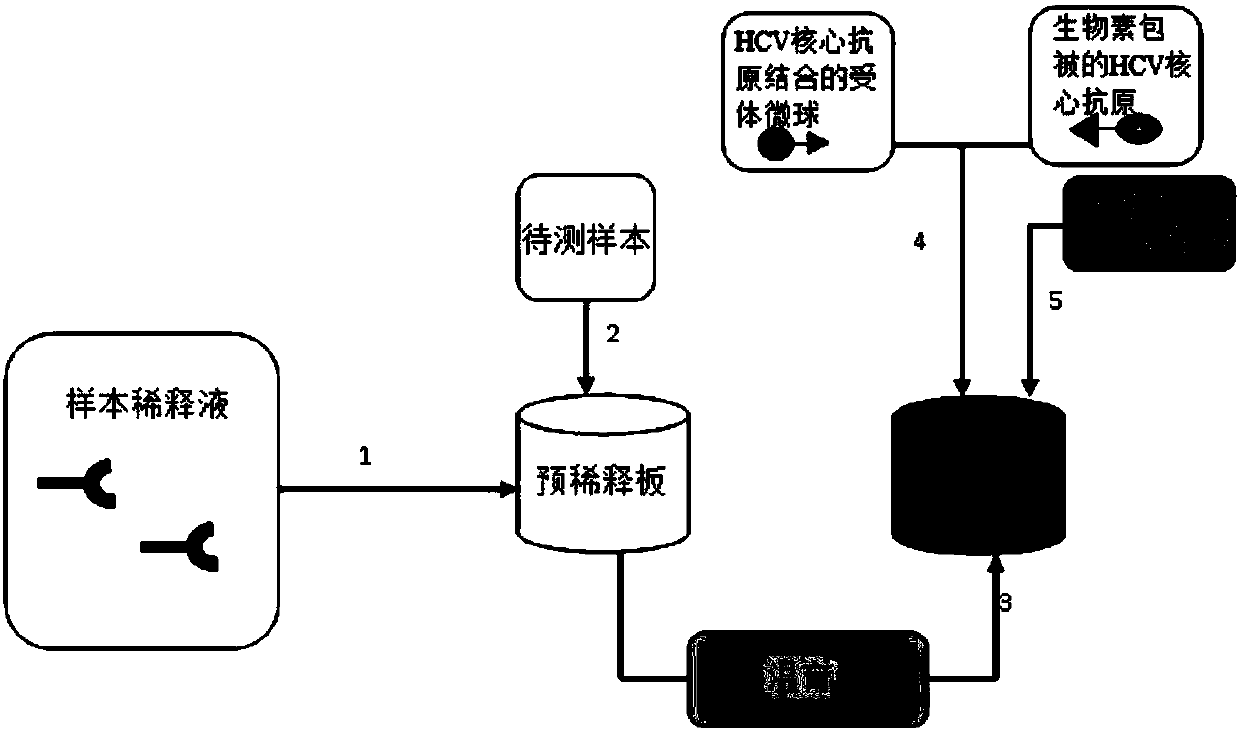
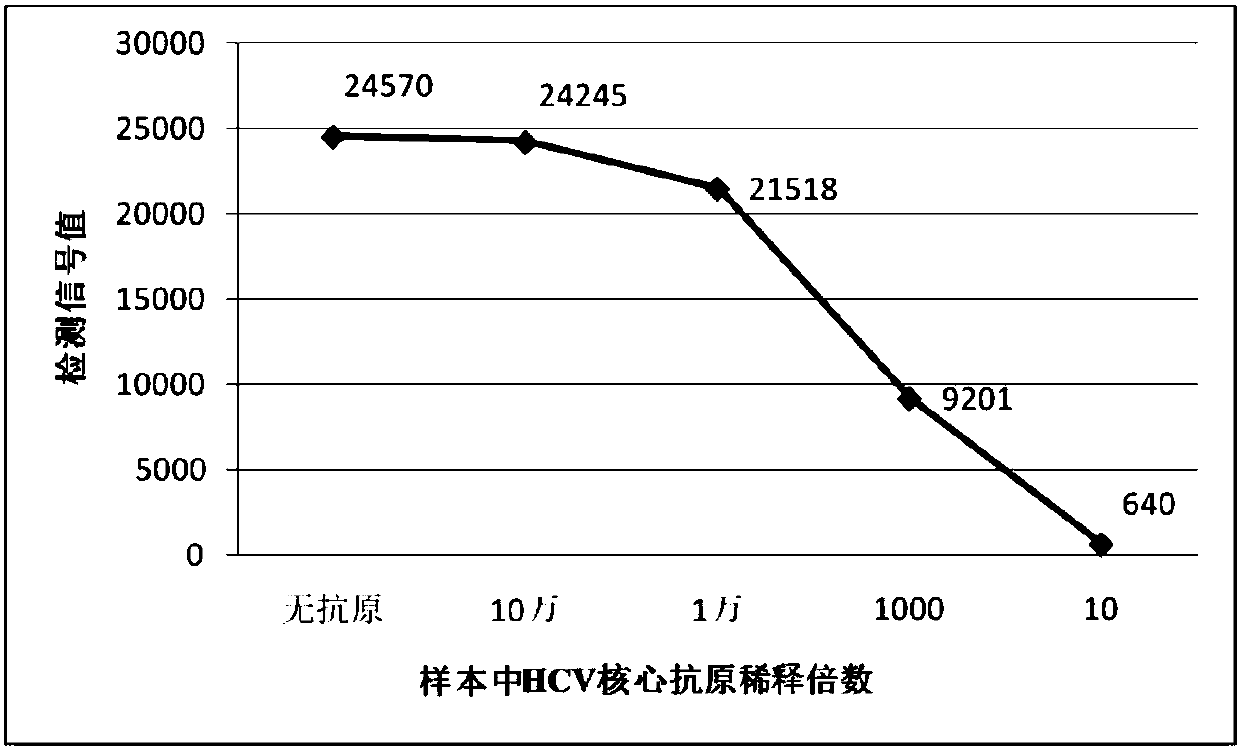
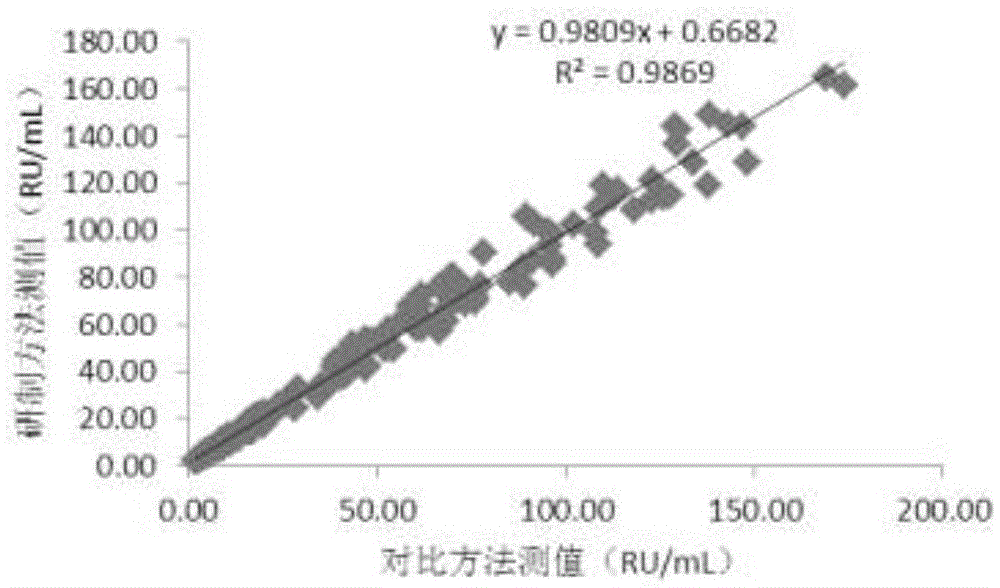
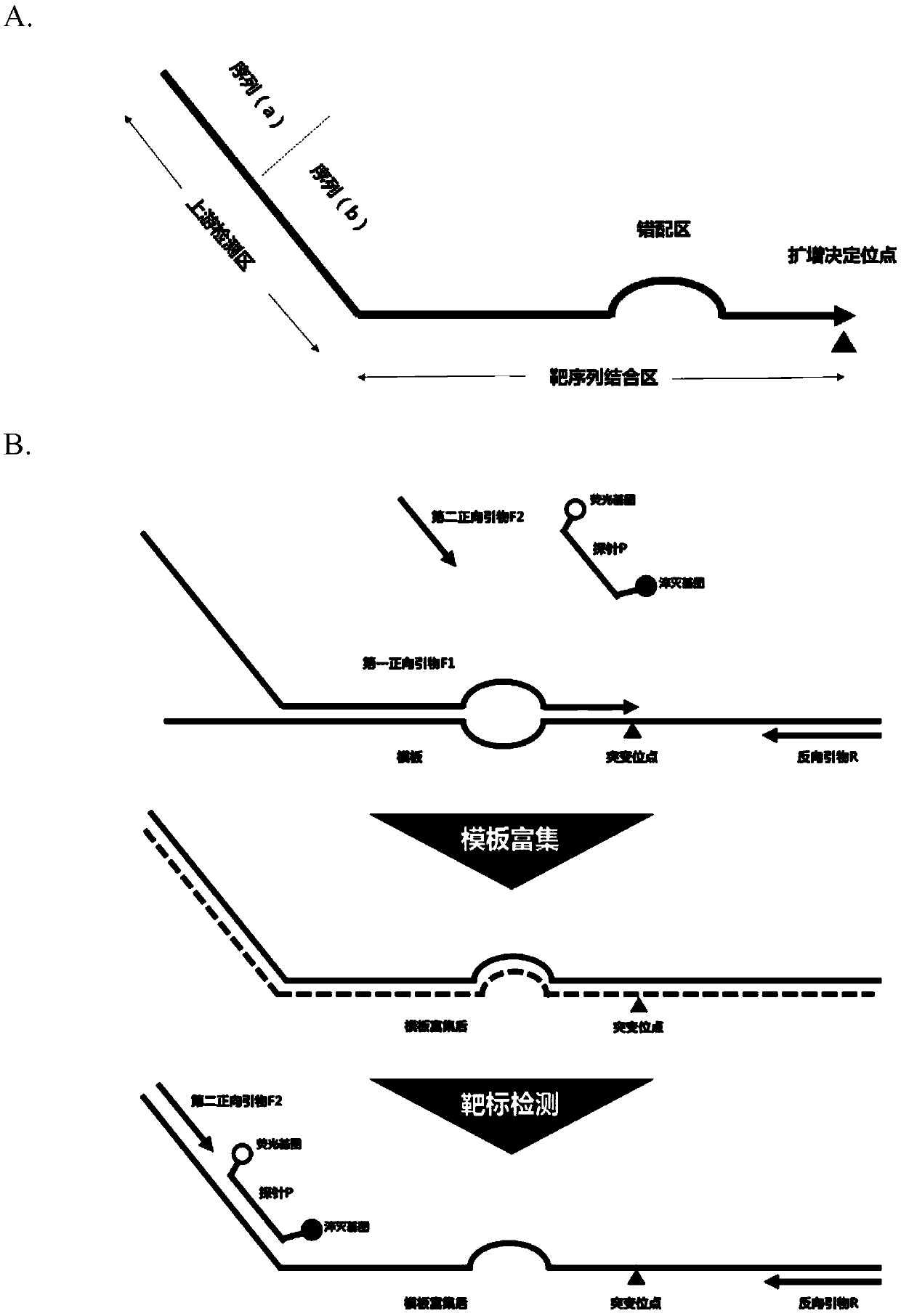
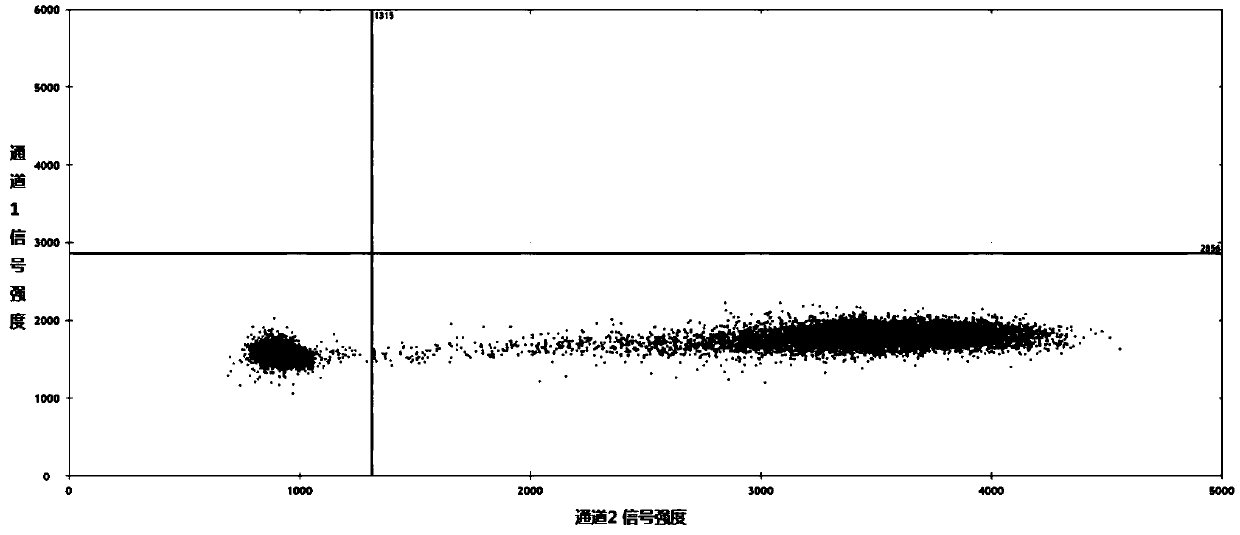
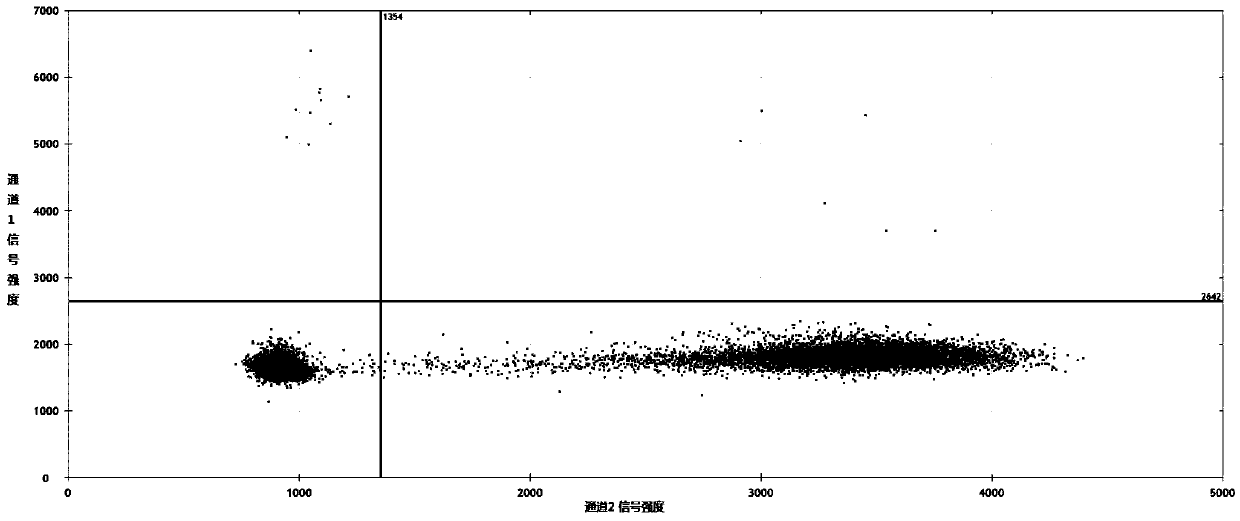
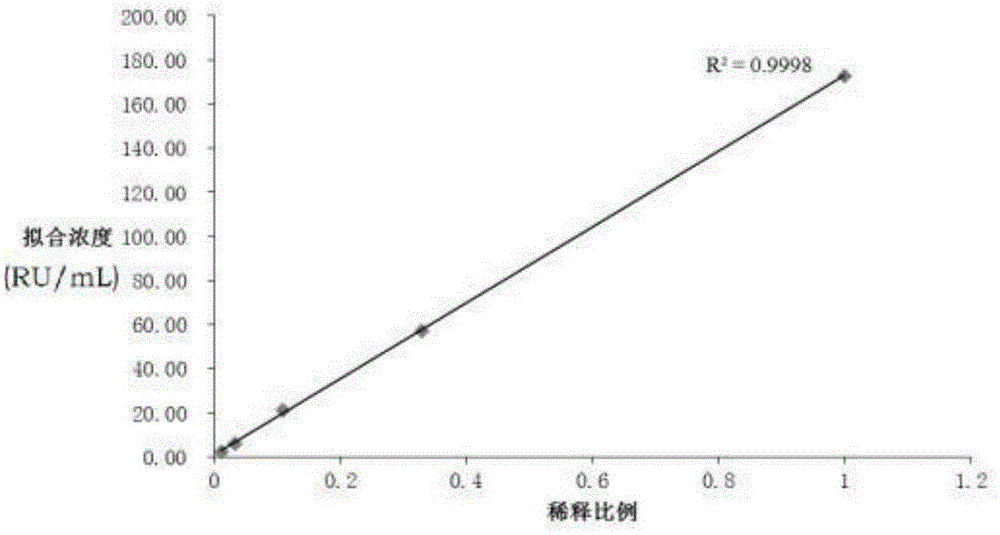
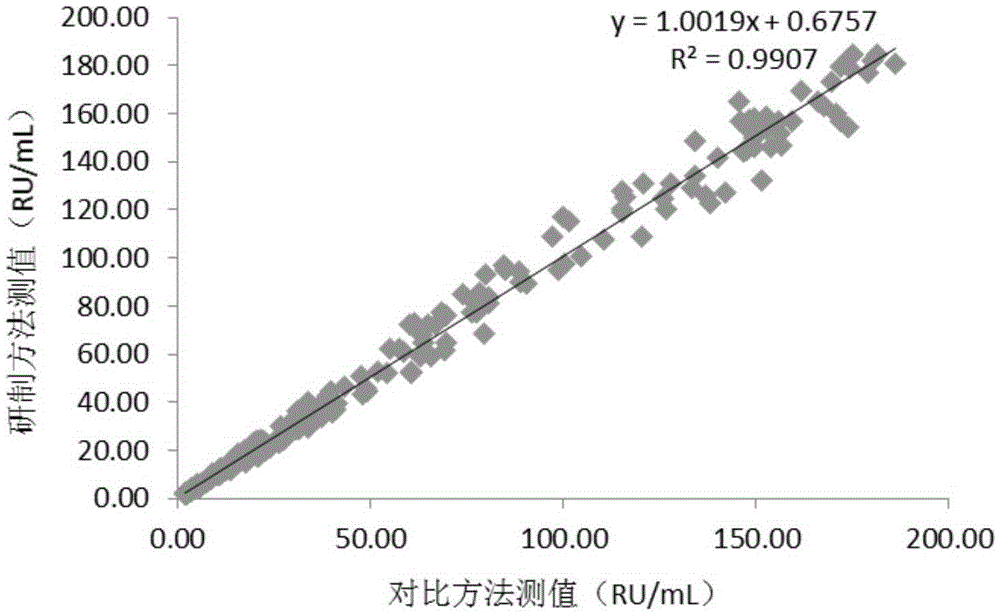
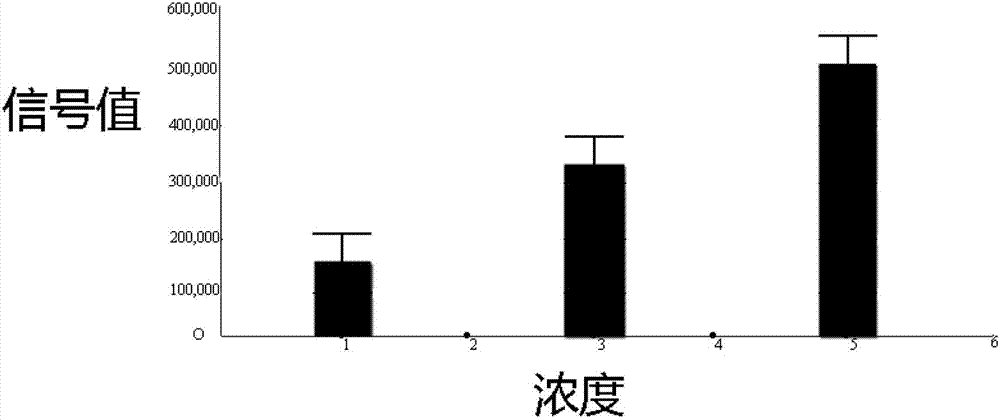
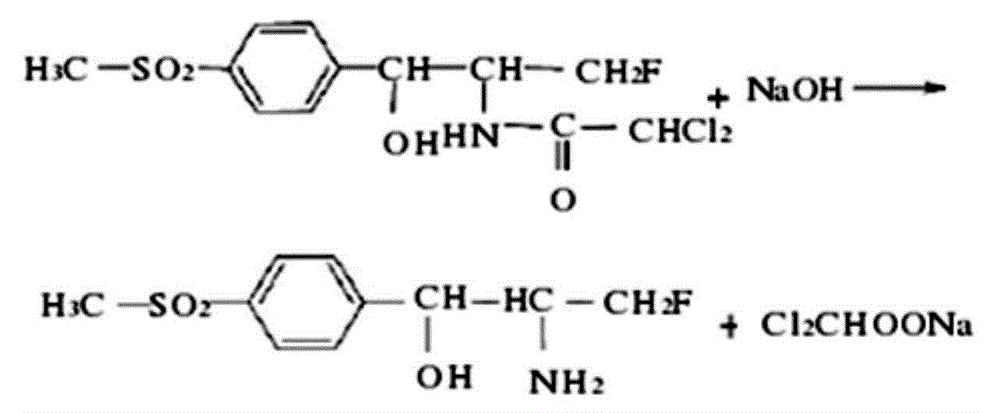
Patents
Literature
315results about How to "Avoid cross reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
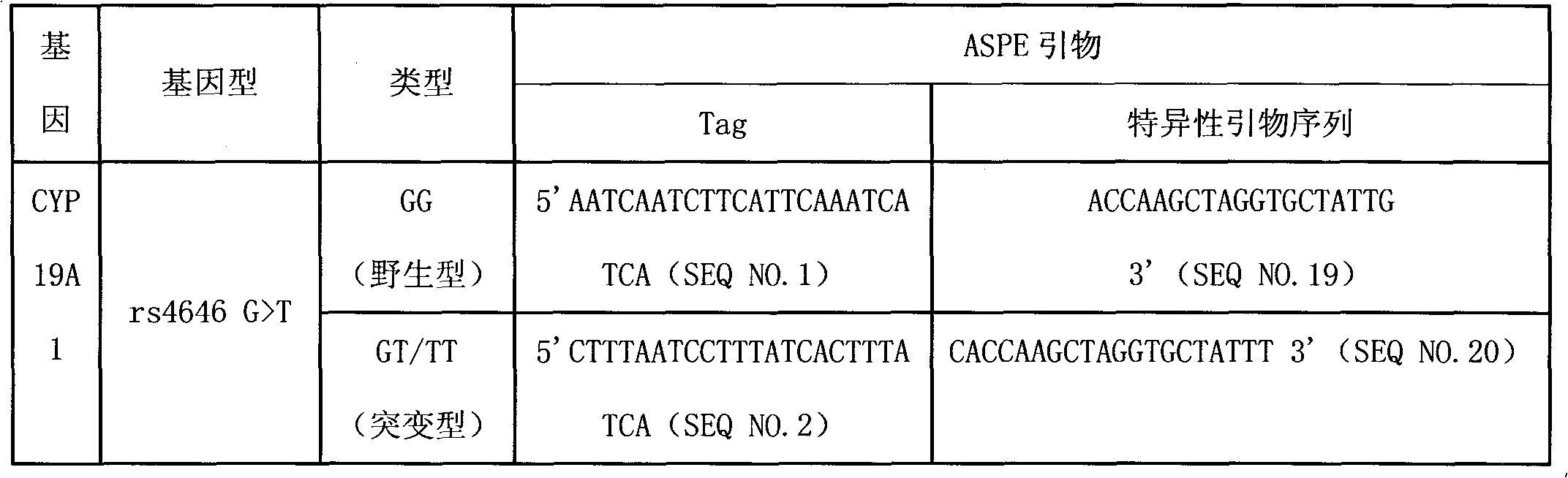
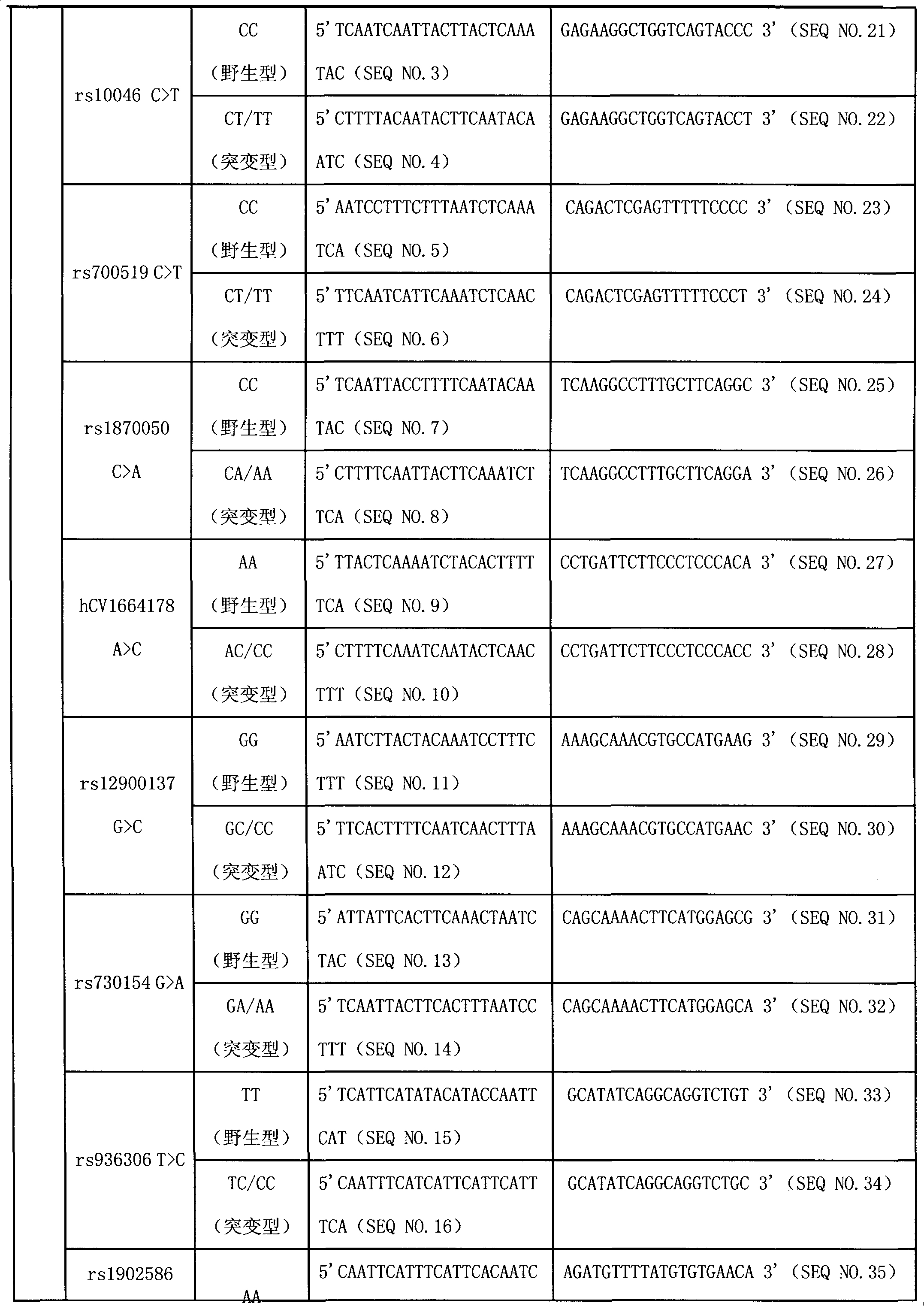
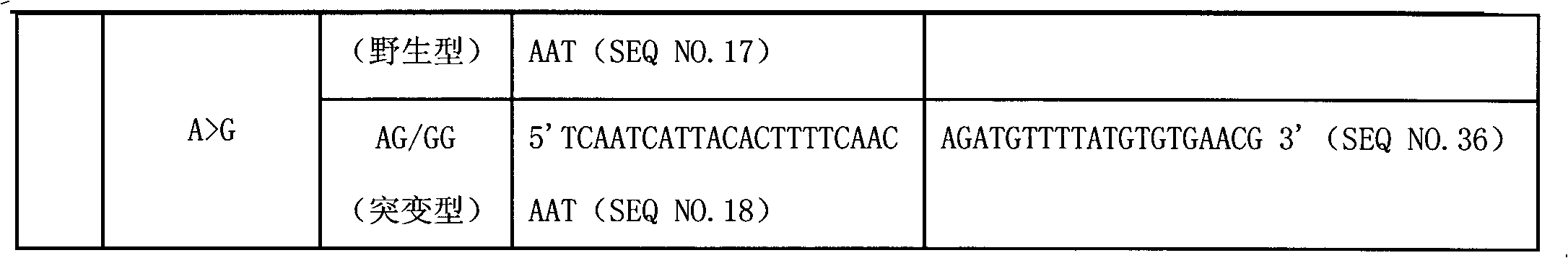
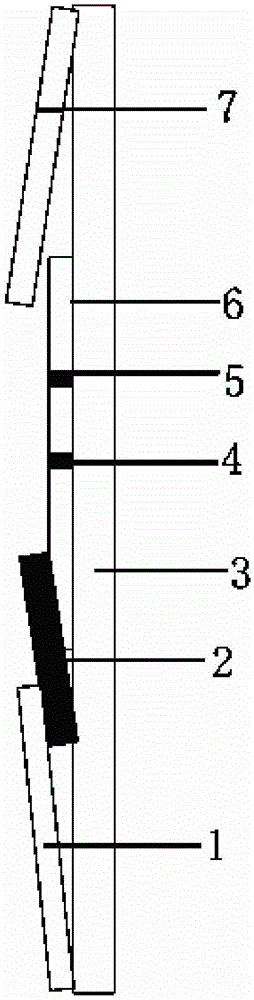
Liquid phase chip for CYP19A1 gene SNP (Single Nucleotide Polymorphism) detection and detection method thereof
ActiveCN101781684AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementType specificWild type
The invention discloses a liquid phase chip for CYP19A1 gene SNP (Single Nucleotide Polymorphism) detection, which comprises wild type and mutant type specific ASPE primers designed targeting CYP19A1 gene SNPs of rs4646, rs10046C>T, rs700519C>T, rs1870050C>A, hCV1664178A>C, rs12900137G>C, rs730154G>A, rs936306T>C and rs1902586A>G, microballoon spheres respectively coated with specific anti-tag sequences and primers used for amplifying CYP19A1 gene SNPs with target sequences to be detected. Each ASPE primer comprises a tag sequence at 5' end and a specific primer sequence at 3' end, wherein the specific primers are selected from SEQ ID NO. 19-36 and the tag sequences are selected from SEQ ID NO.1-18; and the anti-tag sequences can be correspondingly in complementary pairing with the tag sequences. The invention also discloses a CYP19A1 gene SNP detection method. The coincidence rate of the detection method provided by the invention and a sequencing method is as high as 100%; and the prepared liquid phase chip for CYP19A1 gene SNP (Single Nucleotide Polymorphism) detection has excellent signal-to-noise rate, and basically no cross reaction exists between a design probe and the anti-tag sequences.
Owner:SUREXAM BIO TECH
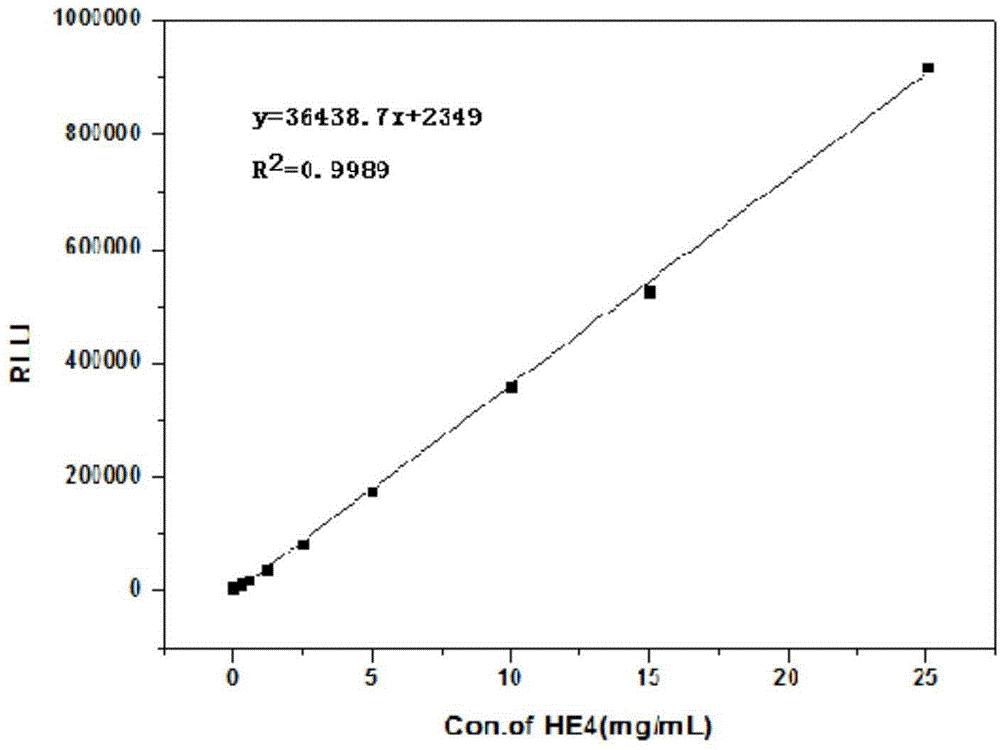
Goldmag particle-based acridinium ester chemiluminescence immunological detection method of HE4
ActiveCN104897901AGood response specificityAvoid cross reactionChemiluminescene/bioluminescenceBiological testingAntigenSolid phases
The invention provides a goldmag particle-based acridinium ester chemiluminescence immunological detection method of human epididymis secretory protein (HE4). The goldmag particle-based acridinium ester chemiluminescence immunological detection method mainly comprises following steps: (1) goldmag particle is taken as an immunoreaction and solid-phase separation carrier, and HE4 coated antibody is connected with the surface of the goldmag particle via coupling; (2) blank sites on the surface of the goldmag particle, which are not combined with the HE4 coated antibody, are blocked with a blocking solution; (3) acridinium ester (AE) is used for marking HE4 labelled antibody; (4) a sample to be detected, and the acridinium ester marked HE4 antibody are added into the blocked HE4 antibody coated goldmag particle for reaction so as to obtain a double-antibody sandwich compound, wherein the acridinium ester marked HE4 antibody is capable of realizing specific binding with HE4 antigen; (5) washing is carried out; and (6) chemiluminescence detection is carried out. The goldmag particle-based acridinium ester chemiluminescence immunological detection method is high in detection sensitivity, specificity, accuracy, and stability, and is simple and rapid; linearity range is wide; no radioactive contamination is caused; and operation is safe.
Owner:XIAN GOLDMAG NANOBIOTECH
Double antibody latex enhanced retinol binding protein detection kit
The invention relates to a double antibody latex enhanced retinol binding protein detection kit. More specifically, the invention discloses a double antibody coating latex enhanced immunoturbidimetry kit for detecting retinol binding protein. The kit contains a reagent 1, a reagent 2 and a calibrator. Paring monoclonal antibody A and B are respectively coated on latex particles. Coated antibody is bonded with RBP to be detected, and a plurality of bonders are aggregated together to form detectable turbidity change. The detection kit provided by the invention has high sensitivity, can be used to detect urine samples and serum samples and can be used as nutritive index to detect RBP in serum. Simultaneously, through the detection of RBP in urine, the kit is sensitive to the damage degree of renal proximal tubule.
Owner:BEIJING STRONG BIOTECH INC
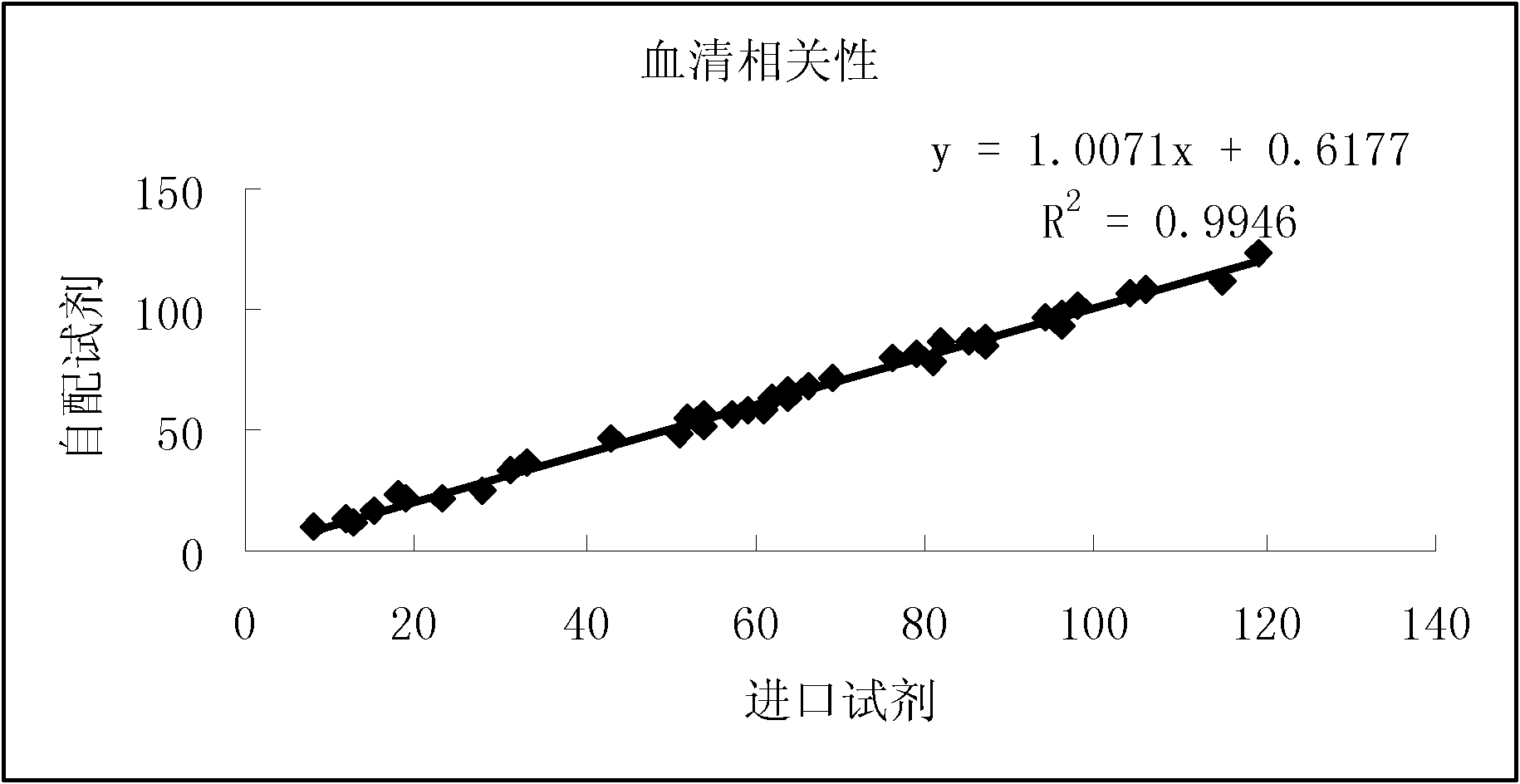
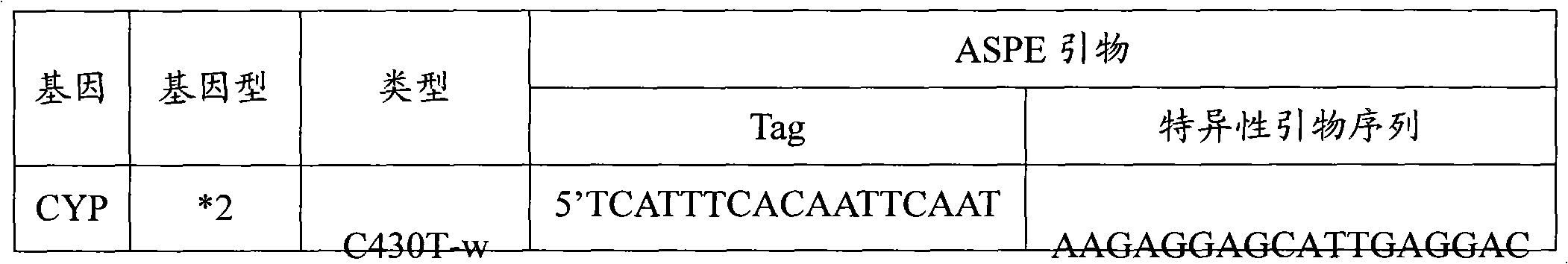
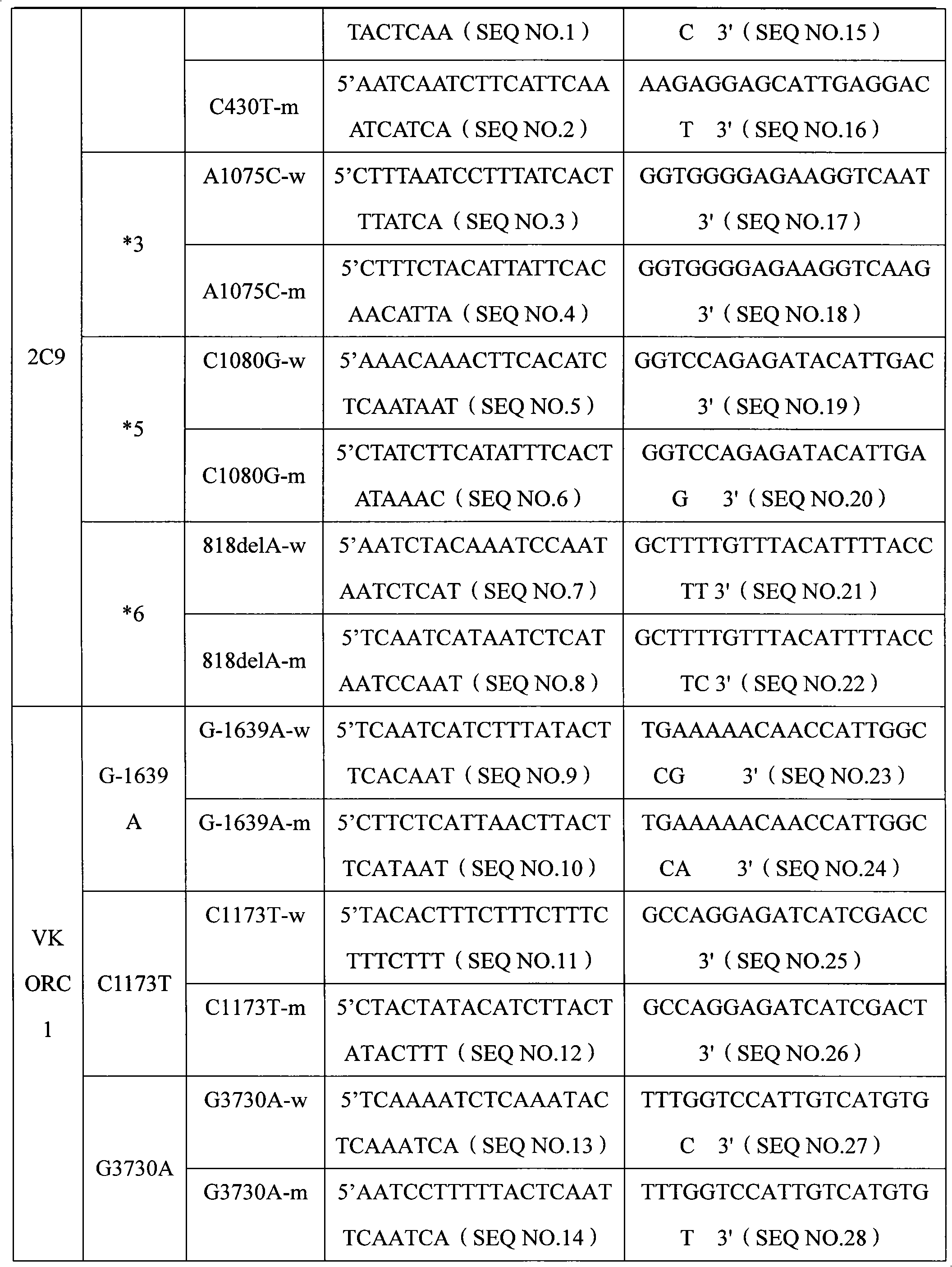
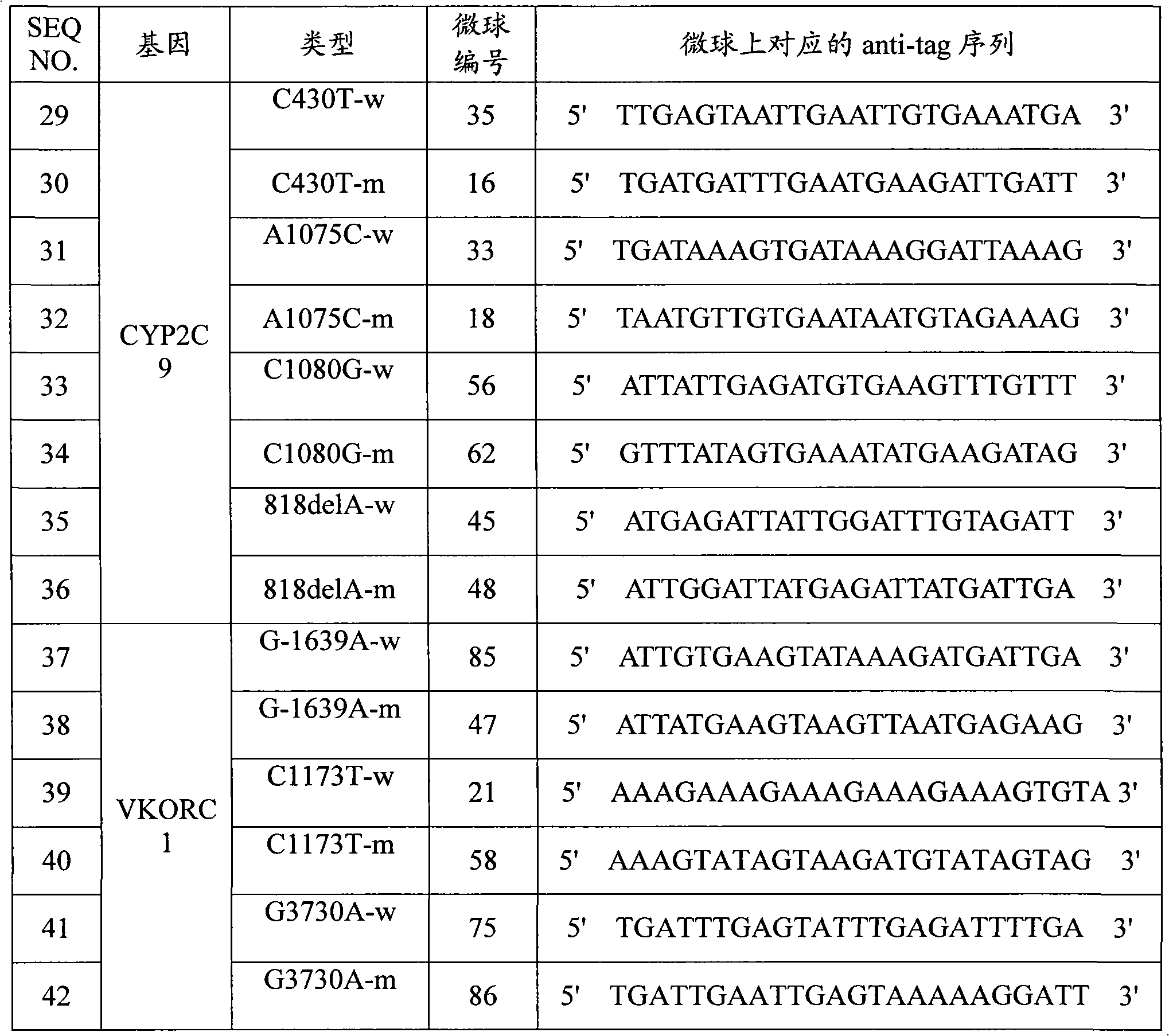
Specific primer, liquid-phase chip and method for SNP detection of CYP2C9 and VKORC1 genes
ActiveCN101824466AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationVKORC1Microsphere
The invention discloses a specific primer, a liquid-phase chip and a method for SNP detection of CYP2C9 and VKORC1 genes. The liquid-phase chip comprises wild-type and mutable-type ASPE primer pairs and microspheres coated by a specific anti-tag sequence respectively, which are designed respectively aiming at each type of mutable loci, primers used for amplifying a CYP2C9 gene target sequence having CYP2C9*2, CYP2C9*3, CYP2C9*5 and CYP2C9*6SNP loci, and / or primers used for amplifying a VKORC1 gene target sequence having G1639A, G1173T and G3730A SNP loci. The liquid phase chip of the invention has a quite good signal-noise ratio, and the cross reaction does not happen between a designed probe and the anti-tag sequence basically; the ASPE primer designed by the invention has quite good specificity, and can accurately differentiate various types of mutable loci; and the detection method has the advantages that: a few steps are adopted, 7 types of SNP loci can be detected in one step, the operation is convenient, a lot of uncertain factors existing in a process of repeated operations can be avoided, and the detection accuracy is greatly improved.
Owner:SUREXAM BIO TECH
Homogeneous immunoassay kit, detection method and application thereof
ActiveCN108051585AReduce difficultyAvoid cross reactionChemiluminescene/bioluminescenceBiological material analysisBiotin-streptavidin complexAntigen
The invention relates to a homogeneous immunoassay kit, detection method and application thereof in the technical field of biology. The kit comprises a reagent I, a reagent II, a reagent III and a reagent IV, wherein the reagent I contains a first counterpart, and the first counterpart is a known antigen or a known antibody specially combined with an antigen to be detected in a sample to be detected; the reagent II contains a receptor which can react with singlet oxygen to generate a detectable signal and a second counterpart combined with the receptor; the reagent III contains a third counterpart specially combined with the first counterpart; the reagent IV contains a donor which can generate the singlet oxygen in an excitation state; the surface of the third counterpart is coated with biotin, and the surface of the donor is coated with streptavidin. By utilizing combined detection of the kit and the homogeneous immunoassay method of the antigen and the antibody, the screening difficulty of raw materials is reduced, and the detection sensitivity is promoted at the same time.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Quick immune histochemical detection reagent for milk gland cancer lymph node metastasis and its detecting method
InactiveCN1945333AAvoid cross reactionReduce stepsPreparing sample for investigationBiological testingCancer cellLymphatic Spread
The present invention is one kind of reagent for quick immune histohcemical detection of lymphatic metastasis of mammary cancer and corresponding detecting method. Several kinds of directly marked antibody capable of distinguishing metastatic cancer cell and inherent lymph node cell are mixed into reagent and one-step reacted to stain the metastatic cancer cell in lymph node specifically. The present invention is used in the fast pathological diagnosis of early lymphatic metastasis of mammary cancer, and has raised sensitivity, high accuracy and high specificity.
Owner:孙爱静
Cyclic chimeric citrullinated peptide antigen and application thereof
InactiveCN104262489AImprove stabilityIncrease exposureBiological testingHybrid peptidesPeptide antigenDisulfide bonding
The invention discloses a cyclic chimeric citrullinated peptide antigen and an application thereof. The preparation of the cyclic chimeric citrullinated peptide antigen comprises the following steps: firstly connecting and jogging three small-molecular antigen peptides, namely a citrullinated peptide1, a citrullinated peptide 2 and a citrullinated peptide 3 derived from a silk polymerizing protein / an intermediate filament protein, and then synthetizing a cyclic polypeptide with a similar protein beta-corner structure by forming a disulfide bond through two cysteines inserted into the end N and the end C of a chimeric peptide. The cyclic chimeric citrullinated peptide antigen coats a solid-phase vector to prepare an indirect enzyme linked immunosorbent assay kit used for detecting the hypotype of multiple anti-citrullinated protein antibodies contained in RA (Rheumatoid Arthritis) serum. The cyclic chimeric citrullinated peptide antigen and the ELISA kit thereof which are disclosed by the invention have the advantages of simple preparation and experimental operation process, good result repeatability, qualification or quantification and wide clinical application and scientific research value and are outstandingly enhanced in detection sensibility and diagnosis value on RA compared with an international similar kit.
Owner:陈仁奋
Norovirus real-time fluorescent RT-PCR detection kit and application thereof
InactiveCN103131798AEasy to judgeAvoid cross reactionMicrobiological testing/measurementMicroorganism based processesOligonucleotide primersTrue positive rate
The invention relates to a norovirus real-time fluorescent RT-PCR detection kit and an application thereof. The invention belongs to the field of gene detection. The kit provided by the invention comprises a pair of oligonucleotide primers and an oligonucleotide probe aiming at type I norovirus obtained by screening, and / or a pair of oligonucleotide primers and an oligonucleotide probe aiming at type II norovirus obtained by screening. With a one-step real-time fluorescent RT-PCR, detectable minimal concentration of type I and / or type II norovirus is 1.0*10<2>copies / mL. Therefore, sensitivity and specificity of the kit provided by the invention are both high. With the invention, rapid early-stage detection and quantitative analysis of norovirus in samples such as stools and rectal swabs are realized. With the kit provided by the invention, detection period is short, detection efficiency is high, virus detection specificity is high, accuracy is high, and virus qualitative analysis and quantitative analysis can be carried out simultaneously. The sensitivity of the kit is higher than common PCR and immunological detection methods. The operation is simple, and the kit is easy to popularize. Experiment result repeatability is good.
Owner:湖北朗德医疗科技有限公司
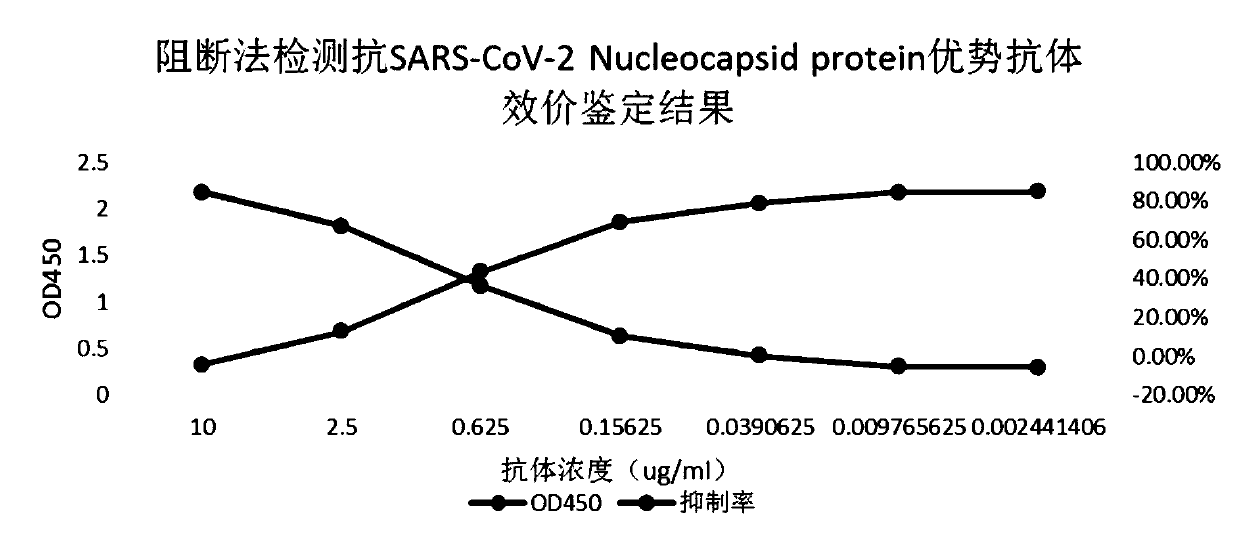
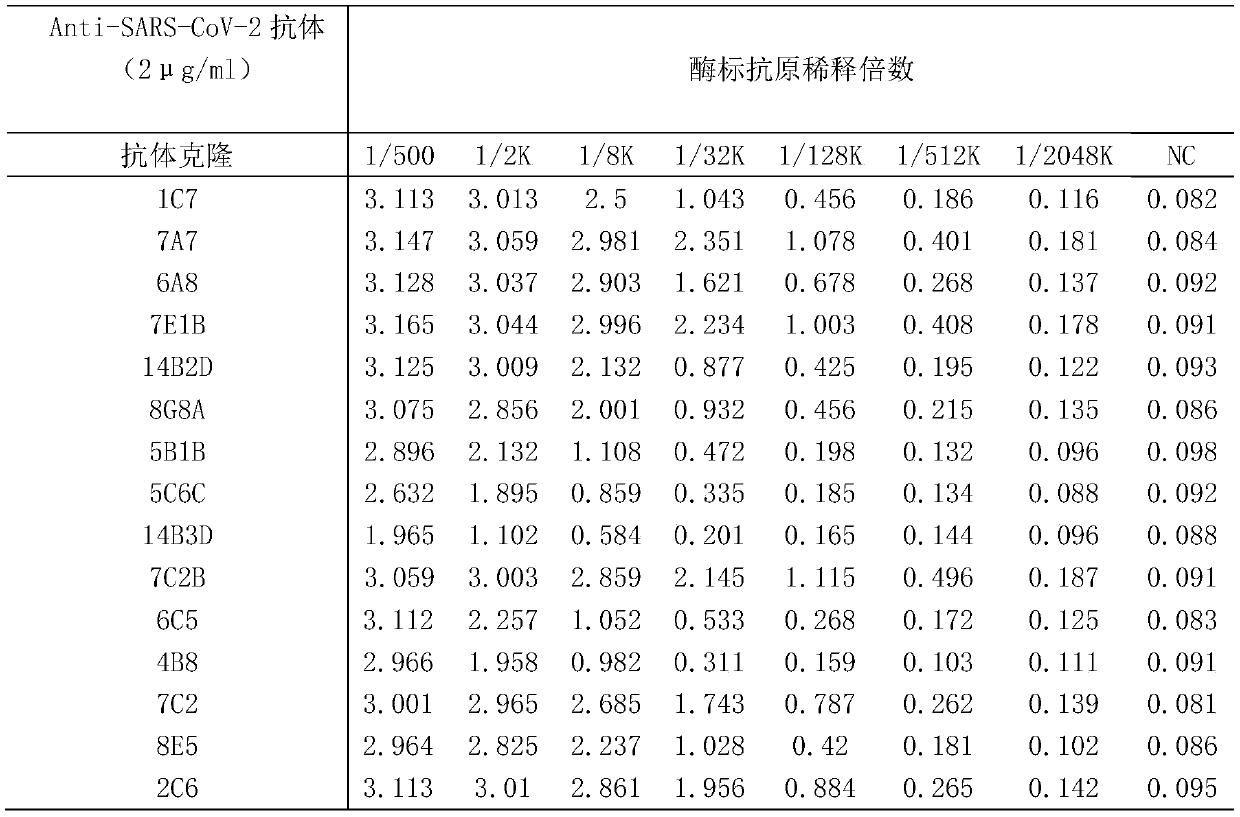
SARS-CoV-2 antibody detection method
PendingCN111474345AQuick checkEasy to detectBiological material analysisAntibody SuppressionAntiendomysial antibodies
The invention discloses an SARS-CoV-2 antibody detection method. The SARS-CoV-2 antibody detection method comprises the following steps: carrying out horseradish peroxidase labeling on recombinant SARS-CoV-2Nucleocapsid protein, meanwhile, selecting an anti-SARS-CoV-2 antibody or a dominant antibody to coat the enzyme-linked plate and sealing; according to a competitive ELISA principle, simultaneously adding a to-be-detected sample and an enzyme-labeled antigen into a test hole of an enzyme-linked plate, performing incubation at room temperature, thoroughly washing the plate, then adding a substrate TMB, performing color development in a dark place, finally, terminating the reaction, measuring a light absorption value at 450 nm, and evaluating the titer of the antibody in the sample by calculating the antibody inhibition rate. According to the method disclosed by the invention, relatively conservative N protein in SARS-CoV-2 and a specific antibody of the N protein are selected as rawmaterials. Compared with a traditional antibody detection method, the detection of the Anti-SARS-CoV-2 specific antibody is completed more quickly, more conveniently and more sensitively, and the method has specificity, sensitivity, accuracy and precision and has important application prospect and value.
Owner:BEIJING BIOSYNTHESIS BIOTECH
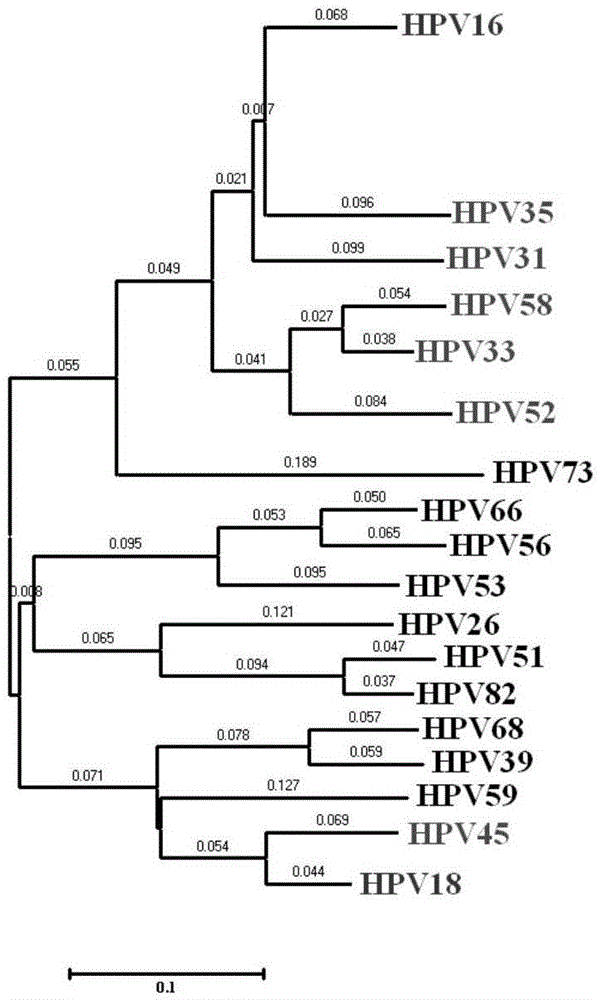
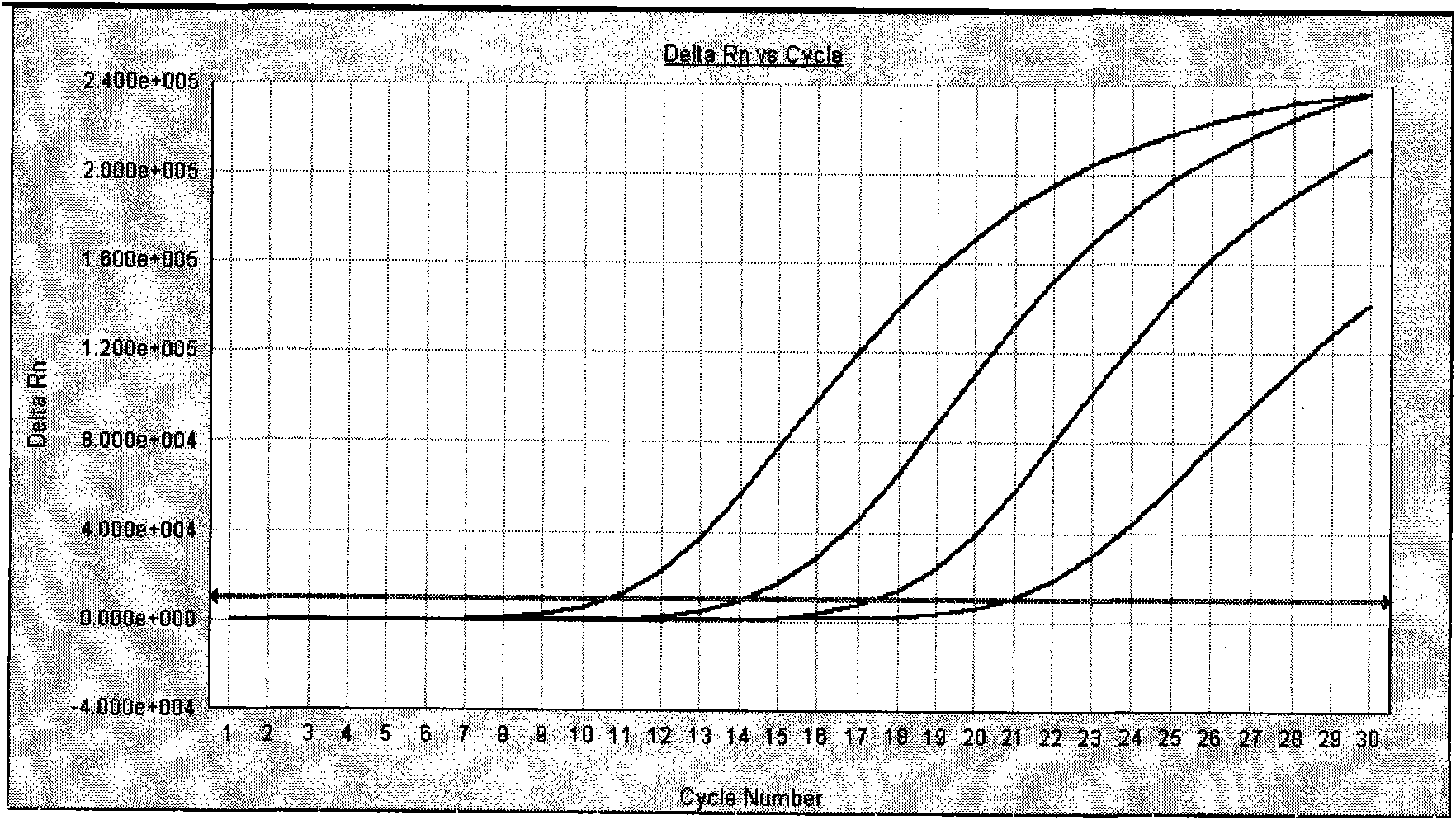
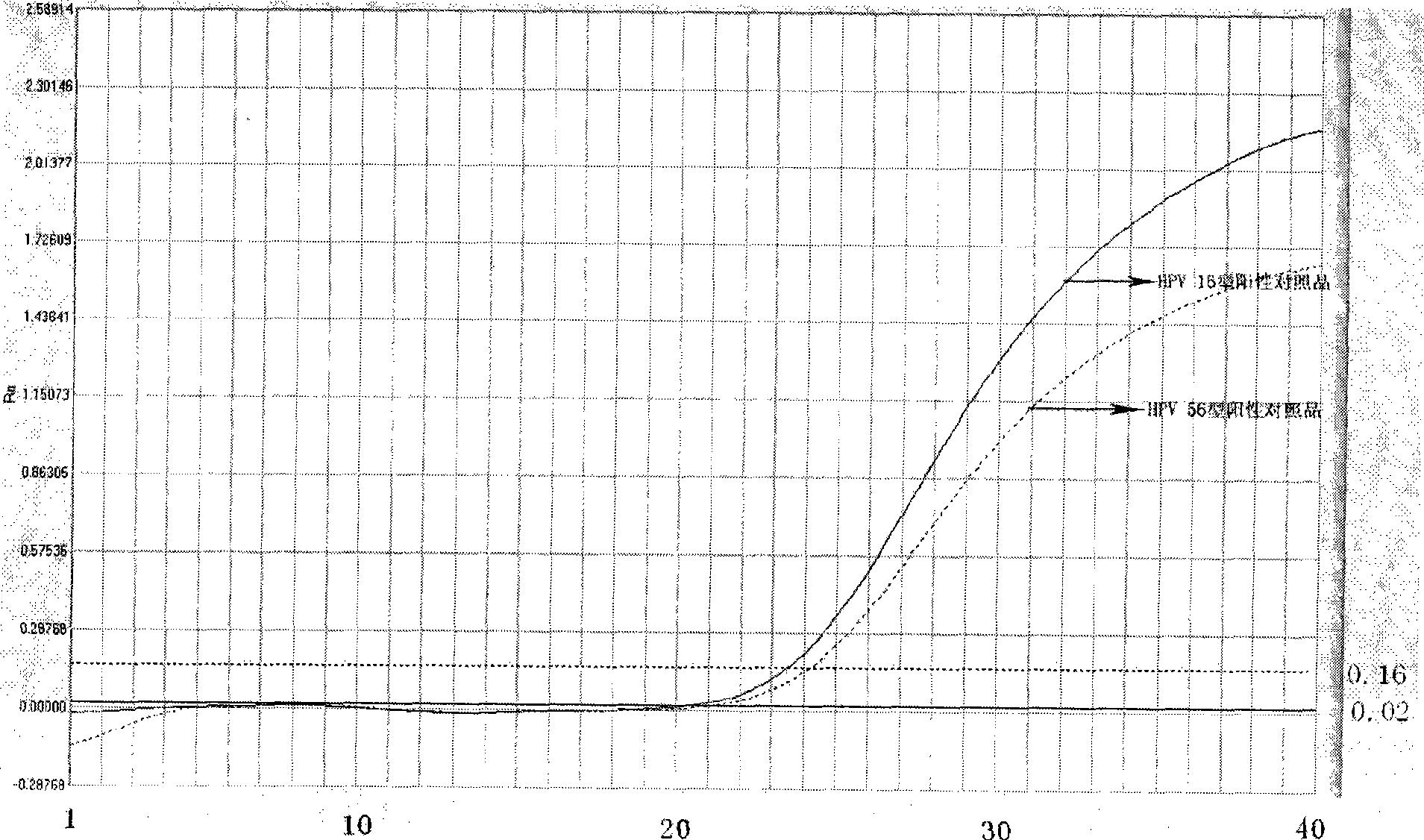
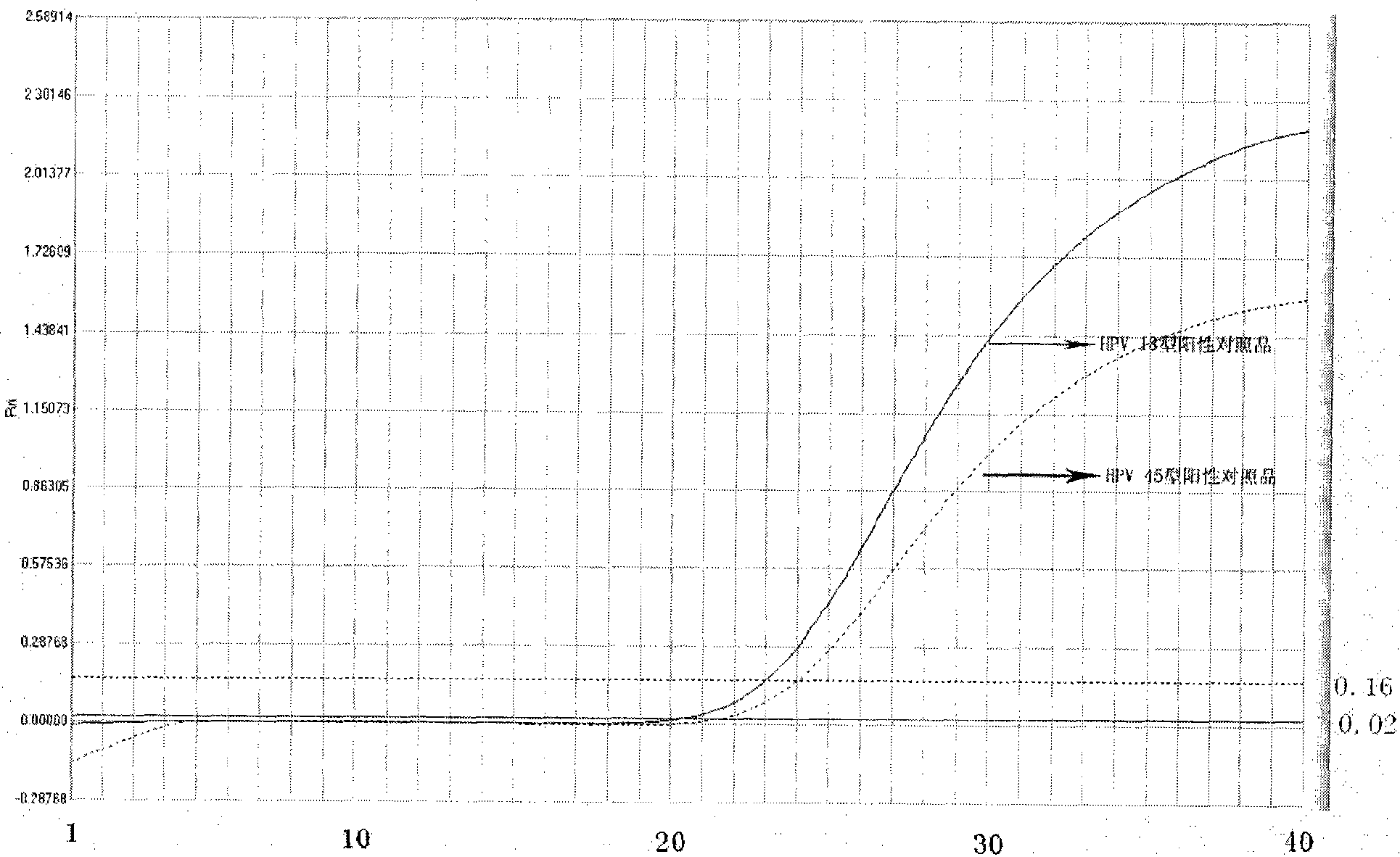
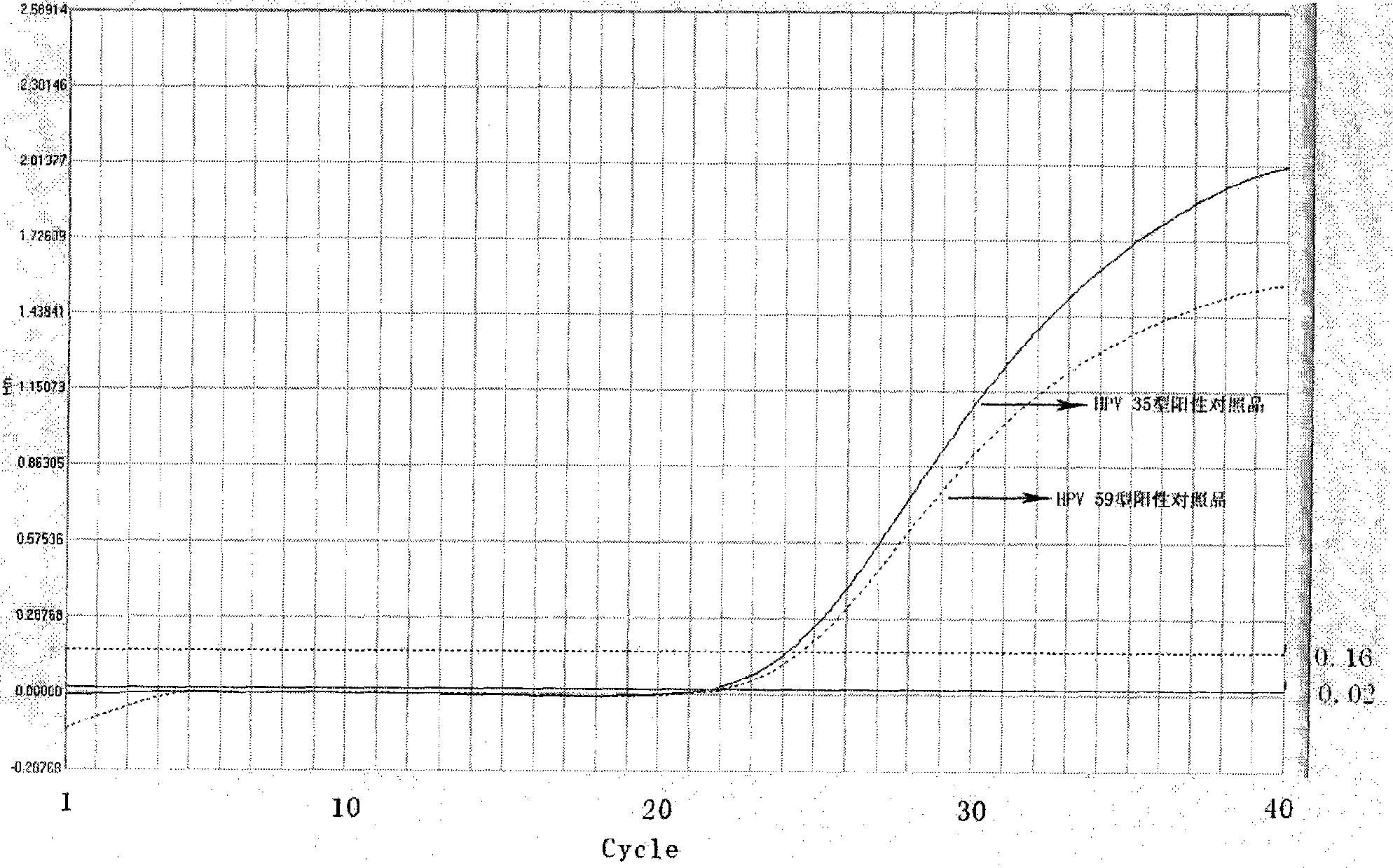
Method, oligonucleotide and kit for detecting high-risk HPV (human papilloma viruses)
ActiveCN105603121AReduce usageGuaranteed SensitivityMicrobiological testing/measurementMicroorganism based processesTrue positive ratePhylogenetic tree
The invention provides a method, oligonucleotide and kit for detecting common high-risk HPV (human papilloma viruses). A fluorescence PCR (polymerase chain reaction) technology is adopted, HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 26, 53, 66, 73 and 82 which possibly exist in a sample are subjected to initial detection and type identification. By the method, oligonucleotide and kit for detecting the common high-risk HPV, 18 high-risk types can be detected simultaneously, and the HPV 16 and 18 can be subjected to genetic typing simultaneously. On the basis of 18 types of high-risk HPV and affinity of other types on a phylogenetic tree, a high-risk HPV primer and a probe are designed, a background fluorescence value is reduced remarkably while detection accuracy, sensitivity and specificity are guaranteed, detection flux is increased remarkably, and detection cost is greatly reduced.
Owner:ACON BIOTECH (HANGZHOU) CO LTD
Syphilis spirochete membrane antigen with shorten expression and uses thereof
InactiveCN101293919ASignificant antigen reactivityAntigen reactivity verificationBacteriaDepsipeptidesAntigenSpiroplasma
The invention discloses a DNA sequence expressing a truncated treponema pallidum membrane antigen and an amino acid sequence The membrane antigen is removed of the part having high homology with human fibronectin, so as to avoid false positive and improve the specificity of the serological test for diagnosis of treponema pallidum infection. The invention also discloses the application of the membrane antigen in preparing diagnostic reagents for detecting treponema pallidum infection.
Owner:ARMY MEDICAL UNIV
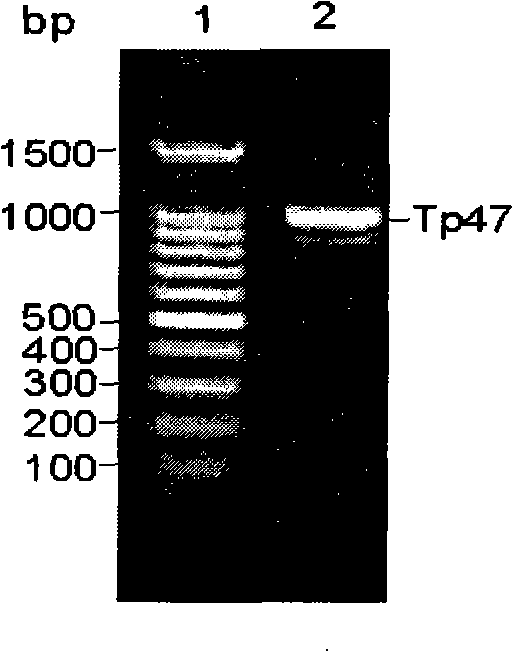
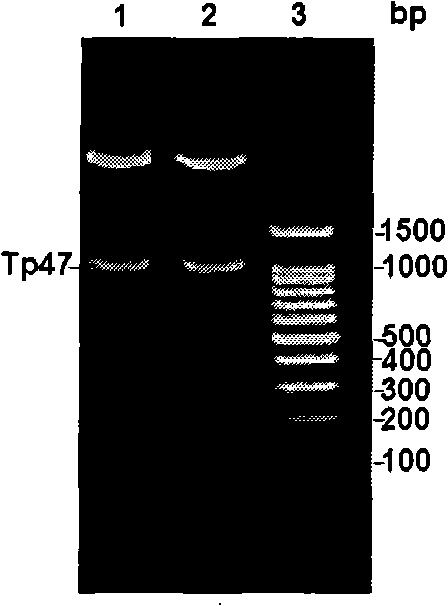
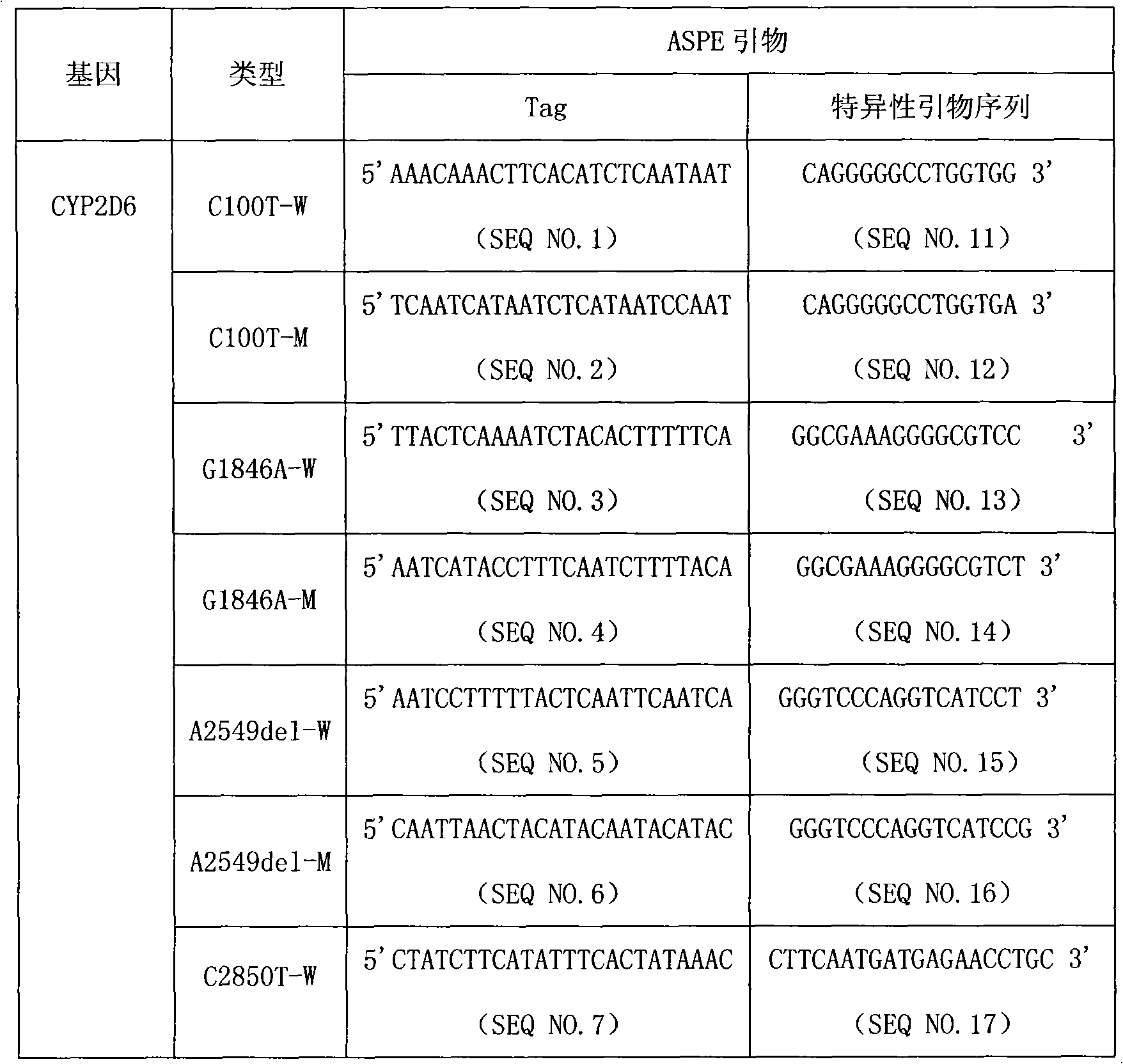
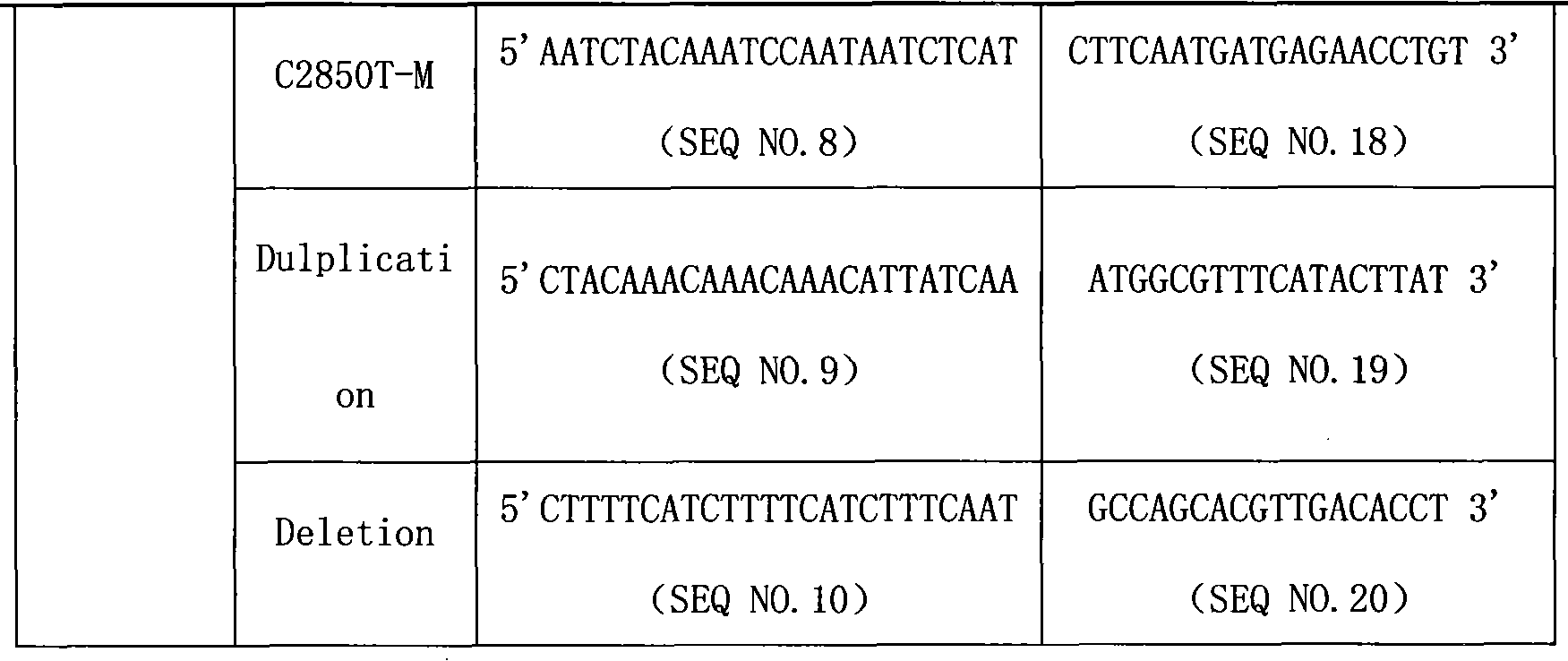
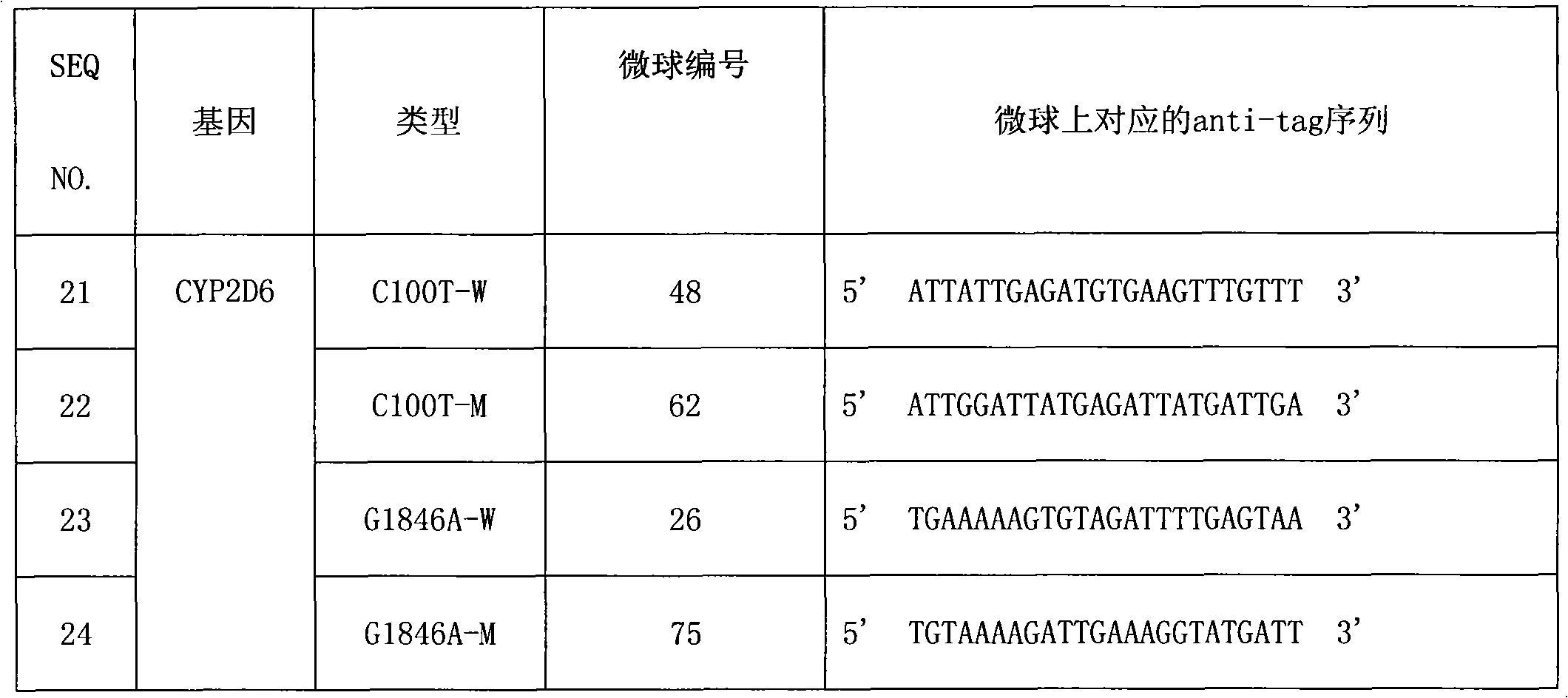
CYP2D6 gene mutation detection liquid-phase chip and detection method
ActiveCN101824467AImplement parallel detectionImprove signal-to-noise ratioMicrobiological testing/measurementSignal-to-noise ratio (imaging)Microsphere
The invention provides a CYP2D6 gene mutation detection liquid-phase chip which comprises ASPE (Allele Specific Primer Extension) primers aiming at CYP2D6 C2850T and CYP2D6Deletion mutational sites, three microballoons respectively enveloped with a specific anti-tag sequence and amplification primers aiming at the CYP2D6C2850T and the CYP2D6 Deletion mutational sites. The CYP2D6 gene mutation detection liquid-phase chip can simultaneously detect aiming at the CYP2D6 C2850T and the CYP2D Deletion mutational sites and has excellent signal to noise ratio. The coincidence ratio with a sequencing method of the CYP2D6 gene mutation detection liquid-phase chip reaches up to 100 percent, and the CYP2D6 gene mutation detection liquid-phase chip has higher specificity and precision compared with intra-class correlation products.
Owner:SUREXAM BIO TECH
Human parainfluenza virus distinguishing and quantitative detection regent kit
ActiveCN101550455AAvoid the "plateau effect"Increased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHuman Parainfluenza VirusFluorescence
The invention relates to three reagent kits for detecting human parainfluenza viruses 1, 2, 3 type real-time fluorescence polymerase chain reaction, which respectively adopts a one-step method RT-PCR, a two-step method RT-PCR and a multicolor fluorescence method RT-PCR to distinguish types of the human parainfluenza viruses of multi-type specimens and fix an amount of the human parainfluenza viruses of the multi-type specimens. The detection methods of the reagent kits have simple operation, short consuming time and high sensitivity and specificity and can be extensively used in a plurality of fields, such as the auxiliary diagnosis of the infection of the human parainfluenza viruses, clinical medicine direction, epidemiology retrospective study, and the like.
Owner:广州达安临床检验中心有限公司
Reagent kit for detecting high-risk human mammilla papillomavirus, as well as preparation and application thereof
ActiveCN101503741AAvoid False Positive ResultsIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescent pcrBiology
The invention relates to a kit for auxiliary diagnosis of cervical carcinoma and high-grade cervical intraepithelial lesion, and discloses a kit for detecting high-risk HPV. The kit for detecting the high-risk HPV comprises a high-risk HPV nucleic acid fluorescent PCR detection mixture and Taq enzyme. The invention also discloses a method for preparing the kit for detecting the high-risk HPV and a method for using the same. The kit can be used for detecting the high-risk HPV and overcomes the defects of high false positive rate, low specificity, high cost and the like of typing detection of the high-risk HPV in the prior art.
Owner:SHANGHAI ZJ BIO TECH
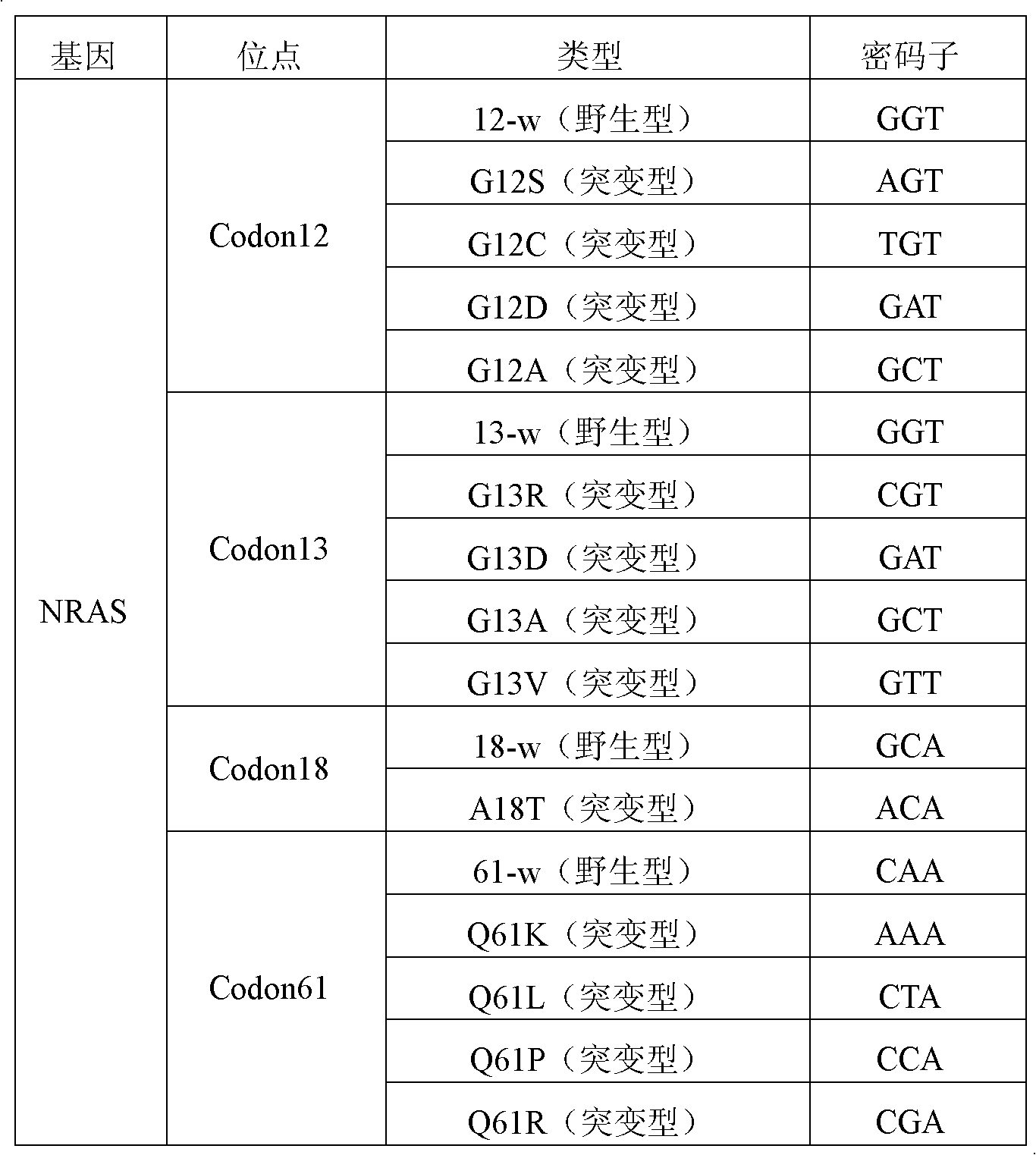
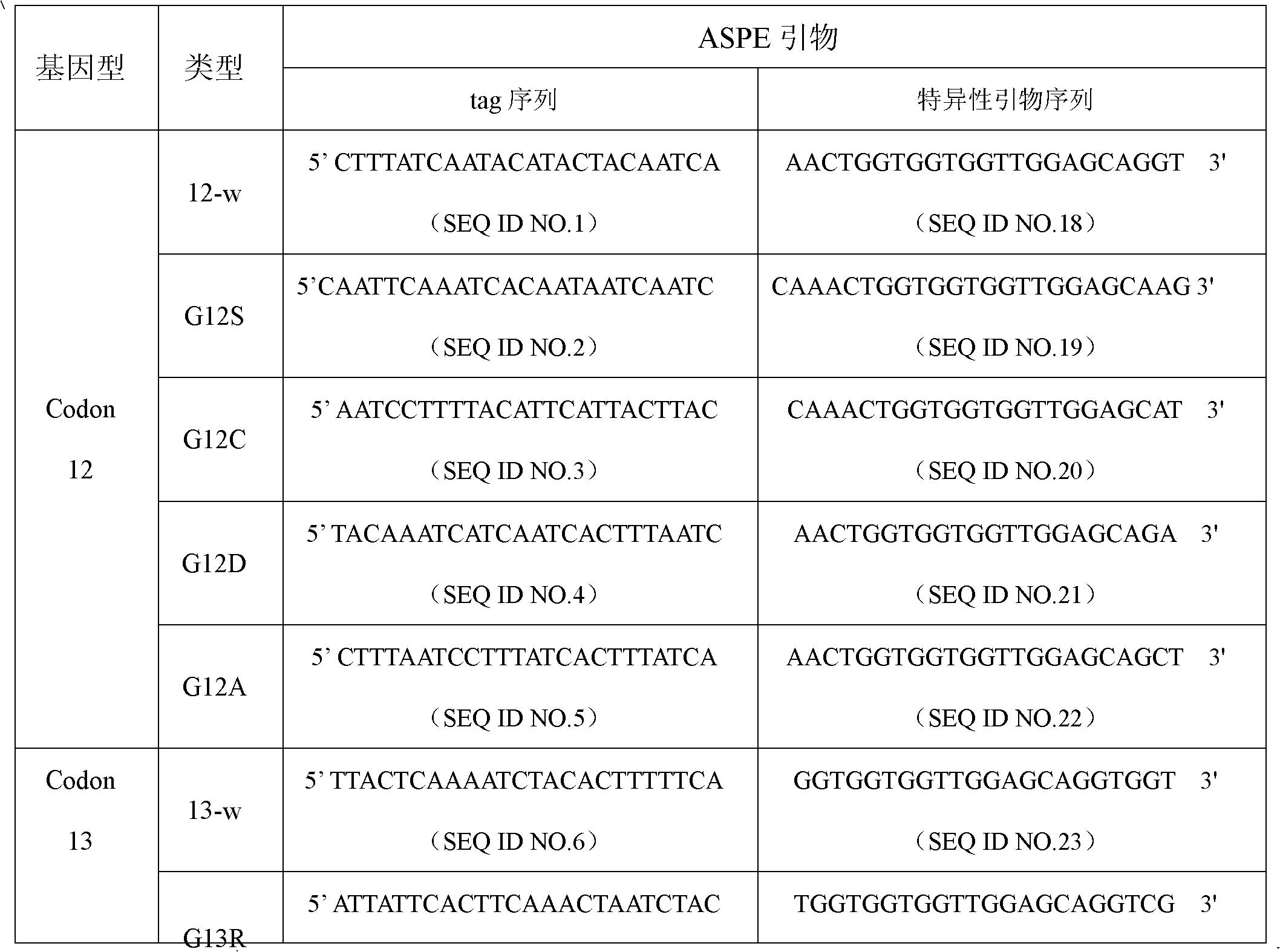
NRAS gene mutation detection specificity primer and liquid chip thereof
ActiveCN102994619AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementFluorescence/phosphorescenceMicrosphereWild type
The invention discloses an NRAS gene mutation detection specificity primer and a liquid chip thereof. The liquid chip mainly comprises: an ASPE primer composed of a 5'-terminal tag sequence and 3'-terminal specificity primer sequences focused on target gene mutation sites, wherein the specificity primer sequences comprise SEQ ID NO.18, SEQ ID NO.19, SEQ ID NO.20, SEQ ID NO.21 and / or SEQ ID NO.22 focused on a Codon12 site, SEQ ID NO.23, SEQ ID NO.24, SEQ ID NO.25, SEQ ID NO.26 and / or SEQ ID NO.27 focused on a Codon13 site, SEQ ID NO.28 and SEQ ID NO.29 focused on a Codon18 site, and / or SEQ ID NO.30, SEQ ID NO.31, SEQ ID NO.32, SEQ ID NO.33 and / or SEQ ID NO.34 focused on a Codon61 site; a microsphere coated by an anti-tag sequence; and an amplimer. The consistency between the detection result of the detection liquid chip provided by the invention and the detection result of a sequencing method is high to 100%, and the wild-type and mutant parallel detection of a plurality of mutation sites is realized.
Owner:SUREXAM BIO TECH
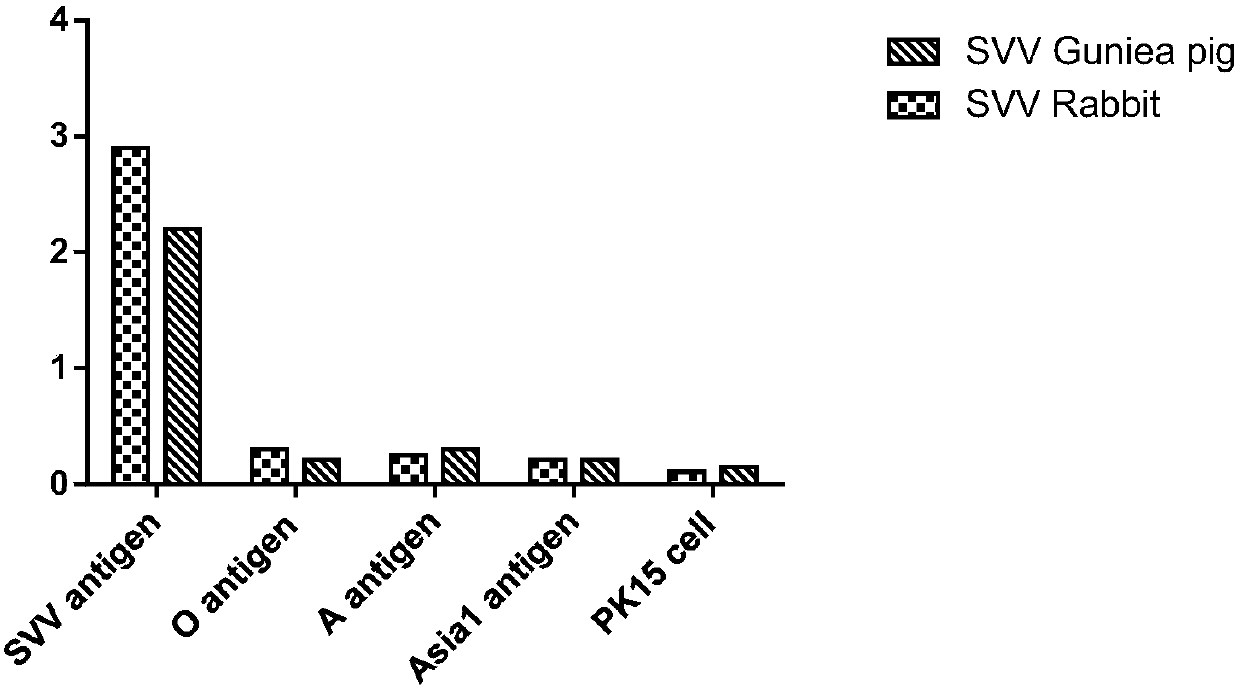
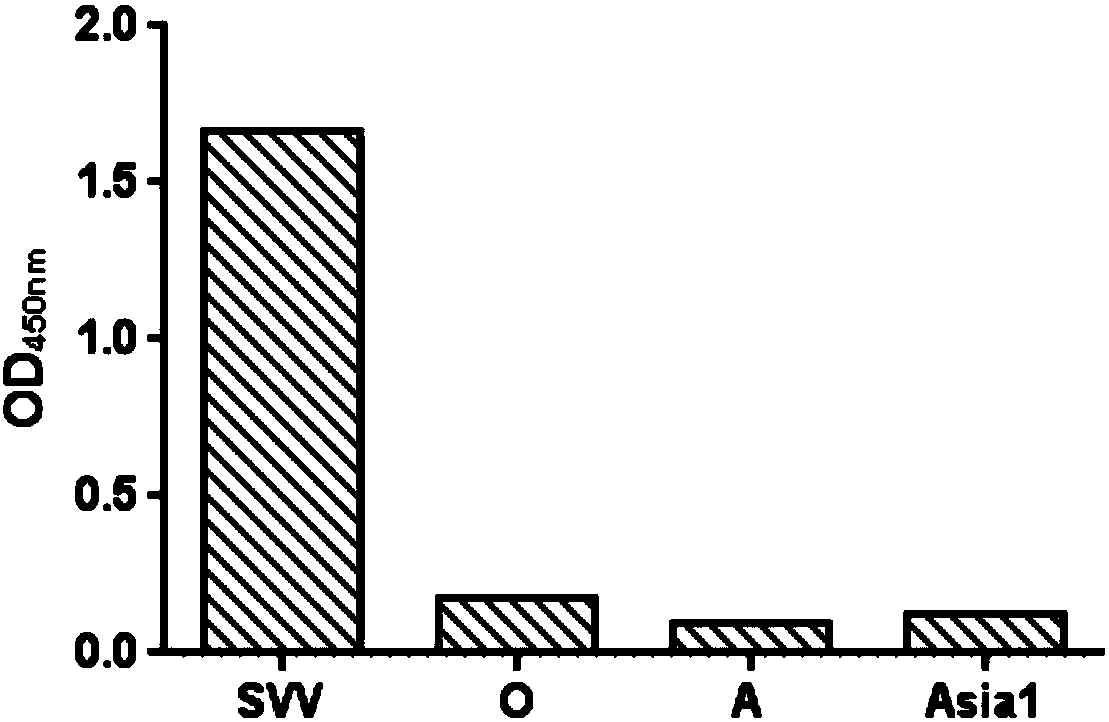
Double antibody sandwich ELISA kit used for Seneca viral antigen detection and application thereof
InactiveCN107741495AAvoid cross reactionShorten detection timeBiological material analysisElisa kitSenecavirus
The invention discloses a double antibody sandwich ELISA kit used for Seneca viral antigen detection and application thereof. The kit comprises a Seneca viral guinea pig anti-IgG enveloped elisa plateand an HRP marked Seneca viral rabbit anti-IgG. The HRP marked rabbit anti-IgG is used for replacing primary antibodies and secondary antibodies in traditional ELISA, so that the operating steps aresimplified. Simultaneously, the SVV guinea pig anti-IgG is enveloped to the surface of a solid-phase carrier by changing an enveloping stabilization technology, the preparation technology of an enzyme-labeled antibody diluent is changed, an enzyme-labeled antibody working solution can be stored stably without changing the activity and valence, and a double antibody sandwich ELISA kit for SVV antigen specific detection and a detection method thereof are established. The vacancy of making up SVV ELISA antigen detection is proposed, the problem that existing virus separation and detection for SVVantigens are low in repeatability and sensitivity and complex in operational program is overcome, and effective technical means is provided for SVV antigen detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Rapid phosphate detection tablet
InactiveCN102879241AImprove stabilityAvoid cross reactionMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationReaction layerPhosphate
The invention relates to a technology for the onsite semi-quantitative detection of the concentration of phosphates in water or soil, and concretely relates to a rapid phosphate detection tablet. The rapid phosphate detection tablet comprises a three-layer structure, the upper layer is a molybdate-containing chromogenic layer, the middle layer is a paraffin isolation layer, the lower layer is a reaction layer containing a reducing agent, and the tablet having a thickness of 5-6mm and a diameter of 8-20mm is prepared by a tablet press, is preserved in a sealing and photophobic manner and has an effective period of three months. The use method of the tablet comprises the following steps: immersing the rapid phosphate detection tablet in 10ml of a water sample or a soil dispersion liquid, mixing for 5-20min, contrasting the upper layer of the detection tablet with phosphate standard color columns, and estimating the concentration of the phosphates. The rapid phosphate detection tablet is suitable for the detection of the phosphates in domestic sewage, industrial wastewater, soil and the like, the background color and the turbidity of the sample have small influences on the detection result, and the tablet has the characteristics of convenient carrying, low price and the like, and provides a simple and rapid method for detecting the phosphates in water and the effective phosphorous in farmlands.
Owner:上海绿帝环保科技有限公司
Immunofluorescence double labeling method based on same species source first antibody
InactiveCN103994911AAvoid cross reactionAvoid immune cross-reactivityPreparing sample for investigationMolecular biologyStain method
The invention discloses an immunofluorescence double labeling method based on the same species source first antibody. The method comprises the steps of: (1) conducting gradient dilution of anti A antibody, and determining anti A protein antibody and measuring the minimum working concentration of the A protein to be detected by using an indirect immunofluorescence staining method; (2) developing the A protein by using the concentration and an SABC method (using luorescein to label streptavidin); (3) applying anti B protein antibody, and developing the B protein by an indirect immunofluorescence staining method; and (4) conducting microscopic examination by a fluorescence microscope, taking photos, overlaying the microscopic examination photos of the A protein and B protein to be detected, and analyzing the expression of the two proteins. The method is simple and easy for operation, realizes high research requirements with low experiment cost, and provides a wide range of applications for the chemical study of clinical immunofluorescence tissue.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Kit for quantitative detection of anti-Jo-1 antibody IgG by using magnetic particle chemiluminescence, and preparation method and detection method thereof
InactiveCN105466913AEasy to useGuaranteed detection effectChemiluminescene/bioluminescenceChemistryAnti jo 1
The invention relates to the technical field of immunology detection, and particularly relates to a kit for quantitative detection of an anti-Jo-1 antibody IgG by using magnetic particle chemiluminescence, and a preparation method and a detection method thereof. The kit for quantitative detection of the anti-Jo-1 antibody IgG by using magnetic particle chemiluminescence includes an Anti-Jo-1 IgG calibrator, an Anti-Jo-1 IgG reagent No. 1, an Anti-Jo-1 IgG reagent No. 2, an Anti-Jo-1 IgG magnetic separation reagent, an Anti-Jo-1 IgG control material and a cleaning solution. The invention also discloses a preparation method and a detection method of the above kit. Based on the traditional film strip immunoassay and enzyme-linked immunosorbent assay, the detection method increases the sensitivity and linearity range by 3-5 orders of magnitude, achieves the quantitative detection, and has the advantages of high sensitivity and specificity, good accuracy, low cost, simple operation and the objective judgment of results. The method cooperates with an automated chemiluminescence immunoassay analyzer to reach complete automation usage, and has broad application prospects.
Owner:北京贝尔医疗设备有限公司
Primer used for detecting nucleotide sequence variation, composition thereof, and method
PendingCN110964814ATroubleshoot cross-reactivity issuesHigh precisionMicrobiological testing/measurementDNA/RNA fragmentationForward primerGenetics
The invention relates to the molecular biology field. Specifically, the invention provides a digital PCR (polymerase chain reaction) primer pair used for measuring nucleotide sequence variation, a composition of the digital PCR primer pair and a method for detecting the nucleotide sequence variation. The forward primer of the improved digital PCR primer pair comprises an upstream detection domainand a target sequence binding domain, wherein the target sequence binding domain contains an amplified decision locus and an upstream mispairing domain; and the upstream detection domain independentlycomprises a domain which has the same sequence with a probe, and a domain which has the same sequence with a second forward primer. Compared with the prior art, sensitivity and high specificity are obviously improved, in addition, primer cost and test cost are greatly lowered, and the invention has an extremely high application value.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Lateral flow immune test strip based on ordered micro-nano structure
PendingCN111751525AAvoid cross reactionPrecise electromagnetic field confinement effectMaterial analysisAnalyteNanoparticle
The invention discloses a lateral flow immune test strip based on an ordered micro-nano structure. The lateral flow immune test strip is used for detecting a target in an analyte. According to the test strip, a base is used as a substrate, a sample pad, a conjugate pad, a chromatography pad, an ordered micro-nano structure detection pad and an absorption pad are sequentially distributed on the substrate from left to right, the sample pad is a target loading area, nanoparticles capable of being coupled with a target are combined on the conjugate pad, the ordered micro-nano structure detection pad is arranged on the chromatography pad, and a detection area and a quality control area are distributed on the ordered micro-nano structure detection pad; the sample pad and the conjugate pad are overlapped together, the conjugate pad and the chromatography pad are overlapped together, the ordered micro-nano structure detection pad is fixed on the chromatography pad, and the chromatography pad and the absorption pad are overlapped together; in practical application, when a sample reaches a detection area, multi-target detection can be realized by collecting signals; the test strip has the advantages of short detection time, simplicity and convenience in operation, low cost, simultaneous detection of multiple targets and the like, and is expected to be widely applied to the field of rapiddetection.
Owner:SOUTHEAST UNIV
Detection primer for rcnobcterium pyogenes and detection kit
ActiveCN108048588AAvoid cross reactionMeet testing needsMicrobiological testing/measurementMicroorganism based processesDiseasePcr method
The invention belongs to the field of molecular biology, and specifically relates to a detection primer for rcnobcterium pyogenes and a detection kit. A specific and sensitive PCR method is established for a hemolysin PLO gene sequence of rcnobcterium pyogenes, so that the rcnobcterium pyogenes can be accurately detected, and other pathogens for goat pyogenic infection are distinguished. Positivecontrol bacteria and negative control bacteria are provided for a user to determine the quality of the kit and the accuracy of an assessment method. The kit and the method provided by the invention can be used in fundamental conventional animal epidemic disease diagnose laboratories, target stripes are clear and easy to distinguish, a PCR reaction can be completed within one hour, and the kit andthe method are significant for monitoring the reproduction of bacterium, occurrence and propagation of diseases and timely prevention and curing of diseases.
Owner:CHONGQING ACAD OF ANIMAL SCI
Immune lateral chromatographic detection system as well as preparation method and application thereof
InactiveCN105974110AData is accurate and controllableIncrease distanceMaterial analysisQuality control systemBiology
The invention discloses an immune lateral chromatographic detection system as well as a preparation method and application thereof, and belongs to the field of medicine. The detection system comprises a substrate, wherein the substrate comprises a near-sample end and a far-sample end; the substrate is provided with a sample pad, a nitrocellulose membrane and a water-absorbing pad in sequence from the near-sample end to the far-sample end; the nitrocellulose membrane is provided with a quality control belt and a detection belt; the quality control belt is arranged between the detection belt and the sample pad; the quality control belt is a biotin-avidin system or a quality control system different from the species of a mouse antibody; the nitrocellulose membrane is provided with a view window used for collecting data. The detection system provided by the invention has higher sensitivity, precision and accuracy.
Owner:北京康思润业生物技术有限公司
PDGFRA gene mutation detection liquid-phase chip
ActiveCN101984072AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementBioinformaticsSignal-to-noise ratio
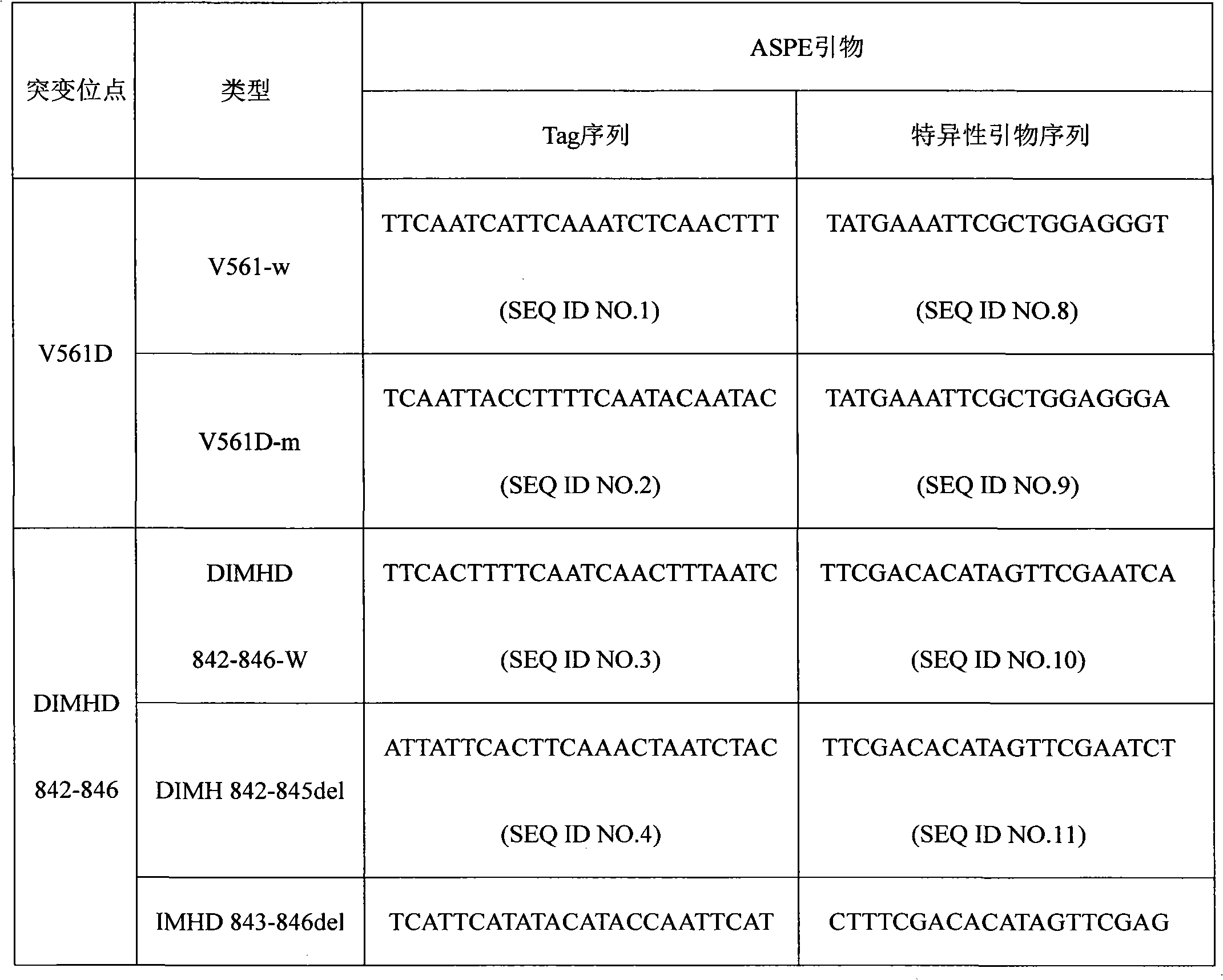
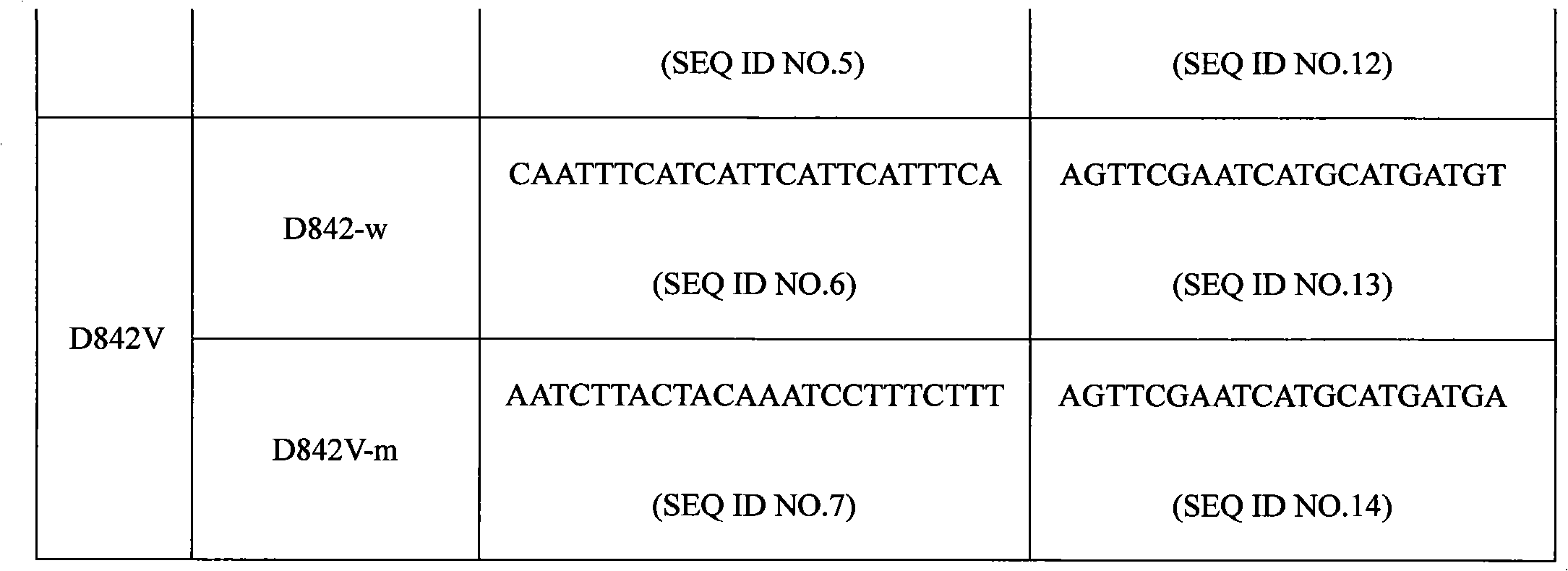
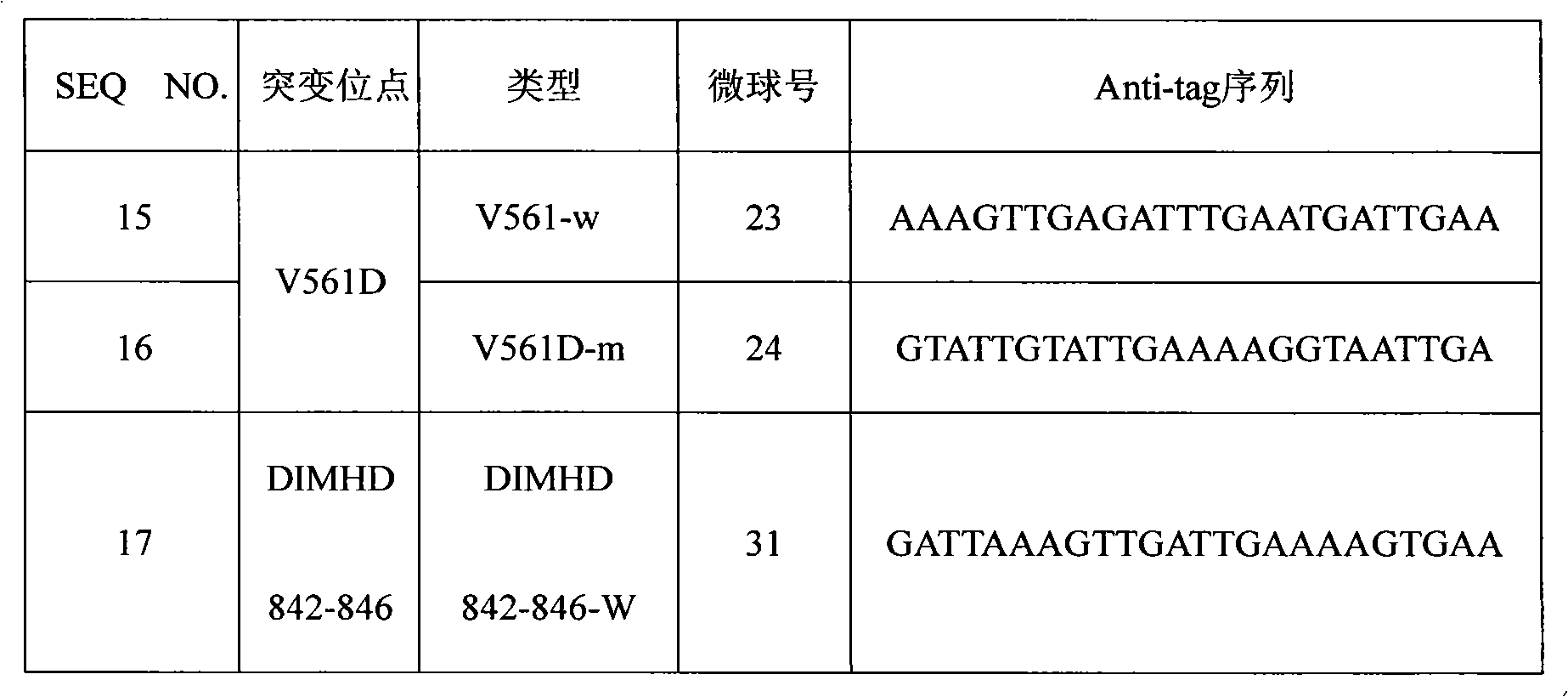
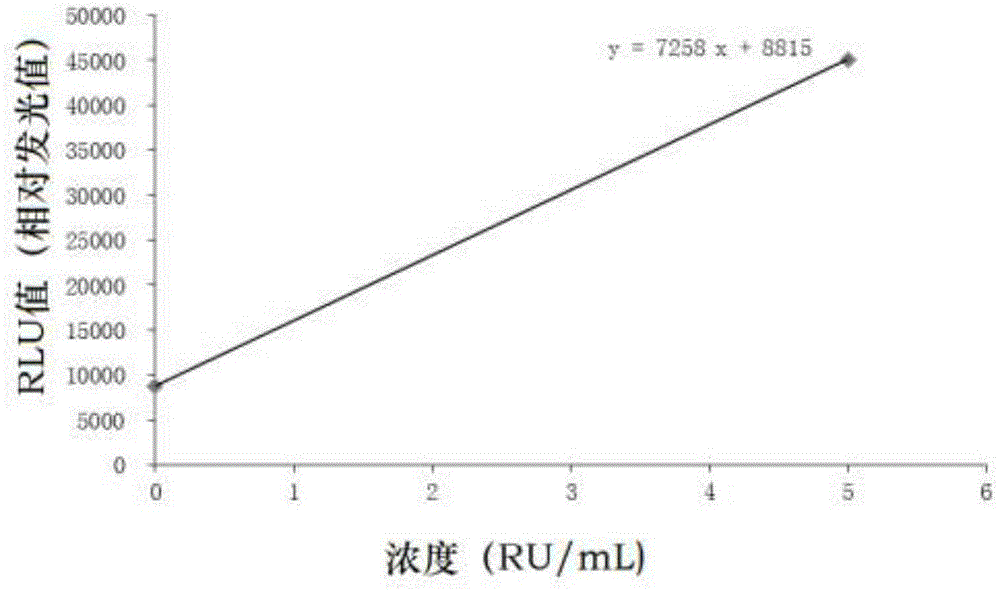
The invention discloses a PDGFRA gene mutation detection liquid-phase chip, which comprises tag sequence at 5' terminal and ASPE (Allele Specific Primer Extension) primers specific to mutational sites at 3' terminal. The ASPE primers consist of sequences shown in SEQ ID No.8-SEQ ID No.9 specific to V561D sites, sequences shown in SEQ ID No.10-SEQ ID No.12 specific to DIMH 842-845 and / or IMHD 843-846 deletion mutational sites, and / or sequences shown in SEQ ID No.13-SEQ ID No.14 specific to D842V site, the tag sequence which is selected from sequences shown in SEQ ID No.1- SEQ ID No.7, color coding microballoons which are respectively enveloped with a specific anti-tag sequence and amplification primers. The coincidence ratio with a sequencing method of the PDGFRA gene mutation detection liquid-phase chip reaches up to 100 percent. The PDGFRA gene mutation detection liquid-phase chip can simultaneously detect aiming at a plurality of deletion mutational sites with excellent signal to noise ratio.
Owner:SUREXAM BIO TECH
Kit for magnetic particle chemiluminescent quantitative detection of anti-SS-B antibody IgG and its preparation method and detection method
ActiveCN105352946AEasy to useGuaranteed detection effectChemiluminescene/bioluminescenceQuantitative determinationOrder of magnitude
The invention relates to the technical field of immunological detection and concretely relates to a kit for magnetic particle chemiluminescent quantitative detection of an anti-SS-B antibody IgG and its preparation method and detection method. The kit for magnetic particle chemiluminescent quantitative detection of an anti-SS-B antibody IgG comprises an Anti-SS-B IgG calibration material, an anti-SS-B IgG regent 1, an anti-SS-B IgG regent 2, an anti-SS-B IgG magnetic separation regent, an anti-SS-B IgG quality control material and a cleaning liquid. The invention also discloses a preparation method and detection method of the kit. Based on the traditional membrane stripe immunization method and enzyme-linked immunosorbent assay, the detection method improves sensitivity and a linear range by 3-5 orders of magnitudes, realizes quantitative determination, has the advantages of high sensitivity, good specificity, good accuracy, low cost, simple operation and objective result determination, realizes full automation by a full-automatic chemiluminescence immunity analyzer and has a wide application prospect.
Owner:北京贝尔医疗设备有限公司
Primer probe and kit for detecting oncogene of cervical cancer and detection method for non-detection disease purpose
ActiveCN103114132AAvoid false positivesAvoid cross reactionMicrobiological testing/measurementDNA/RNA fragmentationDiseaseNon detection
The invention relates to the technical field of biologics, and in particular relates to a primer probe and kit for detecting an oncogene of a cervical cancer and a detection method for a non-detection disease purpose. The probe is high in specificity; and the false positive rate is reduced. By the method, quantified detection for nucleic acid to be detected is realized by hybridization, signal magnification and enzymatic chemiluminescence; the magnification factor is determined without a magnification process for increasing an index; and the repetitiveness and the stability are high.
Owner:ZHENGZHOU KODIA BIOTECHNOLOGY CO LTD
THADA gene mutation detection specific primer and liquid phase chip
InactiveCN103451271AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationMicrosphereMutation detection
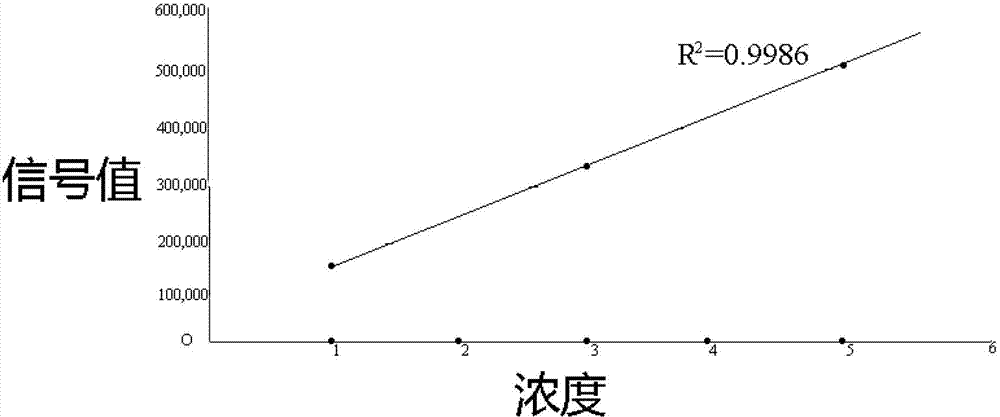
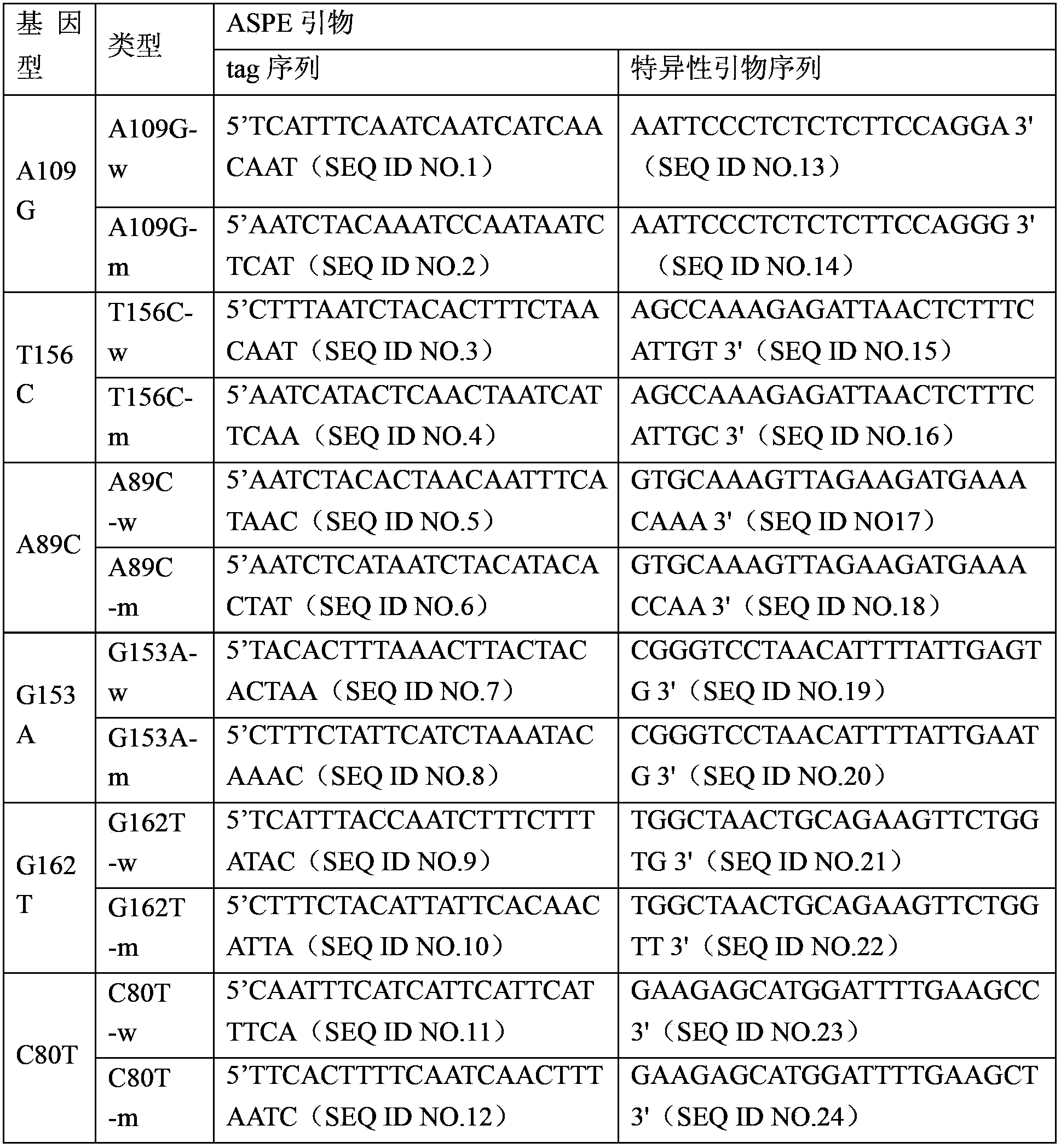
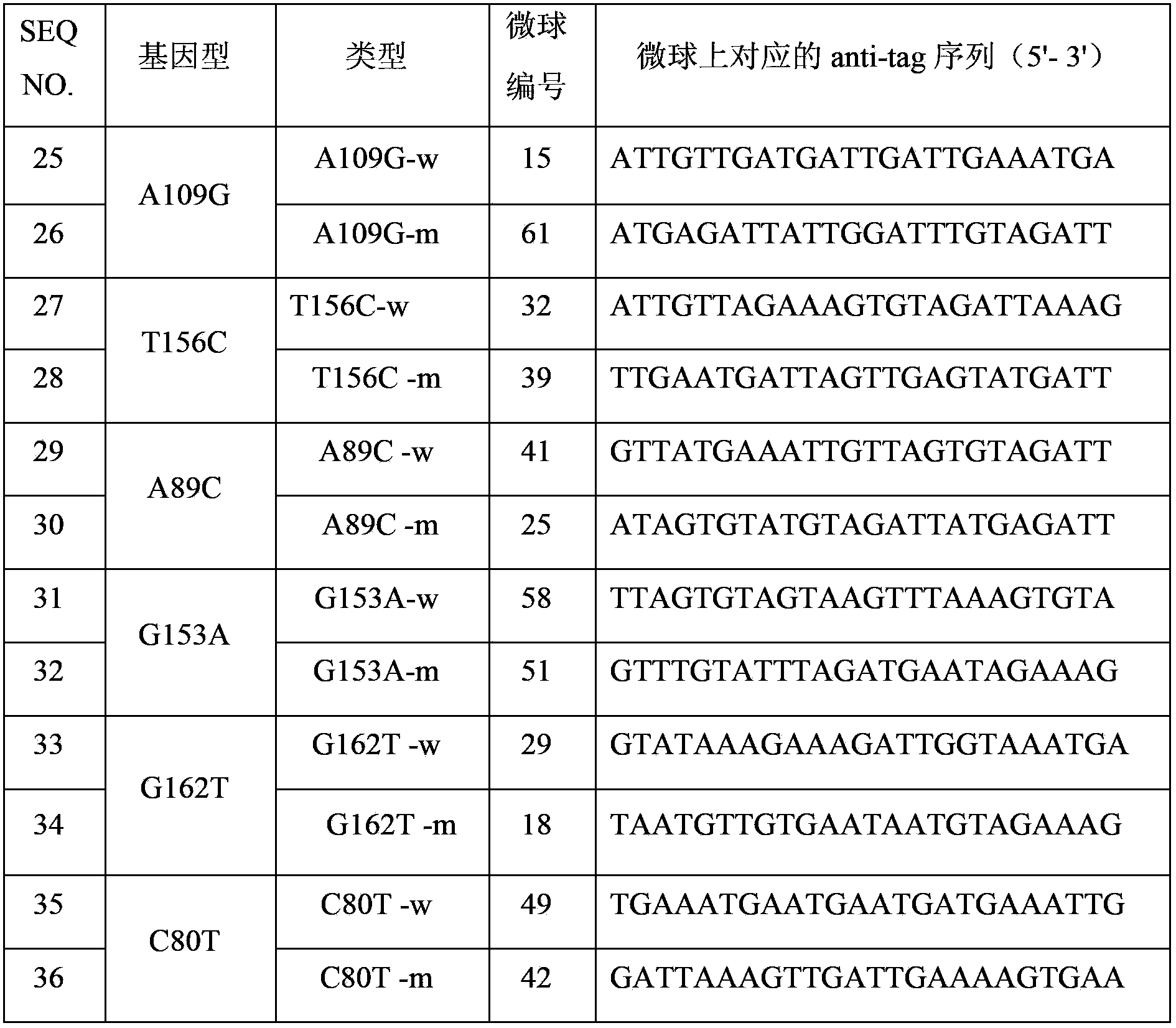
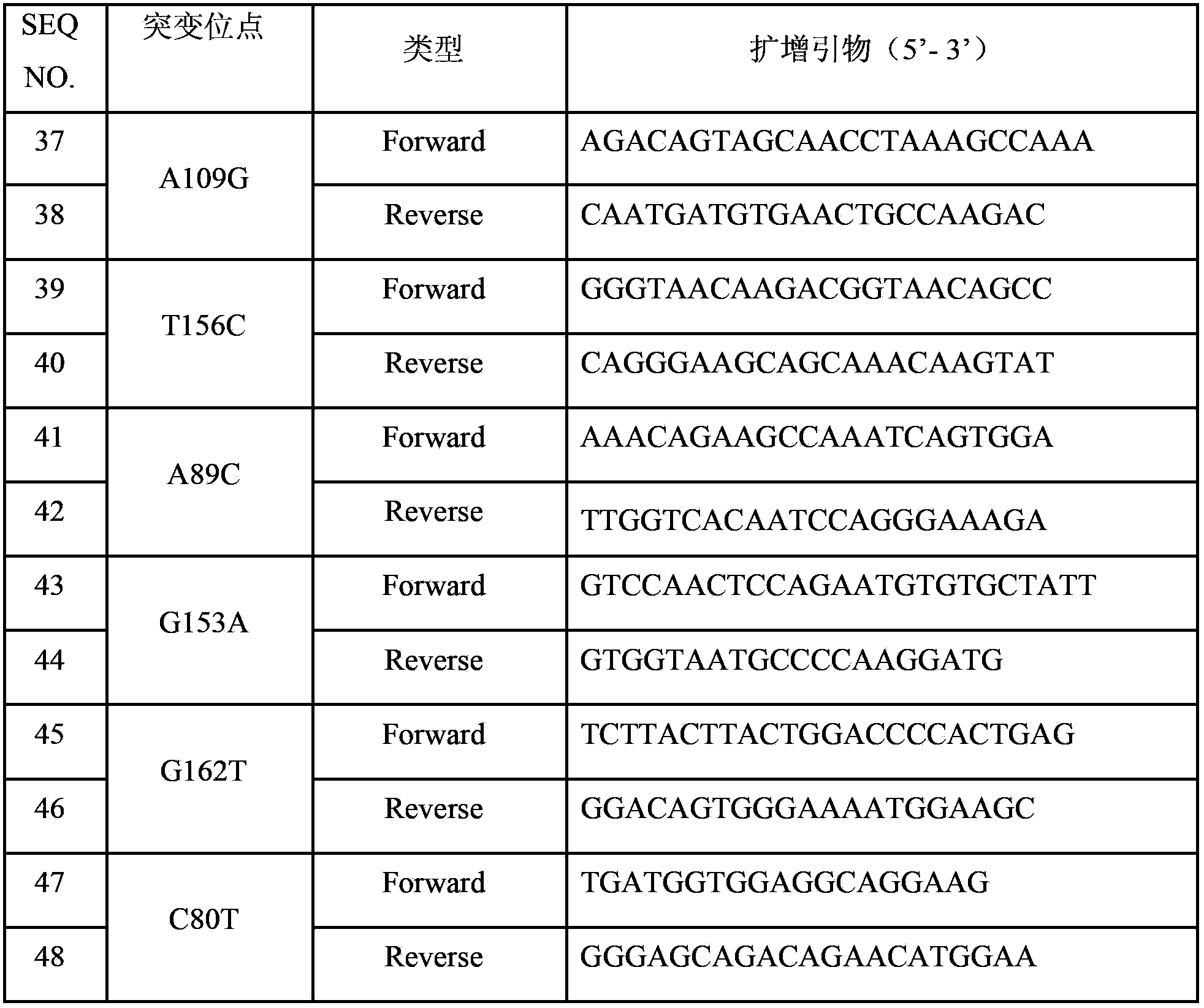
The invention discloses a THADA gene mutation detection liquid phase chip and a specific primer. The liquid phase chip mainly comprises: an ASPE primer composed of a tag sequence at 5'-terminal and specific primer sequences against the mutation site of a target gene at 3'-terminal, wherein the specific primer sequences comprise SEQ ID NO.13 and SEQ ID NO.14 against an A109G site, SEQ ID NO.15 and SEQ ID NO.16 against a T156C site, SEQ ID NO.17 and SEQ ID NO.18 against an A89C site, SEQ ID NO.19 and SEQ ID NO.20 against a G153A site, SEQ ID NO.21 and SEQ ID NO.22 against a G162T site, and / or SEQ ID NO.23 and SEQ ID NO.24 against a C80T site; a microsphere coated with an anti-tag sequence; and an amplimer. The coincidence rate between the detection result of the detection liquid phase chip and a sequencing method is 100%, and the wild and mutant parallel detection of a plurality of mutation sites can be realized.
Owner:SUREXAM BIO TECH
Florfenicol on-site test paper, and preparation and use methods thereof
The invention particularly relates to florfenicol on-site test paper, and preparation and use methods thereof and belongs to the field of immunology. The test paper includes anti-florfenicol amine secreted from hybridoma FFA-C which is assigned the accession number CCTCC-C201575, a monoclonal antibody C marked by colloidal gold and a carrier plate having adhesive sticker, wherein a sample adding end water absorption layer and a hand holding end water absorption layer are formed at two ends of the carrier plate respectively. A test layer is arranged on the middle of the carrier plate. A glass fiber layer block, on which the monoclonal antibody C marked by colloidal gold is supported, is arranged at the boundary between the test layer and the sample adding end water absorption layer. One end of the glass fiber layer block is arranged under the sample adding end water absorption layer and the other end of the glass fiber layer block is arranged above the test layer. A test line and a quality control line are formed on the test layer extended from the glass fiber layer block, wherein the test line and the quality control line are respectively coated by an anti-florfenicol amine polyclonal antibody and sheep-anti mouse IgG. The test paper quickly, simply and accurately detects the florfenicol and the metabolite, florfenicol amine, thereof on site, and can be used for crude screening test of drug residue of the florfenicol.
Owner:LUDONG UNIVERSITY
Hepatitis C virus recombination protein and gene sequence
ActiveCN102321179AReduce the risk of missed detectionAvoid the risk of false positivesHybrid peptidesVector-based foreign material introductionEscherichia coliSingle-Chain Antibodies
The invention discloses hepatitis C virus recombination protein, characterized in that: the hepatitis C virus recombination protein is fusion protein formed by fusing hepatitis C virus main antigenic determinant and hepatitis C virus core protein single chain antibody, and the amino acid sequence is represented as SEQ ID No.1. According to the invention, gene engineering recombination technology is utilized, the hepatitis C virus main antigenic determinant-core protein single chain antibody fusion protein is expressed in escherichia coli system, and the advantages of short production period, high yield and low cost are achieved. The recombination protein can be used as a part of an immunodiagnostic kit for hepatitis C virus.
Owner:杭州博林生物技术有限公司
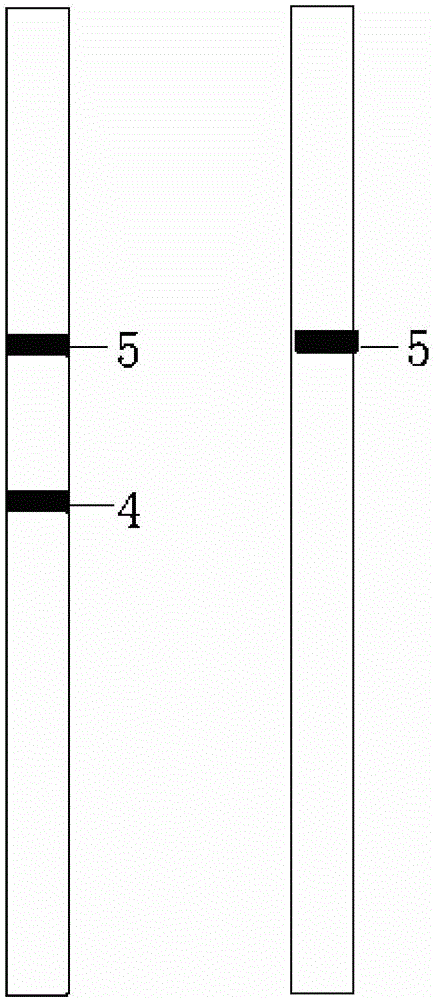
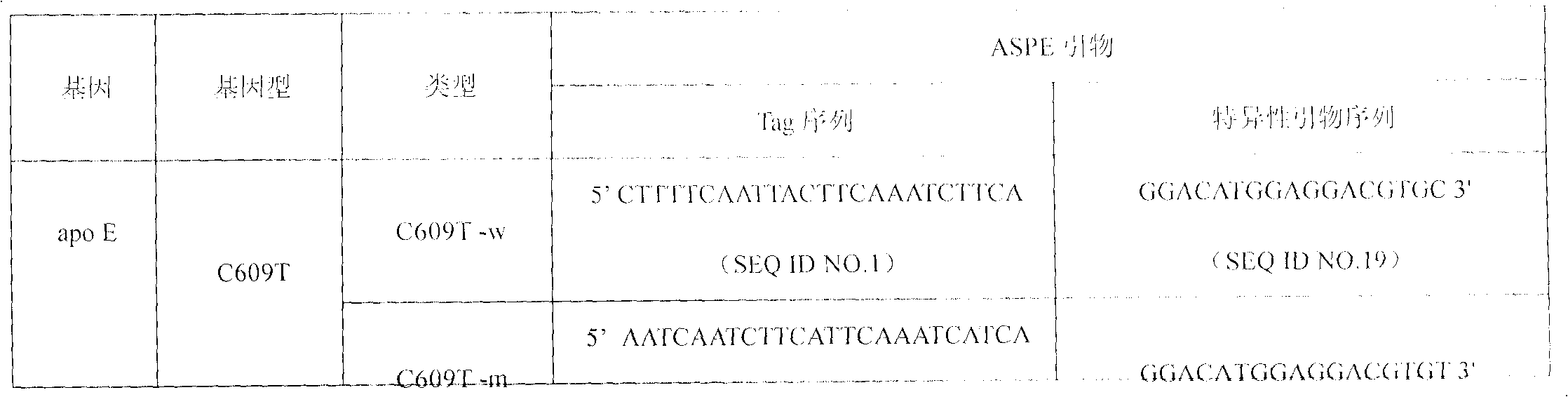
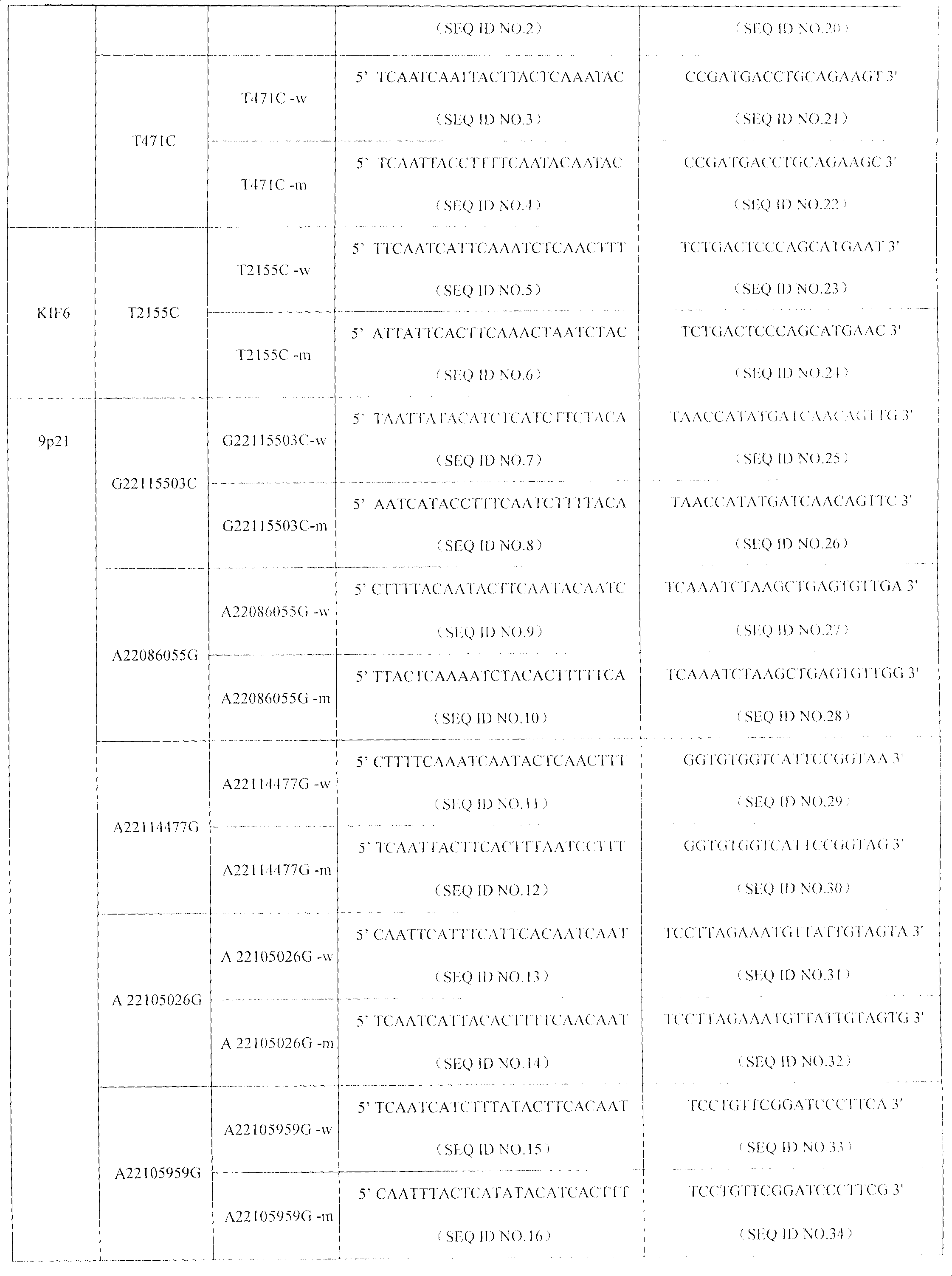
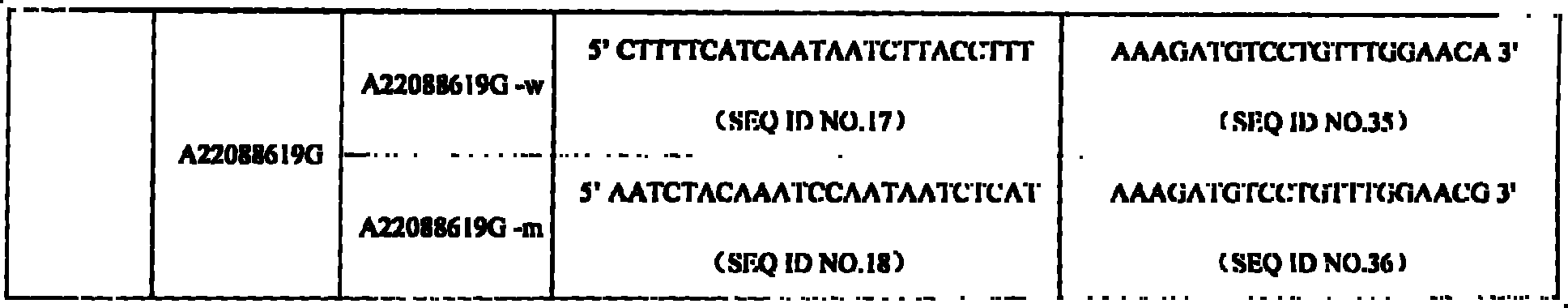
SNP (Single Nucleotide Polymorphism) detection liquid phase chip for KIF6 and apo E genes and chromosome 9p21 section
ActiveCN102010897AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementKIF6Solid phase extraction
The invention discloses an SNP (Single Nucleotide Polymorphism) detection liquid phase chip for KIF6 gene, mainly comprising an ASPE (Application Solid Phase Extraction) primer pair, microballons and an amplification primer, wherein each ASPE primer consists of a 5'-end tag sequence and 3'-end specific primers aiming at T2155C SNP locus; the 3'-end specific primers comprise SEQID NO.23 and SEQ IDNO.24; the tag sequence is selected from SEQID NO.1 to SEQID NO.18; and the microballons are respectively coated by a specific anti-tag sequence and have codes in different colors. The invention also discloses the SNP detection liquid phase chip for apoE and KIF6 genes and the chromosome 9p21 section. The occlusion rate of the detection method and the sequencing method which are provided in the invention is as high as 100 percent. The prepared SNP detection liquid phase chip for the apoE and KIF6 genes and the chromosome 9p21 section has favorable single-noise ratio.
Owner:SUREXAM BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com