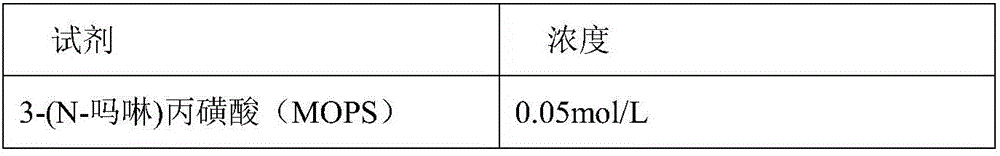
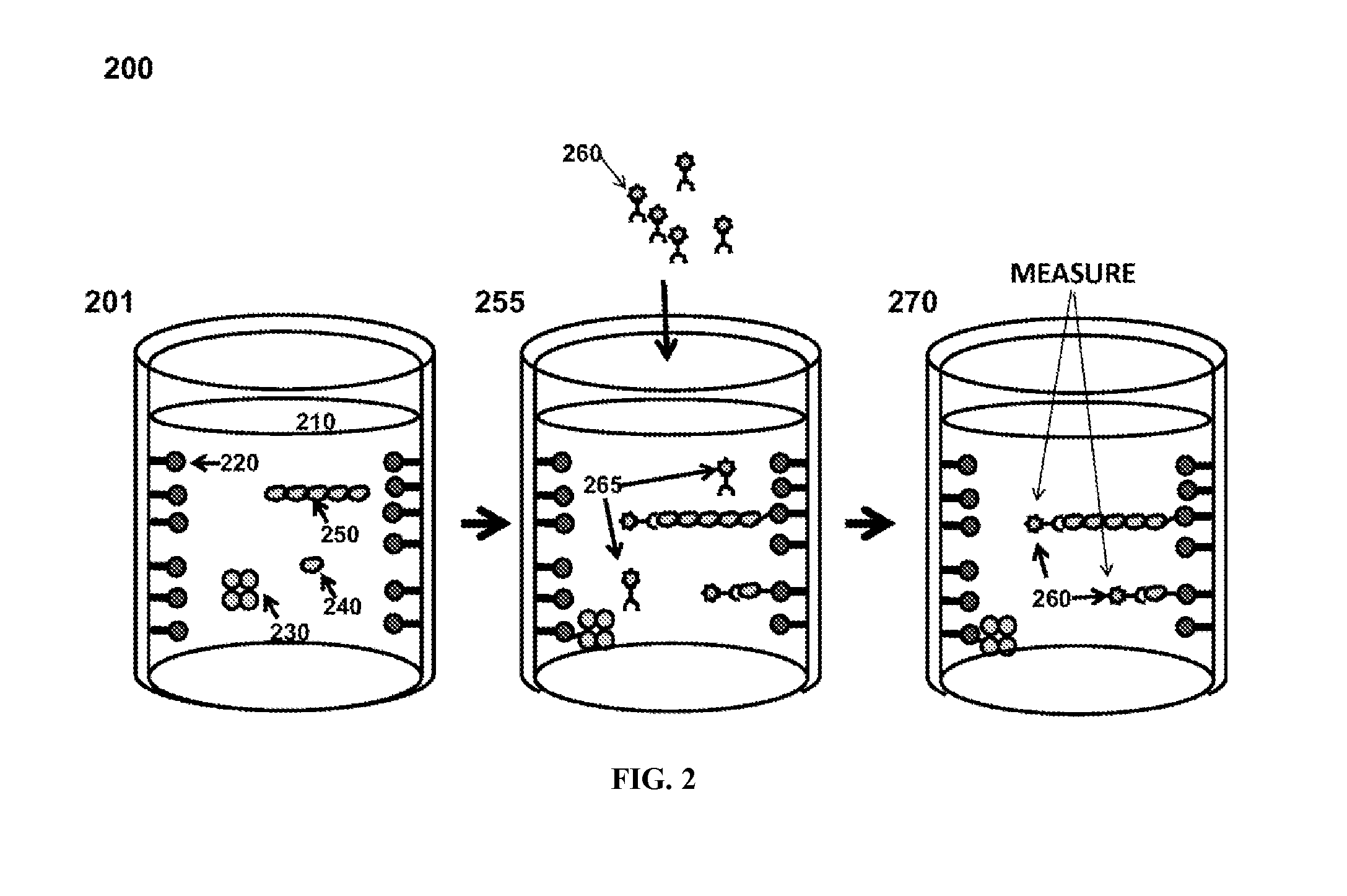
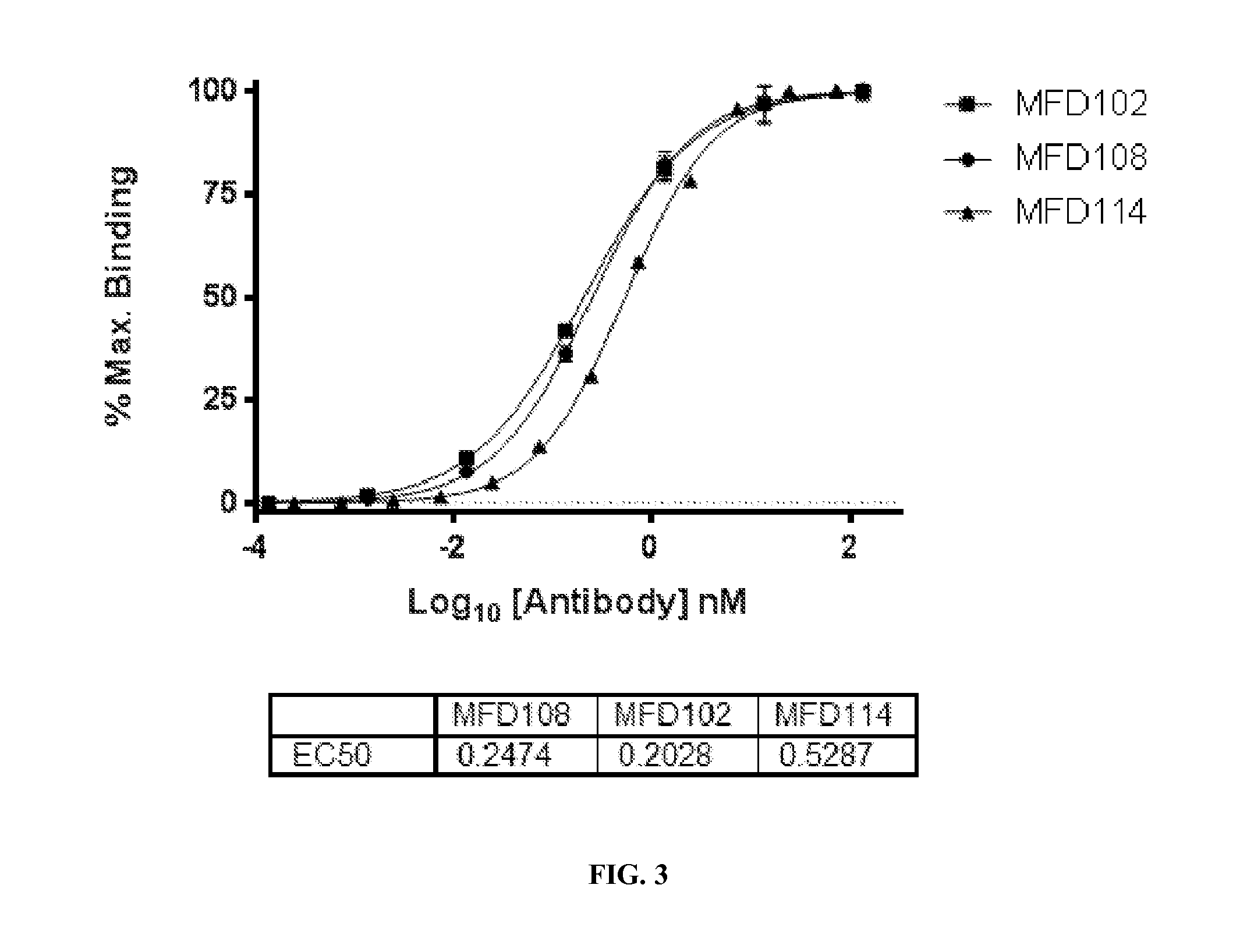
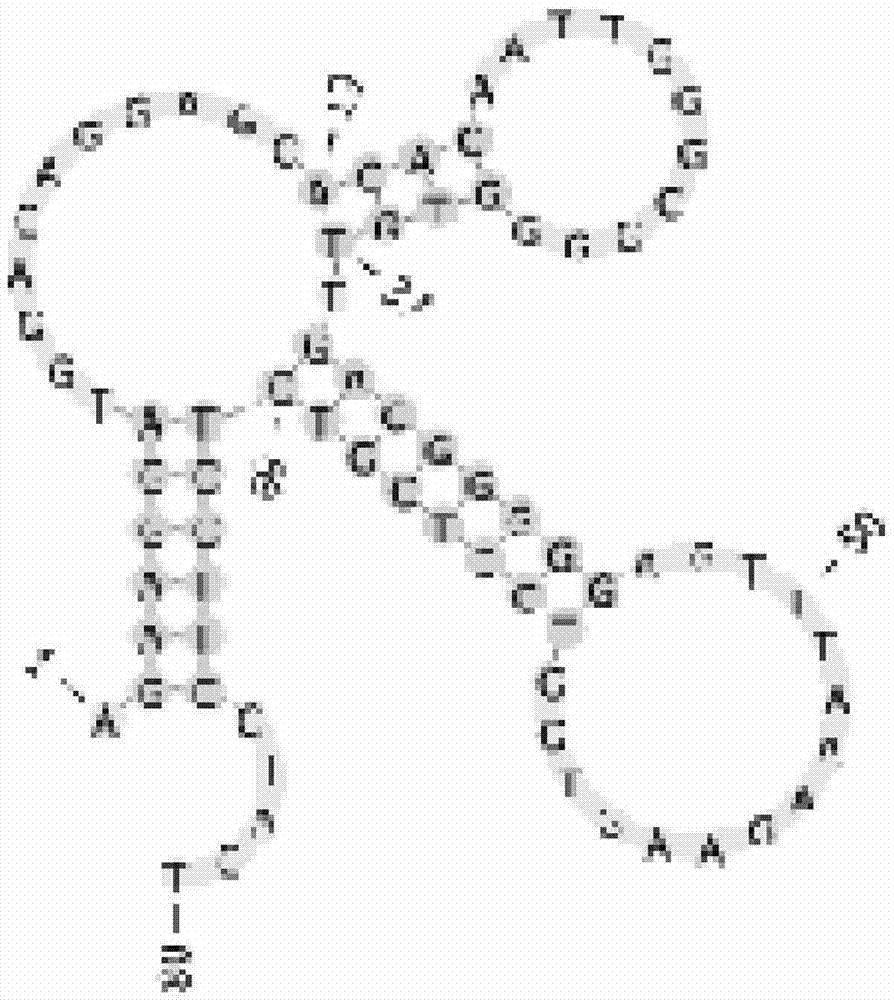
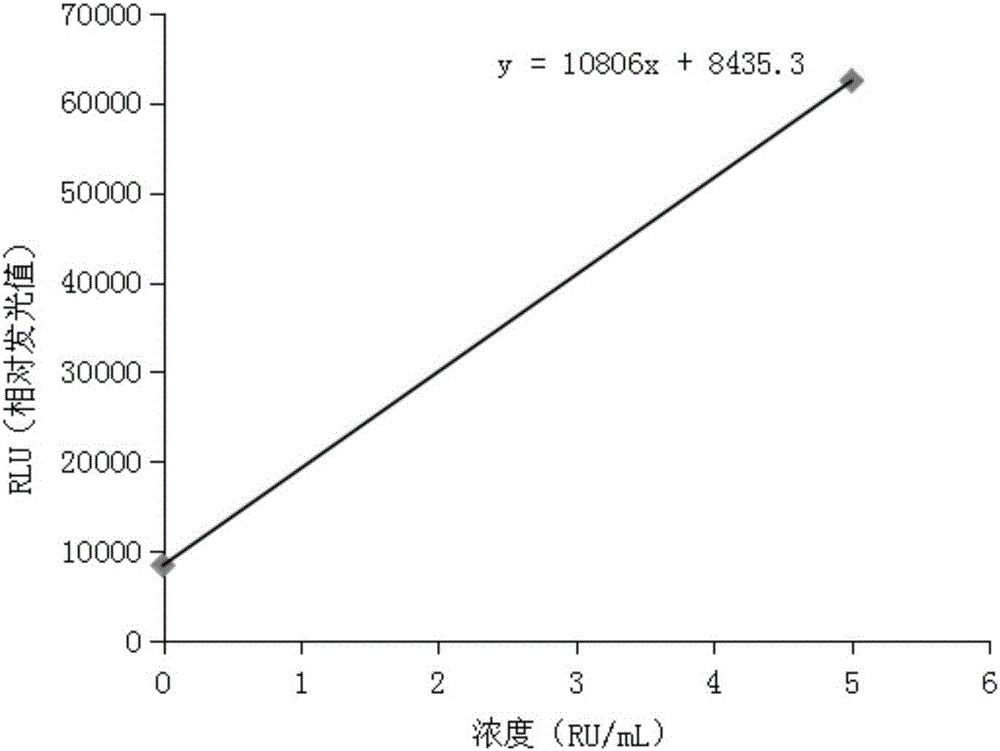
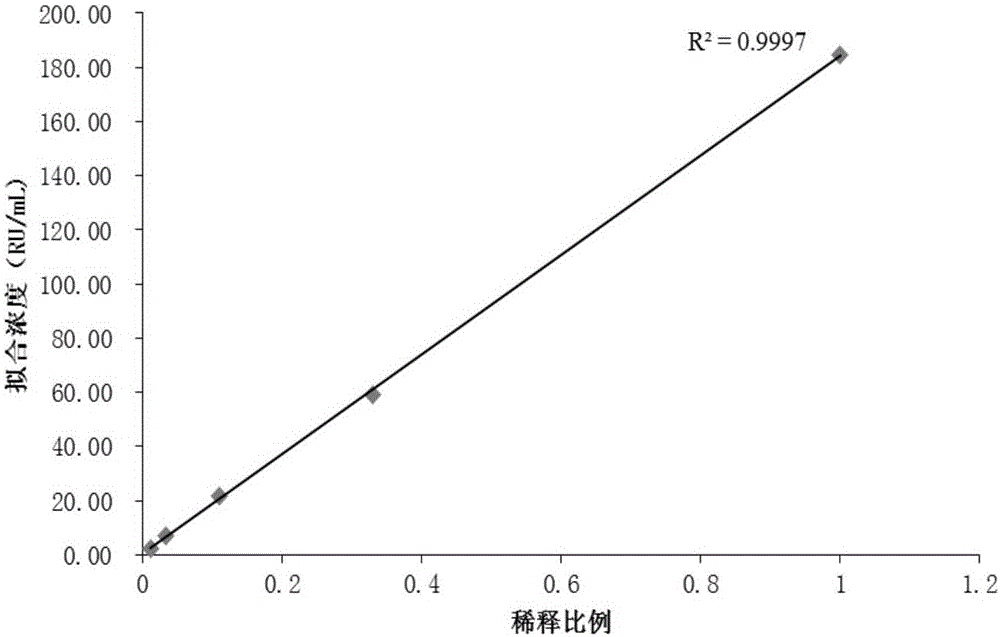
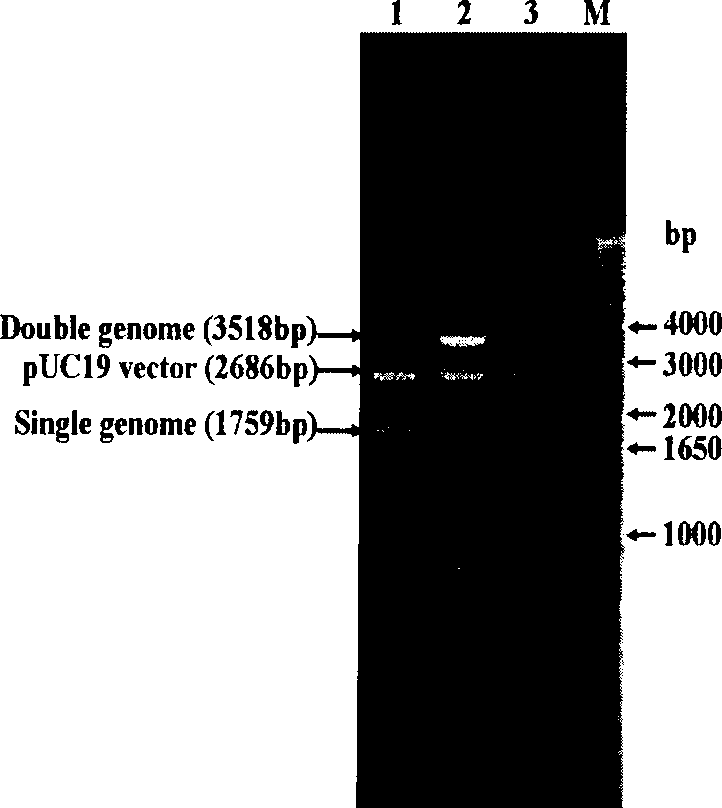
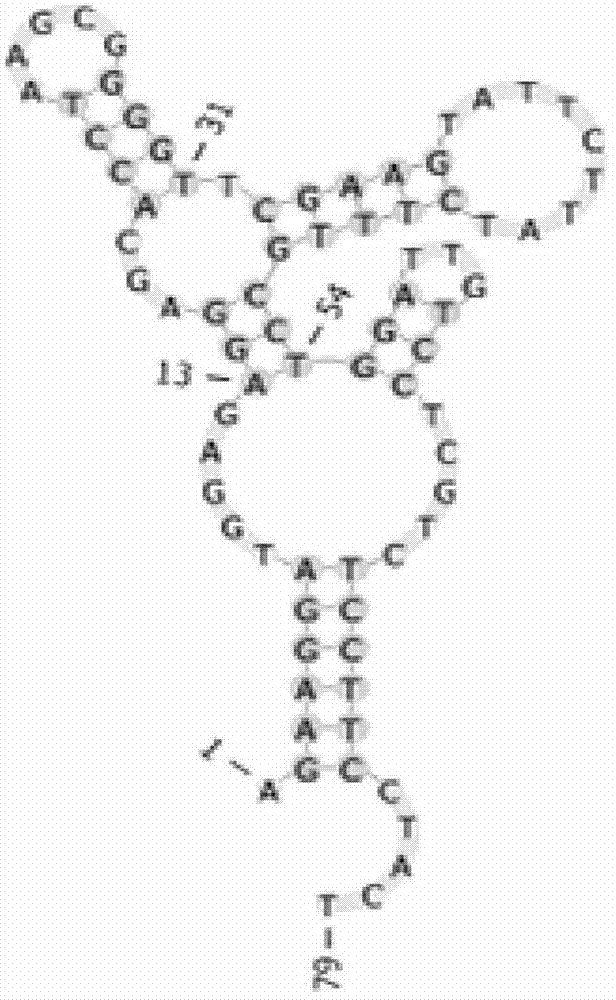Patents
Literature
650 results about "Protein antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Antibodies and related molecules that bind to 161P2F10B proteins
Antibodies and molecules derived therefrom that bind to 161P2F10B protein and variants thereof, are described wherein 161P2F10B exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, 161P2F10B provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The 161P2F10B gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with 161P2F10B can be used in active or passive immunization.
Owner:AGENSYS
Antibody drug conjugates (ADC) that bind to 191p4d12 proteins
ActiveUS20120078028A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProtein antibodyTissue specific
Antibody drug conjugates (ADC's) that bind to 191P4D12 protein and variants thereof are described herein. 191P4D12 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, the ADC's of the invention provide a therapeutic composition for the treatment of cancer.
Owner:AGENSYS +1
Antibodies to interleukin-1 receptor intracellular ligand proteins
InactiveUS6899878B2Inhibit inflammationPeptide/protein ingredientsAntipyreticInterleukin-1 receptorProtein antibody
Antibodies to IL-1-R intracellular ligand proteins are disclosed. The antibodies arc useful for inhibiting the binding between IL-1-R and IL-1-R intracellular ligand proteins.
Owner:GENETICS INST INC
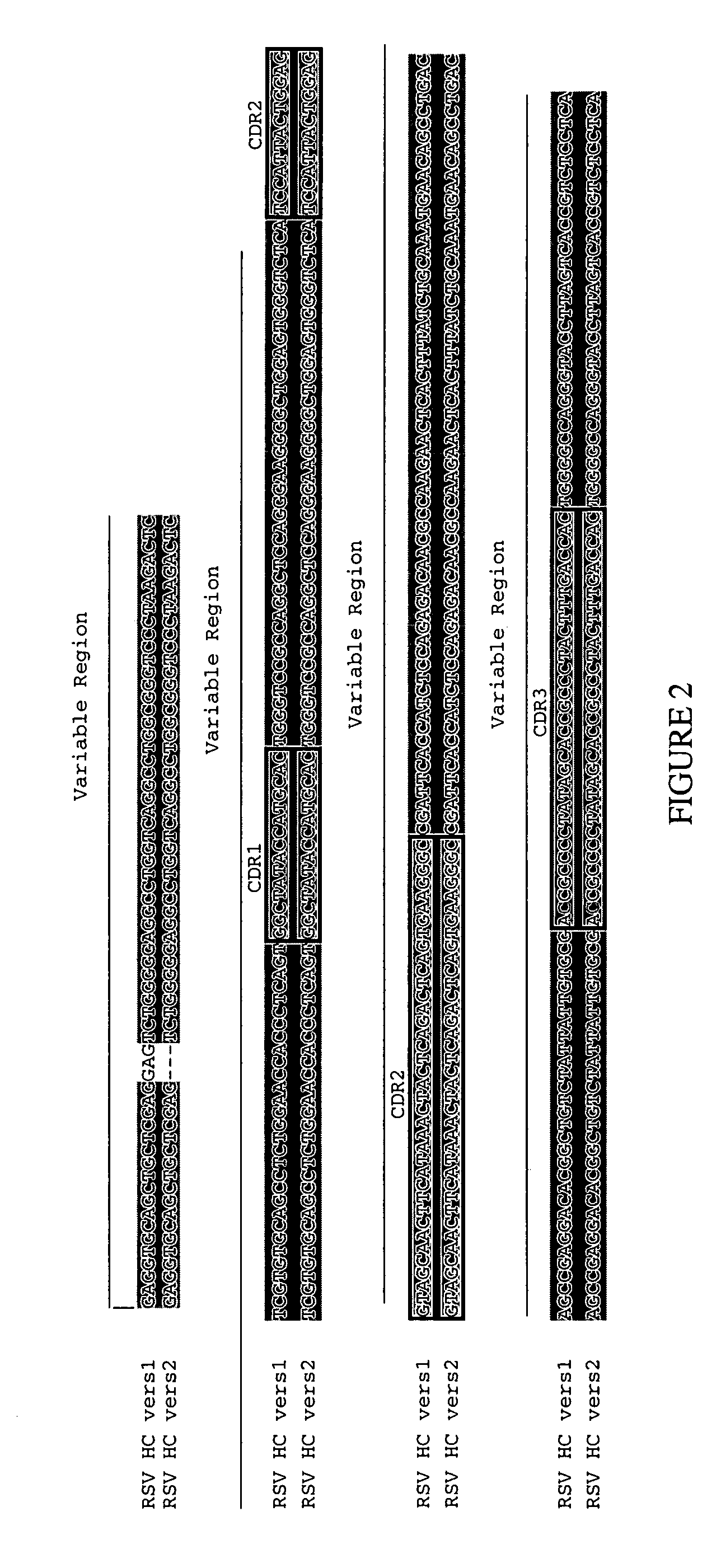
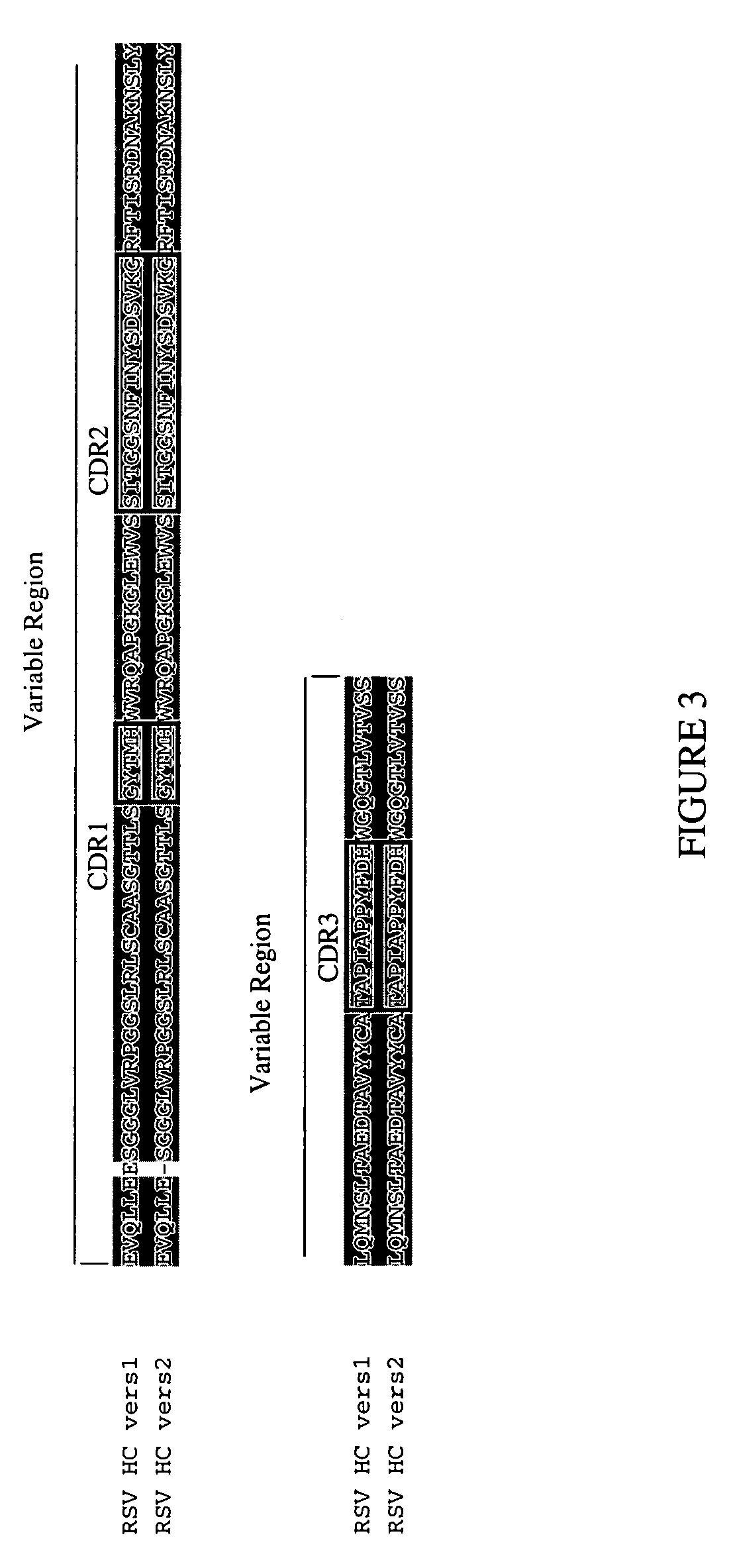
RSV proteins, antibodies, compositions, methods and uses
The present invention relates to at least one novel RSV proteins, antibodies, including isolated nucleic acids that encode at least one RSV protein or antibody, RSV vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:CENTOCOR
Antibody drug conjugates (ADC) that bind to 24P4C12 proteins
ActiveUS8309093B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTissue specificProtein antibody
Antibody drug conjugates (ADC's) that bind to 24P4C12 protein and variants thereof are described herein. 24P4C12 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, the ADC's of the invention provide a therapeutic composition for the treatment of cancer.
Owner:SEAGEN INC +1
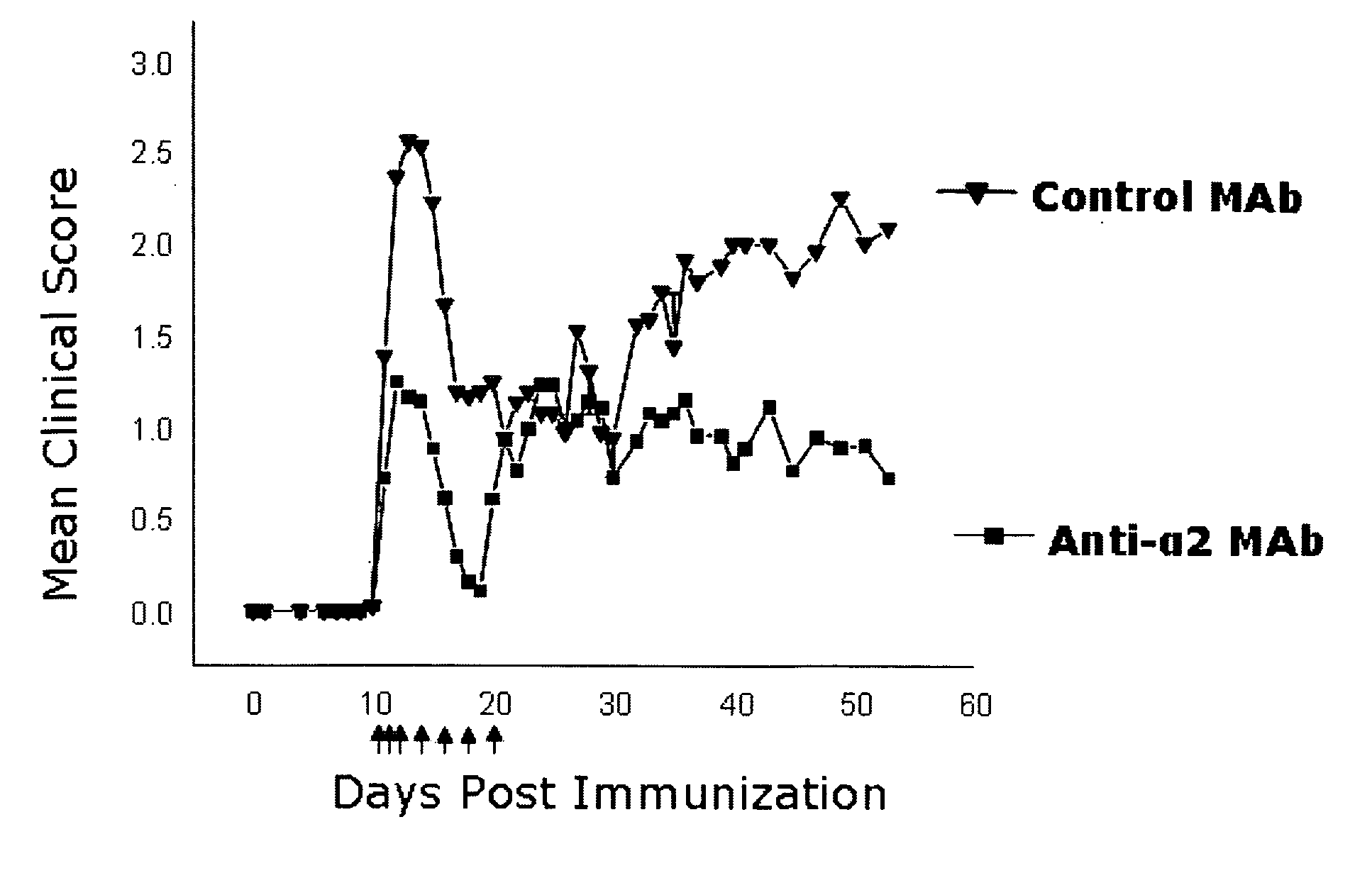
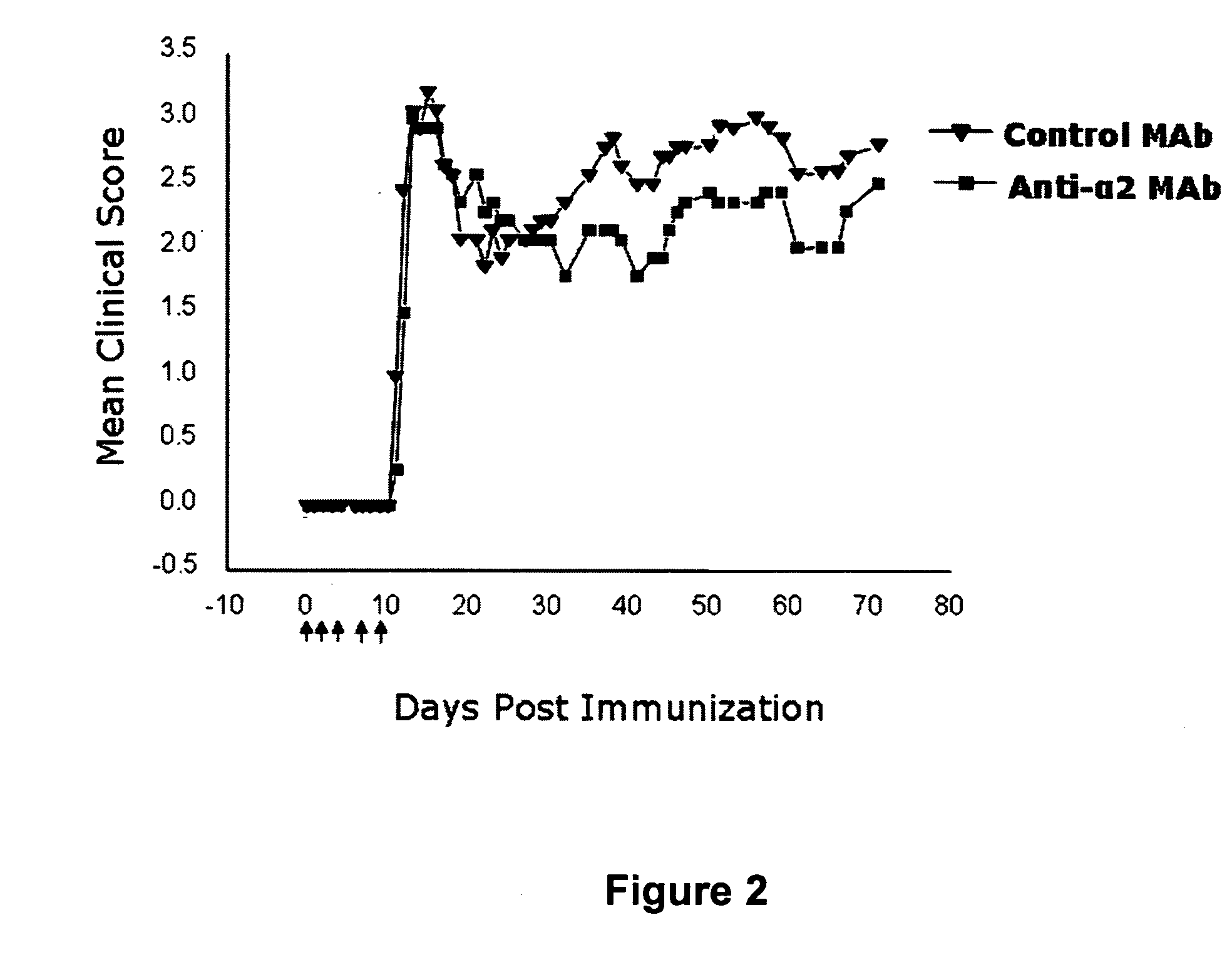
Anti-alpha2 integrin antibodies and their uses
InactiveUS20070128190A1Increased transgene transfer efficiencyEffective absorptionSenses disorderNervous disorderDiseaseAntiendomysial antibodies
The invention relates to anti-α2 integrin antibodies and their uses. Humanized antibodies are disclosed that bind to the I domain of α2 integrin and inhibit the interaction of α2β1 integrin with collagen. Also disclosed are therapeutic uses of anti-α2 integrin antibodies in treating α2β1-mediated disorders, including anti-α2 integrin antibodies that bind to α2 integrin without activating platelets.
Owner:ICHNOS SCI SA
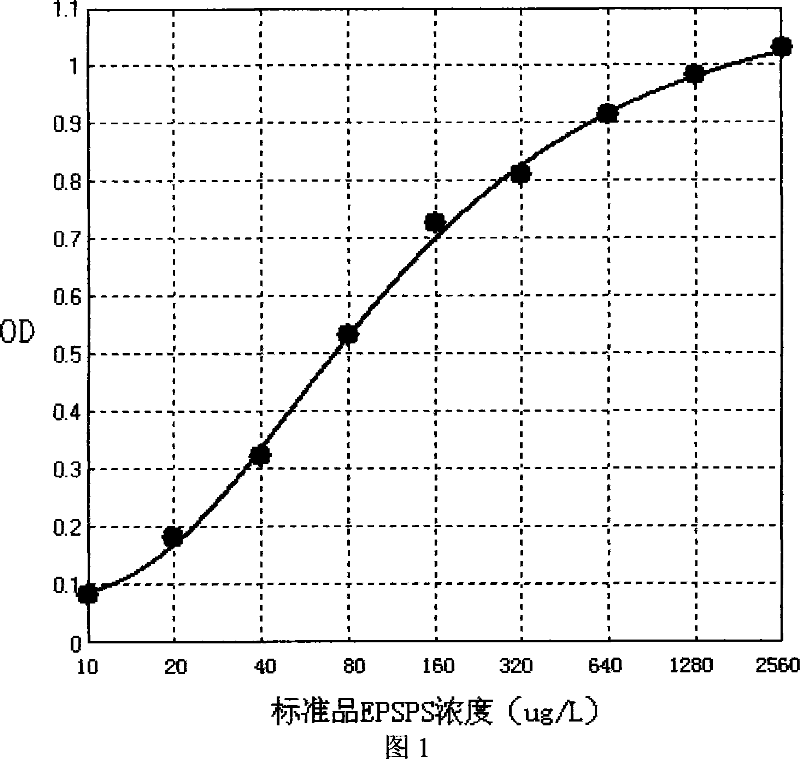
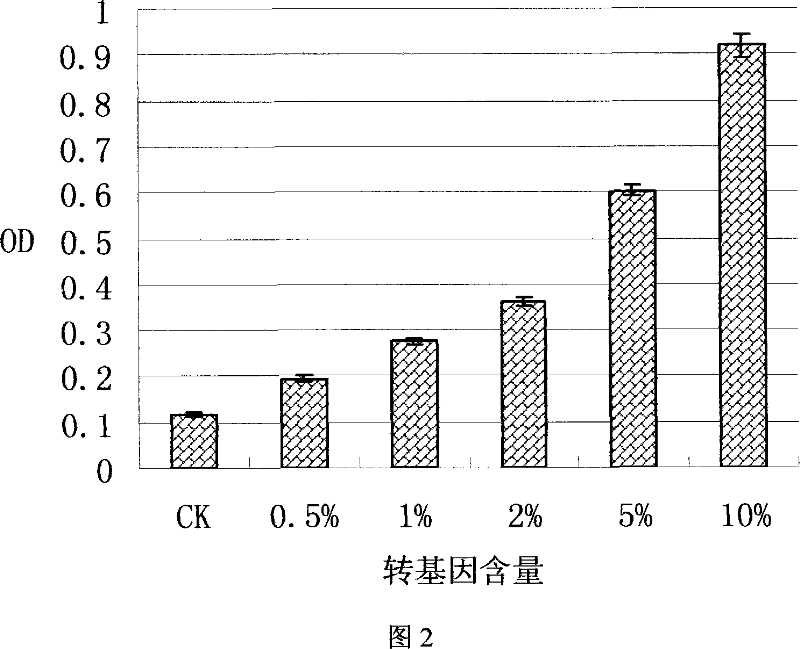
ELISA kit for detecting EPSPS gene in herbicide-tolerance soybeans and method of use thereof
This invention discloses one EPSPS gene enzyme immune agent case and its use method in the anti-herbicide bean, wherein, the case comprises anti-EPSPS multi-clone antigen, enzyme board with the antigen, enzyme antigens for two, gene switch bean standard product, non-transfer gene bean standard, intense wash liquid, develop liquid and terminal liquid. This invention method comprises the following steps: cloning CP4-EPSPS gene from the gene switch bean expressed in the bacillus coli; then through protein purification complex property to regroup EPSPS protein antibody immune animal to get special single clone antigen and multi-clone antigen; establishing double antigen clamp enzyme immune system test sample to determine its EPSPS protein content.
Owner:SOUTH CHINA AGRI UNIV
Nucleic acid aptamer and screening method thereof, and application of nucleic acid aptamer in prostate cancer cell strain detection
ActiveCN103642810AHigh affinityImprove featuresMicrobiological testing/measurementIndividual particle analysisProstate cancer cellChemical synthesis
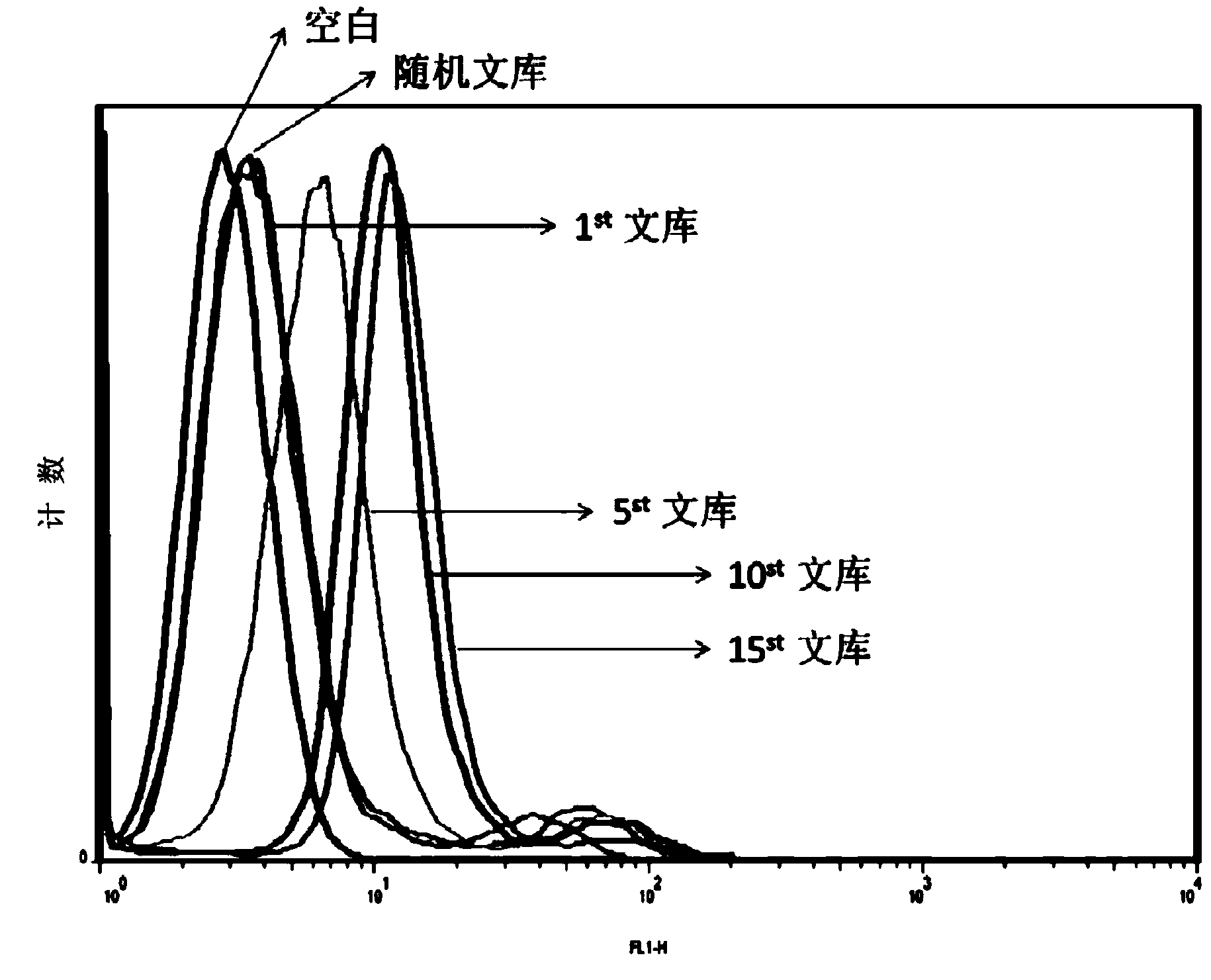
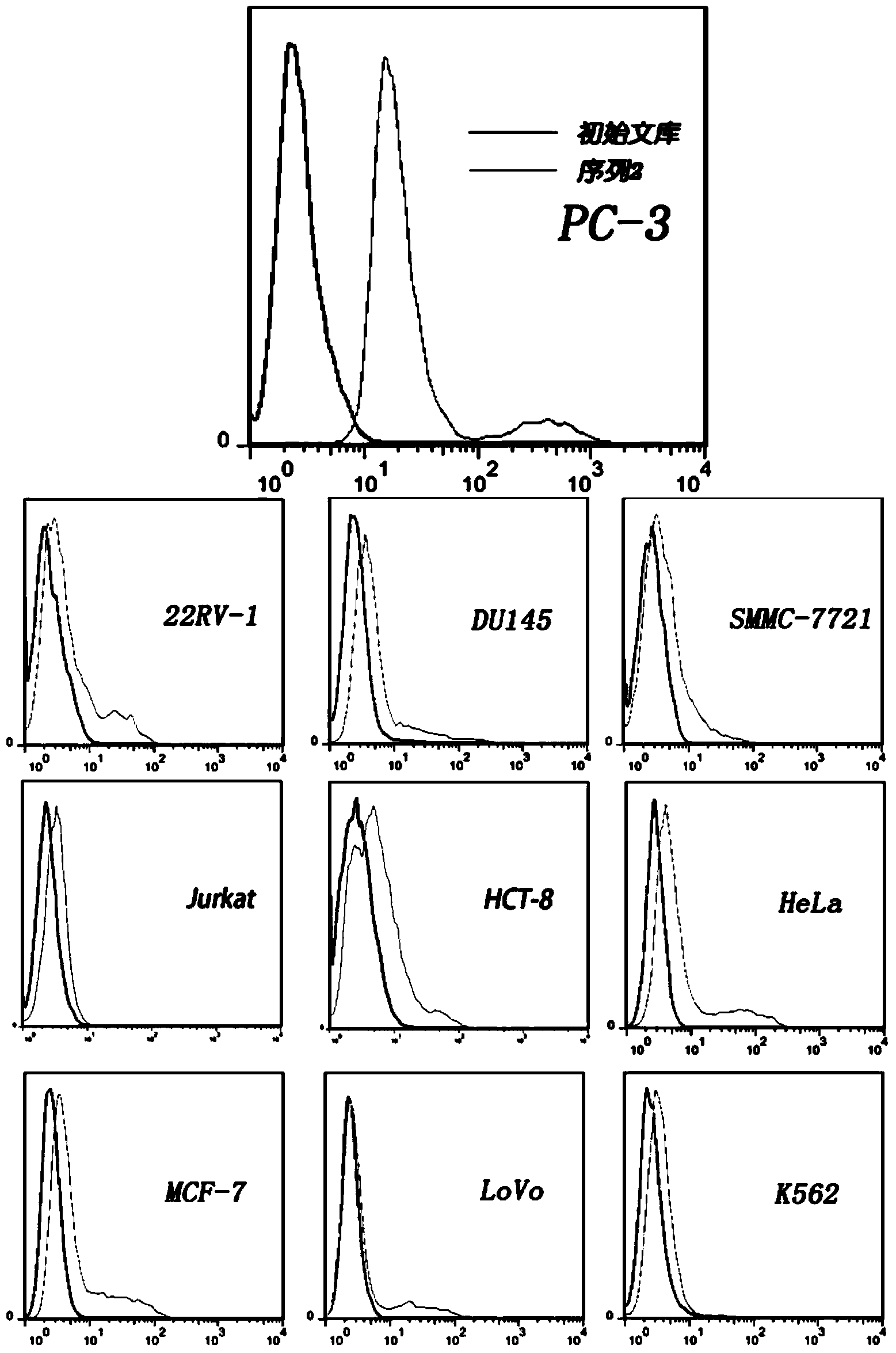
The present invention discloses a nucleic acid aptamer, wherein the sequence of the nucleic acid aptamer comprises a DNA segment represented by any one sequence selected from a sequence 1, a sequence 2 and a sequence 3. The nucleic acid aptamer can further be various similar sequences with high homology or a derivative obtained from the sequence of the present invention. The invention further discloses a nucleic acid aptamer screening method, which comprises: synthesizing a random single-stranded DNA library and primers, carrying out SELEX screening, carrying out PCR amplification of library, preparing a DNA single strand library, and finally carrying out repeated screening, negative screening and multi-round screening to obtain the nucleic acid aptamer. The nucleic acid aptamer and the derivative thereof can be used in recognition of the prostate cancer cell strain PC-3 or preparation of kits, molecular probes and targeted mediums for prostate cancer detection, and can further be used in design and preparation of prostate cancer treatment drugs. Compared with the protein antibody, the nucleic acid aptamer of the present invention has advantages of high affinity, high specificity, no immunogenicity, capability of being chemically synthesized, small molecular weight, stability, easy storage, easy labeling and the like.
Owner:GUANGZHOU SHIWEN BIOTECHNOLOGY CO LTD
Human epidermal growth factor receptor Her-2/neu quantitative detection kit and preparation method and application thereof
InactiveCN106932583AExcellent binding efficiencyLow self-crosslinkingChemiluminescene/bioluminescenceHuman epidermal growth factorHeterophil antibody
The invention provides a human epidermal growth factor receptor Her-2 / neu quantitative detection kit and a preparation method and application thereof. The kit is prepared through the magnetic particle chemiluminescent immunoassay, wherein a reconstructed Her-2 / neu antigen serves as a standard substance antigen and is diluted to a protein buffer component containing a non-ionic surfactant, the dispersity of the antigen is improved, autoagglutination is prevented, and the activity is maintained; besides, the Her-2 / neu protein antigen and magnetic particles are coupled, and the stability of a magnetic separation component is good by means of the optimized curing method and the blocking method; besides, by means of combined use of a multi-component immune complex and Roche active interference-eliminating proteins MAK33, the heterophil antibody interference in tumor marker sandwich method detection can be solved, and specificity of a finished product is improved. The kit is excellent in property, low in cost and long in validity period.
Owner:BEIJING DIACHA BIO ENG
Novel vaccine for preventing COVID-19 and preparation method thereof
ActiveCN111939250AHighly conservativeAntibody induction ability is weakSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleotideReceptor
Provided is a novel vaccine for preventing COVID-19, the nucleotide sequence of an antigen of the novel vaccine is SEQ NO: 1, the amino acid sequence of the antigen of the novel vaccine is SEQ NO: 2,and the antigen of the vaccine comprises two functional parts: an S protein receptor binding structural domain capable of inducing a specific neutralizing antibody and a T cell related N protein truncated peptide fragment capable of inducing and activating effector T cells; The vaccine disclosed by the invention has the characteristics that the T cell related N protein truncated peptide fragment has weak capability of inducing the generation of the N protein antibody, so that a vaccine inoculator and a COVID-19 infected patient can be identified by using the N protein antibody, and the vaccineantigen does not induce the generation of the N protein antibody, so that lung injuries can be reduced, and the vaccine is safer. The cell vaccine disclosed by the invention is low in manufacturing cost, and can induce generation of virus-specific neutralizing antibodies and T cell immune response.
Owner:ZHENGZHOU UNIV
African swine fever virus p72 recombinant protein, monoclonal antibody and test paper
ActiveCN110642926AImproving immunogenicityStrong specificityVirus peptidesBiological material analysisClassical swine fever virus CSFVNucleotide
The invention provides an African swine fever virus p72 recombinant protein, a monoclonal antibody and test paper. An amino acid sequence of the African swine fever virus p72 recombinant protein is obtained by linking amino acid sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2 through flexible amino acid fragments, and has strong immunogenicity and specificity. The invention also provides a nucleotide sequence for encoding the recombinant protein. The invention also provides the monoclonal antibody against the African swine fever virus p72 recombinant protein obtained by immunizing animals byusing the recombinant protein. The invention also provides hybridoma cells 7A7 and 3E5 with preservation numbers of CGMCC No. 18540 and CGMCC No. 18539, respectively, and the monoclonal antibody secreted by the hybridoma cells has high sensitivity and specificity to African swine fever virus p72. The invention also provides the latex microsphere test paper containing the recombinant protein antibody and the colloidal gold test paper containing the recombinant protein antibody, and the test paper has the advantages of small batch difference, high detection sensitivity and simple operation.
Owner:北京纳百生物科技有限公司 +1
Hmgi proteins in cancer and obesity
InactiveUS20030051260A1Peptide/protein ingredientsMicrobiological testing/measurementProtein regulationEmbryonic Stage
The present invention pertains to a method for treating obesity in a mammal which comprises reducing the biological activity of HMGI genes in the mammal. In another embodiment, the invention pertains to a method for treating a tumor in a patient by reducing the biological activity of normal HMGI genes which comprises administering to the patient a therapeutically effective amount of an inhibitor compound active against normal HMGI-C or HMGI(Y) genes. In another embodiment, the invention pertains to a method of producing a transgenic non-human mammal, the germ cells and somatic cells of which contain an inactivated HMGI gene sequence introduced into the mammal at an embryonic stage. In another embodiment, the invention pertains to a method for screening candidate compounds capable of inhibiting the biological activity of normal HMGI proteins. In another embodiment, the invention pertains to a method for screening candidate compounds capable of inhibiting the biological activity of normal HMGI genes. In another embodiment, the invention pertains to a method for detecting normal HMGI proteins as a diagnostic marker for a tumor using a probe. that recognizes normal HMGI proteins, which comprises the steps of (a) contacting normal HMGI proteins from a sample from a patient with a probe which binds to HMGI proteins; and (b) analyzing for normal HMGI proteins by detecting levels of the probe bound to the normal HMGI proteins, wherein the presence of normal HMGI proteins in the sample is positive for a tumor. In another embodiment, the invention pertains to a method for detecting antibodies to normal HMGI proteins using a probe that recognizes antibodies to HMGI normal proteins, which comprises the steps of (a) treating a sample from a patient with a probe which binds to antibodies to normal HMGI proteins; and (b) analyzing for antibodies to HMGI proteins by detecting levels of the probe bound to the antibodies to HMGI proteins, wherein the presence of antibodies to normal HMGI proteins in the sample is positive for a tumor. In another embodiment, the invention pertains to HMGI genes and proteins for use as a starting point to isolate downstream target genes regulated by the HMGI genes and proteins.
Owner:MEDICINE & DENTISTRY OF NEW YORK UNIV OF
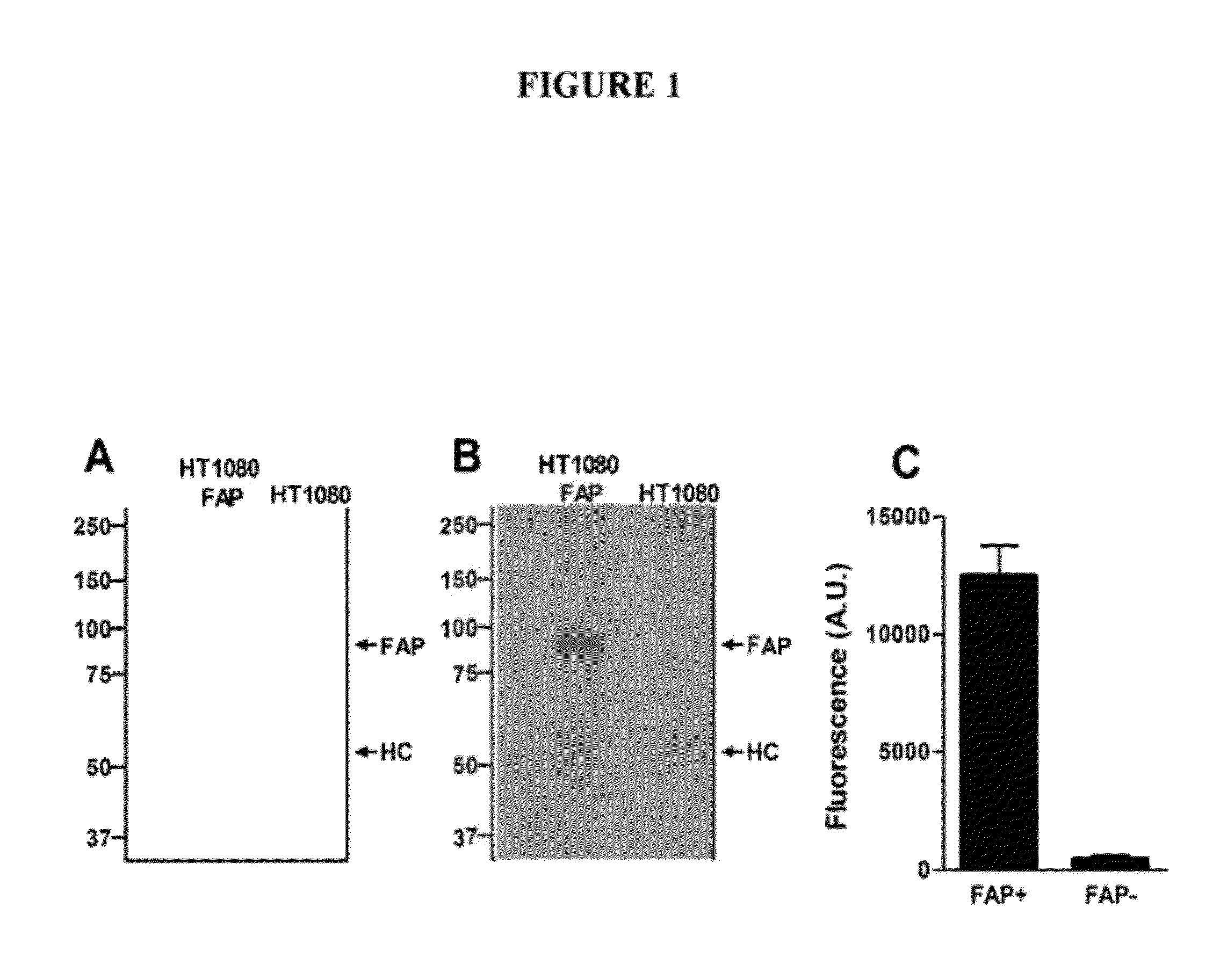
Anti-fibroblast activation protein antibodies and methods and uses thereof
InactiveUS20120258119A1Reduce the amount requiredReduce the numberSugar derivativesAntibody ingredientsAnticarcinogenFibroblast activation protein, alpha
Specific binding members, particularly antibodies and fragments thereof, which bind to Fibroblast Activation Protein (FAP) are provided, particularly recognizing both human and mouse FAP. These antibodies are useful in the diagnosis and treatment of conditions associated with activated stroma, including wound healing, epithelial cancers, osteoarthritis, rheumatoid arthritis, cirrhosis and pulmonary fibrosis. The anti-FAP antibodies, variable regions or CDR domain sequences thereof, and fragments thereof may also be used in therapy in combination with chemotherapeutics, immune modulators, or anti-cancer agents and / or with other antibodies or fragments thereof. Antibodies of this type are exemplified by the novel antibodies ESC1 1 and ESC 14 whose sequences are provided herein.
Owner:LUDWIG INST FOR CANCER RES
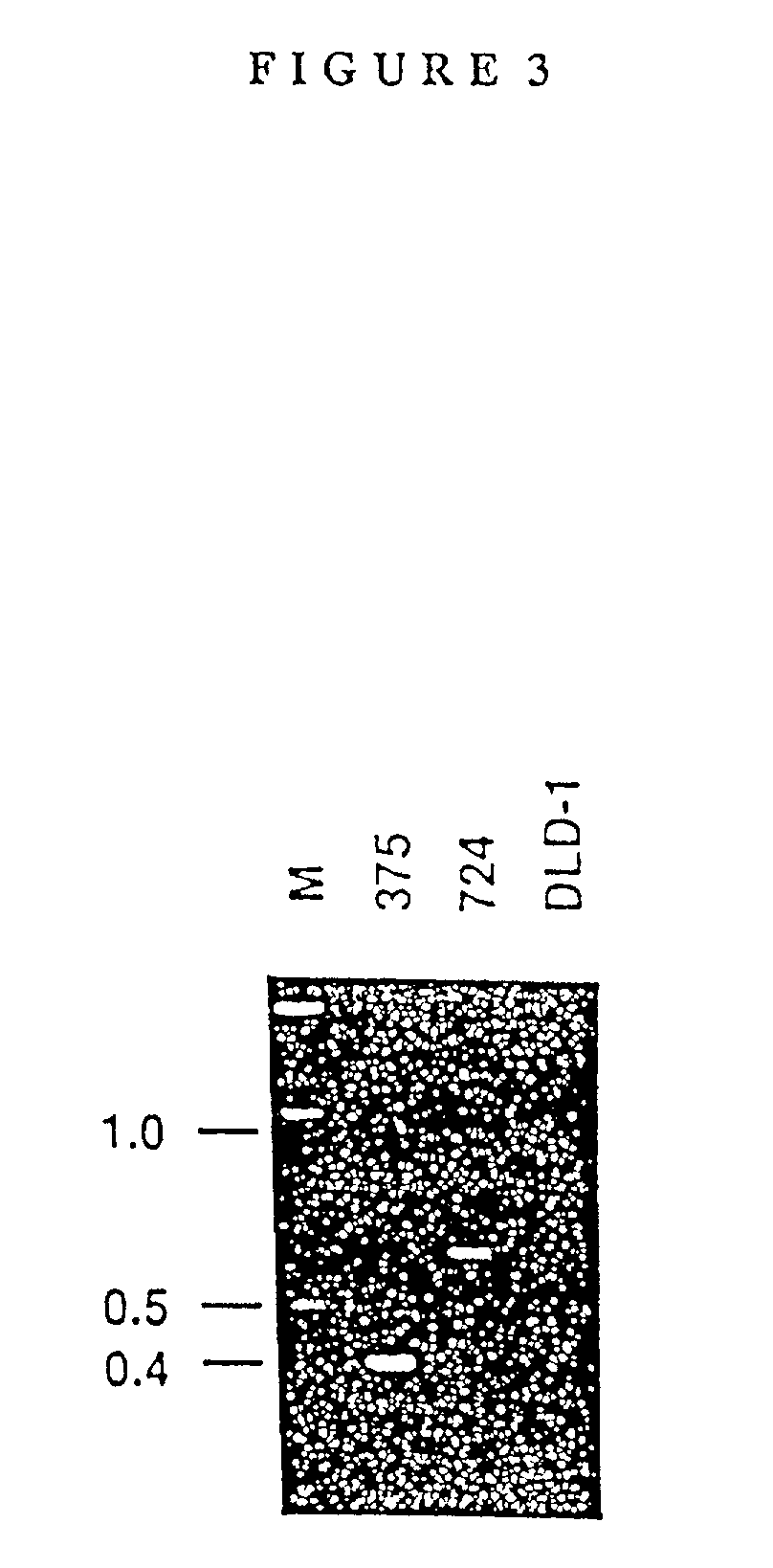
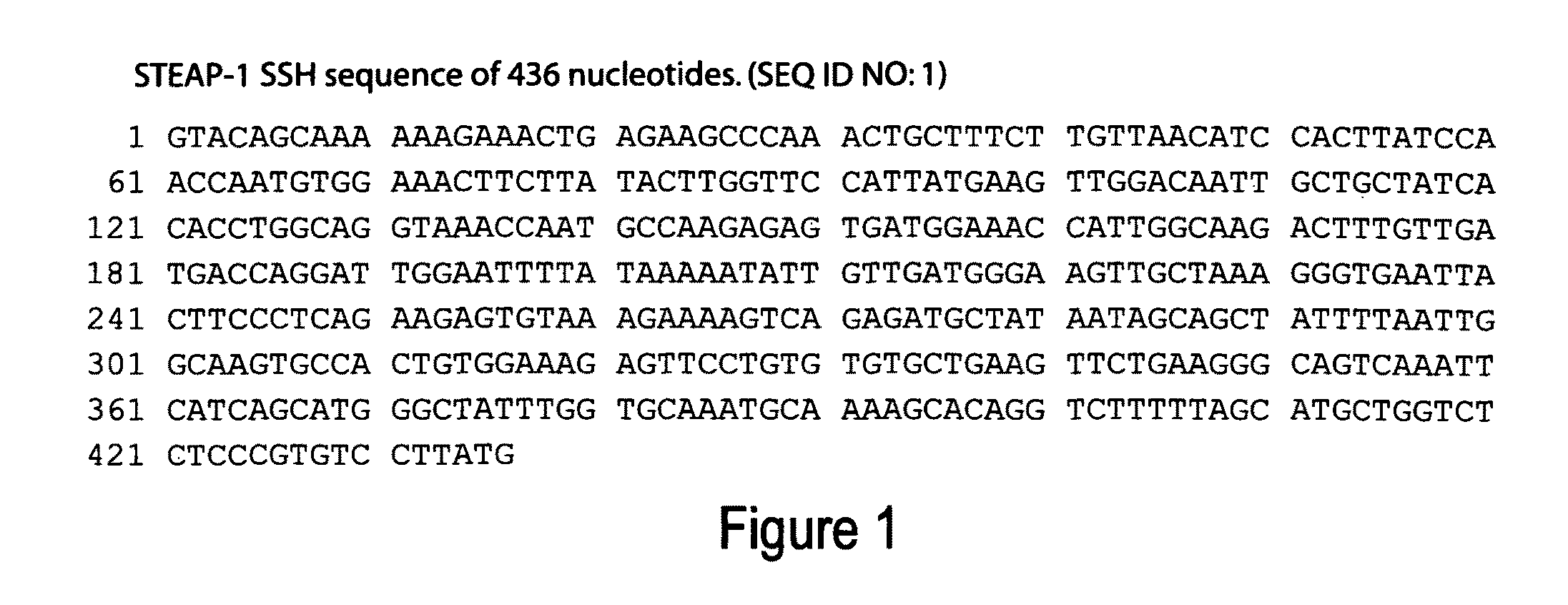
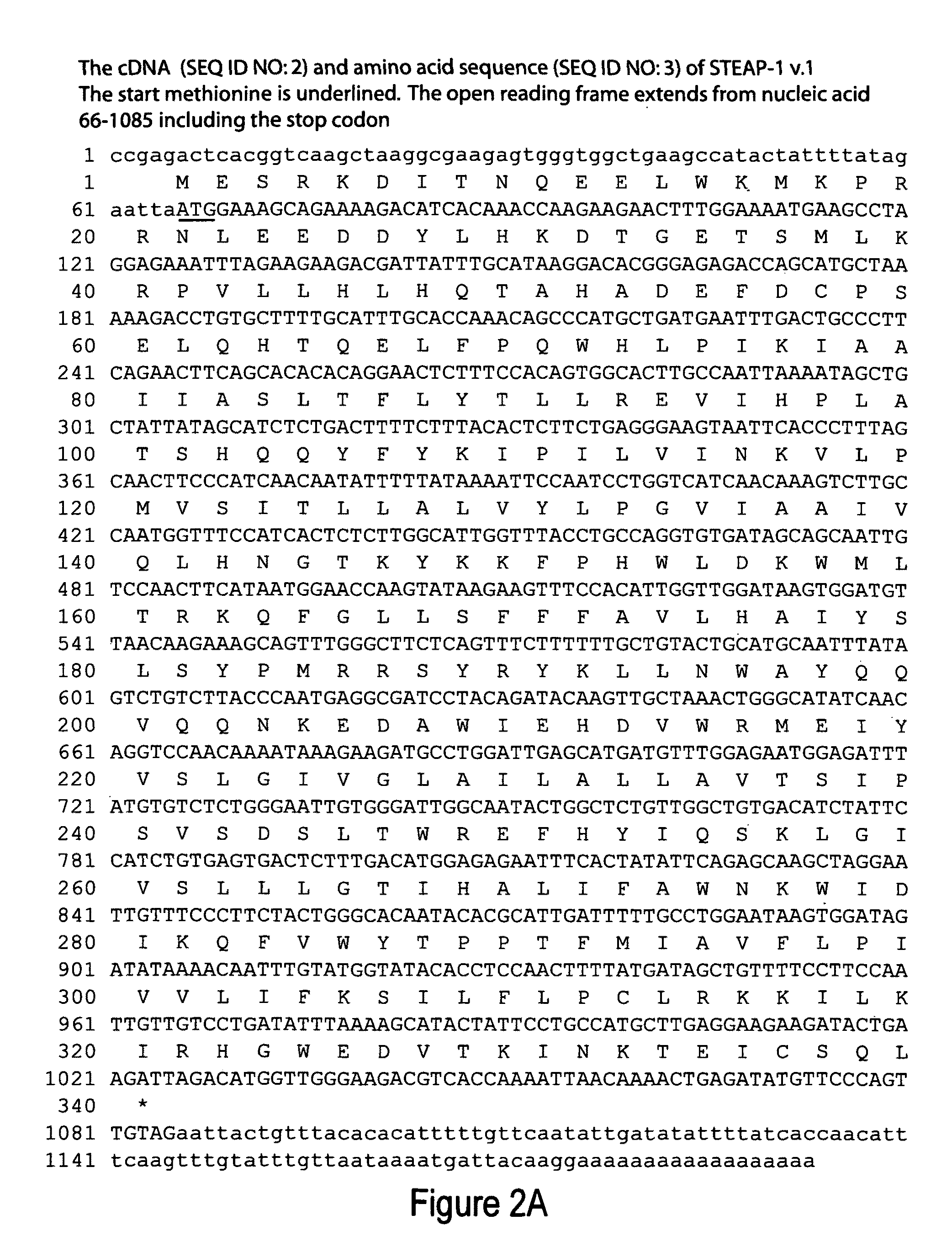
Antibodies and molecules derived therefrom that bind to STEAP-1 proteins
Antibodies and molecules derived therefrom that bind to novel STEAP-1 protein, and variants thereof, are described wherein STEAP-1 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, STEAP-1 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The STEAP-1 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with STEAP-1 can be used in active or passive immunization.
Owner:AGENSYS
Kit for detecting serum amyloid protein and application thereof
InactiveCN106353507AEnhanced detection signalHigh detection sensitivityBiological testingChemistryAntibody
The invention provides a kit for detecting serum amyloid protein and application thereof. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from a buffer solution, inorganic salt, a surfactant, a preservative, a stabilizer and interference elimination protein; the reagent R2 is prepared from the buffer solution, the inorganic salt, the surfactant, the preservative, the stabilizer and a polystyrene latex particle mixture; the polystyrene latex particle mixture is cross-linked with an SAA (Serum Amyloid A) antibody; the polystyrene latex particle mixture is a mixture of large-diameter polystyrene latex particles and small-diameter polystyrene latex particles. The kit disclosed by the invention is based on PETIA (Particle-enhanced Turbidimetric Immunoassay) and can be generally used for analysis of all kinds of full-automatic biochemical analyzer; during use, the required determining time is short, the specificity is high, the precision degree is high, and the accuracy degree is high.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Neutrophil gelatinase-associated lipocalin (NGAL) protein level ELISA kit
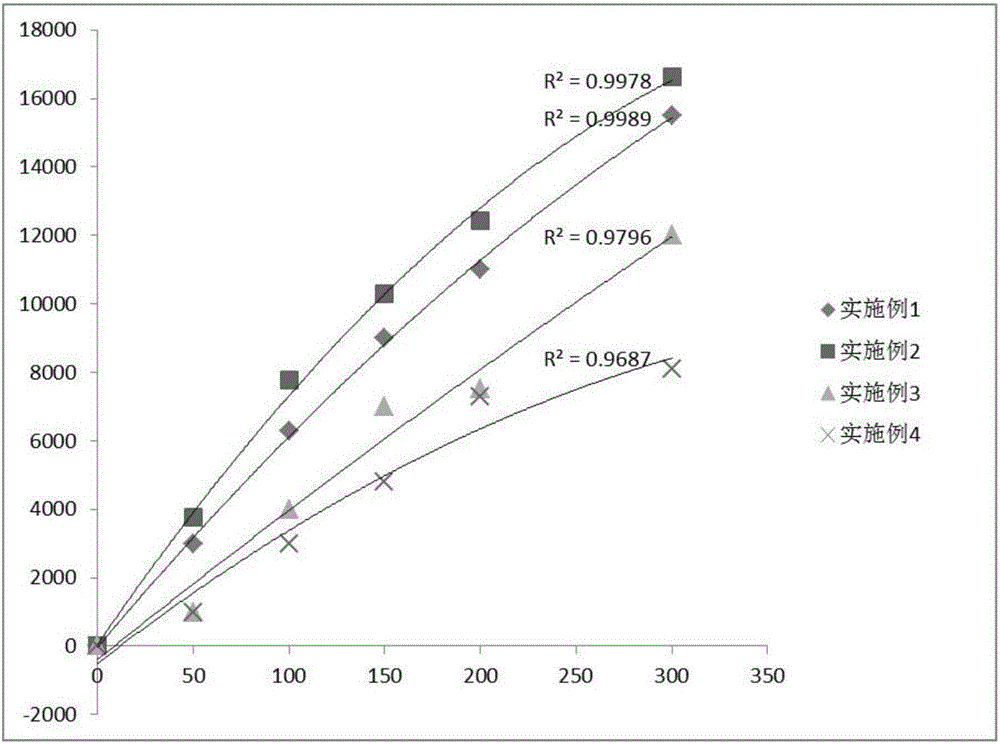
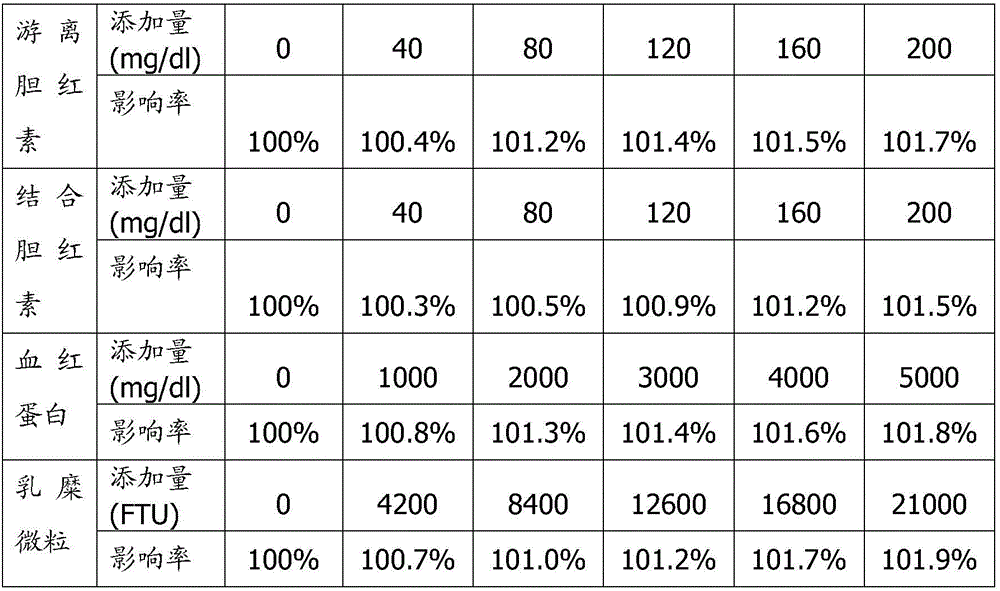
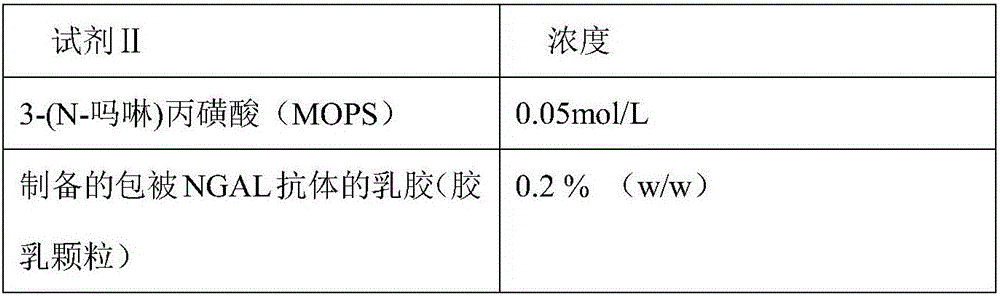
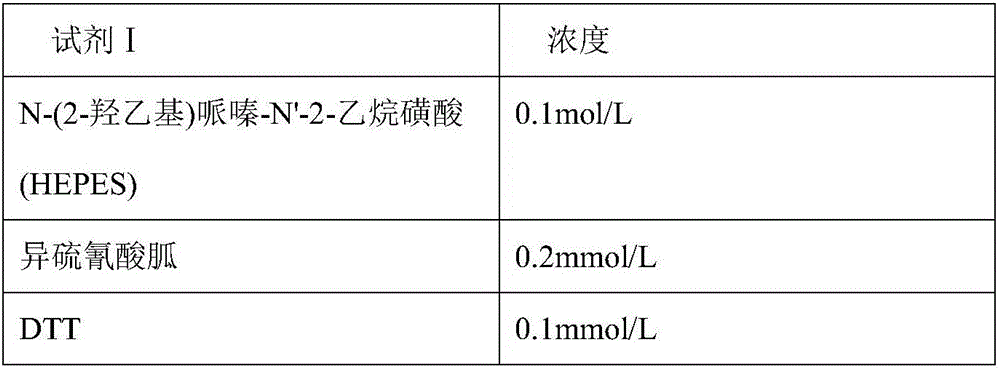
ActiveCN106814193ASpeed up dissociationColor/spectral properties measurementsBiological testingElisa kitBinding site
Disclosed is a neutrophil gelatinase-associated lipocalin (NGAL) protein level ELISA kit which is composed of a reagent I and a reagent II. The reagent I comprises a slow-release agent and a denaturant; the reagent II comprises latex particles coated with NGAL antibodies. Aggregated protein is denatured to some extent after being added with the denaturant, physical and (or) chemical binding site is exposed, chemical binding action of the chemical binding site is fractured through sulfydryl dissociation agent , while physical binding action of the physical binding site is dispersed by surface active agent, so that the aggregated protein is disaggregated. Therefore, it is quite important to choose the appropriate types and concentrations of denaturant, sulfydryl dissociation agent, and surface active agent; the aggregate is disaggregated without interference on following immunological detection. According to the method, the the appropriate types and concentrations of denaturant, sulfydryl dissociation agent and surface active agent are determined and chosen specifically, which solves the technical problem above.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Electrochemiluminescence immunosensor for detecting beta-amyloid protein and construction of electrochemiluminescence immunosensor
ActiveCN107192749AEffectively fixedStrong and efficient covalent bindingChemiluminescene/bioluminescenceColor/spectral properties measurementsDisease markersGold nanorod
The invention relates to an electrochemiluminescence immunosensor for detecting beta-amyloid protein and construction of the electrochemiluminescence immunosensor. The sensor comprises a magnetic glassy carbon electrode and Fe3O4-expoxy, a mesoporous carbon @Ru(bpy)32+ / beta-amyloid protein antibody composite nanometer composite material, beta-amyloid protein to be detected with different concentrations and a gold nanorod-beta- beta-amyloid protein aptamer nanometer composite material, wherein the Fe3O4-expoxy, the mesoporous carbon @Ru(bpy)32+ / beta-amyloid protein antibody composite nanometer composite material, beta-amyloid protein to be detected with different concentrations and the gold nanorod-beta- beta-amyloid protein aptamer nanometer composite material are synthesized on the surface of the magnetic glassy carbon electrode in sequence. Compared with the prior art, the electrochemiluminescence immunosensor has the advantages that the constructing method is simple, detection on Alzheimer's disease markers (beta-amyloid protein and the like) is high in speed, high in sensitivity, high in specificity, low in detection limit, wide in detection range and the like, and the application of the electrochemiluminescence immunosensor provides a new thought for early diagnosis of the Alzheimer's disease and detection of markers of other diseases.
Owner:SHANGHAI NORMAL UNIVERSITY
Cyclic chimeric citrullinated peptide antigen and application thereof
InactiveCN104262489AImprove stabilityIncrease exposureBiological testingHybrid peptidesPeptide antigenDisulfide bonding
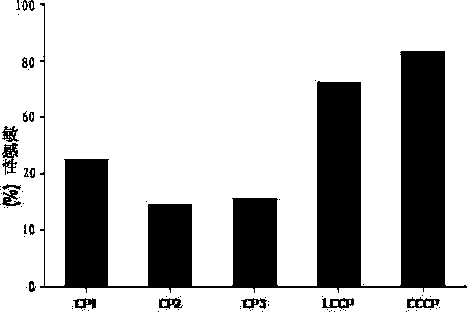
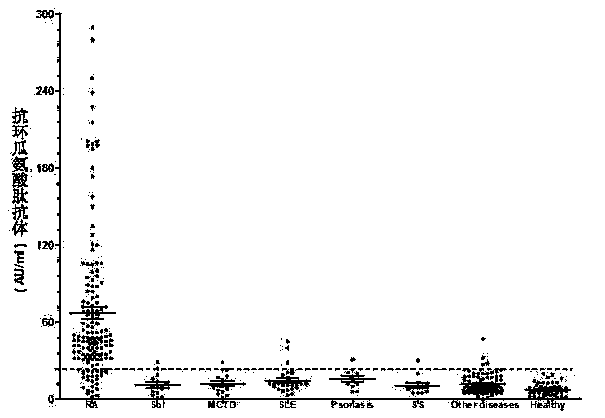
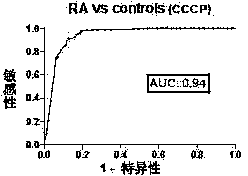
The invention discloses a cyclic chimeric citrullinated peptide antigen and an application thereof. The preparation of the cyclic chimeric citrullinated peptide antigen comprises the following steps: firstly connecting and jogging three small-molecular antigen peptides, namely a citrullinated peptide1, a citrullinated peptide 2 and a citrullinated peptide 3 derived from a silk polymerizing protein / an intermediate filament protein, and then synthetizing a cyclic polypeptide with a similar protein beta-corner structure by forming a disulfide bond through two cysteines inserted into the end N and the end C of a chimeric peptide. The cyclic chimeric citrullinated peptide antigen coats a solid-phase vector to prepare an indirect enzyme linked immunosorbent assay kit used for detecting the hypotype of multiple anti-citrullinated protein antibodies contained in RA (Rheumatoid Arthritis) serum. The cyclic chimeric citrullinated peptide antigen and the ELISA kit thereof which are disclosed by the invention have the advantages of simple preparation and experimental operation process, good result repeatability, qualification or quantification and wide clinical application and scientific research value and are outstandingly enhanced in detection sensibility and diagnosis value on RA compared with an international similar kit.
Owner:陈仁奋
Transthyretin antibodies and uses thereof
The present invention provides compositions comprising anti-transthyretin antibodies. The compositions are particularly useful for diagnosis, prognosis and / or treatment of amyloid diseases or symptoms thereof.
Owner:PROTEGO BIOPHARMA INC
Nucleic acid aptamer and derivatives thereof, screening method of nucleic acid aptamer, application of nucleic acid aptamer and derivatives in detecting human biliary duct carcinoma cell line
ActiveCN103205431AHigh affinityImprove featuresMicrobiological testing/measurementDNA preparationAptamerChemical synthesis
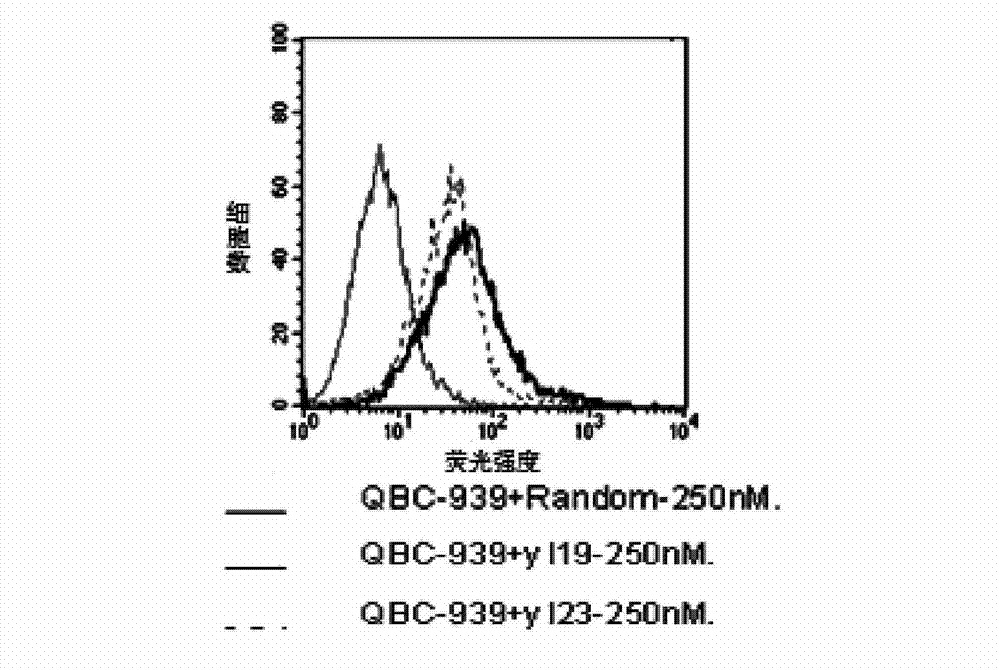
The invention discloses a nucleic acid aptamer with a sequence containing DNA (deoxyribonucleic acid) segments shown by any of a sequence 1 and a sequence 2. The nucleic acid aptamer can also be derivatives obtained by various similar sequences high in homology or the sequence. The invention further discloses a screening method of the nucleic acid aptamer. The method includes: synthesizing a random single-strand DNA library and a primer, performing Cell-SELEX screening and PCR (polymerase chain reaction) library amplification, preparing a DNA single-strand library, and obtaining the nucleic acid aptamer through repeated screening, negative screening and several rounds of screening. The nucleic acid aptamer and derivatives thereof can be applied to recognizing human biliary duct carcinoma cell line QBC-939 or preparing a reagent box for detecting the human biliary duct carcinoma cell line QBC-939, has affinity and specificity higher than anti-keratin antibodies, and is free of immunogenicity, capable of being chemically synthesized, small in molecular weight, stable, easy to store and mark, and the like.
Owner:HUNAN UNIV
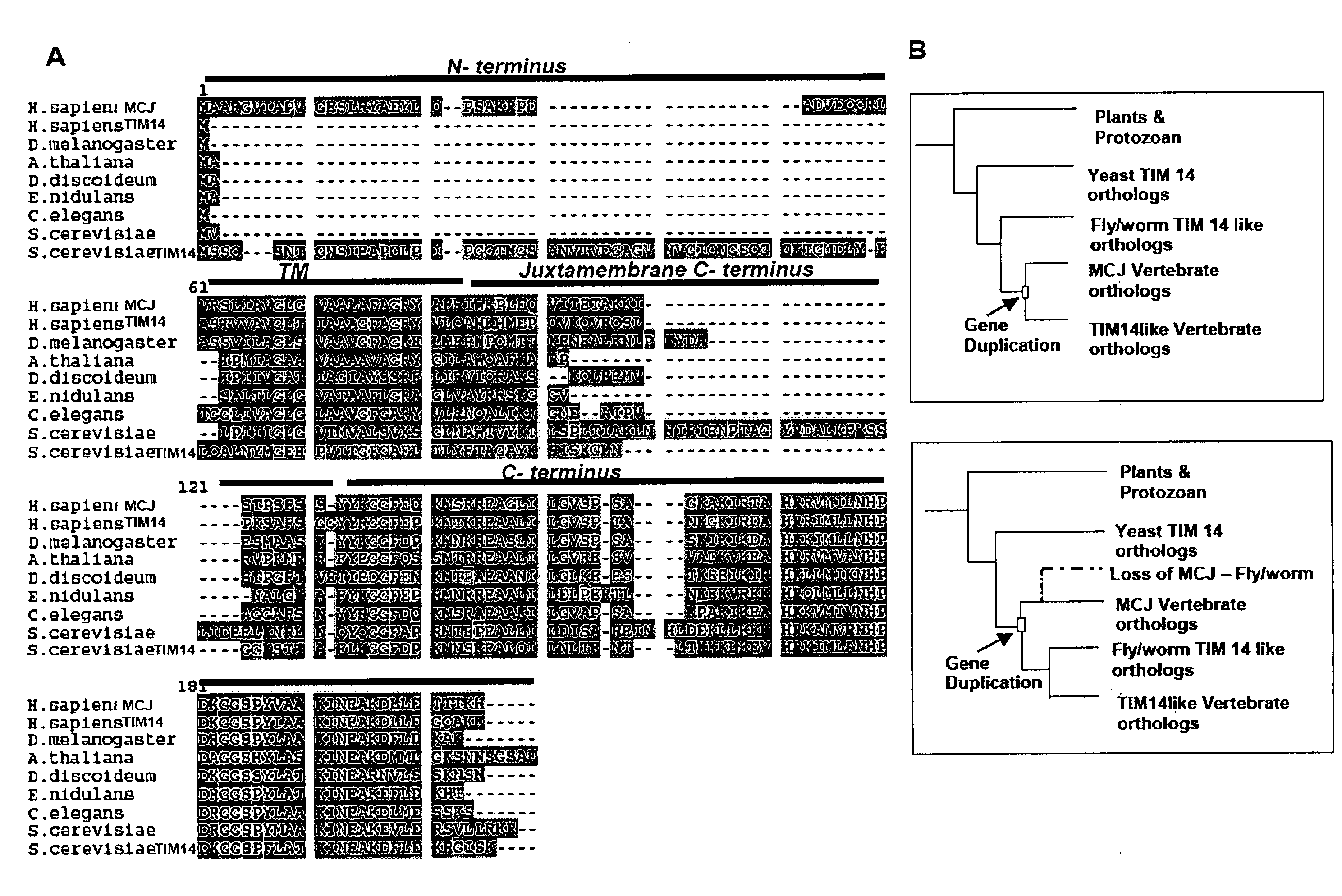
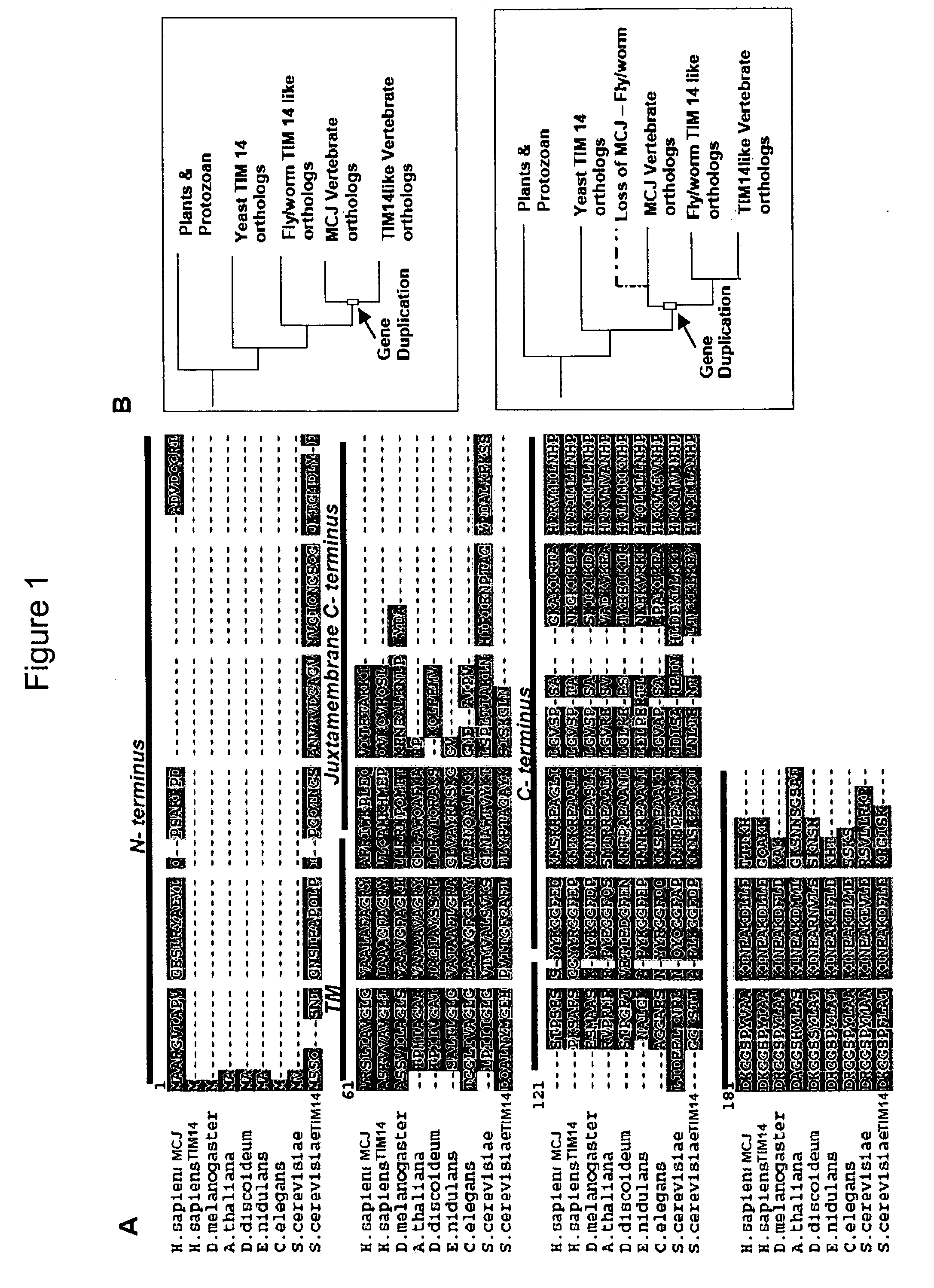
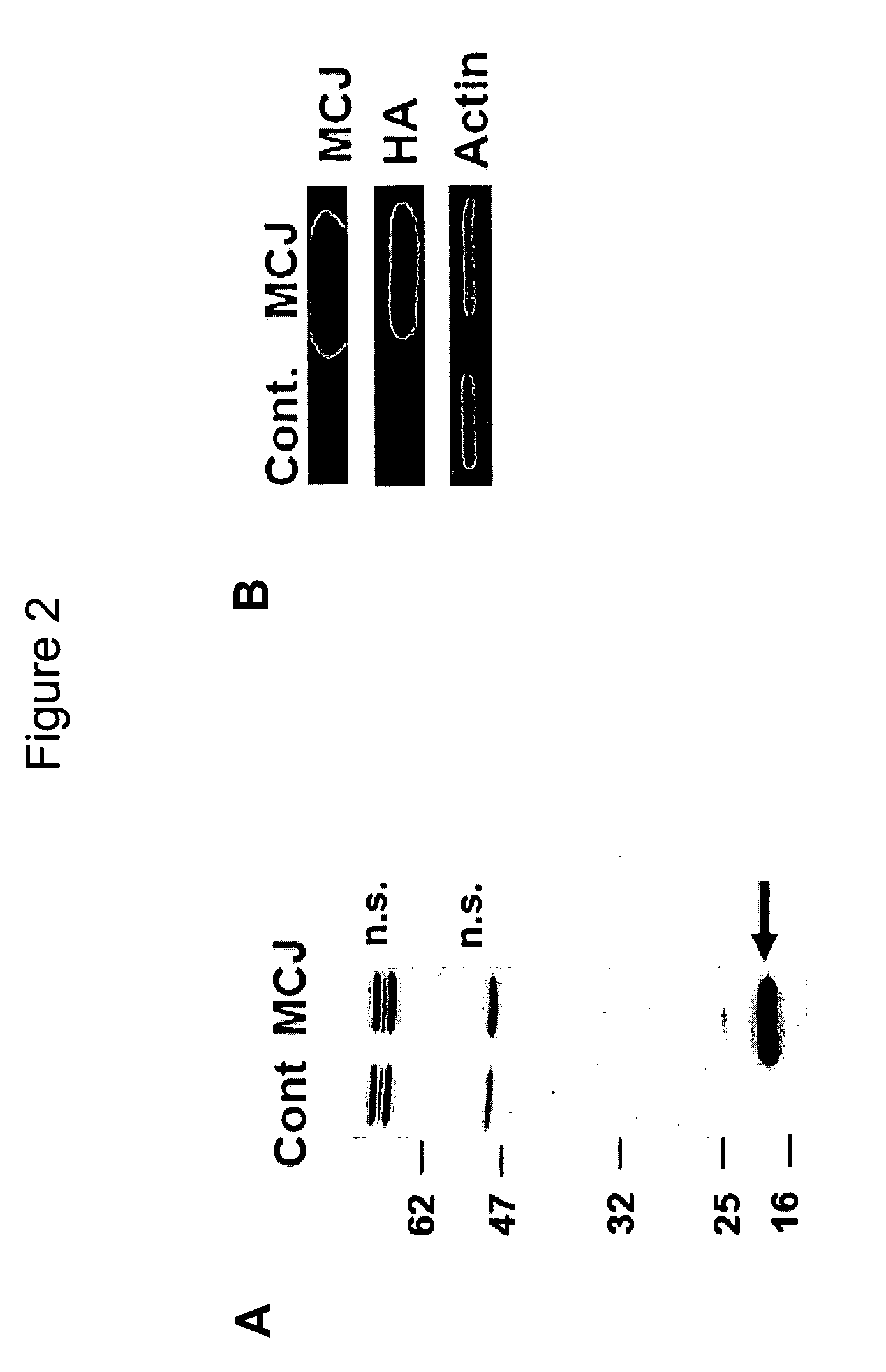
Anti-Methylation-Controlled J Protein Antibodies and Uses Thereof
ActiveUS20100129931A1Easy to analyzeLower Level RequirementsPeptide/protein ingredientsFermentationAntigen Binding FragmentAntigen binding
This application includes, in part, methods of preparing antibodies that specifically bind to tnethylation-controlling J (MCJ) polypeptide. In some aspects, the application also includes, hybridoma cell lines that produce antibodies that specifically MCJ polypeptide; antibodies and antigen-binding fragments thereof produced with the methods of the application,—and methods of using antibodies and antigen-binding fragments that specifically bind MCJ polypeptide for diagnosis and treatment of cancer.
Owner:UNIVERSITY OF VERMONT
Immunochromatographic kit for rapidly detecting novel coronavirus N protein as well as preparation method and application of immunochromatographic kit
PendingCN111398589AThe detection process is fastEasy to operateImmunoassaysAlveolar lavage fluidSaliva sample
The invention relates to an immunochromatographic kit for rapidly detecting novel coronavirus N protein as well as a preparation method and application of the immunochromatographic kit. The immunochromatographic kit comprises a test strip, the test strip comprises a detection line and a quality control line, the detection line is coated with an anti-novel coronavirus N protein antibody, and the quality control line is coated with a goat anti-rabbit polyclonal antibody. The immunochromatographic kit for rapidly detecting the novel coronavirus N protein provided by the invention can be used fordetecting the novel coronavirus N protein in a nasopharynx swab, an oropharynx swab, an alveolar lavage fluid, an oral swab or a saliva sample so as to be used for diagnosing novel coronavirus pneumonia.
Owner:BEIJING ELCOTEQ BIO TECH
Serum-free high density suspension perfusion culture technology of hybridoma cells
ActiveCN102391995AProcess control detection parameters are simpleEasy to operateMicroorganism based processesTissue culturePerfusion CultureBiology
The invention discloses a technology for producing a recombinant antibody protein through the high density perfusion culture of hybridoma cells. The technology which aims at the metabolic characteristic of the hybridoma cells screens a specific culture component for the perfusion culture, and allows metabolic substrates to be fully supplied in the culture process and the concentration of metabolic byproducts to be simultaneously and effectively reduced, so objects of high efficiency and high yield is reached. In the process control aspect, a specific perfusion rate CSPR perfusion culture calculating model is established to carry out real time calculation on culture parameters, and concentrated medium subsection fed batch is adopted to control the perfusion rate, so important substrates ofglucose and glutamine are supplied and the influence of the byproducts to culture is minimized. The technology which is suitable for the preparation of the production antibody protein through the large scale culture of the hybridoma cells allows a large amount of protein antibodies to be provided in a short time, has the advantages of small scale, low investment, realization of the high efficiency output in a short time, low batch difference, high repeatability, and realization of the production of antibody medicines.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Antibody drug conjugates (ADC) that bind to 161p2f10b proteins
ActiveUS20110217321A1Digestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsProtein antibodyTissue specific
Antibody drug conjugates (ADC's) that bind to 161P2F10B protein are described herein. 161P2F10B exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, the ADC's of the invention provide a therapeutic composition for the treatment of cancer.
Owner:AGENSYS
Magnetic particle-based quantitative chemiluminescent assay kit for anti-ribosome P protein antibody IgG, and preparation and detection methods thereof
InactiveCN105954266AEasy to useGuaranteed detection effectChemiluminescene/bioluminescencePolyclonal antibodiesBovine serum albumin
The invention discloses a magnetic particle chemiluminescence quantitative assay kit for anti-ribosomal P protein antibody IgG. The kit includes: anti-ribosomal P protein antibody IgG calibrator; Ribosomal P protein antigen and bovine serum albumin-labeled Tris buffer; alkaline phosphatase-labeled goat anti-human polyclonal antibody and bovine serum albumin in Tris buffer; streptavidin-labeled magnetic particles and bovine serum albumin Serum albumin in Tris buffer; wash solution. Based on the traditional membrane strip immunoassay and enzyme-linked immunosorbent assay, the detection method of the kit increases the sensitivity and linear range by 3-5 orders of magnitude, and realizes quantitative detection in a real sense, with rapid response, reliable results, and It can be used in conjunction with a fully automatic chemiluminescence immunoassay analyzer to realize fully automatic use, and has irreplaceable important value for clinical diagnosis.
Owner:北京贝尔医疗设备有限公司
Porcine circovirus I type infectious clone and virus rescued thereby and application thereof
InactiveCN101423836ASimple and fast operationInfectious cloneViruses/bacteriophagesFermentationViral antibodyPorcine circovirus type 1
The invention discloses infective clone for porcine circovirus type 1 and rescued virus and application thereof. Two genomes of the porcine circovirus type 1 are connected in cis and inserted into a vector to construct and obtain an infective molecular clone. A Sal I enzyme cutting site as a molecular target is inserted in the infective molecular clone. A rescued recombinant virus (PCV1 / G strains) carries a molecular marker, and the preservation number of the rescued recombinant virus is CGMCC NO.2658. An antigen of the recombinant virus is only reacted with a univalent specific antibody of PCV1 / Cap protein, but has no cross with an antibody against PCV2 / Cap protein. The recombinant virus and a parental virus are identified by combination of PCR and RFLP. The virus strain cultured in vitro has stable propagation property and high virus titer, and the rescued virus strain can be used for detecting the antibody of the virus. The invention lays a foundation for research such as genesis and evolution, genetic variation rule, molecular differential diagnosis, and the like for porcine circovirus in future.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
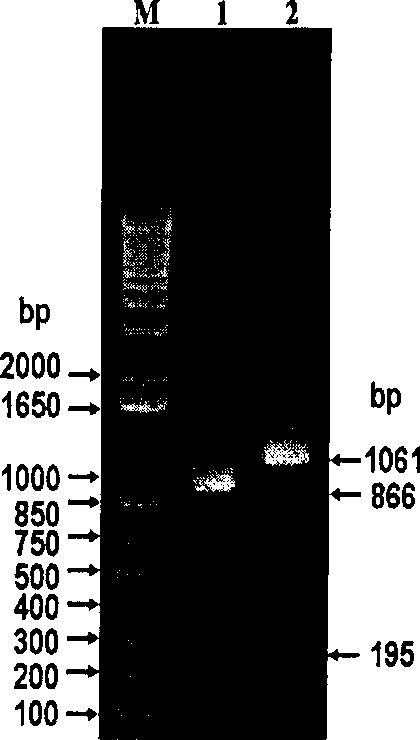
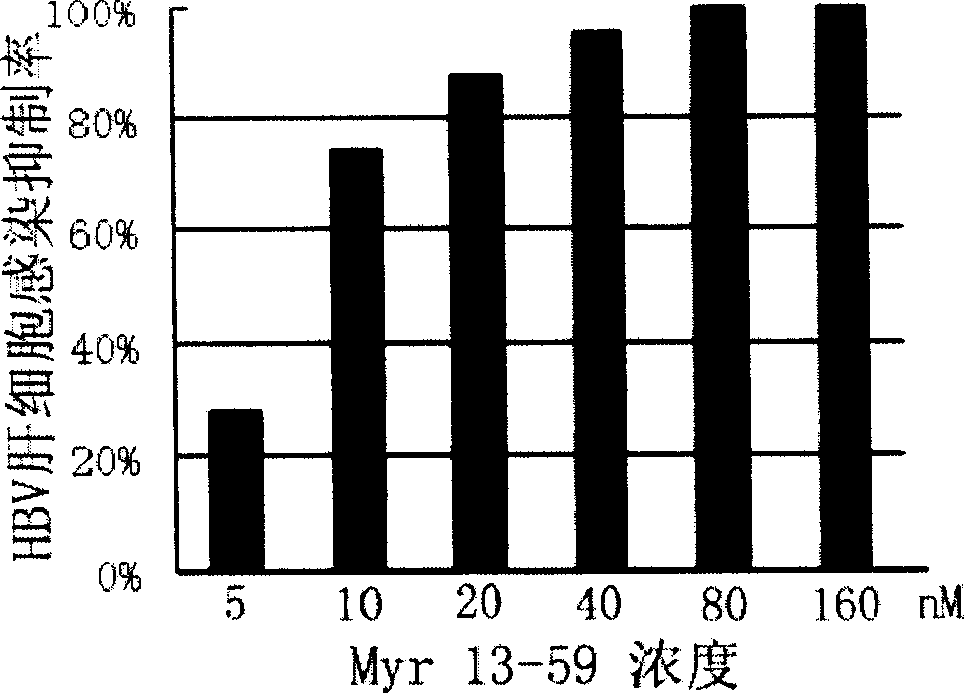
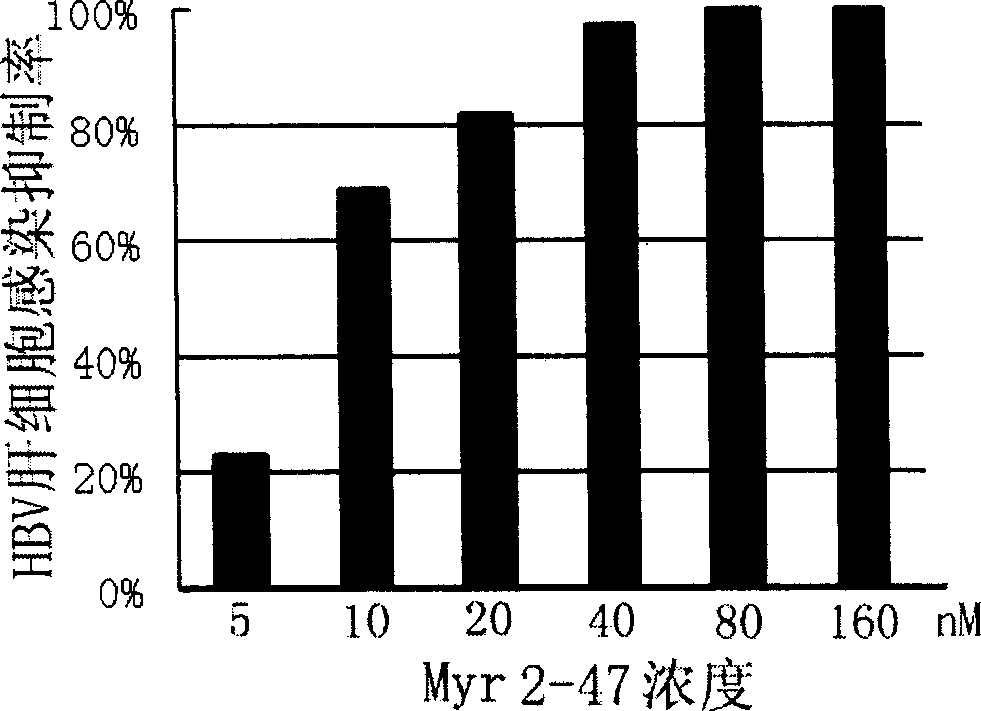
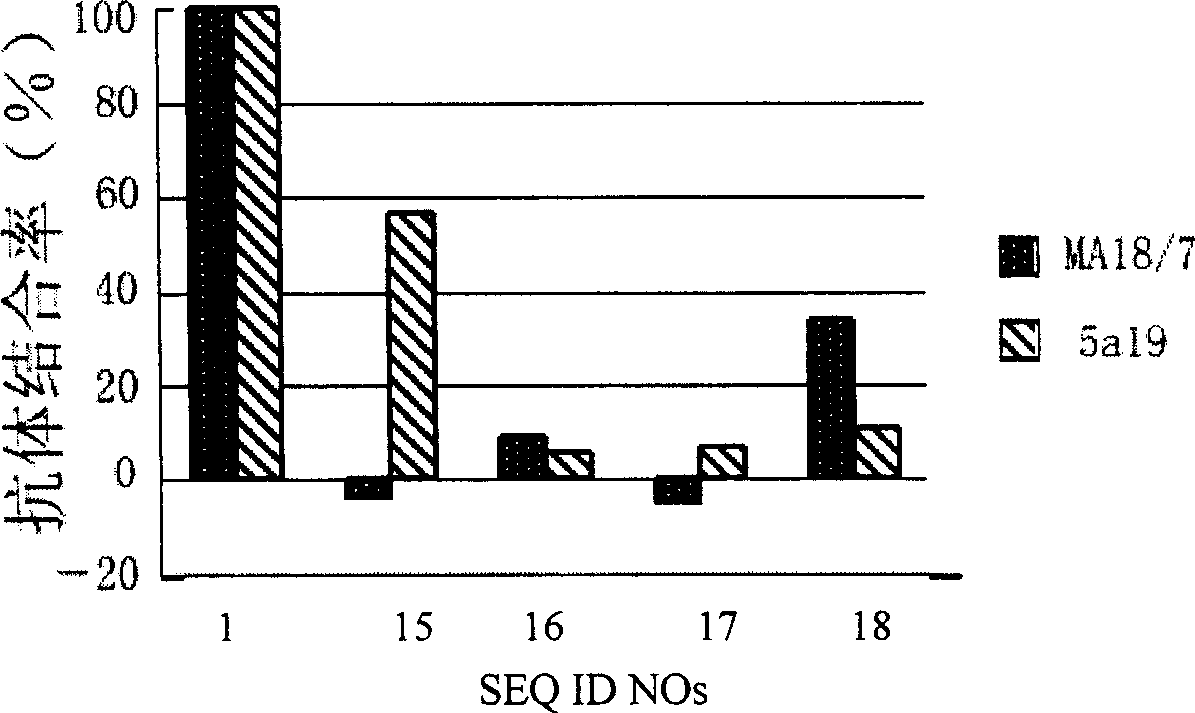
Hepatitis B virus surface L protein related peptide
The invention relates to peptides derived from HBV L protein for the prevention and treatment of Hepatitis B virus (HBV) infection, screening method for removing antigenicity of these peptides and the deantigenic derivative peptide screened through the method, wherein the HBV surface L protein pre-S1 region contains key amino acid sequence of the virus adhesion cell surface acceptor and antigenic amino acid sequence bonding with the anti-L protein antibody. The polypeptides at the HBV surface L protein pre-S1 region can suppress HBV infection to cells. The invention also provides the screening method for removing the antigenicity of these polypeptides.
Owner:SHANGHAI HEP PHARMA
IL-4 mutein proteins, antibodies, compositions, methods and uses
The present invention relates to at least one novel Mut-IL-4 proteins, antibodies, including isolated nucleic acids that encode at least one Mut-IL-4 protein or antibody, Mut-IL-4 vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:CENTOCOR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com