Serum-free high density suspension perfusion culture technology of hybridoma cells
A hybridoma cell and perfusion culture technology, which is applied in the field of hybridoma cell serum-free high-density suspension perfusion culture technology, can solve the problems of difficult to stabilize the culture state and increased substrate consumption, and achieve easy operation, small batch-to-batch difference, Reproducible and continuous effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
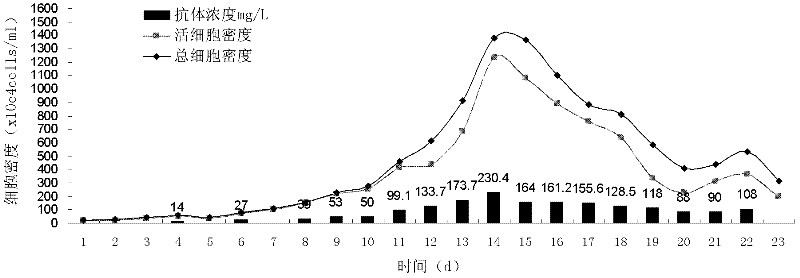
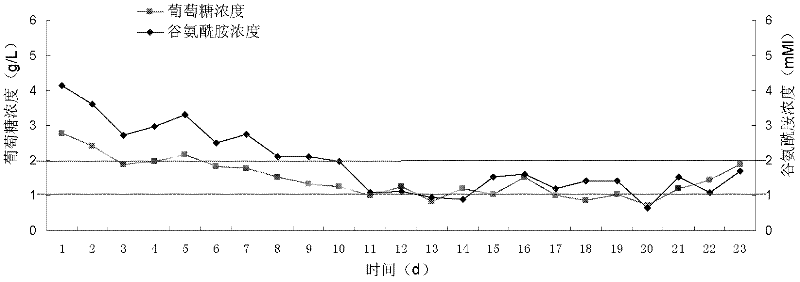
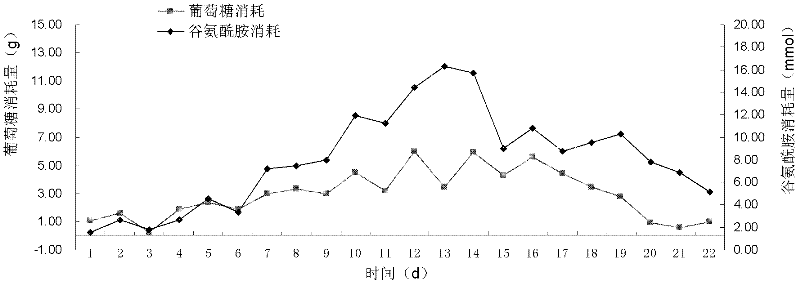
[0051] In view of the existing conditions and previous experience in large-scale cell culture, the applicant optimized the CSPR perfusion culture model and applied it to a hybridoma cell line (CYL-1 colon cancer antibody-expressing cell line). The design idea is to adjust the concentration of the main substrates (glucose and glutamine) in the perfusion medium on the basis of perfusion control. By-products accumulate rapidly. On the other hand, through the related control of perfusion rate and substrate flow rate, the loss of cells and products caused by a large amount of perfusion basal medium is avoided.
[0052] In the specific calculation process, the main control parameters considered are the perfusion rate and the substrate concentration of the perfusion medium, and the substrate concentration is controlled by the amount of concentrated medium added. According to the theory of reaction kinetics, the cell culture process can be divided into three stages, namely logarithmic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com