Novel vaccine for preventing COVID-19 and preparation method thereof
A COVID-19, a new type of technology, applied in the field of genetic engineering and molecular immunology, can solve the problems of exacerbation of lung injury in mice, and achieve the effect of reducing lung injury, weak antibody induction ability, and high conservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A preparation method of a novel vaccine for preventing COVID-19 as described above, comprising the following steps:
[0040] (1) First construct a lentiviral vector capable of secreting and expressing GP96-hFc, infect and screen out a cell line that can stably secrete and express GP96-hFc HEK293T, see SEQ NO: 8 for the nucleotide sequence, and SEQ NO: 8 for the protein sequence 9;
[0041] (2) Construct a lentiviral vector capable of chimerically expressing the S-protein receptor-binding domain of SARS-CoV2 and the truncated N-protein T-cell-associated peptide, and screen out the secreted and expressed GP96- hFc is a HEK293T cell line chimerically expressing the S protein receptor binding domain of SARS-CoV2 and the truncated N protein T cell-associated peptide;
[0042] (3) Collect the cells obtained in step 2) under sterile conditions, and wash them with sterile normal saline or PBS (phosphate buffered saline) to adjust the cell concentration to 1×10 6 ~1×10 7 / 100...
Embodiment 1
[0046] Example 1: Bioinformatics analysis of the S protein of SARS-CoV2
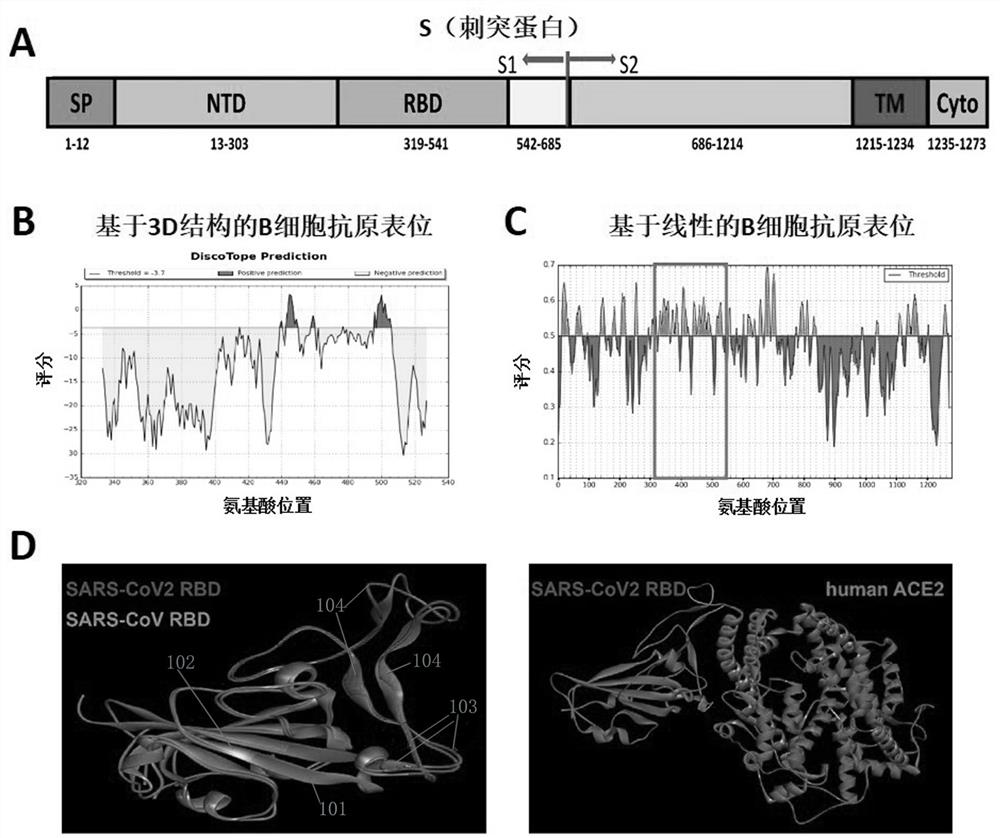
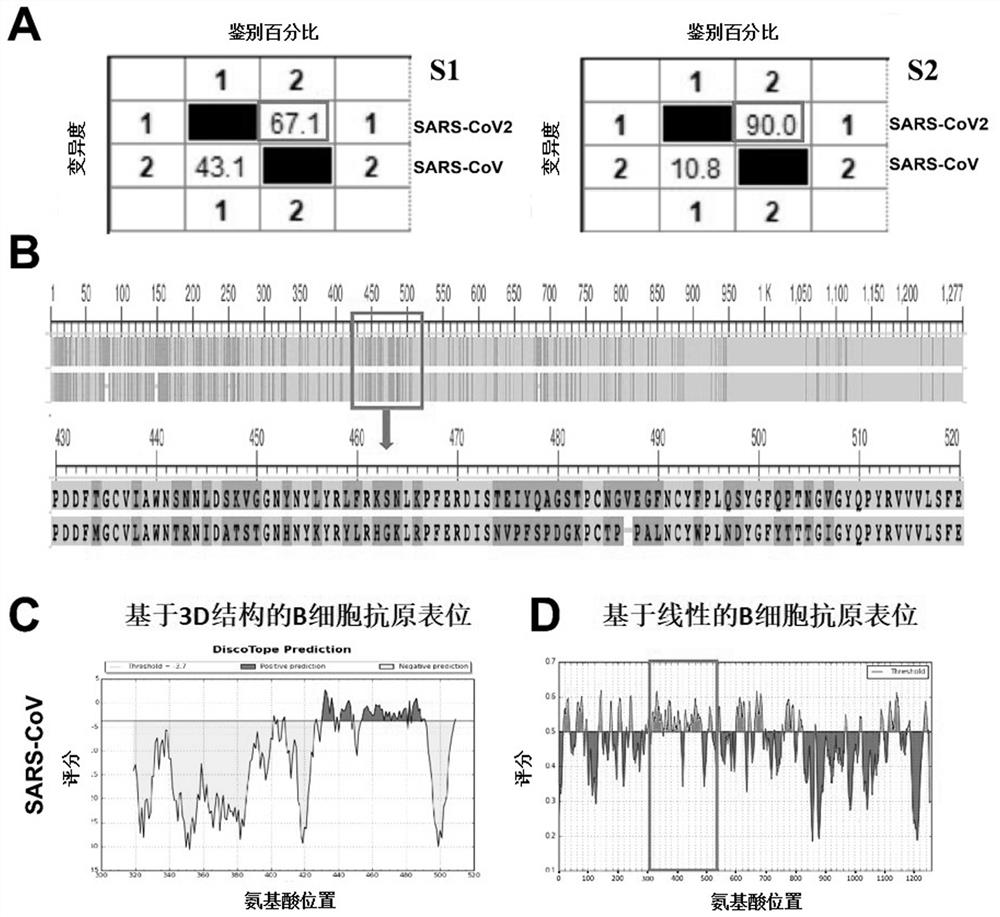
[0047] The S protein of SARS-CoV2 can be divided into multiple functional domains by referring to SARS-CoV related research and UniProt public data; it can be seen that the S protein is mainly composed of S1 and S2 subunits ( figure 1 A), where the receptor-binding domain (RBD) in S1 can bind to angiotensin-converting enzyme 2 (ACE2) to trigger a conformational change of the homotrimer, while the S2 subunit can further form a six-helix bundle, promoting Fusion of virus and host cell membrane. Analysis of potential 3D structure-based B-cell antigens of the receptor-binding domain of the SARS-CoV2 S protein using Discospe software ( figure 1 B), using the IEDB database to analyze the potential linear B cell epitopes of the SARS-CoV2 full S protein ( figure 1 C), showing less structure-based B-antigens and more linear B-cell antigens. The structures of the RBD domains from SARS-CoV2 (PDB: 6LZG) and SARS-...
Embodiment 2
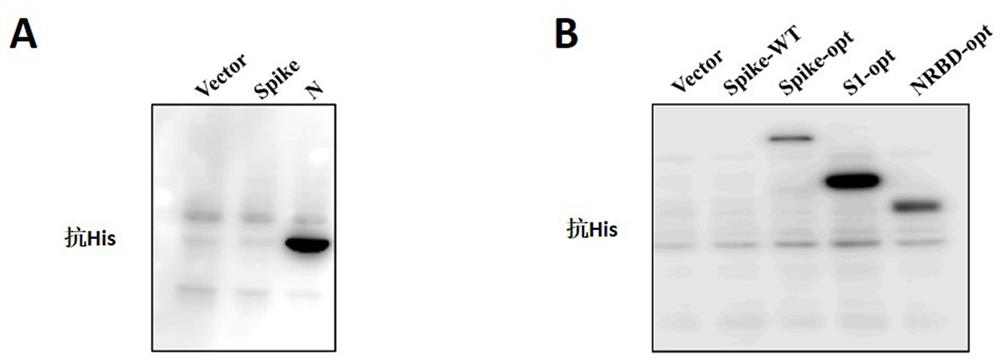
[0049] Example 2: Verification of the expression of SARS-CoV2 spike protein and nucleocapsid protein
[0050] The S protein gene (SEQ NO:11) of SARS-COV and the nucleocapsid protein (SEQ NO:12) of SARS-COV2 were synthesized by Sangon Biotech according to the sequence published by the NCBI database ((MN908947.3), and constructed into pcDNA3.1 -his tag (hygro) vector. The codon-optimized SARS-CoV2 plasmid was purchased from Sino Biological. The following primers were used to construct PCDNA3.1-his-optS, PCDNA3.1-his-optS1, PCDNA3.1-his- optNRBD (from N-terminus to RBD domain) expression.
[0051] Spike-optF (codon optimized): ACTTAATTAAGCCACCATGTTTGTGTTCCTGGTGCTGCT,
[0052] Spike-opt-R: TAACCGGTGGTGTAGTGCAGTTTCACTCCTTTCA;
[0053] Spike-optS1-R: TAACCGGTGCTGTTGGTCTGGGTCTGGTAGG;
[0054] Spike-optNRBD-R: TAACCGGTTCCATTGAAGTTGAAGTTCACACACTT;
[0055] The corresponding expression plasmids were transfected into HEK-293T cells using PEI, and the expression of corresponding genes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com