Epitope polypeptide combination capable of inducing immunity and application thereof
A technology of epitope polypeptide and antigen epitope, which is applied in the direction of application, medical preparations containing active ingredients, peptides, etc., can solve the problems of aggravating immunopathological damage, imbalance of cellular immunity, non-neutralization or inhibition of virus replication, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0062] Example 2 result
[0063] 2.1 Determination of new coronavirus candidate vaccine strains
[0064] The surface glycoprotein [Severe acute respiratory syndrome coronavirus 2] (NC_045512.2) Spike protein published in the NCBI database was selected as the template for cell epitope prediction. The currently uploaded 579 amino acid sequences of different Spike proteins were collected through the NCBI database as templates for conservation analysis, and all protein data were collected in FASTA file format.
[0065] 2.2 Preliminary screening of novel coronavirus epitopes
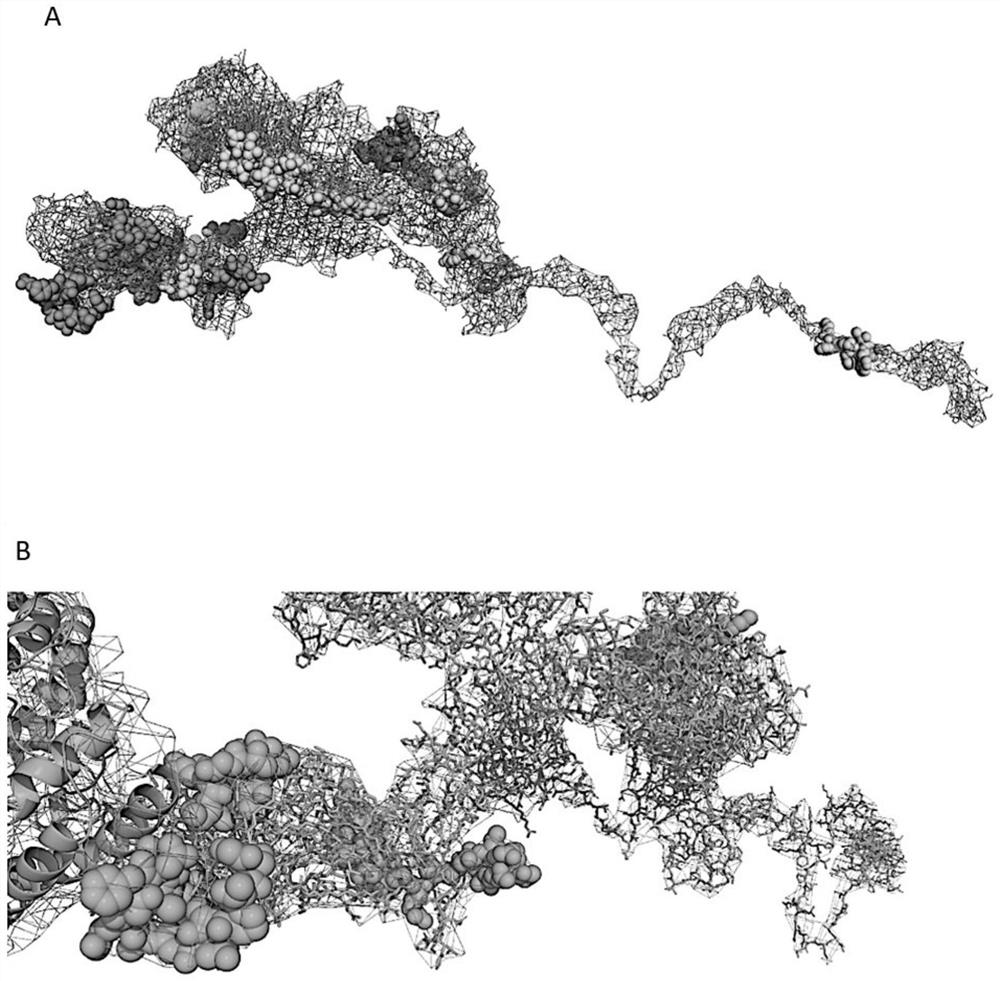
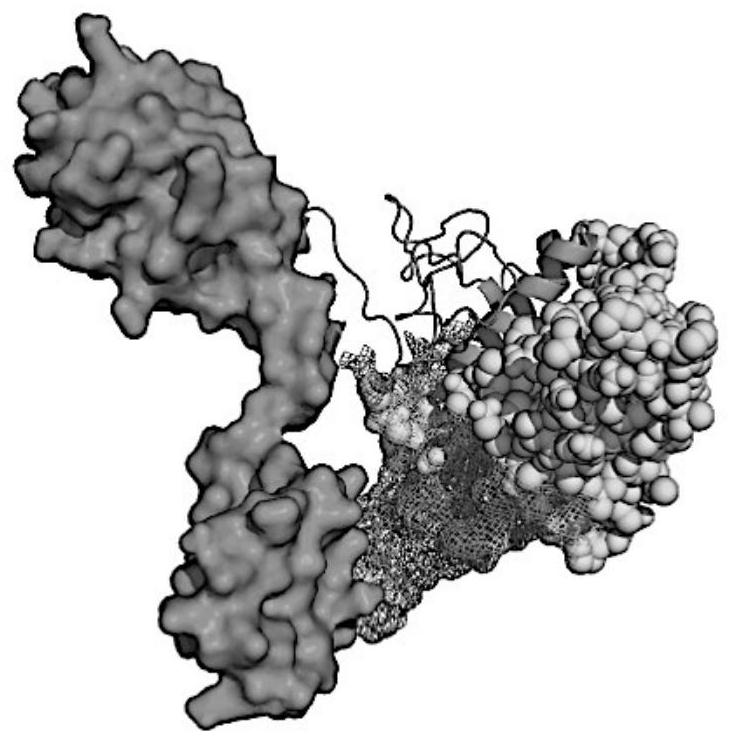
[0066] Table 1 lists five identified B cell epitopes (SEQ ID No. 1-5). Table 2 lists 6 Th epitopes (SEQ ID No.6-11), and Table 3 lists 5 CTL epitopes (SEQ ID No.12-17). The conformational positions of all candidate epitopes in the S protein are as follows figure 1 As shown in A, it is of great significance to obtain neutralizing antibodies that can neutralize the binding domain of Spike protein and ACE2. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com