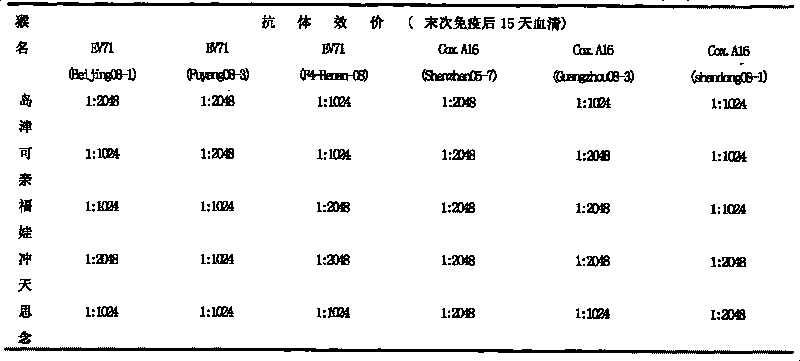
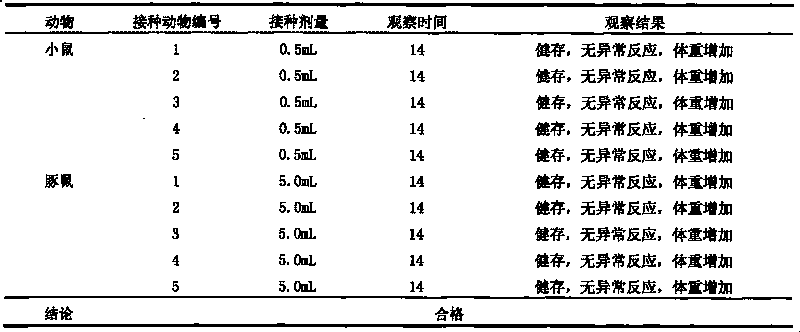
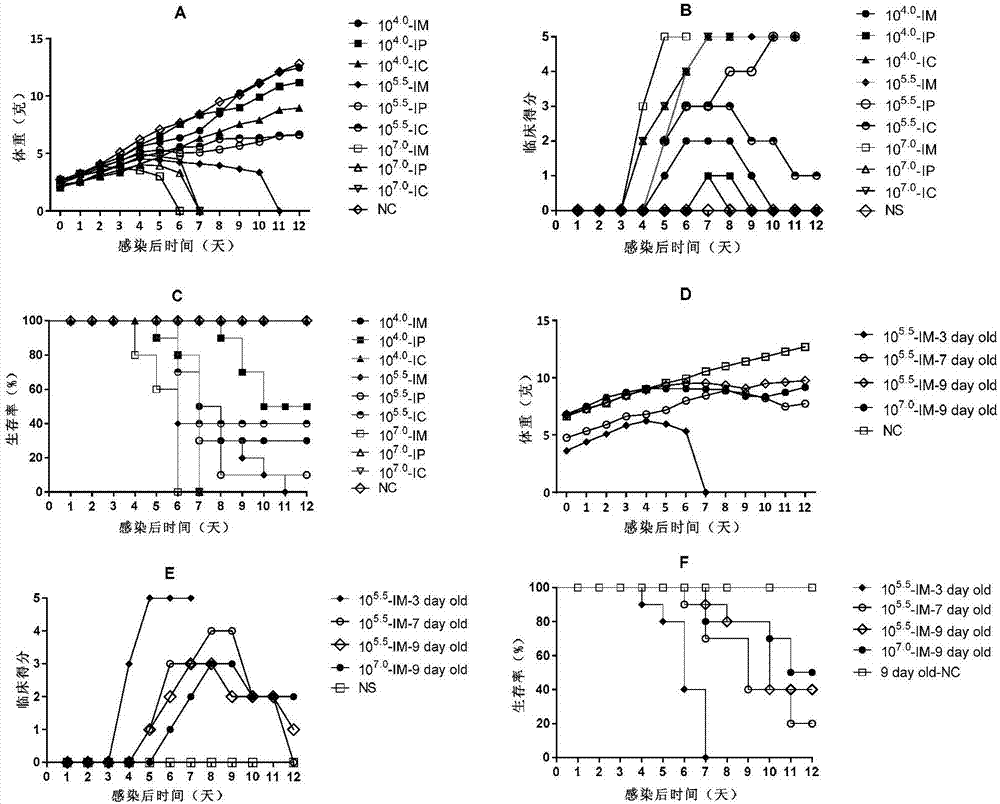
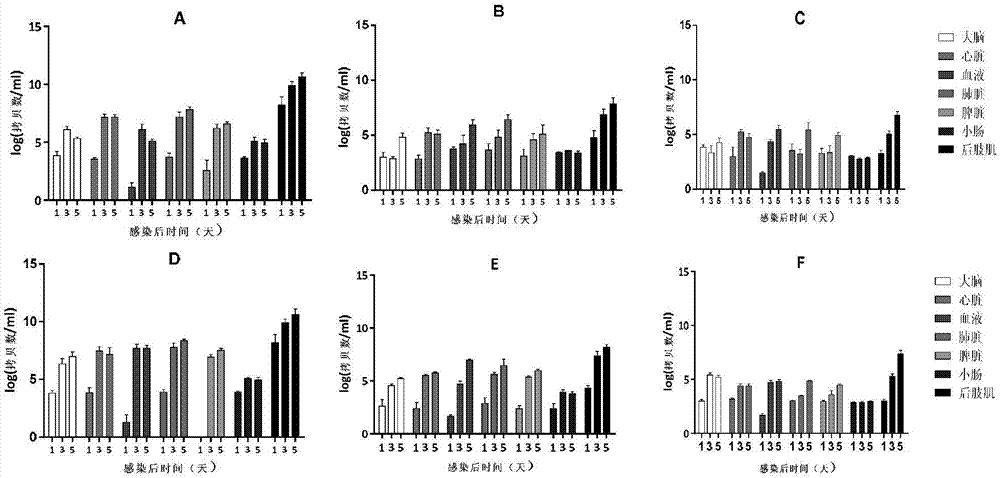
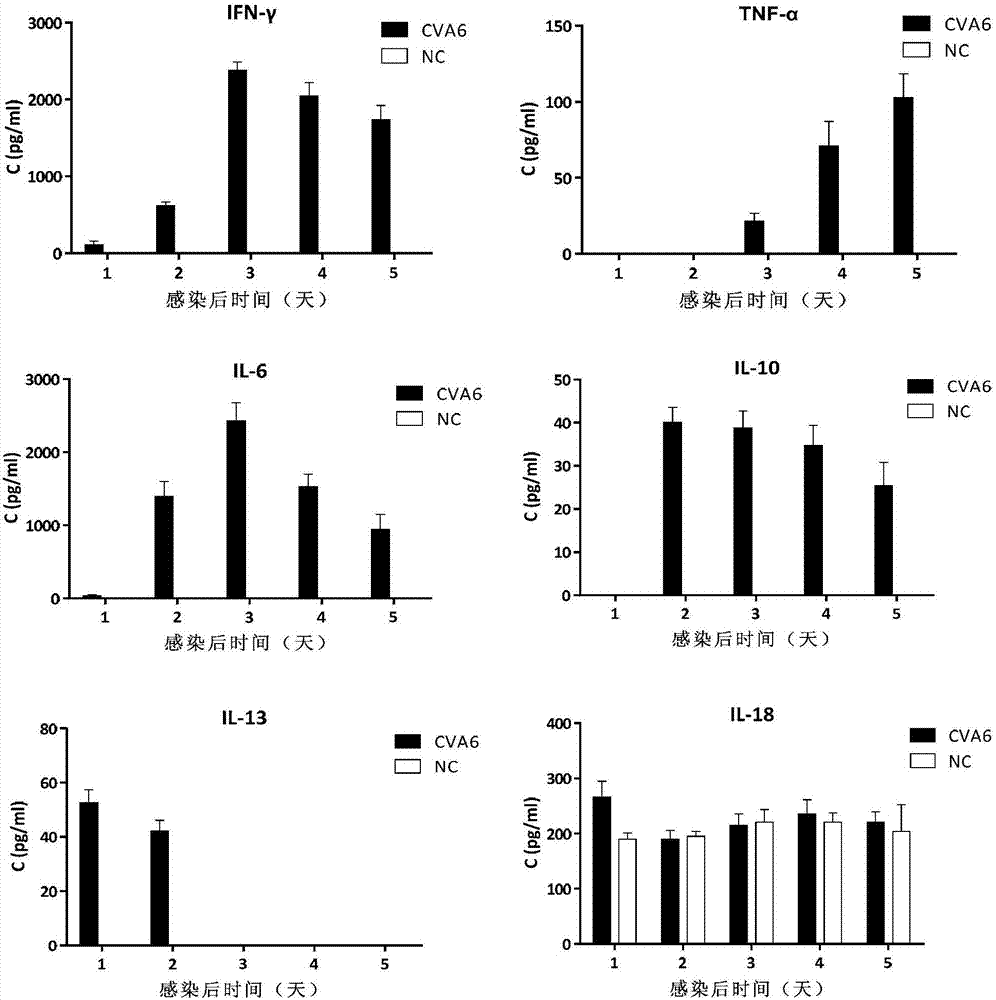
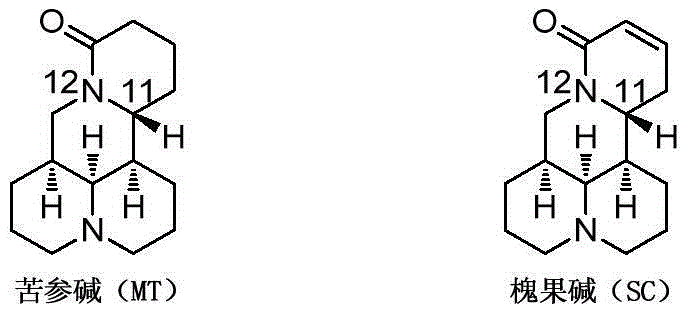Patents
Literature
185 results about "Coxsackie Viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
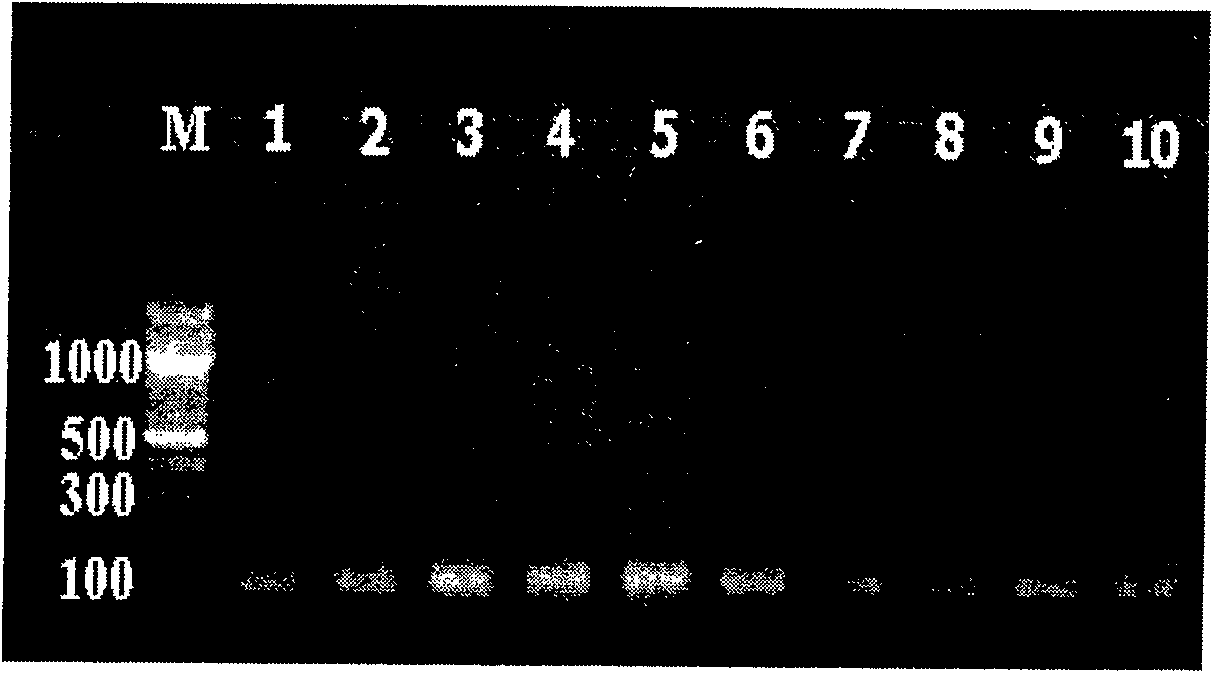
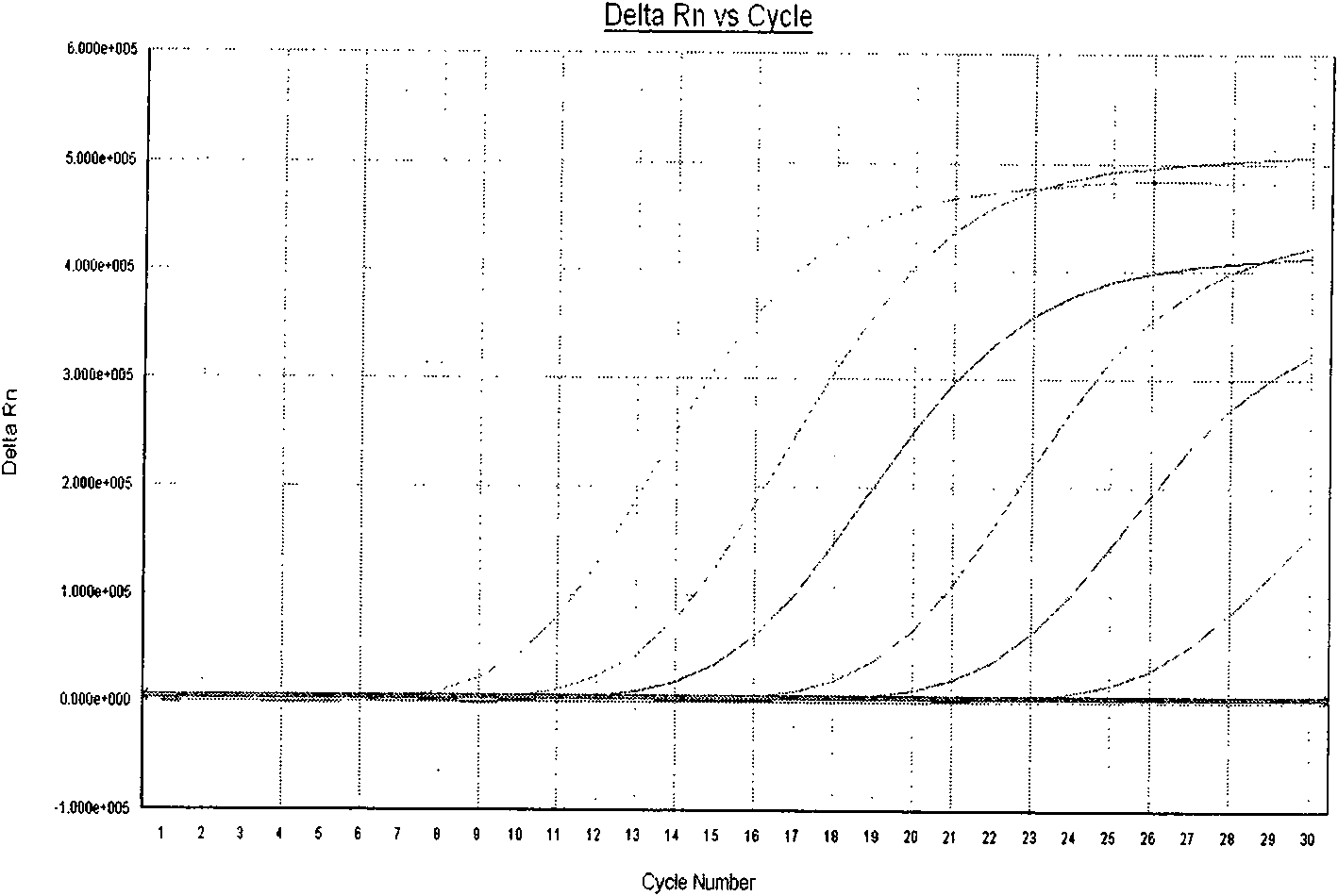
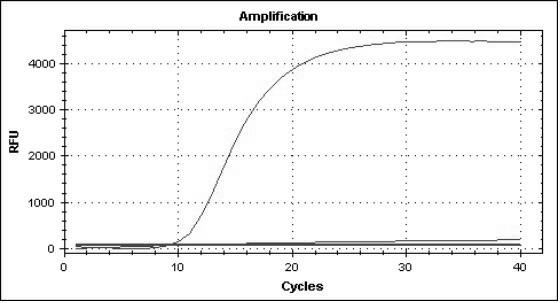
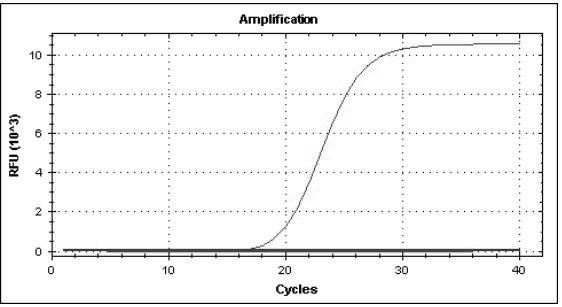
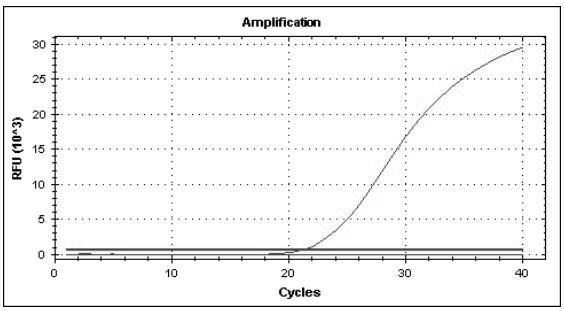
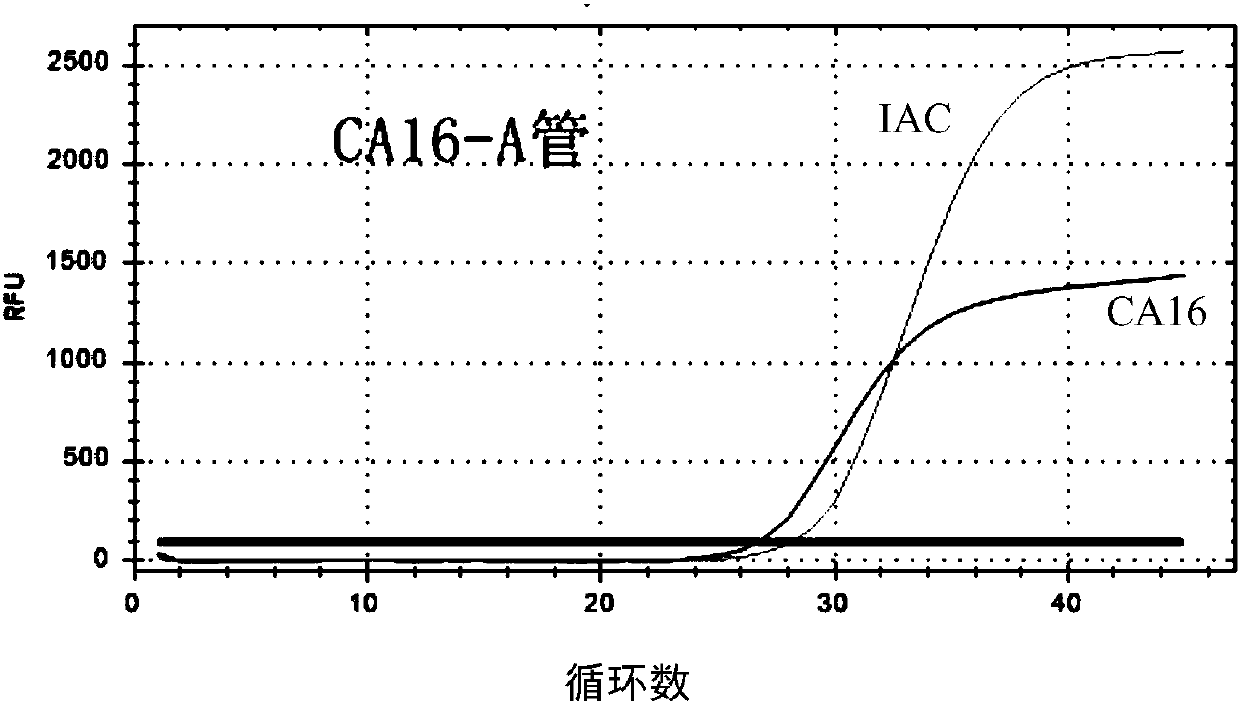
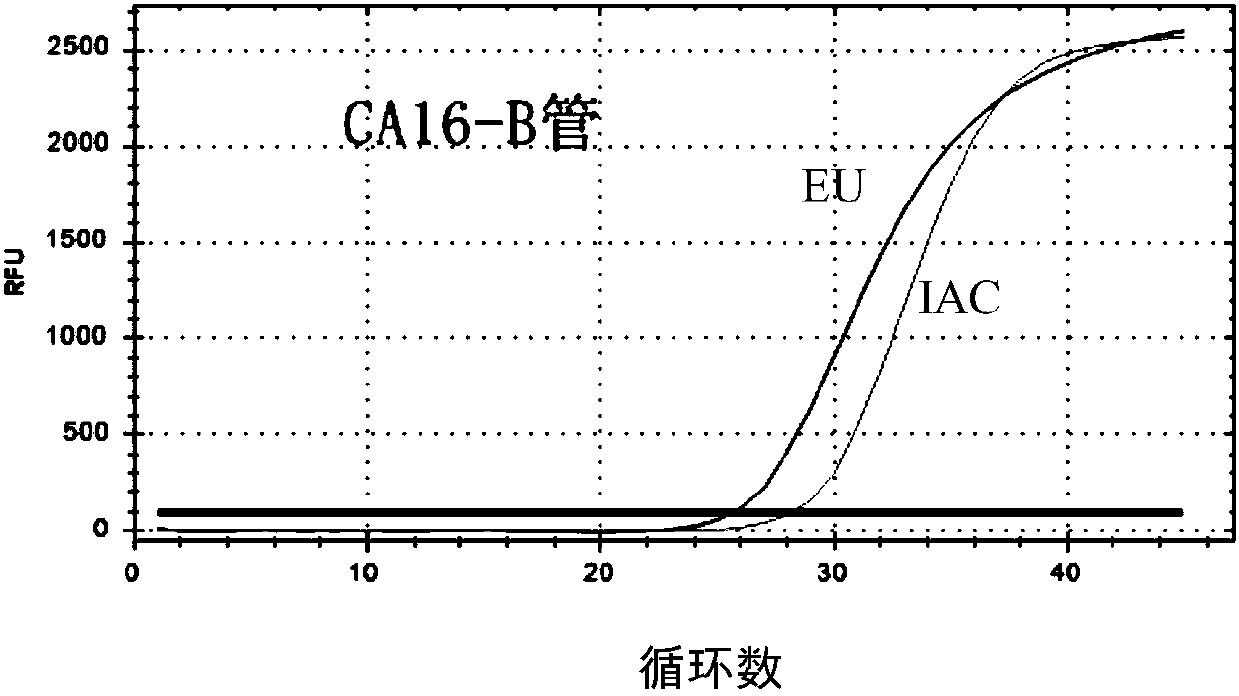
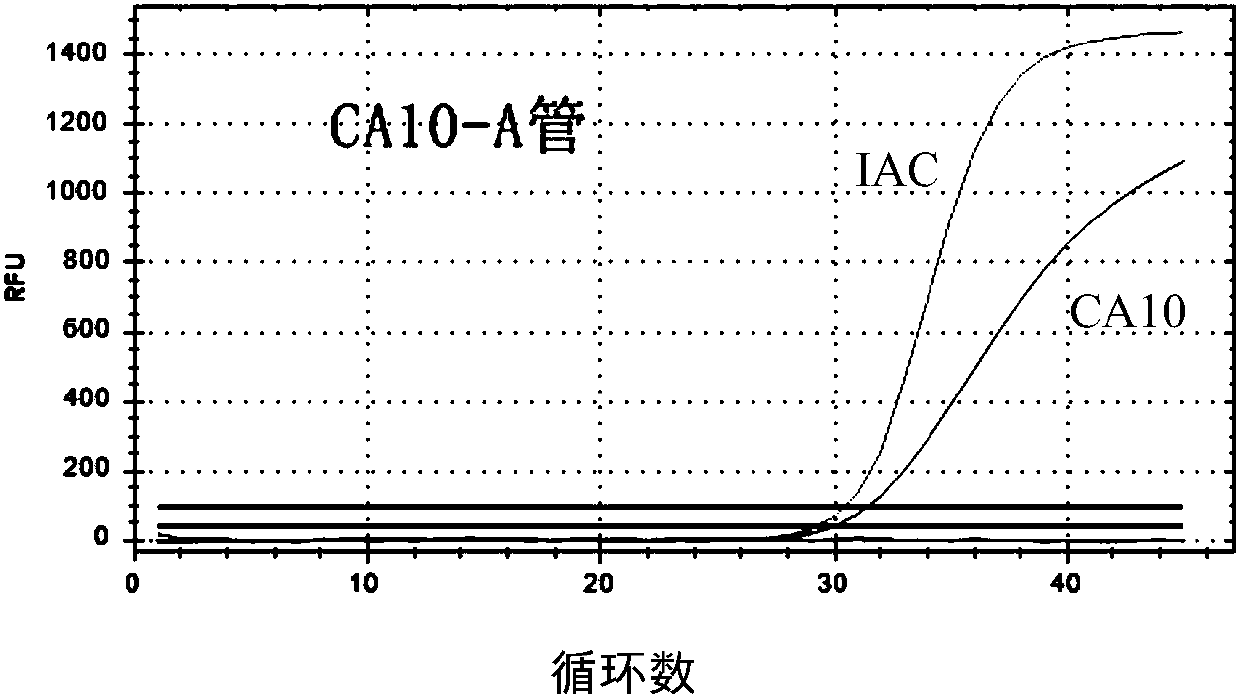
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Preparation method for recombinant coxsackie virus A16 like particle and applications thereof
InactiveCN102533797AIncrease productionFungiInactivation/attenuationYeastHand-foot-and-mouth disease
The invention discloses a preparation method for a recombinant coxsackie virus A16 like particle, which comprises the following steps: (1) cloning a P1 gene and a 3CD gene of a coxsackie virus A16 to a target plasmid to obtain a recombinant expression vector; (2) transforming a target yeast cell by using the recombinant expression vector obtained in the step (1) to obtain a recombinant yeast cellfor expressing the P1 gene and the 3CD gene; and (3) cracking the recombinant yeast cell obtained in the step (2), and separating to obtain the recombinant coxsackie virus A16 like particle. The recombinant coxsackie virus A16 like particle can be prepared in a yeast expression system by the method provided by the invention. Compared with a wild-type P1 gene and a wild-type 3CD gene, the yield ofthe coxsackie virus A16 like particle in the yeast expression system is greatly increased through the optimization of codons of the P1 gene and the 3CD gene, and the recombinant coxsackie virus A16 like particle can be further used for producing candidate preventive vaccines and pharmaceutical compositions for infant hand-foot-and-mouth diseases.
Owner:BEIJING UNIV OF TECH
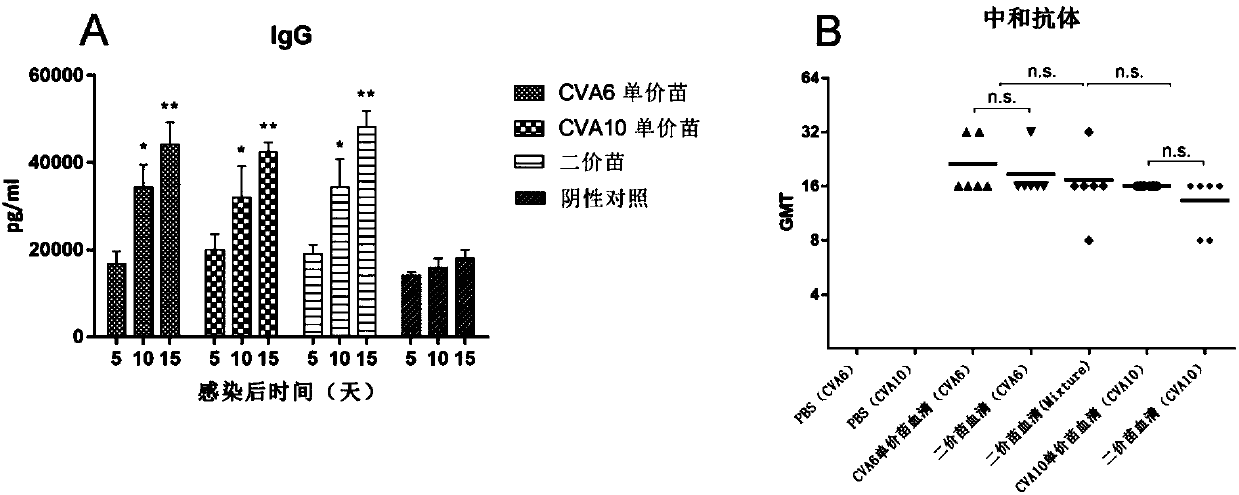
Fluorescent quantitative kit for detecting coxsackie viruses A6 and A10
The invention provides a fluorescent quantitative kit for detecting coxsackie viruses A6 and A10. The fluorescent quantitative kit is composed of a quantitative reverse transcription-polymerase chain reaction (RT-PCR) reaction liquid, enzyme mixed liquor, primer probe mixed liquor, CVA6 and CVA10 standard substances, CVA6 and CVA10 strong positive reference substances, CVA6 and CVA10 weak positive reference substances, and a negative reference substance. Existence of CVA6 and CVA10 is simultaneously detected from a stool specimen by adopting a one-step real-time fluorescent quantitative RT-PCR technology, and CVA6 and CVA10 primers and fluorescently-labeled probes with high specificity, compared with a single fluorescent quantitative PCR method, the fluorescent quantitative kit is more convenient and faster, real-time in detection, and accurate to quantitate; the cost is saved; early diagnosis is provided clinically; a reference frame is provided for formulation of a clinical treatment scheme; the fluorescent quantitative kit can be applied to laboratory emergency diagnosis, rapid screening and clinical diagnosis of epidemic outbreak caused by coxsackie viruses A6 and A10, and research of epidemiology of a hand-foot-and-mouth disease.
Owner:ZHEJIANG UNIV
Coxsackie virus A16 virus strain, uses of strain, vaccine and preparation method of vaccine
ActiveCN104099301AEffective immune activityObvious paralysisSerum immunoglobulinsImmunoglobulins against virusesCoxsackievirus a16Antiviral drug
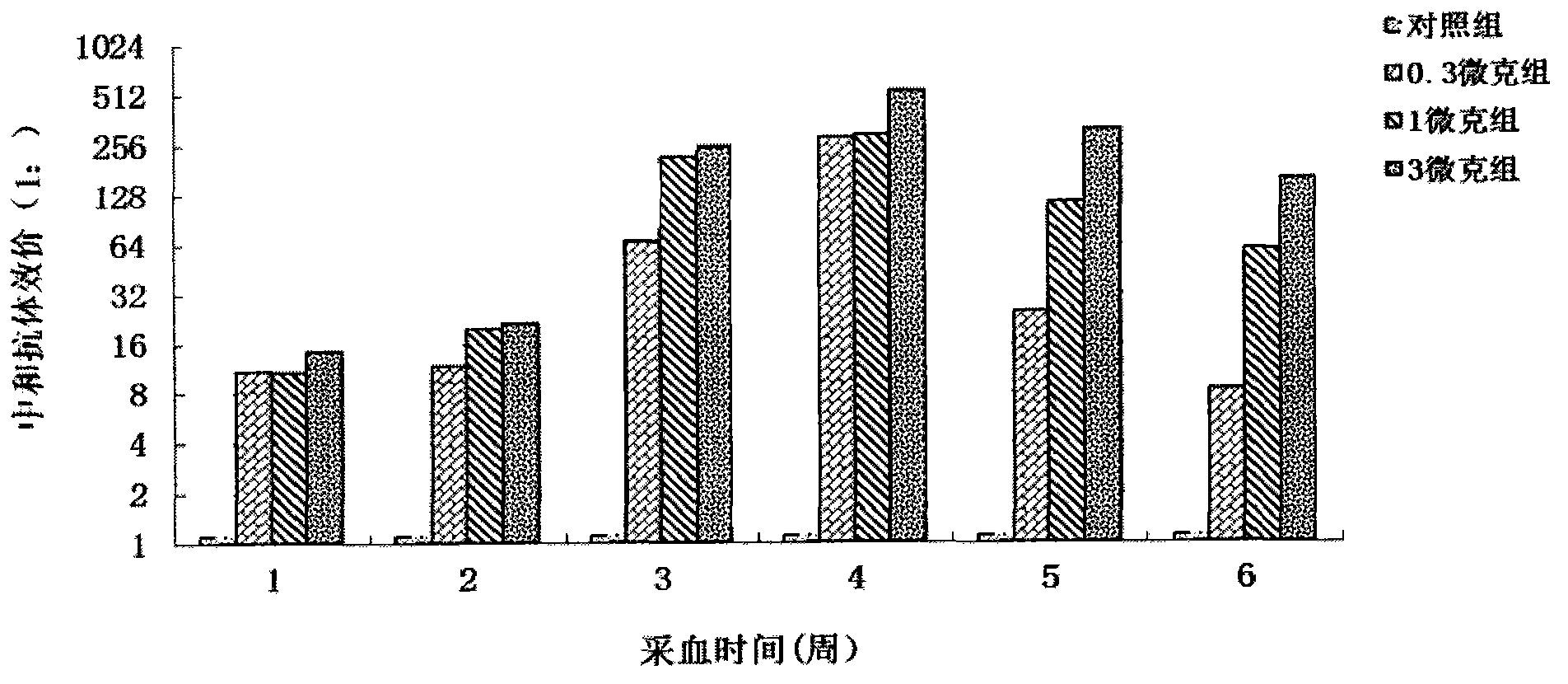
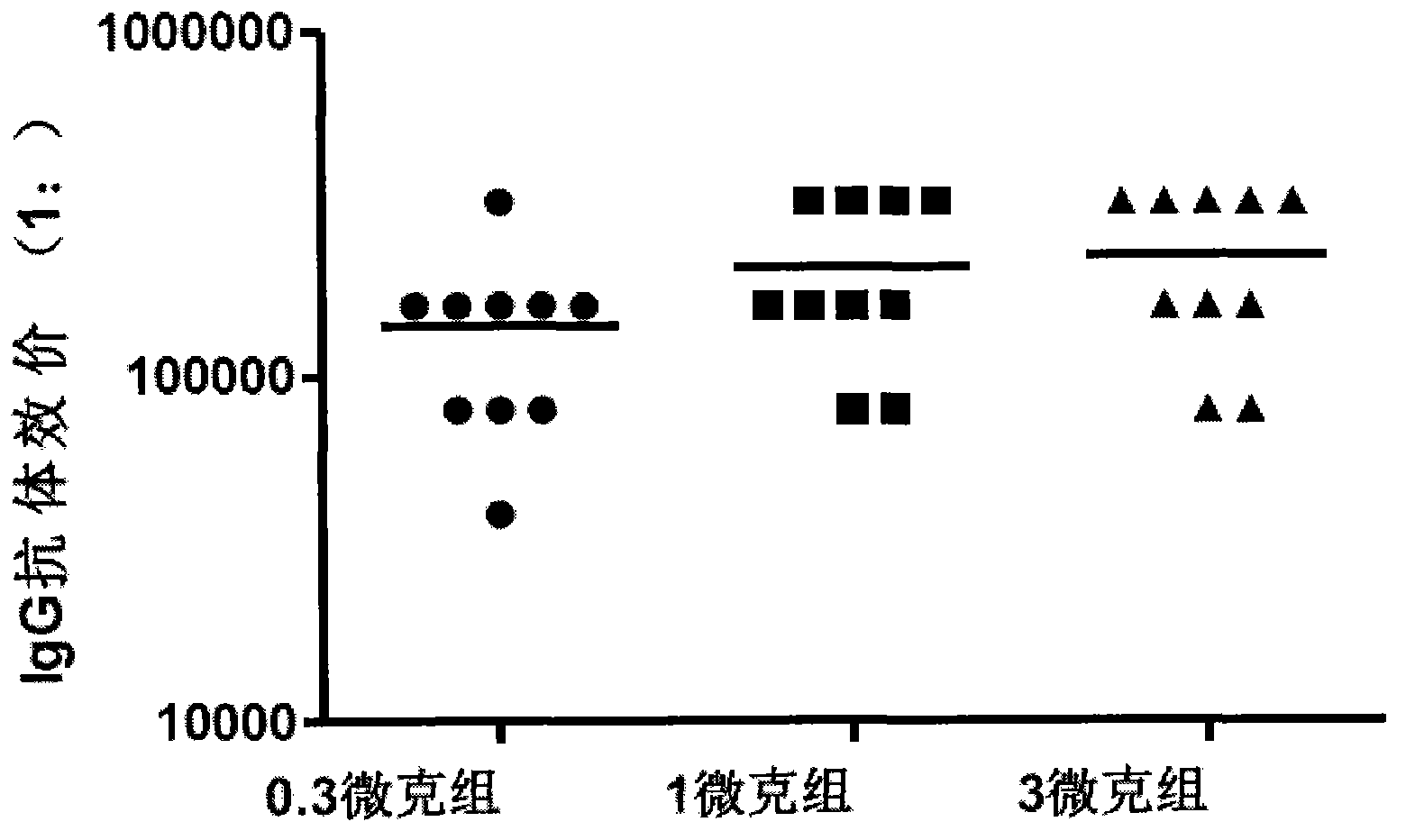
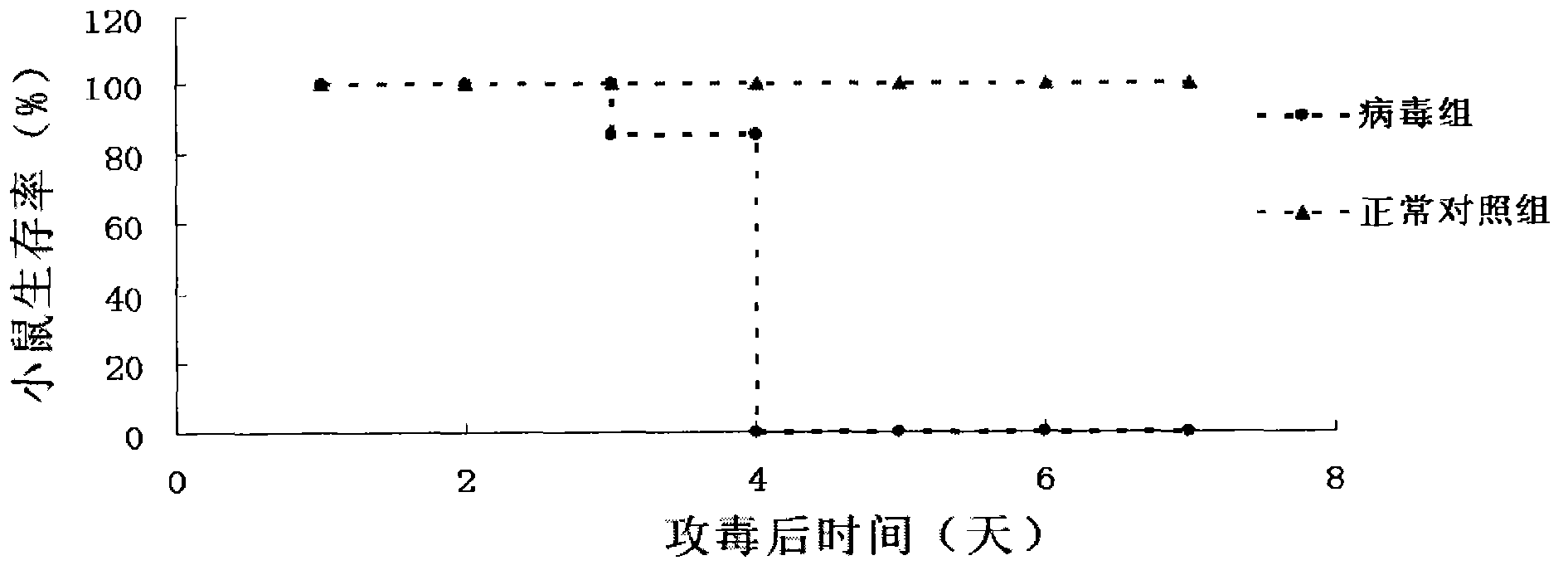
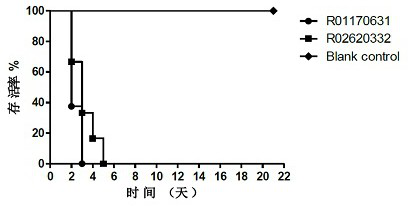
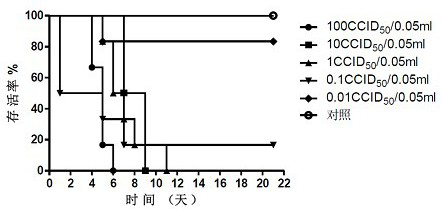
The invention provides a Coxsackie virus A16 virus strain, uses of the strain, a vaccine and a preparation method of the vaccine. The preservation number of the Coxsackie virus A16 virus strain is CGMCC No.6954. The CA16 virus strain has strong virulence, can be used for evaluating the CA16 vaccine, and can also be used for researching the CA16 virus infection mechanism. A method for establishing a Coxsackie virus A16 infected animal model provided by the invention can provide a stable animal model, and provides a foundation for the development of the Coxsackie virus A16 vaccine, the screening of antiviral drugs and the researches of the CA16 virus infection mechanism. The vaccine prepared by using the CA16 virus has effective immune activity.
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
Method for preparing recombinant coxsackievirus A16 type virus-like particles
ActiveCN103436553AUniform shapeStable traitsInactivation/attenuationAntiviralsOpen reading frameCoxsackievirus a16
The invention relates to the field of immunity technology, and particularly relates to a method for preparing recombinant coxsackievirus A16 type virus-like particles. In the method, the PI protein and 3C protein of CA16 are expressed in a double-expression plasmid through a yeast expression system. In order to realize the preparation of CA16 virus-like particles, according to the method provided by the invention, an expression vector and an expression system for CA16 virus-like particles are established, wherein the expression vector comprises two open reading frames which respectively contain a P1 protein expression sequence and a 3C protein expression sequence of CA16. According to the invention, a method for preparing recombinant coxsackievirus-like particle protein by use of co-expression of 3C protease and P1 protein is established through codon optimization; and the method can obtain virus particles with high purity, uniform form and stable characters, is used for preparing a hand-foot-and-mouth disease vaccine, and has a great market value.
Owner:上海博唯生物科技有限公司 +1
Coxsackie virus A6 strain (WF057R) and applications thereof
InactiveCN106947745AProtectiveGood repeatabilityOrganic active ingredientsSsRNA viruses positive-senseTGE VACCINEViral Inactivation
The invention discloses a Coxsackie virus A6 strain (WF057R) and applications thereof. The preservation number of the Coxsackie virus A6 strain (WF057R) in China General Microbiological Culture Collection Center (CGMCC) is CGMCC No.13393. A vaccine and CVA6 antiserum prepared from WF057R can treat and prevent diseases caused by CVA6. Furthermore, the maternal-transferred antibody of WF057R also has a protective effect on newborn mice. WF057R can also be used to establish a stable Coxsackie virus A6 infected animal model with good repeatability, and the animal model can be applied to drug antivirus treatment and immune protective effect evaluation of viral inactivation vaccines.
Owner:TAISHAN MEDICAL UNIV
Coxsackievirus A6 type infected animal model as well as preparation method and application thereof
InactiveCN106867974AGood repeatabilitySsRNA viruses positive-senseMicroorganism based processesMicroorganismViral Vaccine
The invention discloses a coxsackievirus A6 type infected animal model as well as a preparation method and an application thereof. The preparation method of the coxsackievirus A6 type infected animal model provided by the invention comprises a step of infecting an animal with an inactivated coxsackievirus A6 type strain, so that the coxsackievirus A6 type infected animal model is obtained, wherein the coxsackievirus A6 type strain is preserved in China General Microbiological Culture Collection Center with preservation number of CGMCC No.13393. The coxsackievirus A6 type infected animal model, which is stable and is good in repeatability, is constructed on the basis of the CVA6 strain WF057R; and with the application of the model, a research tool is provided for next medicine antiviral therapy and assessment of an immune protective effect of an inactivated viral vaccine.
Owner:TAISHAN MEDICAL UNIV
Hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof
InactiveCN102190725ASignificant effectImprove survival rateImmunoglobulins against virusesAntiviralsTotal proteinPasteurization
The invention relates to a hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof in pharmacy. The preparation method comprises the following steps of: separating component I+II+III, I+III and II precipitates in turn by using a low-temperature methanol protein separation method; performing pasteurization on a component II precipitate; refining and purifying; performing dealcholization; preparing; and sterilizing, packaging and performing low-pH incubated inactivation. The product has antibody titer of not less than 1:640 for enteroviruses (including one or more of coxsackie virus, Echo and EV71), the immunoglobulin content is not less than 95.0 percent of the total protein content, and the sum of IgG monomer and dimer is not less than 95 percent; and the using method is that a specific antibody with titer of 160,000-320,000 is intravenously infused. The invention is suitable for industrial production; and the product has high-titer enterovirus resistant specificity, is safe and reliable, and can become an effective medicine for treating the hand-foot-and-mouth disease.
Owner:HUALAN BIOLOGICAL ENG INC
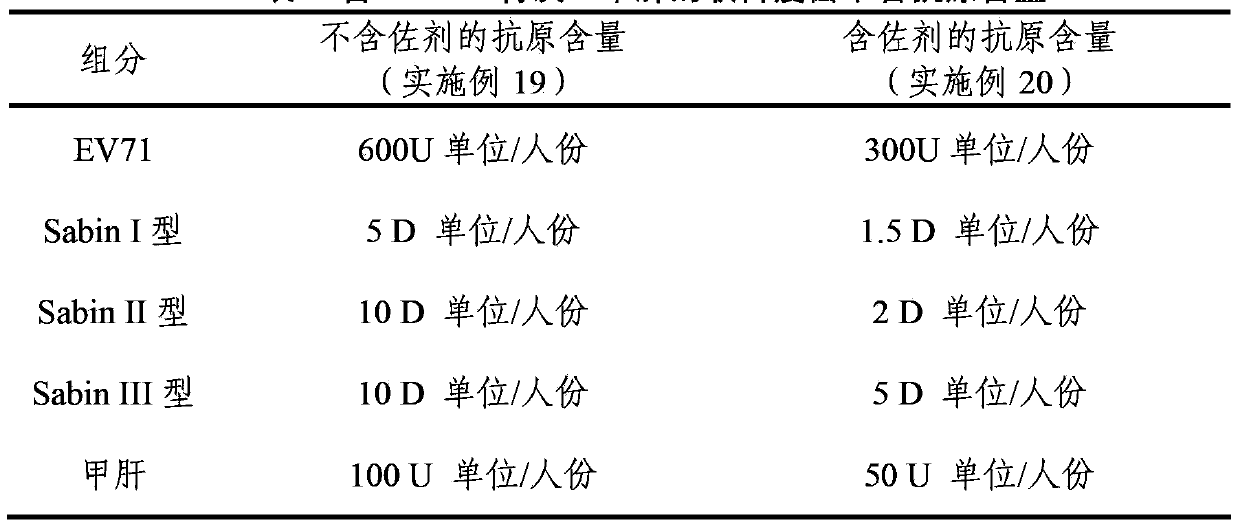
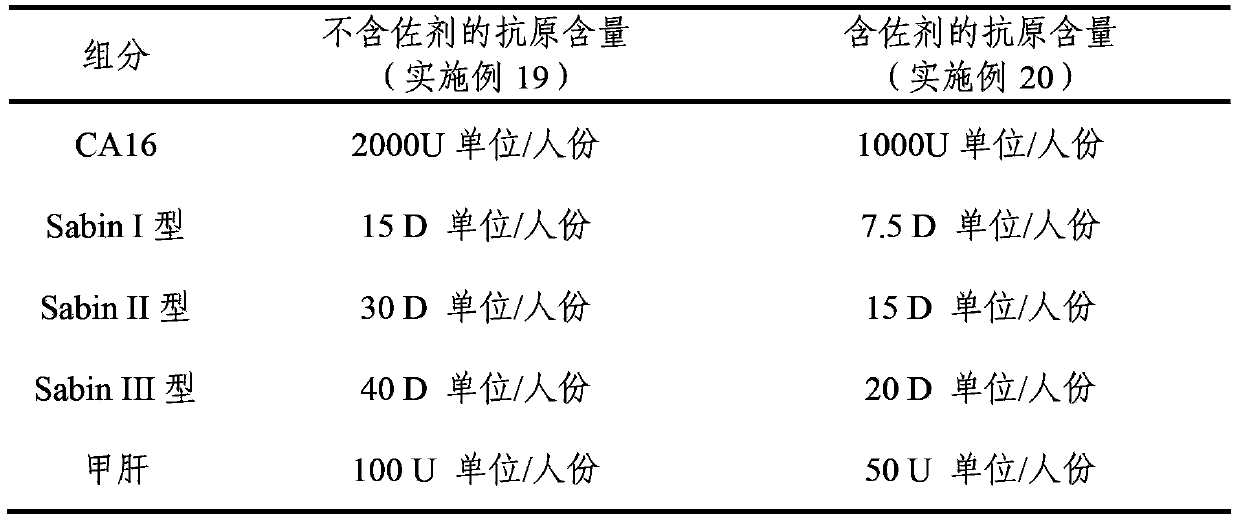
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
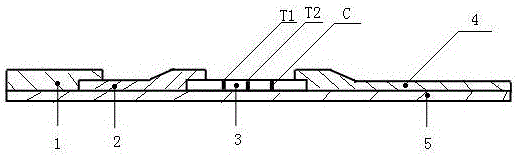
IgM antibody joint detection device and method for coxsackievirus A16 and enterovirus 71
PendingCN105044362ASimple structureNovel ideaMaterial analysis by observing effect on chemical indicatorDisease diagnosisCelluloseCoxsackievirus a16
The invention relates to a joint detection device and a preparation method of helicobacter pylori urease antibodies IgM and IgG. The helicobacter pylori urease antibodies are prepared from a nitrocellulose membrane, a glass fiber adsorbing a colloidal gold labeled helicobacter pylori urease antigen and a mouse IgG, a sample pad, absorbent paper and other auxiliary materials, wherein the materials are adhered together, and the nitrocellulose membrane contains a purified high-specificity mouse-anti-human IgM and IgG antibodies, and a goat-anti-mouse antibody in a solid phase manner. The joint detection device has the advantages that the structure is simple, the conception is novel, the nitrocellulose membrane are coated with the anti-human IgM and IgG antibodies, so that the specificity is strong, and the helicobacter pylori urease antibodies IgM and IgG in a specimen are simultaneously detected without increasing the production operation complexity. Proper gold spray buffer and sample pad treating fluid are matched to effectively improve the reaction sensitivity on the basis of guaranteeing complete release of immunocolloidal gold, and under the same threshold, the use amount of the immunocolloidal gold can be reduced to save the cost. The detection device is high in sensitivity, strong in specificity, simple, convenient and strong in practicability and can realize the time-saving aim during operation.
Owner:吉林双正医疗科技有限公司
Matrine compound derivatives, and preparation method and application thereof
ActiveCN106279167AGood medicineGood anti-CVB virus activityOrganic active ingredientsNervous disorderCompounding drugsMatrine
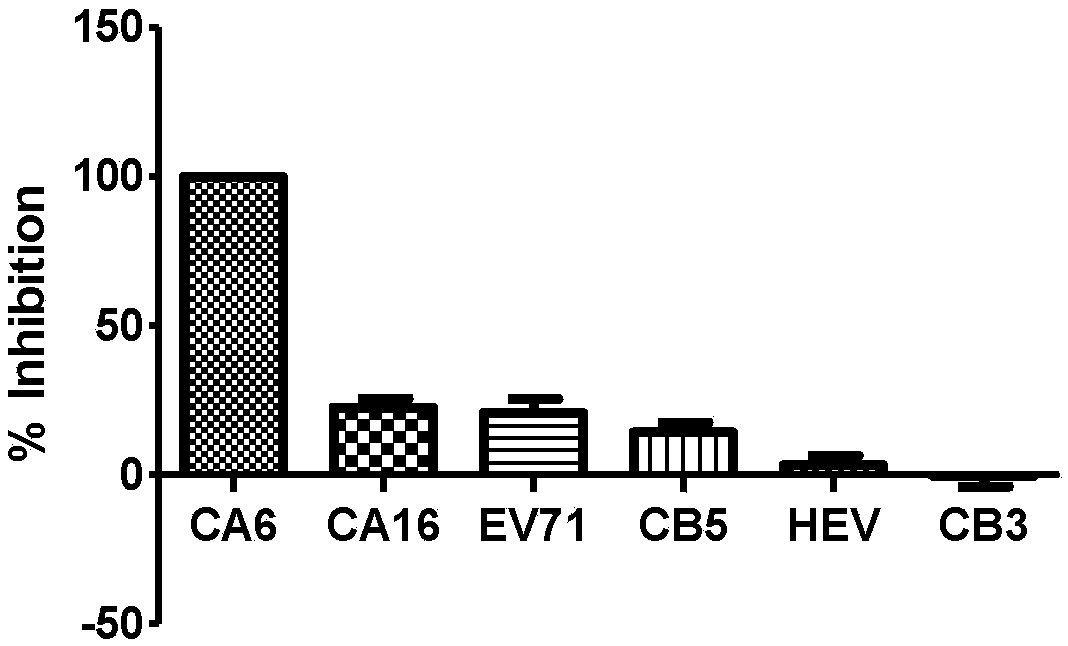
The invention provides a compound shown in Formula I, an isomer, pharmaceutically-acceptable salt or solvate thereof, a preparation method thereof and application thereof in preparation of drugs for preventing and / or treating diseases caused by a Coxsackie virus. According to the invention, the compound keeps the activity of inhibiting the Coxsackie virus group B; and meanwhile, the pharmacokinetic parameters and safety of the compound drug are improved, thus indicating that the compound provided by the invention has favorable druggability and application prospects. The Formula I is shown in the specification.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
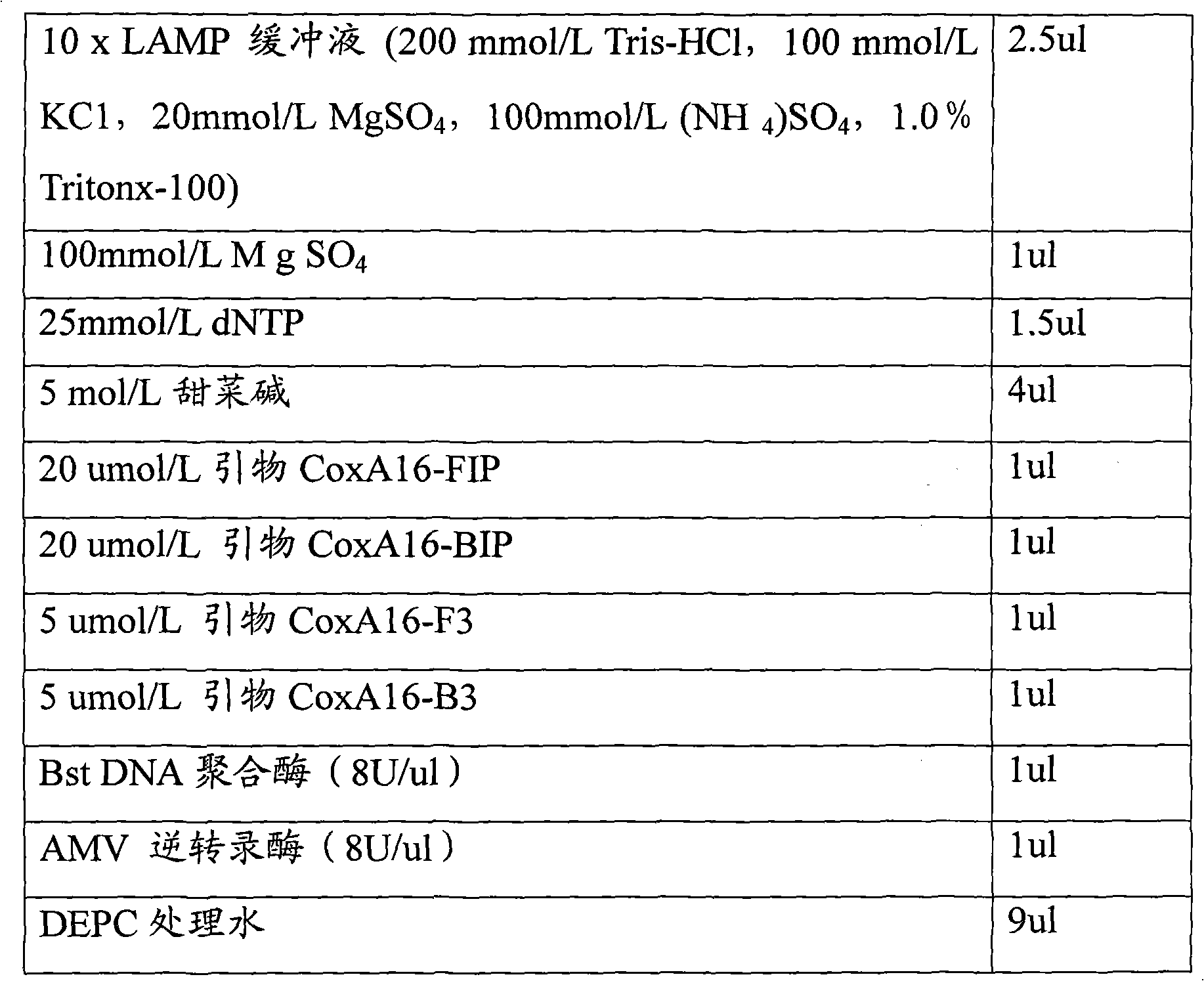
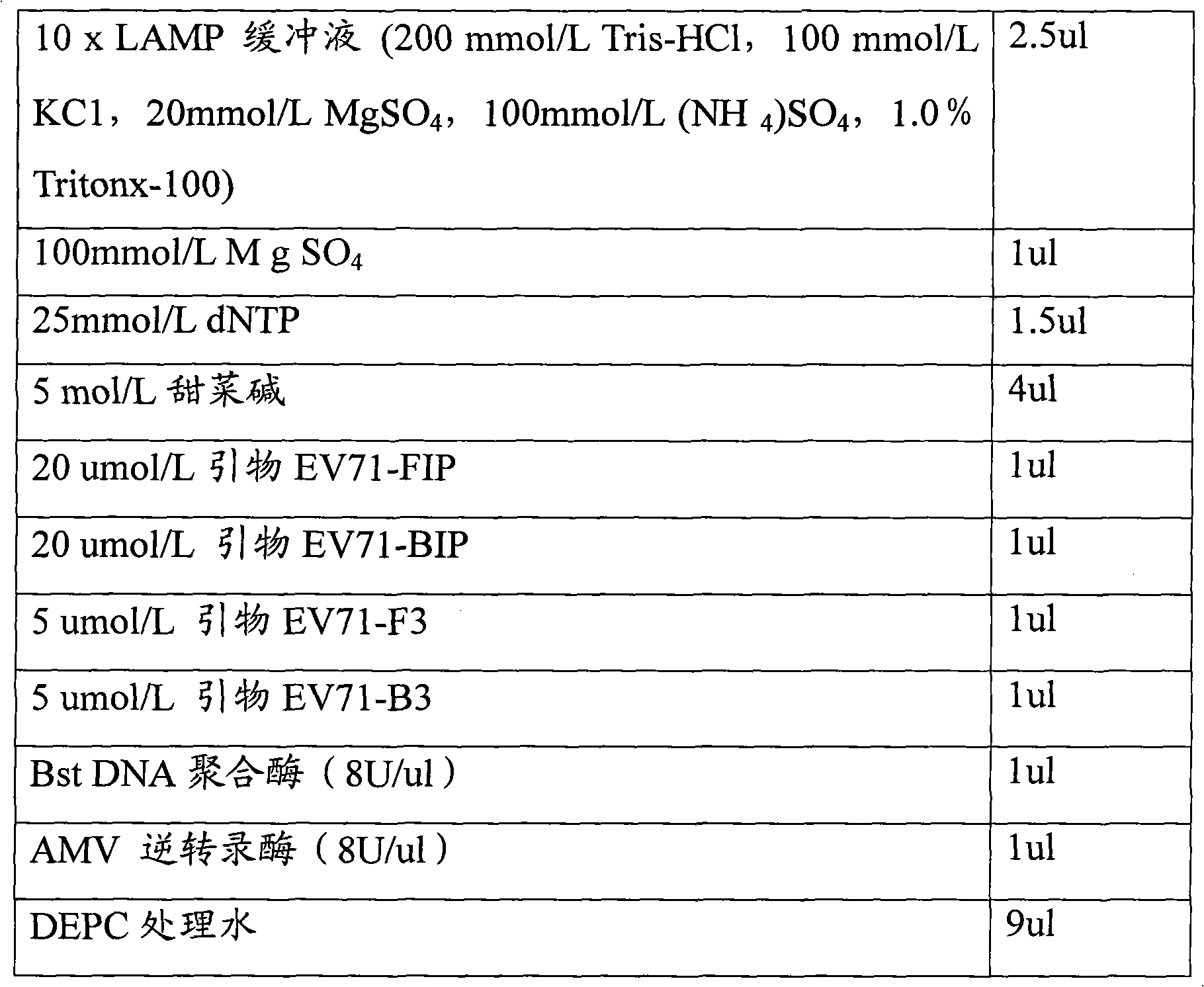
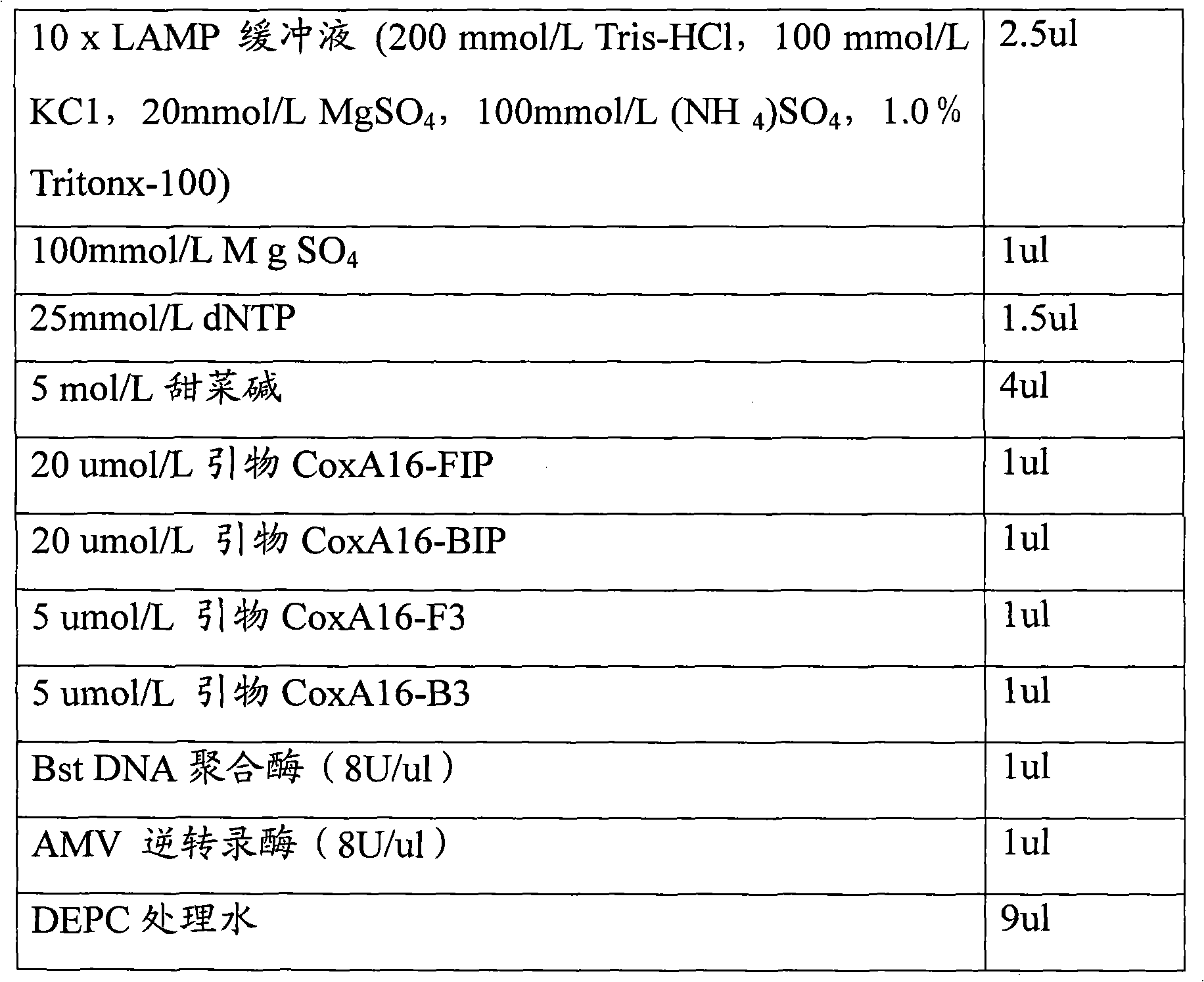
Loop-mediated isothermal amplification assay kit and detection method of hand, foot and mouth disease
InactiveCN102242223ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to the field of biotechnology, and relates to a loop-mediated isothermal amplification (LAMP) assay kit of Coxsackie A16 (Cox A16) and Enterovirus 71 (EV 71) which are main pathogens of hand, foot and mouth disease, and an establishment method and an application of the assay kit. The assay kit comprises four LAMP primers and LAMP reaction liquid for detecting Coxsackie A16, and four LAMP primers and LAMP reaction liquid for detecting Enterovirus 71. Tests prove that the assay kit has the advantages of good specificity and sensitivity, rapid amplification rapid, high efficiency and simple identification. A detection system provided by the invention can rapidly, conveniently, efficiently, high specifically and high sensitively detect Coxsackie A16 and Enterovirus 71 without complex apparatuses thus can satisfy well clinical detection requirements of hand, foot and mouth disease, and is suitable for a large-scale promotion and an application.
Owner:上海吉美生物工程有限公司
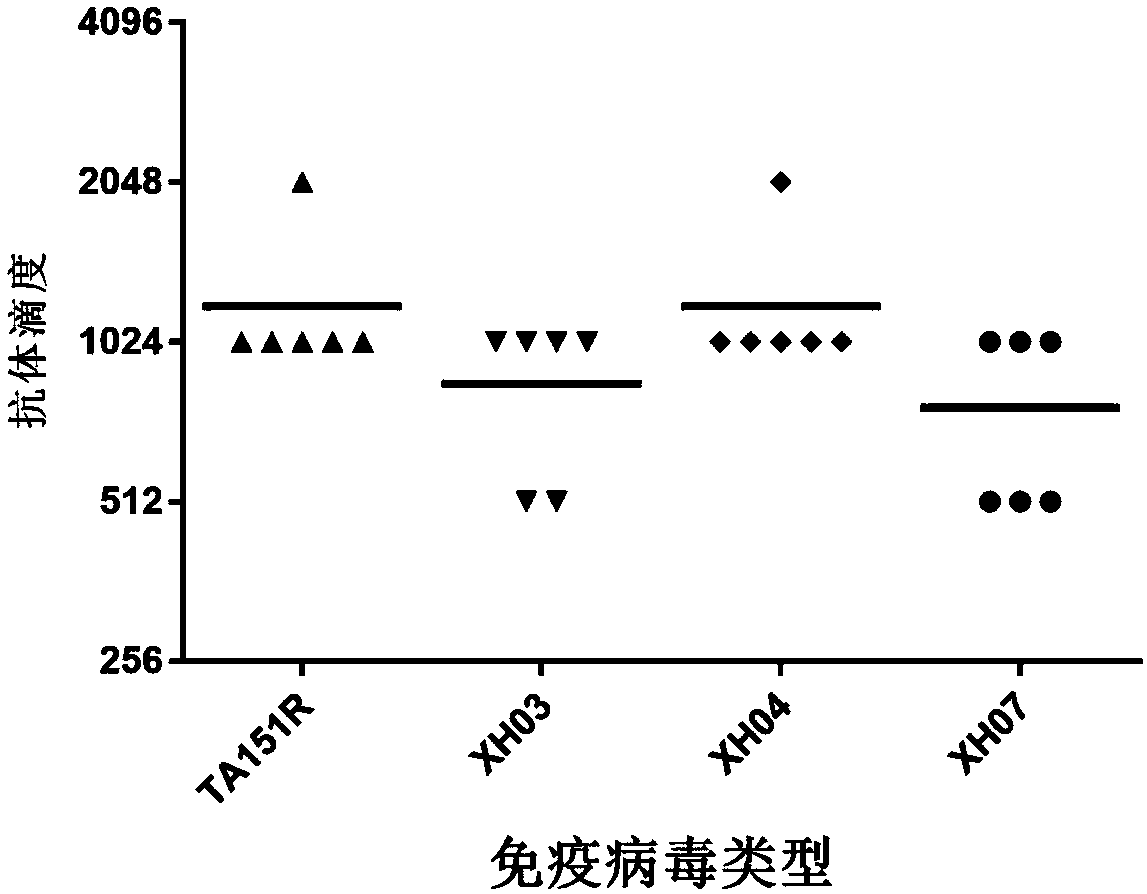
A high-tilter coxsackievirus A10 domesticated strain and applications thereof
InactiveCN107746832APrevent and/or treat diseaseFree from harmSsRNA viruses positive-senseViral antigen ingredientsHep 2 cellCoxsackie meningitis
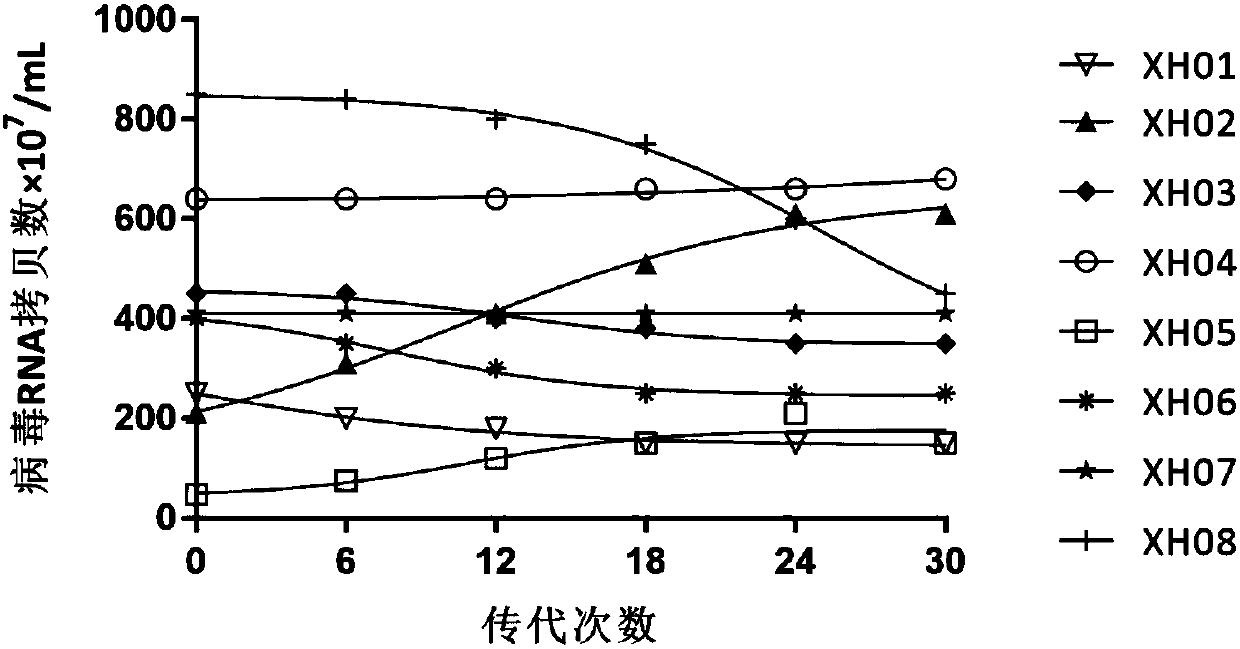
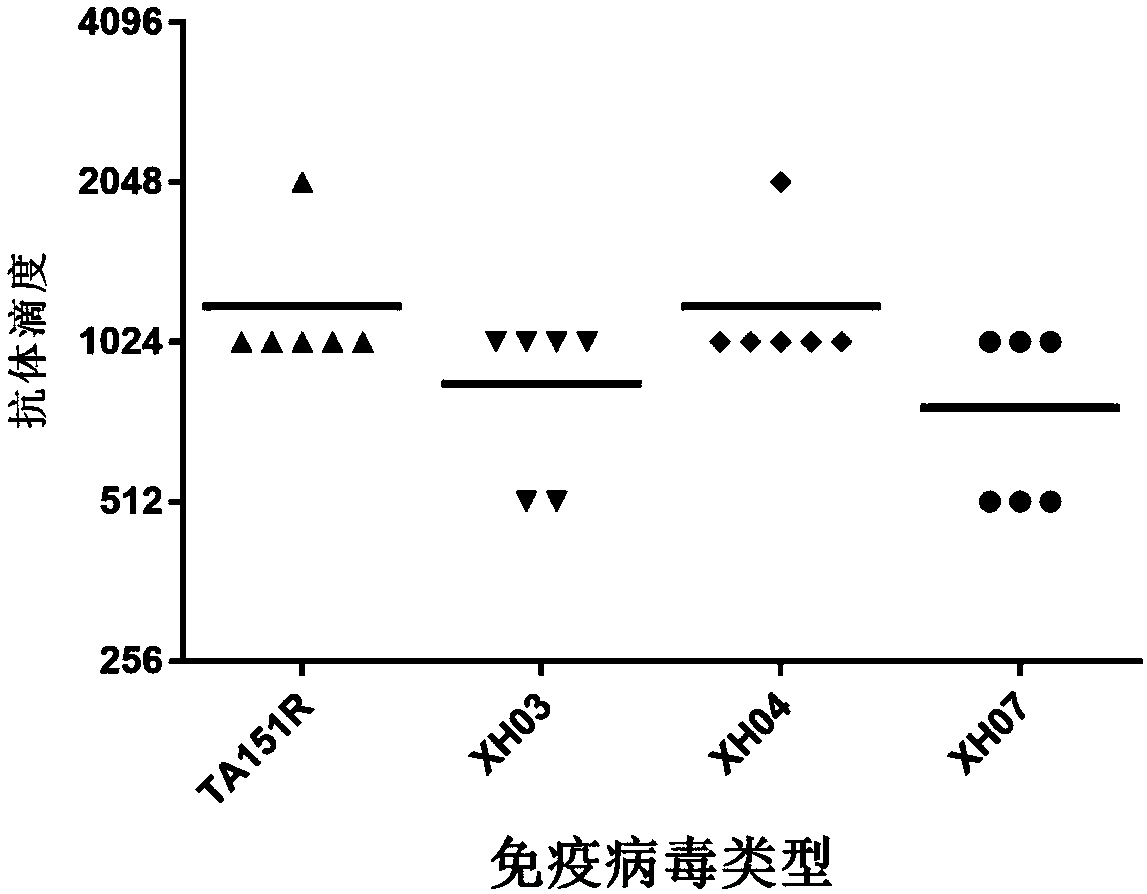
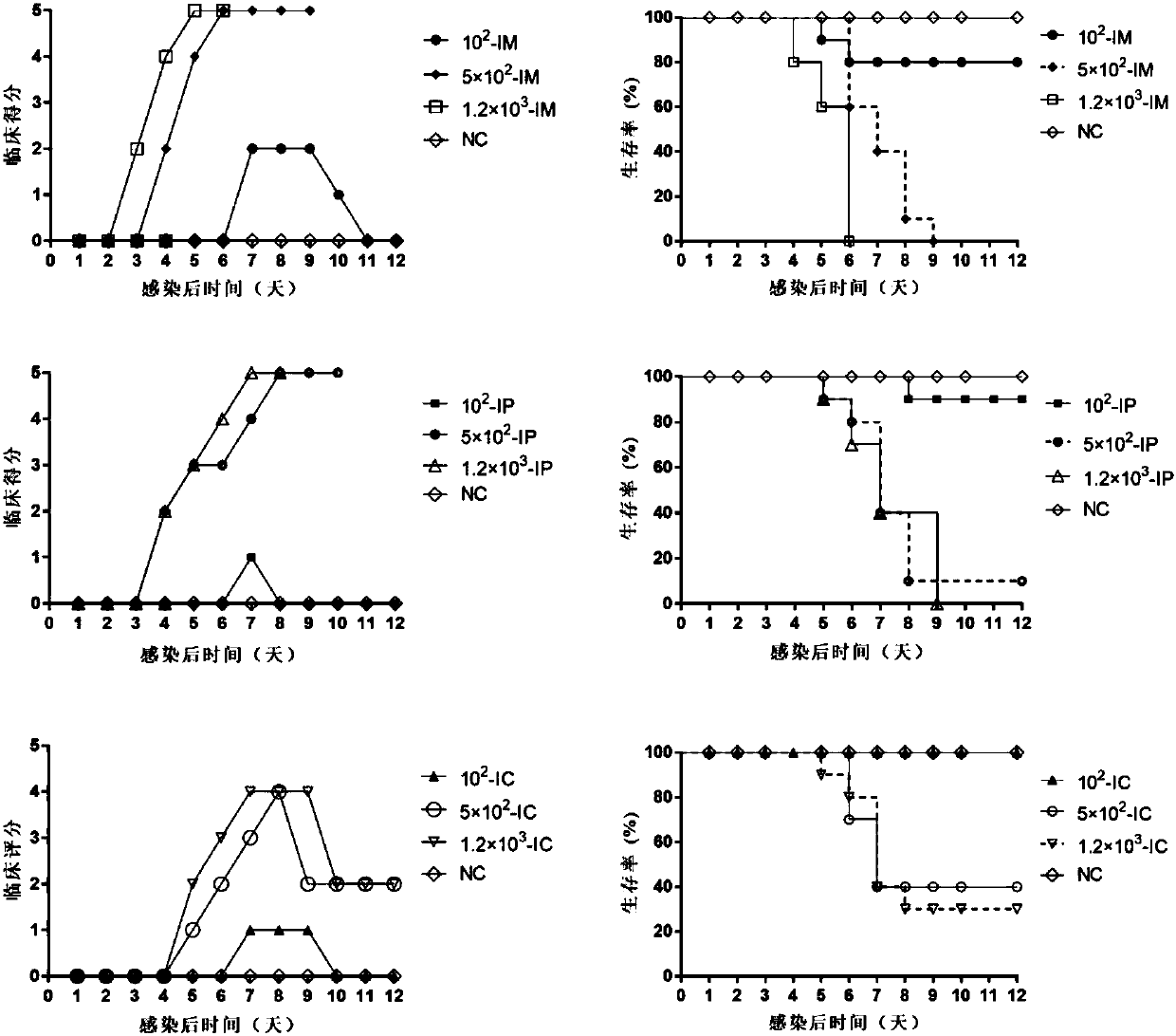
A high-tilter coxsackievirus A10 domesticated strain TA151R-1 stable in passage is disclosed. The virus strain can infect RD cells, HEK293 cells, Vero cells, MRC-5 cells, Hep-2 cells, WI-38 cells, andother cell lines, and can be used for preparing a monovalent or polyvalent vaccine. The prepared vaccine can protect bodies from being harmed by coxsackievirus, can completely protect the body from attack by heterogenous viruses, can effectively prevent and / or treat diseases caused by coxsackievirus infection, and has a wide application prospect.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
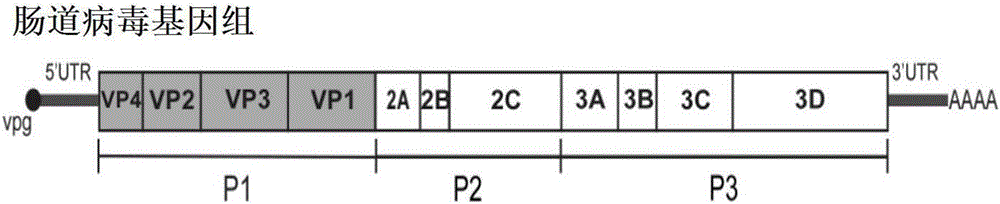
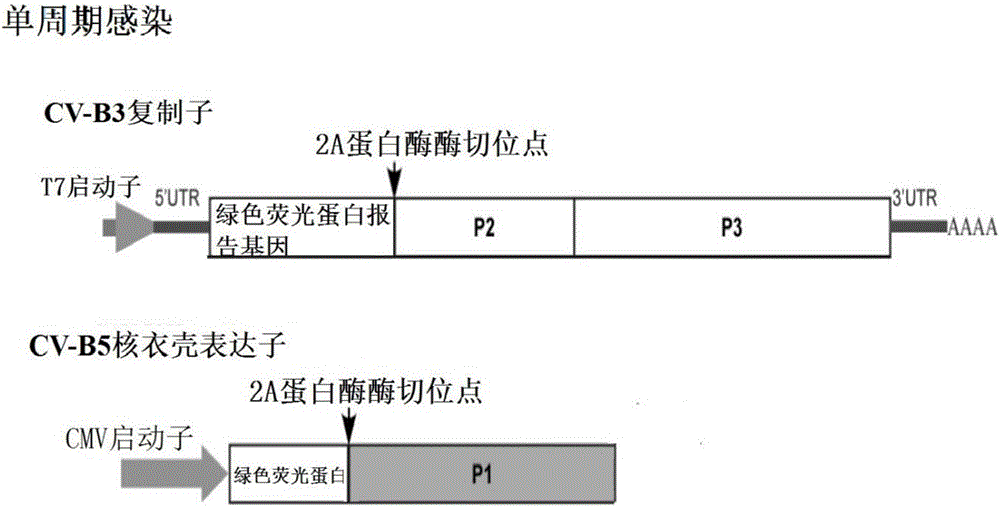
Recombinant expression plasmids used for packaging coxsackievirus B5 (CV-B5) pseudovirus, pseudovirus, kit and method
PendingCN106884017ADetection securityQuick checkViruses/bacteriophagesFermentationCoxsackievirusStructural protein
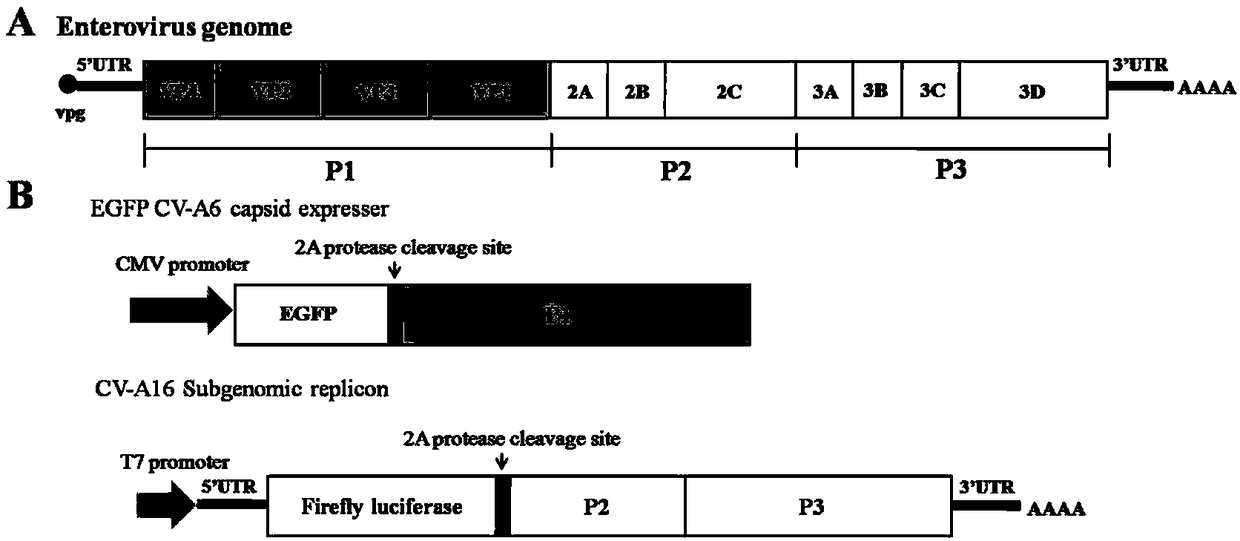
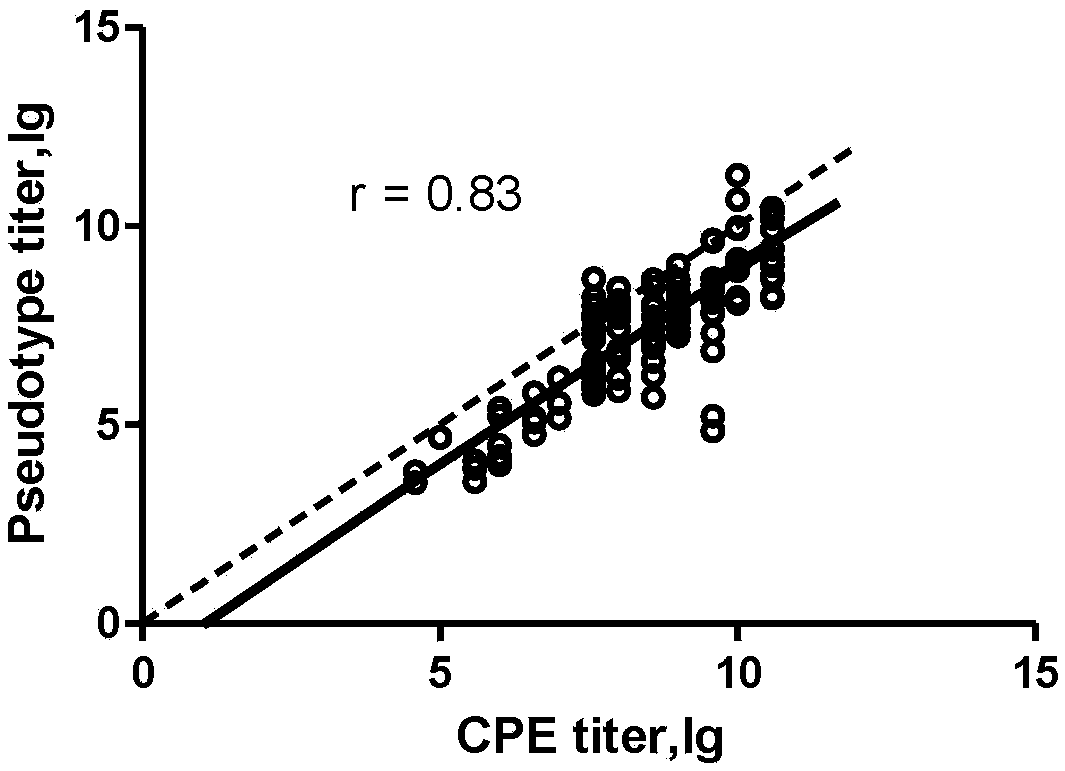
The invention relates to recombinant expression plasmids used for packaging a coxsackievirus B5 (CV-B5) pseudovirus, the pseudovirus, a kit and a method. The recombinant expression plasmids used for packaging the coxsackievirus B5 pseudovirus are respectively named as the pEGFP-CV-B5 (417) plasmid and the pCVB3-replicon, the CV-B5 structural protein expressed by the pEGFP-CV-B5 (417) plasmid can be used for packaging CV-B3 subgenome RNA transcribed by the pCVB3-replicon in the cell, thus the CV-B5 pseudovirus is generated, the pseudovirus can be used for detecting the neutralizing antibody, and since the pseudovirus with single-cycle infection is adopted, the safety problem caused when the live virus is used is avoided. After a plurality of experiments, the result shows that the invention provides the method for detecting the CV-B5 neutralizing antibody which is safe, sensitive, rapid, specific, simple and convenient, and is low in cost. Based on the abovementioned features, the method is particularly suitable for the experiment for rapidly detecting the neutralizing antibody in large scale, and thus the method has the significant application value in developing viral vaccines and detecting the level of the CV-B5 specific neutralizing antibody of individual and group patients.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Preparation and application of gene chip for detecting important enteric causative viruses
InactiveCN102586476AStrong specificityImprove the consistency rateNucleotide librariesMicrobiological testing/measurementRotavirusHybrid system
The invention relates to a gene chip for detecting important enteric causative viruses. The preparation method for the gene chip for detecting important enteric causative viruses comprises the following steps: preparing a specific primer; preparing a virus-specific probe; preparing an oligonucleotide chip; building an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) system; building a hybrid system; preparing a visual detection reagent; and building a color developing method. According to the gene chip prepared by the invention, 10 types of common enteric causative viruses can be simultaneously screened, including poliomyelitis virus I, II and II, enterovirus 71 type, coxsackie virus A16type, coxsackie virus B3, B4 and B5 types, echovirus 30 type and Rotavirus. According to the invention, a new solution for high-flux and quick detection of common enteric causative viruses can be provided, and guidance for monitoring, clinically diagnosing and treating the enteric causative viruses can be provided.
Owner:深圳市普瑞康生物技术有限公司 +1
Building and evaluation of an animal model infected with a coxsackievirus A10 domesticated strain TA151R-1
InactiveCN107744530AImprove replication efficiencyImprove efficiencyCompounds screening/testingViral/bacteriophage medical ingredientsCoxsackievirusPolyvalent Vaccine
A coxsackievirus A10 domesticated strain TA151R-1 high in tilter and stable in passage is disclosed. The virus strain can infect RD cells, HEK293 cells, Vero cells, MRC-5 cells, Hep-2 cells, WI-38 cells, and other cell lines, and can be used for preparing a univalent vaccine or a polyvalent vaccine. The prepared vaccine can protect a body from being harmed by the coxsackievirus, and can completelyprotect the body from attack by heterogenous viruses. An infected animal model can be built efficiently.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Viral myocarditis gene vaccine, its preparation method and application
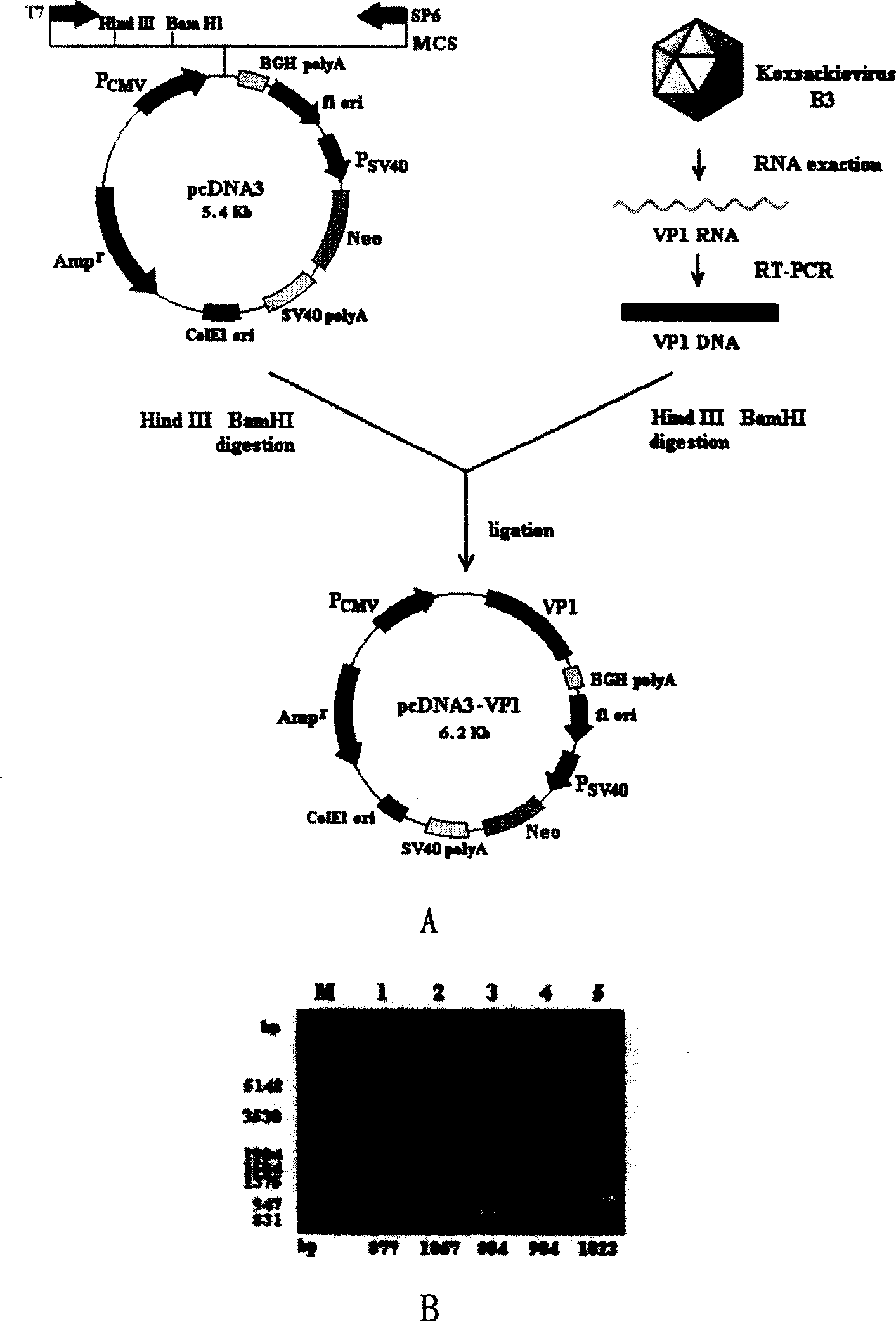
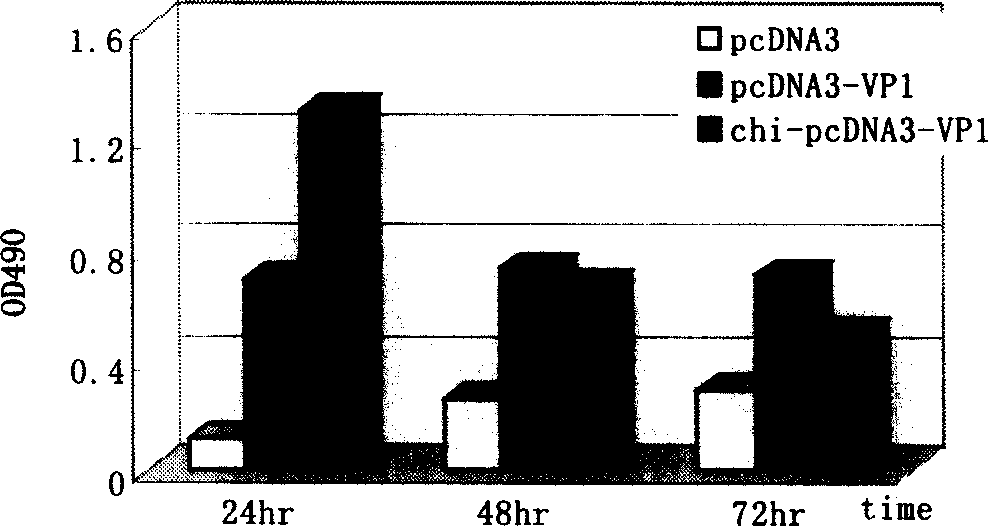
The present invention discloses a viral myocarditis gene vaccine, Said vaccine is constructed by using B3 type Coxsackie virus structural protein VPI gene and pcDNA carrier together, and its exterior is covered with a biological polysaccharide. It also discloses a method for preparing said gene vaccine and the application of said gene vaccine in preparation of medicine for preventing viral myocarditis.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
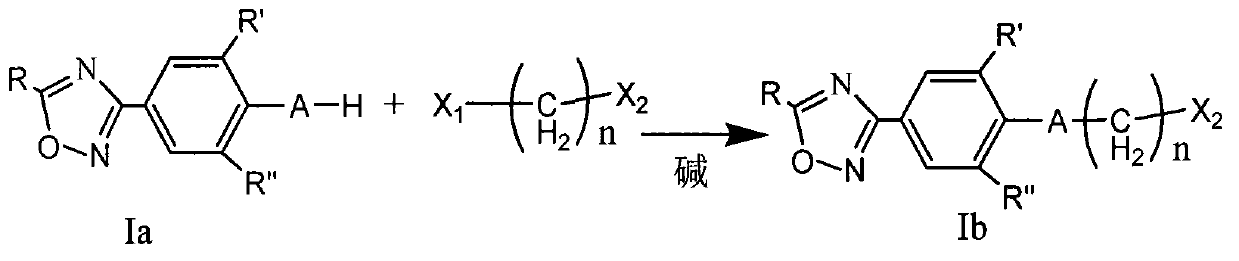
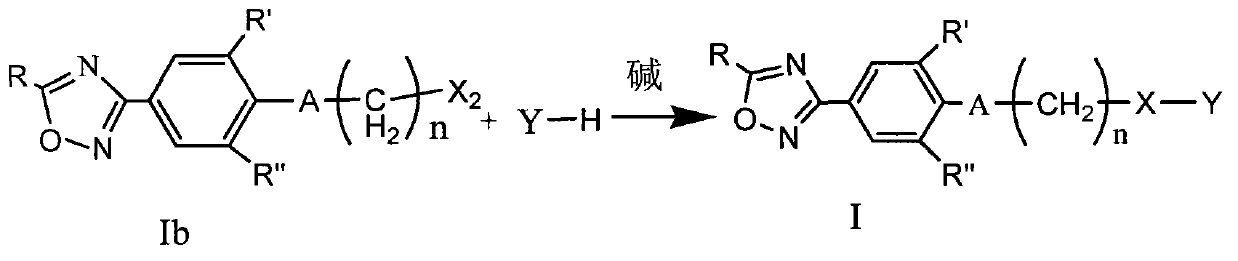
Oxadiazole compound and preparation method thereof, medicine composition and application thereof
ActiveCN103102348AExcellent anti-Coxsackie virus activityLow toxicityOrganic chemistryAntiviralsCoxsackie VirusesChemical compound
The invention provides an oxadiazole compound, a pharmaceutically acceptable salt of the oxadiazole compound, a preparation method of oxadiazole, a medicine composition and application of oxadiazole. The oxadiazole compound contains coxsackie virus and has the structure as shown in formula (I), wherein R is CH3 or CF3, R' and R'' are respectively H, alkyl or halogen; A is O and S; n ranges from 1 to 6; X is O, S or NH; Y is alkyl, unsubstituted naphthenic base, monosubstituted naphthenic base, disubstituted naphthenic base, polysubstituted naphthenic base, unsubstituted aromatic base, monosubstituted aromatic base, disubstituted aromatic base, polysubstituted aromatic base, unsubstituted 5-6 membered heterocyclic radical, monosubstituted 5-6 membered heterocyclic radical, disubstituted 5-6 membered heterocyclic radical or polysubstituted 5-6 membered heterocyclic radical. Compared with the prior art, the oxadiazole compound provided by the invention has high anti-coxsackie virus effect, low toxicity and high safety.
Owner:SHANGHAI JIAOTONG UNIV +1
Primer probe set used for detecting coxsackie viruses, enterovirus 71-type and enterovirus universal type through multiple PCR
InactiveCN108034764AEasy to operateRapid implementation of screeningMicrobiological testing/measurementDNA/RNA fragmentationHand-foot-and-mouth diseaseCoxsackie Viruses
The invention relates to a primer probe set used for detecting coxsackie viruses A6 type / A10 type / A16 type / enterovirus 71-type / enterovirus universal type through multiple PCR. The invention also provides a kit used for detecting coxsackie viruses A6 type / A10 type / A16 type / enterovirus 71-type / enterovirus universal type through multiple quantitative fluorescence PCR. The kit comprises the primer probe set. The above technical scheme provides a complete solution scheme for rapidly screening coxsackie viruses A6 type / A10 type / A16 type / enterovirus 71-type / enterovirus universal type in food, can increase emergent processing for hand-foot-and-mouth disease and comprehensive control capability, provides the necessary technical guarantee for reducing large-scope spreading of hand-foot-and-mouth disease in population, and eliminates the social influence and economic loss due to outbreak of infectious diseases.
Owner:北京卓诚惠生生物科技股份有限公司
Coxsackievirus A6-type strain and application thereof
ActiveCN113564131AImprove abilitiesToxicSsRNA viruses positive-senseViral antigen ingredientsStructural proteinGenotype
The invention relates to the technical field of biology, in particular to a coxsackievirus A6-type strain and application thereof. The amino acid sequence of P1 structural protein of the coxsackievirus A6-type strain is shown as SEQ ID NO.1. The strain has high cross-neutralization capacity in genotypes and between genotypes, is high in toxicity, has high pathogenic and lethal capacity on mice, and has good immunogenicity and high titer and stability. The strain can be used for immunogenicity evaluation or protective evaluation of coxsackievirus A6-type vaccines, the accuracy and repeatability of vaccine immunogenicity evaluation are improved, and the strain can also be used for preparing coxsackievirus infection animal models and has good application prospects.
Owner:BEIJING MINHAI BIOTECH
Method for detecting neutralizing antibody in coxsackievirus A6 (CV-A6), and recombinant virus applied in method
ActiveCN108060172ALower requirementGood linear relationshipSsRNA viruses positive-senseVector-based foreign material introductionViral VaccineNeutralizing antibody
The invention discloses a method for detecting a neutralizing antibody in coxsackievirus A6 (CV-A6), and a recombinant virus applied in the method. According to the method, the neutralizing antibody is detected by using a pseudovirus system packaged by two types of recombinant plasmids; by adopting a single-cycle infected pseudovirus, the safety problem caused by use of live viruses is avoided. The results of a plurality of tests prove that the pseudovirus detection system is a CV-A6 neutralizing antibody detection method which is safe, sensitive, rapid, specific, simple and convenient, and low in cost. Based on the characteristics, the pseudovirus detection system is very suitable for the tests for rapid and large-scale detection of the neutralizing antibody, and has an import applicationvalue for development of a CV-A6 viral vaccine and detection of CV-A6 specific neutralizing antibody level of patients and populations.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Preparation method and application of recombination coxsackie virus B3-type virus-like particles
The invention discloses a preparation method of recombination coxsackie virus B3-type virus-like particles. The method disclosed by the invention comprises the following steps that 1, the P1 gene and 3CD gene of the coxsackie virus B3 type are cloned to a target plasmid to obtain a recombination expression vector; 2, the target yeast cell is transformed by the recombination expression vector which is obtained in the step 1 to obtain a recombination yeast cell which expresses the P1 gene and the 3CD gene; and 3, the recombination yeast cell which is obtained in the step 2 is cracked, and the recombination coxsackie virus B3-type virus-like particles are obtained after the separation. Experiments prove that after the method disclosed by the invention is utilized, the recombination coxsackie virus B3-type virus-like particles are successfully produced in the yeast expression system, and can be further used for candidate prophylactic vaccine and medicine combination for vital myocarditis and diabetes mellitus type I.
Owner:BEIJING UNIV OF TECH
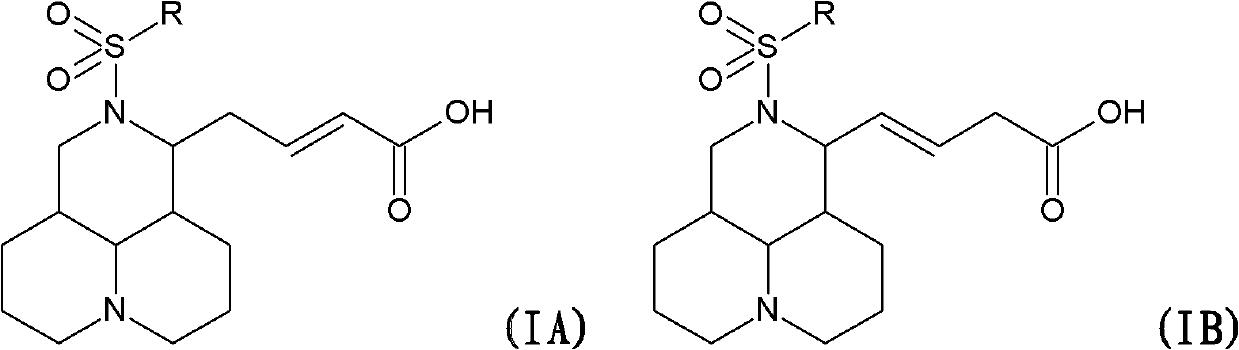
N-substituted sophora flavescens olefine acid derivative as well as preparation method and application thereof
The invention relates to an N-substituted sophora flavescens olefine acid derivative represented by a general formula IA or IB, a preparation method thereof, a medicine composite containing the derivative, a method for utilizing the derivative to treat diseases caused by Coxsackie B viruses, and an application of the derivative to prepare a medicine for treating the diseases caused by the Coxsackie B viruses.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Hand-foot-mouth disease detection reagent kit and its detection method
InactiveCN102643927ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to biological field, and relates to a reagent kit for detecting main pathogen Coxsackievirus A16 (Cox A16) and enterovirus 71 of hand-foot-mouth disease, construction method and uses thereof. The reagent kit comprises reaction solutions for detecting four primers of Coxsackievirus A16 and four primers of enterovirus 71.As detected, the reagent kit has a good performance of specificity, sensitivity, rapid amplification, high efficiency and simple identification. The detection system of the invention can detect Coxsackievirus A16 and enterovirus 71 quickly, conveniently, high-effectively, high-specifically and highly-sensitive under temperature of 64 DEG C without need of complicated instruments, which can preferably satisfy clinical detection for hand-foot-mouth disease and be easily popularized in large scope.
Owner:刘志学 +1
Interfering RNA (Ribonucleic Acid) for suppressing hand-foot-and-mouth disease virogene, vector containing the same and application thereof
InactiveCN102399780AElimination of clinically manifest symptomsImprove targetingGenetic material ingredientsAntiviralsA-DNAExperimental animal
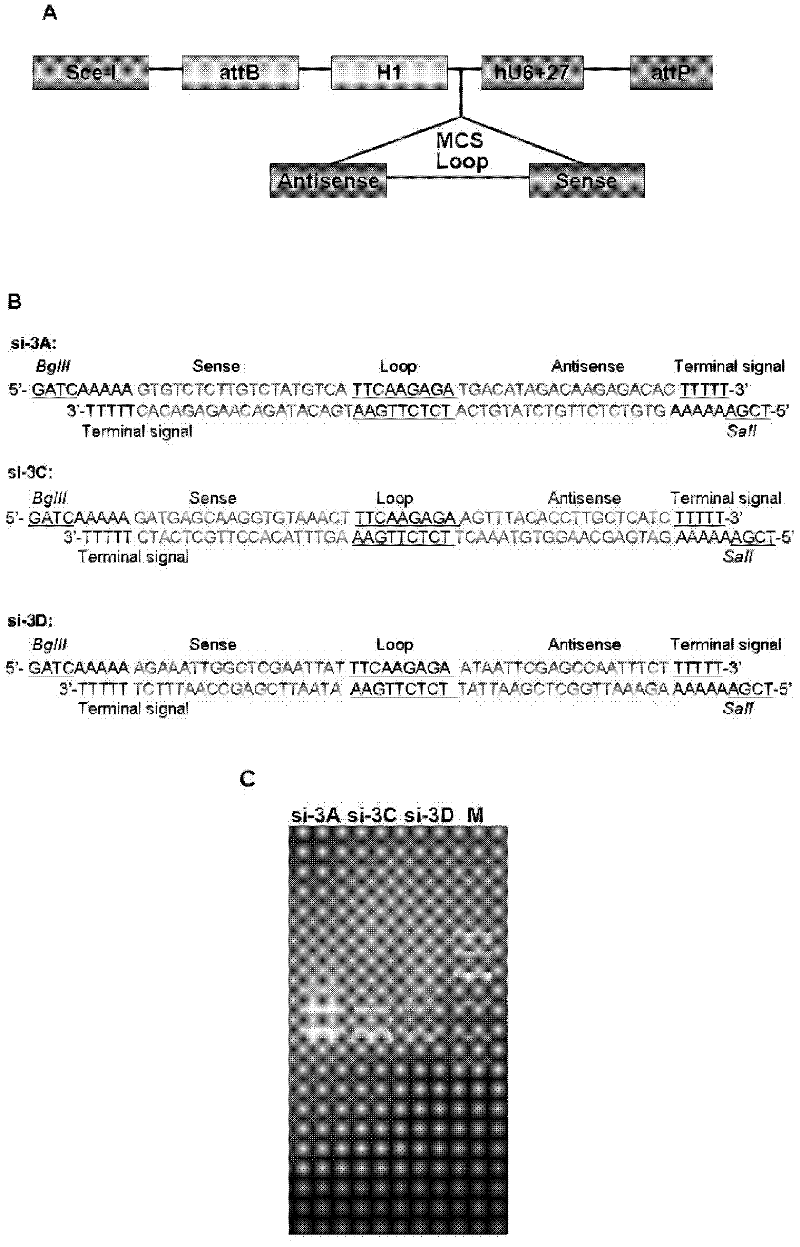
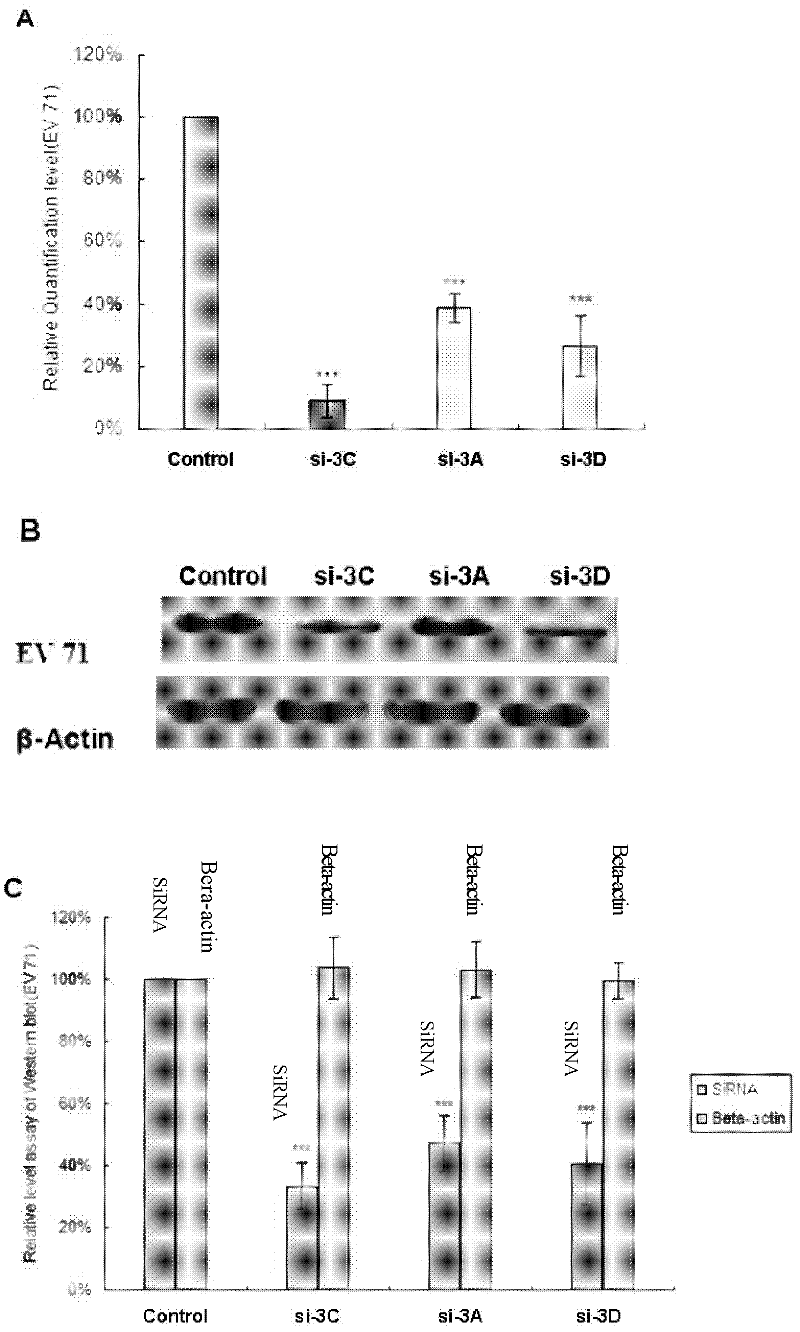
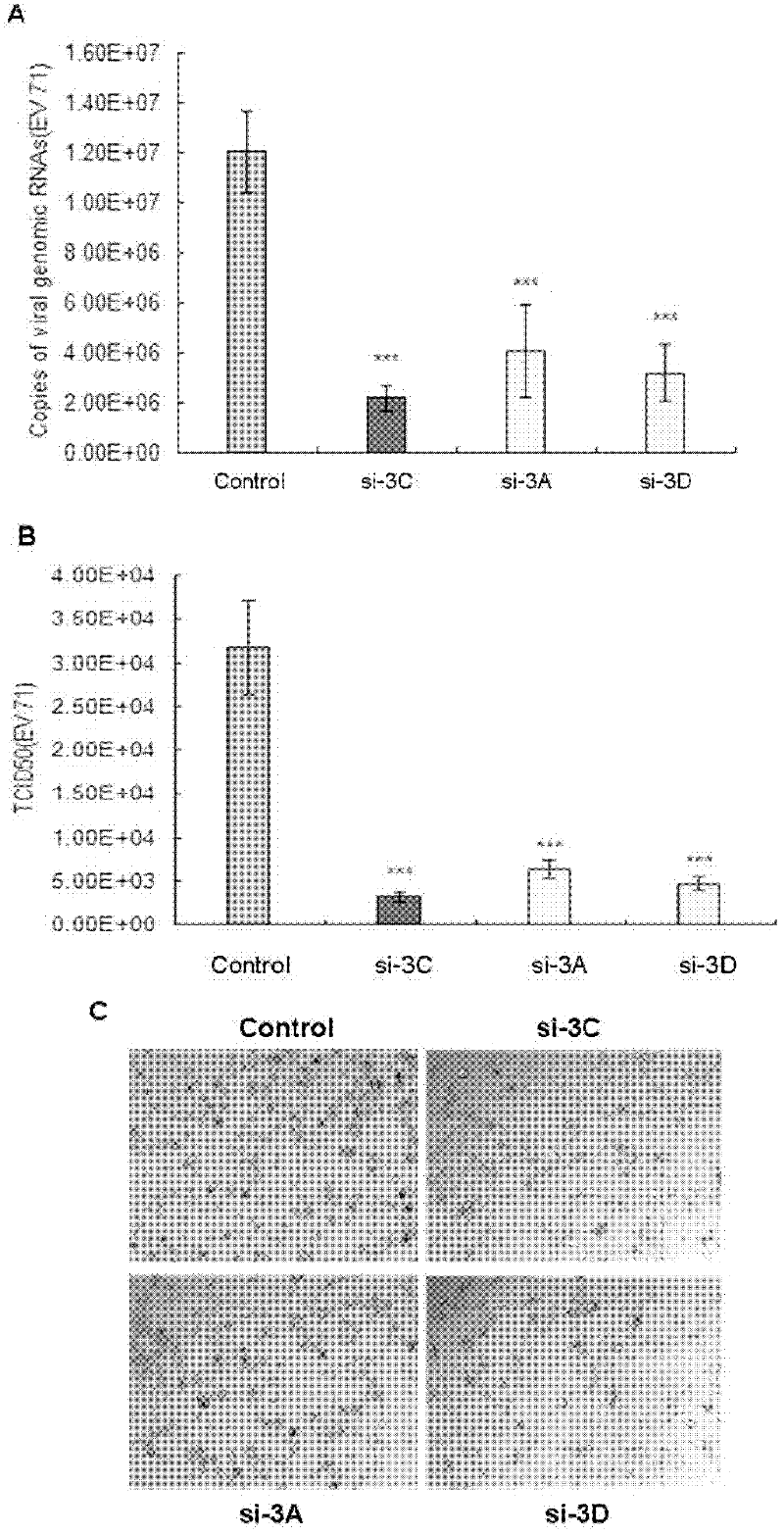
The invention provides a method for suppressing virogene expression causing hand-foot-and-mouth disease, in particular two interfering RNA (Ribonucleic Acid) for two main pathogens of enterovirus 71 and coxsackie virus A16 genomes and a DNA sequence thereof, a carrier containing the interfering RNA and an application of the carrier transcribed interfering RNA in preparation of medicines for preventing and curing hand-foot-and-mouth diseases. It is shown by experiment result that the carrier transcribed interfering RNA can be used for obviously suppressing copying of two viruses and eliminating clinical symptoms of the hand-foot-and-mouth diseases at the same time of reducing pathogen enterovirus 71 and coxsackie virus A16 gene expressions on cell level and experimental animals. The carrier transcribed interfering RNA and the carrier containing the same, provided by the invention, can play am important role in preventing and curing the hand-foot-and-mouth diseases.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Isoflavone derivative and preparation method and application thereof
InactiveCN101723926ASignificant anti-Coxsackie virus effectSimple preparation processOrganic active ingredientsOrganic chemistryCoxsackie VirusesStructural formula
The invention relates to an isoflavone derivative and a preparation method and application thereof, which belong to the field of chemical medicaments. The technical problem solved by the invention is to provide the isoflavone derivative and the preparation and the application thereof. The isoflavone derivative has a structural formula as shown by (I), wherein R1 and R2 in the structural formula are C1-C5 alkyls. The isoflavone derivative has a significant effect of resisting Coxsackie viruses, can be used for preparing medicaments for resisting the Coxsackie viruses, and has an intensive application prospect.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Hand-foot-and-mouth disease pathogen detection primer group and kit
ActiveCN105695633AQuick checkEasy to detectMicrobiological testing/measurementMicroorganism based processesMicrofluidic chipMicro fluidic
The invention discloses a hand-foot-and-mouth disease pathogen detection primer group and a kit, belonging to the technical field of virus detection. The hand-foot-and-mouth disease pathogen detection primer group comprises an RT-LAMP primer of a coxsackie virus type A16 and / or enterovirus type 71. The kit formed by the hand-foot-and-mouth disease pathogen detection primer group comprises a micro-fluidic chip and a detection solution. By adopting the primer system, the amplified reaction background is reduced, and the sensitivity and the specificity are very good. The detection solution system of the kit saves an independent RT-PCR secondary amplification step, so that the detection time is shortened; and moreover, the denaturation and renaturation processes of nucleic acid are avoided, so that the pollution probability of RNA enzyme and amplified nucleic acid is reduced, and the detection sensitivity and the detection safety are improved. By adopting the micro-fluidic chip detection system, the requirement on experimental hardware is lowered, the consumption of a reaction reagent is reduced, the detection cost is decreased, and a detection result can be directly determined by virtue of the color change. The hand-foot-and-mouth disease pathogen detection primer group and the kit have the advantages of safety, specificity, sensitivity and convenience.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Coxsackie virus CA16 VP1 recombinant antigens, and monoclonal antibodies and application thereof
InactiveCN104710537AEase of specific binding reactionsStrong specificityImmunoglobulins against virusesBiological testingCoxsackie VirusesViral test
The invention discloses a set of Coxsackie virus A16 (CA16) VP1 recombinant antigens which are prepared by respectively connecting 8 amino acids disclosed as SEQ ID NO.1-SEQ ID NO.8 with a flexible short peptide amino acid sequence. The invention also discloses monoclonal antibodies capable of being specifically combined with the CA16 VP1 recombinant antigens and application of the recombinant antigens in preparing an A16 Coxsackie virus antigen or antibody detection kit. The 8 recombinant antigens and 4 monoclonal antibodies have the characteristics of high sensitivity, high specificity and the like, and can be used as key raw materials for developing Coxsackie virus CA16 detection reagents.
Owner:汪运山
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com