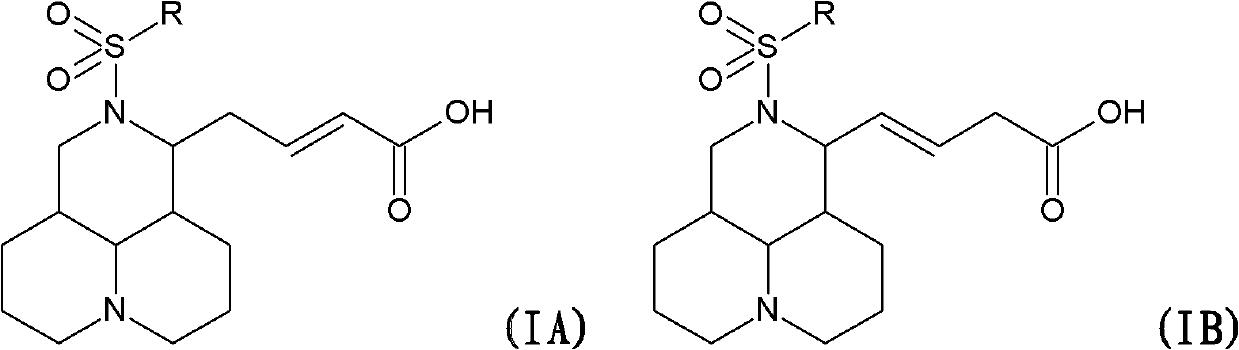
N-substituted sophora flavescens olefine acid derivative as well as preparation method and application thereof
A technology of matrine and compounds, applied in the field of medicine for diseases, can solve the problem of Coxsackie virus with no specific medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Step 2. Preparation of diphenyldiazomethane:
[0061] Take 9.8g (0.05mol) of benzophenone hydrazone, add 120mL of petroleum ether (30-60℃), add 13.05g (0.15mol) of electrolytic manganese dioxide, reflux for 1h, filter with suction, wash the filter cake with petroleum ether, and combine the filtrates , used directly in the next reaction.
[0062] Step 3. Preparation of diphenylmethyl ester of α and β matrine:
[0063] Add the petroleum ether solution of diphenyldiazomethane directly to the methanol solution of SC-1, stir at room temperature until the purple color completely subsides, about 12 hours, concentrate to dryness, add dichloromethane and water layer, dichloromethane layer with anhydrous Na 2 SO 4 After drying, a dichloromethane solution of diphenylmethyl esters of α and β matrine acids was obtained, which was directly used in the subsequent synthesis reaction of N-substituted α and β matrine acids.
[0064] Step 4. Preparation of diphenylmethyl esters of N-s...
Embodiment 1
[0072] The synthesis of embodiment 1.N-benzenesulfonyl matrine acid (SC-16A) and (SC-16B):
[0073]
[0074] Add K 2 CO 3 3.45g (0.025mol), 1.60mL (12.5mmol) of benzenesulfonyl chloride was added dropwise, stirred at room temperature, until TLC detection, the raw material point disappeared, filtered off inorganic salts, concentrated, flash column chromatography, 3g white solid was obtained, added m-formazine 15 mL of phenol was reacted at 80-90°C for 8 hours, 50 mL of methyl ethyl ketone was added, extracted with water three times, the aqueous layers were combined and concentrated to obtain 1.6 g of a white solid. Flash column chromatography yielded 0.6 g of SC-16A and 0.8 g of SC-16B.
[0075] SC-16A:
[0076] HRMS-ESI (M / Z): C 21 h 29 N 2 o 4 S 405.1833(M+1); 406.1909(M+2); 407.1851(M+3);
[0077] 1 H-NMR (CD 3 OD, δppm): 7.73-7.75 (2H, m), 7.50-7.59 (3H, m), 5.42-5.45 (2H, m), 3.79 (1H, dd, J=4.4, 12.4Hz), 3.49-3.54 ( 1H, m), 3.09(1H, t, J=12.4Hz), 2.98(2H, t...
Embodiment 2
[0081]The synthesis of embodiment 2.N-p-toluenesulfonyl matrine acid (SC-18A) and (SC-18B):
[0082]
[0083] Add K 2 CO 3 3.45g (0.025mol), 2.38g (12.5mmol) of p-toluenesulfonyl chloride was added dropwise, stirred at room temperature, until the TLC detection, the raw material point disappeared, filtered off inorganic salts, concentrated, flash column chromatography, to obtain 3.5g white solid, added 15 mL of m-cresol was reacted at 80-90°C for 8 hours, 50 mL of methyl ethyl ketone was added, extracted with water for 3 times, the aqueous layers were combined and concentrated to obtain 1.8 g of white solid. Flash column chromatography yielded 0.7 g of SC-18A and 0.8 g of SC-18B.
[0084] SC-18A:
[0085] HRMS-ESI (M / Z): C 22 h 31 N 2 o 4 S 419.2006(M+1); 420.2083(M+2); 421.2011(M+3);
[0086] 1 H-NMR (CD 3 OD, δppm): 7.62 (2H, d, J = 8Hz), 7.34 (2H, d, J = 8Hz), 5.38-5.49 (2H, m), 3.74 (1H, dd, J = 4.4, 12Hz), 3.43 (1H, t, J=9.6Hz), 2.91-3.05(5H, m), 2.76(1H, s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com