Hyaluronic acid derivative and preparation method for hyaluronic acid hydrogel
A technology of hyaluronic acid and derivatives, which is applied in the field of biomedical material preparation to achieve stable performance, controllable modification reaction and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
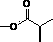
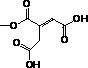
[0059] Hyaluronic acid-butenediolate derivatives were prepared as follows:
[0060] (1) Weigh 0.2 g of hyaluronic acid (about 0.527 mmol, the molecular weight of its structural unit is 379.32 g / mol, the same below) into 100 mL of anhydrous formamide, stir at 50°C until fully dissolved;
[0061] (2) Weigh 0.517 g of maleic anhydride (about 5.27 mmol, molecular weight of 98.06 g / mol) and dissolve it in anhydrous formamide, and add it to the above solution (1) under stirring conditions. The molar number of maleic anhydride is hyaluronic acid 10 times the number of moles of reactive hydroxyl groups on the acid;
[0062] (3) No catalyst is added;
[0063] (4) React at 50°C for 5 h. After the reaction solution is cooled to room temperature, add it dropwise to 500 mL of cold absolute ethanol under stirring to precipitate the reaction product;
[0064] (5) Centrifuge the above solid-liquid mixture at high speed, disperse and centrifuge the precipitate in absolute ethanol, and repeat...
Embodiment 2
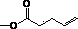
[0068] Hyaluronic acid-4-pentenoate derivatives were prepared as follows:
[0069] (1) Weigh 0.4 g of hyaluronic acid (about 1.06 mmol) into 100 mL of anhydrous tetrahydrofuran, stir at 60°C until fully dissolved;
[0070] (2) Weigh 0.961 g 4-pentene anhydride (about 5.27 mmol, molecular weight 182.22 g / mol) and dissolve it in anhydrous tetrahydrofuran, add it to the above solution (1) under stirring condition, the mole number of 4-pentene anhydride It is 5 times the number of moles of reactive hydroxyl groups on hyaluronic acid;
[0071] (3) Add 0.1g of DMAP catalyst to the above reaction solution;
[0072] (4) React at 60°C for 10 h. After the reaction solution is cooled to room temperature, add it dropwise to 800 mL of cold anhydrous isopropanol under stirring to precipitate the reaction product;
[0073] (5) Centrifuge the above solid-liquid mixture at high speed, disperse and centrifuge the precipitate in anhydrous isopropanol, and repeat this step 2-4 times;
[0074] ...
Embodiment 3
[0076] A single-component hyaluronic acid-butene ester hydrogel was prepared as follows:
[0077] Weigh 20 mg of hyaluronic acid-butenediolate derivatives sealed after freeze-drying, dissolve in 1.0 mL containing 0.1% 2-hydroxy-4'-(hydroxyethoxy)-2-methylpropiophenone ( I2959) Aqueous solution of initiator; after taking an appropriate volume of the solution in a specific mold, at a wavelength of 365nm and a light intensity of 30mW / cm 2 The UV radiation for 120s initiates the polymerization reaction to form hyaluronic acid hydrogel.
[0078] The single-component hyaluronic acid-butenediolate hydrogel obtained by DMA is used for detection, and the dynamic force frequency is 1.0Hz and 10Hz. The detection results are as follows: figure 2 shown. It can be seen from the figure that the average storage modulus of the hydrogel is above 0.2 MPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com