Patents
Literature
108 results about "Alkylating antineoplastic agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An alkylating antineoplastic agent is an alkylating agent used in cancer treatment that attaches an alkyl group (CₙH₂ₙ₊₁) to DNA. The alkyl group is attached to the guanine base of DNA, at the number 7 nitrogen atom of the purine ring.
Process for manufacturing a glutamic acid derivative and a pyroglutamic acid derivative and a novel intermediate in the manufacture thereof
InactiveUS20060009394A1Easy to manufactureEfficient and easy manufactureNervous disorderOrganic compound preparationAlkylating antineoplastic agentHydroxyproline
Glutamic acid derivatives, in particular monatin, may be conveniently prepared by alkylating a 4-protected hydroxyl pyroglutamic acid derivative with an alkylating agent to prepare a 4-protected hydroxyl-4-alkylglutamic acid derivative, followed by the steps of hydrolysis and deprotection. The 4-protected hydroxyl pyroglutamic acid derivative is easy to produce from hydroxyproline. The 4-protected hydroxyl pyroglutamic acid derivative is particularly suitable for use in the efficient manufacture of monatin of high optical purity, since it can be alkylated selectively at the 4-position and stereoselectively and after its alkylation, it can easily be converted to a glutamic acid derivative.
Owner:AJINOMOTO CO INC
Catalytic system for the preparation of polybutadienes and preparation process
InactiveUS7056998B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationRare earthPhosphoric acid
The present invention relates to a catalytic system usable for the preparation of polybutadienes by polymerisation, to a process for the preparation of said catalytic system and to a process for the preparation of polybutadienes by means of this catalytic system.A catalytic system according to the invention is based on at least:a conjugated diene monomer,an organic phosphoric acid salt of one or more rare earth metals, said salt being in suspension in at least one inert, saturated and aliphatic or alicyclic hydrocarbon solvent,an alkylating agent consisting of an alkylaluminium of formula AlR3 or HAlR2, the “alkylating agent:rare earth salt” molar ratio being greater than 5, anda halogen donor which belongs to the family of alkylaluminium halides with the exception of alkylaluminium sesquihalides,and, according to the invention, said catalytic system comprises said rare earth metal(s) in a concentration equal to or greater than 0.005 mol / l.
Owner:MICHELIN RECH & TECH SA
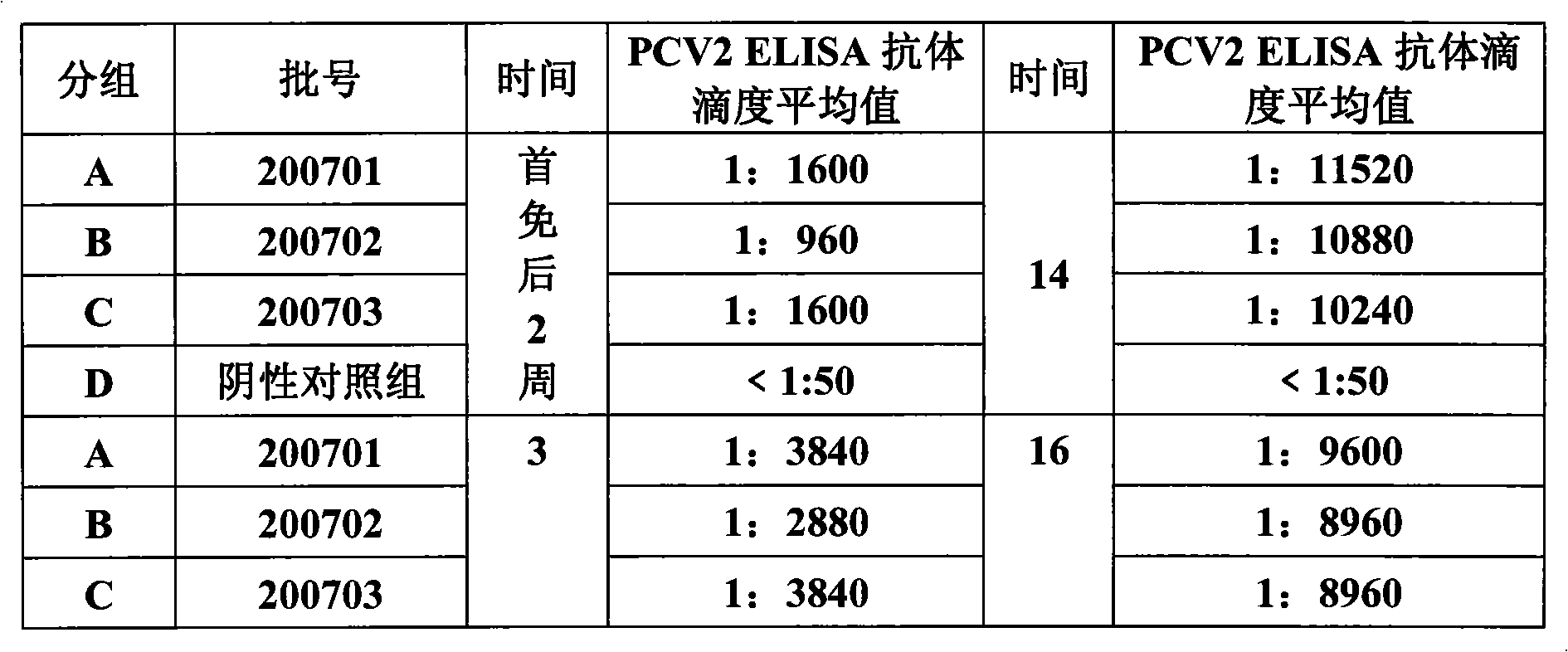
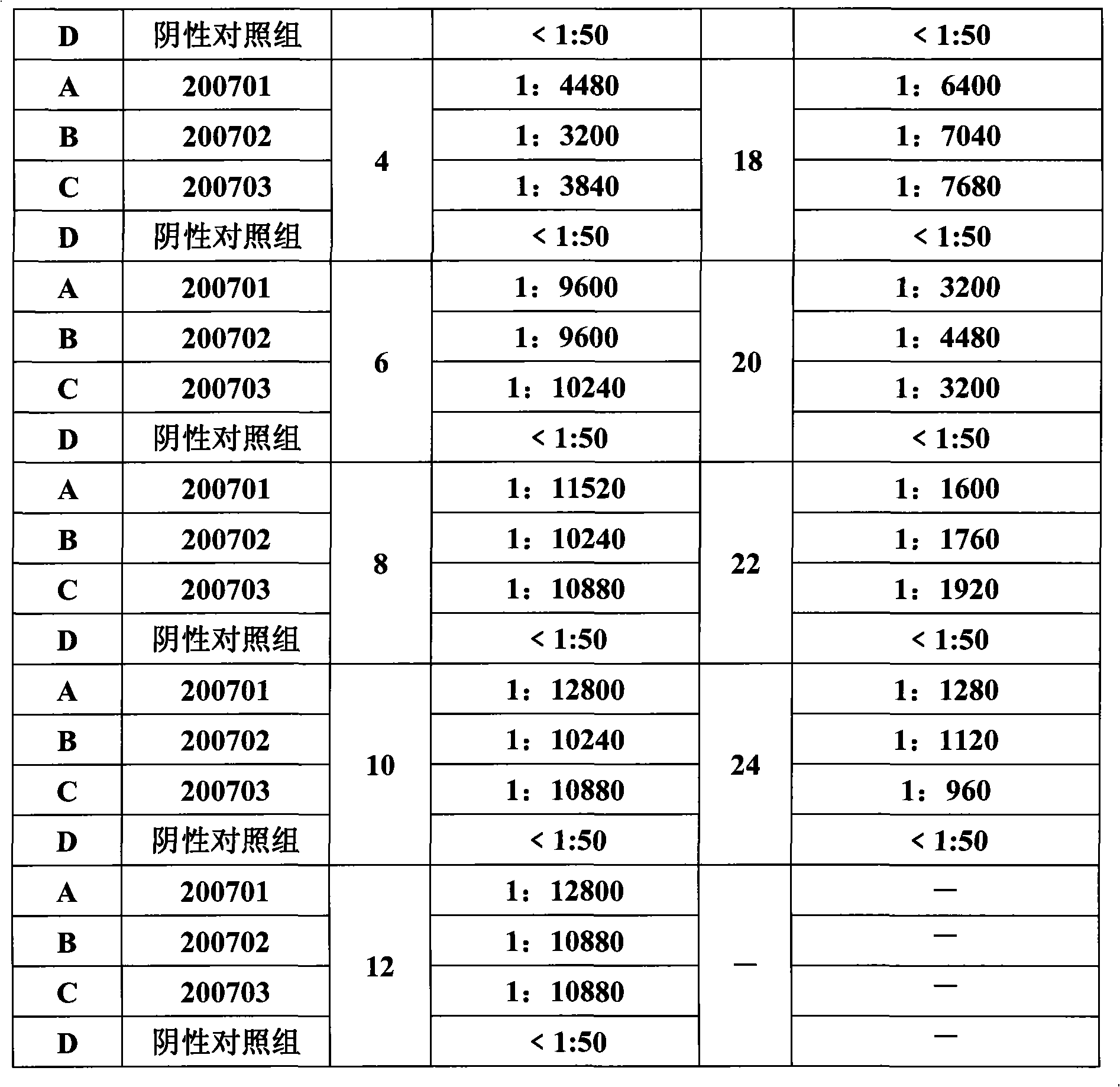
Porcine circovirus type II inactivated vaccine of and method for preparing same
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
Use of dianhydrogalactitol and analogs or derivatives thereof in combination with platinum-containing antineoplastic agents to treat non-small-cell carcinoma of the lung and brain metastases
ActiveUS20160008316A1Increase survivalSuppress growthHeavy metal active ingredientsBiocideTyrosine kinaseCisplatin
The use of dianhydrogalactitol provides a novel therapeutic modality for the treatment of non-small-cell lung carcinoma (NSCLC) and ovarian cancer, as well as other types of malignancy, including brain metastases of NSCLC. Dianhydrogalactitol acts as an alkylating agent on DNA that creates N7 methylation. Dianhydrogalactitol is effective in suppressing the growth of cancer stem cells and is active against tumors that are refractory to temozolomide, cisplatin, and tyrosine kinase inhibitors; the drug acts independently of the MGMT repair mechanism. Dianhydrogalactitol can be used together with other anti-neoplastic agents and can possess additive or super-additive effects.
Owner:DEL MAR PHARMA
Novel cc-1065 Analogs and Their Conjugates
ActiveUS20110207767A1Improve pharmacological propertiesHigh cytotoxic activityAntibacterial agentsBiocideAlkylating antineoplastic agentDisease
This invention relates to novel analogs of the DNA-alkylating agent CC-1065 and to their conjugates. Furthermore this invention concerns intermediates for the preparation of said agents and conjugates. The conjugates are designed to release their (multiple) payload after one or more activation steps and / or at a rate and time span controlled by the conjugate in order to selectively deliver and / or controllably release one or more of said DNA alkylating agents. The agents, conjugates, and intermediates can be used to treat an illness that is characterized by undesired (cell) proliferation. As an example, the agents and the conjugates of this invention may be used to treat a tumor.
Owner:BYONDIS BV
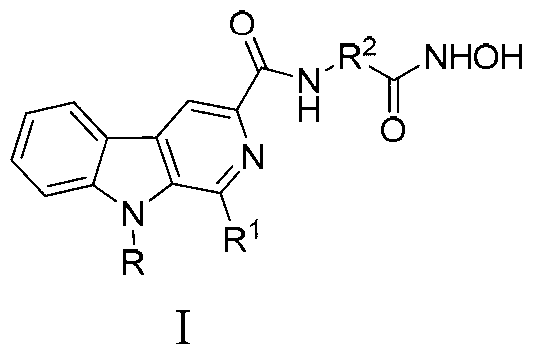
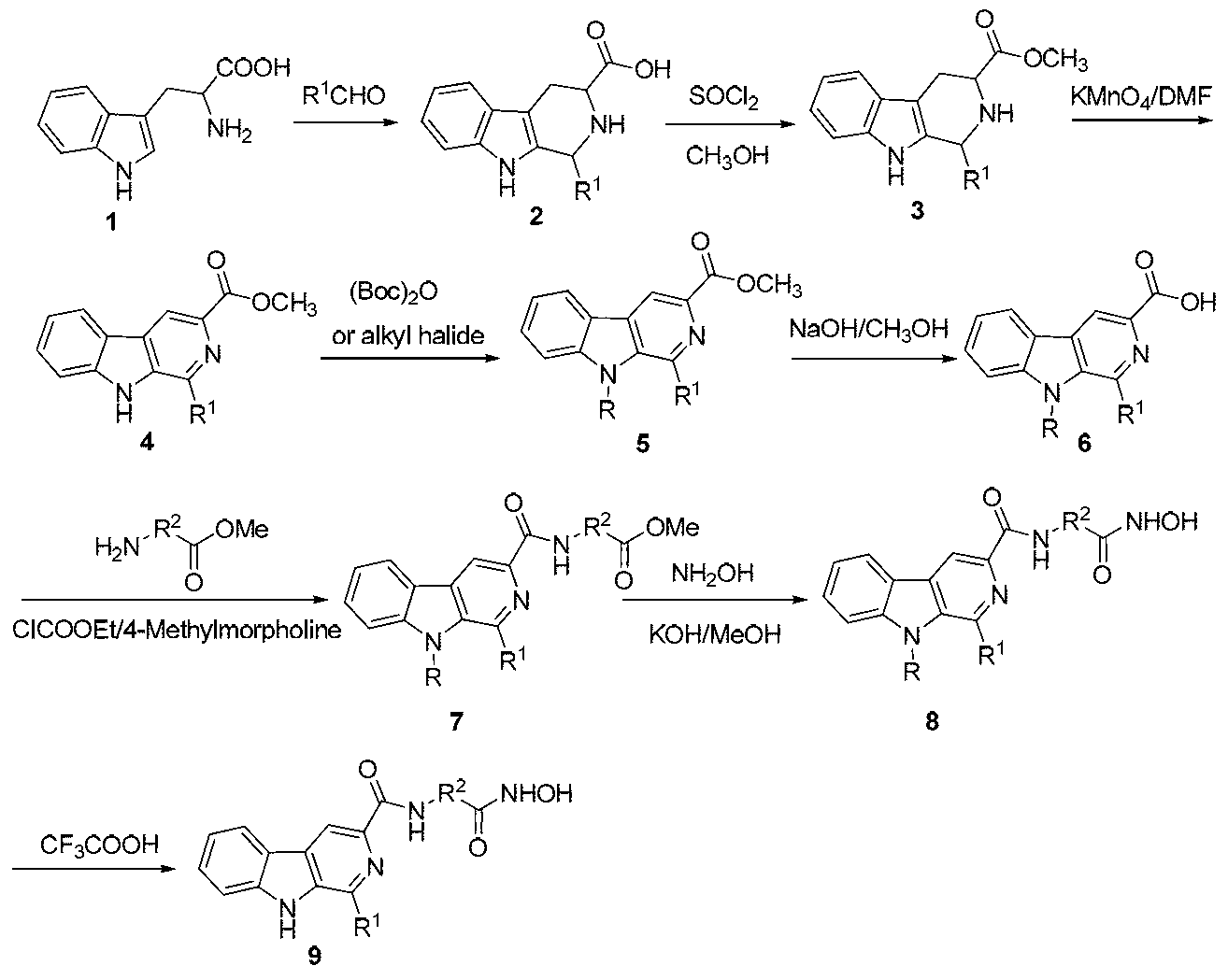
Beta-carboline derivative containing hydroximic acid as well as preparation method and medical application thereof
ActiveCN103304564APromote apoptosisApoptotic promotionOrganic active ingredientsNervous disorderDiseaseTreatment effect
The invention discloses a beta-carboline derivative containing hydroximic acid as well as a preparation method and a medical application thereof. The product has the structure as shown in the general formula I. The compound can be combined with other antitumor drugs such as alkylating agents, antimetabolites, topoisomerase inhibitors, mitotic inhibitors and DNA intercalating agents and additionally can be combined with radiotherapy. The invention also discloses a pharmaceutical composition containing the compound with the general formula I as well as medical application thereof, particularly application of preparation of antitumor medicines, Alzheimer disease medicines and Parkinson disease medicines. The other antitumor medicines or radiotherapy can be administrated with the compound at the same time or in different times. The combination therapy can generate the synergistic effect, so that the treatment effect is improved.
Owner:JIANGSU FANGSHIYUANLVE SCI & TECH CONSULTING CO LTD +1
Cell-derived viral vaccines with low levels of residual cell DNA
ActiveUS20090304729A1Enhance immune responseLess recognizableSsRNA viruses negative-senseViral antigen ingredientsViral VaccineA-DNA
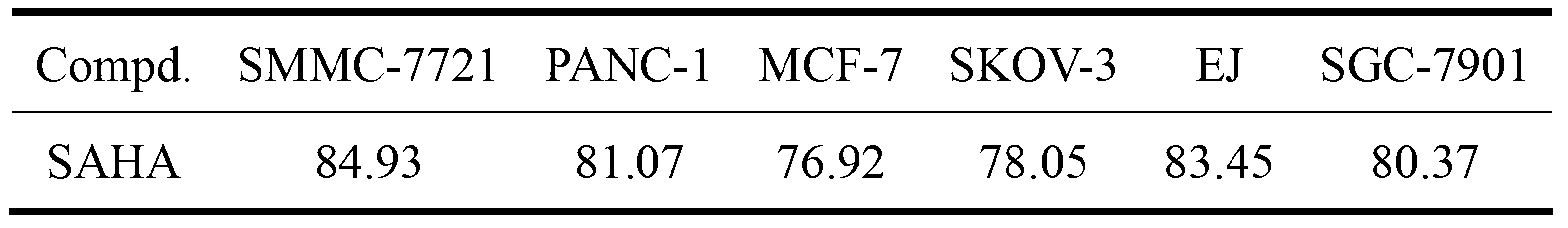
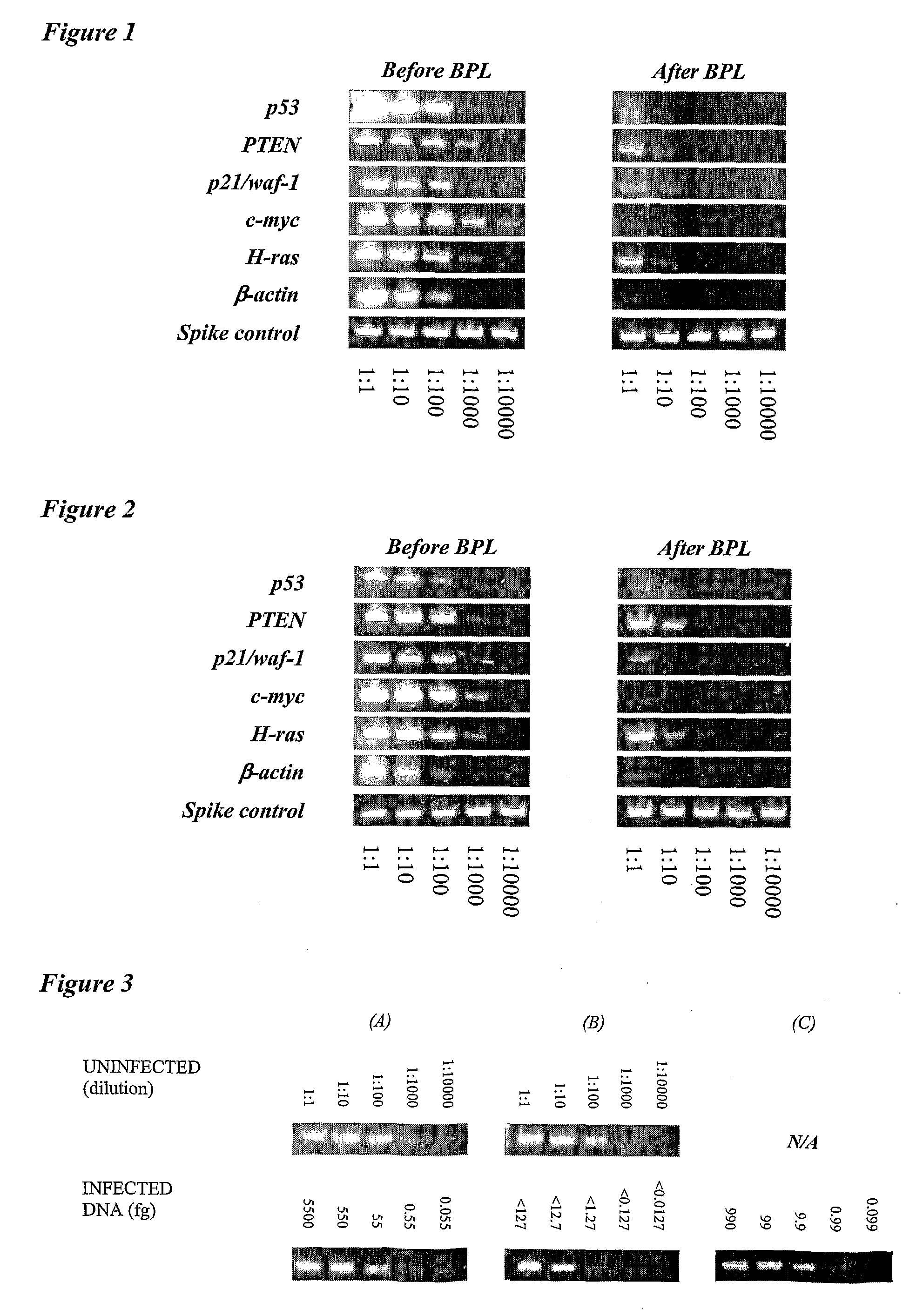
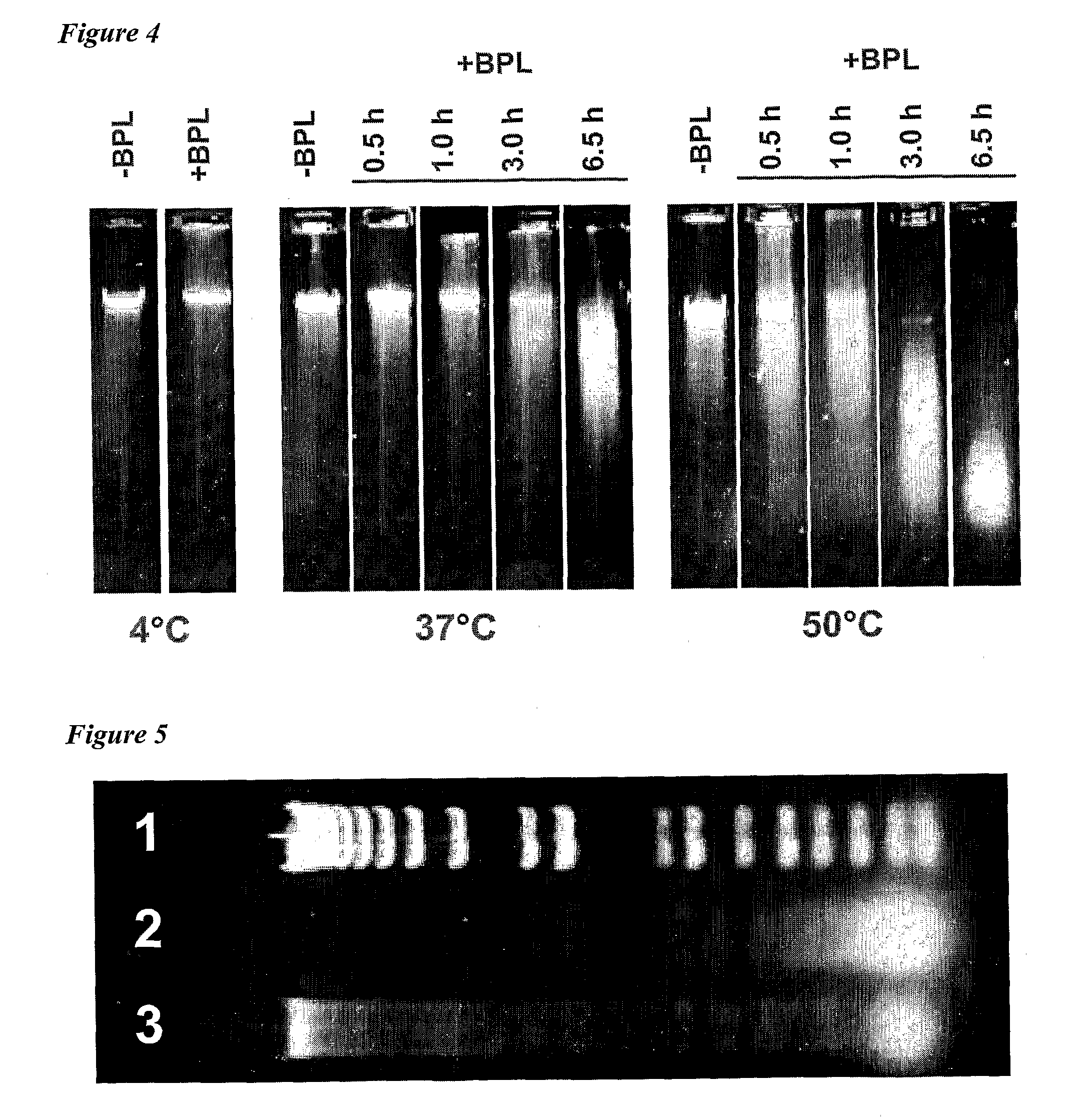
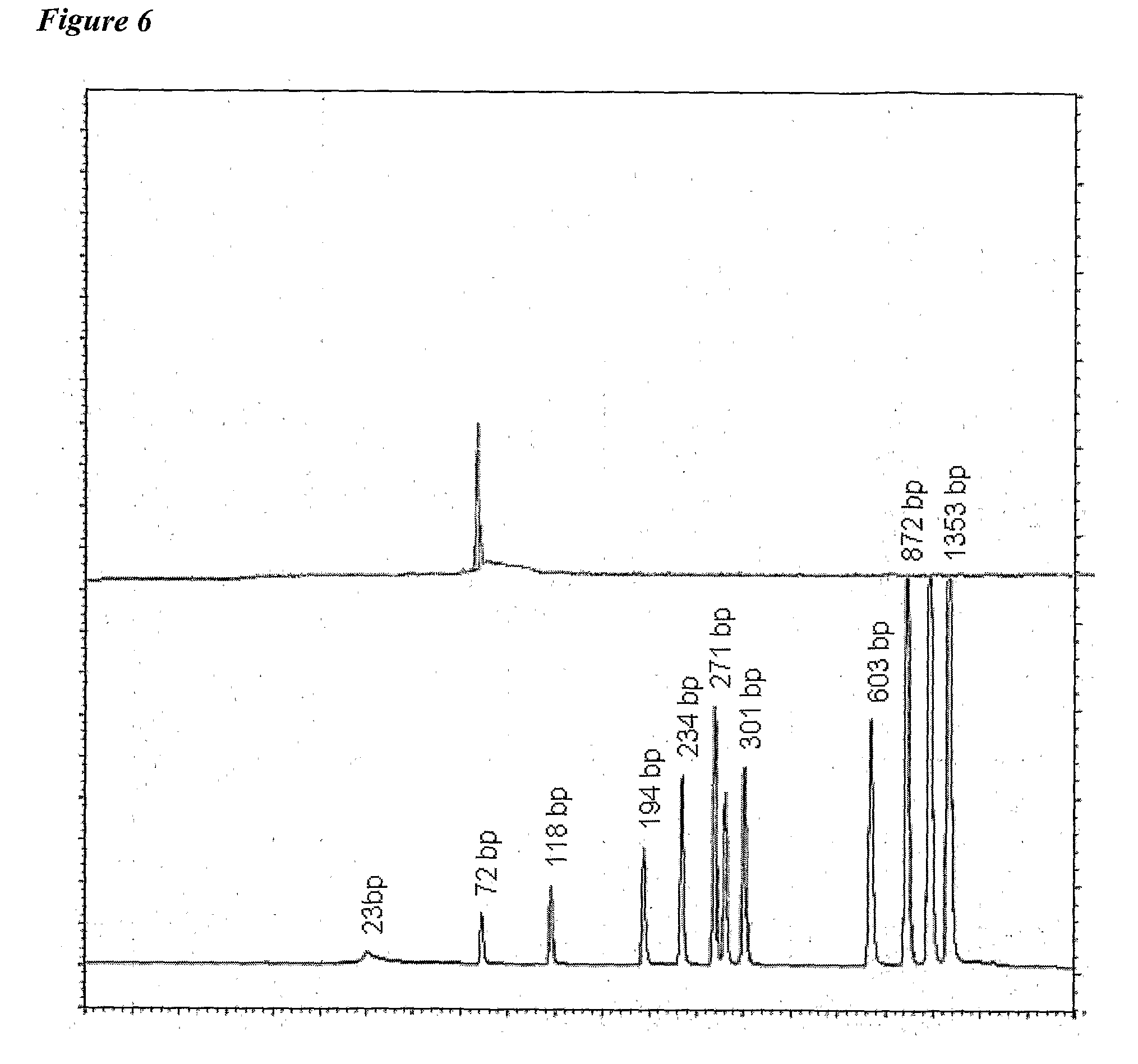
The present invention relates to vaccine products for the treatment or prevention of viral infections. Further provided are methods of reducing contaminants associated with the preparation of cell culture vaccines. Residual functional cell culture DNA is degraded by treatment with a DNA alkylating agent, such as β-propiolactone (BPL), thereby providing a vaccine comprising immunogenic proteins derived from a virus propagated on cell culture, substantially free of residual functional cell culture DNA.
Owner:SEQIRUS UK LTD
CC-1065 analogs and their conjugates
ActiveUS8889868B2High activityPromote aggregationAntibacterial agentsBiocideBiological activationDNA
Owner:BYONDIS BV
Multi drug response markers for breast cancer cells
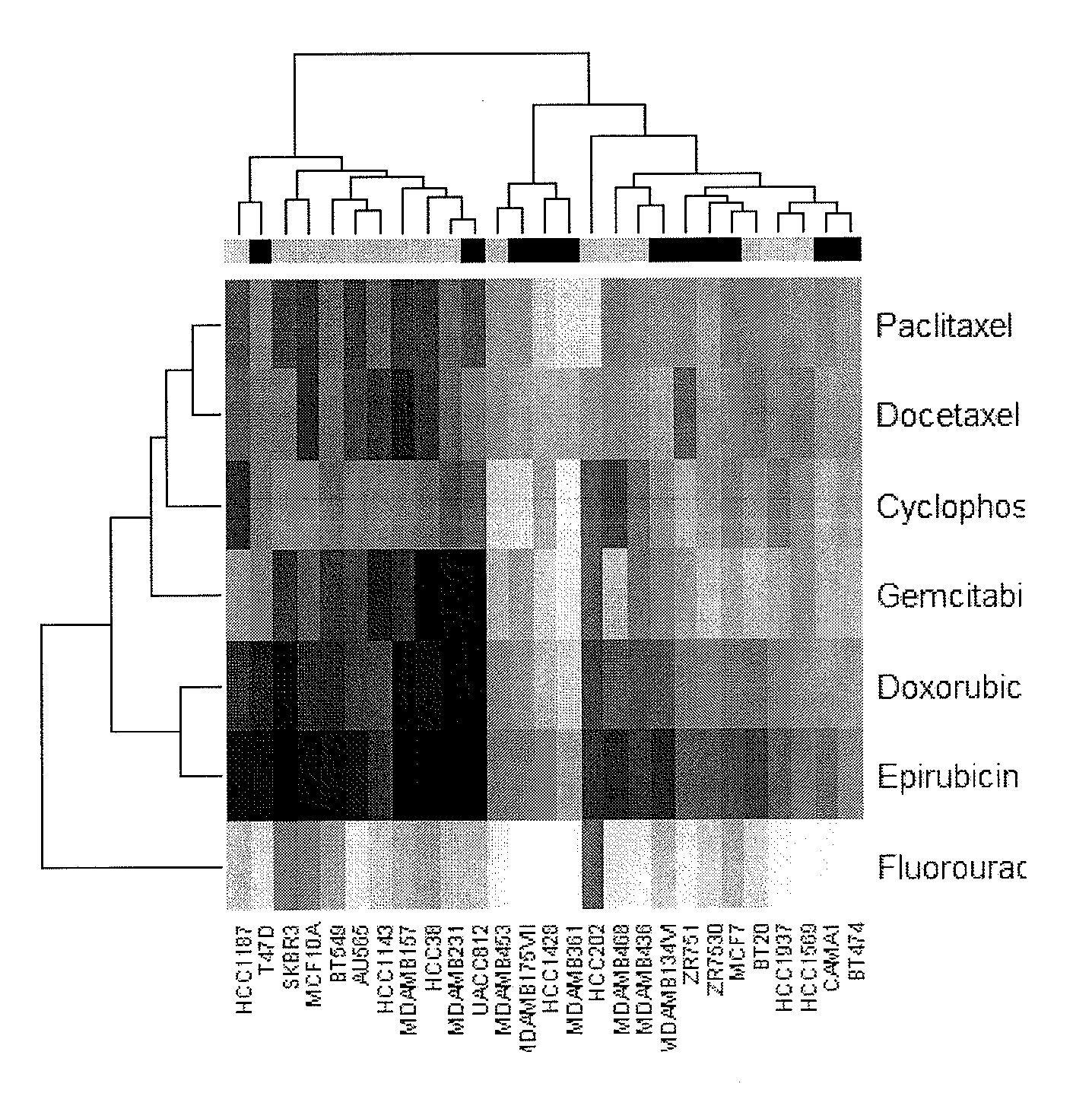
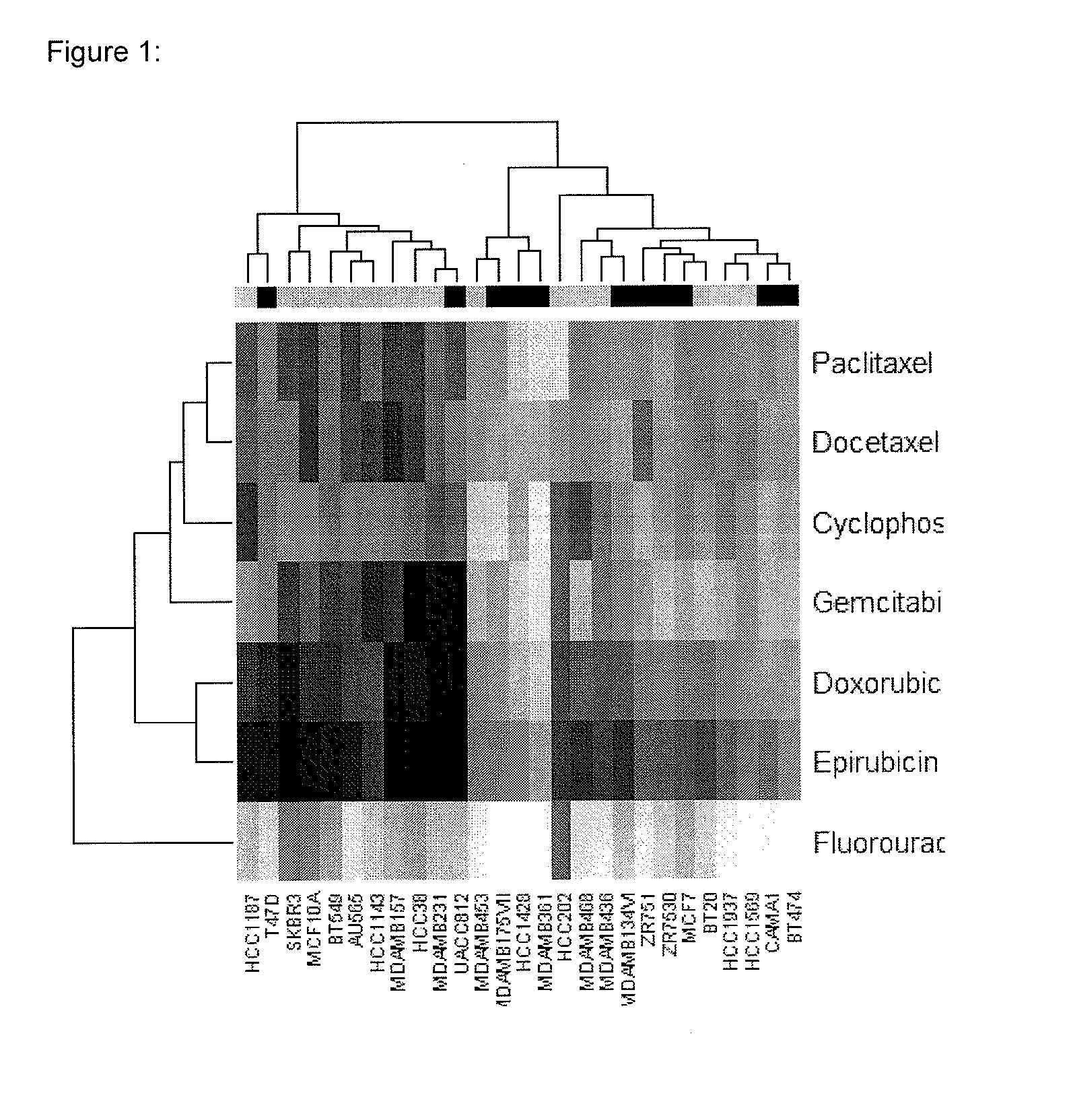
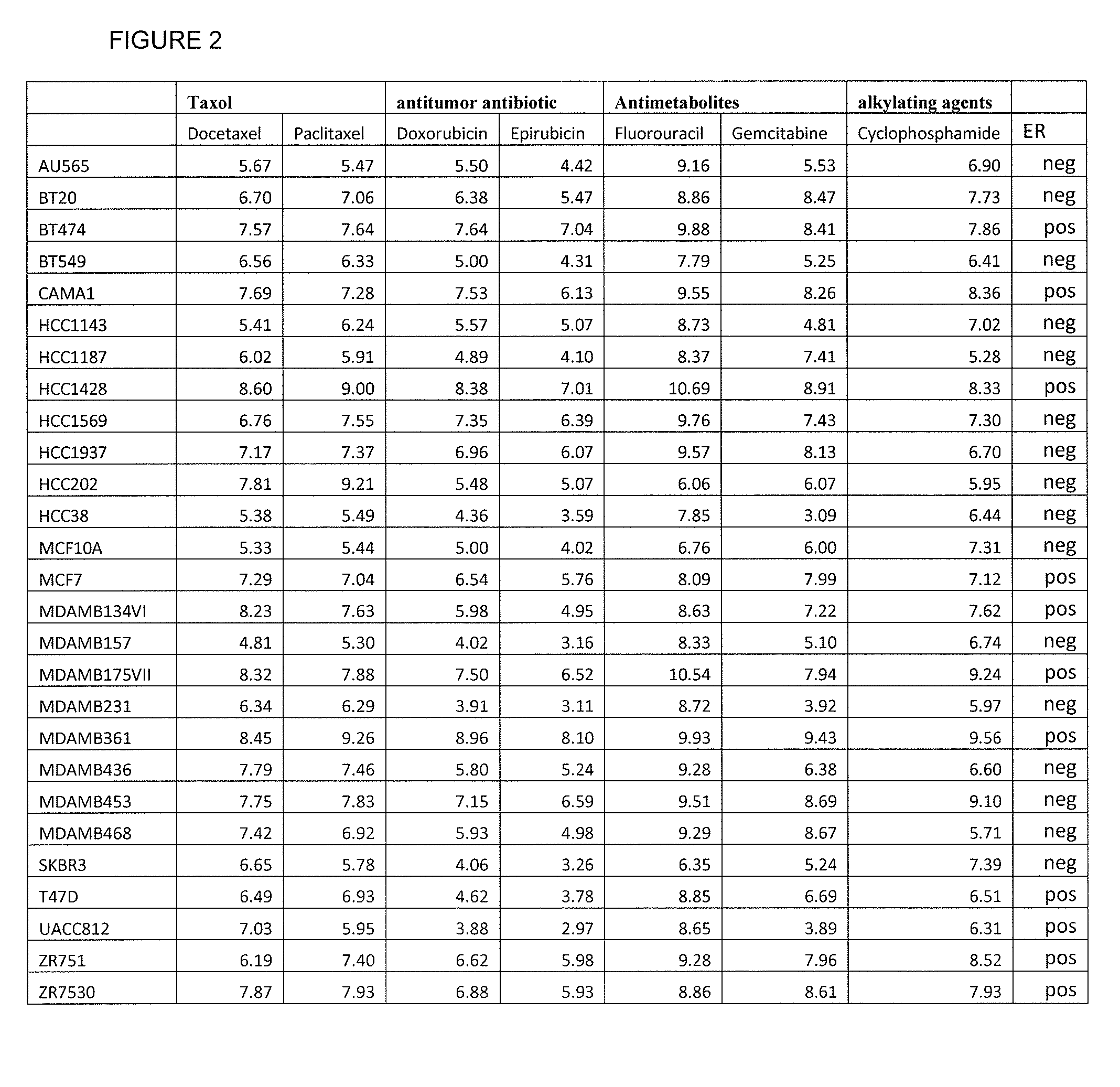
The present invention provides methods for preparing a gene expression profile of a breast cancer cell, tumor, or cell line, where the gene expression profile may be evaluated for one or more gene expression signatures indicative of multidrug resistance. The signature may be indicative of resistance to one or more chemotherapeutic agents selected from a Taxol (e.g., Docetaxel or Paclitaxel), an antibiotic (e.g., Doxorubicin or Epirubicin), an antimetabolite (e.g., Fluorouracil and / or Gemcitabine), and an alkylating agent (e.g., Cyclophosphamide). Generally, the gene expression profile contains the level of expression for a plurality of genes listed in FIGS. 3, 4, and / or 5. Gene expression profiles for evaluating multidrug resistance for ER positive and ER negative breast cancers are also provided.
Owner:PRECISION THERAPEUTICS
Novel conjugates of cc-1065 analogs and bifunctional linkers
ActiveUS20130224227A1Improve propertiesIncrease polarityBiocideTetrapeptide ingredientsAlkylating antineoplastic agentDisease
This invention relates to novel analogs of the DNA-alkylating agent CC-1065 and to their conjugates. Furthermore this invention concerns intermediates for the preparation of said agents and conjugates. The conjugates are designed to release their (multiple) payload after one or more activation steps and / or at a rate and time span controlled by the conjugate in order to selectively deliver and / or controllably release one or more of said DNA alkylating agents. The agents, conjugates, and intermediates can be used to treat an illness that is characterized by undesired (cell) proliferation. As an example, the agents and the conjugates of this invention may be used to treat a tumor.
Owner:BYONDIS BV
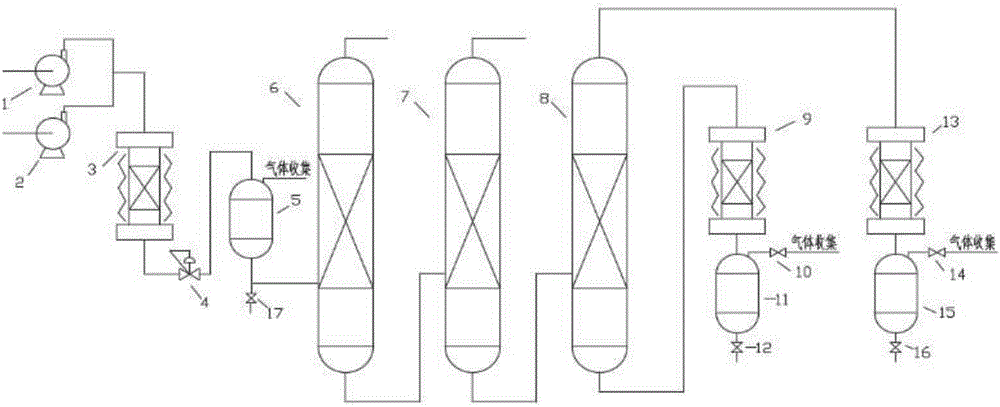
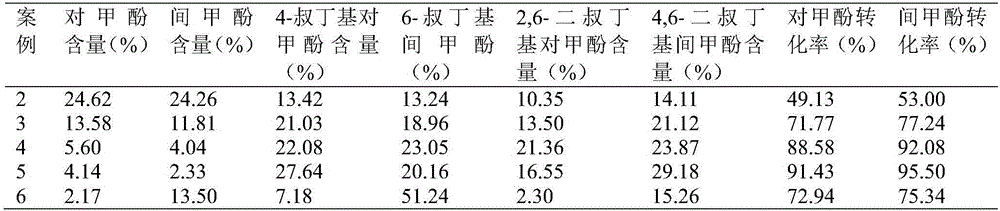
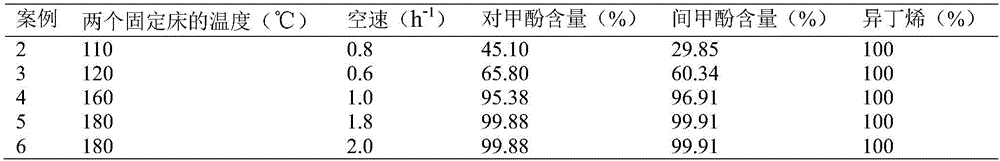
Method for separating m-cresol and p-cresol mixture by liquid-phase alkylation method
InactiveCN106810422AImprove conversion rateIncrease profitOrganic chemistryOrganic compound preparationCresolMetacresol
The invention discloses a method for separating an m-cresol and p-cresol mixture by a liquid-phase alkylation method. According to the method, alkylation reaction between isobutene mixed gas and the m-cresol and p-cresol mixture are performed through a fixed-bed reactor under the liquid phase state, the reacted mixture is separated to obtain metacresol and paracresol, the isobutene mixed gas serves as an alkylating agent, the m-cresol and p-cresol mixture serves as a raw material, the alkylation reaction is performed for the alkylating agent and the m-cresol and p-cresol mixture, and the treated mixture is separated to obtain pure metacresol and pure paracresol. The method overcomes the shortcomings of low utilization rate and serious environmental pollution of alkylating agents in gas-phase alkylation reaction in the prior art and solves the problems that production cost is high as high-purity isobutene serves as the alkylating agent in the gas-phase alkylation reaction.
Owner:HEBEI UNIV OF TECH +1
Compositions of alkylating agents and methods of treating skin disorders therewith
InactiveUS20130184243A1BiocidePharmaceutical delivery mechanismAlkylating antineoplastic agentNitrogen mustard
Owner:ACTELION PHARM LTD
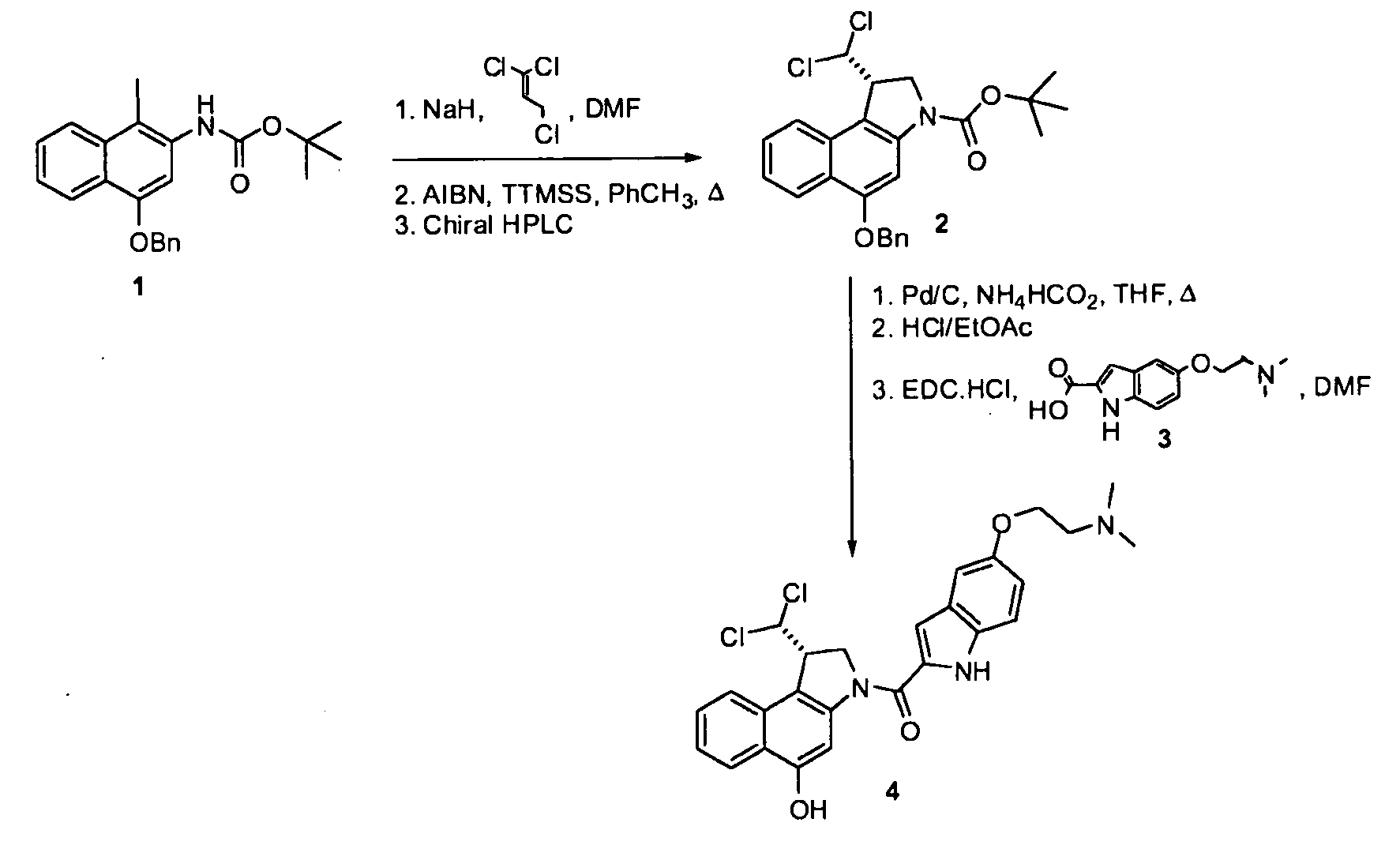
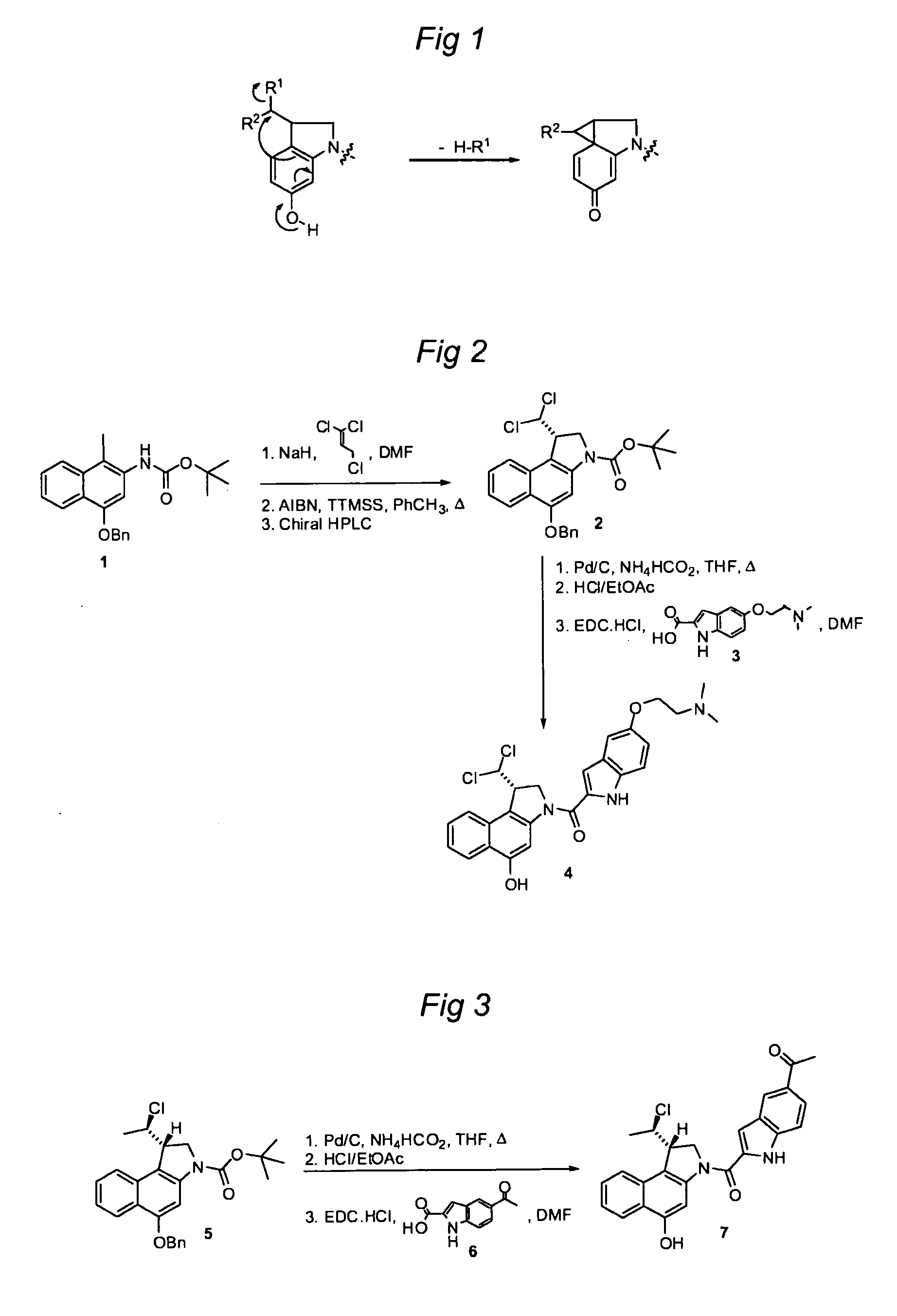
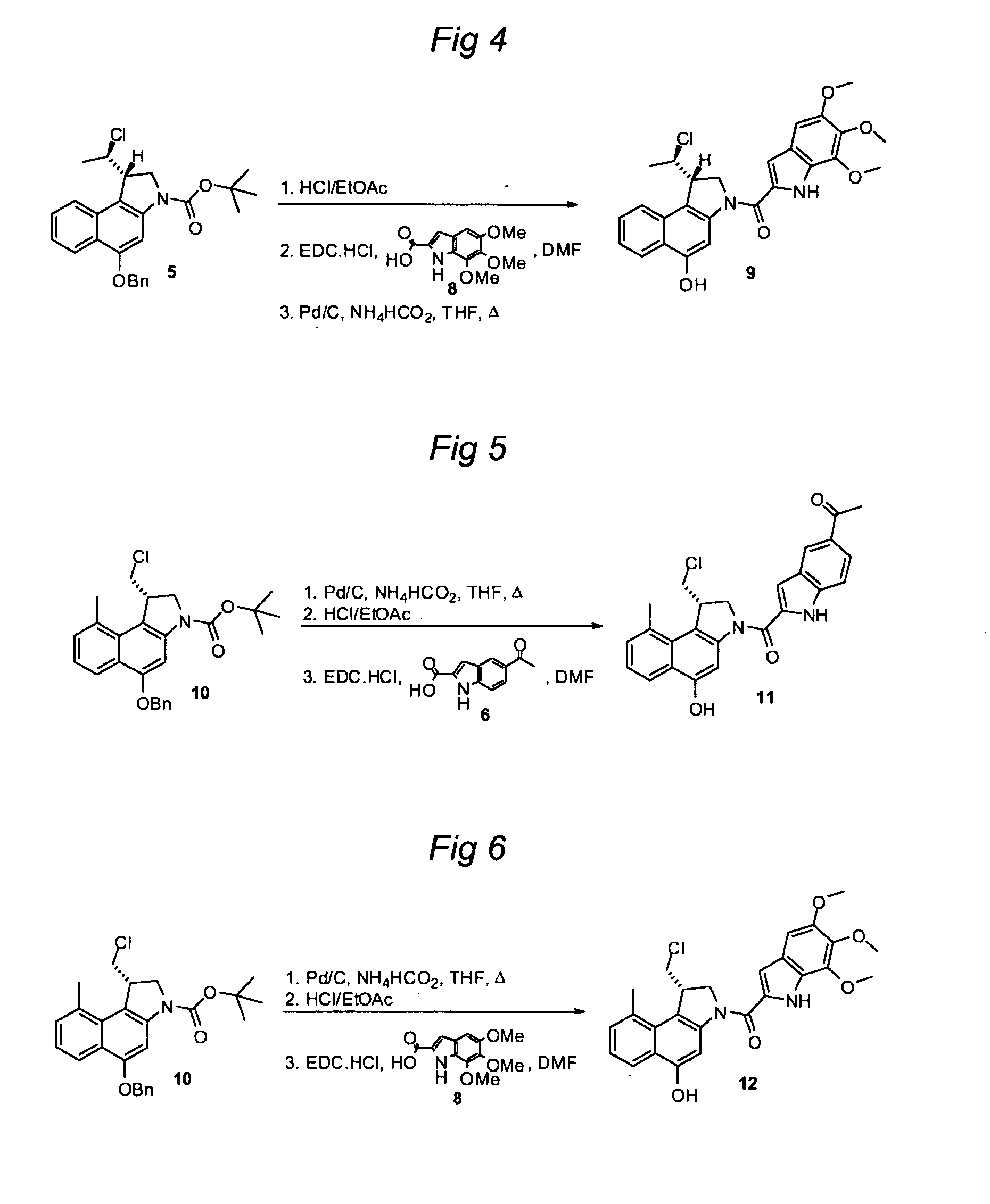
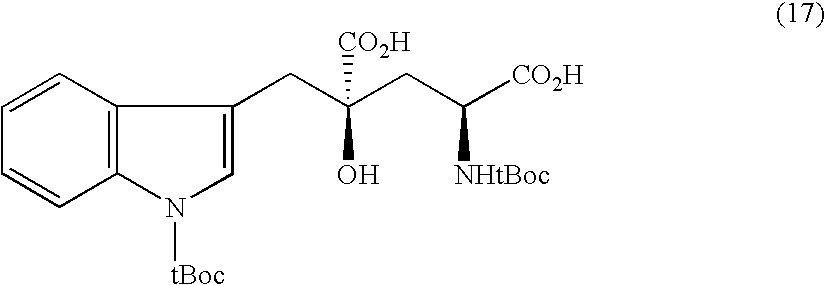
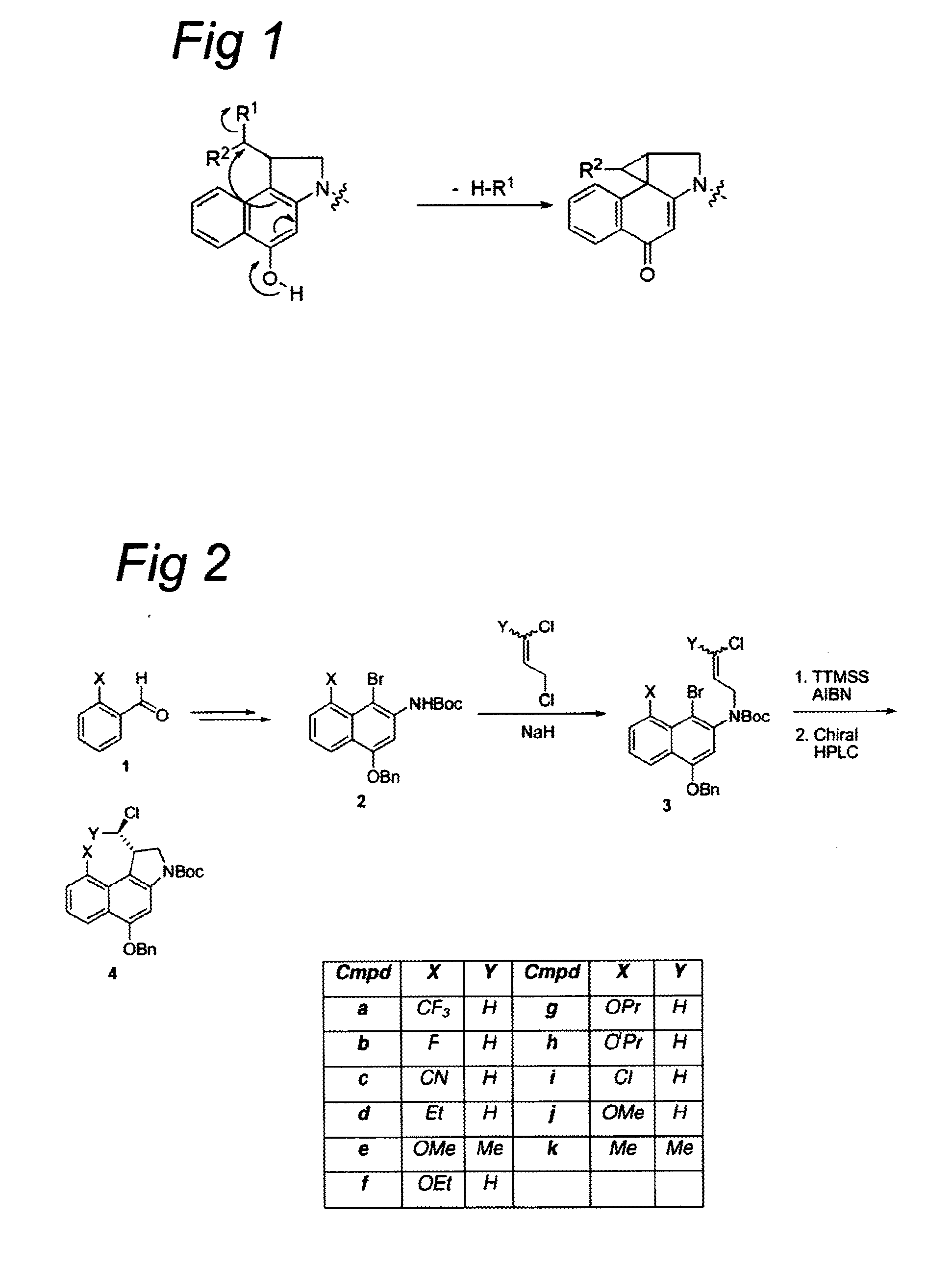
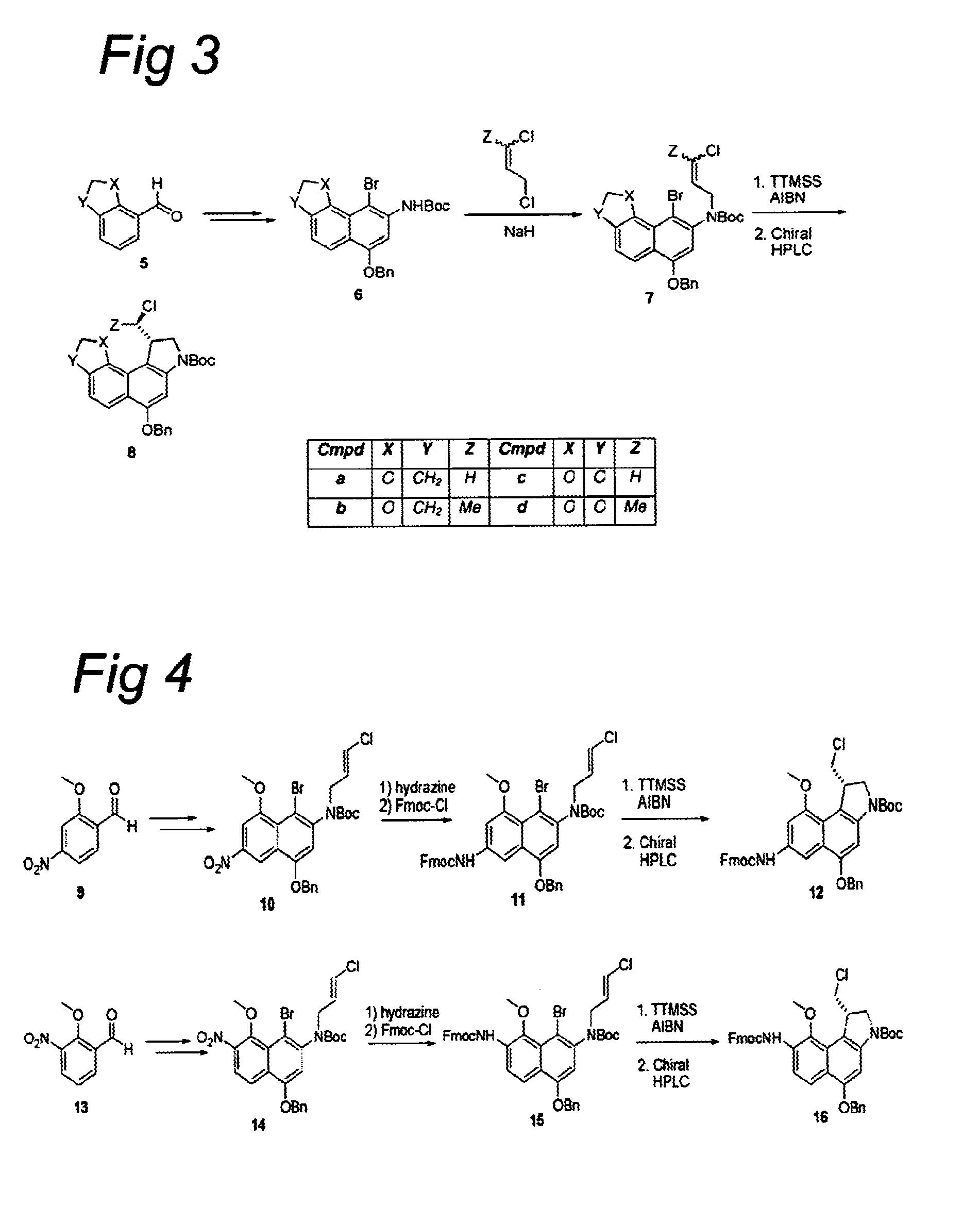
Synthesis of 8h-3a-aza-cyclopenta[a]indenes and 5,10-dihydropyrrolo[1,2-b]isoquinolines derivatives and their use as antitumor therapeutic agents
InactiveUS20090117125A1Potent therapeutic efficacyPotential for clinical applicationBiocideOrganic chemistryCarbamateHuman tumor
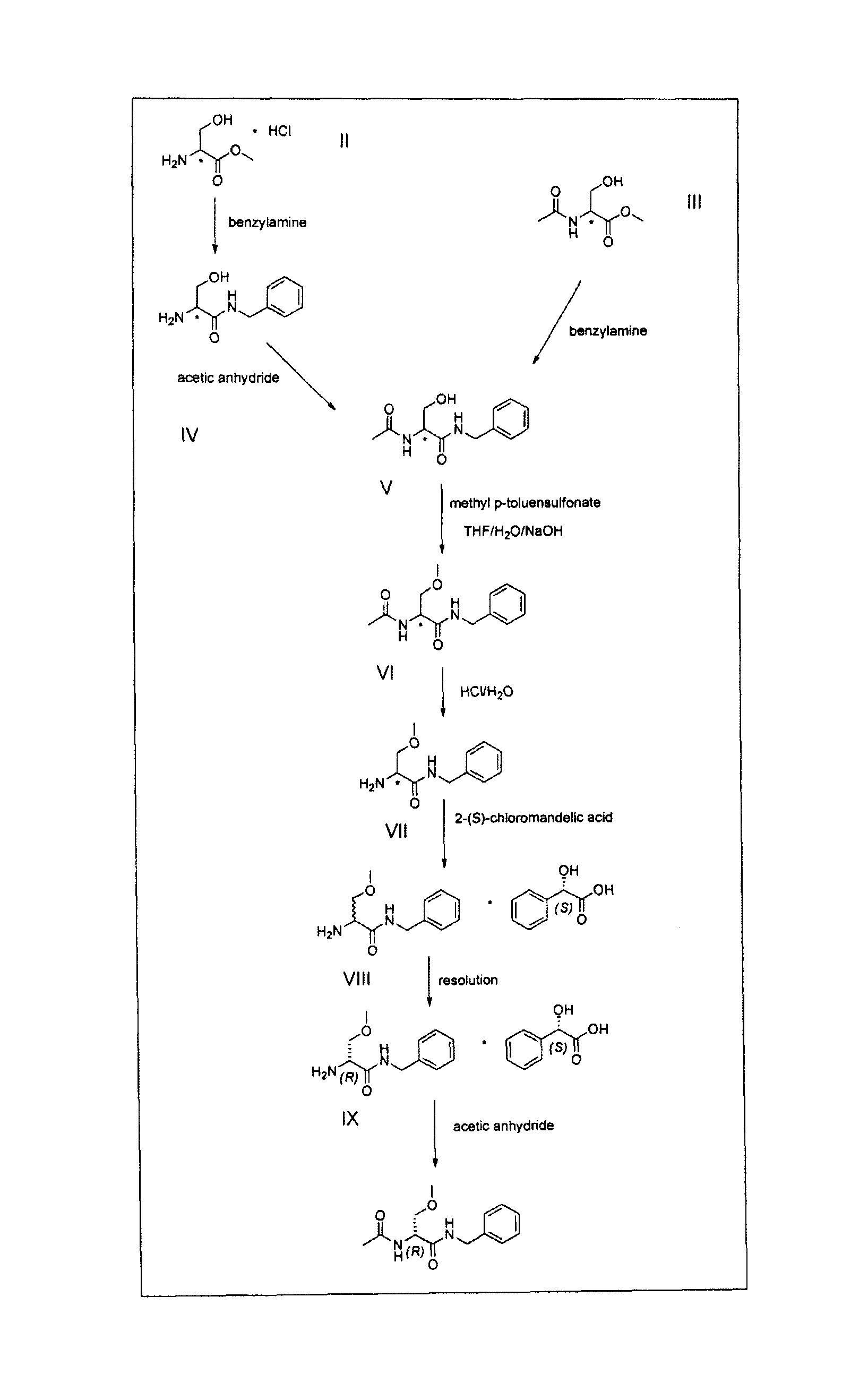
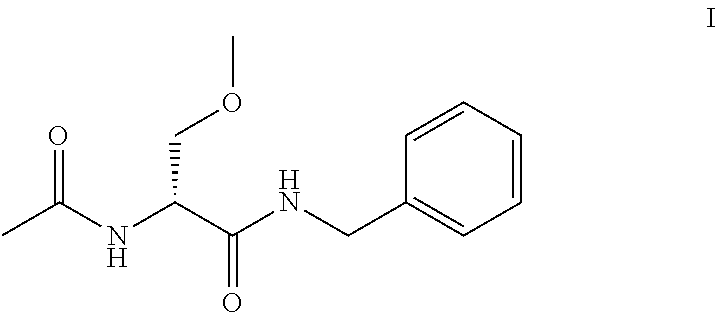
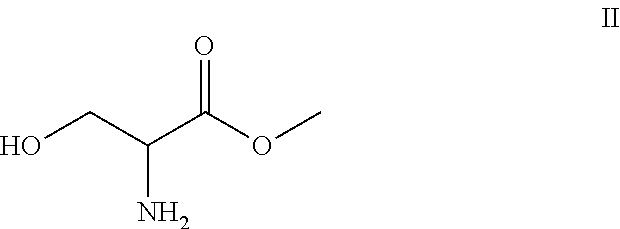
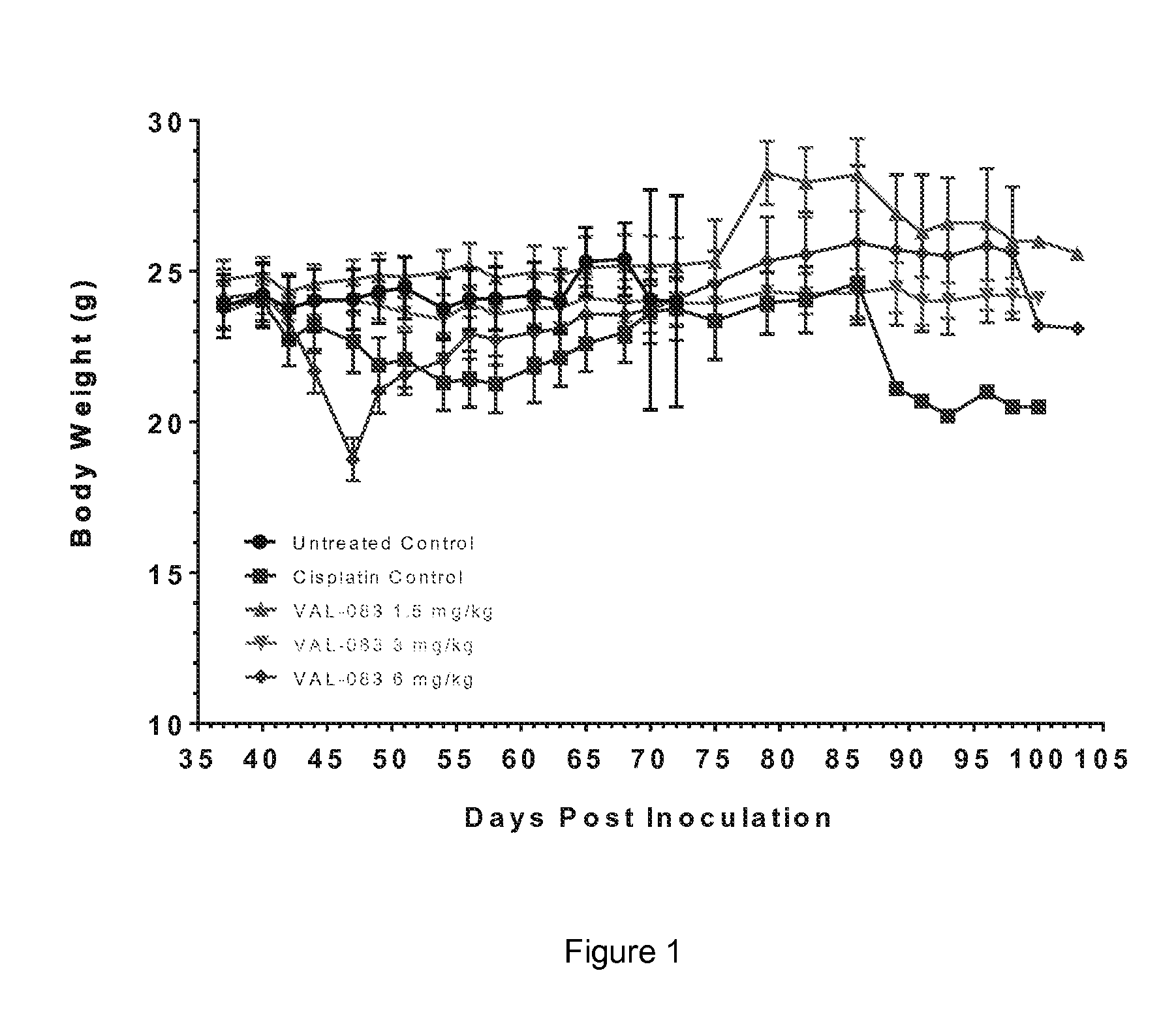
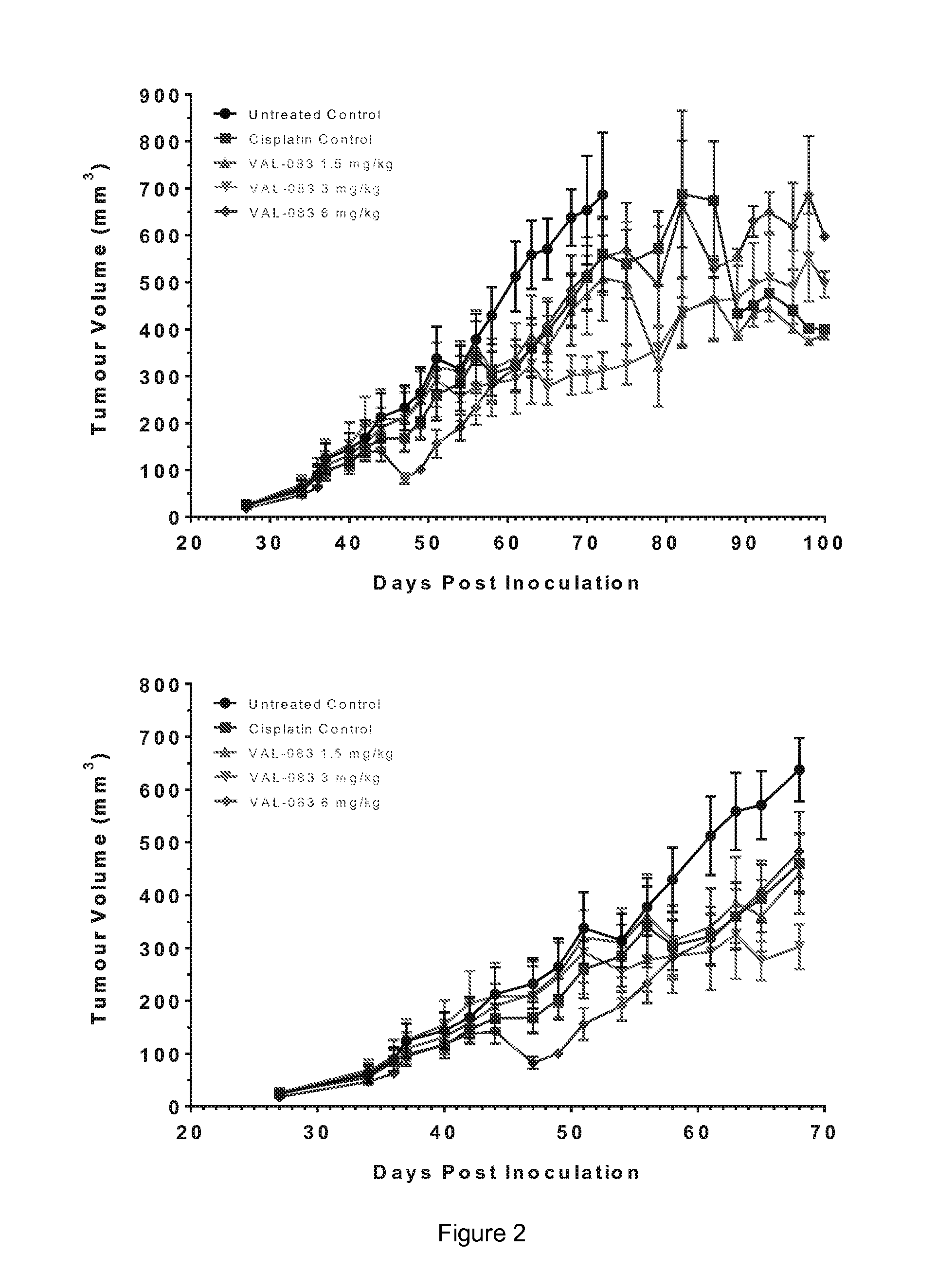
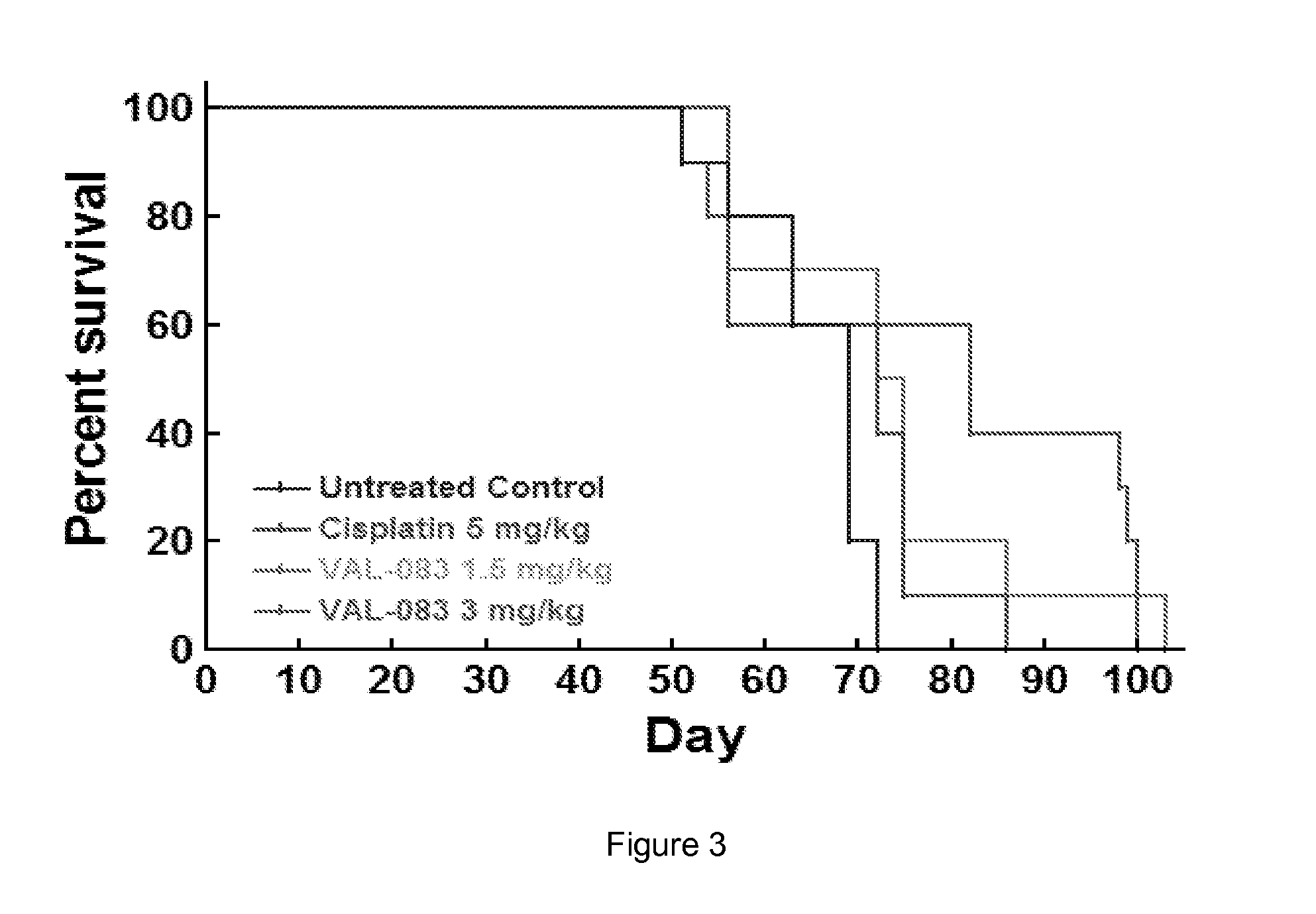
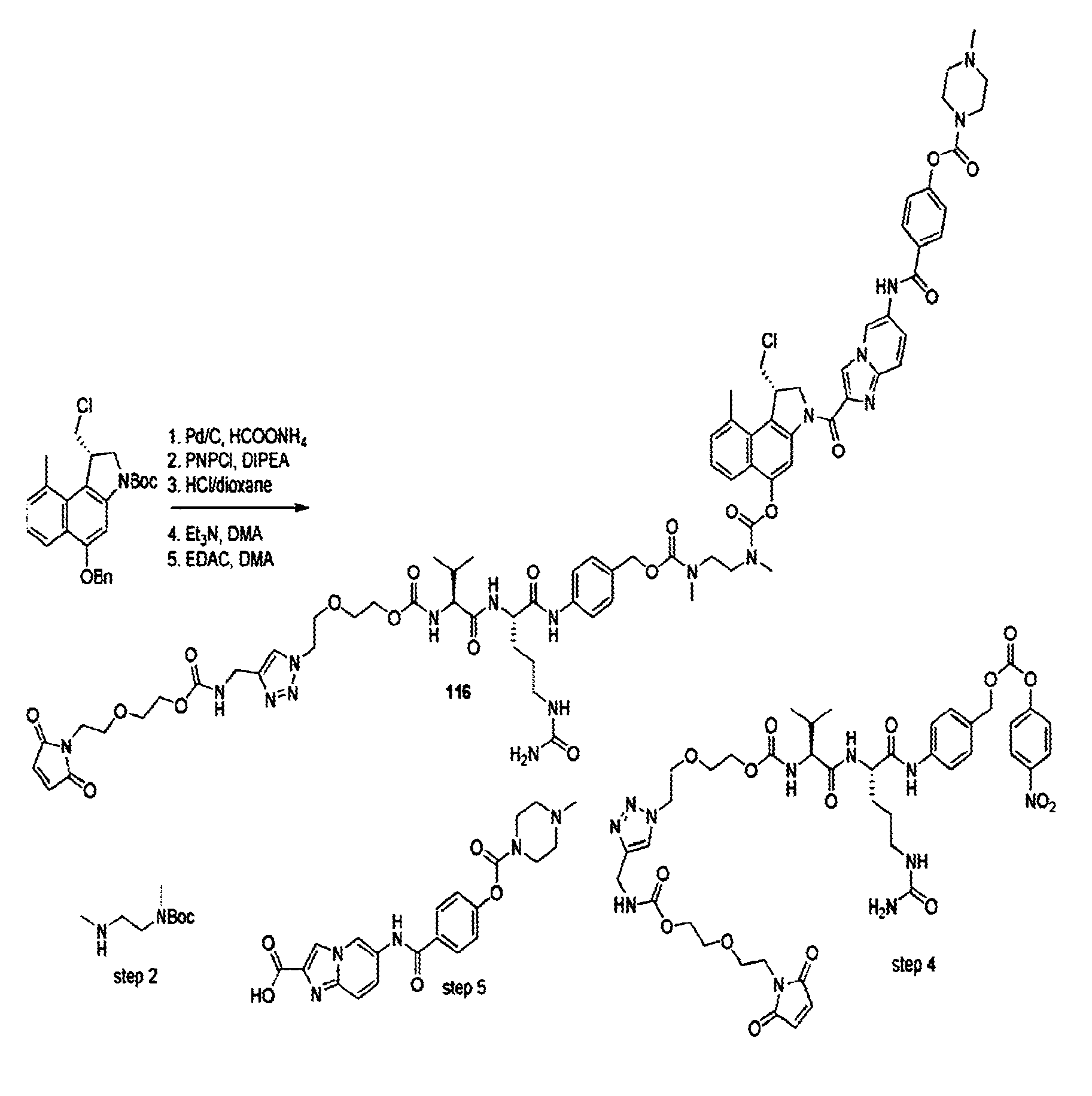
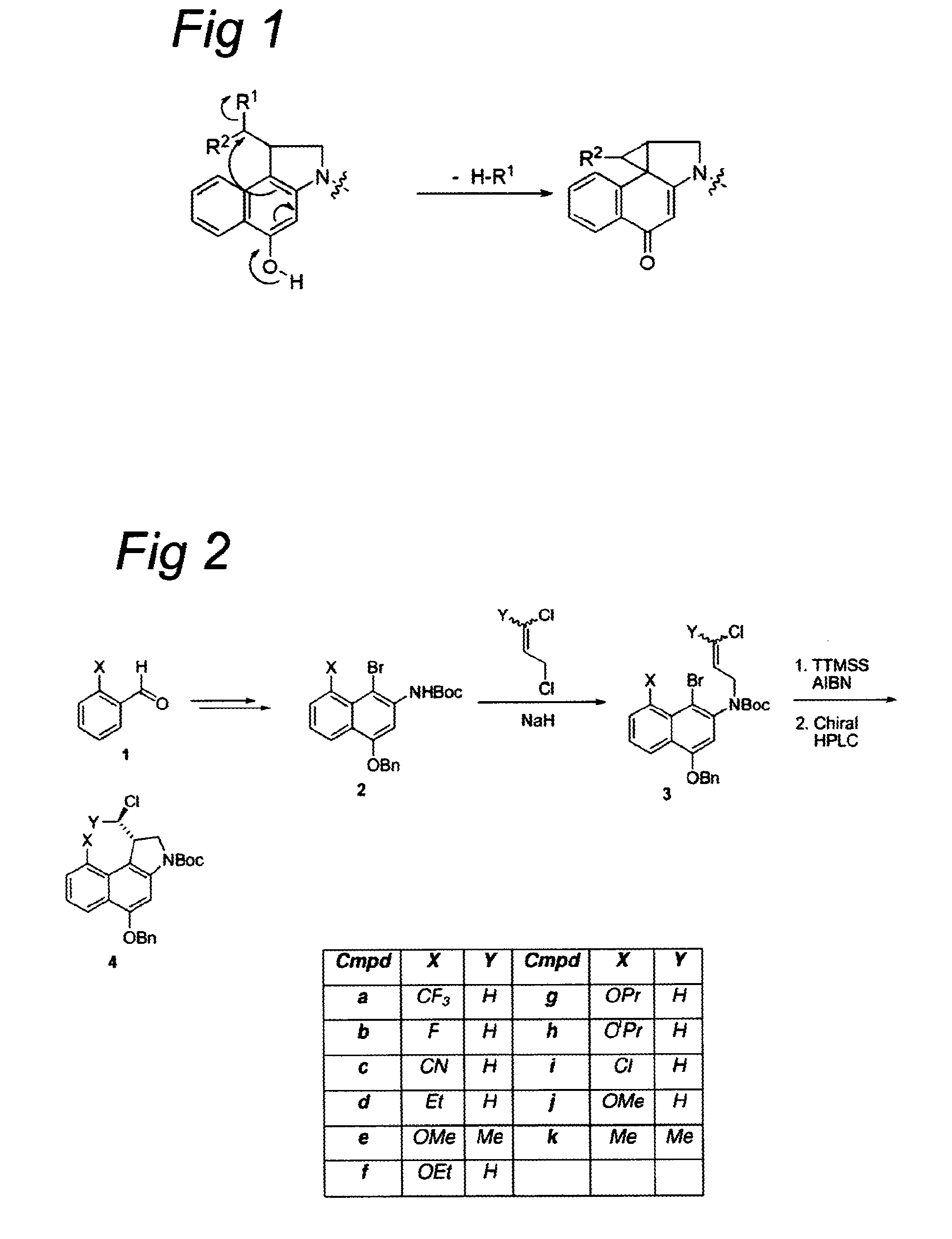
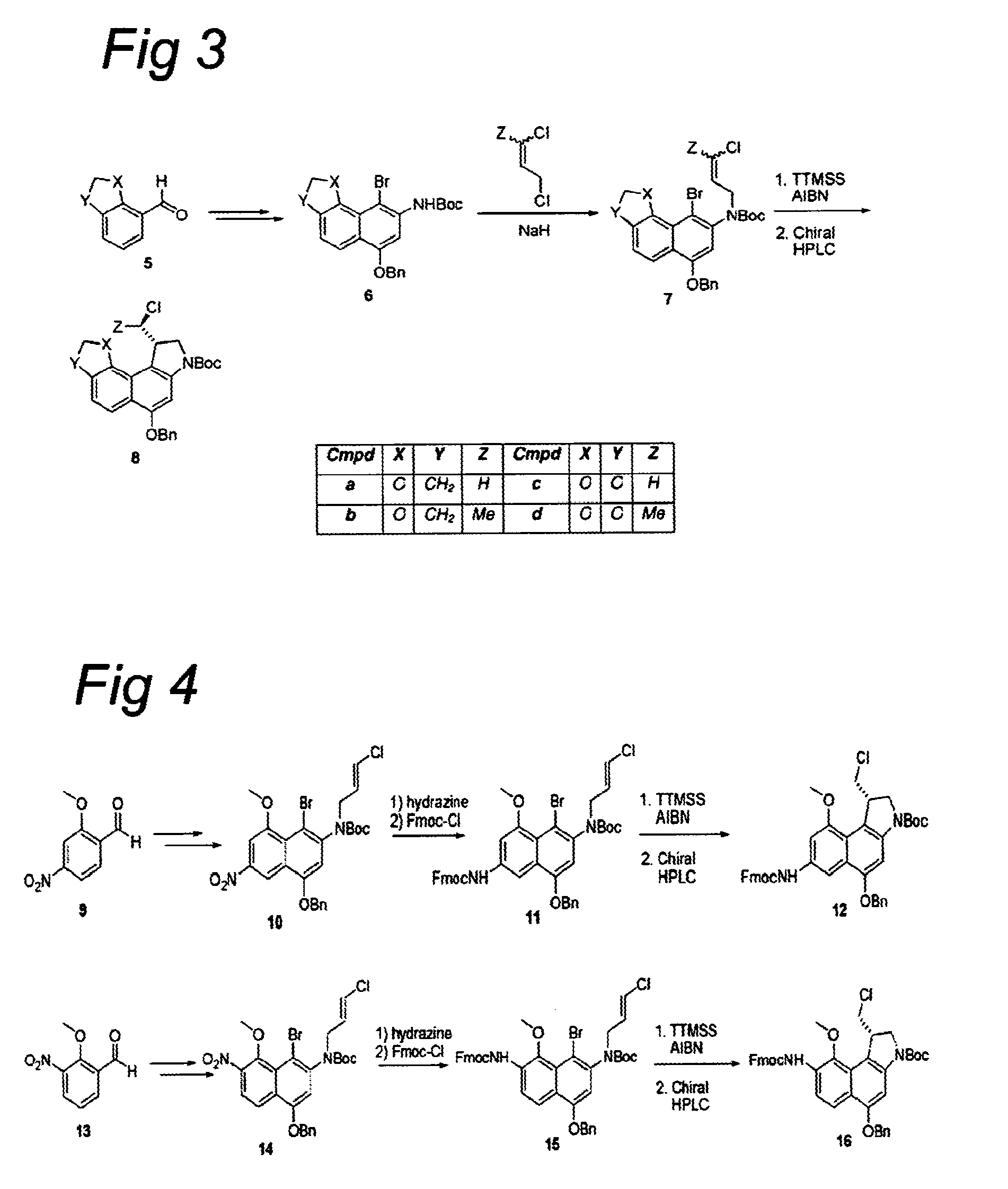
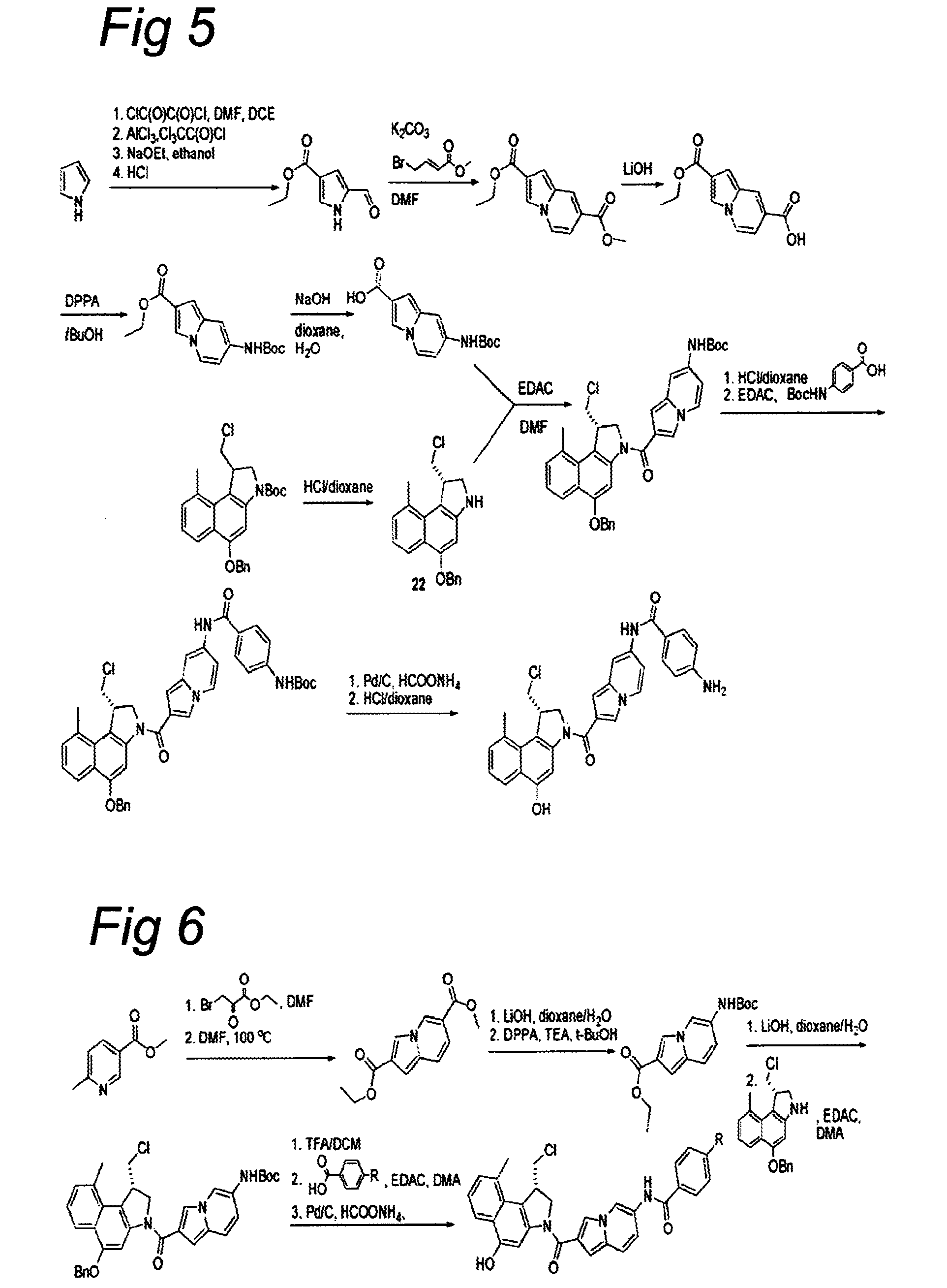
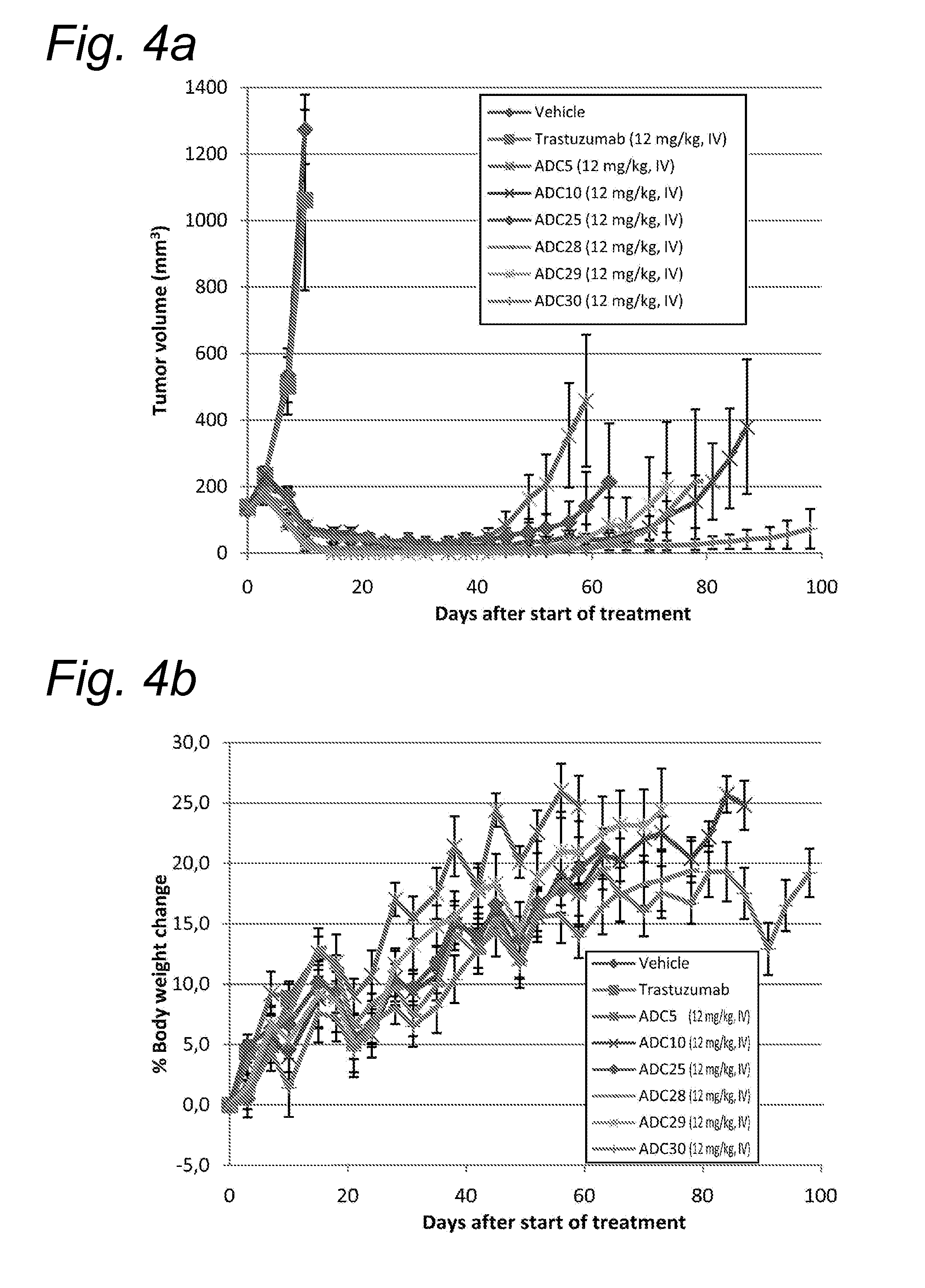
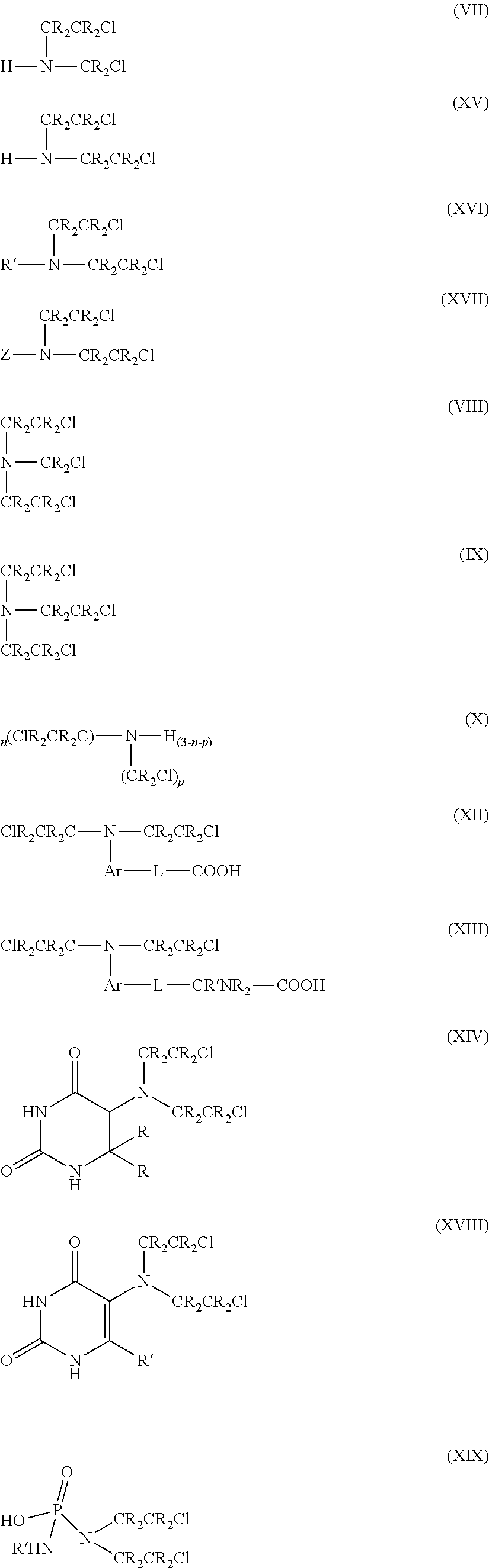
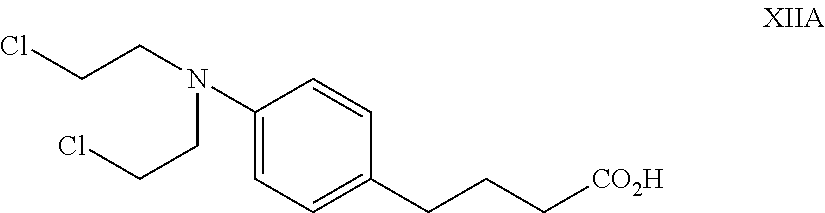
The present disclosure relates to a series of bis(hydroxymethyl) and its bis(carbamate) of 8H-3a-azacyclopenta[a]indene-1-yl and 5,10-dihydropyrrolo-[1,2-b]isoquinolines derivatives (Formula I-Formula IV) as DNA di-alkylating agents. The preliminary antitumor studies indicated that compounds disclosed herein could exhibit potent cytotoxicity in vitro and antitumor therapeutic efficacy in human tumor xenografts and could have little or no cross-resistance to either Taxol or Vinblastine. The results demonstrated that compounds disclosed herein possess potent antitumor therapeutic efficacy and are expected to have potential for clinical applications.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Therapeutic benefit of suboptimally administered chemical compounds
ActiveUS11491154B2Increase profitAvoid repairsOrganic active ingredientsCoatingsAlkylating antineoplastic agentSide effect
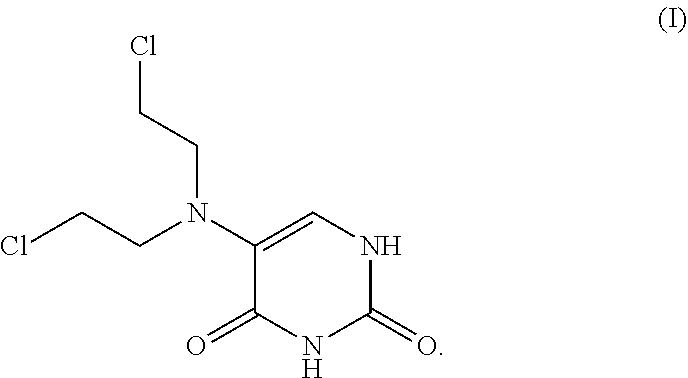
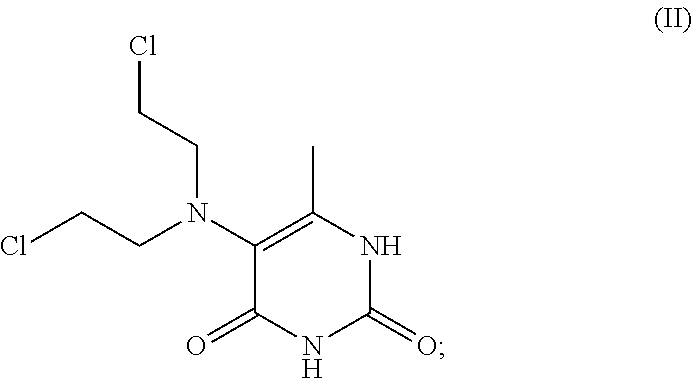
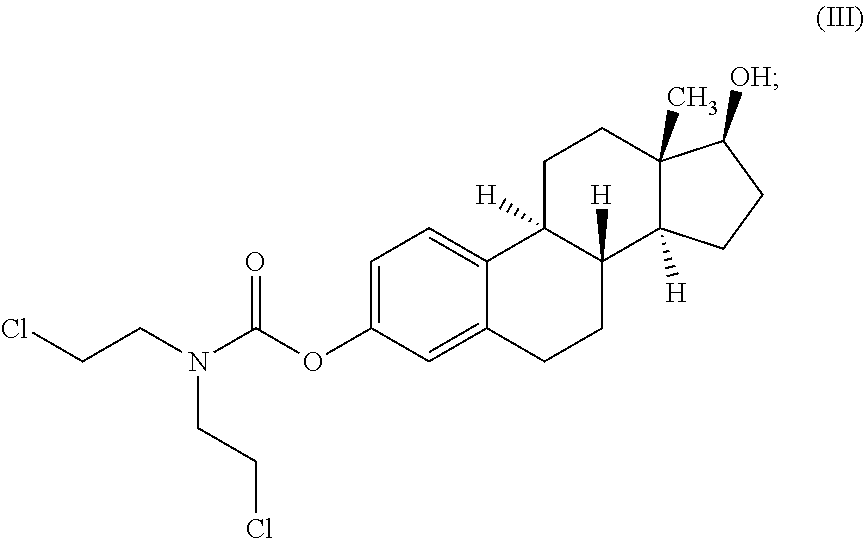
The present invention describes methods and compositions for improving the therapeutic efficacy of therapeutic agents previously limited by suboptimal therapeutic performance by either improving efficacy as monotherapy or reducing side effects. Such methods and compositions are particularly applicable to mustard-based alkylating agents such as uracil mustard and analogs, derivatives, or prodrugs thereof, including 6-methyluracil mustard and 6-ethyluracil mustard.
Owner:BROWN DENNIS M
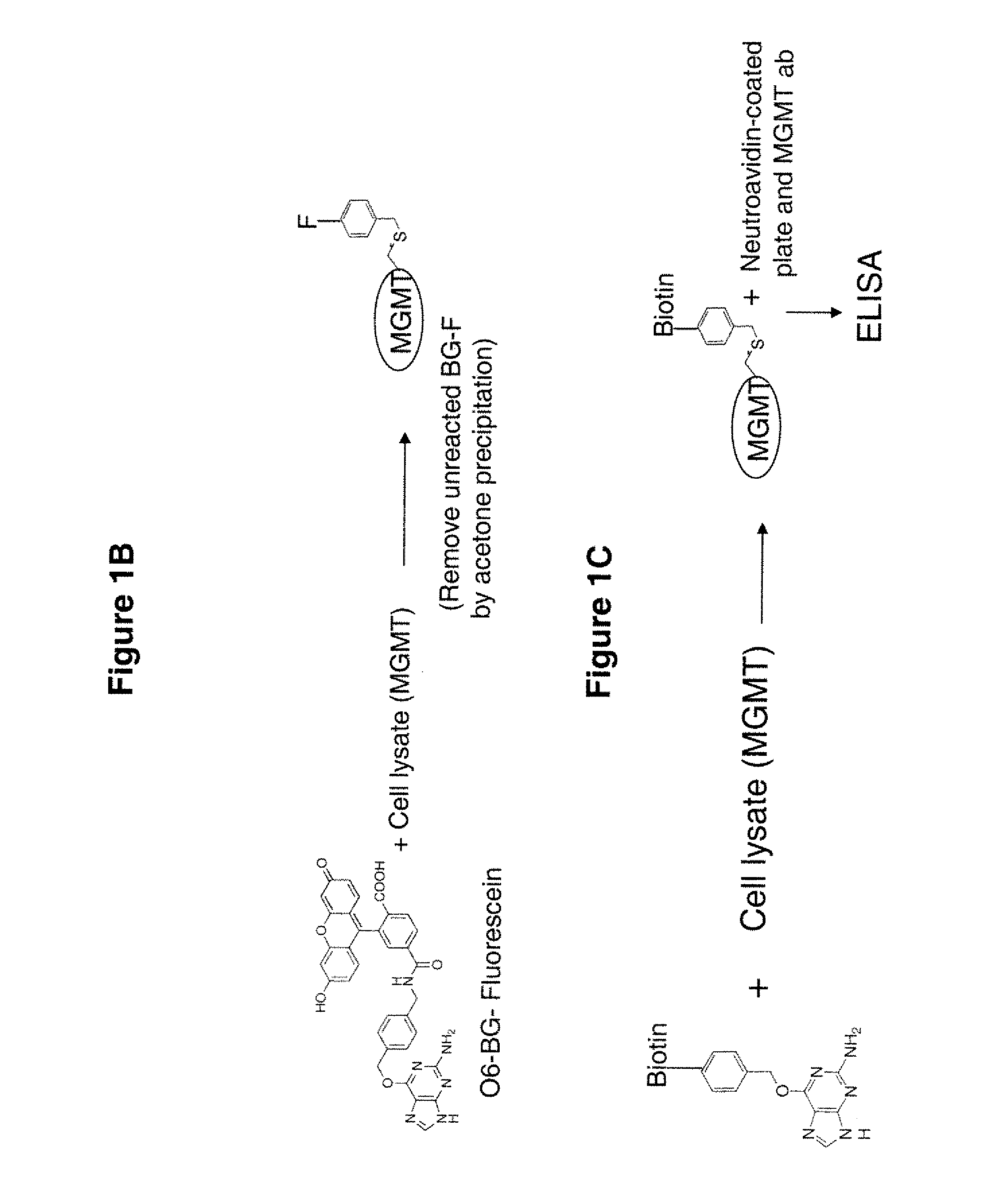
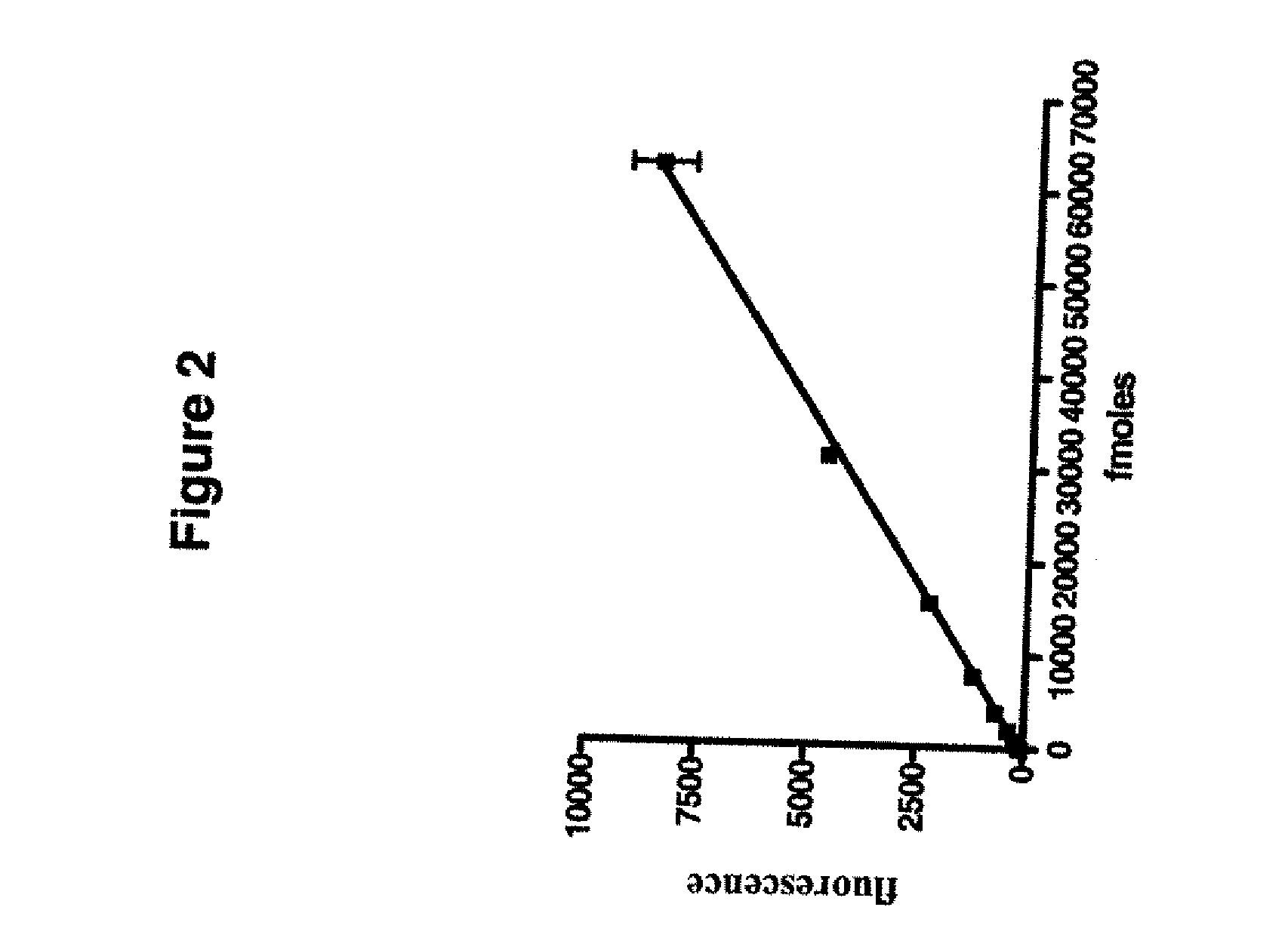
Development of a novel assay for mgmt (methyl guanine methyl transferase)
InactiveUS20070264672A1Shorten the timeLower potentialBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceRepair enzyme
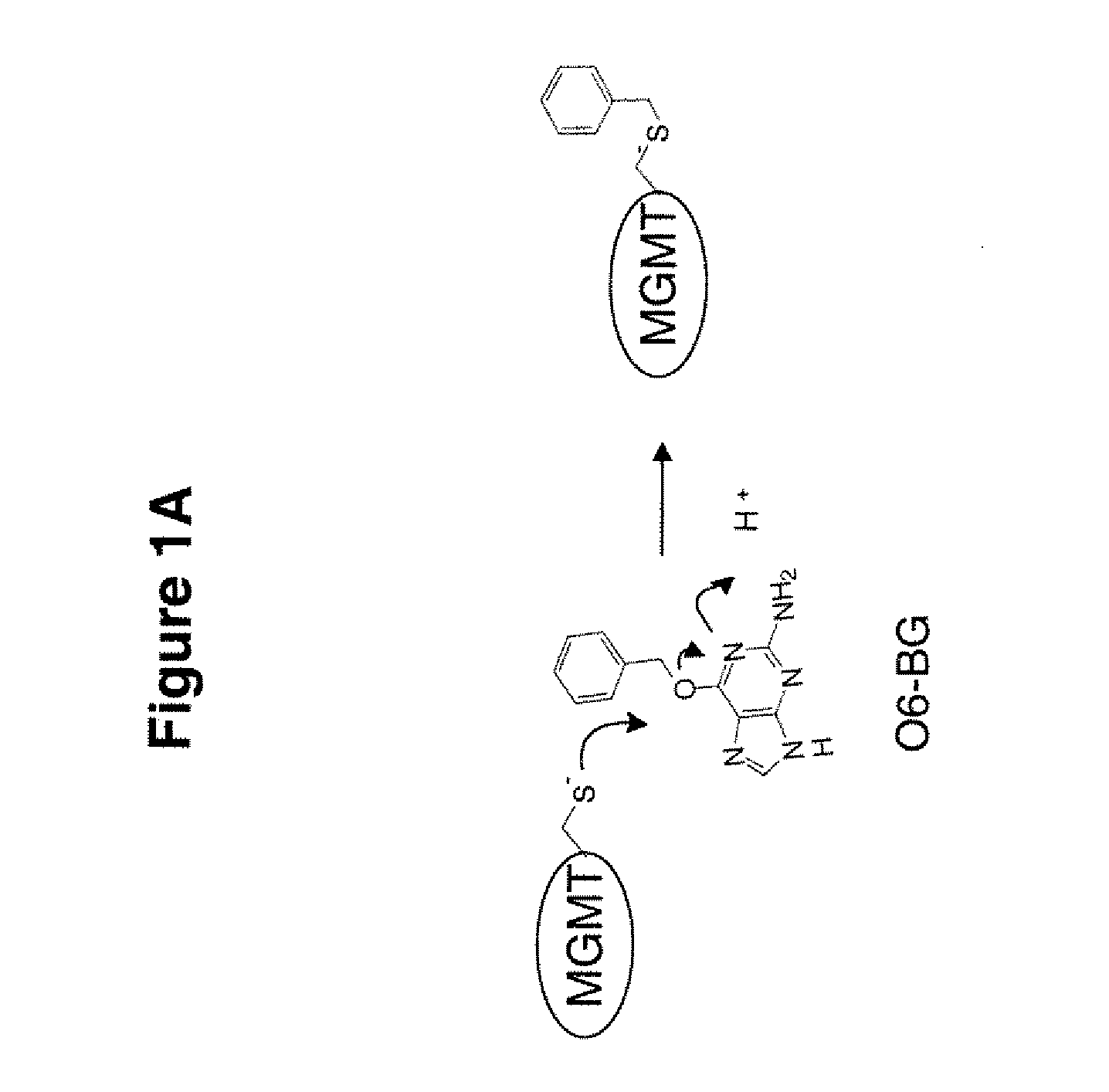
The present invention provides improved methods for assessing the level of MGMT activity in a variety of biological preparations. MGMT, a DNA repair enzyme, can reduce the chemotherapeutic efficacy of alkylating agents by repairing the damage that alkylating agents do to tumor cell DNA. The methods of the present invention can be used, inter alia, to measure MGMT levels and to thereby predict the clinical response to alkylating agents. The present invention includes three preferred assays for assessment of MGMT activity: (1) the immunoassay technique, (2) the labeled O6—BG technique, and (3) the fluorescence polarization technique. Kits useful for the performance of such assays are also provided.
Owner:SCHERING CORP
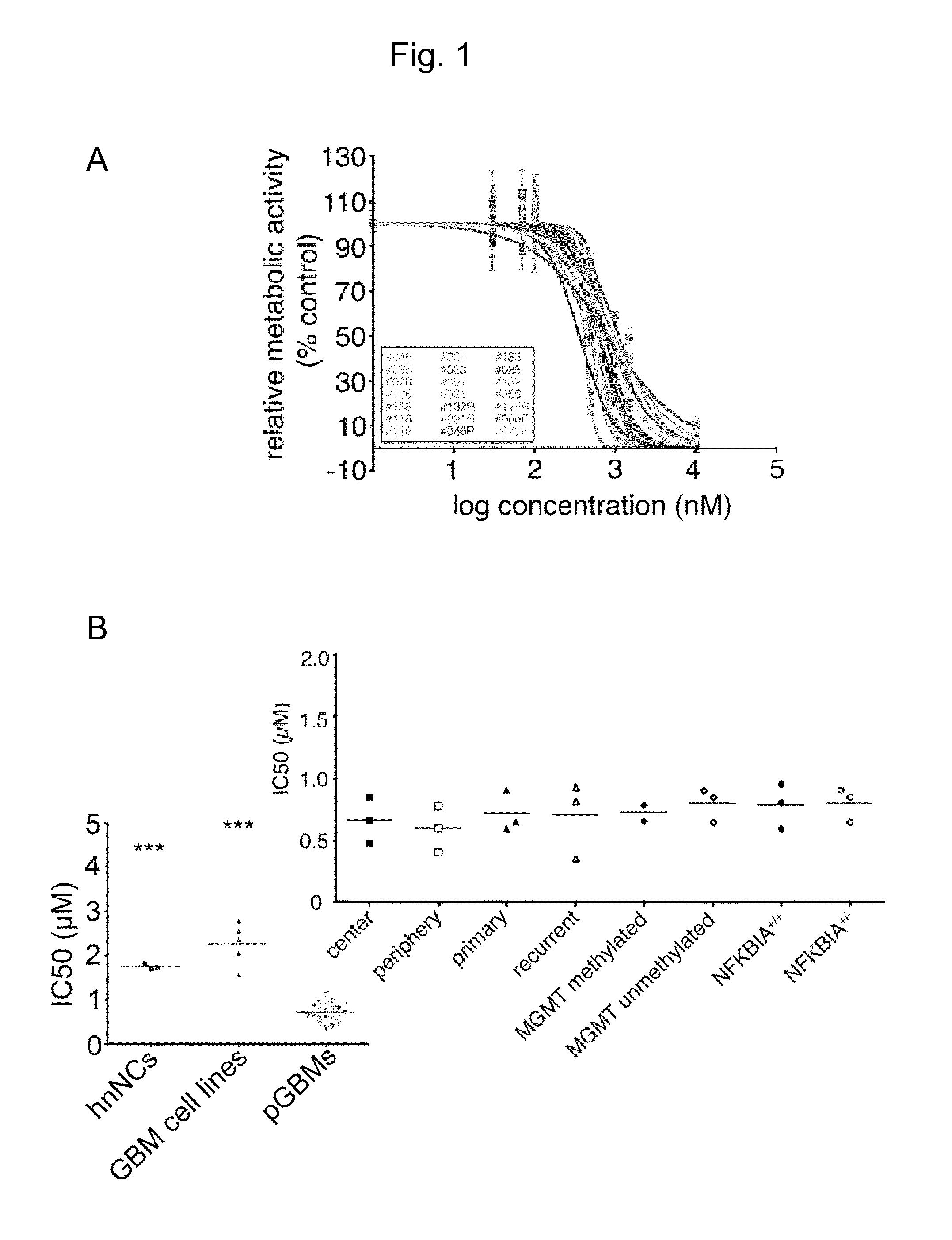
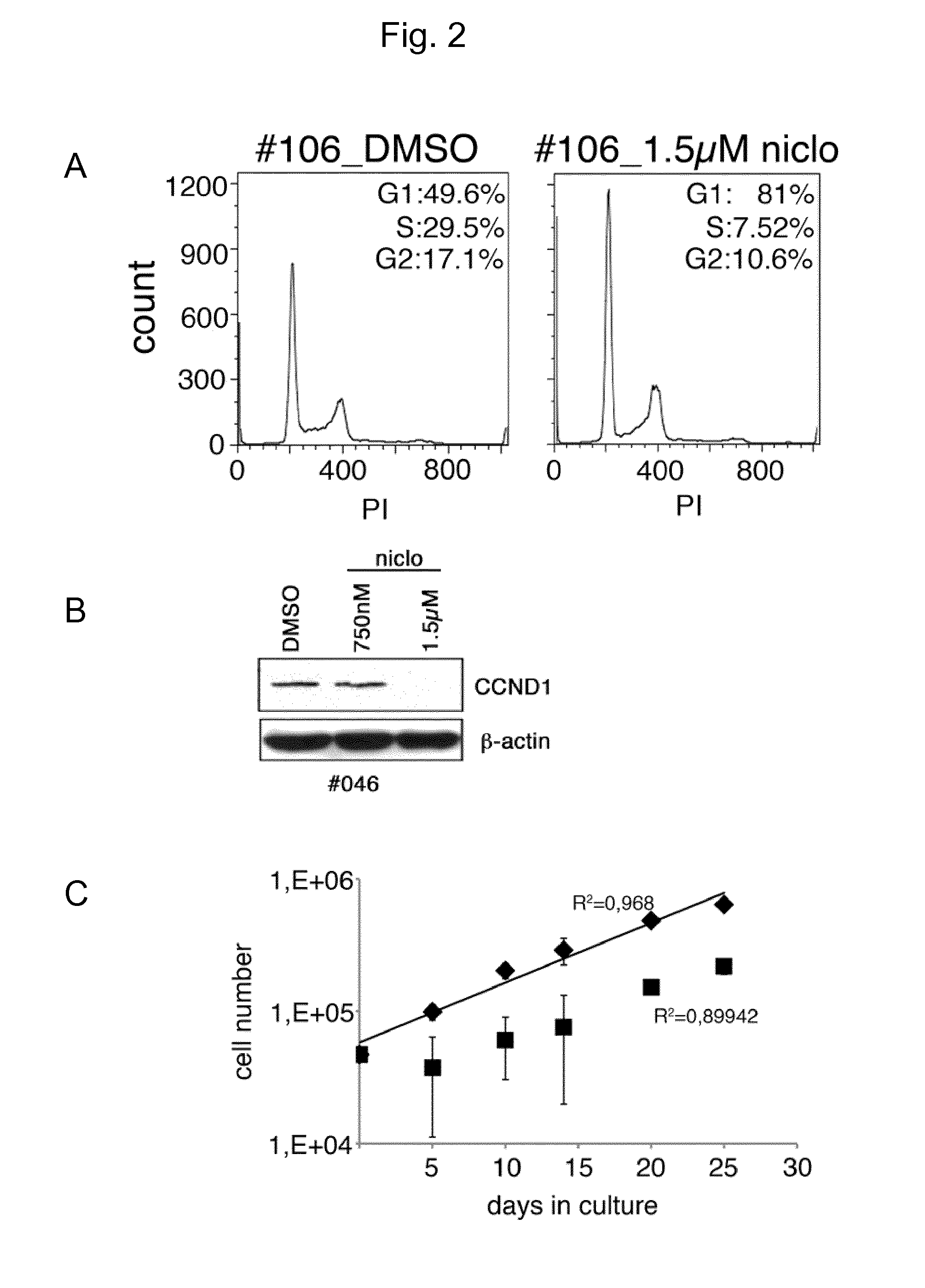
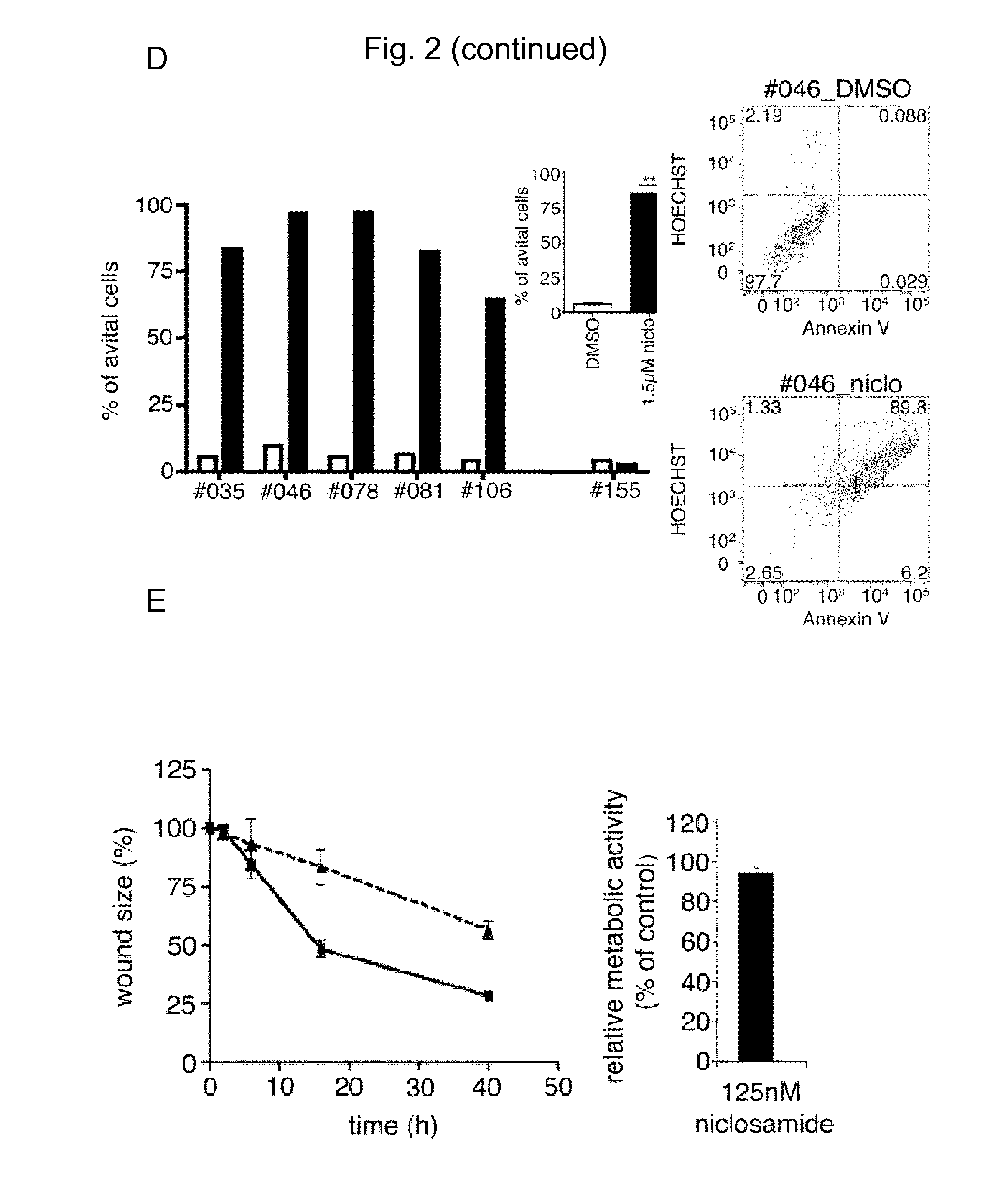
Niclosamide and its derivatives for use in the treatment of sold tumors
InactiveUS20150174086A1Reduced expression levelProlong survival timeOrganic active ingredientsBiocideNiclosamideSolid tumor
The present invention relates to novel therapeutic uses of niclosamide for the treatment of cancer. In particular, a combination of niclosamide or one of its derivatives with an alkylating agent is provided for the treatment of solid tumors. Moreover, niclosamide or one of its derivatives can be used for the treatment of solid tumors characterized by underexpression of NFKBIA. Finally, the invention relates to diagnostic methods for determining whether treatment with niclosamide alone or in combination with an alkylating agent is suitable for a cancer patient.
Owner:UNIVERSITY OF BONN +1
Substituted CC-1065 Analogs and Their Conjugates
InactiveUS20110065767A1BiocideImmunoglobulins against cell receptors/antigens/surface-determinantsNovel agentsBiological activation
This invention relates to novel agents that are analogs of the DNA-alkylating agent CC-1065 and to their conjugates. Furthermore this invention concerns intermediates for the preparation of said agents and their conjugates. The conjugates are designed to release their (multiple) payload after one or more activation steps and / or at a rate and time span controlled by the conjugate in order to selectively deliver and / or controllably release one or more of said DNA alkylating agents. The agents, conjugates, and intermediates can be used to treat an illness that is characterized by undesired (cell) proliferation. As an example, the agents and the conjugates of this invention may be used to treat a tumor.
Owner:SYNTARGA BV
Slow released anticancer medicien containing both platinum compound and its synergist
The slow released injection containing both platinum compound and its synergist consists of slow released microsphere and solvent. The solvent is special solvent containing suspending agent, and the suspending agent is carboxymethyl cellulose sodium, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The effective anticancer component is platinum compound dicycloplatin, etc or the composition of platinum compound and platinum compound synergist selected from antimitotic medicine and alkylating agent. The slow releasing supplementary material is selected from difatty acid-sebacic acid copolymer, poly (erucic acid dipolymer-sebacic acid), poly (fumaric acid-sebacic acid), etc. The slow released microsphere may be also prepared into slow released implanting agent set or injected to in tumor to lower the systemic toxic reaction, raise the local tumor medicine concentration and to raise chemotherapeutic and radiotherapeutic effect.
Owner:JINAN SHUAIHUA PHARMA TECH
Protein modulators of resistance to alkylating agents
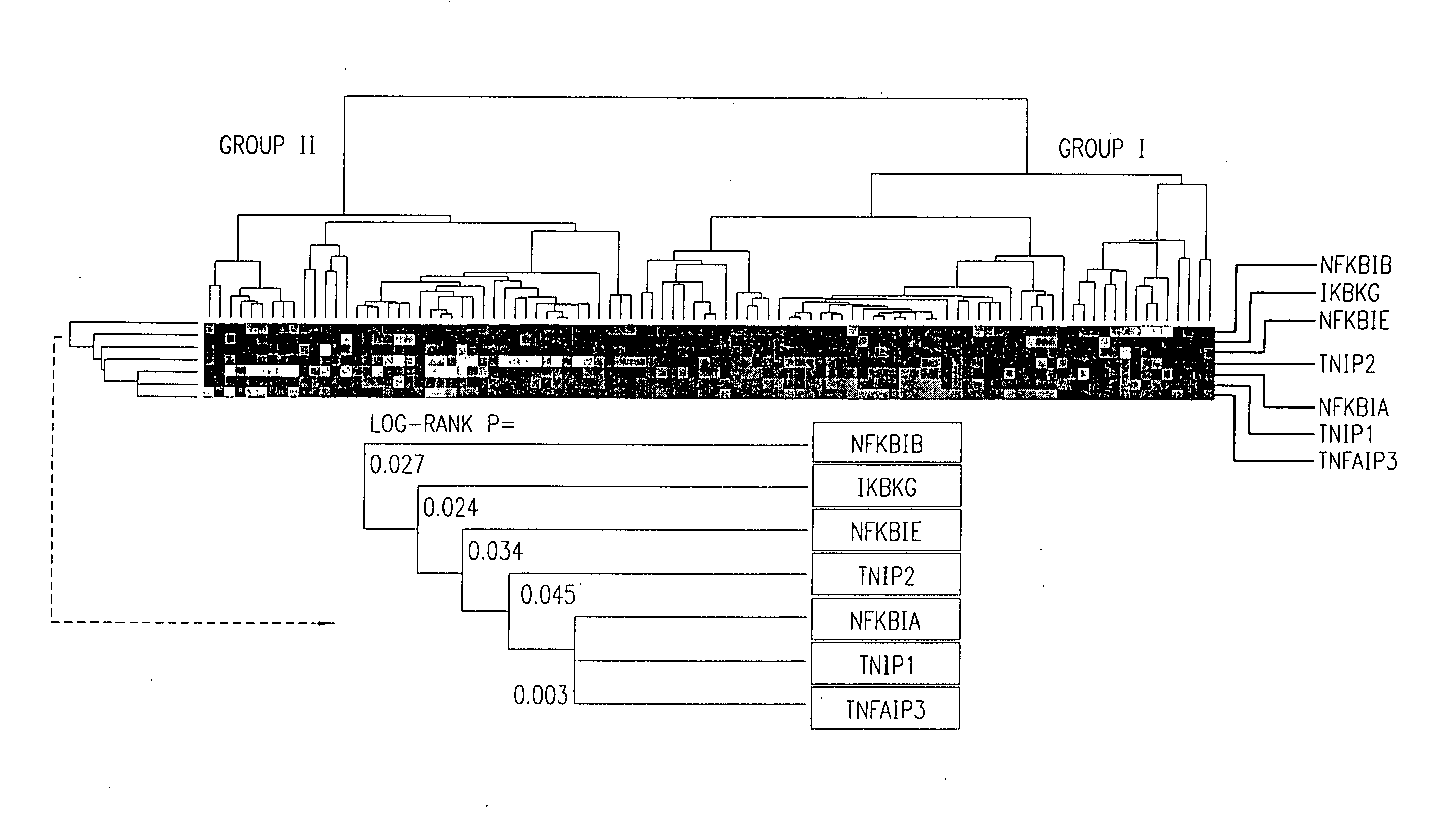
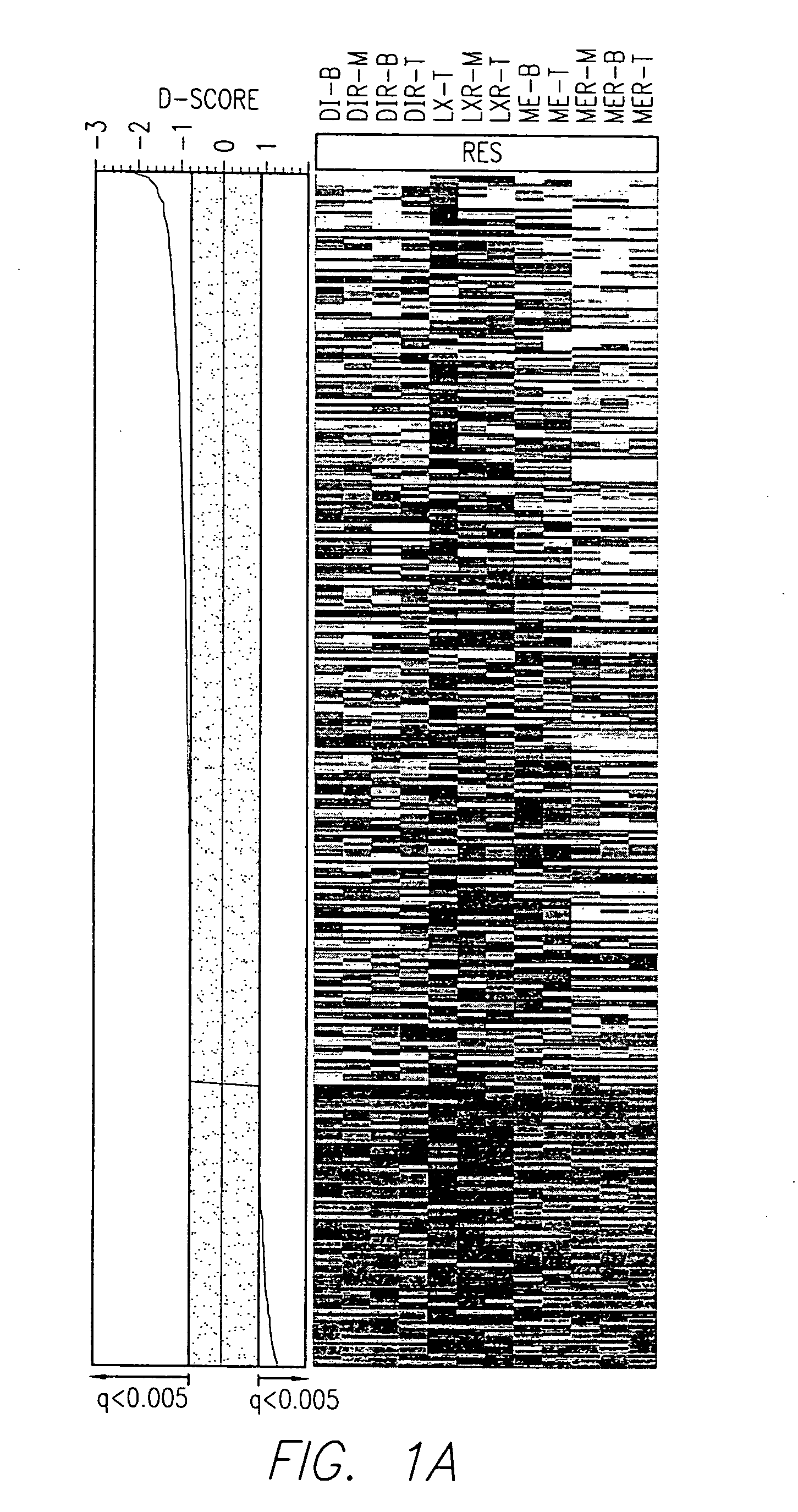
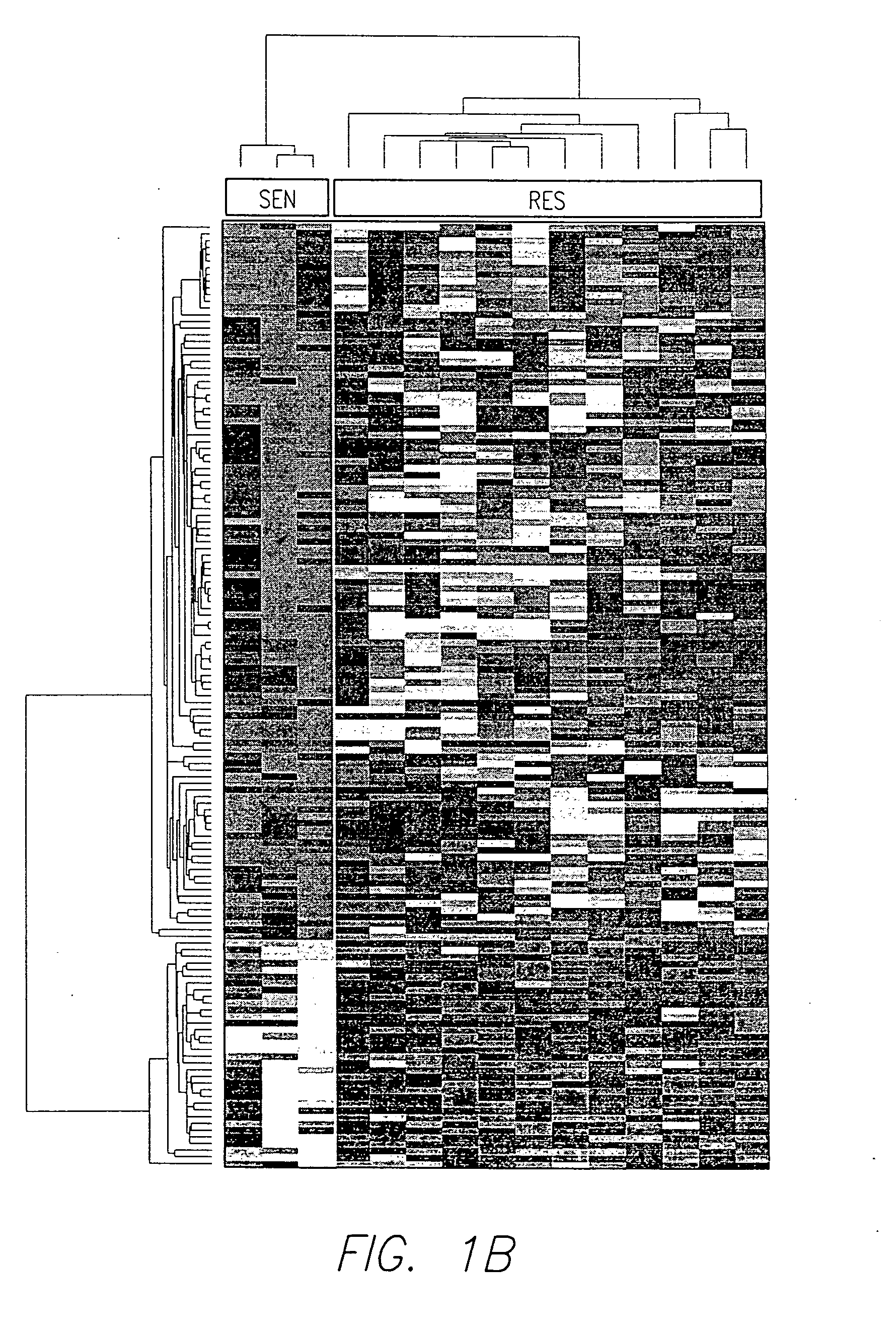
There is disclosed a method for identifying a therapeutically responsive phenotype, as distinguished, e.g. from an alkylating agent resistant phenotype in a cell, which method may be used to evaluate the likelihood of successful outcome of treating a tumor cell with an alkylating agent. The method is directed to the NF-κB activation in response to DNA damage caused by alkylating agents. It comprises the step of measuring a level of expression of a protein, which participates in the NF-κB pathway. Preferably it comprises measuring the expression of TNFAIP3 in the cell, wherein a resistant phenotype has less expression of TNFAIP3 than a sensitive phenotype. Another particularly significant gene, which predicts survival, is NFKBIA. Other genes whose altered expression level is associated with resistance or prognosis are TNIP1, TNIP2, RIP, NFKBIB, Beta4GalNAc-T4, NFKBIE, C8orf4, LIF, CD44, FBXO32, and SDC1, and these are also measured in certain embodiments.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Process for the preparation of lacosamide
Owner:AMRI ITAL SRL
Anticancer combination of artemisinin-based drugs and other chemotherapeutic agents
InactiveUS9023861B2Heavy metal active ingredientsBiocideAlkylating antineoplastic agentChemotherapeutic drugs
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Temperature controlled sustained-release injection comprising alkyl agent and method for preparing the same
InactiveCN101273962APharmaceutical delivery mechanismPharmaceutical non-active ingredientsWater insolubleWhole body
The invention relates to a temperature-controlled sustained-release injection containing an alkylating agent and a preparation method thereof, the temperature-controlled sustained-release injection comprises effective anti-cancer amount of the alkylating agent, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained angiogenesis inhibitor to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months. The sustained-release gel injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug and being used for the treatment of the tumors in different stages. The alkylating agent is selected from cyclophosphamide, melphalan, leukeran, 4H-cyclophosphamide peroxide, norcantharidin, mannosulfan, treosulfan, ritrosulfan, ethoglucid, pipobroman, piposulfan, pumitepa, uredepa, azatepa and so on.
Owner:SHANDONG LANJIN PHARMA +1
Enmein diterpene-melphalan derivative as well as preparation method and application thereof
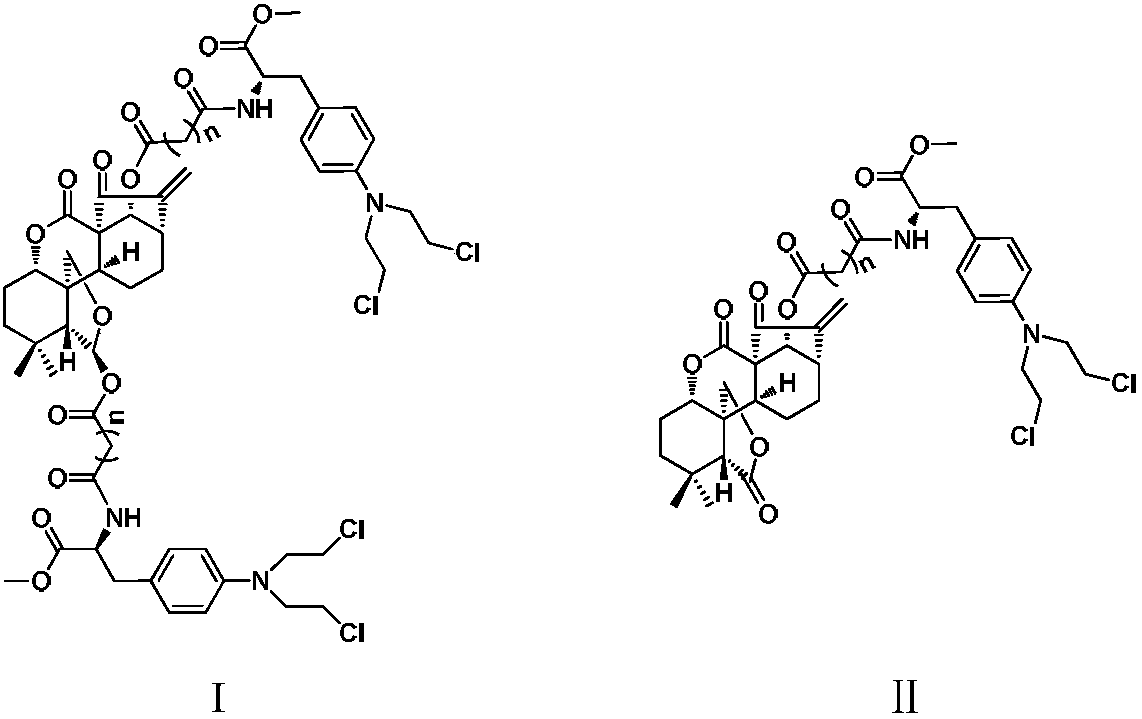
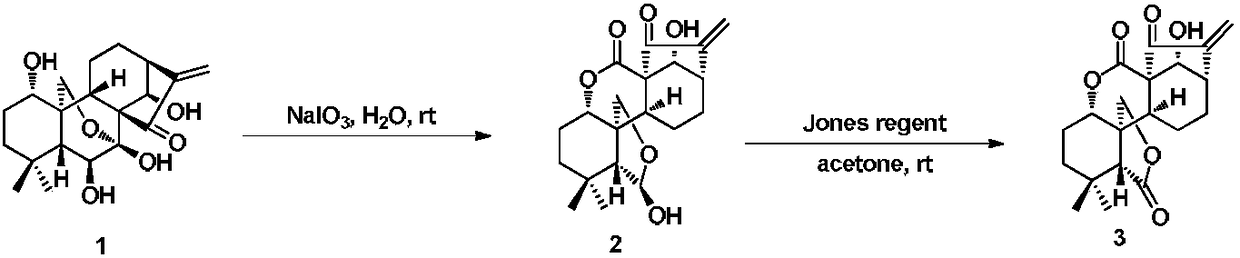
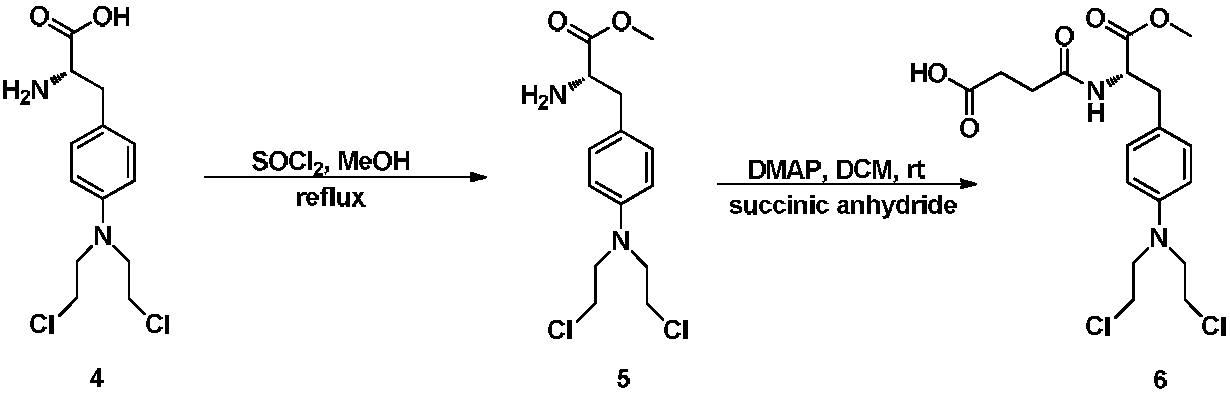
ActiveCN108101925AHigh activityImprove pharmacological activityOrganic active ingredientsOrganic chemistryA-DNADiterpene
The invention relates to the fields of natural drugs and medicinal chemistry, relates to an enmein diterpene-melphalan derivative as well as a preparation method and application thereof and specifically relates to a preparation method of a melphalan derivative with a DNA alkylating agent induced on a 14-OH or 6, 14-OH site in a mother nucleus structure of enmein diterpene and application of the melphalan derivative to an antitumor drug. The structures of enmein diterpene-melphalan derivative and pharmaceutically acceptable salt thereof are shown as the general formula I or the general formulaII, wherein n is described as the claims and the specification.
Owner:SHENYANG PHARMA UNIVERSITY
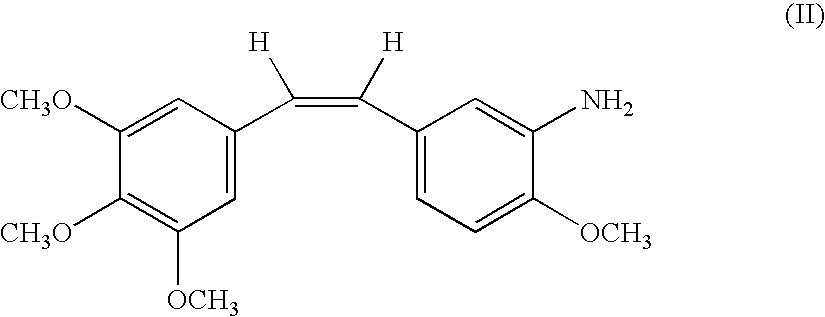
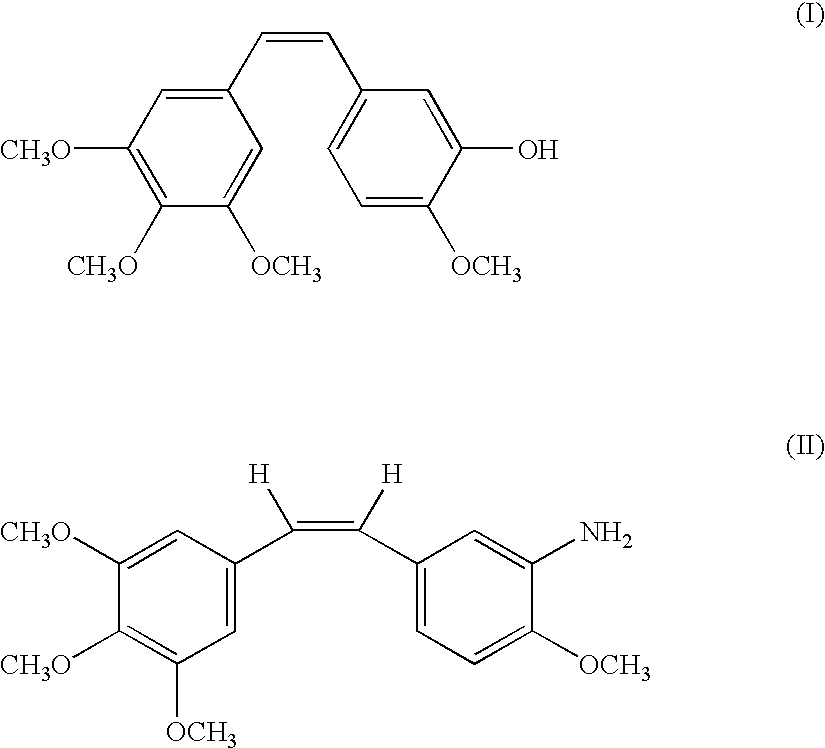
Combination comprising combretastatin and anticancer agents
An antitumor combination comprising a stilbene derivative and an anticancer compound selected from the group consisting of taxanes, alkylating agents, antimetabolites, vinca alkaloids, platinum compounds, epidophylloptoxins, and antibiotics as the active ingredients is provided. Methods of using these pharmaceutical preparations for the treatment of solid carcinomas and the like are also provided.
Owner:AVENTIS PHARMA SA
Anti-cancer composition containing tyrosine kinase inhibitor and alkylating agent
InactiveCN101073554APharmaceutical delivery mechanismPharmaceutical non-active ingredientsProtein-Tyrosine KinasesTreatment effect
The invention is concerned with a kind of sustained and controlled release injection of vanticancer compound of depressor with protein tyrosine kinase and / or alkylating agent, relating to sustained and controlled release microsphere and menstruum. The microsphere has effect component and sustained and controlled release assistant stuff form the depressor with protein tyrosine kinase and / or alkylating agent, and the menstruum is common menstruum or special menstruum with assistant suspending agent. The viscosity of assistant suspending agent is 100cp to 3000cp (20 to 30 degree) selecting form Carboxymethylcellulose sodium, and so on. The sustained and controlled release assistant stuff are seleted from the polyphosphate ester copolymer or copolymer of polyphosphate ester and polylactic acid, polystyrene propionic double fatty acids and sebacic acid, copolymer of poly (erucic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid), such as p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p( BHET-EOP / TC), p(BHDPT-EOP / TC), p(BHDPT-EOP / TC), p(CHDM-HOP) or p(CHDM-EOP). The anticancer compound is made into sustained and controlled release implant and injects or sets in the tumour or on the round of tumour to maintain the effect concentration over 60 days. It reduces the reaction of whole body greatly and increases the cure effect of nonoperative treatments, such as chemoradiotherapy with selectivity.
Owner:JINAN KANGQUAN PHARMA TECH
Pharmaceutical composition of oral suspension of anti-neoplastic alkylating agents
ActiveUS11147810B2Improve palatabilityImprove stabilityOrganic active ingredientsDispersion deliveryAlkylating antineoplastic agentOral suspensions
The present invention provides pharmaceutical composition of antineoplastic alkylating agent in oral suspension dosage form. The oral suspension composition comprises of alkylating agent with other pharmaceutical excipients such as vehicle, preservative, antioxidant, suspending agent, surfactant, sweetener and flavouring agent. The present invention is an oral suspension having improved stability and palatability. The present invention also provides oral solution with flavor that has masked bitter taste of the drug. Further, the present invention also provides a process for preparation thereof.
Owner:FTF PHARMA LTD
Novel method
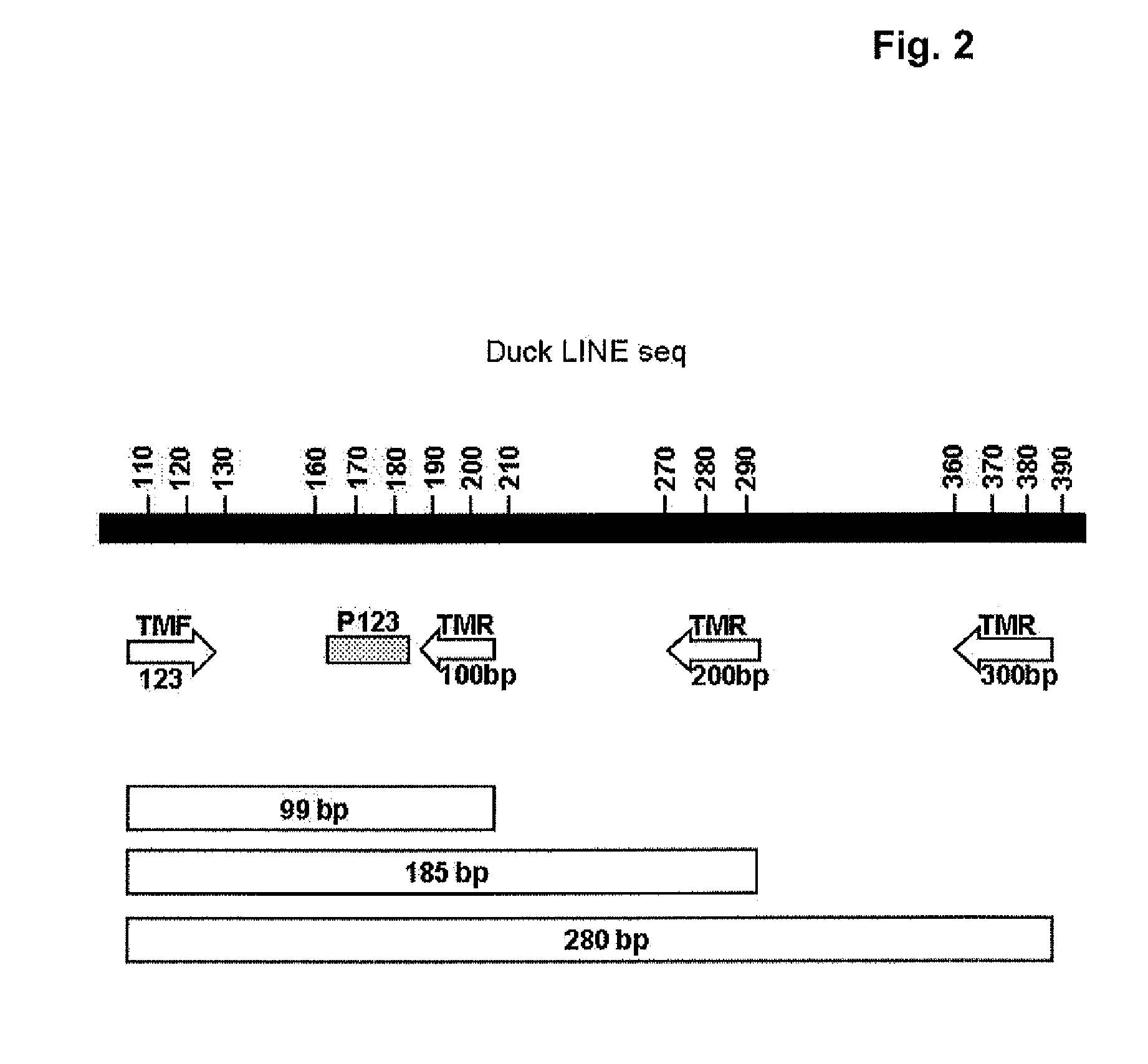
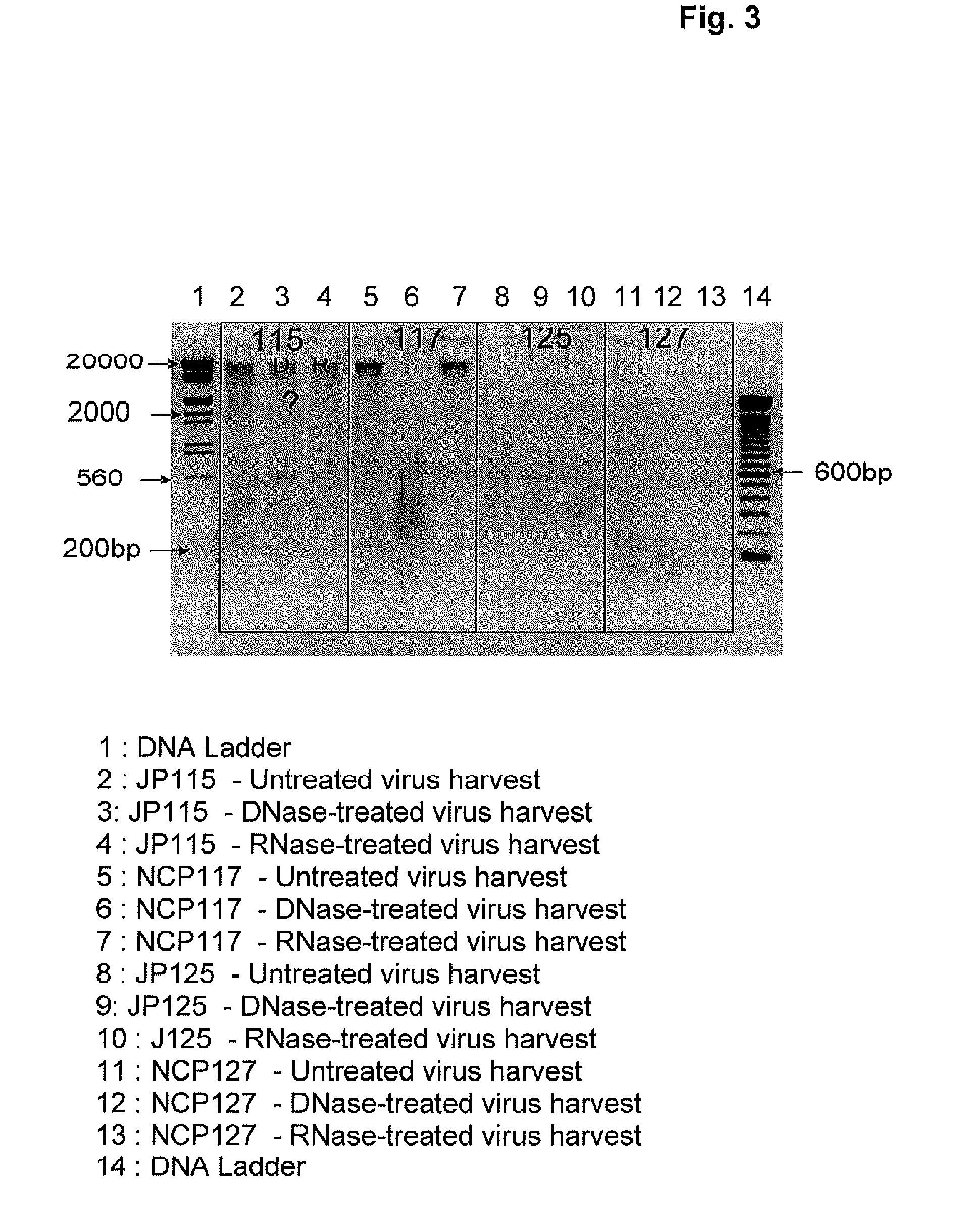
InactiveUS20110217330A1High fragmentationReduce residual contentSsRNA viruses negative-senseViral antigen ingredientsAlkylating antineoplastic agentViral antigens
The present invention relates to a method for degrading host cell nucleic acids associated with a virus or a viral antigen thereof produced by cell culture, the method comprising at least two steps of nucleic acids degradation with a compound selected from i) an endonuclease and ii) a DNA alkylating agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Combined inactivated vaccine for against infectious brunchitis and newcastle disease and preparation method thereof
ActiveCN101480492ASolve protection problemsBroad antigen spectrumViral antigen ingredientsAntiviralsDiseaseOil adjuvant
The invention belongs to the technical field of veterinary biology new medicine and relates to a chicken infectious bronchitis and chicken new castle disease bigeminal inactivated vaccine and a preparation method thereof. Virus seeds used by the vaccine are respectively a chicken infectious bronchitis virus Beijing A2 strain and a chicken new castle disease virus GM strain. The vaccine is prepared by the steps of mixing the chicken infectious bronchitis virus Beijing A2 strain and the chicken new castle disease virus GM strain after respectively being inactivated by an alkylating agent, adding oil adjuvant and emulsifying. The bigeminal inactivated vaccine can play a good role in preventing the novel serological type virus of infectious bronchitis and the novel antigen virus of new castle disease and solve the problem that the two viruses are only partially protected or even not protected by the prior vaccine in many places in China, thereby the occurrence of the chicken infectious bronchitis and the chicken new castle disease is effectively prevented.
Owner:兆丰华生物科技(南京)有限公司
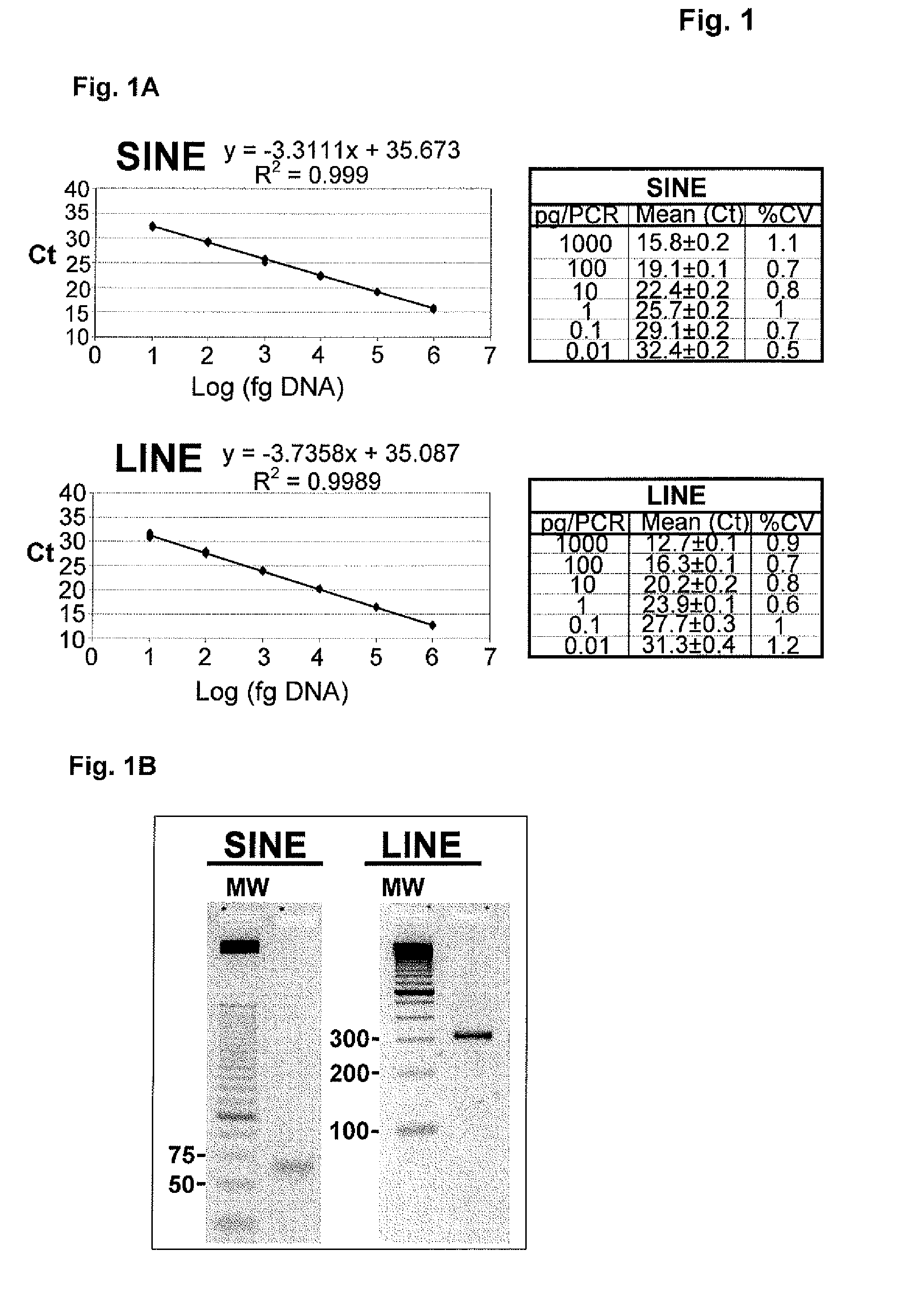
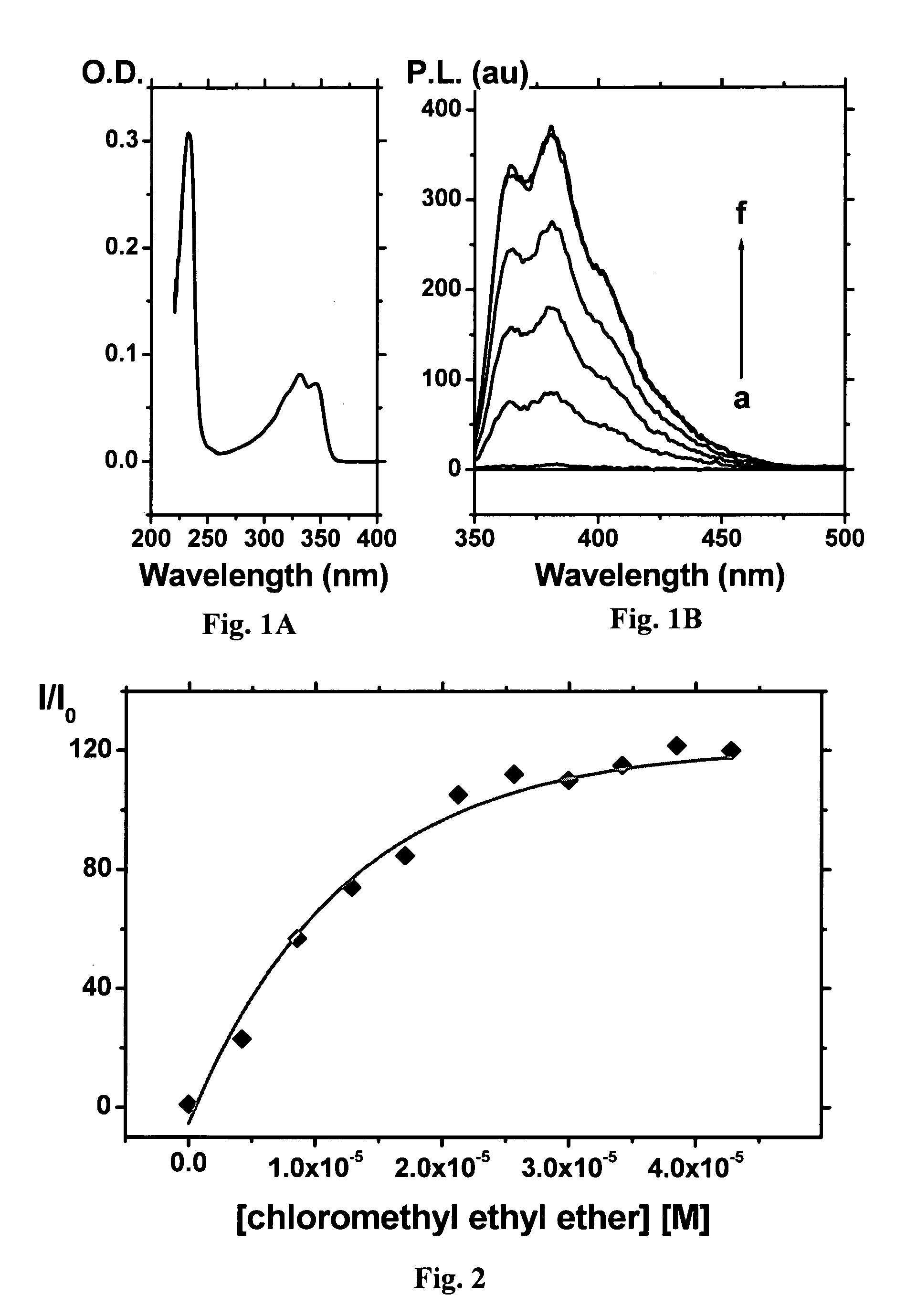
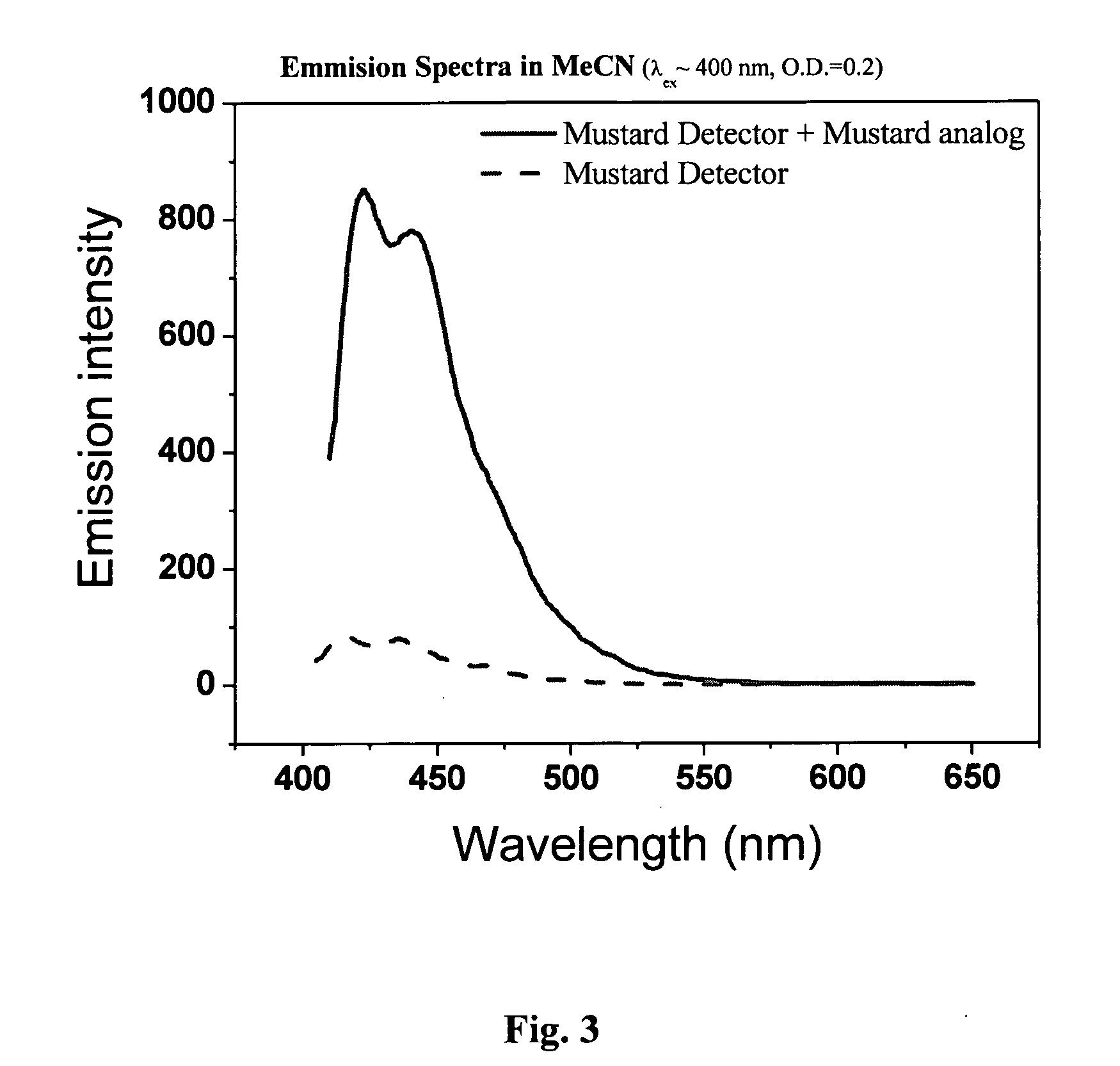
Method for Detecting Alkylating Agents
InactiveUS20090305429A1Simple and complex shapeHigh strengthMaterial thermal conductivityMaterial analysis by optical meansOrganic chemistryChemical sensor
Owner:TECHNION RES & DEV FOUND LTD
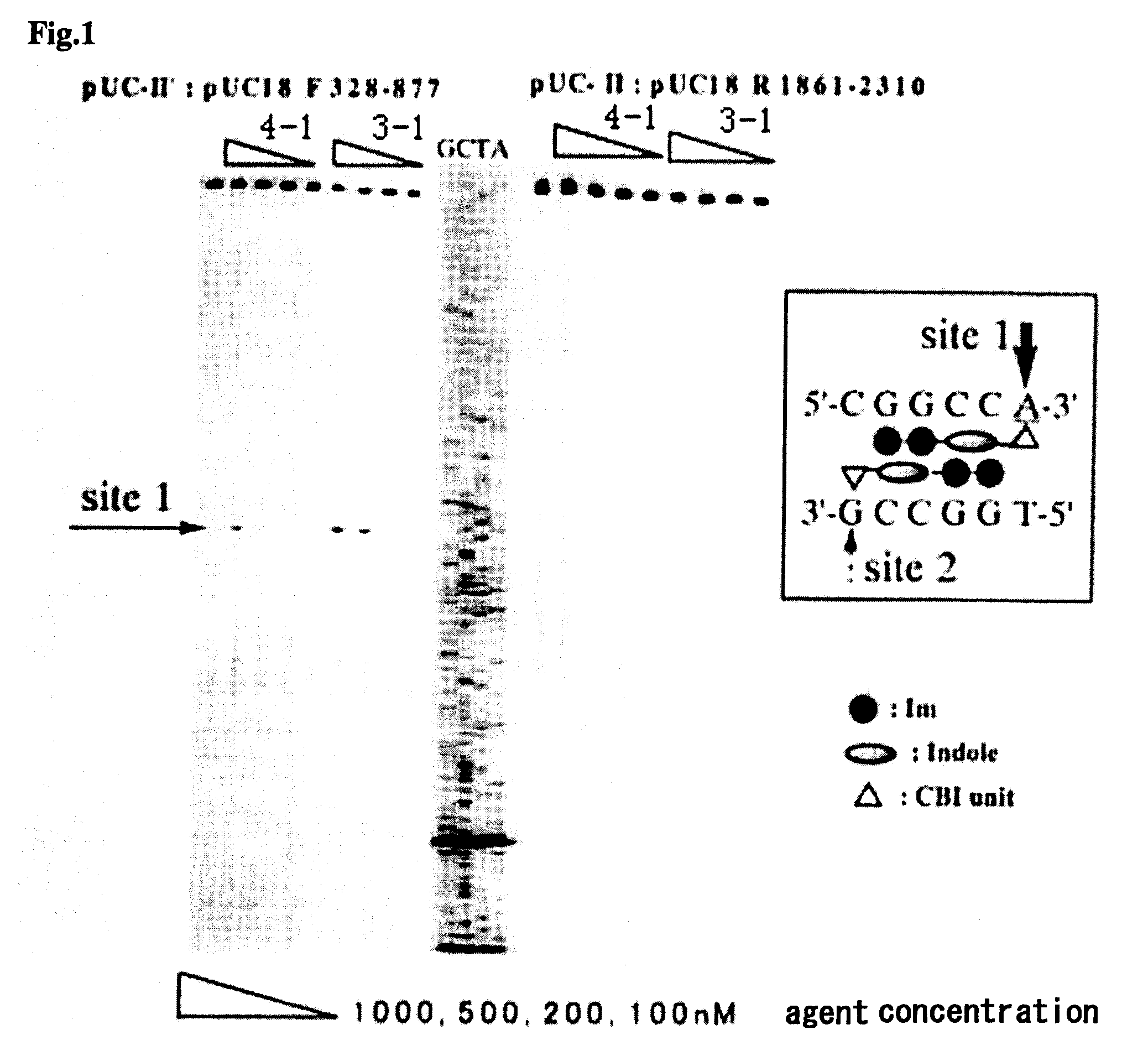
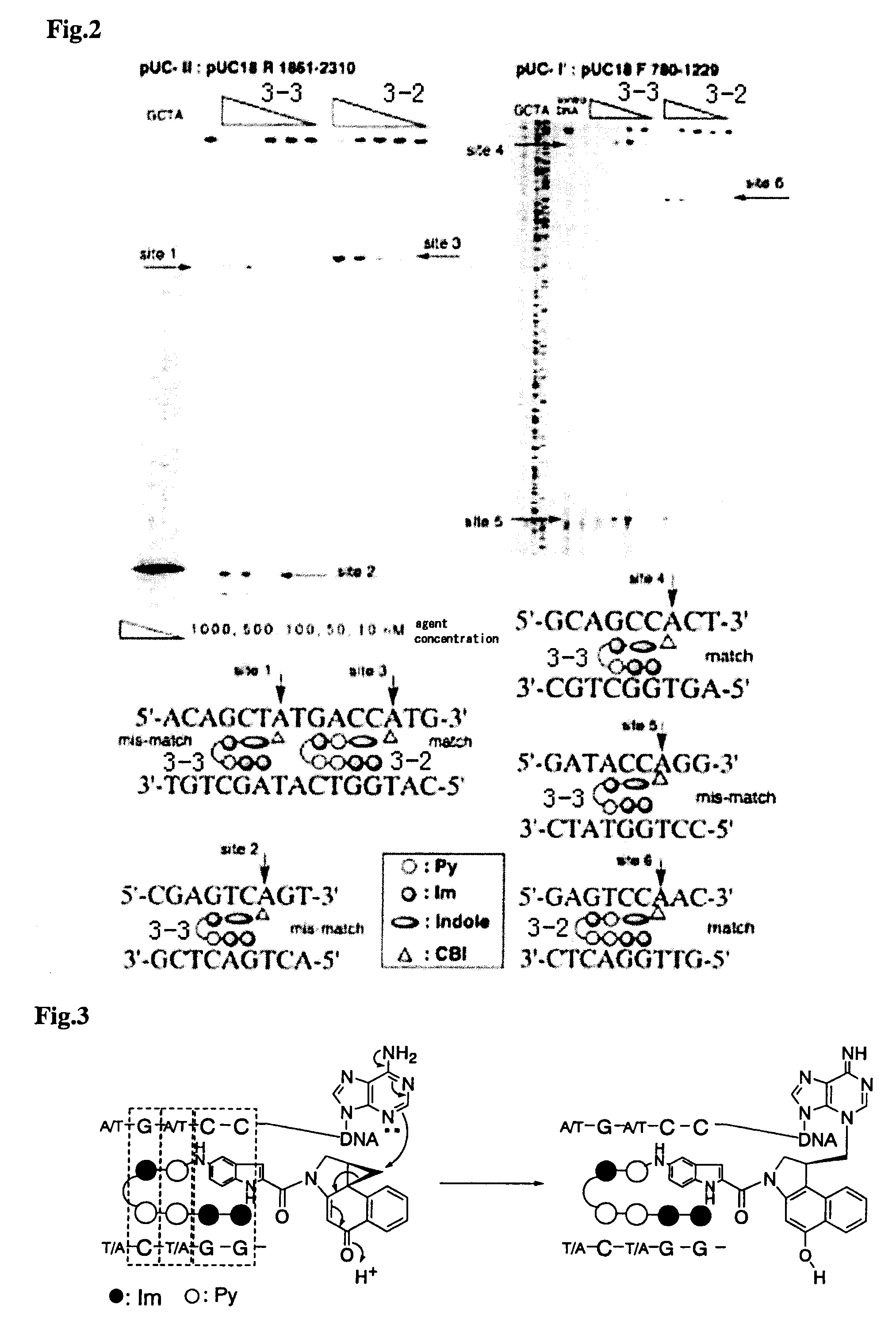
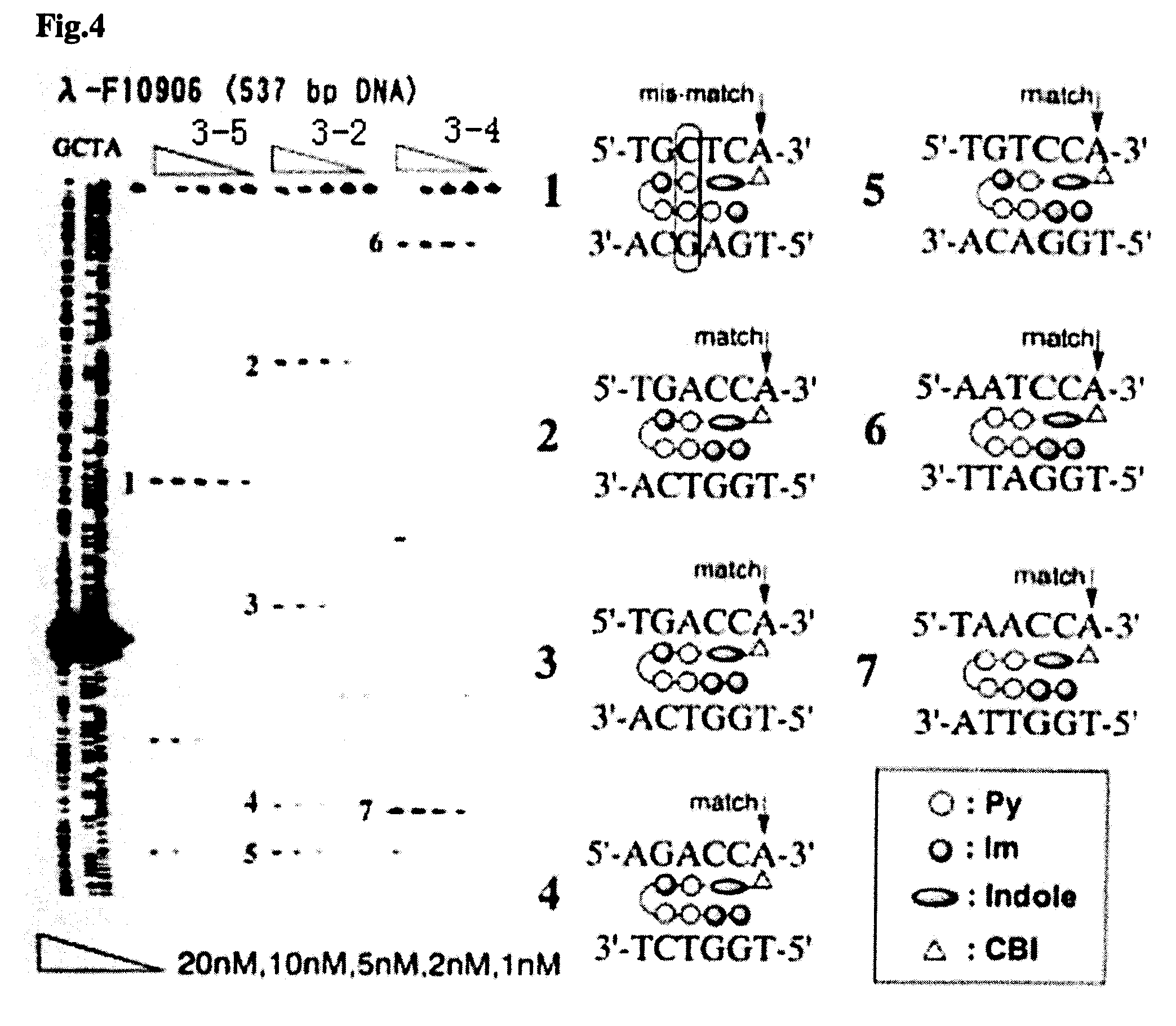
Novel indole derivative for alkylating specific base sequence of dna and alkylating agent and drug containing the derivative
InactiveUS20070191260A1Improve applicationBiocidePeptide/protein ingredientsAlkylating antineoplastic agentAcyl group
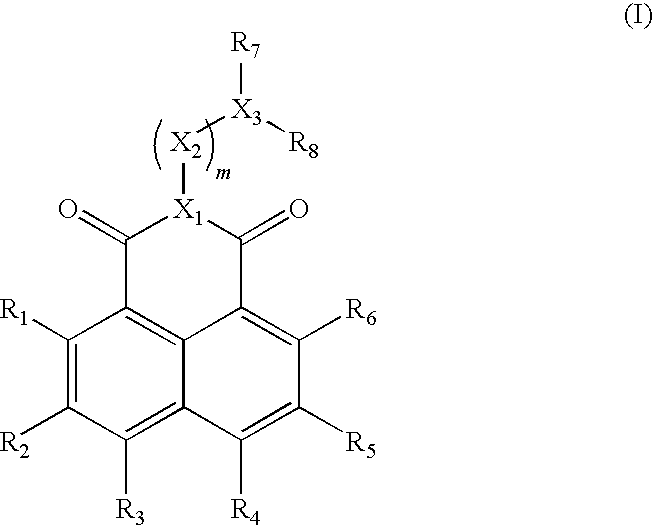
There is provided a novel pyrrole-imidazole polyamide compound for alkylating the specific base sequence of DNA, the polyamide compound being capable of being synthesized through fewer reaction steps than known hybrid molecules and having a combination of a high reactivity in DNA alkylation and the ability to recognize a sequence. Furthermore, there is provided an alkylating agent and a molecule serving as a drug, the alkylating agent and the molecule containing the polyamide compound. An indole derivative is represented by general formula (1): wherein R1 represents a functional group for alkylating DNA; R2 represents a hydrogen atom, an alkyl group, or an acyl group; and X represents a divalent group having one constitutional unit or having two or more constitutional units which may be the same or different, the constitutional unit being represented by the following formula: (wherein m is an integer of 0 to 10), wherein among the constitutional units, a terminal constitutional unit adjacent to R2 may be a constitutional unit represented by the following formula: (wherein k is an integer of 0 to 10).
Owner:KYOTO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
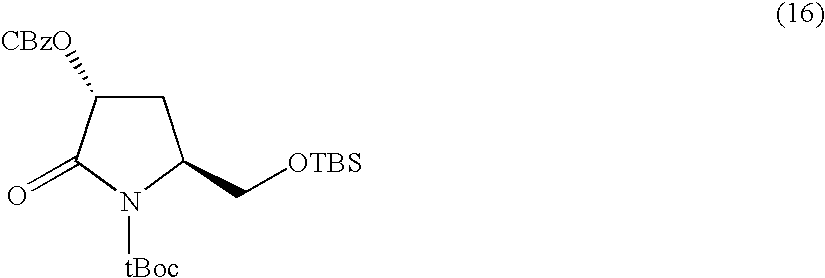


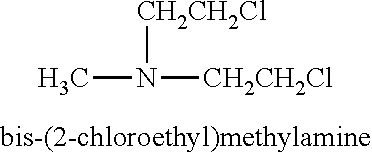
![Synthesis of 8h-3a-aza-cyclopenta[a]indenes and 5,10-dihydropyrrolo[1,2-b]isoquinolines derivatives and their use as antitumor therapeutic agents Synthesis of 8h-3a-aza-cyclopenta[a]indenes and 5,10-dihydropyrrolo[1,2-b]isoquinolines derivatives and their use as antitumor therapeutic agents](https://images-eureka.patsnap.com/patent_img/5ff64d09-3cd6-4b58-a304-bcfd4fcdb6f0/US20090117125A1-20090507-D00001.png)
![Synthesis of 8h-3a-aza-cyclopenta[a]indenes and 5,10-dihydropyrrolo[1,2-b]isoquinolines derivatives and their use as antitumor therapeutic agents Synthesis of 8h-3a-aza-cyclopenta[a]indenes and 5,10-dihydropyrrolo[1,2-b]isoquinolines derivatives and their use as antitumor therapeutic agents](https://images-eureka.patsnap.com/patent_img/5ff64d09-3cd6-4b58-a304-bcfd4fcdb6f0/US20090117125A1-20090507-D00002.png)
![Synthesis of 8h-3a-aza-cyclopenta[a]indenes and 5,10-dihydropyrrolo[1,2-b]isoquinolines derivatives and their use as antitumor therapeutic agents Synthesis of 8h-3a-aza-cyclopenta[a]indenes and 5,10-dihydropyrrolo[1,2-b]isoquinolines derivatives and their use as antitumor therapeutic agents](https://images-eureka.patsnap.com/patent_img/5ff64d09-3cd6-4b58-a304-bcfd4fcdb6f0/US20090117125A1-20090507-D00003.png)