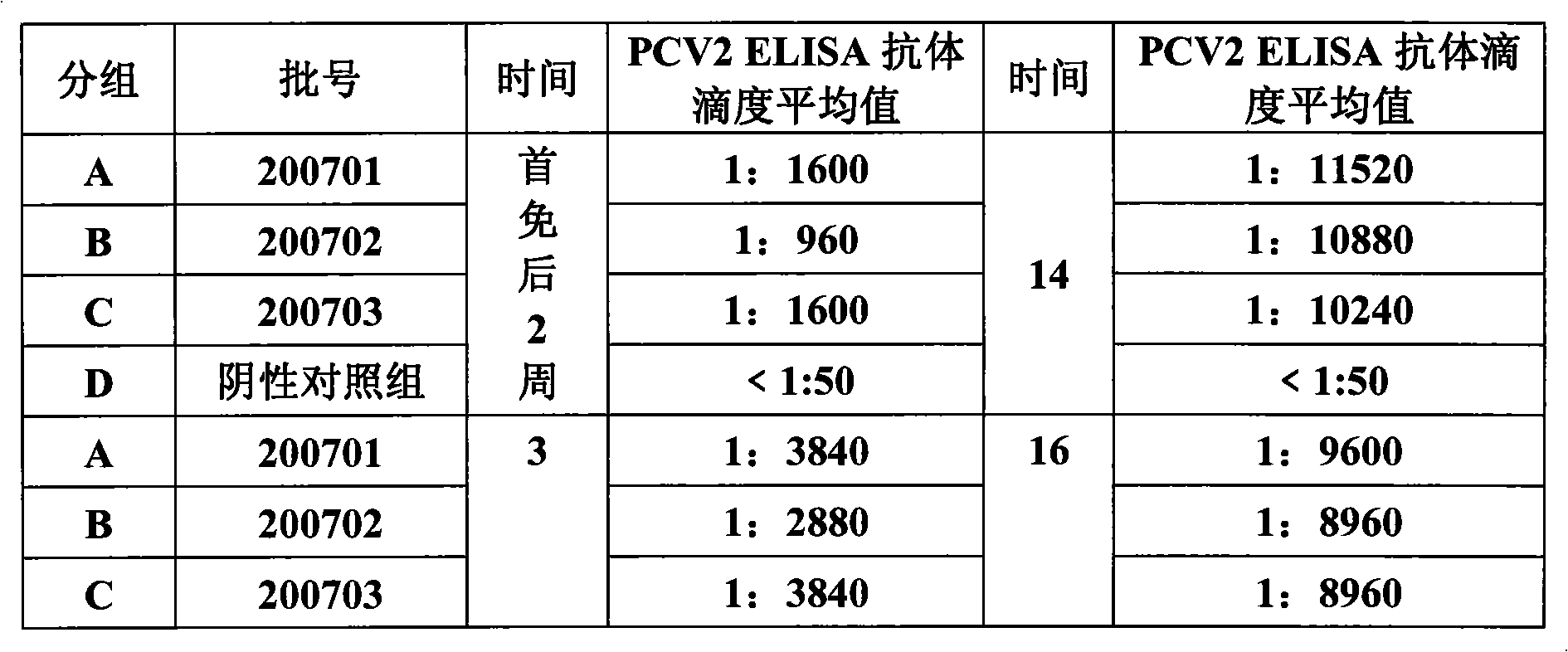
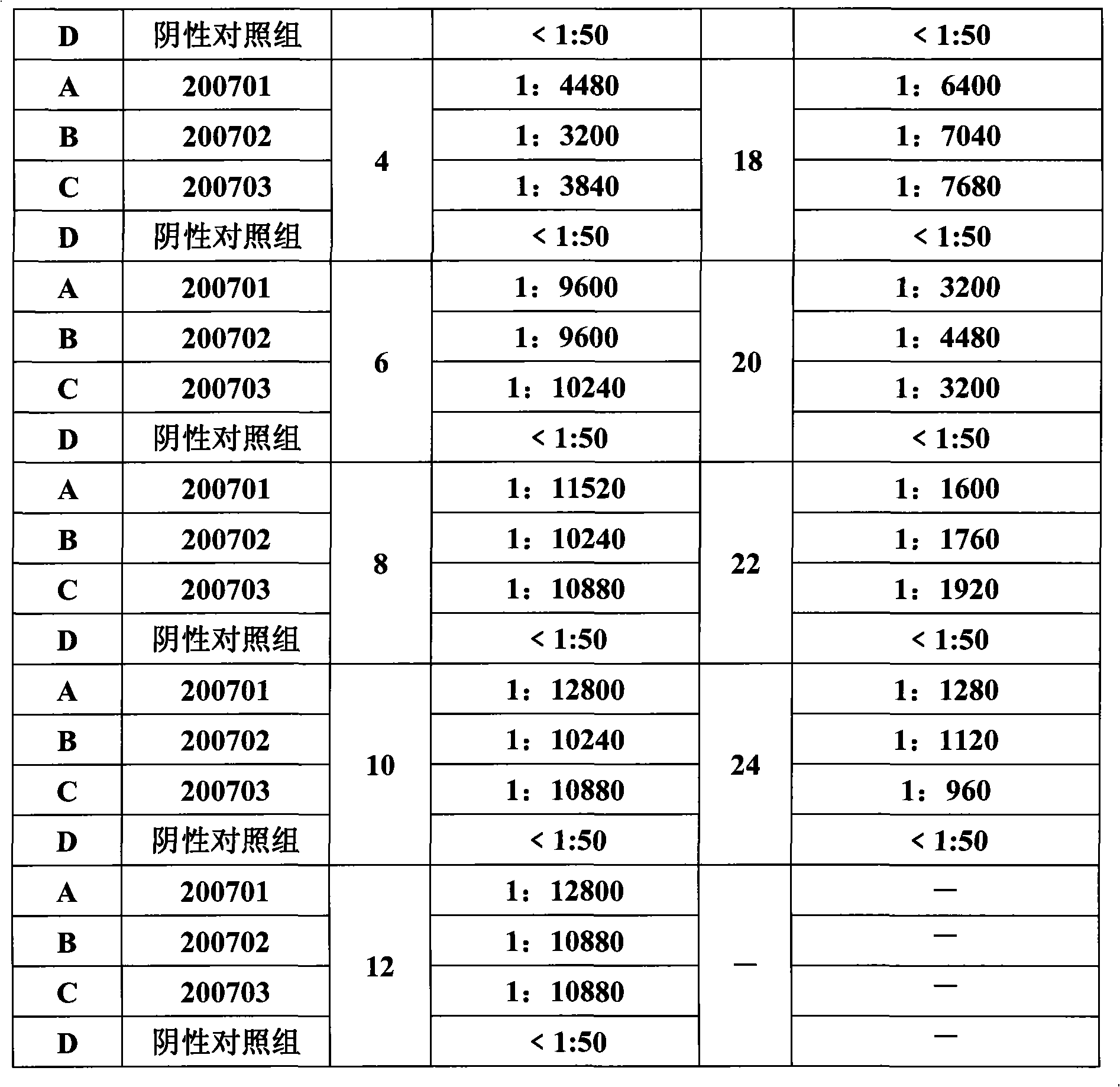
Porcine circovirus type II inactivated vaccine of and method for preparing same
A porcine circovirus and inactivated vaccine technology, applied in antiviral agents, virus antigen components, etc., can solve the problems of destroying the immune activity of PCV2 antigen, rough vaccine production process, and reduced immune effect, and achieve good immunogenicity and low cost. Low, infection prevention effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 porcine circovirus type II DBN-SX07 strain inactivated vaccine
[0033] (1) Preparation of poison for production
[0034] Get the required number of cells with a cell coverage rate of more than 80% and discard the growth medium, insert porcine circovirus type II DBN-SX07 strain virus seeds at a ratio of 5% (V / V), 37 After adsorption at ℃ for 1 hour, maintenance solution was added for incubation. After 24 hours, treat with 300mM D-glucosamine at 37°C for 20min, wash twice with sterile PBS (pH 7.4), add maintenance solution, rotate at 37°C for 48 hours, freeze and thaw three times, centrifuge or Cell debris was removed by filtration and frozen at -40°C.
[0035] (2) Inactivation of poisons used in production
[0036] Add the qualified virus solution to the formaldehyde solution at a ratio of 0.5‰ (V / V), mix well and inactivate at 37°C for 16 hours, shaking 4-5 times during the period.
[0037] (3) emulsification
[0038] The inactivate...
Embodiment 2
[0056] The preparation of embodiment 2 porcine circovirus type II inactivated vaccines
[0057] (1) Preparation of poison for production
[0058] Get the required number of cells with a cell coverage rate of more than 80% and discard the growth medium, insert porcine circovirus type II DBN-SX07 strain virus seeds at a ratio of 5% (V / V), 37 After adsorption at ℃ for 1 hour, maintenance solution was added for incubation. After 24 hours, treat with 300mM D-glucosamine at 37°C for 20min, wash twice with sterile PBS (pH 7.4), add maintenance solution, rotate at 37°C for 48 hours, freeze and thaw three times, centrifuge or Cell debris was removed by filtration and frozen at -40°C.
[0059] (2) Inactivation of poisons used in production
[0060] Add the qualified virus solution to the formaldehyde solution at a ratio of 0.75‰ (V / V), mix well and inactivate at 37°C for 20 hours, shaking 4-5 times during the period.
[0061] (3) emulsification
[0062] The inactivated vaccine is p...
Embodiment 3
[0066] The preparation of embodiment 3 porcine circovirus type II inactivated vaccines
[0067] (1) Preparation of poison for production
[0068] Get the required number of cells with a cell coverage rate of more than 80% and discard the growth medium, insert porcine circovirus type II DBN-SX07 strain virus seeds at a ratio of 5% (V / V), 37 After adsorption at ℃ for 1 hour, maintenance solution was added for incubation. After 24 hours, treat with 300mM D-glucosamine at 37°C for 20min, wash twice with sterile PBS (pH 7.4), add maintenance solution, rotate at 37°C for 48 hours, freeze and thaw three times, centrifuge or Cell debris was removed by filtration and frozen at -40°C.
[0069] (2) Inactivation of poisons used in production
[0070] Add the qualified virus solution to the formaldehyde solution at a ratio of 1‰ (V / V), mix well and inactivate at 37°C for 24 hours, shaking 4-5 times during the period.
[0071] (3) emulsification
[0072] The inactivated vaccine is prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com