Patents
Literature
33 results about "DNA Methyltransferase Inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any substance that inhibits DNA methyltransferase, an enzyme required for DNA methylation. Inhibition of DNA methyltransferase results in hypomethylation of genomic DNA and cell cycle arrest at S-phase. Hypomethylation in malignant cells may also restore suppressor gene expression and re-establish controlled growth and differentiation.
Cyclopentathiophene/cyclohexathiophene DNA methyltransferase inhibitors
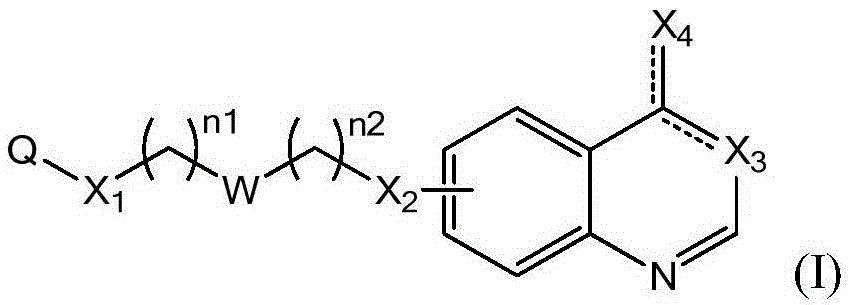
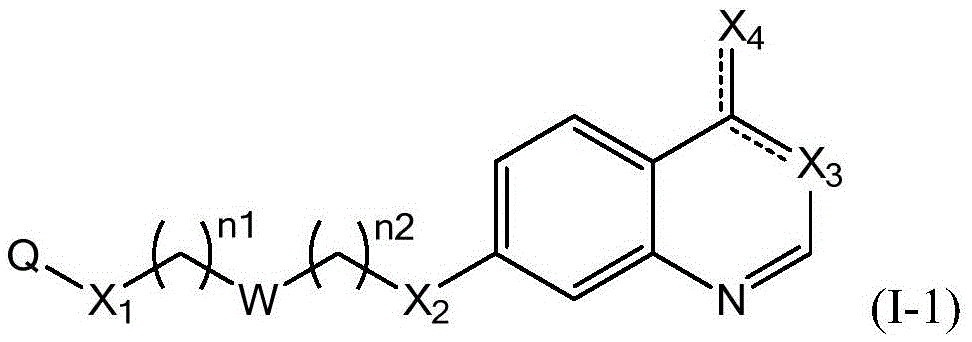
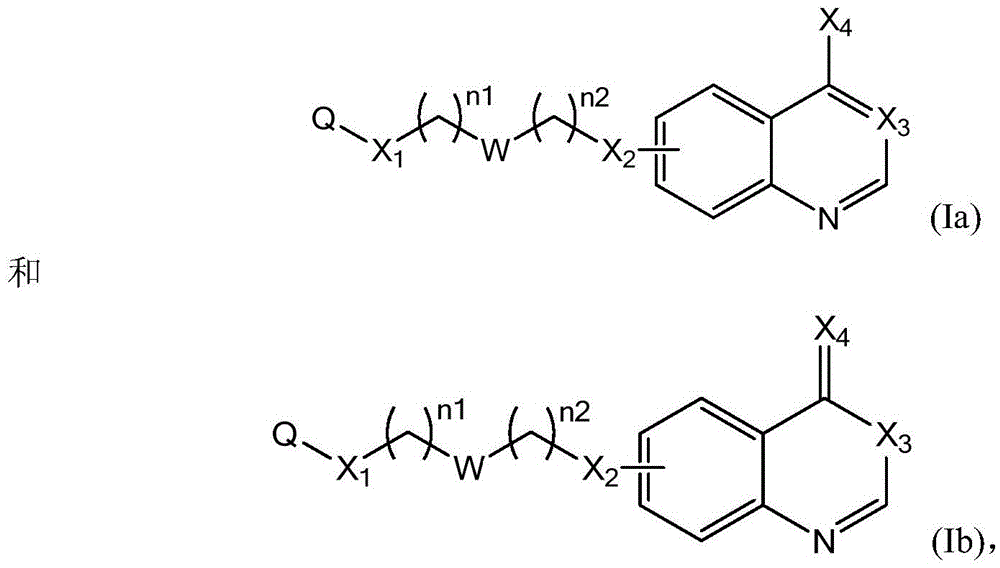
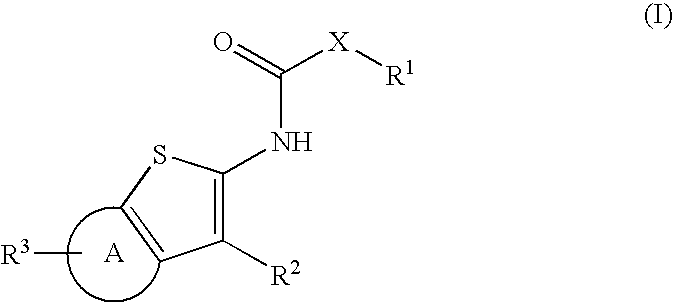
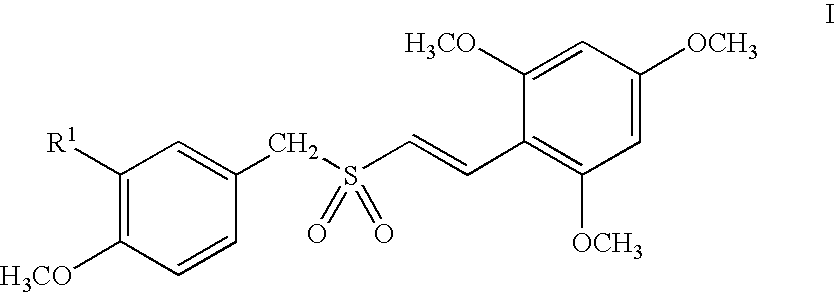
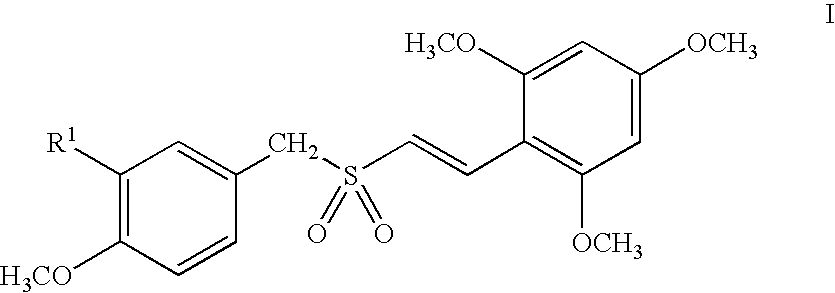
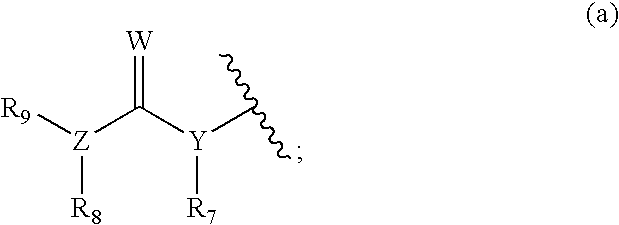
Compounds represented by Formula (I):are useful in treating diseases, such as cancer, that are mediated and / or associated (at least in part) with DNMT3b activity. The compounds can be formulated as pharmaceutically acceptable compositions for administration to a subject in need thereof.
Owner:VANKAYALAPATI HARIPRASAD +2
Composition and methods for the treatment of myelodysplastic syndrome and acute myeloid leukemia
Methods and compositions are provided for treating myelodysplastic syndrome and acute myeloid leukemia, wherein the composition comprises at least one compound according to Formula I:wherein R1 is selected from the group consisting of —NH2, —NH—CH2—CO2H, —NH—CH(CH3)—CO2H, and —NH—C(CH3)2—CO2H, or a pharmaceutically acceptable salt of such a compound; and a DNA methyltransferase inhibitor, or a pharmaceutically acceptable salt thereof.
Owner:MT SINAI SCHOOL OF MEDICINE +1
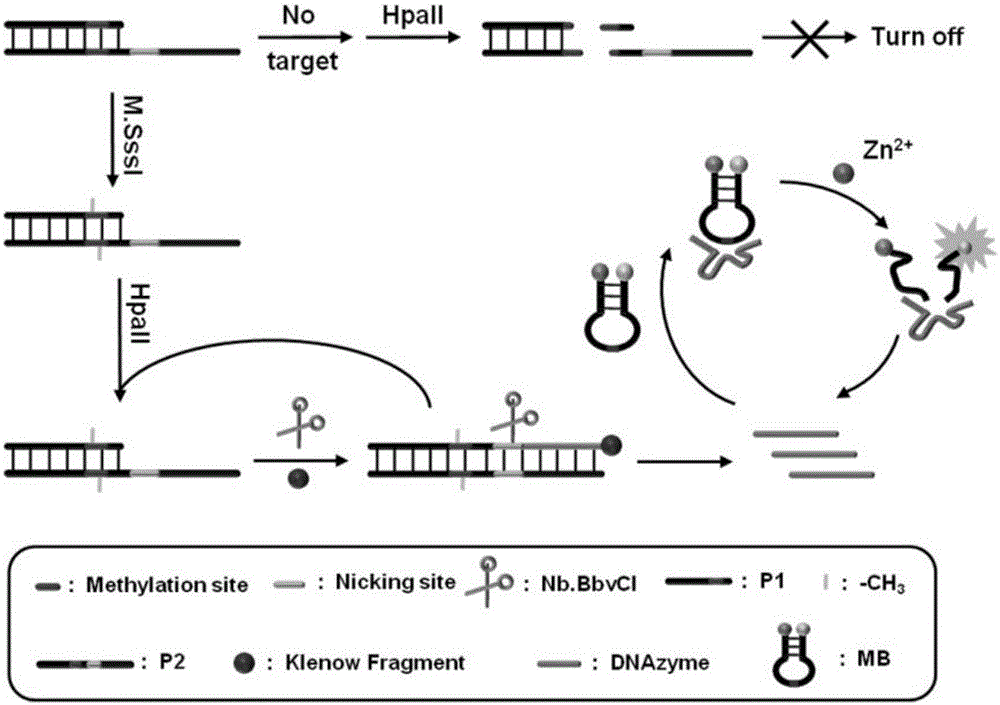
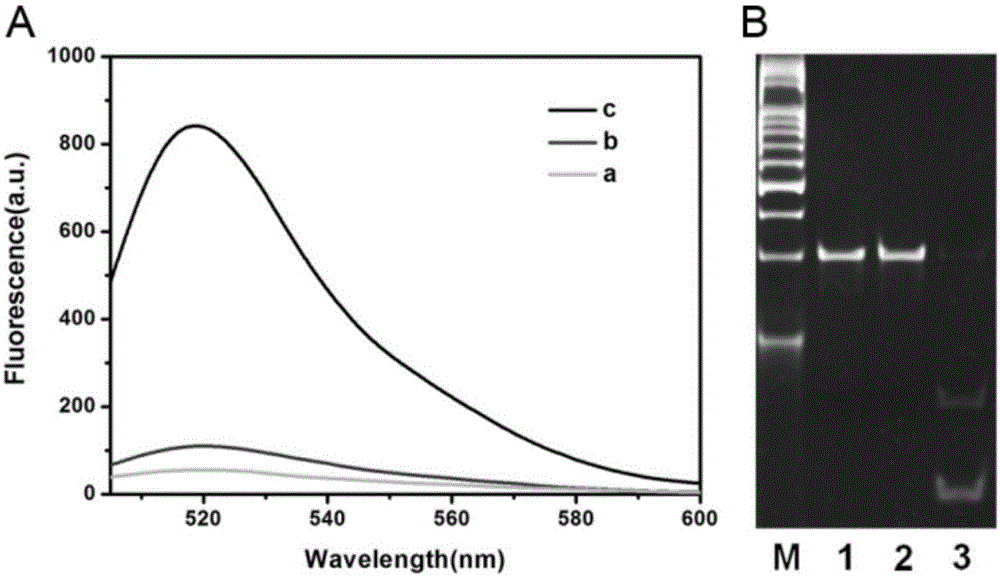
Method for detecting DAN methyltransferase activity based on strand displacement amplification and DNAzyme amplification
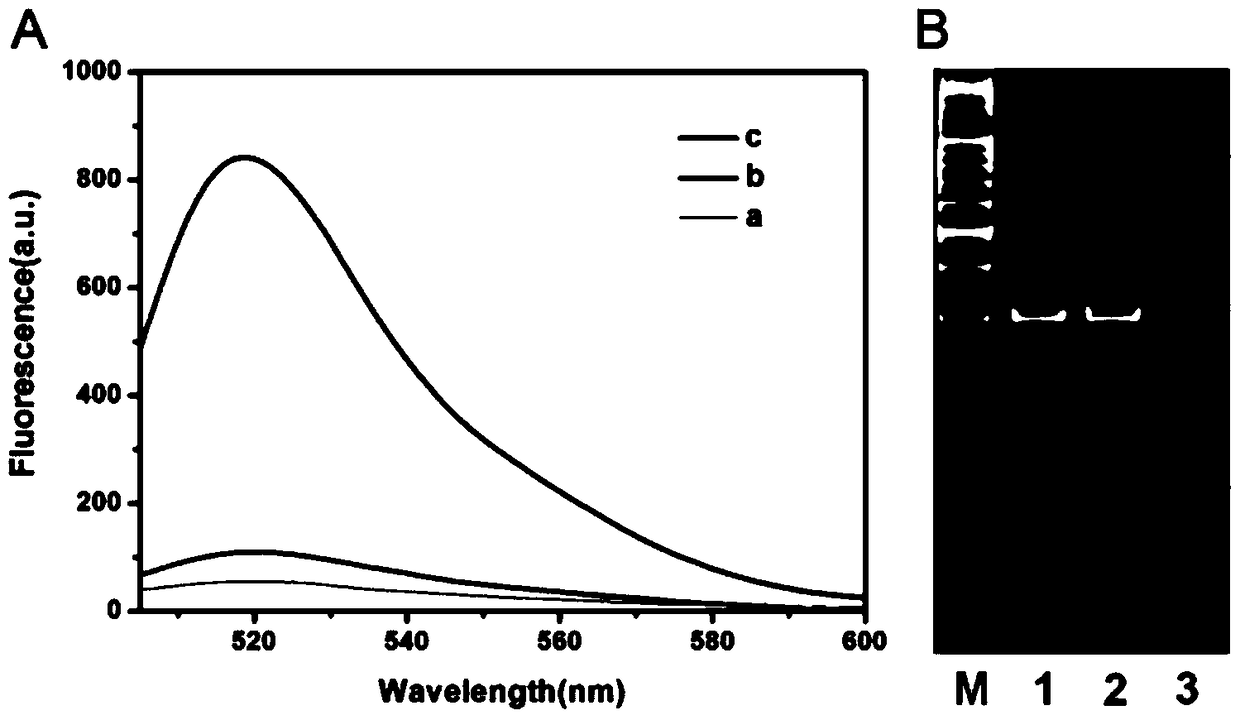
ActiveCN105112540AHighly sensitive detection activityLow detection limitMicrobiological testing/measurementBiological material analysisFluorescenceDNA Methyltransferase Inhibitor
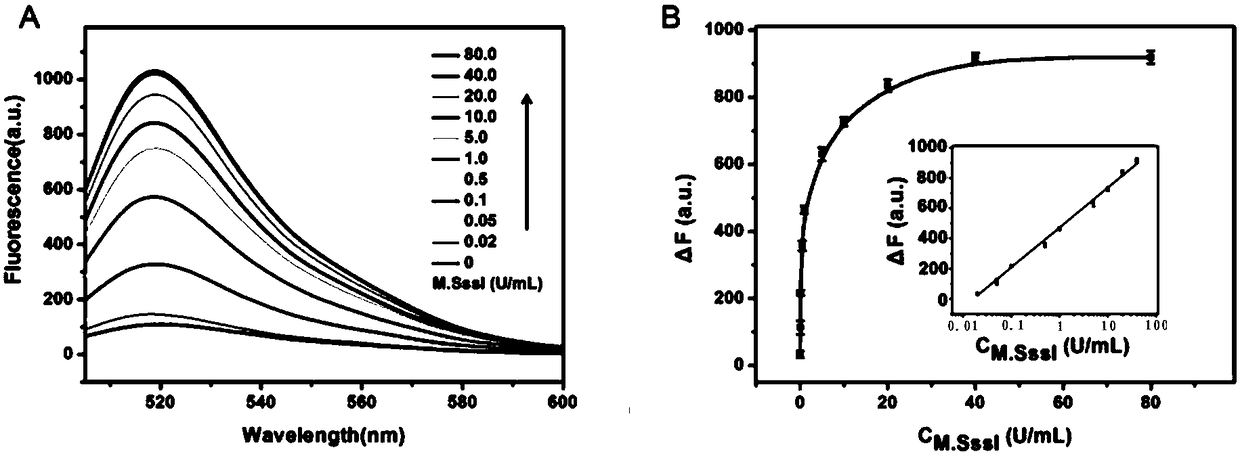
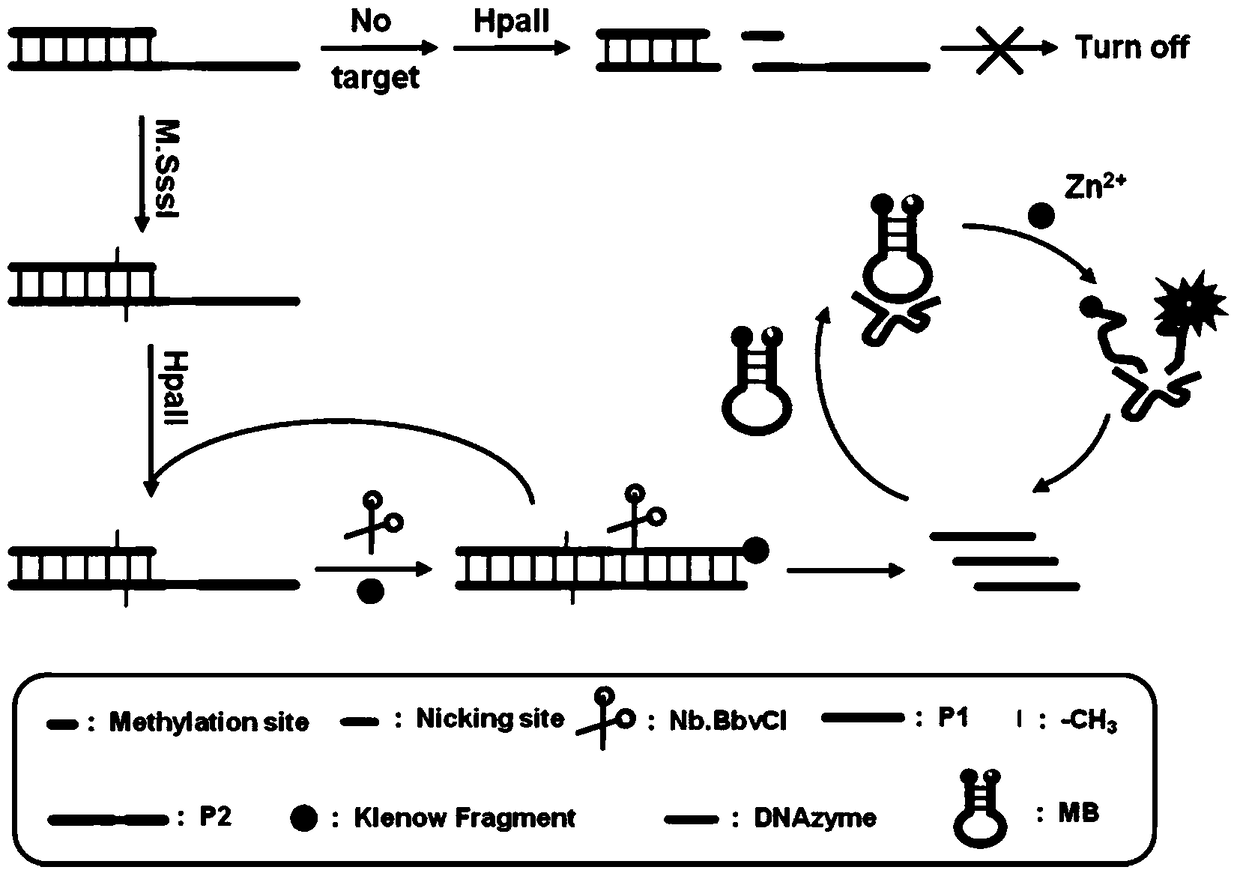
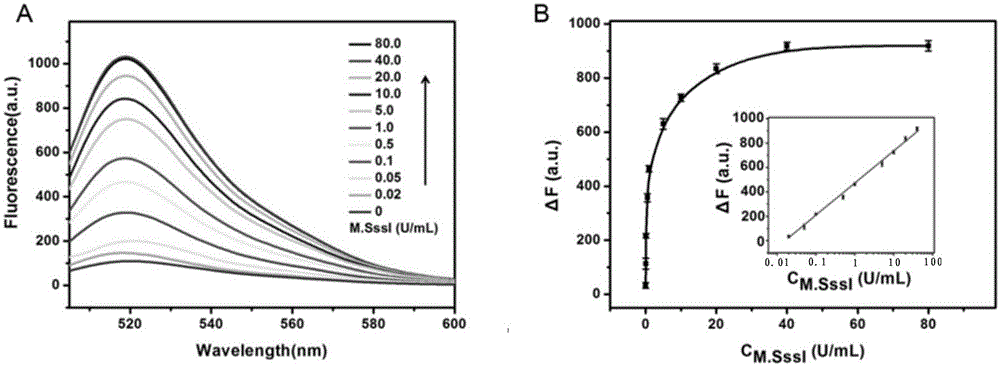
The invention relates to a method for detecting DAN methyltransferase activity based on strand displacement amplification and DNAzyme amplification. A three-function double-stranded DNA probe is designed. Methylation happening to the three-function double-stranded DNA probe is specifically recognized through DAN methyltransferase. Remaining non-methylated double-stranded DNA is specifically cut through HpaII restriction enzymes, methylated double-stranded DNA triggers a strand displacement reaction, a large amount of 8-17 DNAzyme is released, the 8-17 DNAzyme catalyzes cutting of a large number of hairpin-line molecular beacon substrates, and remarkable fluorescent enhancement is triggered. The method can sensitively detect the DAN methyltransferase activity, and the detection limit is 0.0082 U / mL. The method has potential application in research of influences of anti-cancer drugs on DAN methyltransferase activity inhibition and screening of DAN methyltransferase inhibitors.
Owner:SHANDONG UNIV
Combination therapies for treatment of cancer
ActiveUS20180256580A1Organic active ingredientsOrganic chemistryDNA Methyltransferase InhibitorA-DNA
Owner:SUMITOMO PHARMA ONCOLOGY INC
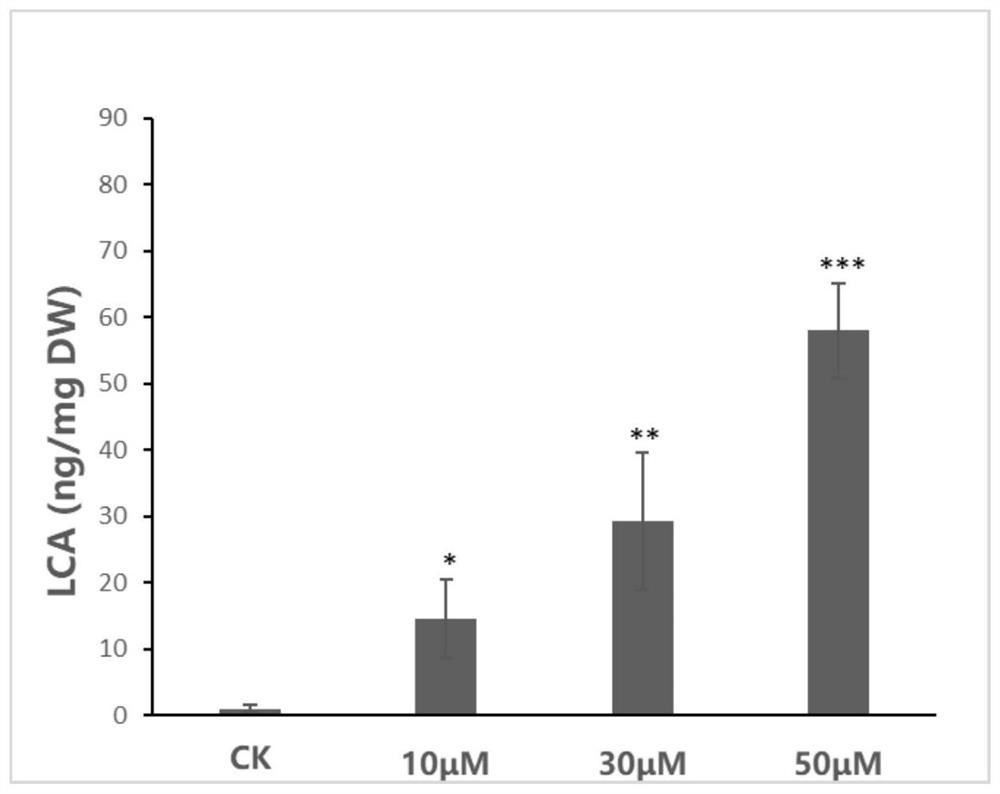
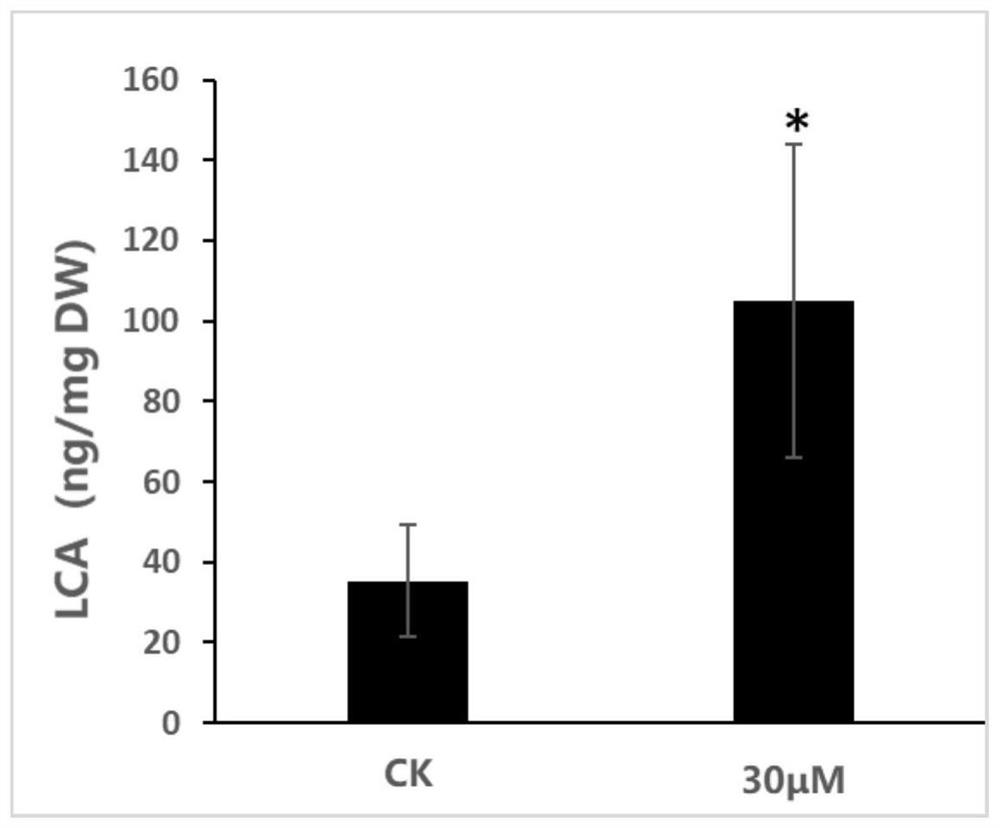
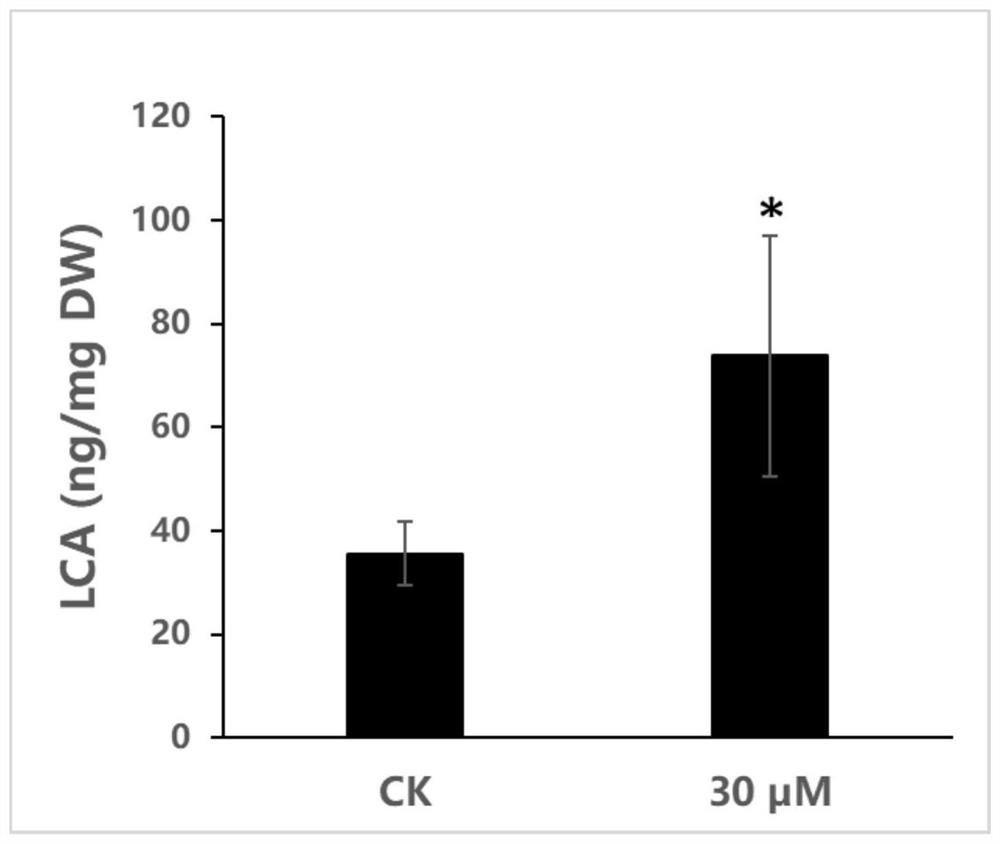
Method for preparing licochalcone A
ActiveCN112898146AFast contentIncrease contentFabaceae cultivationCultivating equipmentsBiotechnologyGlycyrrhiza lepidota
The invention provides a method for preparing licochalcone A. The method comprises the following steps: culturing glycyrrhiza inflata hairy roots or glycyrrhiza inflata seedlings in a culture medium containing a DNA methyltransferase inhibitor for not less than three days, collecting roots of the glycyrrhiza inflata hairy roots or glycyrrhiza inflata seedlings, and extracting the licochalcone A. The culture medium containing the DNA methyltransferase inhibitor is used for culturing glycyrrhiza inflate seedlings or glycyrrhiza inflate hairy roots, so that the DNA methylation degree of the glycyrrhiza inflate seedlings or glycyrrhiza inflate hairy roots can be reduced; and genes which are originally silent or low in expression quantity and are beneficial to accumulation of licochalcone A are reactivated, so that the content of licochalcone A is quickly and effectively increased.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI
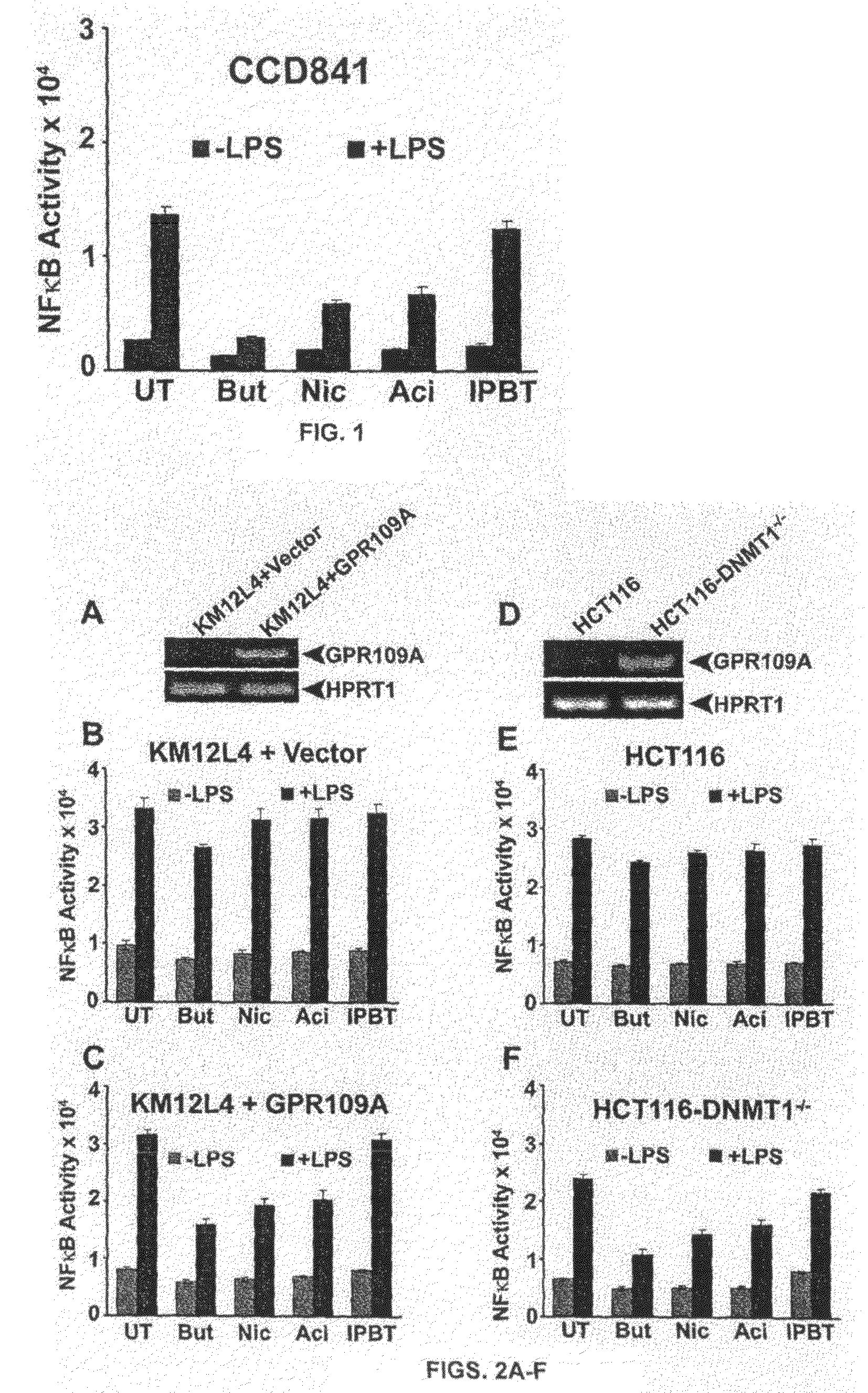
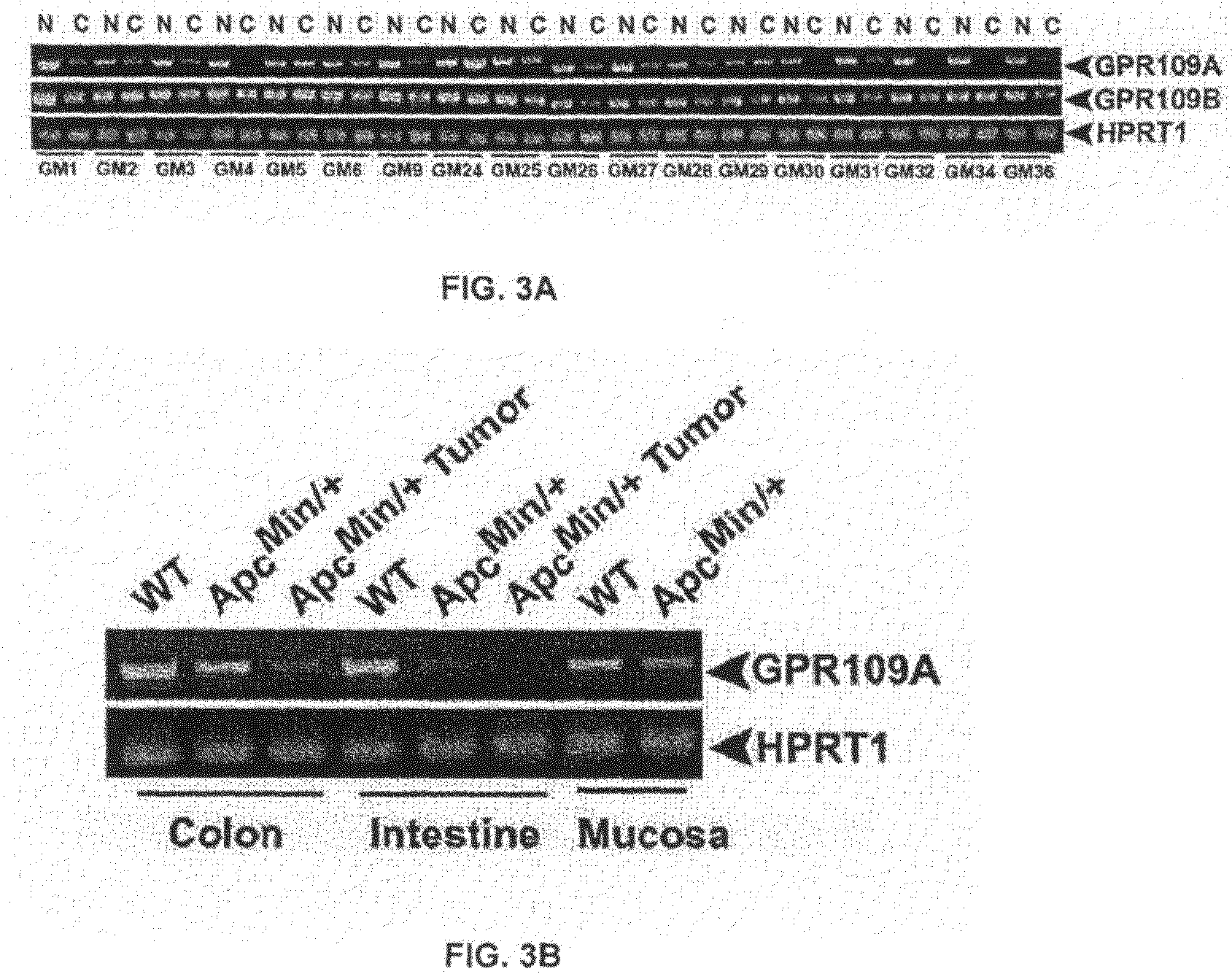
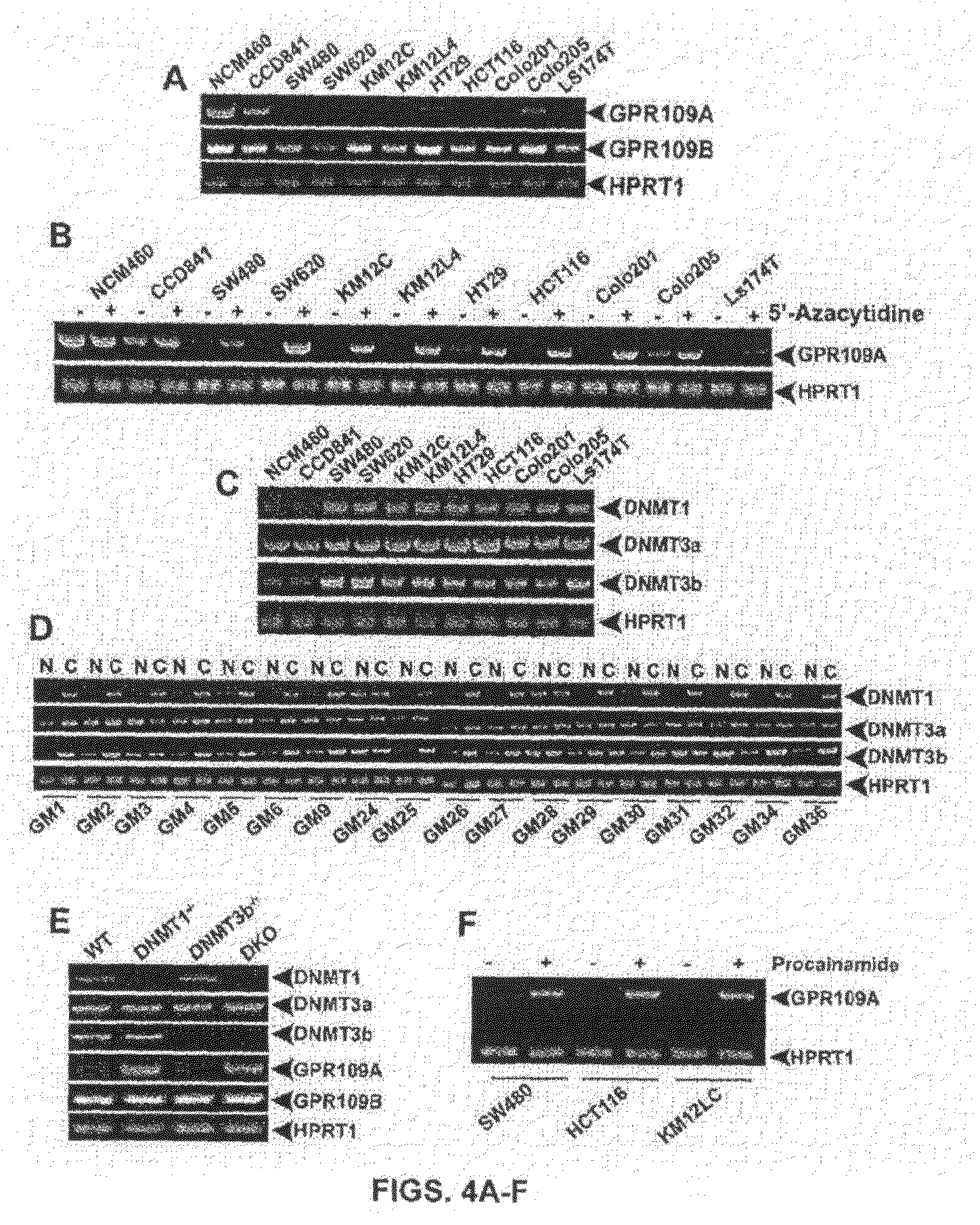
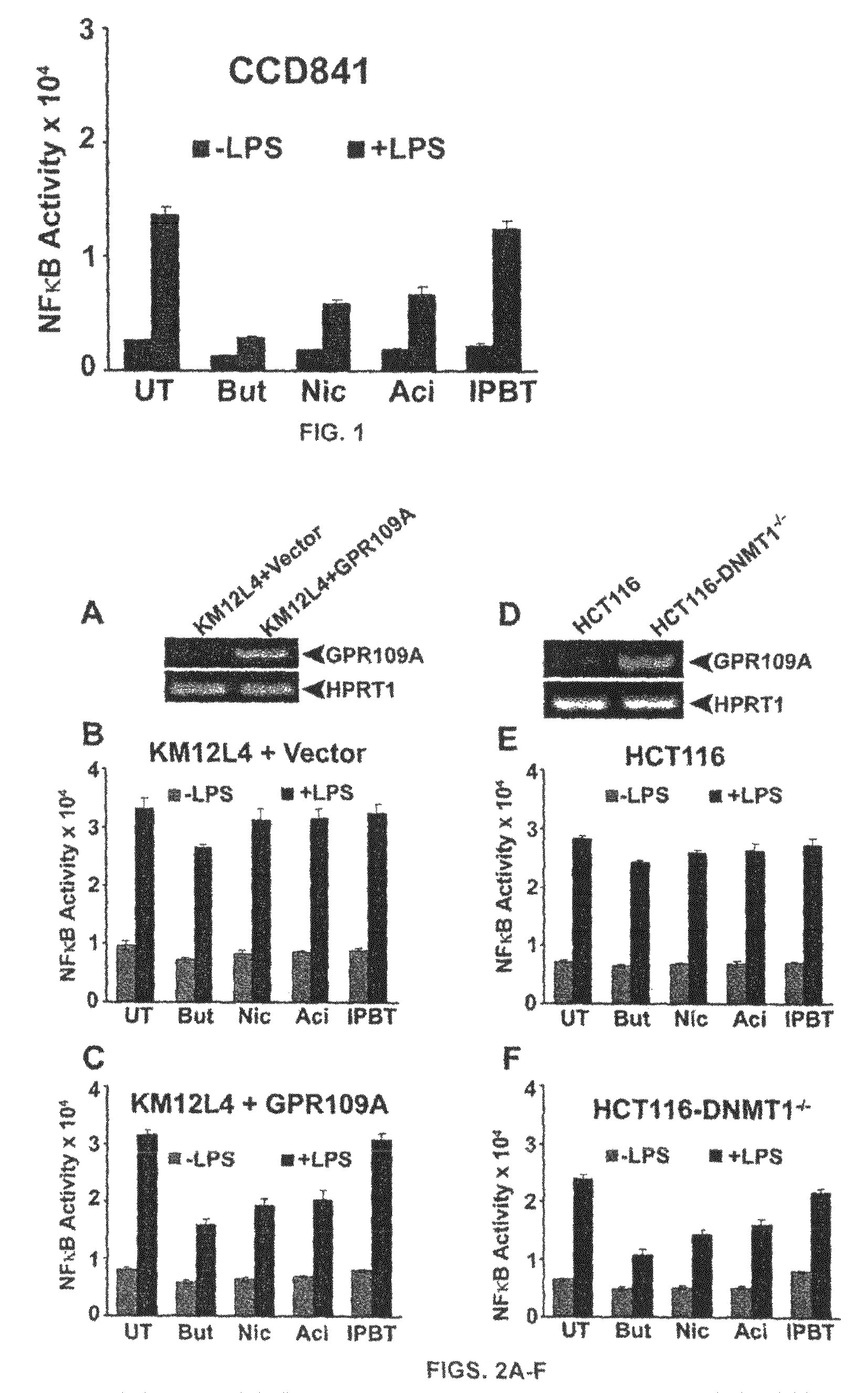
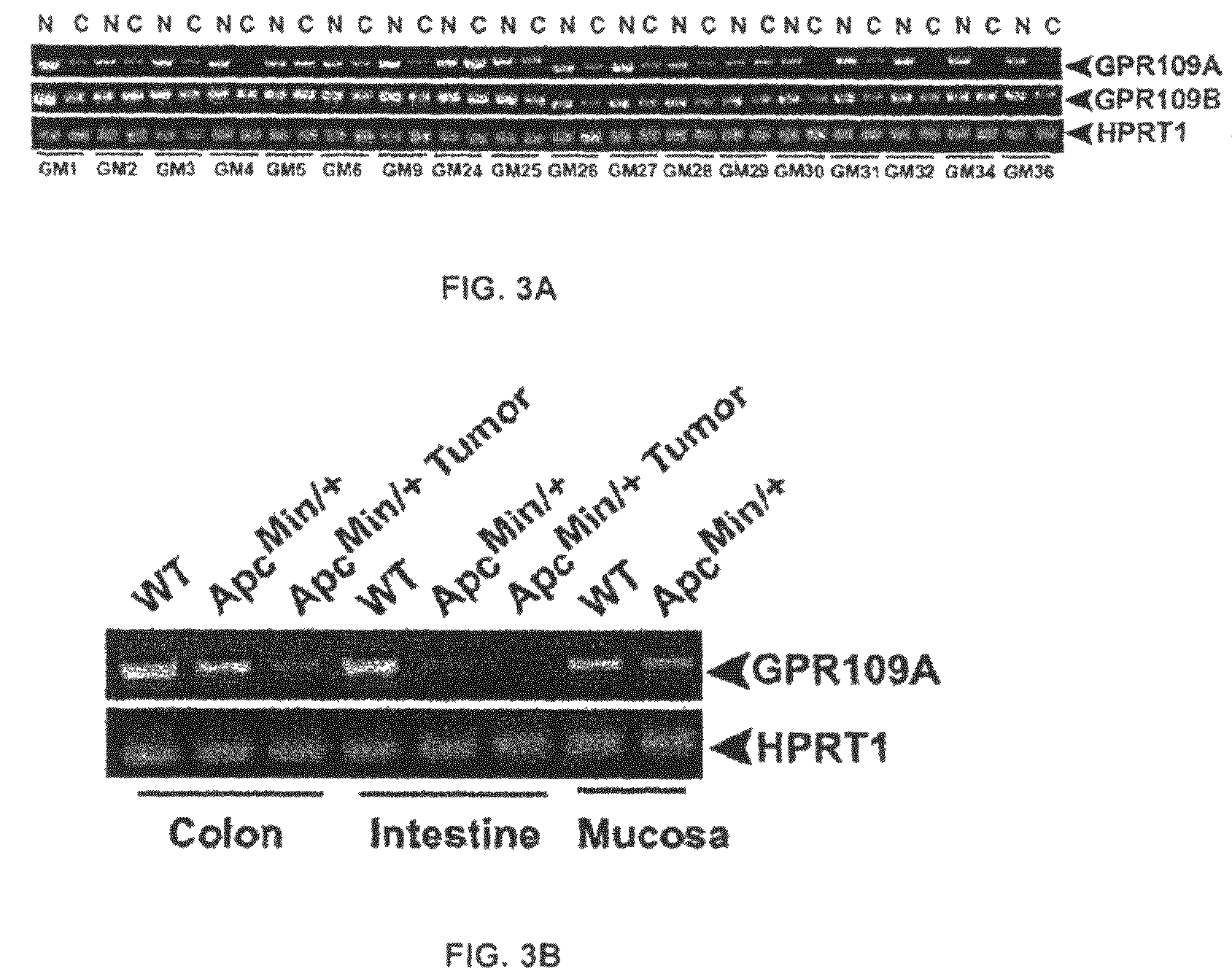
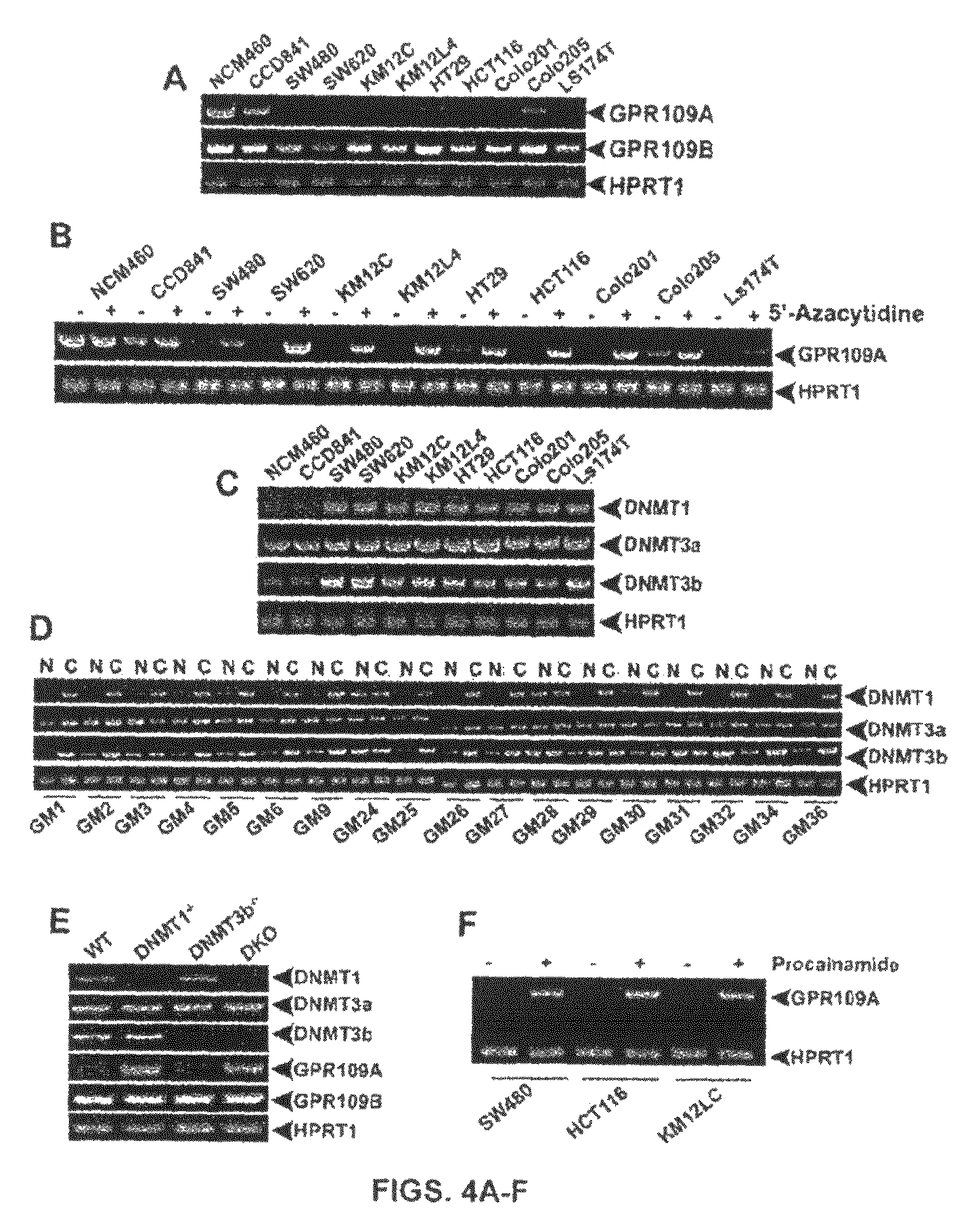
Compositions Comprising a GPR109 Ligand For Treating Disorders of the Digestive Tract and/or Cancer
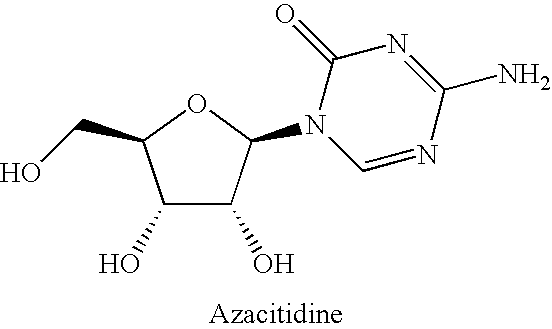
Pharmaceutical compositions containing an effective amount of a ligand for GPR109 to decrease intracellular cAMP levels of a subject in combination with an effective amount of a DNA methyl transferase inhibito to reduce or inhibit downregulation of GPR109 in the intestinal epithelial cells of the subject relative to a control are provided. It has been discovered that ligands for GPR109 can be used to treat one or more symptoms of cancer, inflammatory disorders, and diarrhea. Representative CPR 109 ligands include, but are not limited to butyrate, β-hydroxybutyrate, nicotinic acid, acifran, and octanoate. Suitable DNA methyl transferase inhibitors include 5-azacytidine, 5-aza-2′-deoxytidine, 1-β-D-arabinfαmosyl-5-azacytosine and dihydro-5-azacytidine. Typically, the compositions are formulated to achieve a GPR 109 ligand serum blood level of about 1 to about 1000 μM. The compositions are useful for the treatment of one or more symptoms of cancer. Preferred cancers that can be treated using the disclosed compositions include, but are not limited to colon cancer, breast cancer and leukemia. Methods for treating cancer, inflammatory disorders, and diarrhea are also provided.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
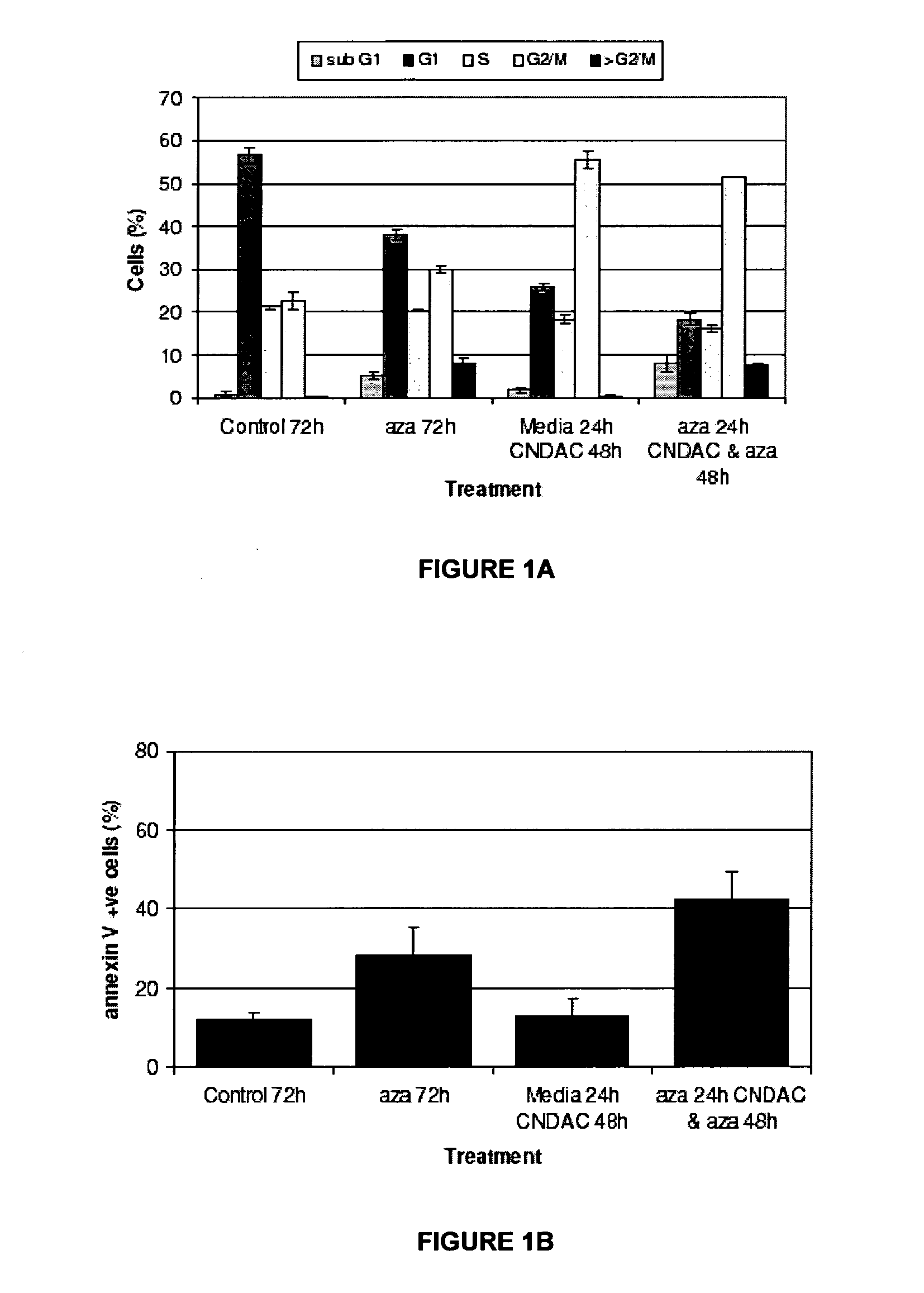
Combinations of sapacitabine or CNDAC with DNA methyltransferase inhibitors such as decitabine and procaine
InactiveUS8530445B2Good effectEase of preparation and detectabilityBiocideCarbohydrate active ingredientsProcaineSapacitabine
A first aspect of the invention relates to a combination comprising a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof. A second aspect of the invention relates to a pharmaceutical product comprising a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof, as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect of the invention relates to a method of treating a proliferative disorder, said method comprising simultaneously, sequentially or separately administering a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof, to a subject.
Owner:CYCLACEL
DNA methyl transferase inhibitors containing a zinc binding moiety
InactiveUS20080234355A1Effective for treating diseaseHigh activityBiocideOrganic chemistryDiseaseDNA Methyltransferase Inhibitor
The present invention relates to inhibitors of DNMT containing zinc moiety derivatives that have enhanced or unique properties as inhibitors of DNA methyl transferases (DNMT) and their use in the treatment of DNMT related diseases and disorders such as cancer. The said derivatives may further act as HDAC inhibitors.
Owner:CURIS INC
Pharmaceutical composition and uses thereof
InactiveCN103861107ABroad antigen spectrumComprehensive antigen spectrumOrganic active ingredientsPeptide/protein ingredientsProcaineDNA Methyltransferase Inhibitor
The invention discloses a pharmaceutical composition and uses thereof. The pharmaceutical composition comprises a DNA methyltransferase inhibitor and a histone deacetylase inhibitor. The invention also discloses new uses of the pharmaceutical composition. The pharmaceutical composition is used to treat tumor cells together to obtain an exosomes tumor vaccine. The invention also discloses a method for preparing a tumor vaccine by using the pharmaceutical composition. The method comprises the following steps of using procaine and MS-275 to treat the tumor cells, and separating and purifying exosomes secreted by the tumor cells. The pharmaceutical composition disclosed by the invention improves the therapeutic effect of the exosomes tumor vaccine, and has an important clinical application value.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
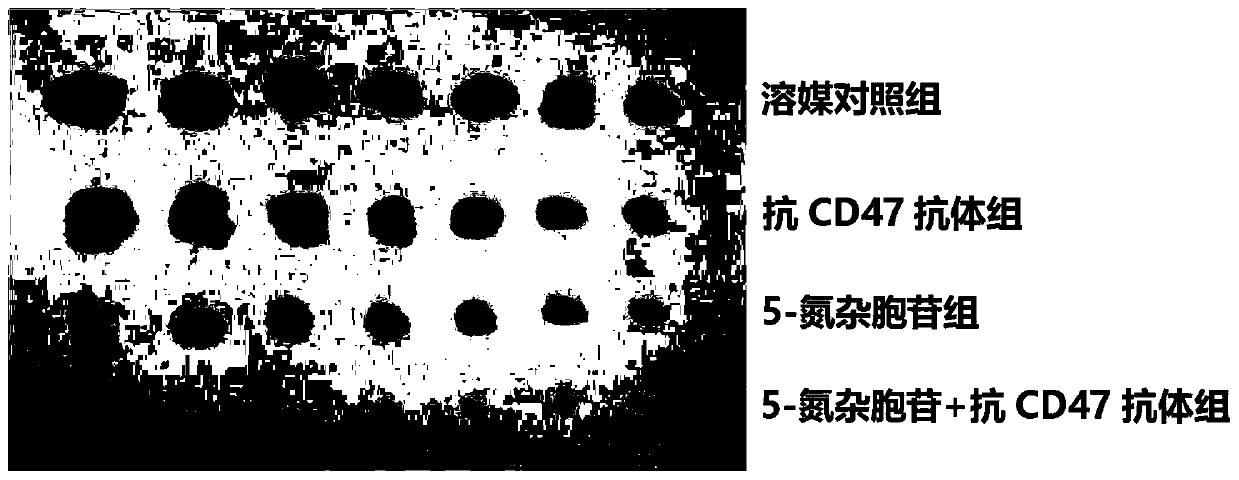
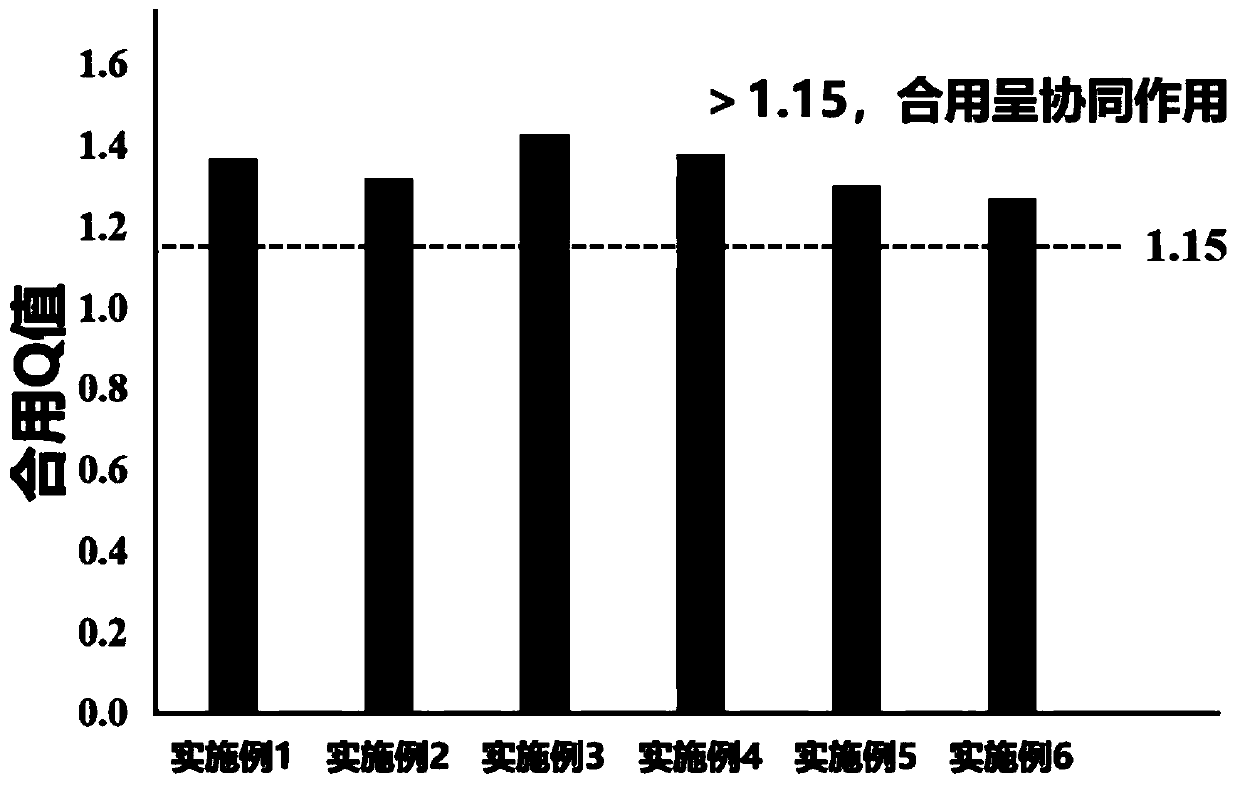
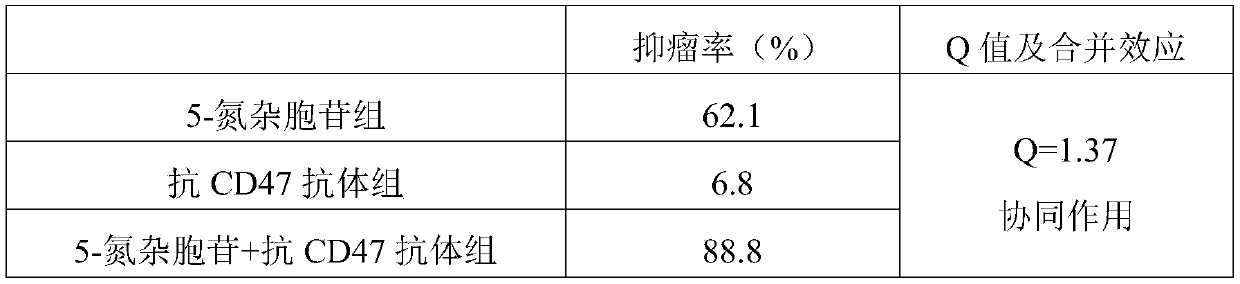
Medical application of DNA methyltransferase inhibitor in combination with anti-CD47 antibody for preparing antineoplastic drugs
ActiveCN109224079AImprove anti-tumor efficacyReduce financial burdenOrganic active ingredientsAntibody ingredientsPhagocyteDNA Methyltransferase Inhibitor
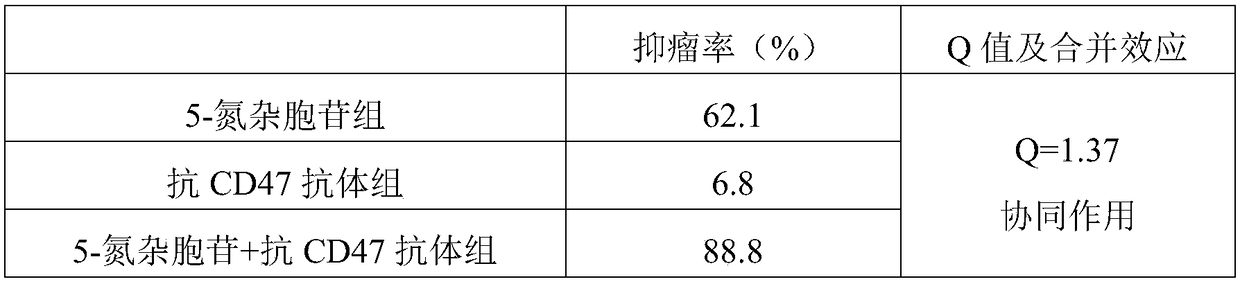
The invention discloses a medical application of DNA methyltransferase inhibitor combined with anti-CD47 antibody for preparing antineoplastic drugs, which will be appreciated by those skilled in theart, CD47 in physiological state can maintain the immune tolerance of the body's own cells, and in pathological state, it can be overexpressed in a variety of hematological tumors and solid tumors, through the combination of phagocyte ligands to initiate a series of inhibitory signal transduction to avoid phagocytosis, and inhibit the presentation of tumor antigens phagocytes, anti-CD47 antibody has anti-tumor effect. However, anti-CD47 antibodies alone are expensive and have limited efficacy. DNA methyltransferase inhibitors play an anti-tumor role by restoring the activity of tumor suppressor genes. As that DNA methyltransferase inhibitor and the anti-CD47 antibody combine to exhibit obvious synergistic effect on anti-tumor, the DNA methyltransferase inhibitor and the anti-CD47 antibodycan be combined to be use for anti-tumor, thereby enhancing the anti-tumor effect of the anti-CD47 antibody and reducing the economic burden of the patient.
Owner:CHINA PHARM UNIV
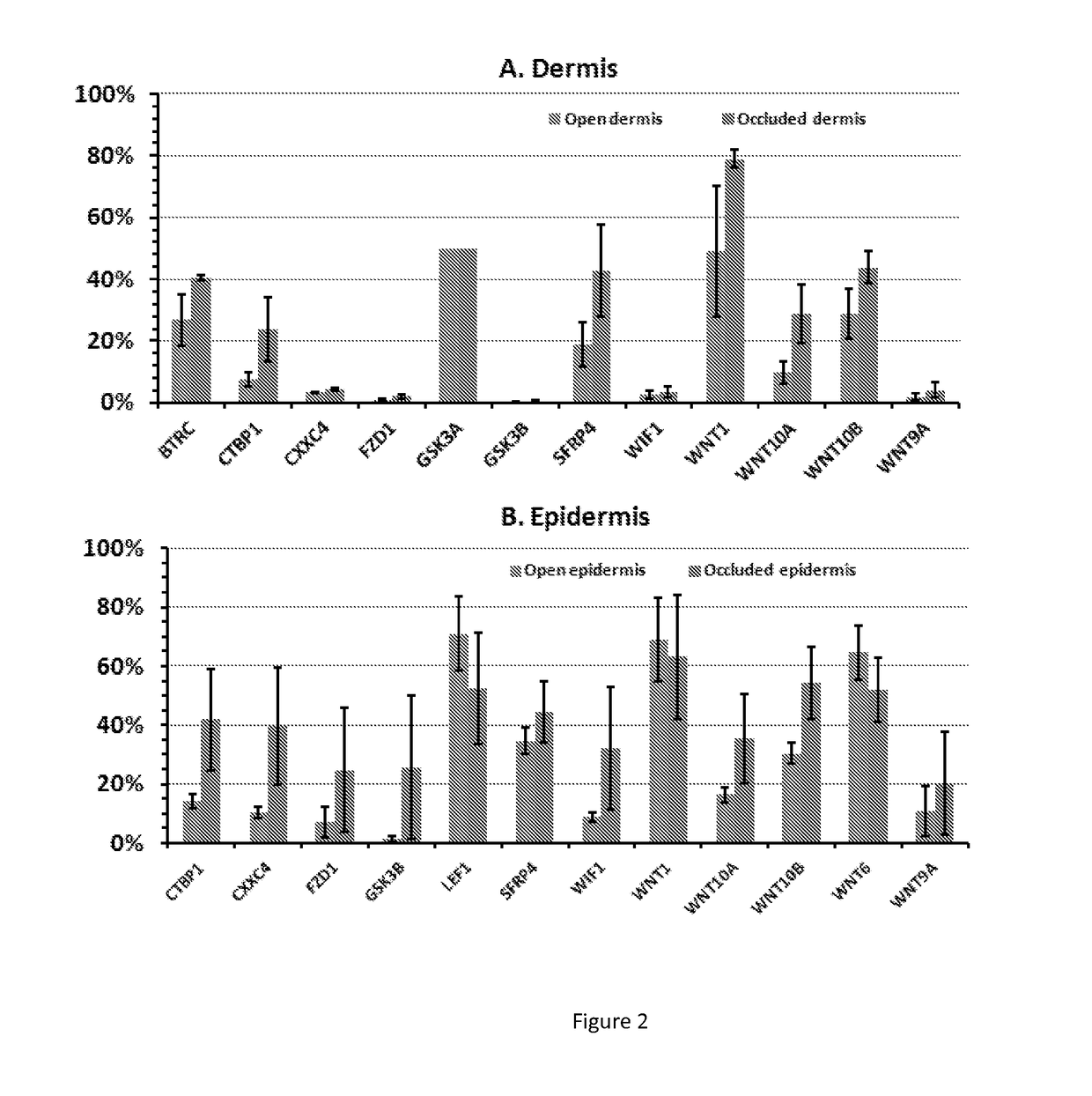
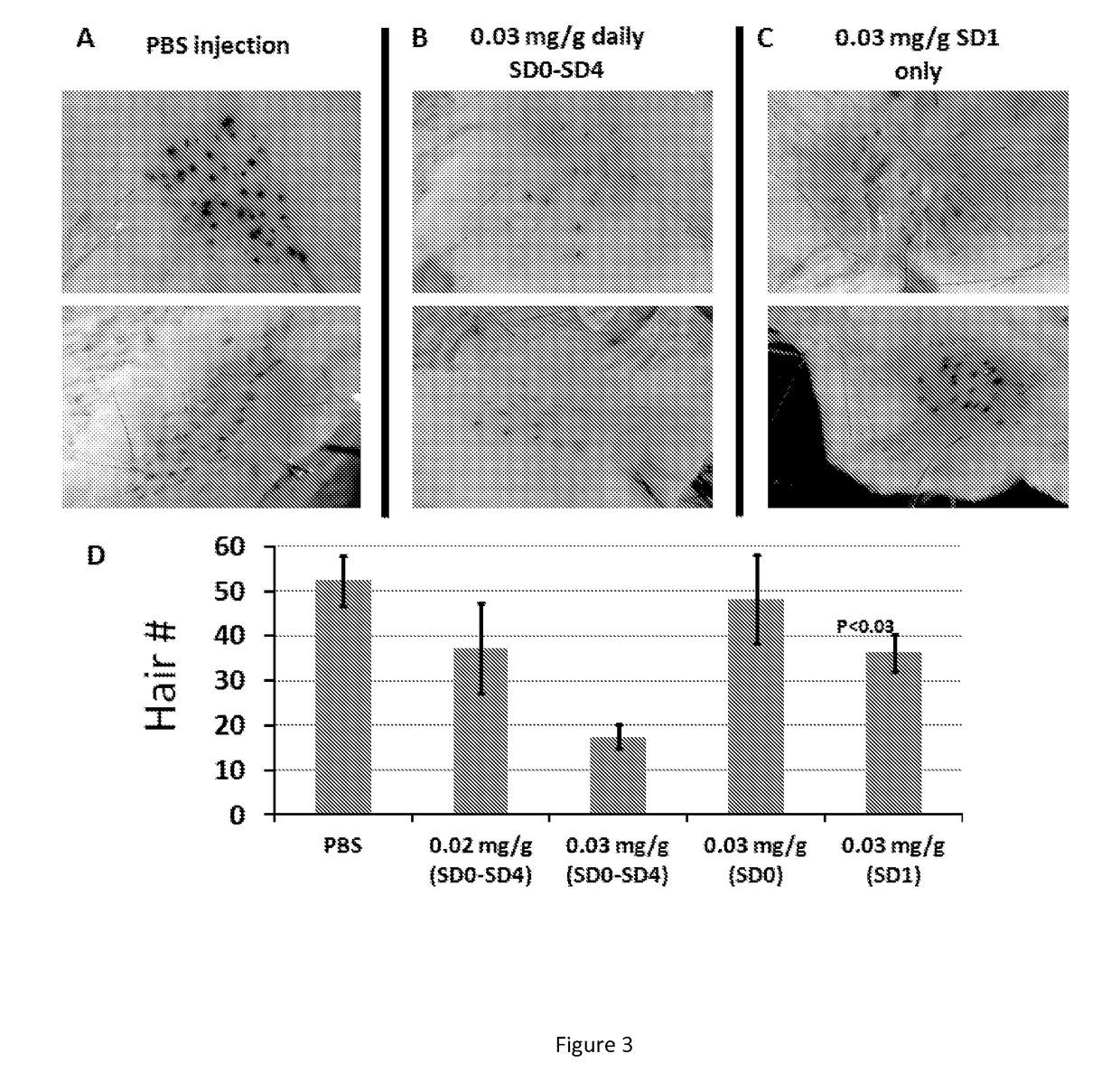
5-aza-2'- deoxycytidine and methods of use thereof for promoting wound healing and regeneration
ActiveUS20170209475A1Promote wound healingCosmetic preparationsOrganic active ingredientsTransferase inhibitorDNA Methyltransferase Inhibitor
This invention is directed to compositions for promoting wound healing and regeneration and methods of use thereof. In one aspect, methods for promoting wound healing in a subject are provided, the methods comprising: administering a therapeutically effective amount of a DNA methyl transferase inhibitors (DNMT) to the subject. In another aspect, methods for reducing scarring during healing of a skin wound are provided, the methods comprising: administering a therapeutically effective amount of a DNMT inhibitor.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
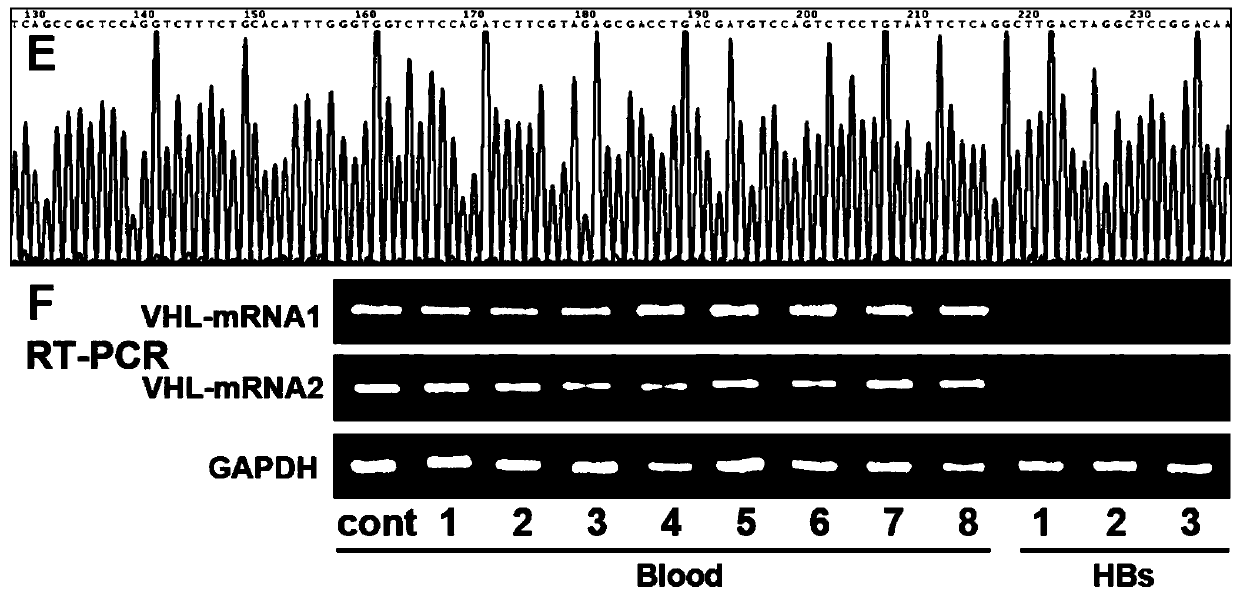
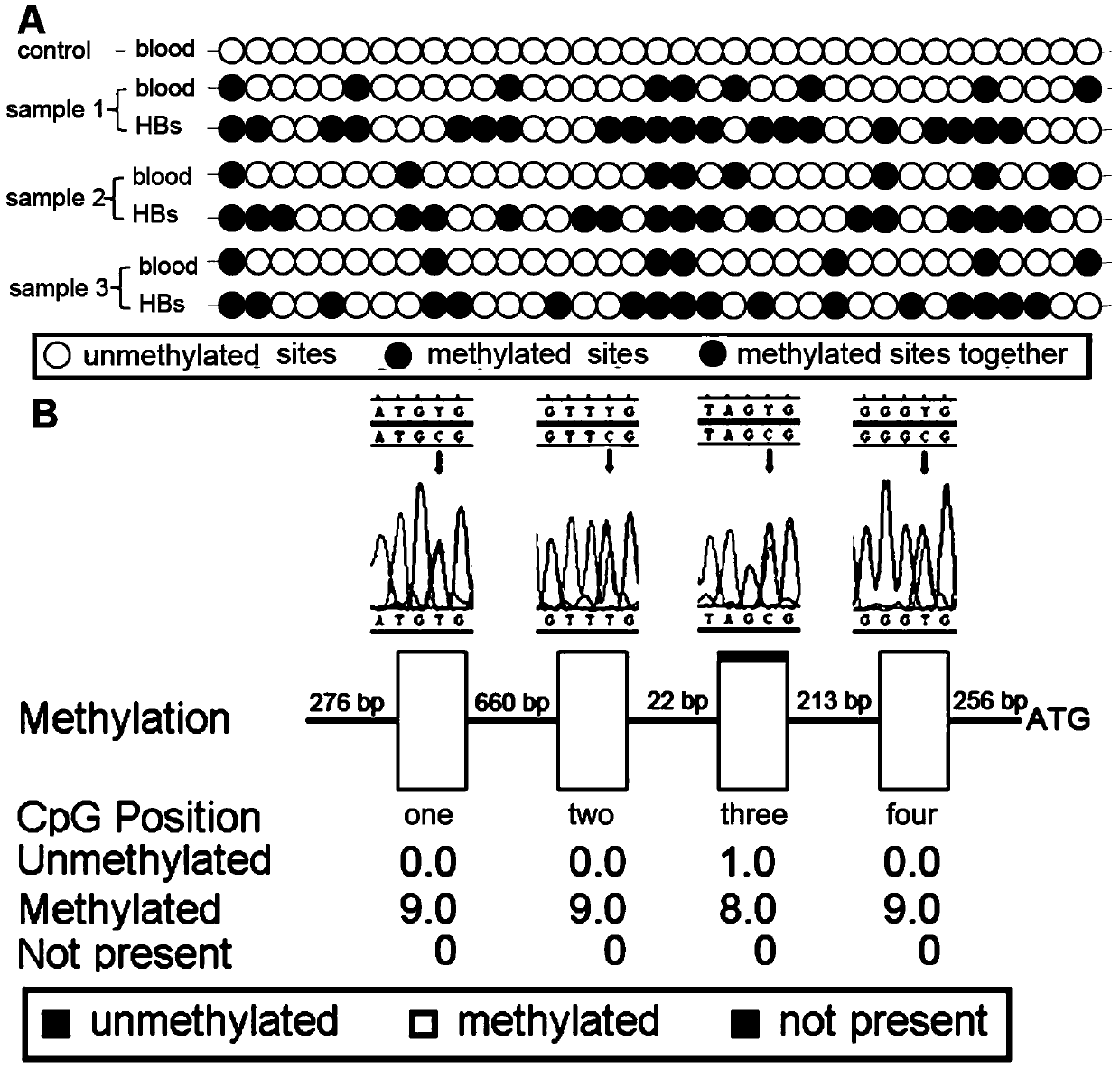
Medicine used for treating genetic imprinting subtype hemangioblastoma
InactiveCN110075114AGood treatment effectGood prevention effectOrganic active ingredientsAntineoplastic agentsHigh risk populationsMedicine
The invention provides a medicine used for treating genetic imprinting subtype hemangioblastoma. The medicine used for treating genetic imprinting subtype hemangioblastoma is characterized by being prepared from a DNA methyltransferase inhibitor. Through the DNA methyltransferase inhibitor, demethylation treatment can be conducted on genetic imprinting subtype hemangioblastoma patients and geneticimprinting subtype hemangioblastoma high-risk populations, so that VHL mRNA is re-expressed, and excellent treatment and prevention effects are achieved.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Compositions comprising a GPR109 ligand for treating disorders of the digestive tract and/or cancer
Pharmaceutical compositions containing an effective amount of a ligand for GPR109 to decrease intracellular cAMP levels of a subject in combination with an effective amount of a DNA methyl transferase inhibito to reduce or inhibit downregulation of GPR109 in the intestinal epithelial cells of the subject relative to a control are provided. It has been discovered that ligands for GPR109 can be used to treat one or more symptoms of cancer, inflammatory disorders, and diarrhea. Representative CPR109 ligands include, but are not limited to butyrate, β-hydroxybutyrate, nicotinic acid, acifran, and octanoate. Suitable DNA methyl transferase inhibitors include 5-azacytidine, 5-aza-2′-deoxytidine, 1-β-D-arabinfarnosyl-5-azacytosine and dihydro-5-azacytidine. Typically, the compositions are formulated to achieve a GPR109 ligand serum blood level of about 1 to about 1000 μM. The compositions are useful for the treatment of one or more symptoms of cancer. Preferred cancers that can be treated using the disclosed compositions include, but are not limited to colon cancer, breast cancer and leukemia. Methods for treating cancer, inflammatory disorders, and diarrhea are also provided.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
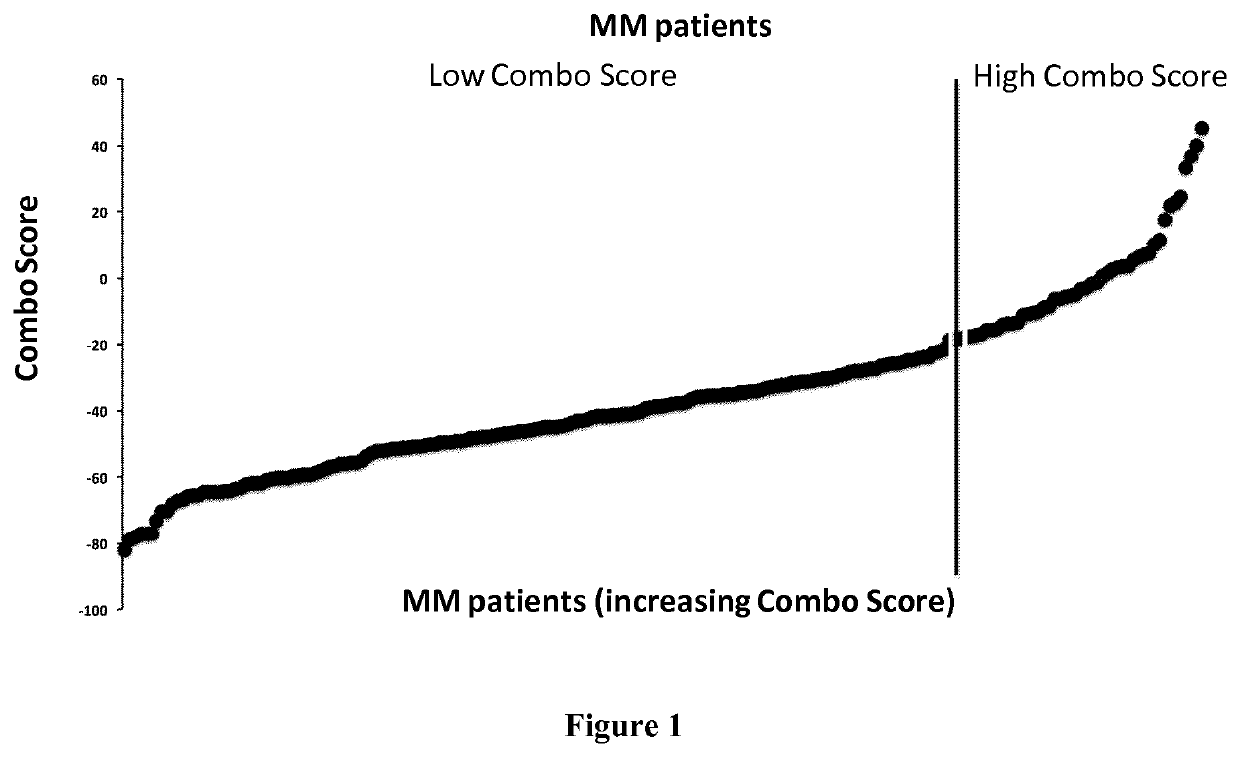
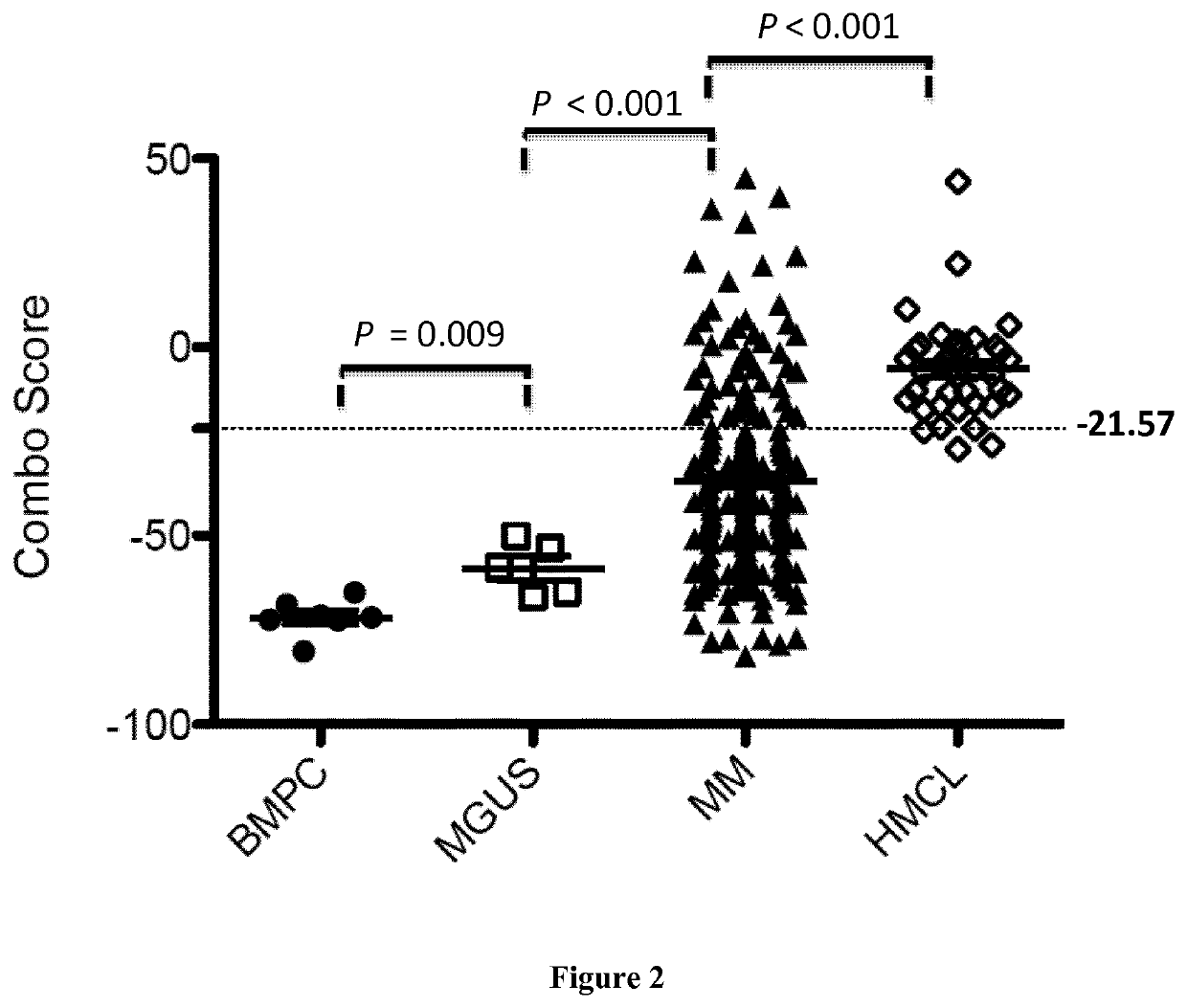
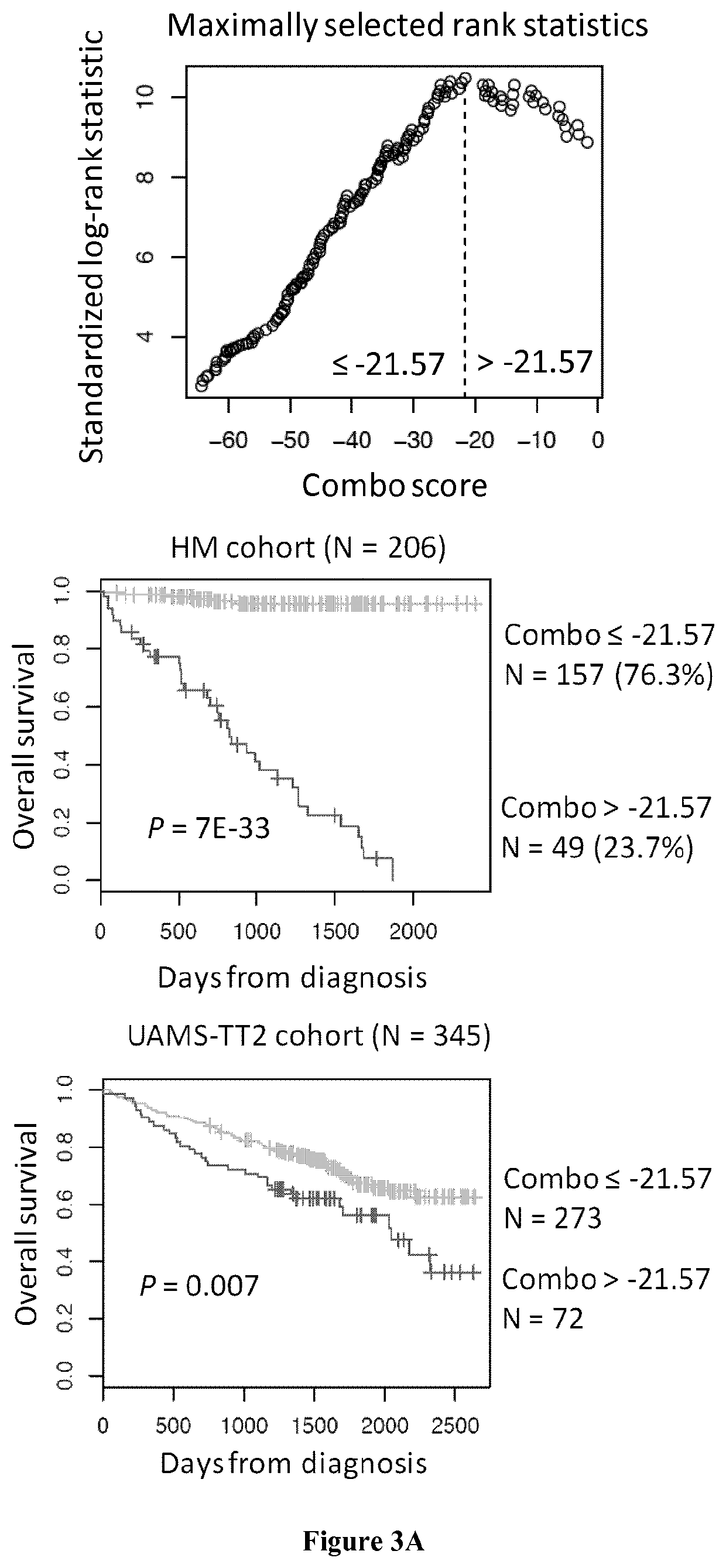
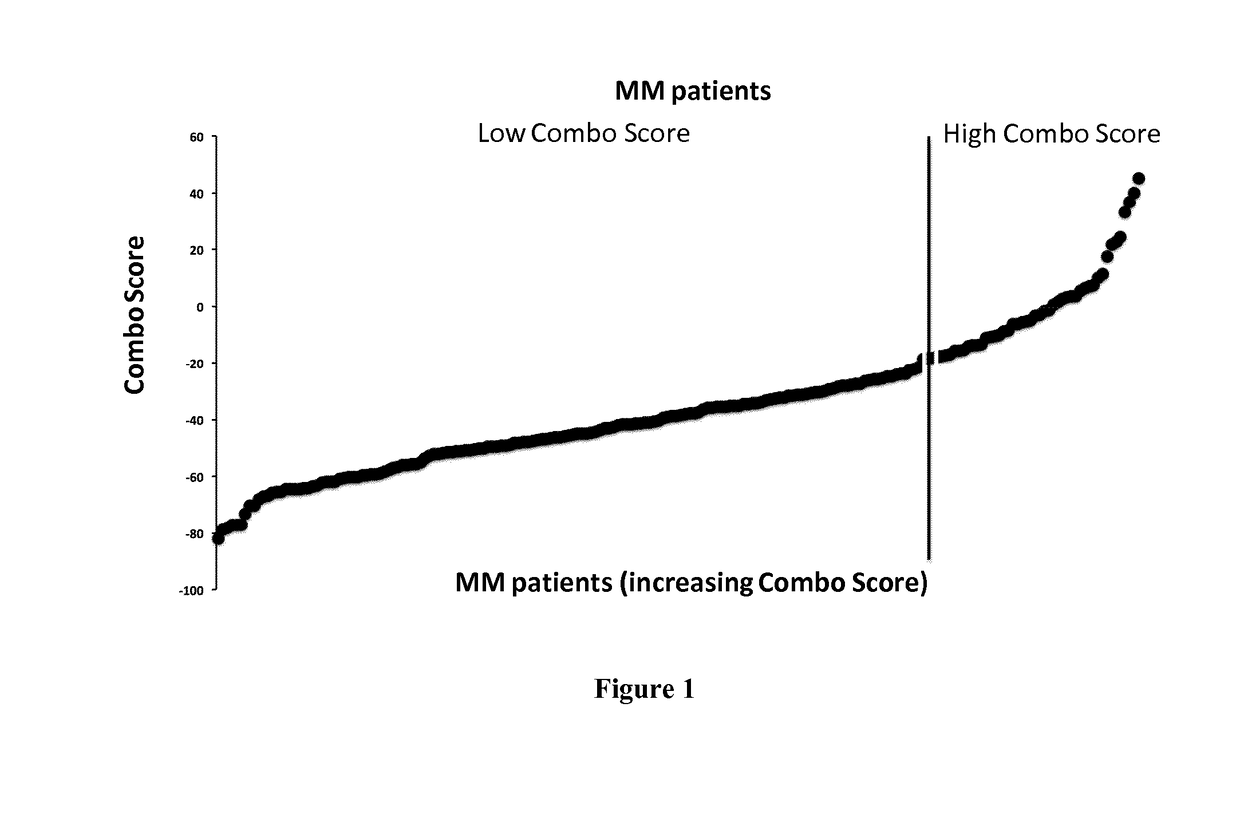
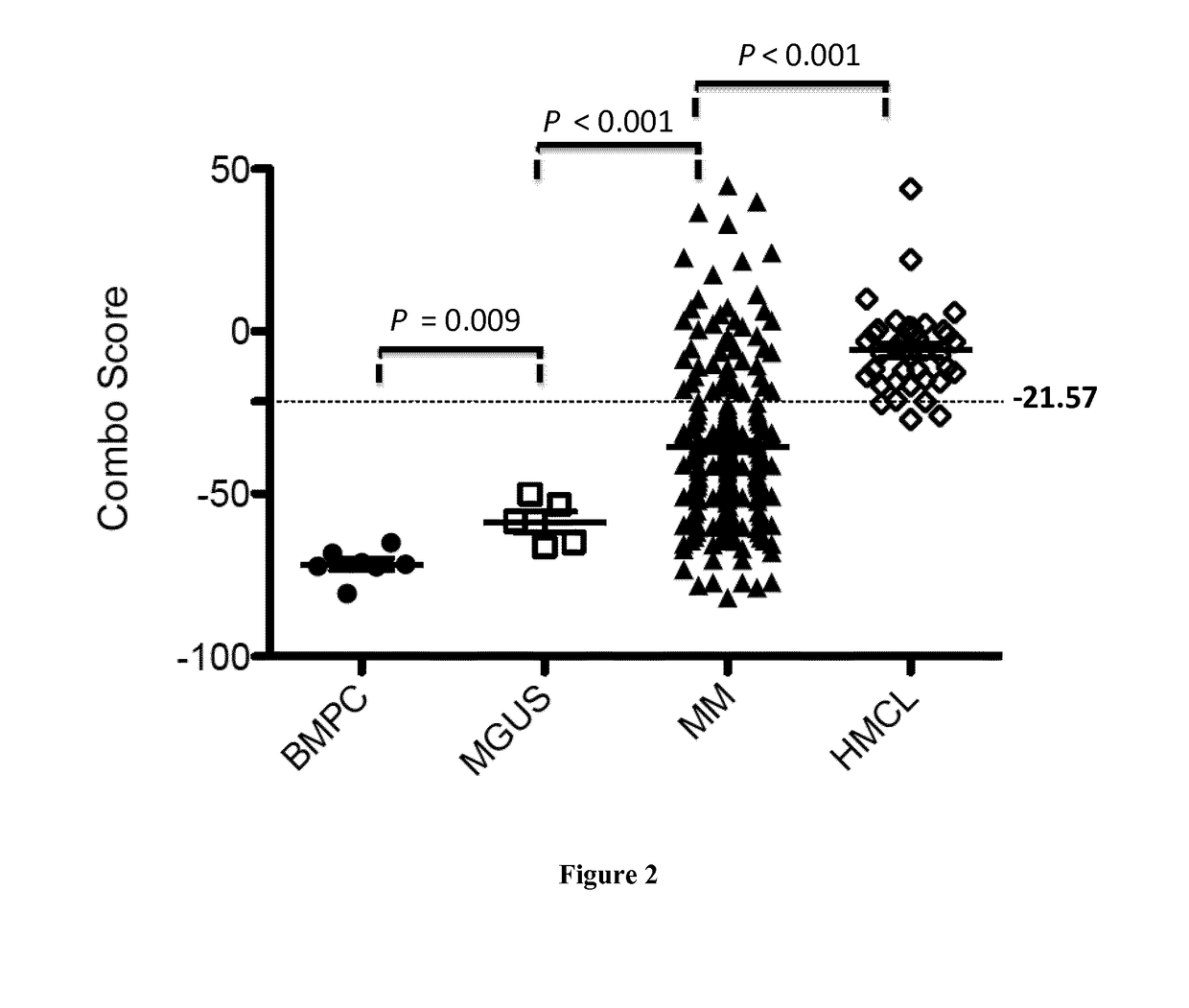
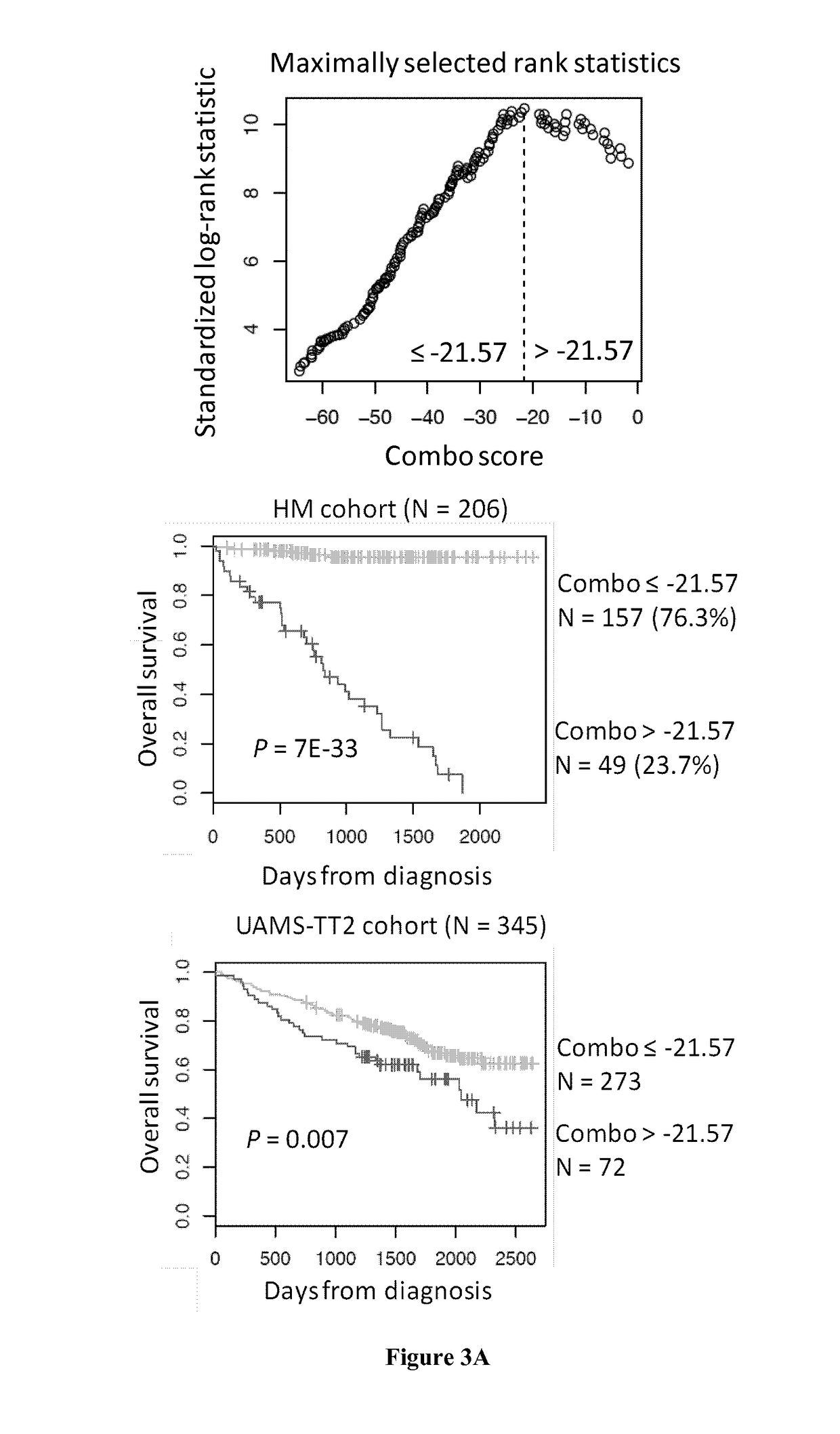
Methods for predicting response to HDACi/DNMTi combination in multiple myeloma
ActiveUS10662481B2Organic active ingredientsHealth-index calculationTransferase inhibitorDNA Methyltransferase Inhibitor
The present invention relates to a method of testing whether a patient suffering from multiple myeloma will respond or not to a combination treatment consisting of at least one histone deacetylase inhibitor (HDACi) with at least one DNA methyltransferase inhibitors (DNMTi) comprising: i) determining the expression level (ELi) of several genes G1-Gn selected from table A in a biological sample obtained from said patient ii) comparing the expression level (ELi) determined at step i) with a predetermined reference level (ELRi) iii) calculating the HADMS score trough the following formula (I) wherein βi represent the regression β coefficient reference value for the gene Gi and Ci=1 if the expression of the gene Gi (ELi) is higher than the predetermined reference level (ELRi) or Ci=−1 if the expression of the gene (ELi) is lower than or equal to the predetermined reference level (ELRi) iv) comparing the score HADMS determined at step iii) with a predetermined reference value HADMSR v) and concluding that the patient will respond to the combination treatment when the HADMS score is higher than the predetermined reference value HADMSR or concluding that the patient will not respond to the combination treatment when the HADMS score is lower than the predetermined reference value HADMSR.HADMS=∑i=1nβi×Ci(I)
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Methods of treating neurodegenerative disorders comprising DNA methyltransferase inhibitors
ActiveUS10842807B2Lower Level RequirementsReduce neurotoxicityOrganic active ingredientsNervous disorderDNA Methyltransferase InhibitorNeuro-degenerative disease
The present disclosure provides a method of treating a neurodegenerative disorder, the method comprising administering a DNA methyltransferase inhibitor.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Methods for predicting response to hdaci/dnmti combination in multiple myeloma
ActiveUS20170096710A1Organic active ingredientsHealth-index calculationDNA Methyltransferase InhibitorGene
The present invention relates to a method of testing whether a patient suffering from multiple myeloma will respond or not to a combination treatment consisting of at least one histone deacetylase inhibitor (HDACi) with at least one DNA methyltransferase inhibitors (DNMTi) comprising: i) determining the expression level (ELi) of several genes G1-Gn selected from table A in a biological sample obtained from said patient ii) comparing the expression level (ELi) determined at step i) with a predetermined reference level (ELRi) iii) calculating the HADMS score trough the following formula (I) wherein βi represent the regression β coefficient reference value for the gene Gi and Ci=1 if the expression of the gene Gi (ELi) is higher than the predetermined reference level (ELRi) or Ci=−1 if the expression of the gene (ELi) is lower than or equal to the predetermined reference level (ELRi) iv) comparing the score HADMS determined at step iii) with a predetermined reference value HADMSR v) and concluding that the patient will respond to the combination treatment when the HADMS score is higher than the predetermined reference value HADMSR or concluding that the patient will not respond to the combination treatment when the HADMS score is lower than the predetermined reference value HADMSR.HADMS=∑i=1nβi×Ci(I)
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
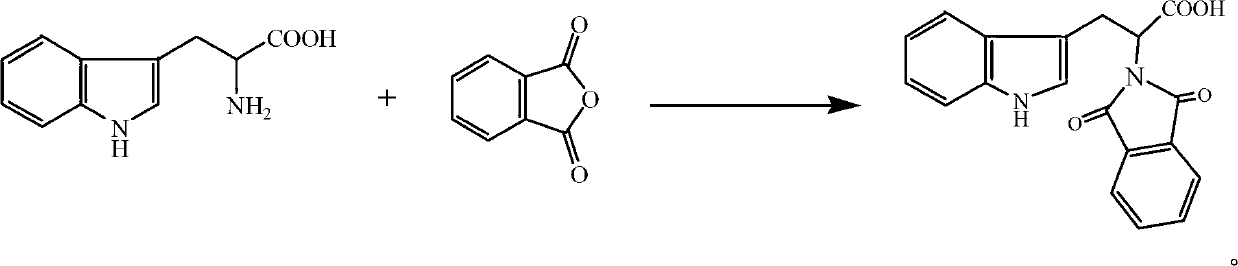
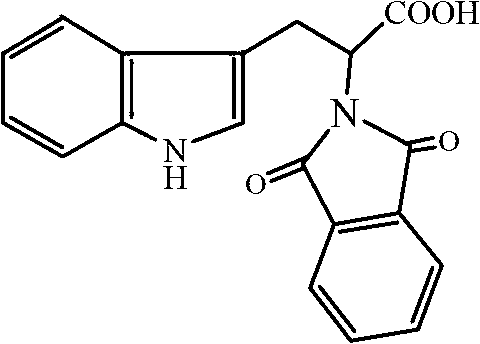
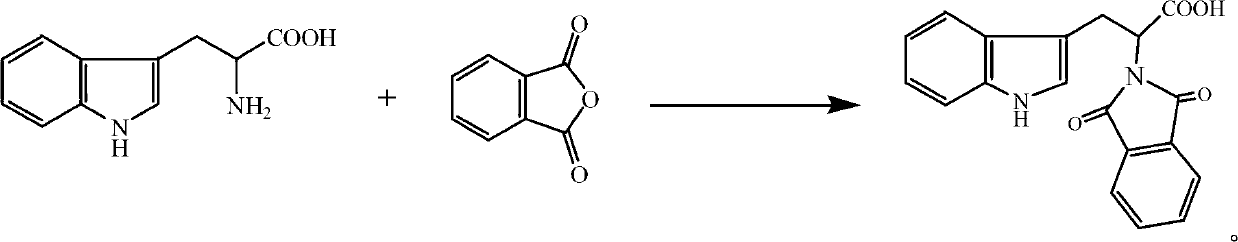
Synthetic method of deoxyribonucleic acid (DNA) methyl transferase inhibitor
InactiveCN102863372ARaw materials are cheap and easy to getThe synthetic route is simpleOrganic chemistryPropanoic acidReaction rate
The invention discloses a synthetic method of a DNA methyl transferase inhibitor. The method includes that tryptophan and phthalic anhydride serve as raw materials, and acylation reaction is conducted to obtain a compound 2-(1,3-)-dioxo-1,3-dihydro-2H-isobenzazole)-3-(1H- isobenzazole) propionic acid. According to the method, the raw materials are cheap and available, a synthetic route is simple, a catalyst is added into a reaction system, acid generated during reaction is neutralized, and the reaction rate is improved; and besides, a dehydrating agent is added to the reaction system, the reaction temperature is reduced greatly, and the reaction rate is accelerated at the same time. The 2-(1,3-)-dioxo-1,3-dihydro-2H-isobenzazole)-3-(1H- isobenzazole) propionic acid is synthetized by using the method, and the yield rate can exceed 80%.
Owner:SHAANXI XUEQIAN NORMAL UNIV
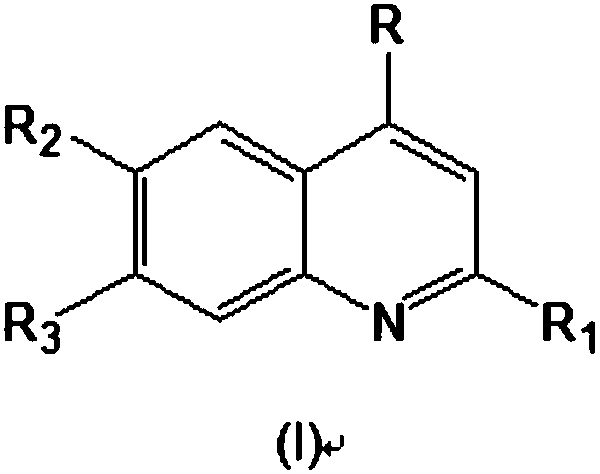
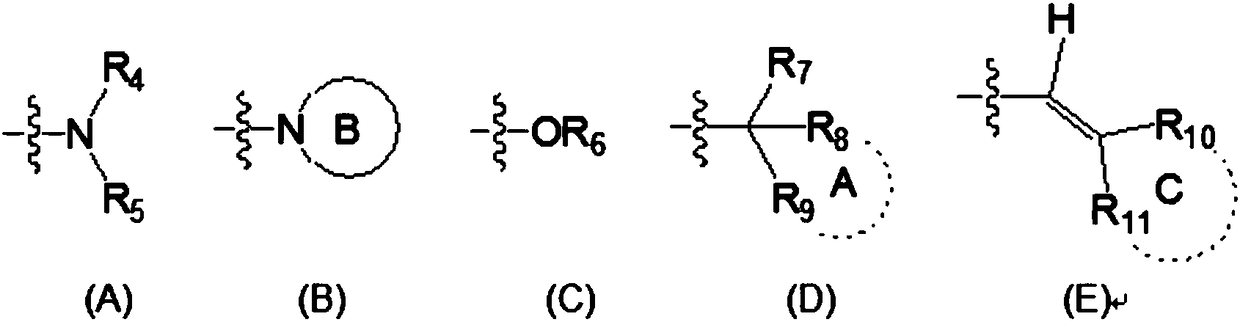
2,4,6,7-tetrasubstituted quinoline compounds as inhibitors of DNA methyltransferases
InactiveCN108602798AEffective treatmentOrganic active ingredientsOrganic chemistryDNA Methyltransferase InhibitorImmunomodulations
The invention relates to the compounds of formula (I), or their pharmaceutically or veterinary acceptable salts, or their stereoisomers or mixtures of stereoisomers, wherein R is a radical selected from the group consisting of formula (A), formula (B), formula (C), formula (D), and formula (E), and R1, R2, and R3 are as defined herein, which are inhibitors of one or more DNMTs selected from the group consisting of DNMT1, DNMT3A and DNMT3B. It also relates to pharmaceutical or veterinary compositions containing them, and to their use in medicine, in particular in the treatment and / or preventionof cancer, fibrosis and / or immunomodulation.
Owner:FUNDACION PARA LA INVESTIGACION MEDICA APLICADA
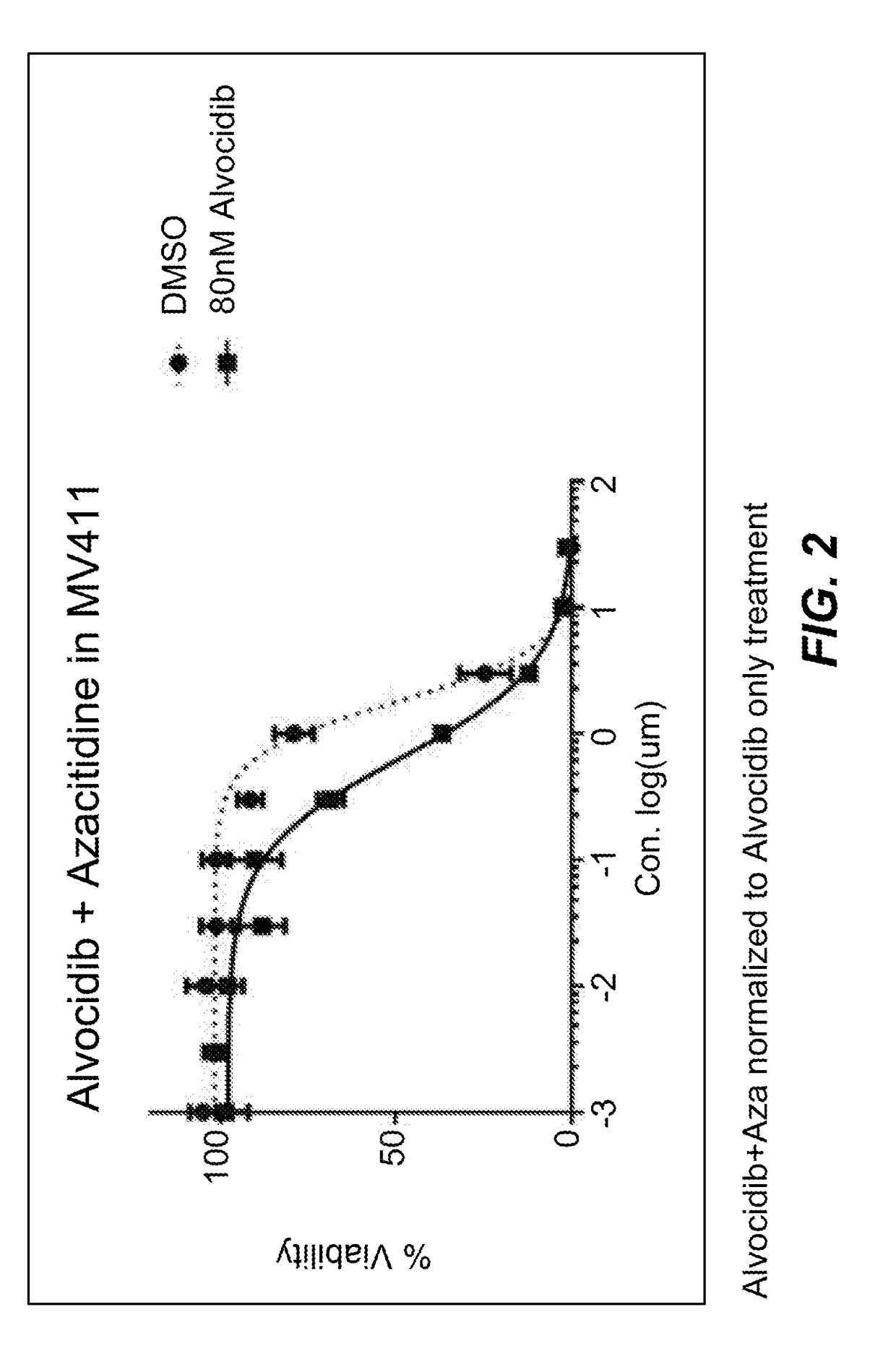
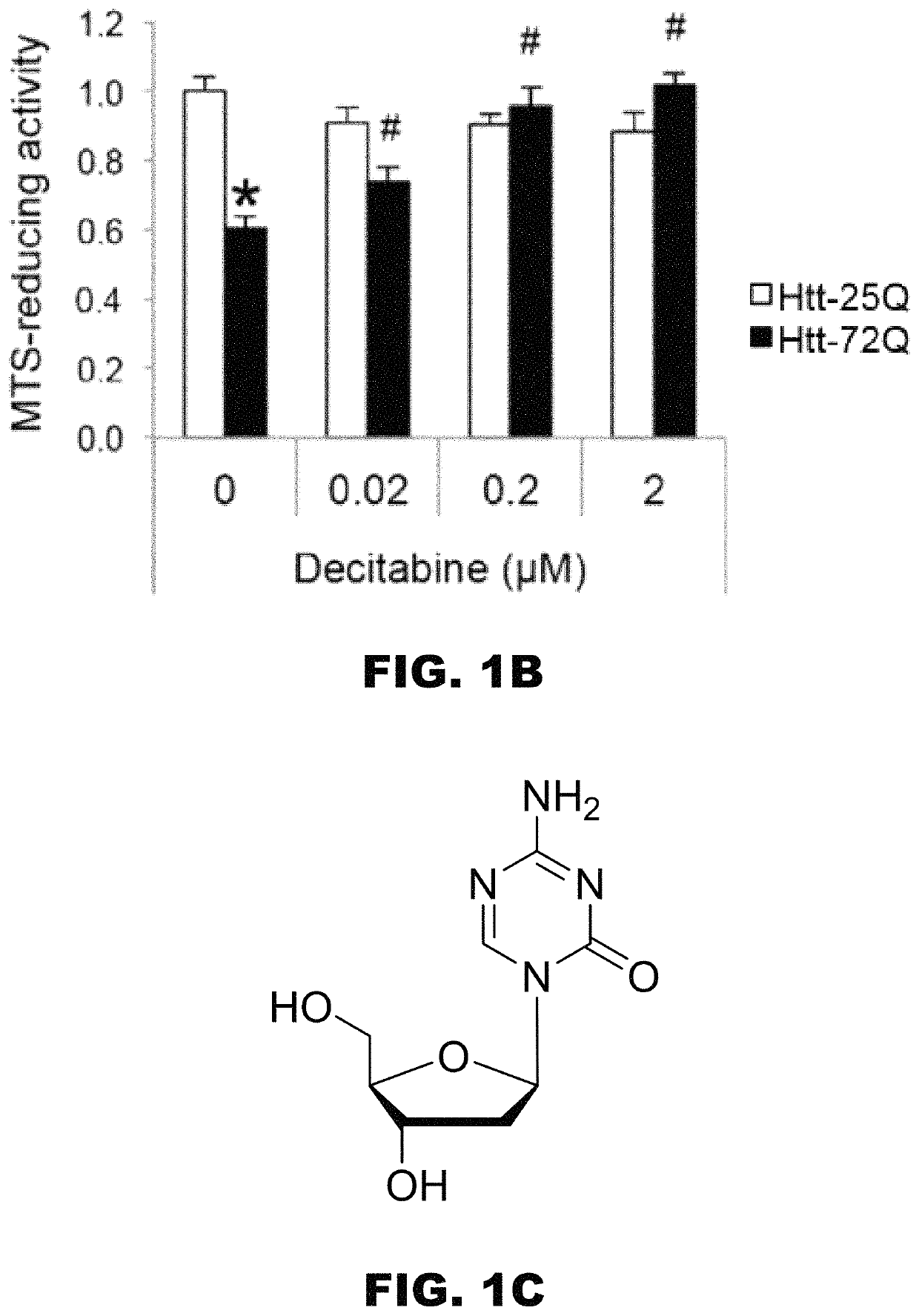
Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy
InactiveUS20130102556A1BiocideCarbohydrate active ingredientsDNA Methyltransferase InhibitorCutaneous T-cell lymphoma
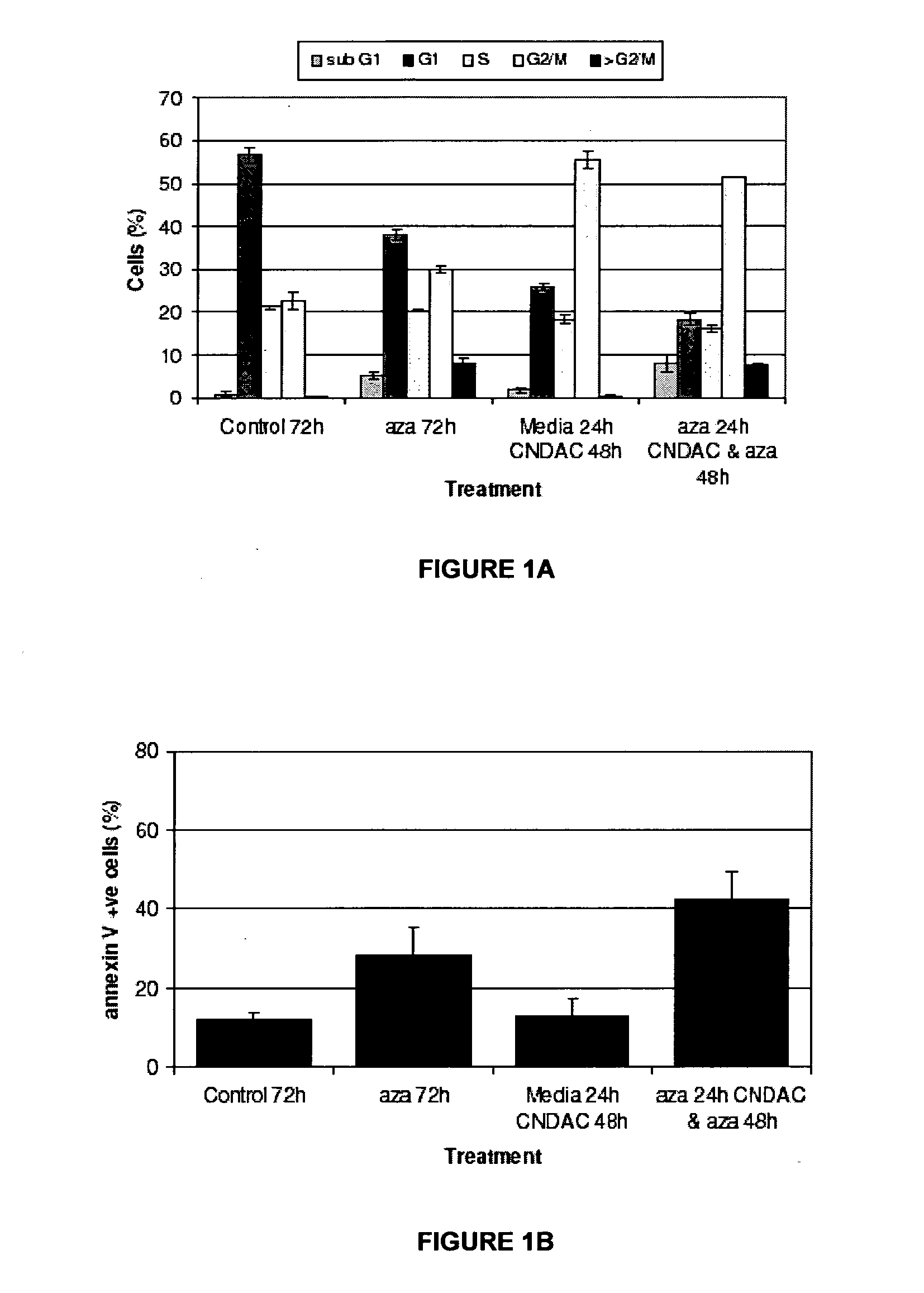
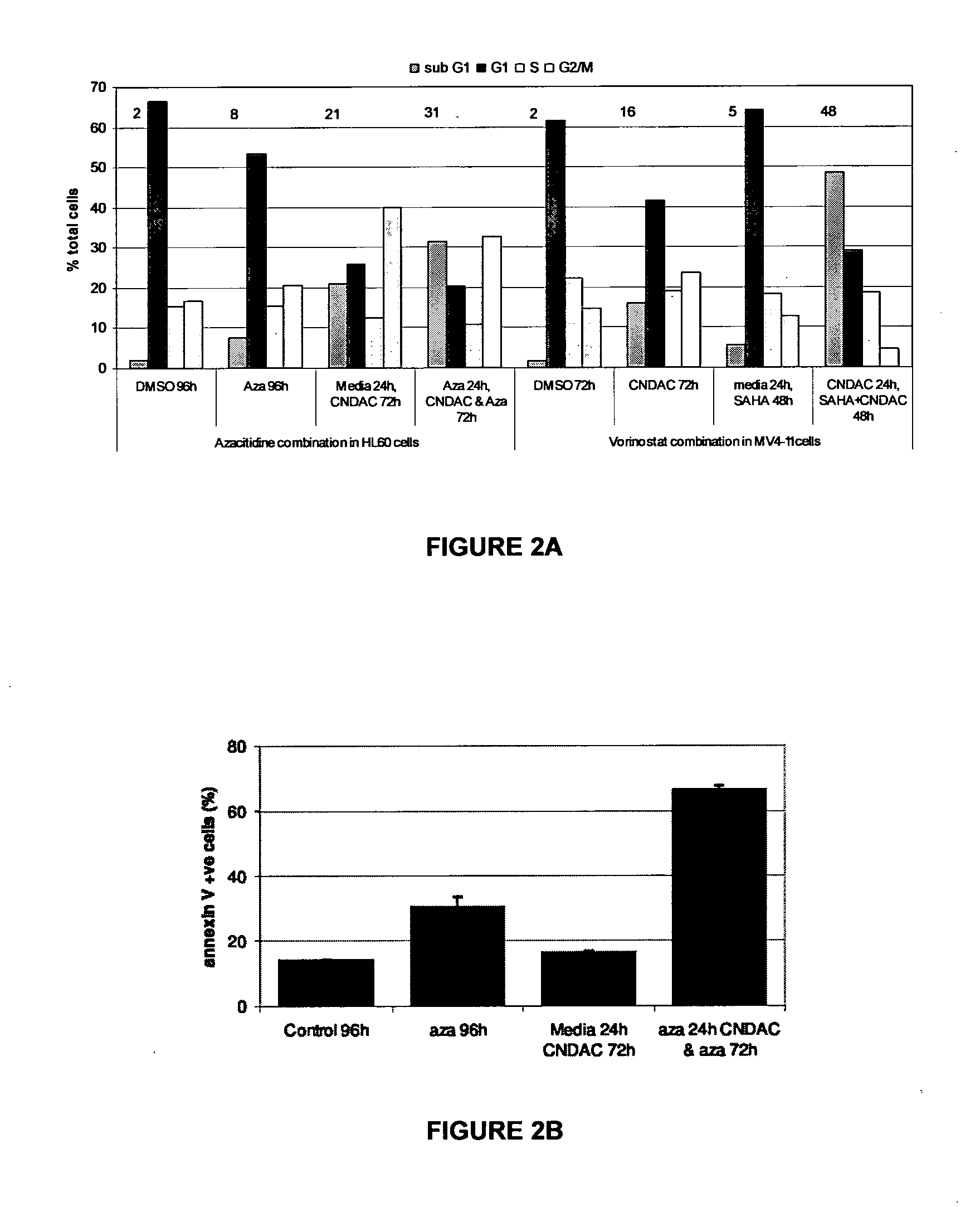
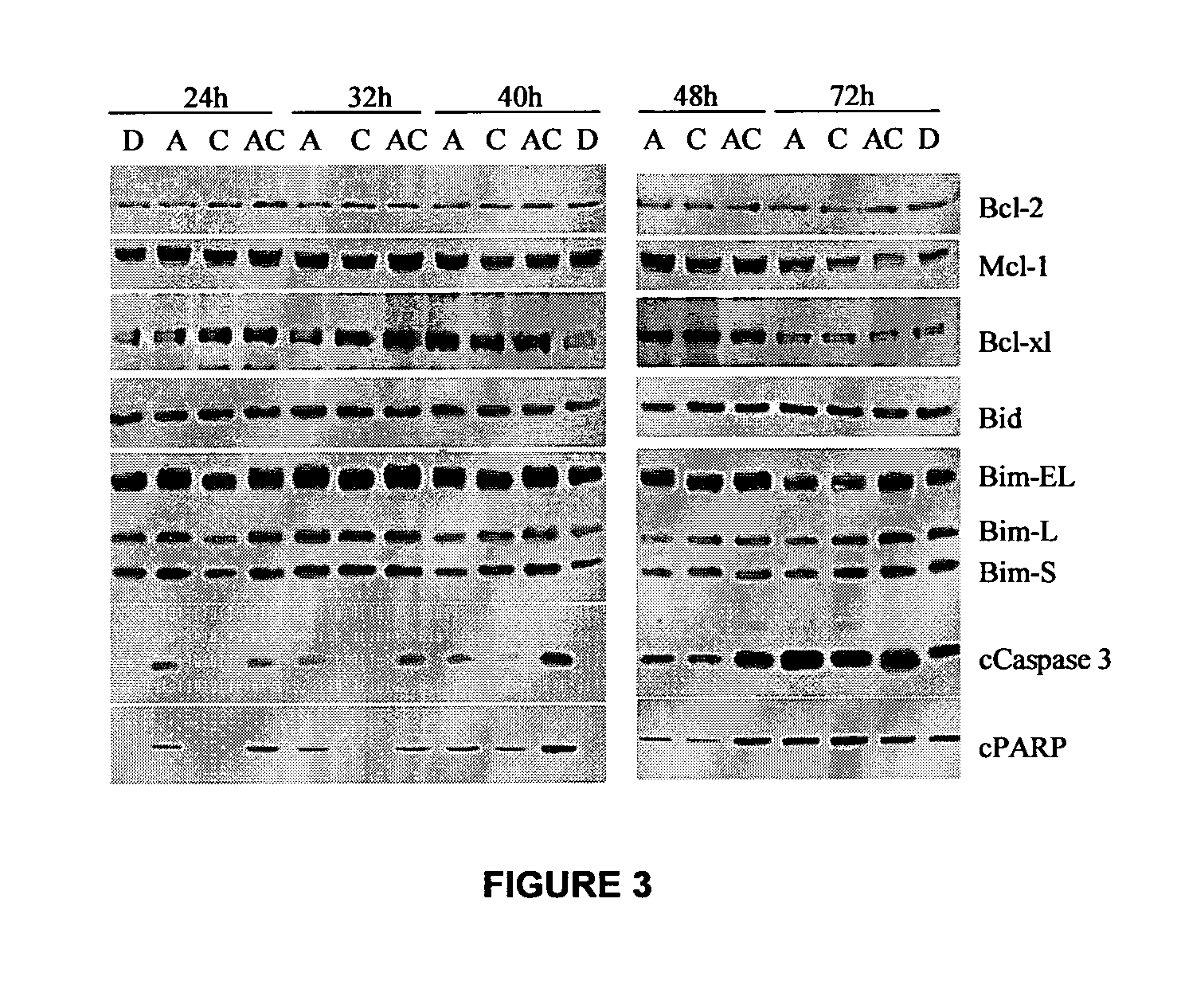
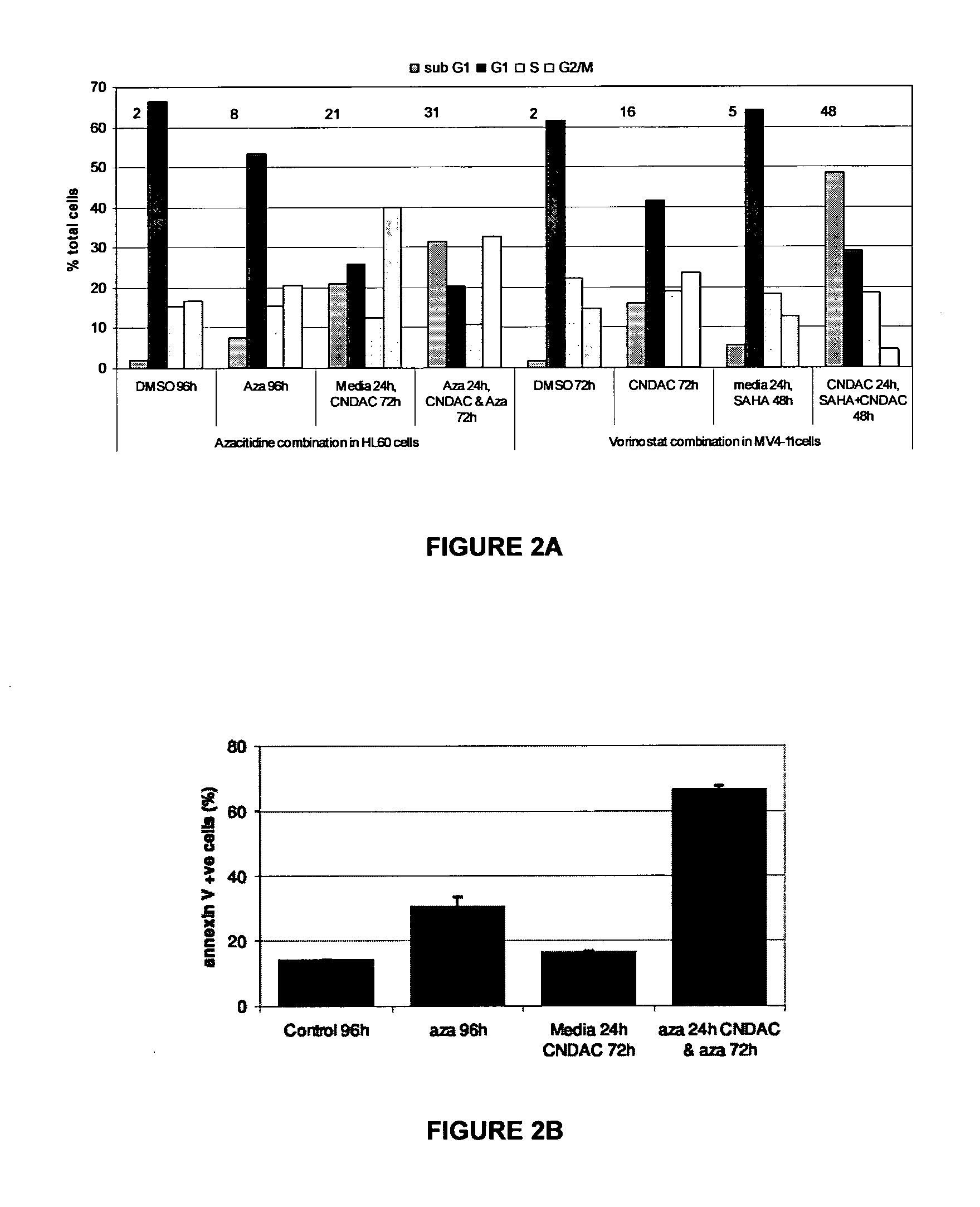
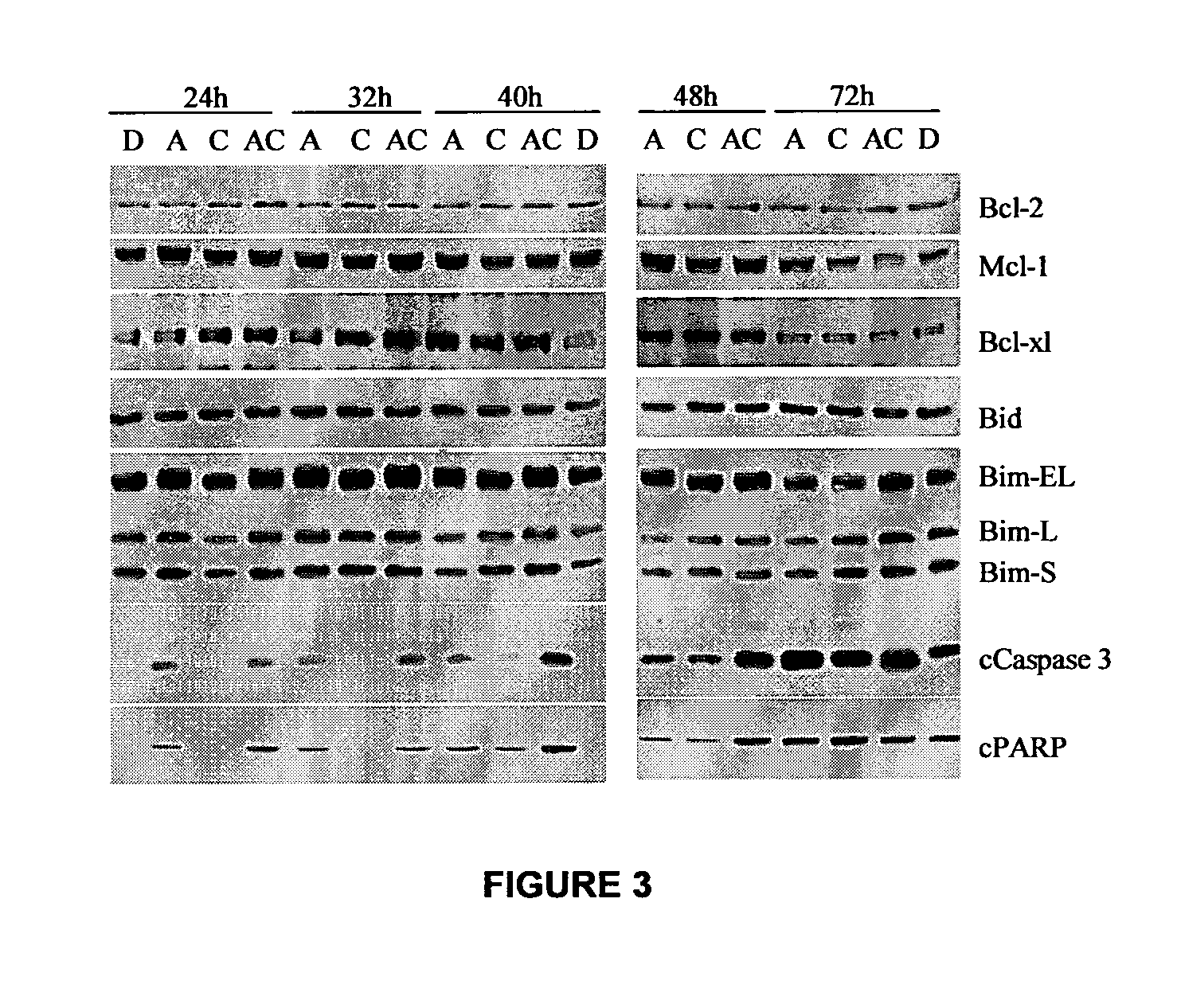
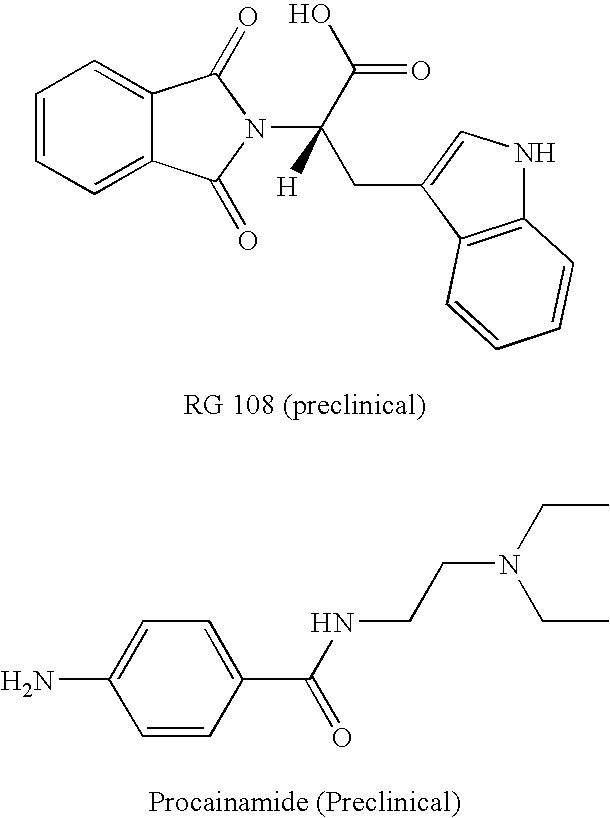
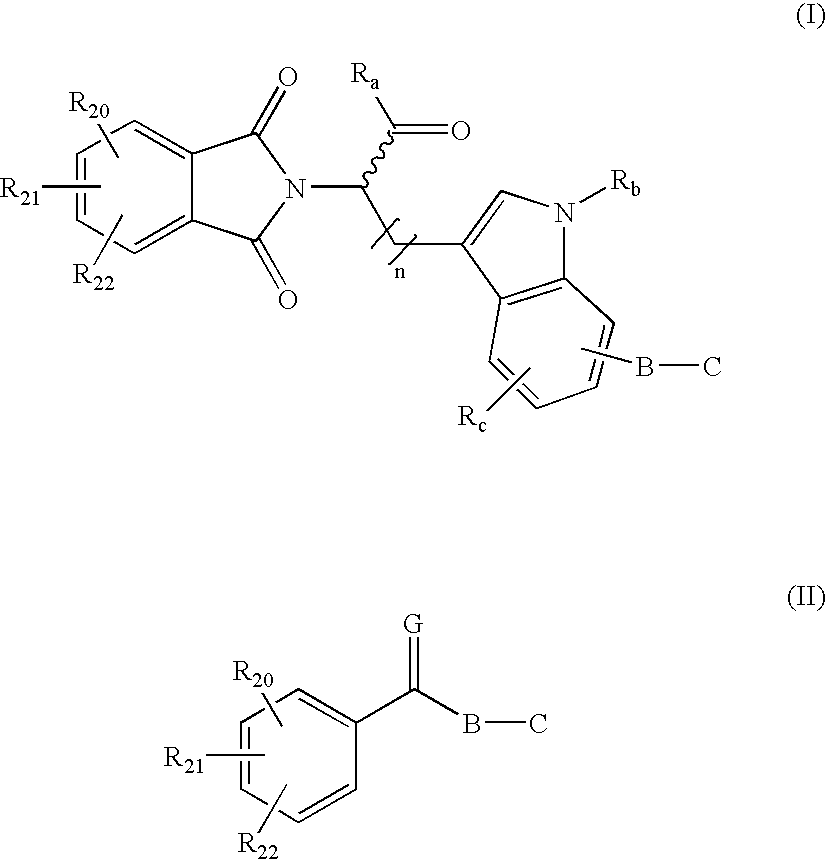
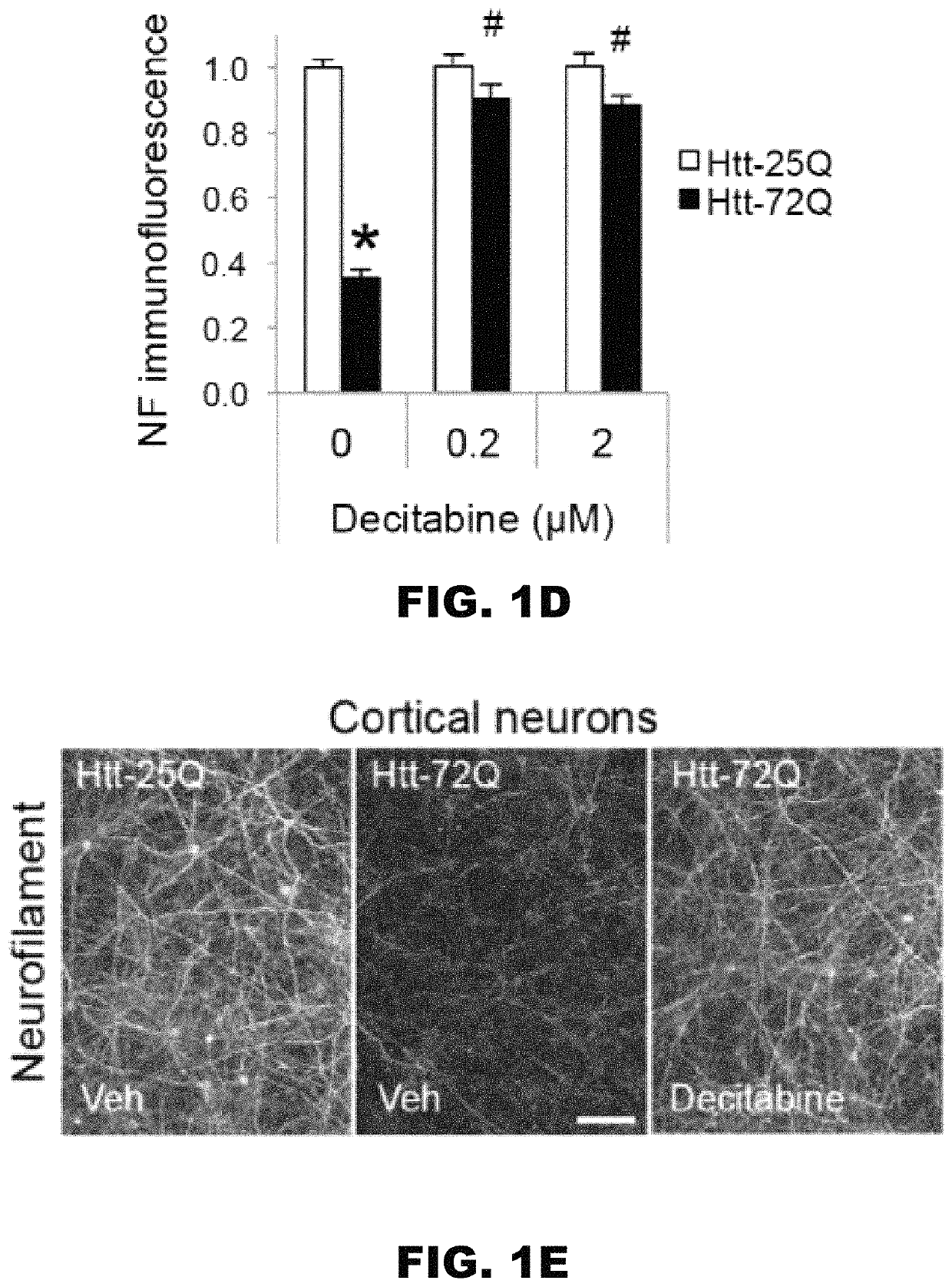
Combination methods for treatment of cancer and of blood disorders, using PG-11047 ((2Z)-N1,N4-bis[3-(ethy-lammo) propyl]-2-butene-1,4-diamine) and PG-11048 ((2E)-N1,N4-bis[3-(ethylamino)propyl]-2-butene-1,4-diamine) in combination with DNA methyltransferase (DNMT) inhibitors, histone deacetylase (HDAC) inhibitors, or both DNA methyltransferase inhibitors and histone deacetylase inhibitors, are disclosed. Hematopoietic cancers, lung cancers, mesothelioma, cutaneous T-cell lymphoma (CTCL), multiple myeloma, solid tumors, and blood disorders such as myelodysplastic syndromes can be treated using the methods of the invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
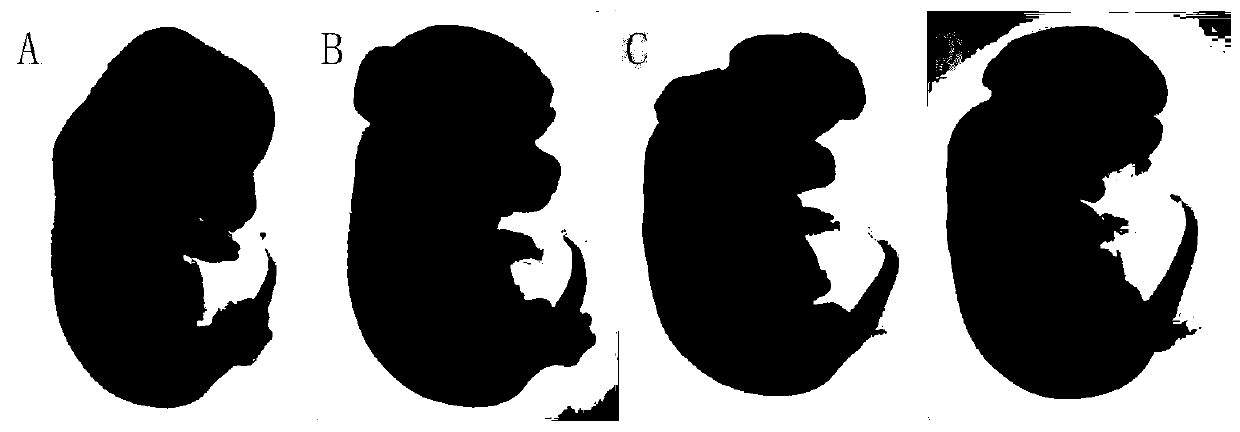
Application of DNA methyltransferase inhibitor to construction of neural tube defect mouse model
InactiveCN109771647AUnderstand the causeUnderstanding the pathogenesisOrganic active ingredientsAnimal husbandryDNA Methyltransferase InhibitorA-DNA
The invention relates to a construction method for a mouse neural tube defect model. When the neural tube of a pregnant mouse fetus grows, a DNA methyltransferase inhibitor is injected into a pregnantmouse, then, the fetus of the mouse has methylated metabolism abnormity, and accordingly the neural tube defect mouse mode is established; the mouse model can be used for biomedical research on neural tube defects, is beneficial to research on the nosetiology and pathogenesis of the neural tube defects, and is especially provided as a good animal model for research on all molecules and cells related to the neural tube defects. The method is easy to operate, high in feasibility, stable in effect and high in teratogenesis rate.
Owner:首都儿科研究所
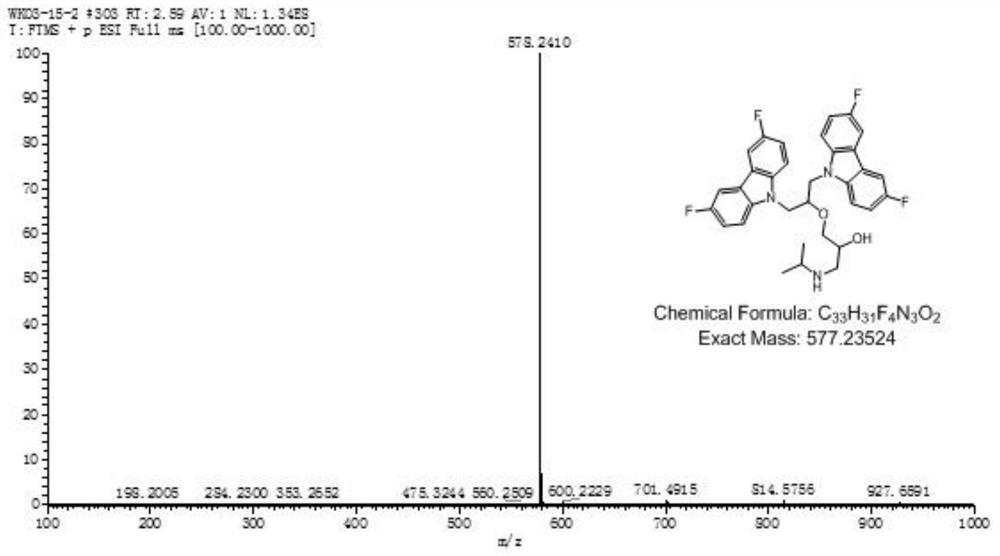
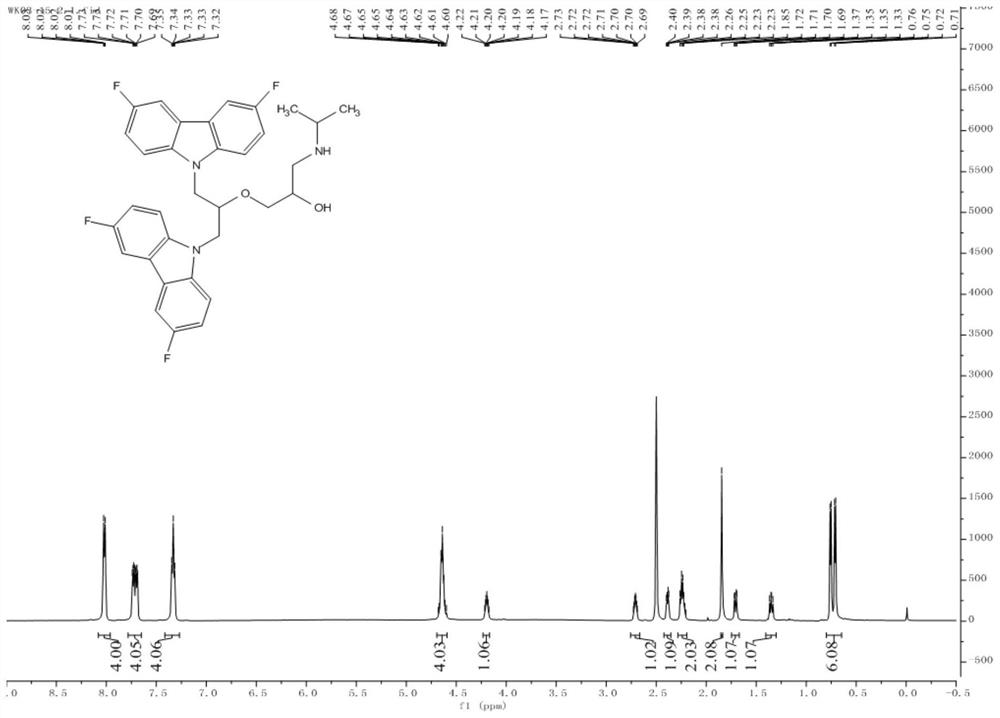
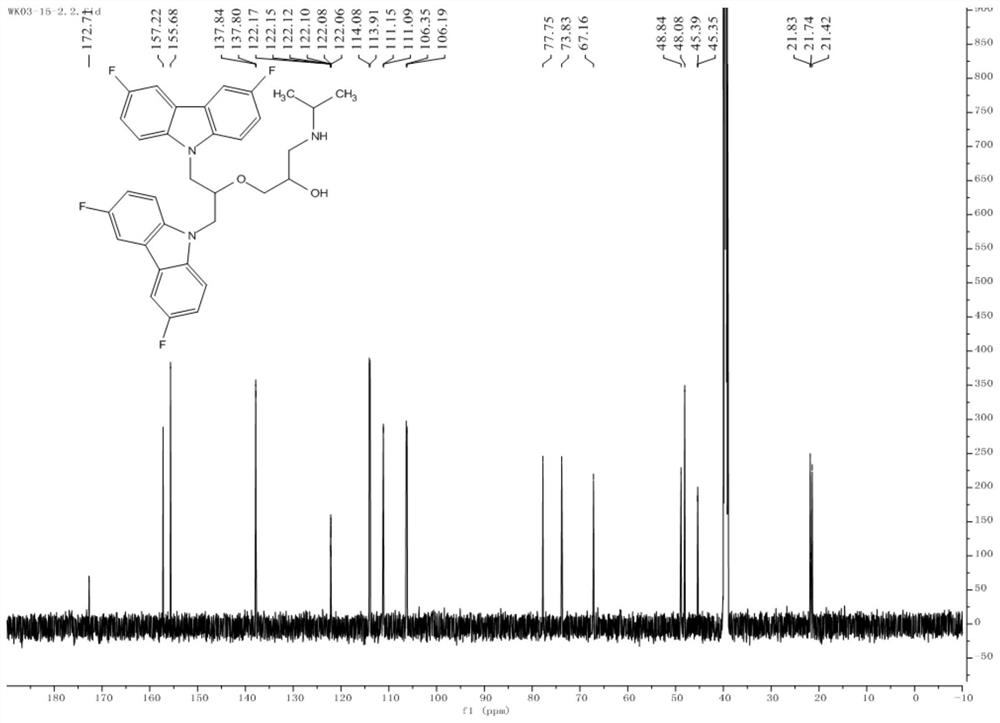
Fluorine-substituted bimolecular carbazole derivatives, preparation method and application thereof
The invention discloses a fluorine-substituted bimolecular carbazole derivative represented by formula (I) or a pharmaceutically acceptable salt thereof, R 1 , R 2 , R 3 , R 4 , R 5 as defined in the specification. The fluorine-substituted bimolecular carbazole derivatives provided by the present invention or their pharmaceutically acceptable salts can be used to prepare DNA methyltransferase inhibitors or histone demethylase inhibitors, which are confirmed by in vitro cancer cell anti-proliferation tests, The fluorine-substituted bimolecular carbazole derivative of the present invention has anti-proliferation activity on cancer cells.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
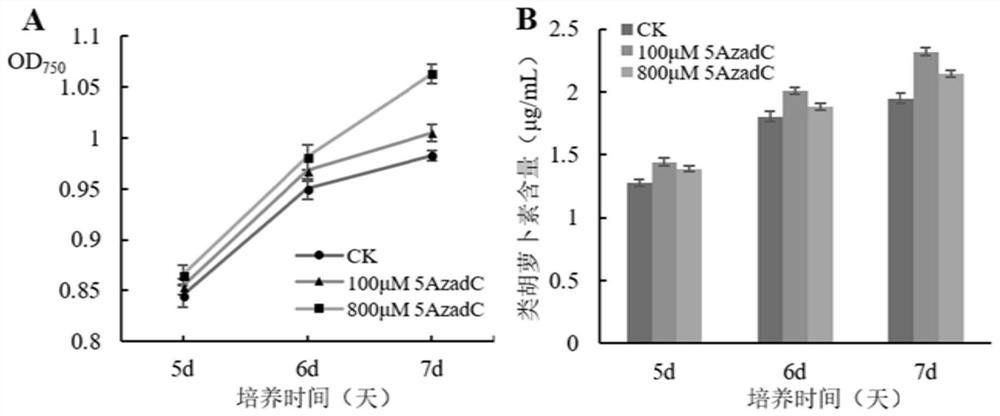
A method for increasing the biomass and carotenoid content of Chlamydomonas reinhardtii by using DNA methyltransferase inhibitors
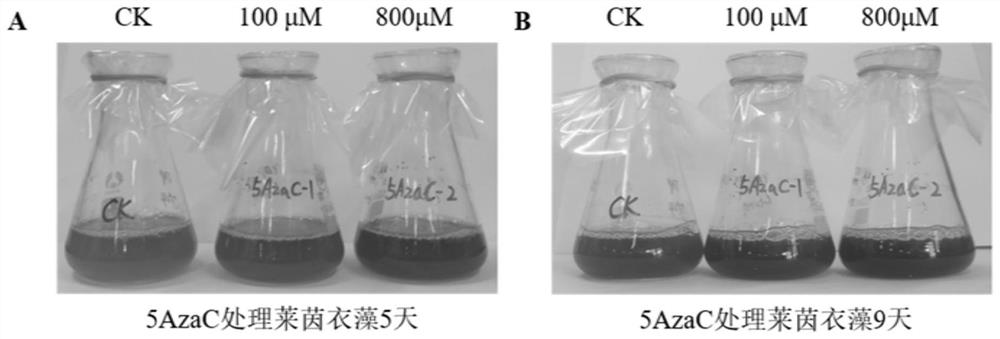
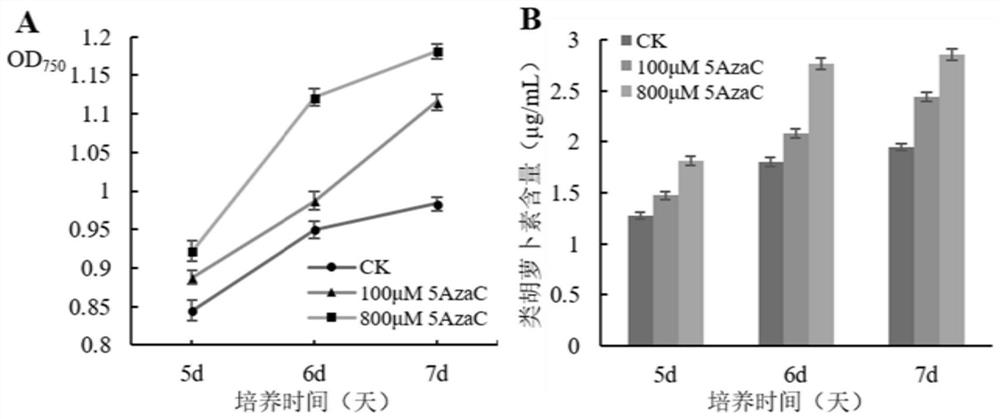
ActiveCN110857448BEasy to operateReduce dosageUnicellular algaeMicroorganism based processesBiotechnologyChlamydomonas reinhardtii
The invention discloses a method for improving the biomass and carotenoid content of Chlamydomonas reinhardtii by using a DNA methyltransferase inhibitor, and belongs to the field of food science andtechnology. According to the method, after the Chlamydomonas reinhardtii cells are cultured in a liquid medium containing a DNA methyltransferase inhibitor for a certain period of time, the biomass ismonitored, the algae cells are collected and the pigments are extracted every day. The method of the invention is simple and feasible, has a good effect, is convenient to operate, and can help quickly and effectively improve the biomass and carotenoid content of Chlamydomonas reinhardtii after adding of the DNA methyltransferase inhibitor. In the invention, the dosage of the DNA methyltransferaseinhibitor is very small, the effect is remarkable, and the operation is convenient.
Owner:SOUTH CHINA UNIV OF TECH
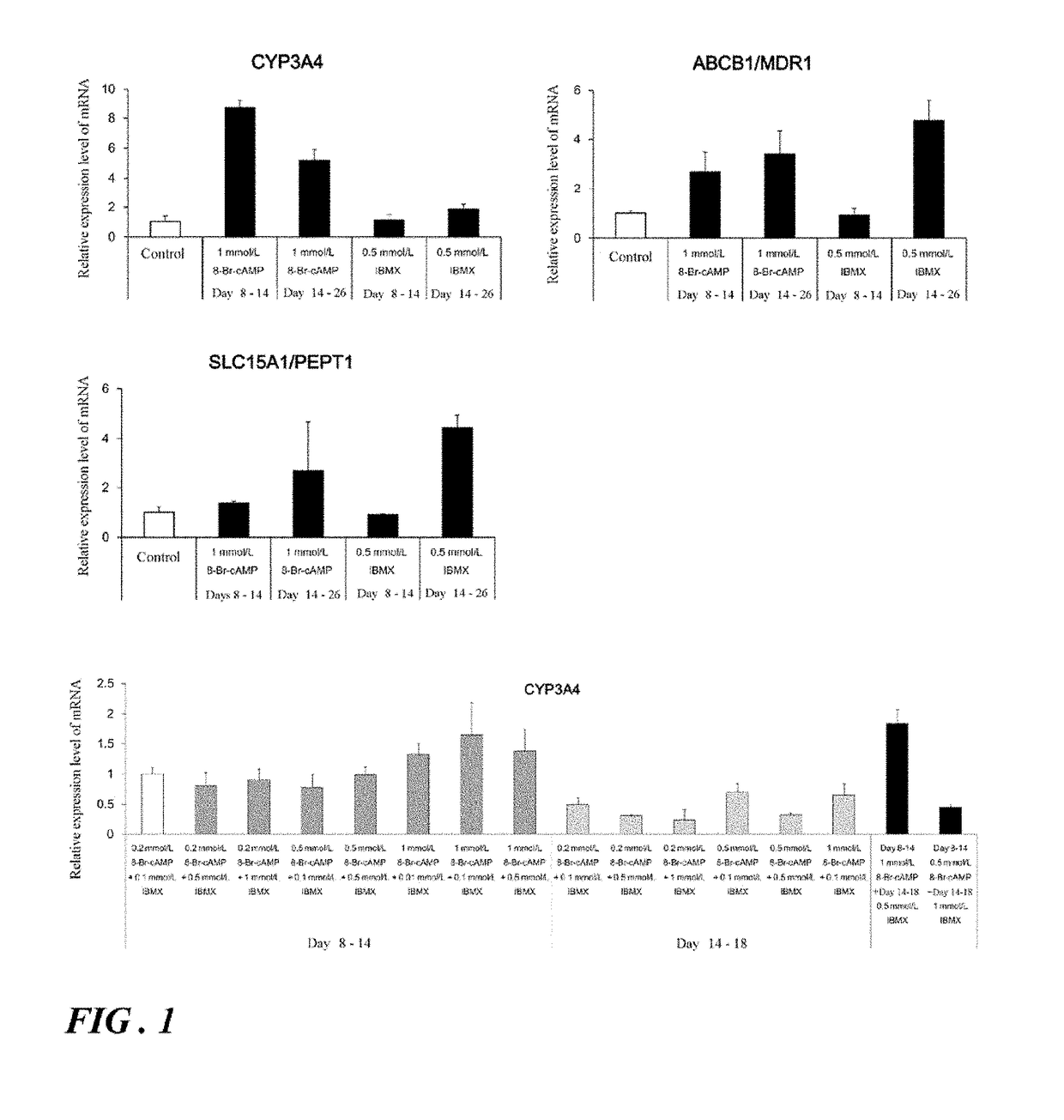
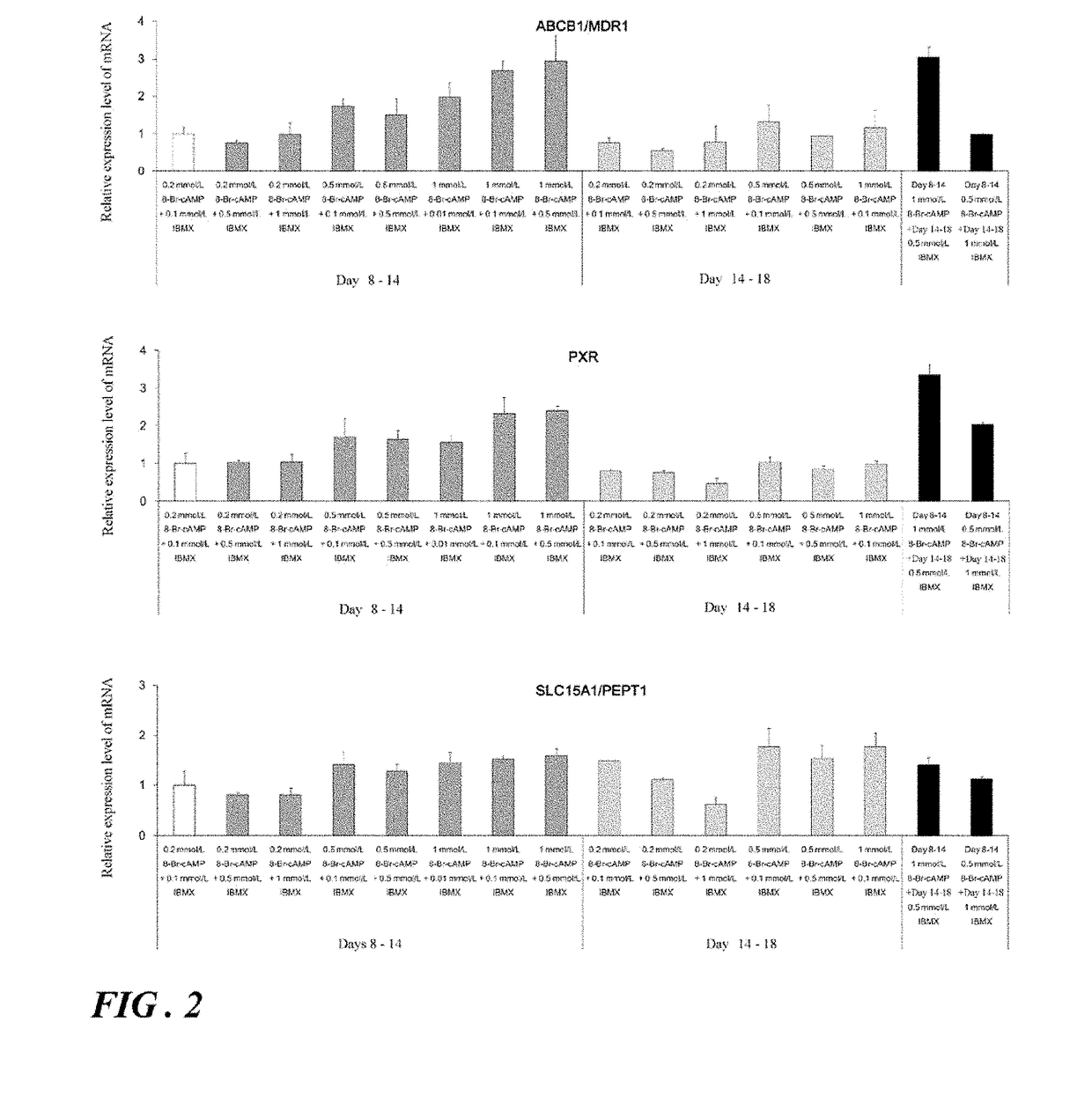
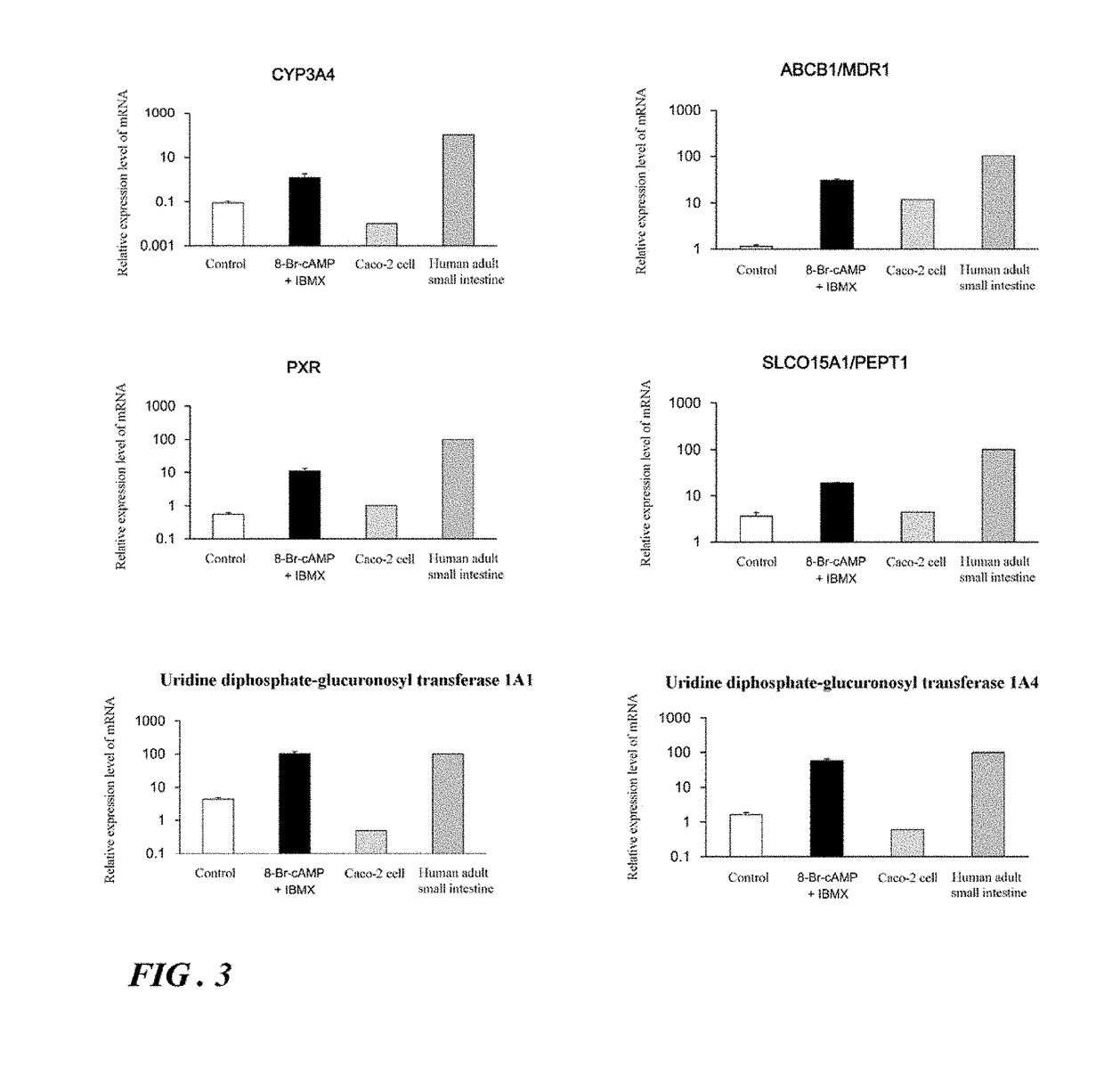
Induction of differentiation of induced pluripotent stem cells into intestinal epithelial cells
ActiveUS20190079076A1Decreasing concentration of cAMPReduce concentrationGastrointestinal cellsDigestive systemDNA Methyltransferase InhibitorA-DNA
An object of the present invention is to provide a novel method which enables convenient preparation of cells exhibiting functions close to that of intestinal epithelial cells of living bodies, and use of the method. The differentiation of induced pluripotent stem cells into intestinal epithelial cells is induced by step of differentiating induced pluripotent stem cells into endoderm-like cells; step of differentiating the endoderm-like cells obtained in step into intestinal stem cell-like cells; and step of differentiating the intestinal stem cell-like cells obtained in step into intestinal epithelial cell-like cells, wherein step includes culture in the presence of a MEK1 inhibitor, a DNA methyltransferase inhibitor, a TGF-β receptor inhibitor, and EGF and under the condition that cAMP is supplied to the cells.
Owner:NAGOYA CITY UNIVERSITY
Fluorine-substituted monocarbazole derivatives, preparation method and application thereof
ActiveCN111217741BOrganic active ingredientsOrganic chemistryDNA Methyltransferase InhibitorCancer cell proliferation
The invention belongs to the field of medicinal chemistry and relates to a fluorine-substituted monocarbazole derivative, which is one or more of the compounds shown in formula (I) and formula (II), or formula (I) or formula (II) One or more of the medically acceptable soluble salts formed by the indicated compounds. The fluorine-substituted monocarbazole derivatives obtained in the present application can be used to prepare DNA methyltransferase inhibitors or histone demethylase inhibitors. The fluorine-substituted monocarbazole derivatives of the invention have inhibitory effect on the proliferation of cancer cells and can be used to prepare medicines for treating and / or preventing cancer. The in vitro cancer cell proliferation inhibition test shows that the fluorine-substituted monocarbazole compounds obtained in the present invention exhibit inhibitory effects on human lung cancer cells (A549), human colon cancer cells (HCT116), human gastric cancer cells (MNK-45), human liver cancer Antiproliferative activity of cells (HepG2), RPMI‑8226 and Karpas 299.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Quinazoline derivatives and their use as DNA methyltransferase inhibitors
InactiveCN105555781AOrganic active ingredientsOrganic chemistryDNA Methyltransferase InhibitorQuinazoline
Owner:PIERRE FABRE MEDICAMENT SAS +1
A method for detecting dna methyltransferase activity based on strand displacement amplification and dnazyme amplification
ActiveCN105112540BHighly sensitive detection activityLow detection limitMicrobiological testing/measurementBiological material analysisFluorescenceDNA Methyltransferase Inhibitor
The invention relates to a method for detecting DAN methyltransferase activity based on strand displacement amplification and DNAzyme amplification. A three-function double-stranded DNA probe is designed. Methylation happening to the three-function double-stranded DNA probe is specifically recognized through DAN methyltransferase. Remaining non-methylated double-stranded DNA is specifically cut through HpaII restriction enzymes, methylated double-stranded DNA triggers a strand displacement reaction, a large amount of 8-17 DNAzyme is released, the 8-17 DNAzyme catalyzes cutting of a large number of hairpin-line molecular beacon substrates, and remarkable fluorescent enhancement is triggered. The method can sensitively detect the DAN methyltransferase activity, and the detection limit is 0.0082 U / mL. The method has potential application in research of influences of anti-cancer drugs on DAN methyltransferase activity inhibition and screening of DAN methyltransferase inhibitors.
Owner:SHANDONG UNIV
Combinations of sapacitabine or cndac with DNA methyltransferase inhibitors such as decitabine and procaine
InactiveUS20110207692A1Good effectEase of preparation and detectabilityBiocideCarbohydrate active ingredientsSapacitabineProcaine
A first aspect of the invention relates to a combination comprising a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof. A second aspect of the invention relates to a pharmaceutical product comprising a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof, as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect of the invention relates to a method of treating a proliferative disorder, said method comprising simultaneously, sequentially or separately administering a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof, to a subject.
Owner:CYCLACEL
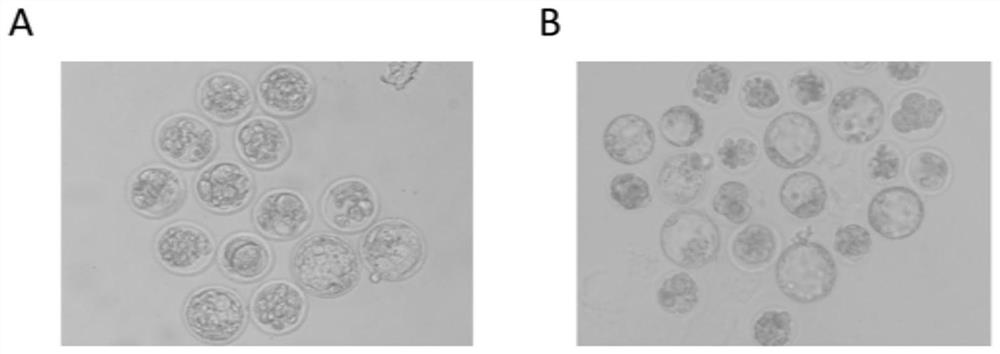
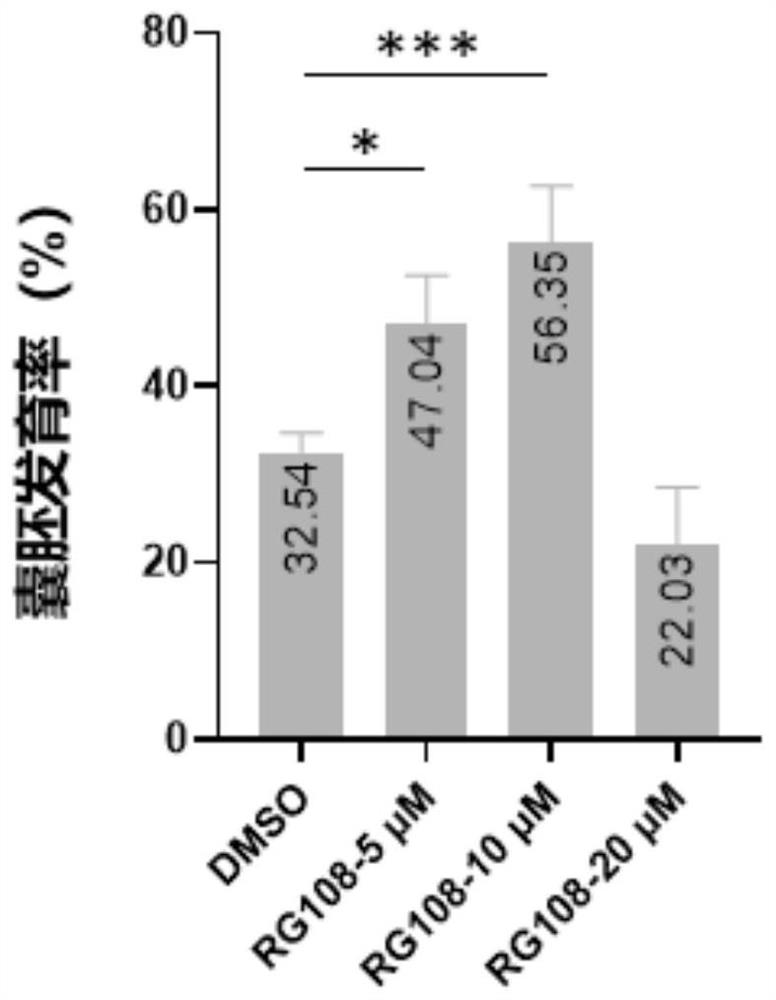
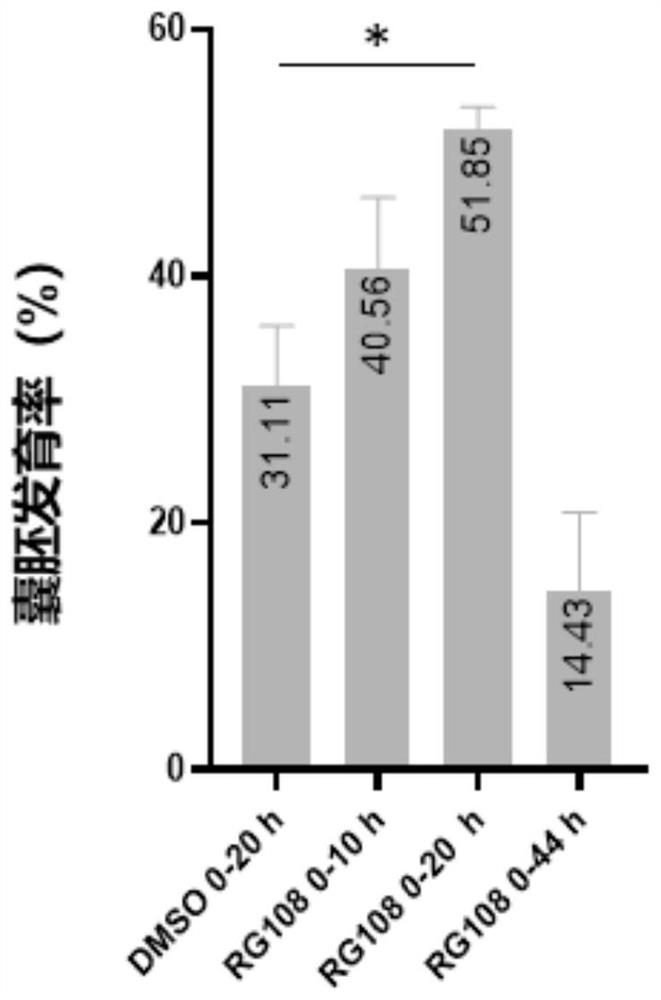
Application of DNA methyltransferase inhibitor in improvement of animal round sperm injection embryo development efficiency
ActiveCN113186152AImprove developmental efficiencyIncreased rate of blastocyst developmentCulture processCell culture active agentsPhysiologyDNA Methyltransferase Inhibitor
The invention belongs to the field of medicines, and discloses application of a DNA methyltransferase inhibitor to improvement of animal round sperm injection embryo development efficiency. The invention discloses the application of the DNA methyltransferase inhibitor in improving the development efficiency of the animal round sperm injection embryo for the first time; compared with an existing round sperm injection technology, the blastocyst development rate of the round sperm injection embryo can be remarkably improved through the DNA methyltransferase inhibitor under the condition that transgenic risks are not introduced; furthermore, excellent round sperm injection embryo in-vitro culture system is established, and the feasibility of a round sperm injection technology is improved.
Owner:SOUTHERN MEDICAL UNIVERSITY
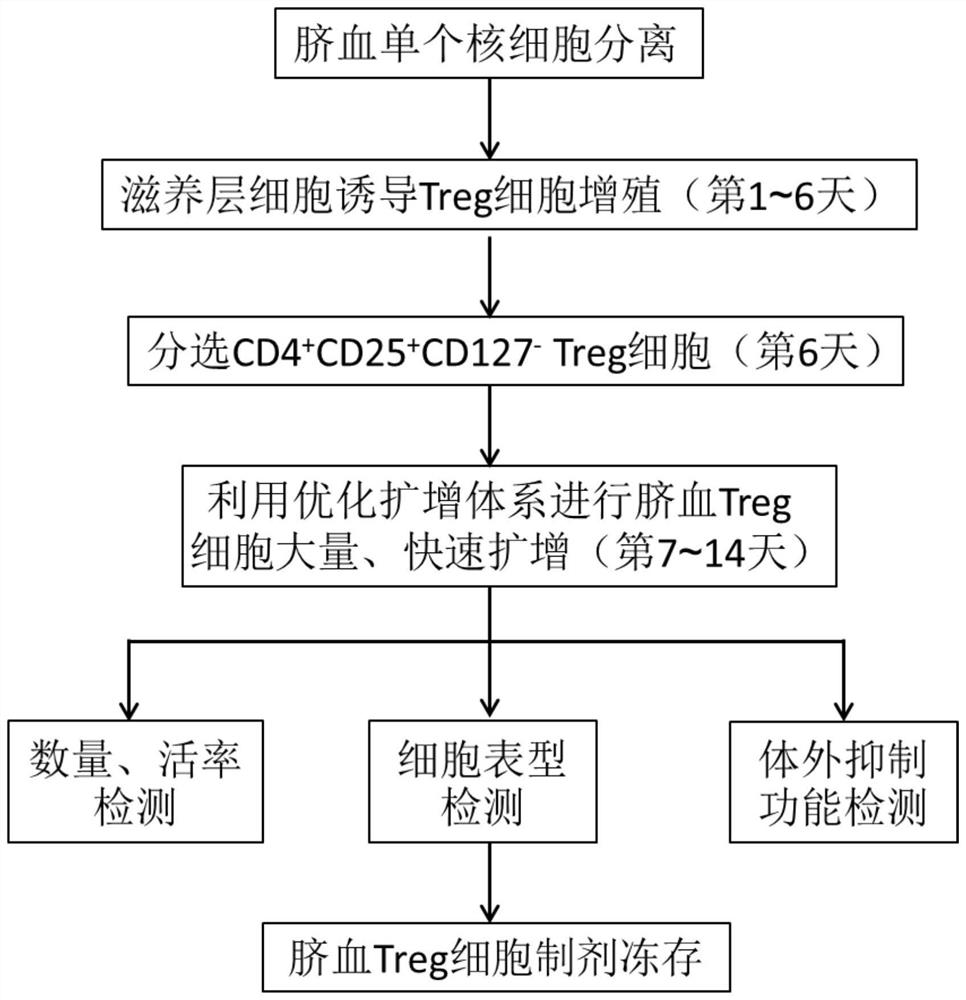
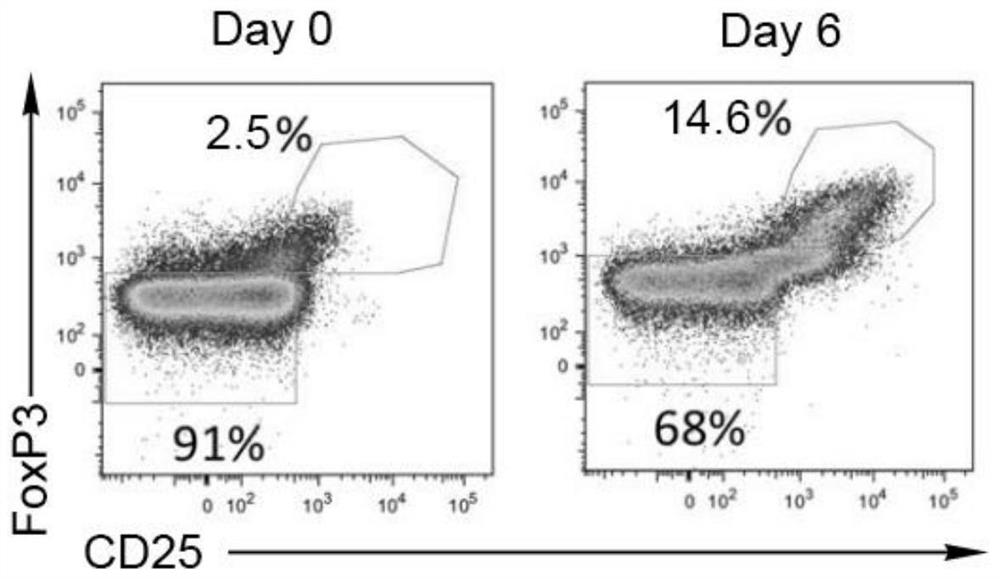
A method and application of umbilical cord blood Treg cell expansion in vitro based on trophoblast cells
ActiveCN112458053BPromote amplificationRaise the ratioAntipyreticDigestive systemAutoimmune conditionCord blood stem cell
The invention discloses an in vitro amplification method and application of umbilical cord blood Treg cells based on trophoblast cells. The specific technical method is as follows: firstly, using umbilical cord Wharton's jelly mesenchymal stem cells as trophoblast cells to induce Treg in umbilical cord blood mononuclear cells Initial proliferation of cells; then purer Treg cells were obtained by magnetic bead sorting; finally, optimized expansion factors were used to stimulate the rapid expansion of Treg cells. The present invention adopts human AB plasma, IL-2, rapamycin, RARA agonist and DNA methyltransferase inhibitor as optimized amplification factors, and can prepare a large amount, high purity and high activity within 2 weeks. Strong cord blood Treg cells. Using umbilical cord blood as the raw material for Treg cell expansion can be prepared in batches, and can reduce the quality fluctuation of Treg cells caused by individual differences in samples. Umbilical cord blood Treg cells have low immunogenicity and can be used as general-purpose cells for clinical research, such as autoimmune diseases and graft-versus-host disease.
Owner:成都云测医学生物技术有限公司
Medicinal use of dna methyltransferase inhibitor combined with anti-cd47 antibody for the preparation of anti-tumor drugs
ActiveCN109224079BImprove anti-tumor efficacyReduce financial burdenOrganic active ingredientsAntibody ingredientsAntiendomysial antibodiesDNA Methyltransferase Inhibitor
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy](https://images-eureka.patsnap.com/patent_img/74da7ed5-a69a-45be-a6e5-212fe27d97c2/US20130102556A1-20130425-D00001.png)
![Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy](https://images-eureka.patsnap.com/patent_img/74da7ed5-a69a-45be-a6e5-212fe27d97c2/US20130102556A1-20130425-D00002.png)
![Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy Use of N1,N4-bis[3-(Ethylamino)Propyl]-2-Butene-1,4-Diamine Compounds in Combination with Epigenetic-Acting Pharmaceuticals for Enhanced Cancer Therapy](https://images-eureka.patsnap.com/patent_img/74da7ed5-a69a-45be-a6e5-212fe27d97c2/US20130102556A1-20130425-D00003.png)