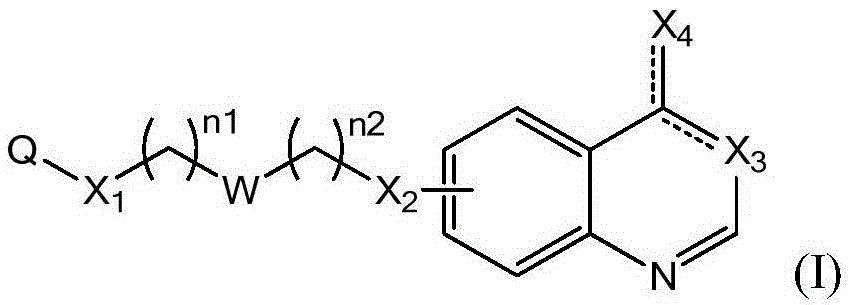
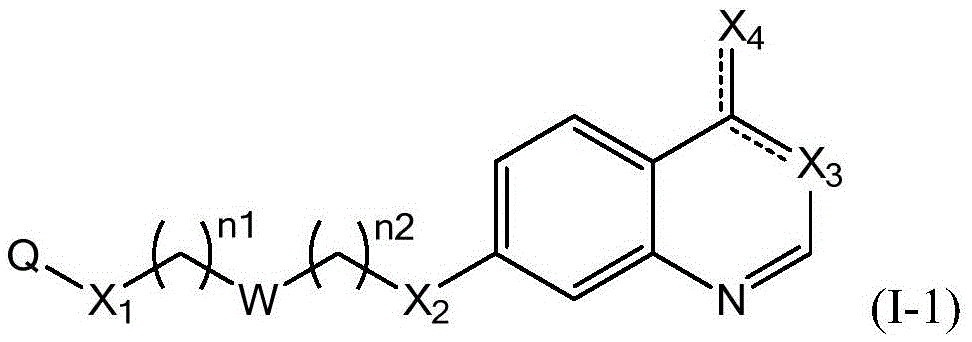
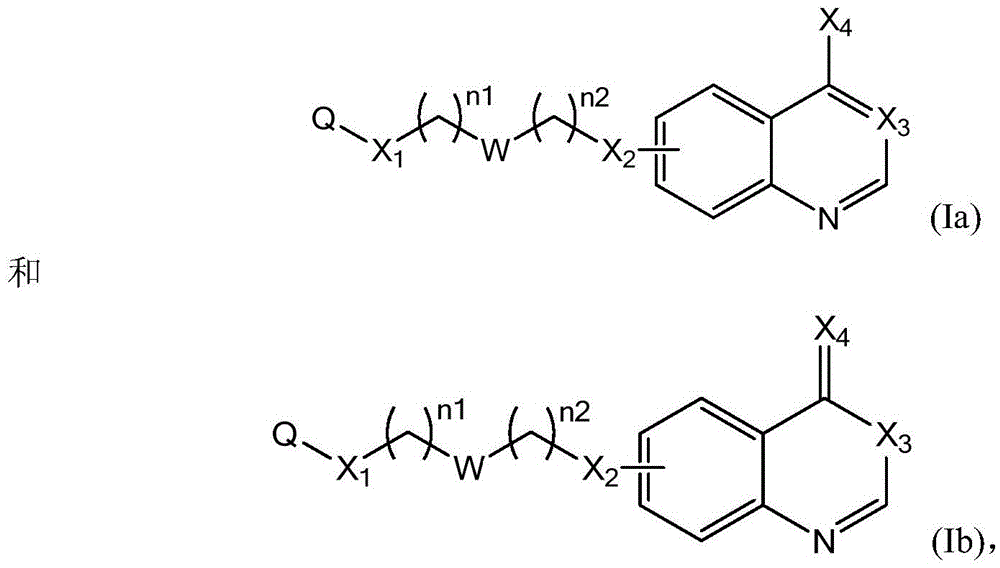
Quinazoline derivatives and their use as DNA methyltransferase inhibitors
An alkyl and aryl technology, applied in the field of quinazoline derivatives, can solve problems such as lack of specificity, low activity, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0364] Example 1: Compound F
[0365]
[0366] a) i) SOCl 2 , 110°C, 1h.ii) Amphetamine, DMF, K 2 CO 3 ,RT,2h,85%.
[0367] 4-(3-phenylpropylamino)-7-(2-chloroethoxy)quinazoline (17)
[0368] A solution of 16 (440 mg; 2.01 mmol) in thionyl chloride (10 mL) and a catalytic amount of DMF was boiled for 30 min. The solvent was removed, and the crude product was dissolved in a DMF solution of amphetamine (570 μL; 4.0 mmol), and the mixture was stirred at room temperature for 2 h. The mixture was diluted with ethyl acetate and saturated Na 2 CO 3 solution, the organic phase was washed with brine, and dried over magnesium sulfate. The solvent was removed and the residue was purified by silica gel flash chromatography using a linear gradient of ethyl acetate in cyclohexane (0→100% ethyl acetate) to afford 17 as a pale brown solid (607 mg; 1.70 mmol; yield 85 %).
[0369]
[0370] 1 HNMR (500MHz, CDCl 3 )δ8.58 (s, 2H, Ha1), 7.35-7.13 (m, 7H, Ha7, Ha15, Ha13 and Ha14...
Embodiment 2
[0407] Example 2: Compounds A, B, C, D, E, T and U
[0408]
[0409] a) 3-Boc aminopropylamine or 4-Boc aminobutylamine, DMF, 125°C, 4h, 78% for n=1 and 73% for n=2. b) TFA, 91% for n=1 and 84% for n=2. c) NosCl, TEA, DMAPcat., DMF, RT, 6h, 66% for n=1 and 71% for n=2. d) i) 17, K 2 CO 3 , KIcat., DMF, RT, 80°C, 6h, 71% for n=1 and 75% for n=2.ii) PhSH, K 2 CO 3 , MeCN, RT, 24h, 85% for n=1 and 75% for n=2. e) TFA, water, RT, 1h. Or e) i) methyl 4-bromobutyrate, TEA, DMF, 90°C, 12h, 44% for n=1 and 45% for n=2. f) 0.5N NaOH, dioxane, 18h, 78% for n=1 and 79% for n=2.
[0410] 4-((2-Bocaminopropyl)amino)quinoline (27)
[0411] 4-((2-Bocaminobutyl)amino)quinoline (28)
[0412] N-boc aminopropylamine (2.06g, 11.84mmol) or N-boc aminobutylamine (2.25g, 12mmol), 4-chloroquinoline (1.525g, 9.32mmol) and DiPEA (1.8mL, 10.3mmol) in 12mL normal The solution in amyl alcohol was stirred at reflux for 6h. The solvent was removed and the residue was diluted in DCM. The organi...
Embodiment 3
[0487] Example 3: Compound G
[0488]
[0489] a) N-Boc-piperidine-4-methanol, NaH, DMF, 110℃, 3h, 67%. b) POCl 3 , Triazole, TEA, MeCN, RT, 18h. c) 3-amphetamine, TEA, DMF, RT, 2h, two steps 80%. d) TFA, RT, 1h, 96%. e) 26, K 2 CO 3 , KI, DMF, 65℃, 12h, 62%.
[0490] 7-O-((N-Boc)piperidin-4-ylmethoxy)quinazolinone (43)
[0491] To a mixture of (N-Boc)piperidin-4-ylmethanol (1.12 g; 5.2 mmol) in DMF (2 mL) was added sodium hydride (125 mg, 5.2 mmol) at 0 °C under argon. The mixture was stirred at 0 °C for 15 min, then 42 (162 mg; 1 mmol) was added portionwise. The mixture was stirred at 0 °C for 10 min, then at room temperature for 10 min, at 60 °C for 15 min, and finally at 110 °C for 2 h. The reaction mixture was diluted with ethyl acetate and washed with water and brine. The organic phase was dried over magnesium sulfate and the solvent was removed. The crude product was purified by flash chromatography on silica gel using a linear gradient of ethyl acetate in c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com