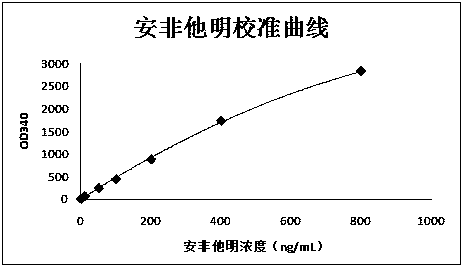
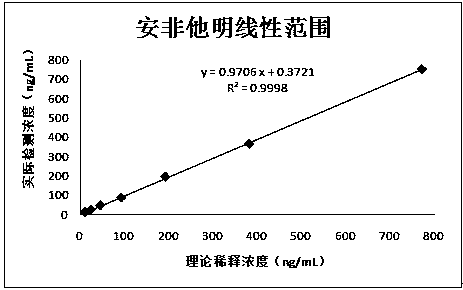
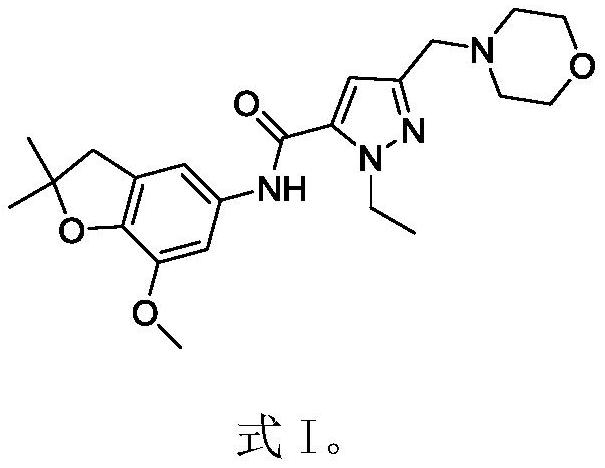
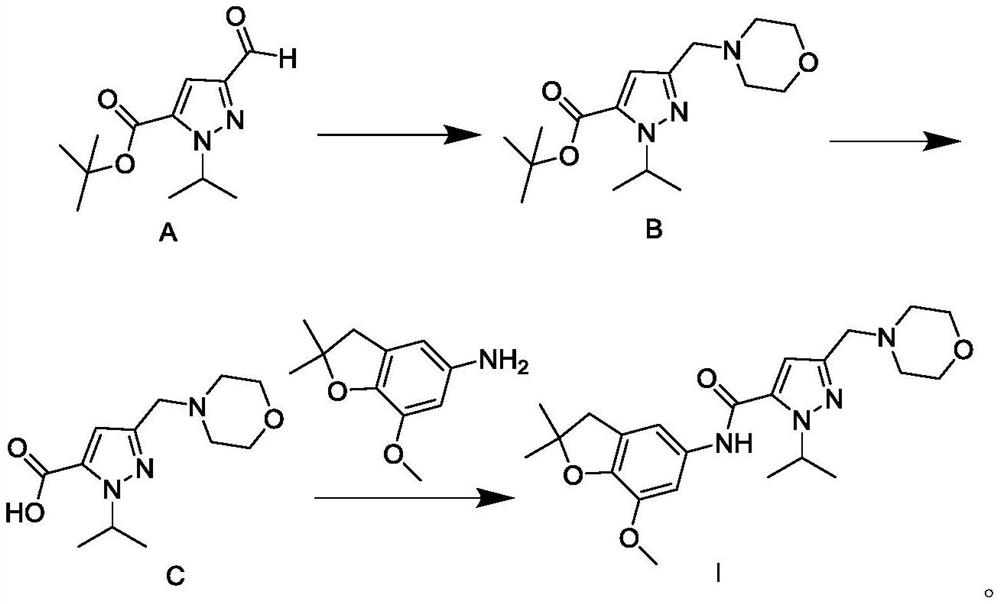
Patents
Literature
37 results about "Amphetamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat attention deficit hyperactivity disorder - ADHD.
Method and synergistic composition for treating attention deficit/hyperactivity disorder
InactiveUS6541043B2Minimize side effectsBiocideHydroxy compound active ingredientsBeta-CaroteneBetaine
A composition and method for treating Attention Deficit / Hyperactivity Disorder (ADHD) is provided which can be used both with and without ethical drugs now used to treat ADHD. The composition contains dimethylaminoethanol (DMAE), omega 3-fatty acids, betaine, oligomeric proanthocyanidins (OPC), folic acid, vitamins C, E, B12, B6, B5 and beta-carotene and minerals (calcium, magnesium, zinc and selenium). Ethical drugs such as amphetamines, methylphenidate HCl and pemoline are known to control ADHD, but each has significant side effects when used in their therapeutic dose. When combining the composition with such ethical drugs, the amount of the ethical drug can be lowered below a level which causes undesirable side effects which is an important feature. Preferred compositions contain one or more of lecithin, choline, 5-hydroxytryptophan, tyrosine, Reishi Extract, Kava Extract, Gingko, Ginseng and St. John's Wort.
Owner:PHILIP C LANG
Exo-R-mecamylamine formulation and use in treatment
InactiveUS20020016370A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationStimulantS syndrome
A pharmaceutical composition includes a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-S-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-S-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
Silicone-containing acrylic polymers for transdermal drug delivery compositions
Described herein are silicone-containing acrylic polymers useful, for example, in transdermal drug delivery compositions, to methods of making and using them, to transdermal drug delivery compositions comprising them, and to methods of making and using such transdermal drug delivery compositions. The polymers are particular suitable for formulating amine drugs, such as amphetamine, methylphenidate, rivastigmine, paroxetine and clonidine.
Owner:NOVEN PHARMA
Non-standard amino acid conjugates of amphetamine and processes for making and using the same
ActiveUS20080139653A1Diminish and eliminate pharmacological activityInhibition effectBiocideNervous disorderAmphetamineAmino acid
Disclosed are amphetamine prodrug compositions comprising at least one non-standard amino acid conjugate of amphetamine, a salt thereof, a derivative thereof, or a combination thereof. Methods of making and using the same are also disclosed.
Owner:TAKEDA PHARMA CO LTD
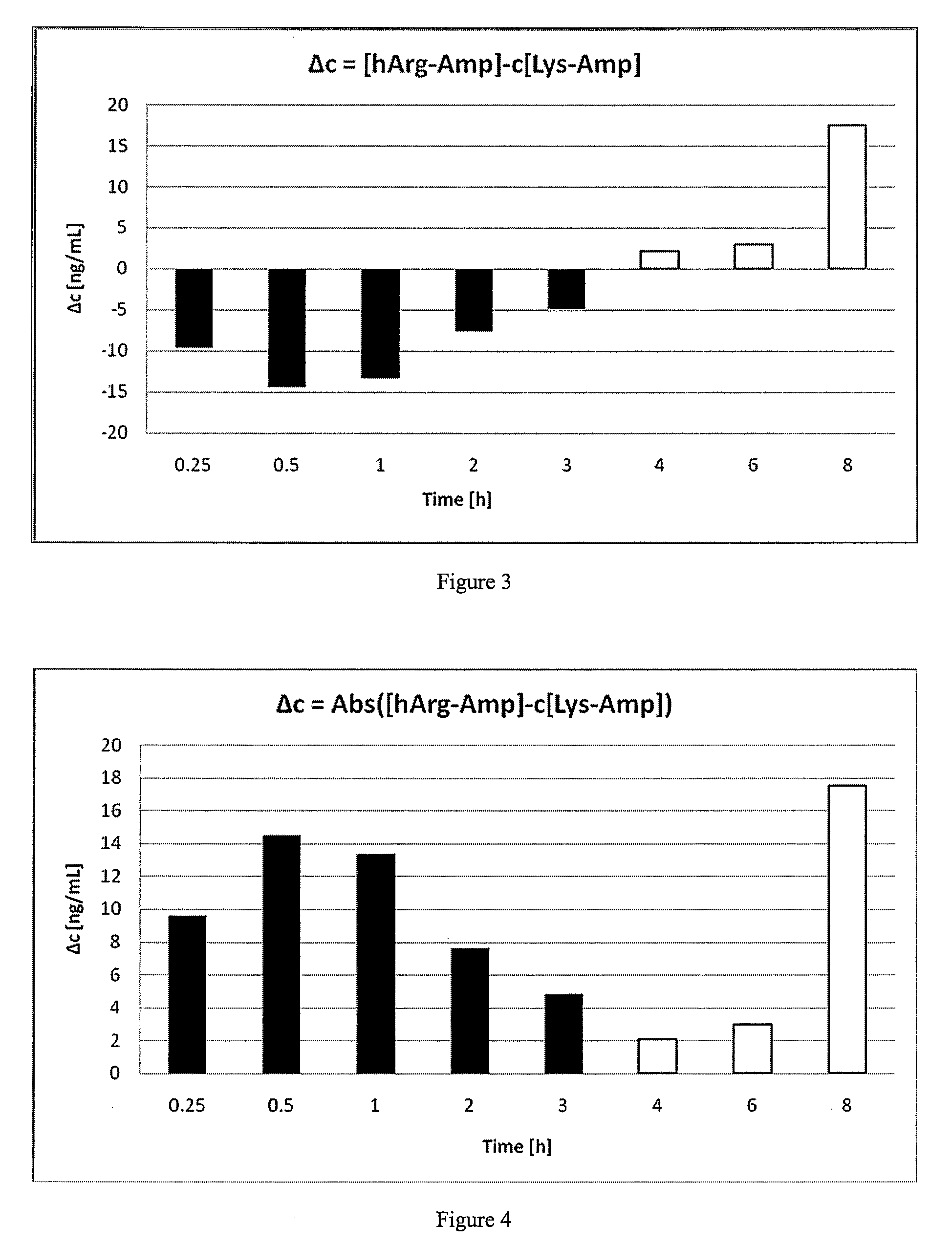
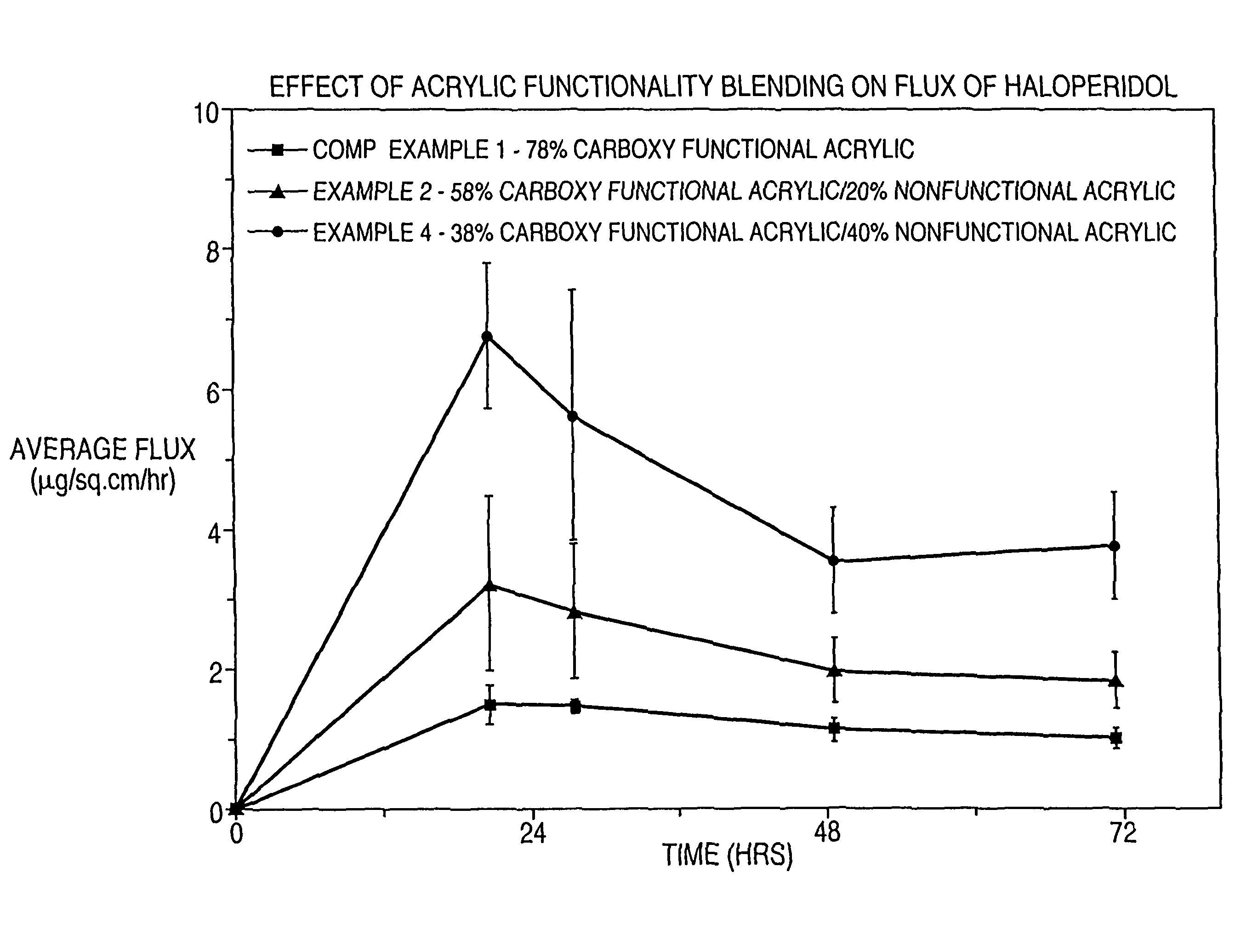
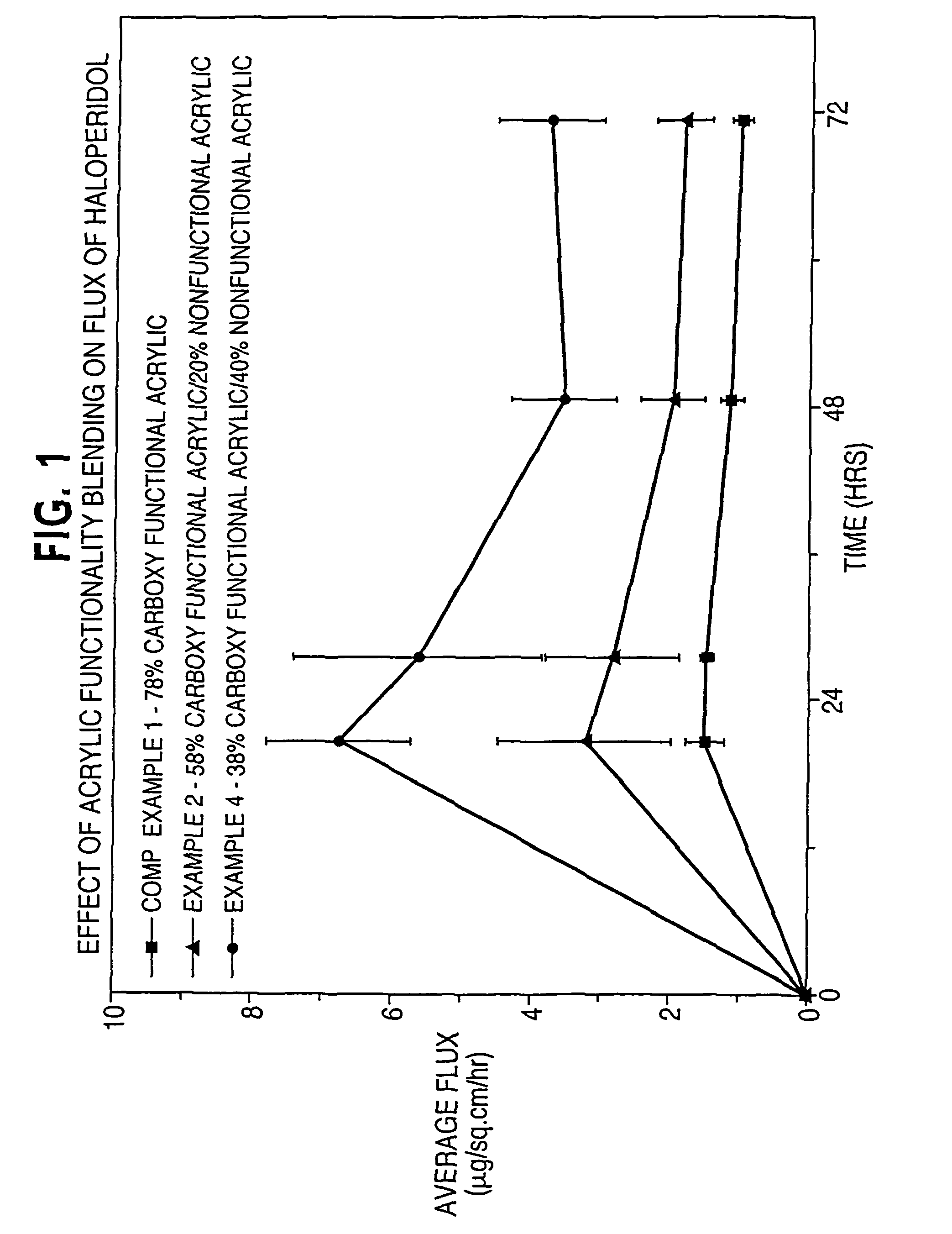
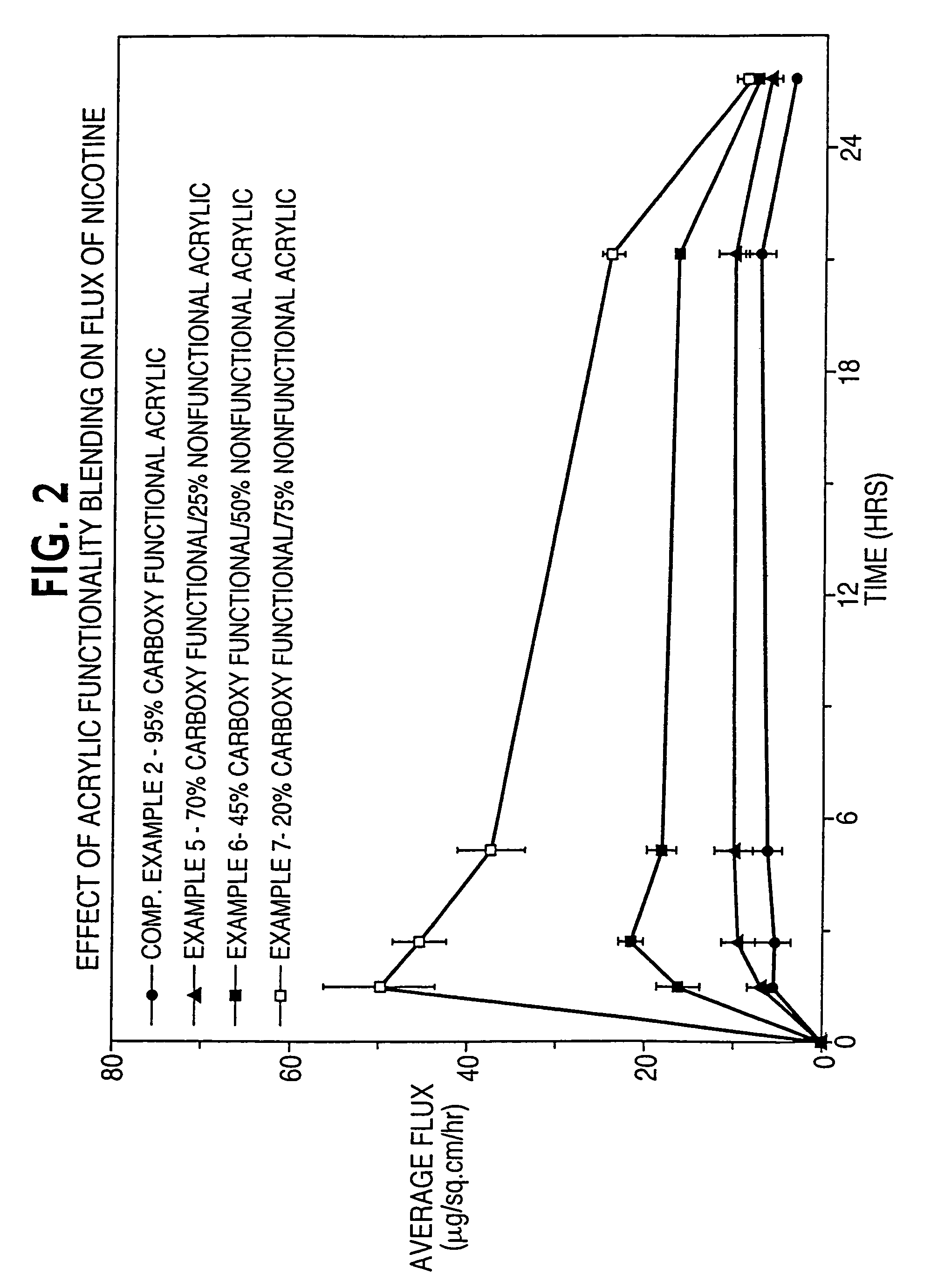
Dermal composition for controlling drug flux comprising two acrylic adhesive polymers having different functionalities and different solubility parameters
InactiveUS8187628B2Simple and inexpensiveDesirable in adhesiveCosmetic preparationsBiocideControlled drugsAmphetamine
A dermal composition for administration of an amphetamine drug comprising a blend of two or more acrylic-based polymers having differing functionalities so as to modulate the drug solubility in the polymer matrix and the delivery rate of the drug, and methods therefor.
Owner:NOVEN PHARMA
Multi-in-one combined detection kit for drug and preparation process and enclosed reagent thereof
InactiveCN1632583AAppropriate detection sensitivityHigh precisionMaterial analysisTest agentBiochemical engineering
It is a poison multiple and combination test agent box and its process method, which comprise plastic shell and test paper bar. The plastic shell comprises upper cover and down cover, wherein, the upper cover comprises specimen adding holes, test result display hole and fix mark hole; the down cover is set with flute. The test paper comprises test specimen area, test result display area and water absorption area, wherein, the test specimen area comprises glue gold and filter paper; the test result display area comprises pyroxylin film, C line control area with sheep and rabbit polyclonal antibody, test display area with heroin antigen and test display area with methyl amphetamine antigen. The water absorption area is set with absorption paper and fix mark.
Owner:梅里埃(上海)生物制品有限公司
Compositions Comprising Enzyme-Cleavable Amphetamine Prodrugs and Inhibitors Thereof
InactiveUS20130059914A1Eliminate side effectsPatient compliance is goodBiocideOrganic chemistryControlled releaseAmphetamine
Pharmaceutical compositions and their methods of use are provided, where the pharmaceutical compositions comprise an amphetamine prodrug that provides enzymatically-controlled release of amphetamine or an amphetamine analog. The composition can further comprise an enzyme inhibitor that interacts with the enzyme(s) that mediates the enzymatically-controlled release of amphetamine or the amphetamine analog from the amphetamine prodrug so as to attenuate enzymatic cleavage of the amphetamine prodrug.
Owner:SIGNATURE THERAPEUTICS
Treatment of addiction and dependency
The present invention relates to methods of treating or preventing addiction and relapse use of addictive agents and treating or preventing addictive or compulsive behavior and relapse practice of an addictive behavior or compulsion. The methods and compositions of the invention are useful in the treatment or prevention of addiction to any agent, including alcohol, nicotine, marijuana, cocaine, and amphetamines, as well as compulsive and addictive behaviors, including pathological gambling and pathological overeating.
Owner:TACA JR ARTURO C
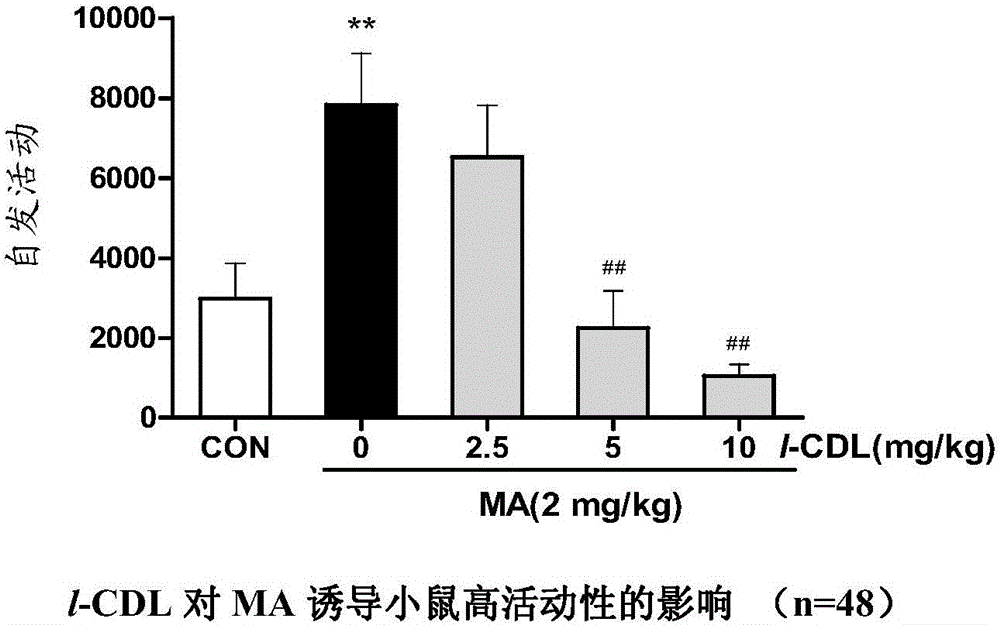
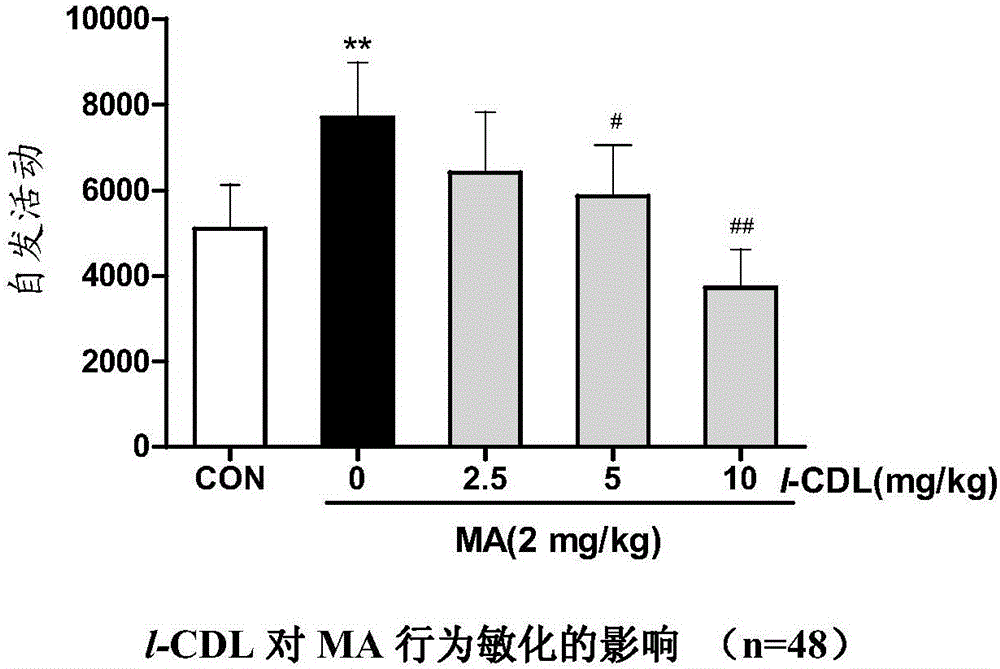
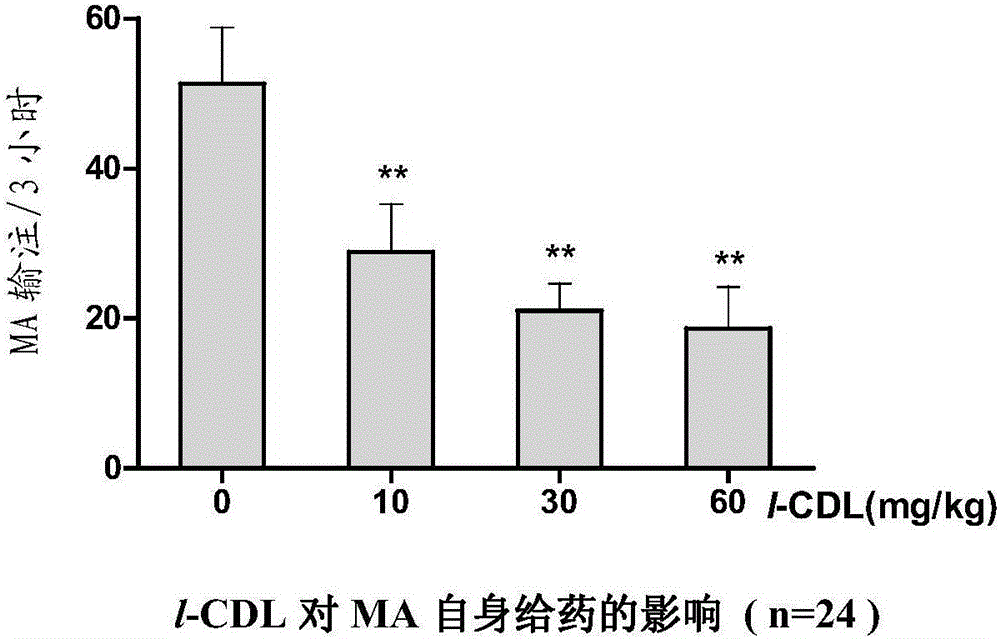
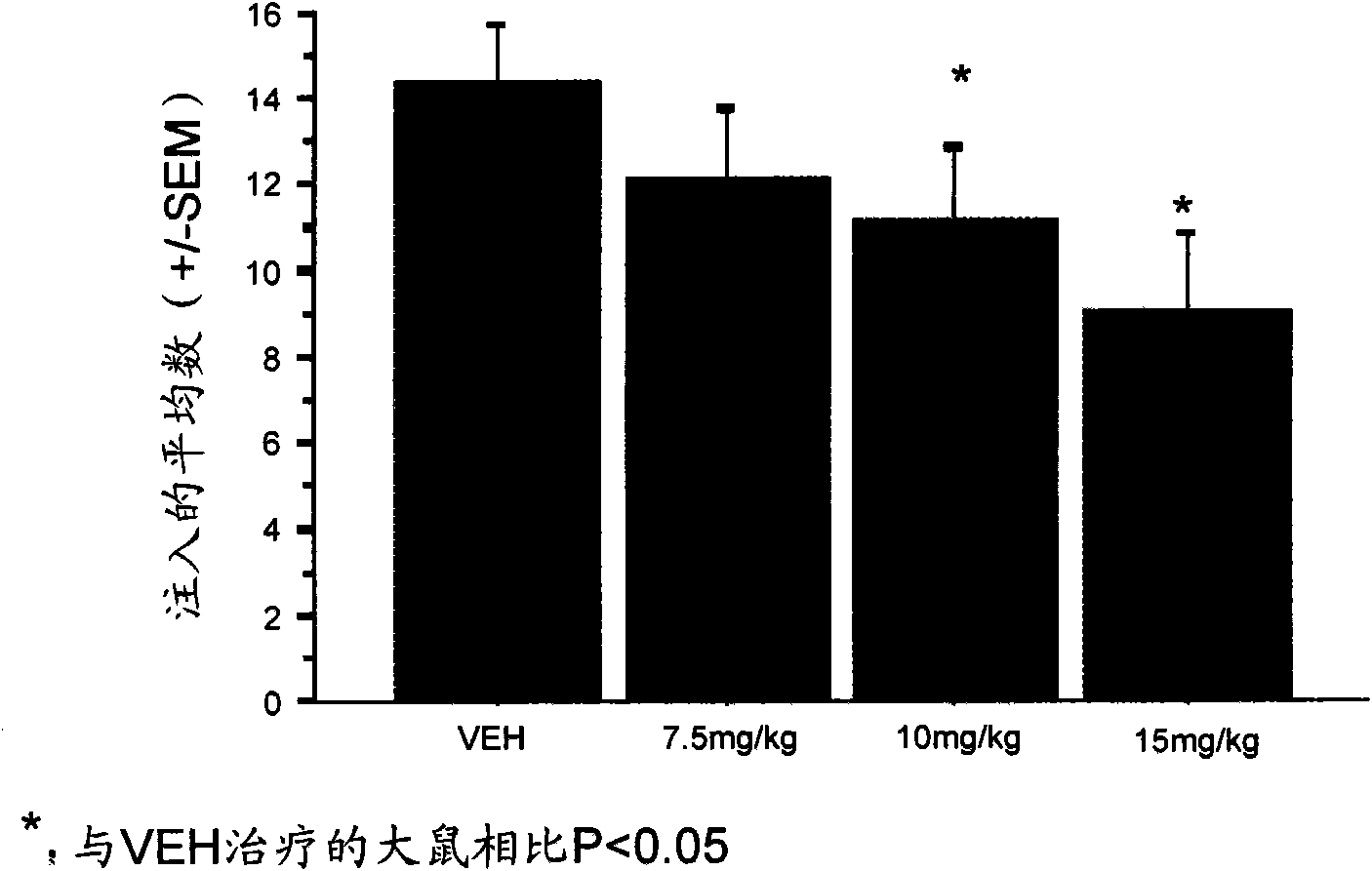
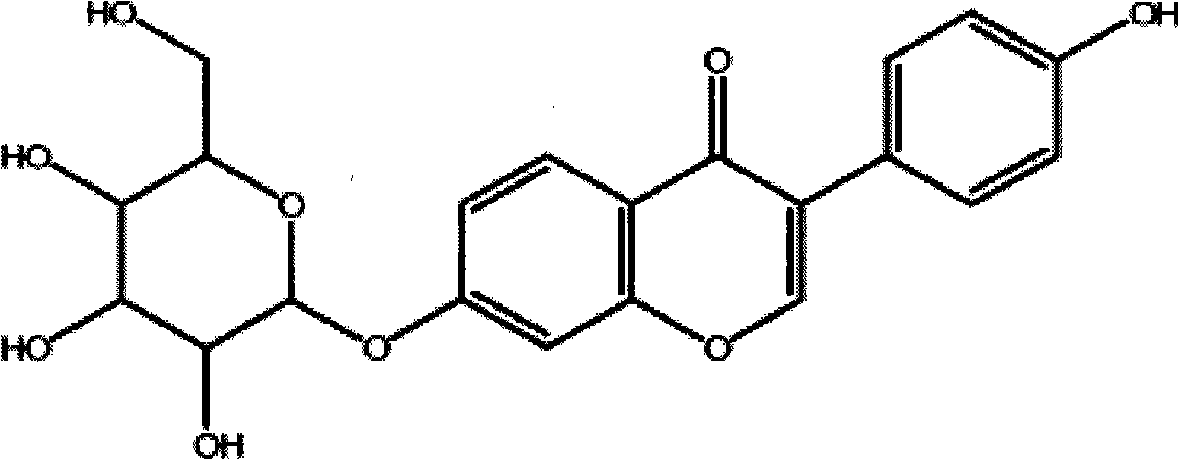
Anti-addiction medical application of L-corydalmine (L-CDL)
ActiveCN106176740AReduced reinforcementIncrease mobilityOrganic active ingredientsNervous disorderDiseaseIsoquinoline
The invention relates to an application of a lead compound namely L-corydalmine (L-CDL) of isoquinoline compounds on treating diseases related with addiction. The diseases comprise drug addiction (heroin, opium, methyl amphetamine, morphine, marihuana, cocaine, and the like), nicotine addiction, and alcohol addiction, and also comprise novel drug addiction (stimulant type novel drugs) and brain damage caused by novel drugs.
Owner:杨征
Method for the detection of compounds comprising methylenedioxyphenyl and testing kit for the same
InactiveUS20050130312A1Analysis by subjecting material to chemical reactionBiological testingStrong acidsAmphetamine
Process and a test kit for the detection of drugs of methylenedioxy amphetamine family and derivatives, are disclosed. The process comprises (a) sampling sufficient amount of a material, suspected to be examined comprise of said methylenedioxyphenyl group; (b) admixing said sample with a sufficient amount of a strong acid reagent; and (c) detecting color gradual appearance and determining accordingly the presence of said methylenedioxy amphetamine drugs in predetermined interval of time. The test kit comprises a strong acid reagent; a color vs time chart comprising means for the detection of a specific drug by means of color and time of appearance, a reaction chamber wherein suspected material and said strong acid reagent are admixed and color is indicated; a sampler having means to collect sufficient amount of suspected material to be tested and to insert it to the reaction chamber.
Owner:BARUCH GLATTSTEIN
Aldh-2 inhibitors in treatment of addiction
InactiveCN101925590AAvoid disintegrationIntact accessOrganic active ingredientsNervous disorderAlcoholMorphine
Owner:GILEAD PALO ALTO +1
Production method of novel efficient rat poison
The invention provides a production method of a novel efficient rat poison which is prepared from the following raw materials in part by weight: 5-50 parts of corn meal or wheat meal or soybean meal, 10-40 parts of bran or fine bran powder, 6-36 parts of industrial paraffin at 60 DEG C, 1-30 parts of rat excrement, 1.0-10 parts of common fish meal, 0.5-10 parts of animal giblets, 0.5-5 parts of animal fat, 0.1-3.0 parts of vegetable oil, 0.01-0.5 part of table salt, 0.01-0.5 part of saccharin, 0.05-0.5 part of amphetamine, 0.001-0.25 part of warfarin or bromadiolone or brodifacoum, 0.001-0.05 part of diethyl ether, 0.001-0.1 part of nitrate or nitrite and 0.01-0.3 part of red pigment or blue pigment. The preparation comprises the steps of sequentially placing the raw materials into a container; stirring for 25-35 minutes at normal temperature under normal pressure to uniformly mix the materials; and preparing the rat poison with a feed granulator.
Owner:文群海
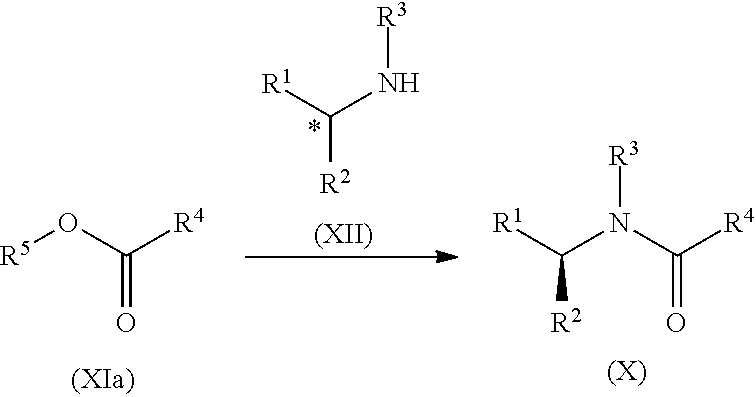
Process for the preparation of lisdexamfetamine and related derivatives
The present invention is directed to processes for the preparation of lisdexamfetamine and related derivatives, wherein the processes comprise coupling to racemic or enantiomerically enriched amphetamine and wherein the resulting product is advantageously enantiomerically or diastereomerically enriched in the desired stereoisomer.
Owner:NORAMCO LLC
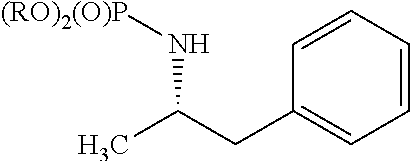
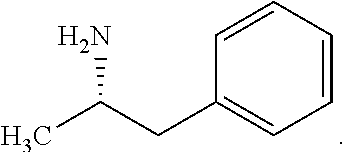
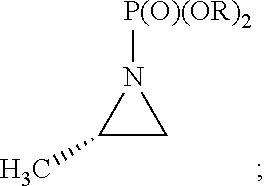
Synthesis of chiral amphetamine derivatives by stereospecific, regioselective cuprate addition reaction with aziridine phosphoramidate compounds
The invention includes processes for the synthesis of amphetamine, dexamphetamine, methamphetamine, derivatives of these, including their salts, and novel precursors and intermediates obtained thereby, by synthesizing aziridine phosphoramidate compounds in specified solvents at specified temperatures, and then converting to a novel aryl or aryl-alkyl phosphoramidate precursors using an organometallic compound such as a copper salt, where the novel aryl or aryl-alkyl phosphoramidate precursor is then easily converted to the target compounds using known reactions.
Owner:CHEMAPOTHECA
Hapten compounds and compositions and uses thereof
ActiveUS20100143391A1Reduce concentrationAntibody mimetics/scaffoldsSnake antigen ingredientsAmphetamineMDMA
The invention generally relates to hapten compounds comprising either (+)methamphetamine or (+)amphetamine conjugated to a linker. Generally speaking, hapten compounds of the invention may be used to elicit an immune response to one or more of (+)methamphetamine, (+)amphetamine, or (+)MDMA.
Owner:BIOVENTURES LLC
Treatment of addiction and dependency
The present invention relates to methods of treating or preventing addiction and relapse use of addictive agents where the method comprises: (a) administering to a subject a co-therapy treatment with an auricular or peri-auricular electro-acupuncture or neurostimulation device, (b) co-treatment with at least one non-narcotic detoxification agent and (c) administering to the subject an opioid antagonist. The methods and compositions of the invention are useful in the treatment or prevention of addiction to any agent, including alcohol, nicotine, marijuana, cocaine, and amphetamines, as well as compulsive and addictive behaviors, including pathological gambling and pathological overeating.
Owner:TACA JR ARTURO C
Hapten compounds and compositions and uses thereof
The invention generally relates to hapten compounds comprising either (+)methamphetamine or (+)amphetamine conjugated to a linker. Generally speaking, hapten compounds of the invention may be used to elicit an immune response to one or more of (+)methamphetamine, (+)amphetamine, or (+)MDMA.
Owner:BIOVENTURES LLC
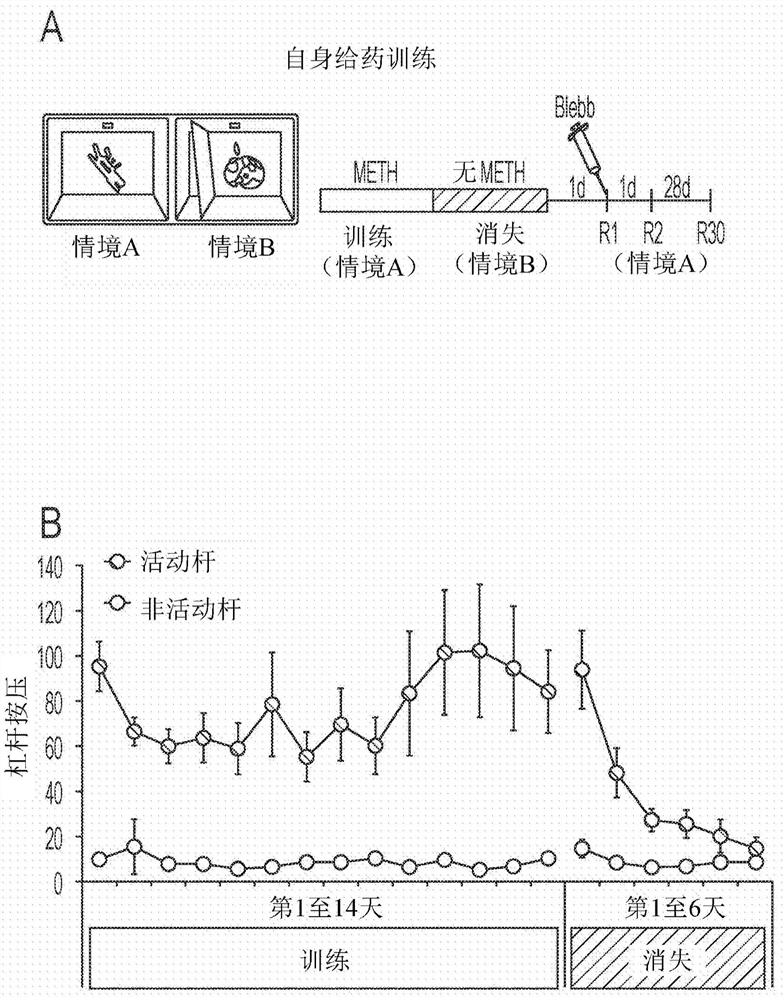
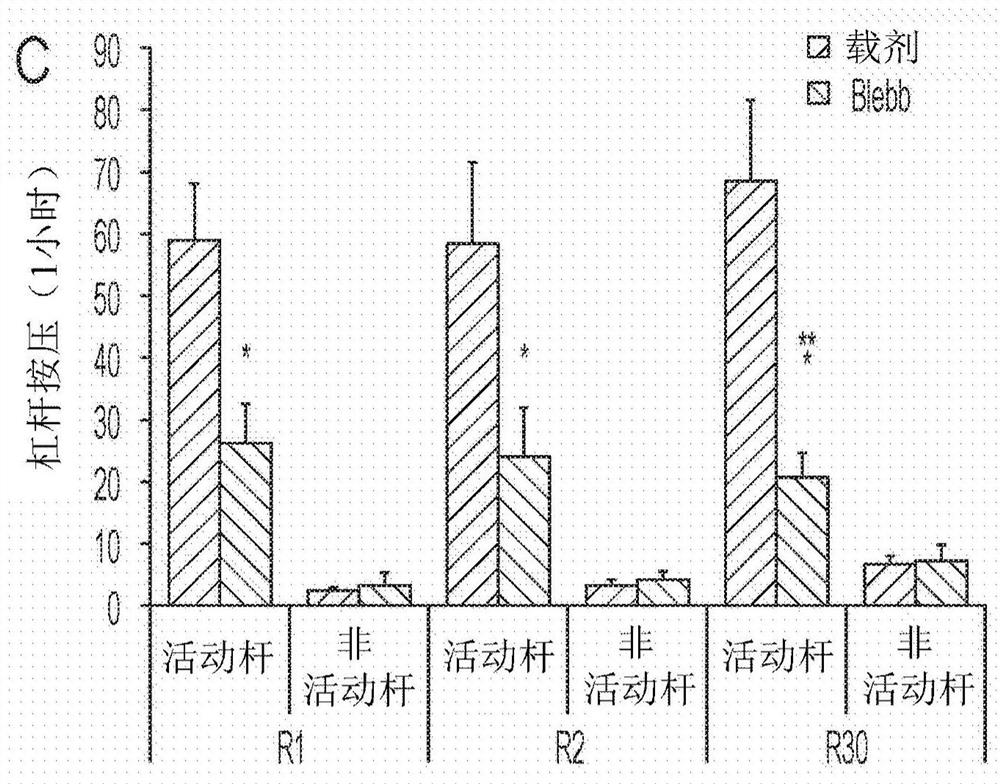
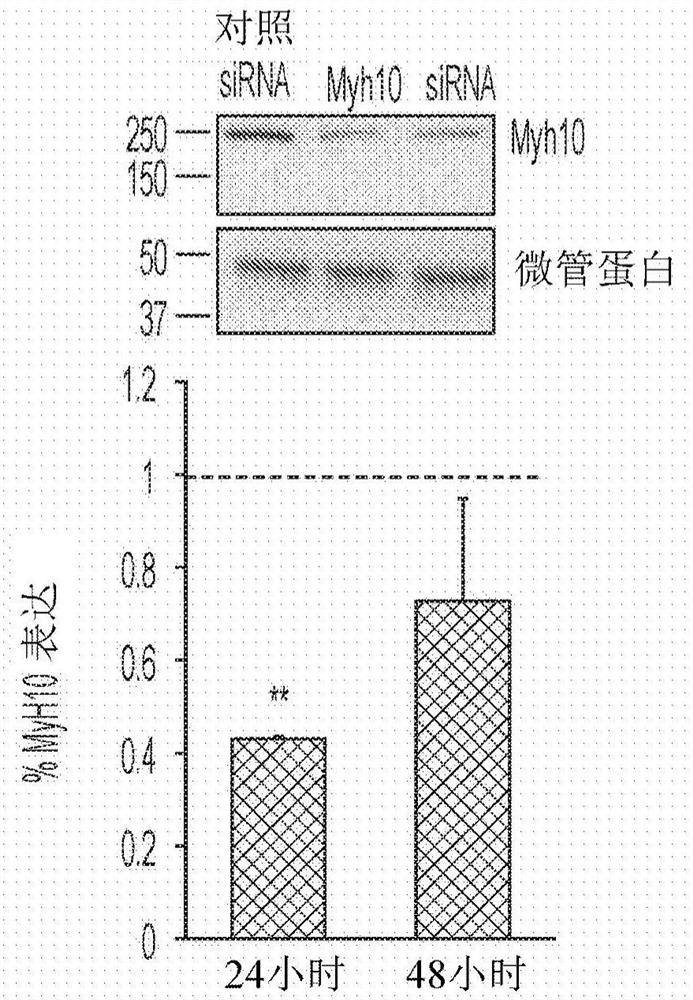
Nonmuscle myosin ii inhibitors for substance use relapse
The invention can provide compounds, analogs of blebbistatin, effective and selective inhibitors of nonmuscle myosin II relative to cardiac myosin II. Compounds can be used in the method of treating adisease, disorder, or medical condition in a patient, comprising modulating myosin II ATPase, such as treatment of substance abuse relapse disorder, or of renal disease, cancer and metastasis, benignprostate hyperplasia, hemostasis or thrombosis, nerve injury including retinal damage, lung fibrosis, liver fibrosis, arthrofibrosis, wound healing, spinal cord injury, periodontitis, glaucoma and immune-related diseases including multiple sclerosis; or wherein the disease, disorder, or medical condition comprises addiction including abuse of or addiction to anything classified as a Substance-Related or Addictive Disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM), such as, but not limited to, cocaine, opioids, amphetamines, ethanol, cannabis / marijuana, nicotine, and activities including gambling Compounds are of general formula (I) with substituents as defined herein.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Emulsified metal cutting fluid and preparation method thereof
InactiveCN104263477AImprove antibacterial propertiesImprove solubilityLubricant compositionSODIUM NAPHTHALENESULFONATEAmphetamine
The invention discloses an emulsified metal cutting fluid and a preparation method thereof. The emulsified metal cutting fluid is prepared from the following raw materials in parts by weight: 15-70 parts of lauric acid, 40-90 parts of epoxyethane, 5-60 parts of polyacrylic acid, 35-60 parts of nekal, 55-80 parts of fatty acid polyoxyethylene ester, 45-90 parts of ethylene glycol, 45-60 parts of citric acid, 50-80 parts of methyl amphetamine, 20-60 parts of sodium tripolyphosphate, 35-65 parts of sodium borate, 15-70 parts of sodium sulfide, 5-20 parts of diffusant and 10-40 parts of leveling agent. The preparation method comprises the following steps: (1) reacting the citric acid, lauric acid, epoxyethane, polyacrylic acid, nekal and fatty acid polyoxyethylene ester at 80-100 DEG C for 1-2 hours, and cooling to 20-30 DEG C; and (2) adding the rest of components into a closed reaction kettle, uniformly mixing, adding the mixture of the step (1), and stirring uniformly. The emulsified metal cutting fluid has the advantages of high antimicrobial capacity and long shelf life.
Owner:JIANGSU KANG BAISI MECHANICAL TECH
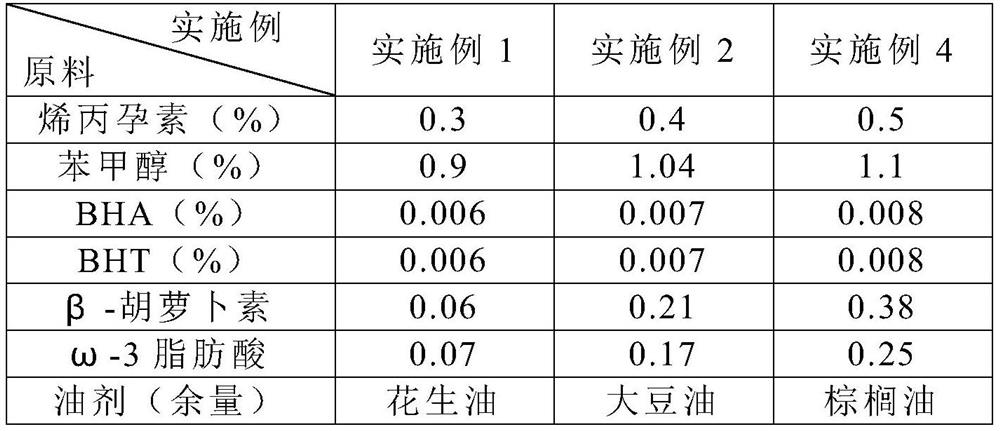
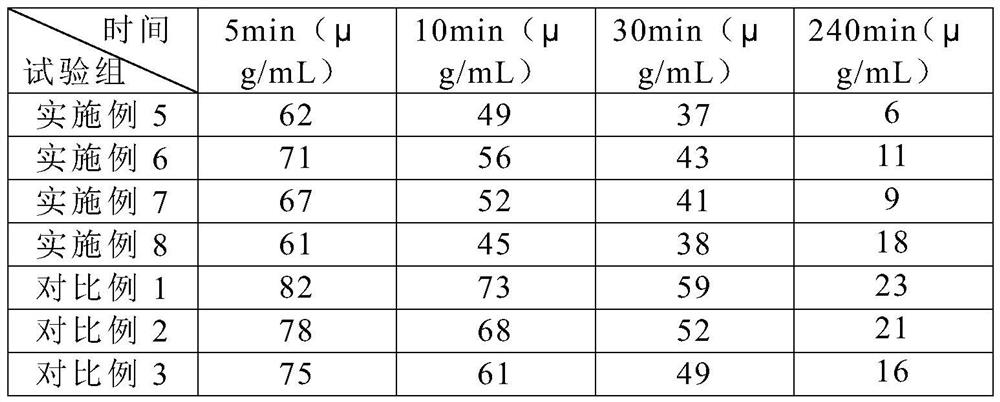
A kind of veterinary allyl progesterone preparation and preparation method thereof
ActiveCN109260208BEffectively regulate endocrineControl regular estrusOrganic active ingredientsOrganic non-active ingredientsAmphetamineVeterinary Drugs
The invention relates to a veterinary allylgestrine preparation and a preparation method thereof, belonging to the technical field of veterinary medicine. The veterinary allylgestrine preparation of the present invention is made of raw materials comprising the following mass percentages, 0.3-0.5% allylgestrine, 0.9-1.1% benzyl alcohol, 0.012-0.016% antioxidant, 0.06-0.38% β- Carotene, 0.07~0.25% omega-3 fatty acid. The allylgestrine preparation of the invention has good absorption effect, high bioavailability and good dispersion effect of the preparation.
Owner:NINGBO SANSHENG BIOLOGICAL TECH CO LTD
A rapid detection method for amitraz residues in agricultural products
ActiveCN114486876BLess side effectsHigh yieldMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationBiotechnologyXylylene
The invention discloses a rapid detection method for amitraz residue in agricultural products, comprising the following steps: step a, preparing a homogeneous sample to be tested; step b, extracting and concentrating; step c, redissolving; step d, adding phthalate The chloroform solution of formic anhydride is subjected to protection reaction; step e, deprotection reaction to generate xylidine; step f, color reaction; step g, result judgment. The principle of the method of the present invention is simple and easy to understand, with strong specificity, high sensitivity, intuitive detection results, high accuracy of detection results, no need for large-scale analysis instruments, relatively less time-consuming, and extremely low average analysis cost, and is specially suitable for agricultural products. The on-site rapid detection of amitraz residues in Chinese medicine has strong practicability.
Owner:广东江门中医药职业学院
Compositions and methods for prophylaxis and treatment of addictions
ActiveUS11241420B2Many symptomReduce oneNervous disorderPeptide/protein ingredientsAmphetamineAddictive behavior
Owner:OMEROS CORP
Methylamphetamine detection card capable of preventing urine sample from being counterfeited as well as preparation method and application of methyl amphetamine detection card
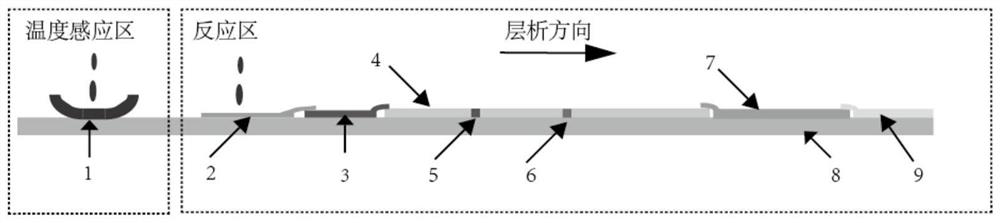
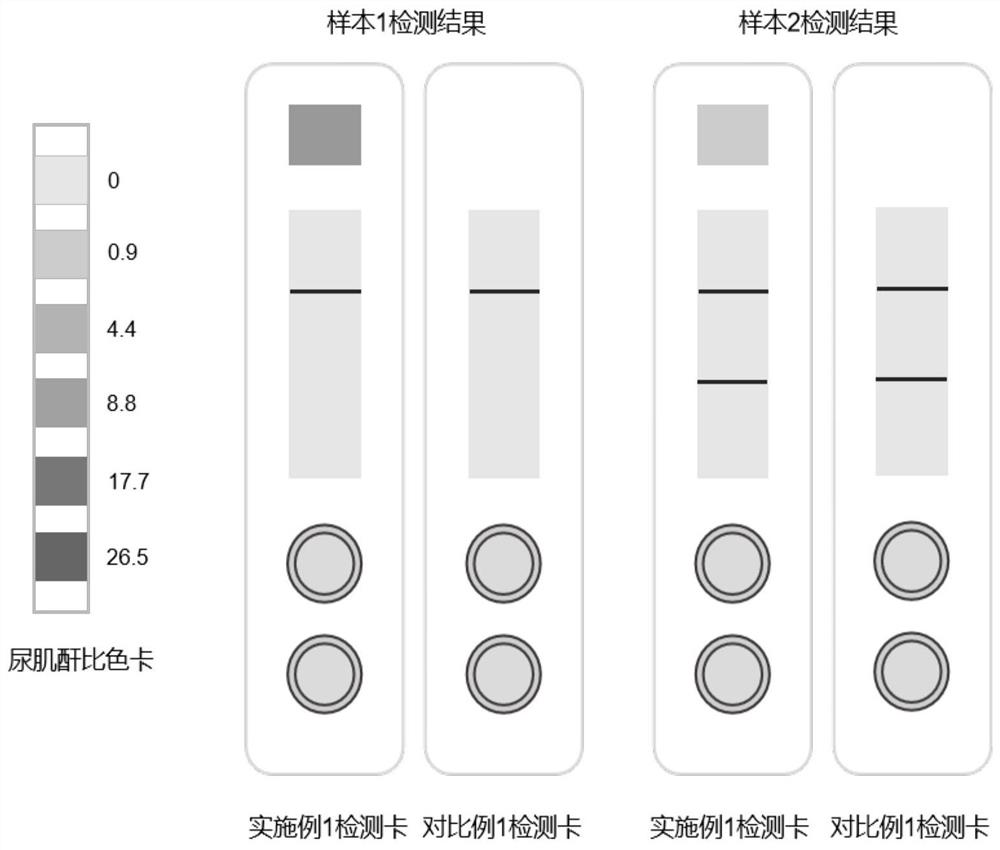
The invention relates to the technical field of biological detection, in particular to a methylamphetamine detection card for preventing urine samples from being counterfeited as well as a preparation method and application of the methylamphetamine detection card. The methylamphetamine detection card comprises a temperature sensing area, a methylamphetamine detection area and a urine property detection area, wherein the temperature sensing area is used for detecting the temperature of urine, the methylamphetamine detection area is used for detecting the content of methylamphetamine, and the urine property detection area is used for detecting the physicochemical properties of the urine; the detection card can be used for simultaneously detecting the content of methylamphetamine in a urine sample and detecting whether the urine sample is diluted or not, and has higher specificity and sensitivity on the detection of the counterfeiting of the urine sample and the detection of the content of methylamphetamine; the detection card can effectively prevent leak detection caused by the counterfeiting of the urine sample due to dilution and the like, improves the accuracy of methylamphetamine detection, and has important application value in methylamphetamine detection.
Owner:戴国华
D-amphetamine compounds, compositions, and processes for making and using the same
ActiveUS20200131130A1Eliminate the effects ofReduce stressOrganic active ingredientsNervous disorderOrganic acidAmphetamine
Disclosed are d-amphetamine compounds and compositions comprising at least one organic acid covalently bound to d-amphetamine, a salt thereof, a derivative thereof, or a combination thereof. Methods of making and using the same are also disclosed.
Owner:KEMPHARM INC
Compositions Comprising Enzyme-Cleavable Amphetamine Prodrugs and Inhibitors Thereof
ActiveUS20160235694A1Eliminate side effectsPatient compliance is goodOrganic chemistryPharmaceutical delivery mechanismControlled releaseAmphetamine
Pharmaceutical compositions and their methods of use are provided, where the pharmaceutical compositions comprise an amphetamine prodrug that provides enzymatically-controlled release of amphetamine or an amphetamine analog. The composition can further comprise an enzyme inhibitor that interacts with the enzyme(s) that mediates the enzymatically-controlled release of amphetamine or the amphetamine analog from the amphetamine prodrug so as to attenuate enzymatic cleavage of the amphetamine prodrug.
Owner:SIGNATURE THERAPEUTICS
Simple Mechanical Procedure and Product for Deterring Substance Abuse.
InactiveUS20120301405A1Improve securityProblem can be addressedBiocidePharmaceutical delivery mechanismBenzodiazepineSubstance abuser
A drug-formulating method, a drug commercial-distribution method, and a drug formulation improve safety of a drug that is at risk for abuse—such as methylphenidate, or amphetamine, or an amphetamine-like central-nervous-system stimulant, and particularly a benzodiazepine. The drug is formulated into a form (not a transdermal patch) that tends to deter conversion to powder; and in this form commercially distributed—preferably enclosed in, or dissolved or dispersed into or onto, a nontoxic carrier such as a capsule, for example a gel, e.g., methylcellulose, hydroxymethylcellulose, carbomer polymer, or other gelatinous pharmaceutical agent that is FDA-acceptable. The carrier is preferably water-insoluble, to deter dissolving in water for injection, and may be an oil or a solid—for example paper or other thin medium broadly extended in two dimensions, or a sponge or other medium having generally coarse cellular structure.
Owner:KULLI JOHN C
Matrix-based pulse release pharmaceutical formulation
The present invention relates to an oral pulse release pharmaceutical composition, which comprises a polymer matrix core, wherein at least one pharmaceutically active ingredient is distributed within the core and on the outer surface of the core. Amphetamine salts, among a number of other pharmaceutically active ingredients, can be formulated as a pharmaceutical composition described herein. The present invention also provides a method for preparing an immediate release component on a solid pharmaceutical formulation.
Owner:SPECGX LLC
Amphetamine immunodetection reagent and preparation and detection methods thereof
InactiveCN108061796AHigh sensitivityStrong specificityBiological testingAmphetamineGlucose dehydrogenase
The invention discloses an amphetamine immunodetection reagent and preparation and detection methods thereof. The amphetamine immunodetection reagent comprises enzyme labeled amphetamine and an indication reagent for detecting an amphetamine antibody-enzyme labeled amphetamine compound, wherein the enzyme labeled amphetamine is formed by coupling amphetamine with glucose dehydrogenase. The amphetamine immunodetection reagent can accurately and quickly determine the content of amphetamine in such samples as human blood. Compared with a conventional detection reagent on the market, the amphetamine immunodetection reagent has the advantages of being convenient and quick, high in sensitiveness, strong in specificity and accurate in quantification, and facilitates clinical popularization.
Owner:太原瑞盛生物科技有限公司
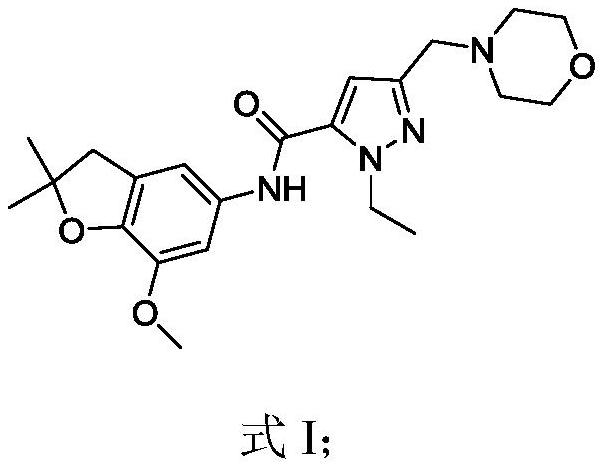
Pyrazole derivative and application thereof as PDE10 inhibitor
ActiveCN114524808ABroaden MAO inhibitory activityEnhanced inhibitory effectOrganic active ingredientsNervous disorderDiseaseOpiate
The invention relates to a pyrazole derivative and application thereof as a PDE10 inhibitor. Meanwhile, the compound can be used for treating PDE10 mediated diseases; the disease is selected from schizophrenia, such as disorder, tension, unclassified or residual schizophrenia; schizophrenia-like conditions; emotional schizophrenia, such as delusional disorder or depressive type; delusional disorders; a substance-induced psychiatric disorder, such as a psychiatric disorder induced by alcohol, amphetamine, cannabis sativa, cocaine, a rosemary, an inhalant, an opium-like substance, or benxacidine; a delusional personality disorder; and schizophrenia personality disorder.
Owner:SHENZHEN CHILDRENS HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com