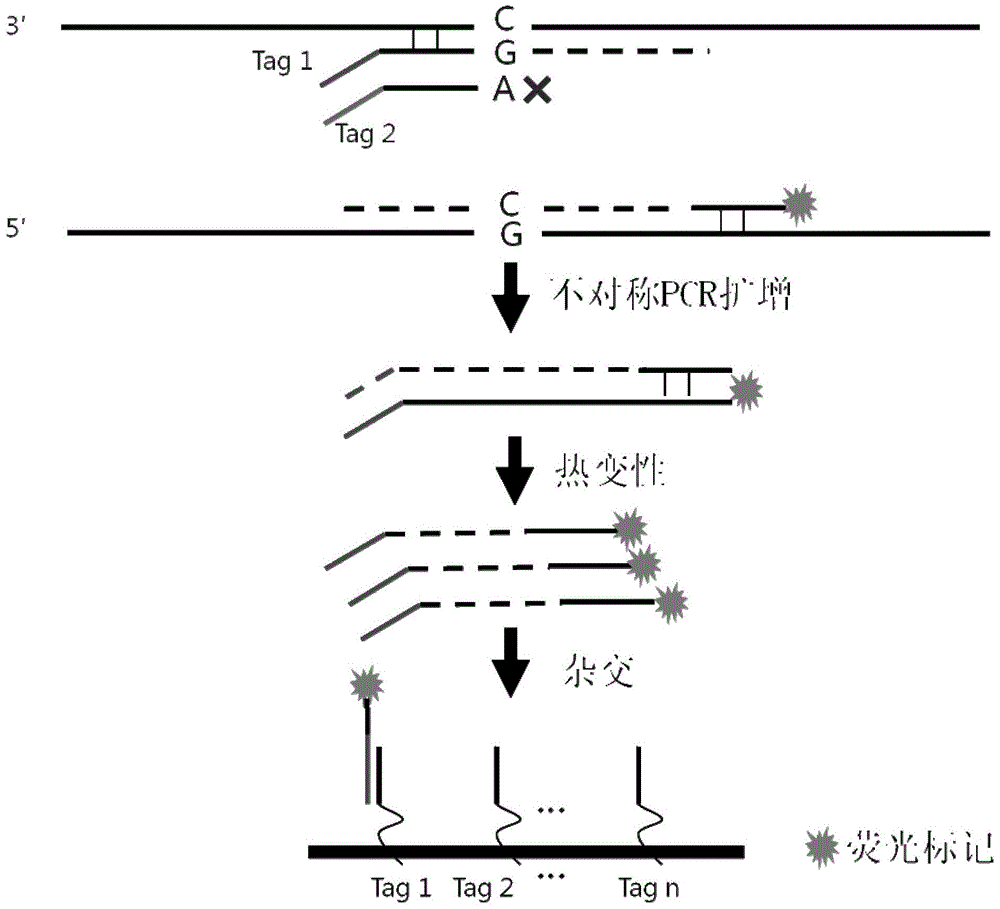
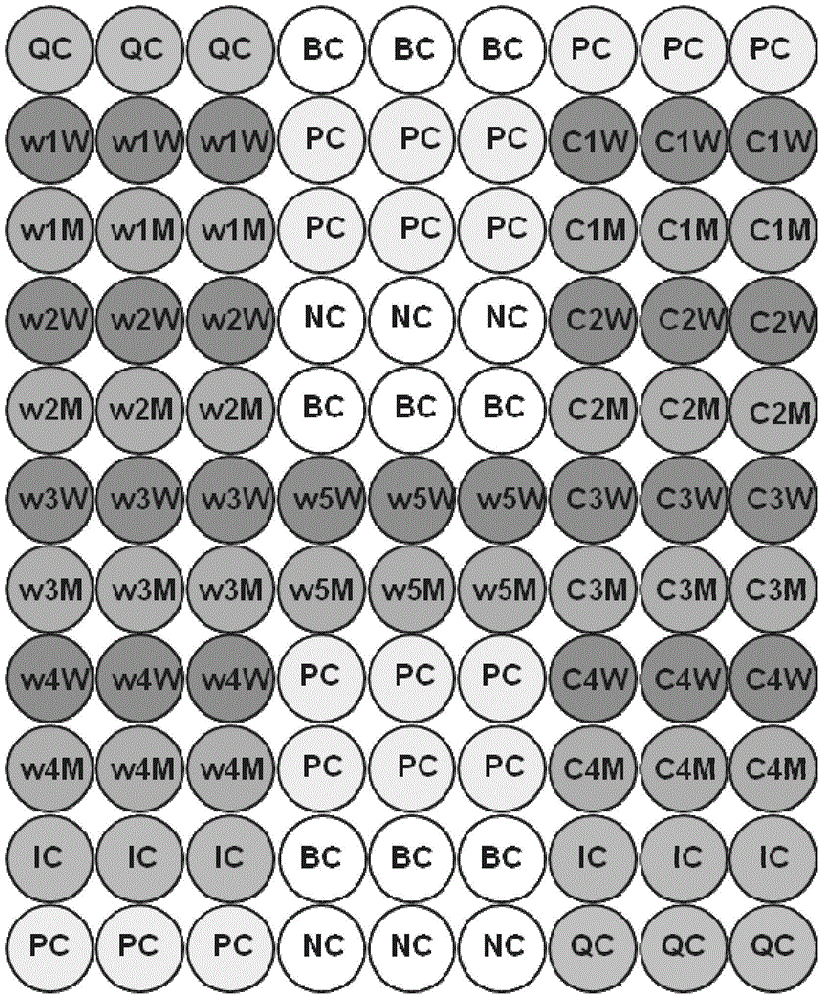
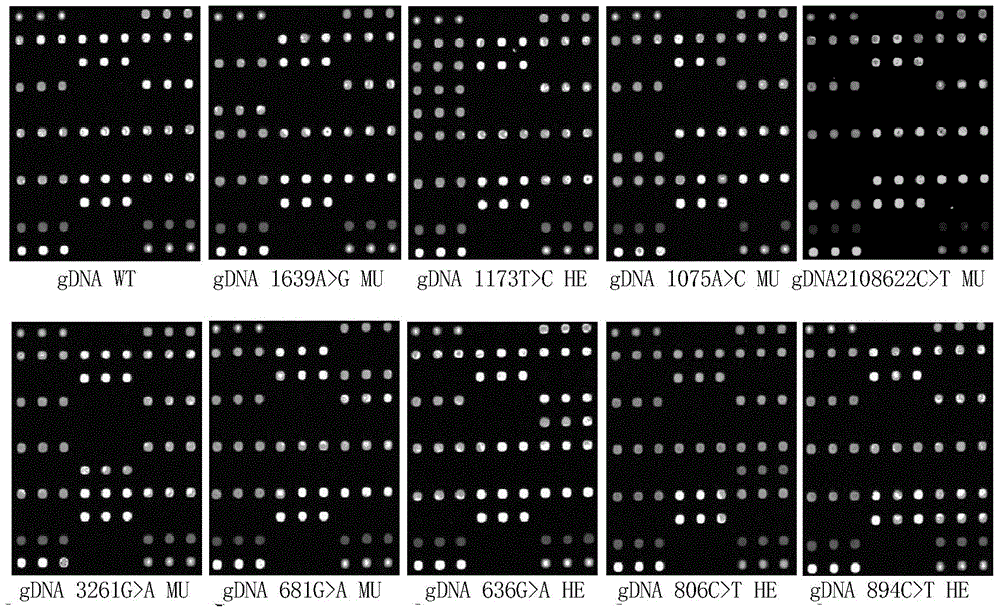
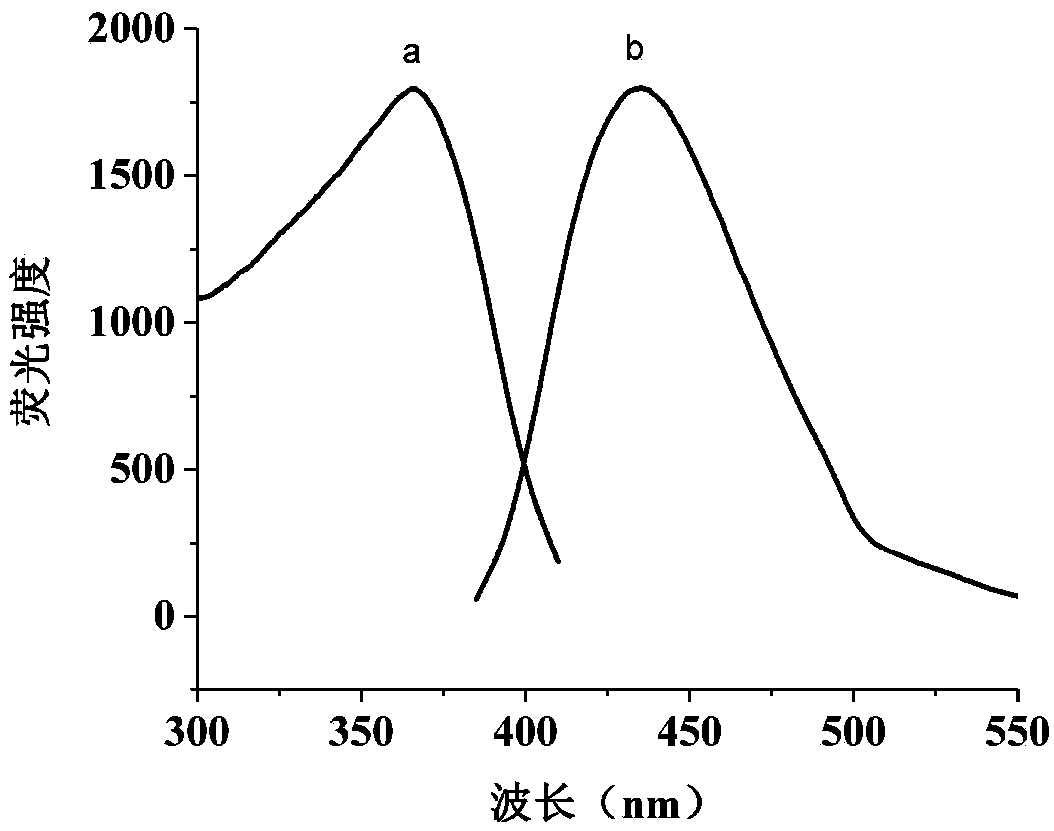
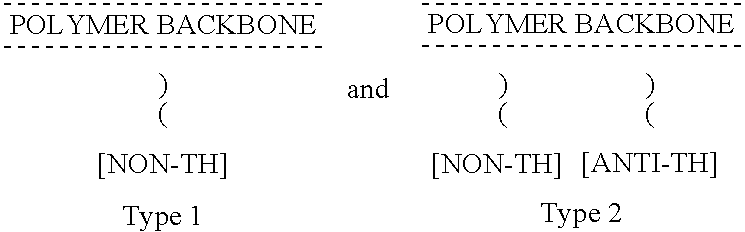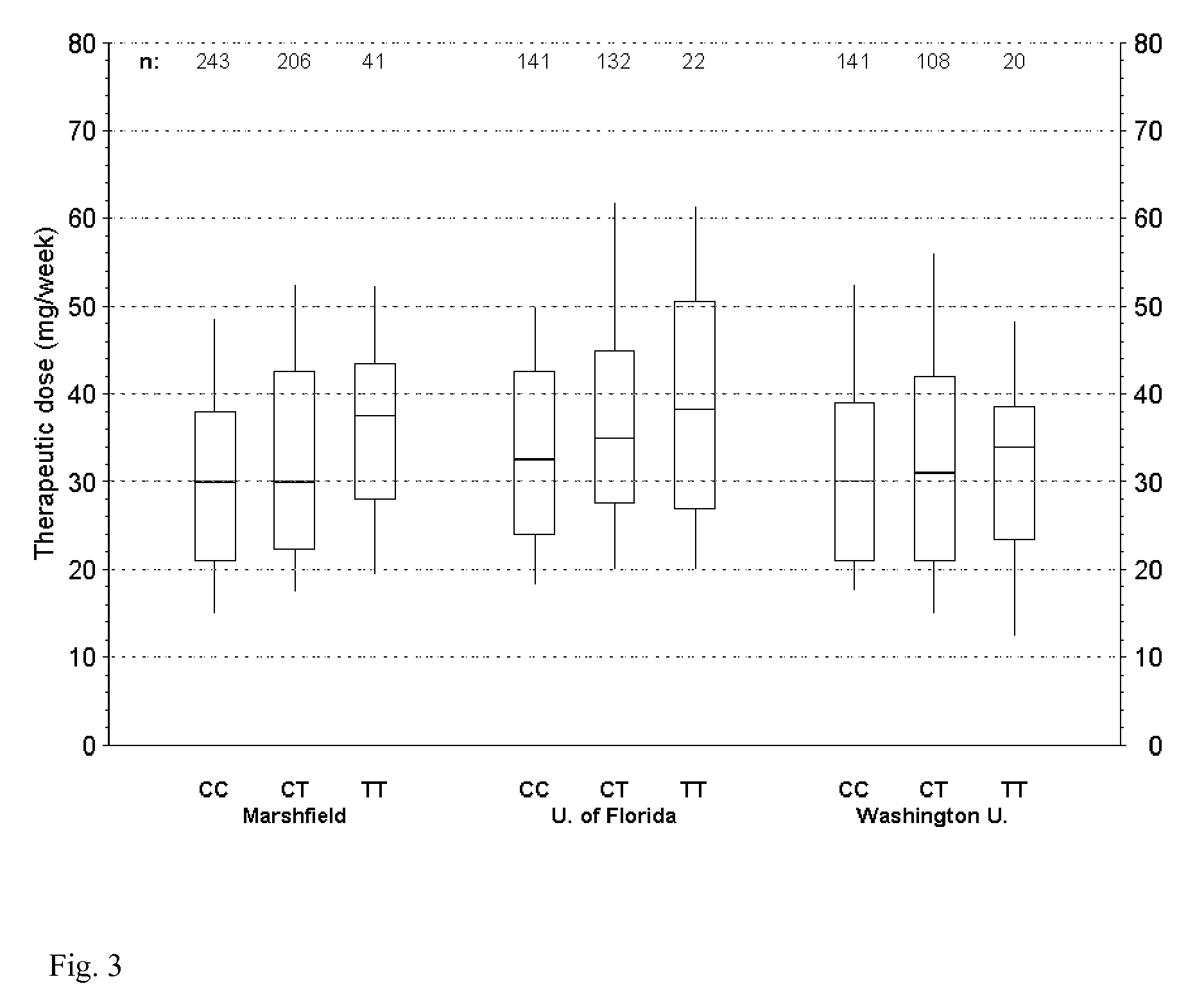Patents
Literature
136 results about "Warfarin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat blood clots (such as in deep vein thrombosis-DVT or pulmonary embolus-PE) and/or to prevent new clots from forming in your body.
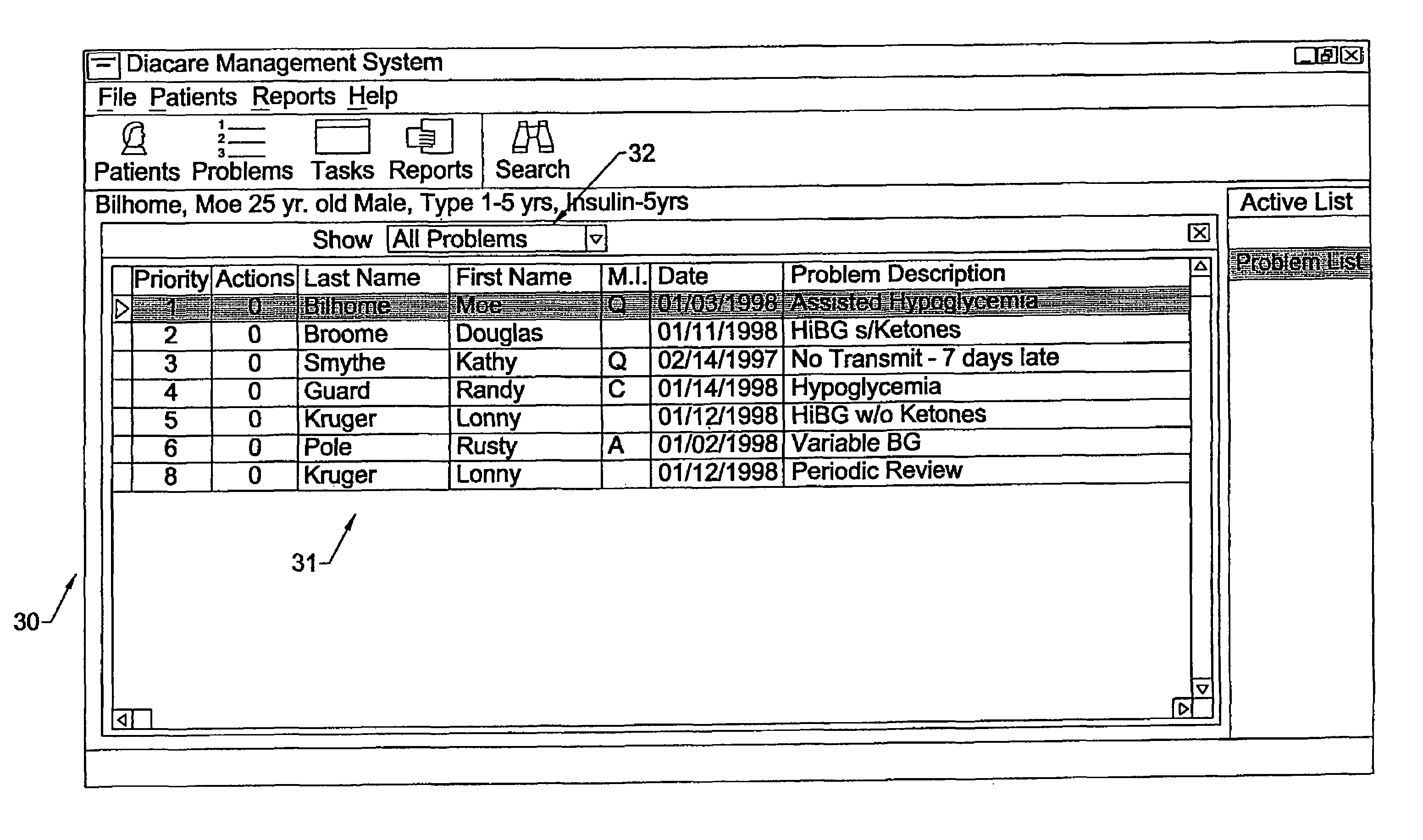
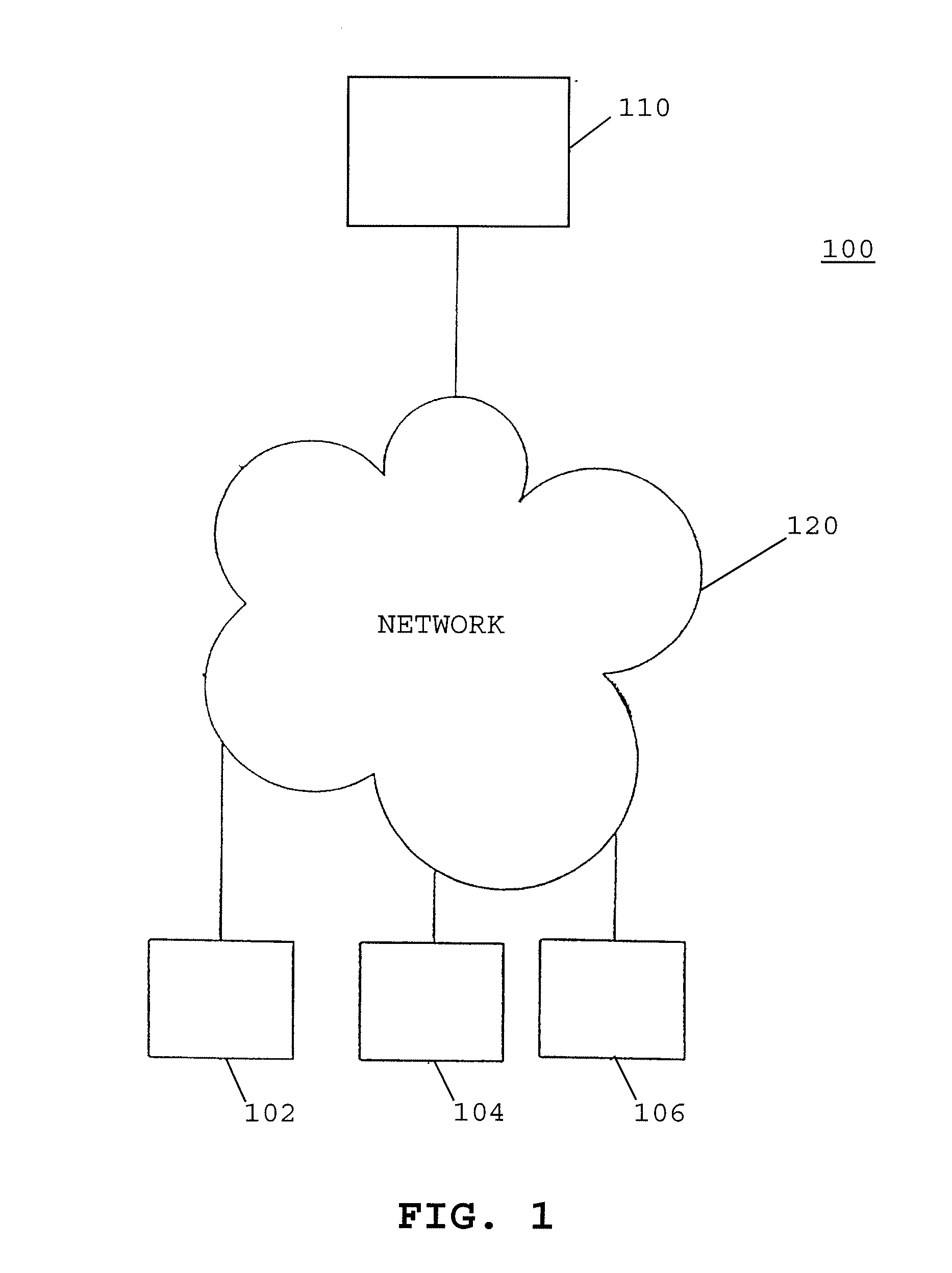
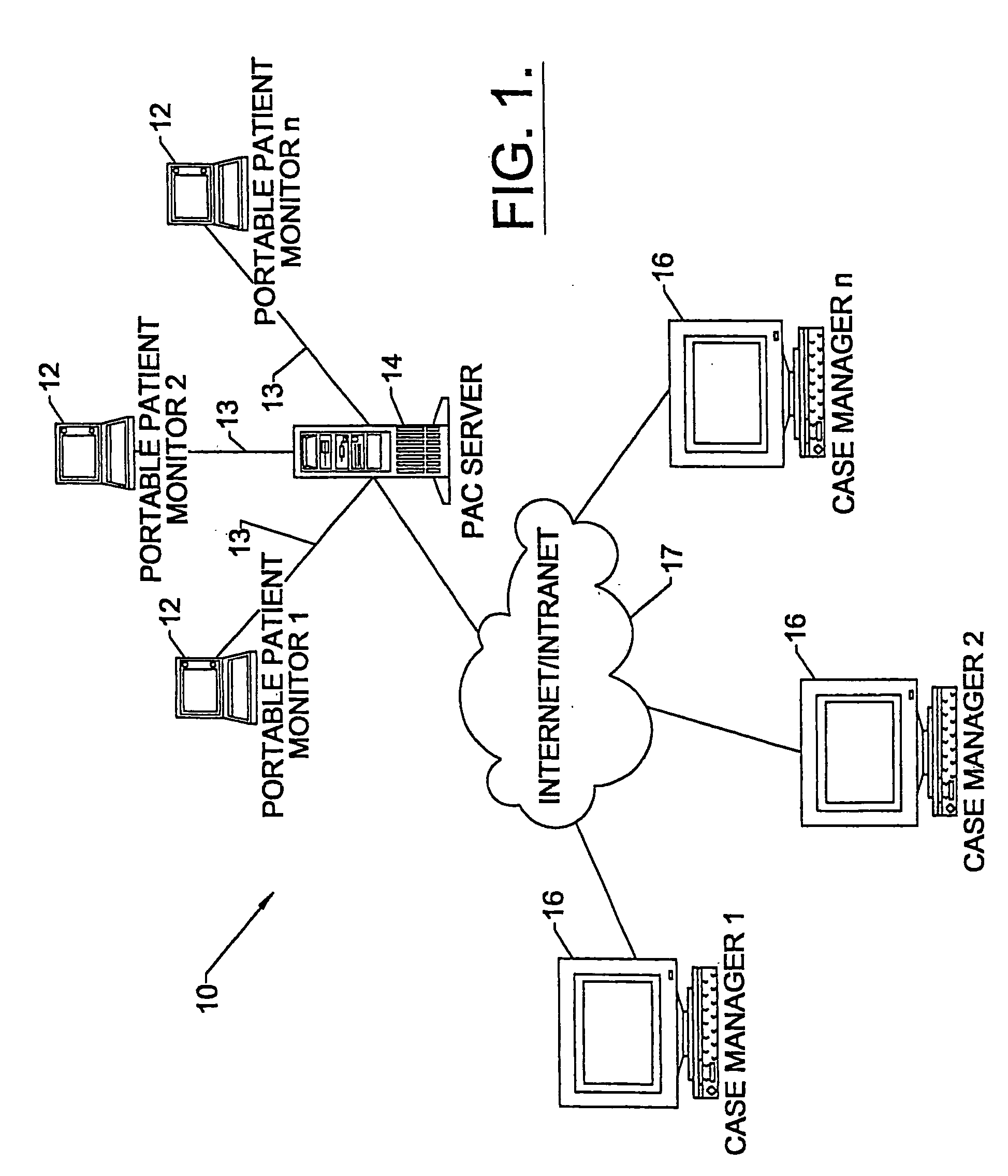
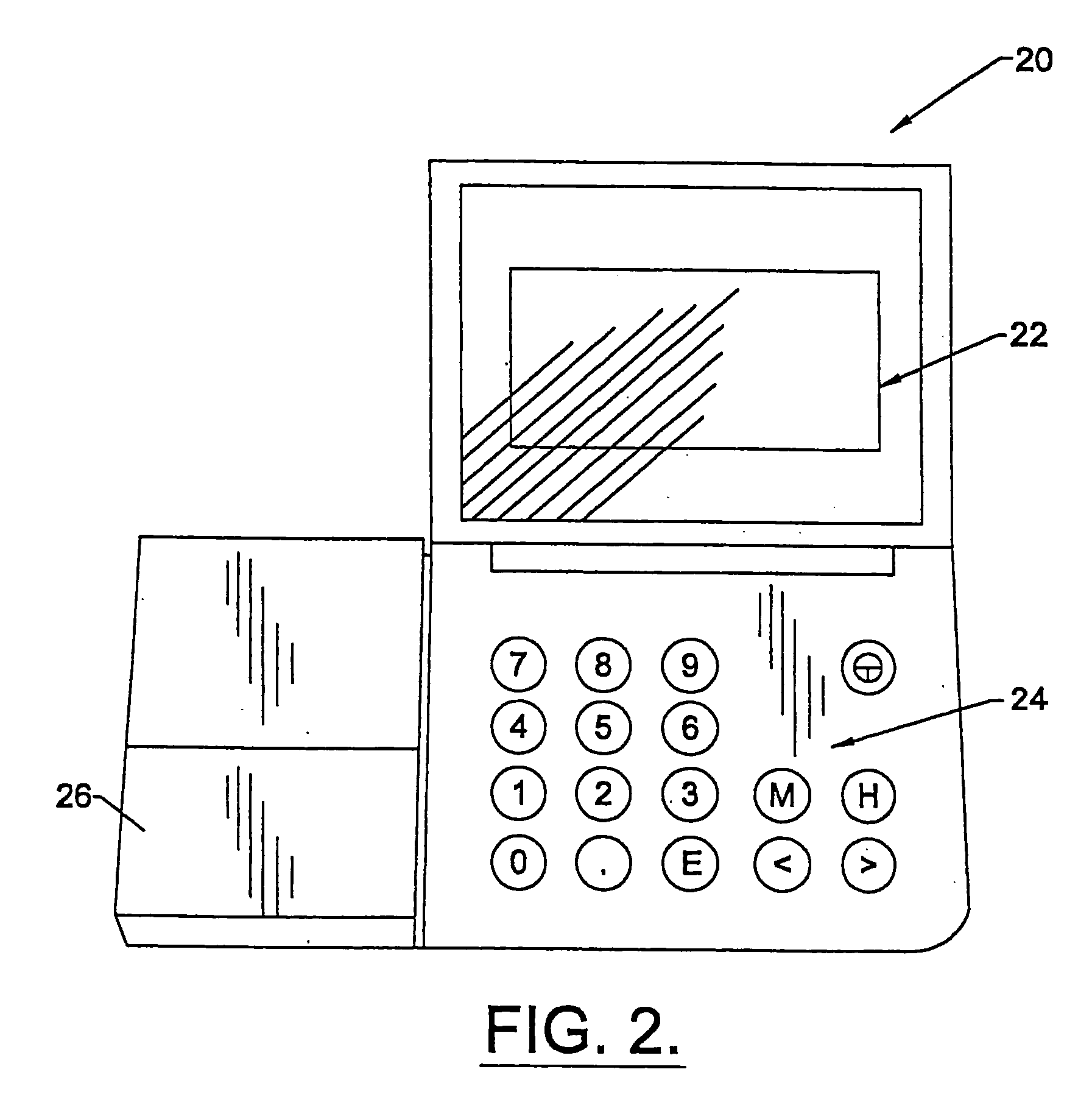
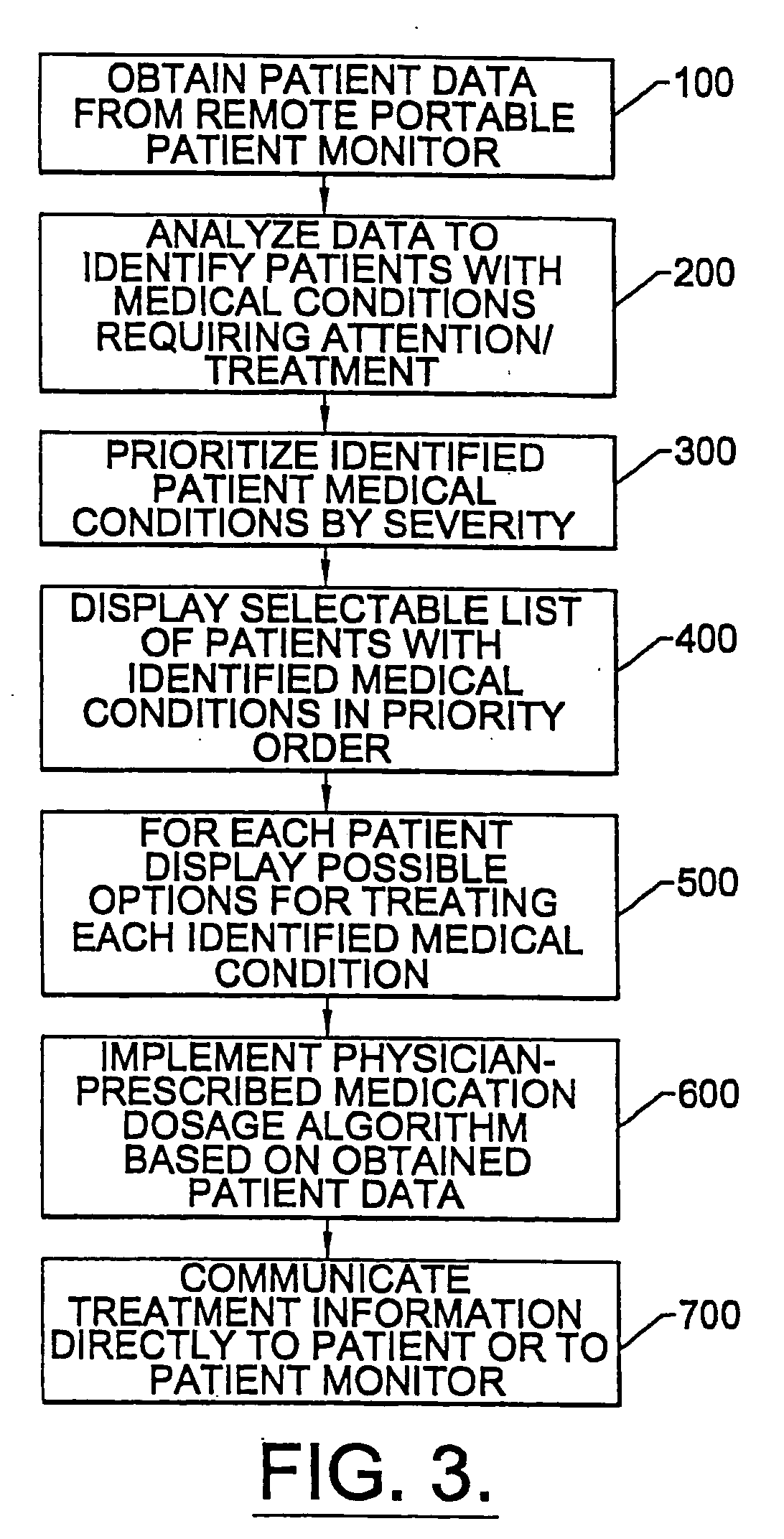
Apparatus and methods for monitoring and modifying anticoagulation therapy of remotely located patients
InactiveUS6980958B1Significant comprehensive benefitsCost-effectiveDrug and medicationsComputer-assisted medical data acquisitionWarfarinRegimen
A patient apparatus is configured to receive and analyze information regarding patient compliance with anticoagulation medication and self-test coagulation regimens related to anticoagulation therapy. In addition, a patient apparatus is configured to receive data from a patient, including physiological data, pathophysiological data, biological data, psychological data, neuropsychological data, and / or behavioral data. Utilizing the received patient data, a patient apparatus can modify a warfarin regimen using an algorithm contained within the apparatus. The apparatus can communicate the modified warfarin regimen to the patient and to third parties, such as remotely located healthcare providers. In addition, the apparatus can prompt a patient when to perform a self-test and can prompt a patient to seek immediate medical attention, or to directly contact medical help, when so warranted.
Owner:GENERAL ELECTRIC CAPITAL AS AGENT
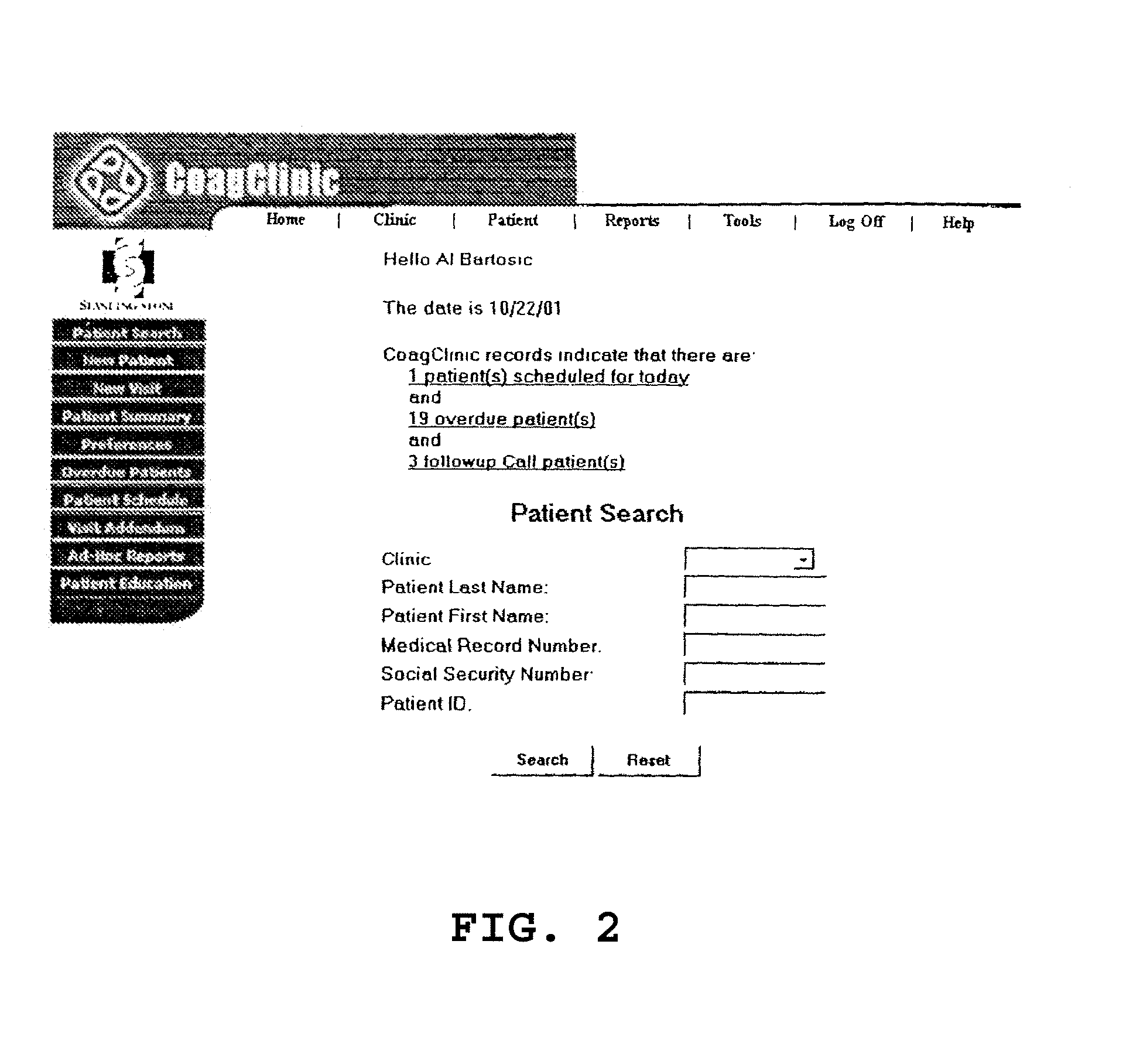
Method and system for administering anticoagulation therapy
InactiveUS20090234674A1Efficient managementOptimizationDrug and medicationsOffice automationWarfarinTime schedule
A method and system for effectively administering anticoagulation therapy, including providing a warfarin dose weekly schedule and converting a total weekly requirement into daily dosages based on the number of milligrams in the pills selected for treatment. A default pill size can be selected as well as other customizable features. Medications can be recorded simultaneously and potential drug interactions are highlighted. Dates on which the patient should return to the clinic are automatically calculated for review. The patient is provided with a hardcopy of the visit as well as the recommended warfarin dose schedule. The user is provided with several customizable options for recording pertinent visit data.
Owner:STANDING STONE
Apparatus and methods for monitoring and modifying anticoagulation therapy of remotely located patients
InactiveUS20050191716A1Maximizes therapeutic benefitRisk minimizationBiocideDrug and medicationsWarfarinRegimen
A patient apparatus is configured to receive and analyze information regarding patient compliance with anticoagulation medication and self-test coagulation regimens related to anticoagulation therapy. In addition, a patient apparatus is configured to receive data from a patient, including physiological data, pathophysiological data, biological data, psychological data, neuropsychological data, and / or behavioral data. Utilizing the received patient data, a patient apparatus can modify a warfarin regimen using an algorithm contained within the apparatus. The apparatus can communicate the modified warfarin regimen to the patient and to third parties, such as remotely located healthcare providers. In addition, the apparatus can prompt a patient when to perform a self-test and can prompt a patient to seek immediate medical attention, or to directly contact medical help, when so warranted.
Owner:GENERAL ELECTRIC CAPITAL AS AGENT
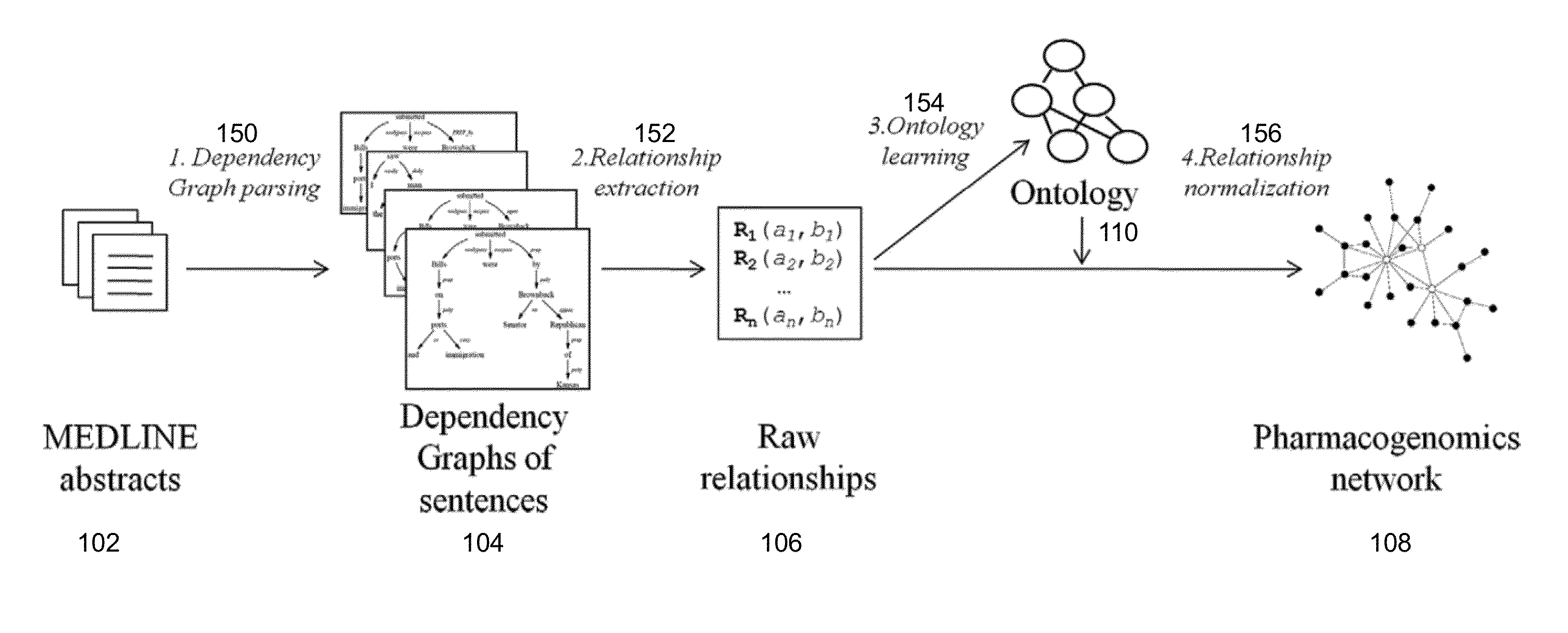
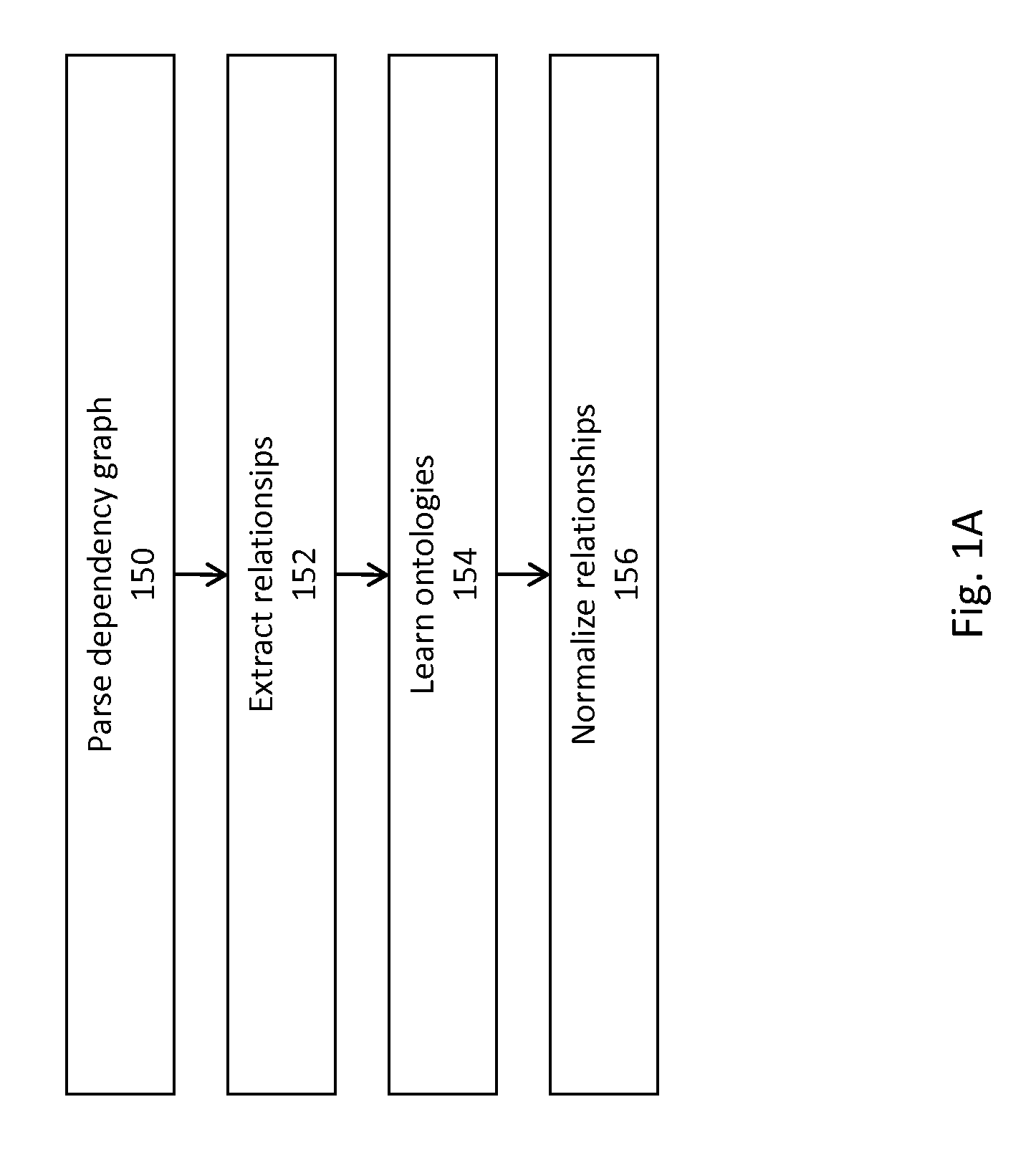
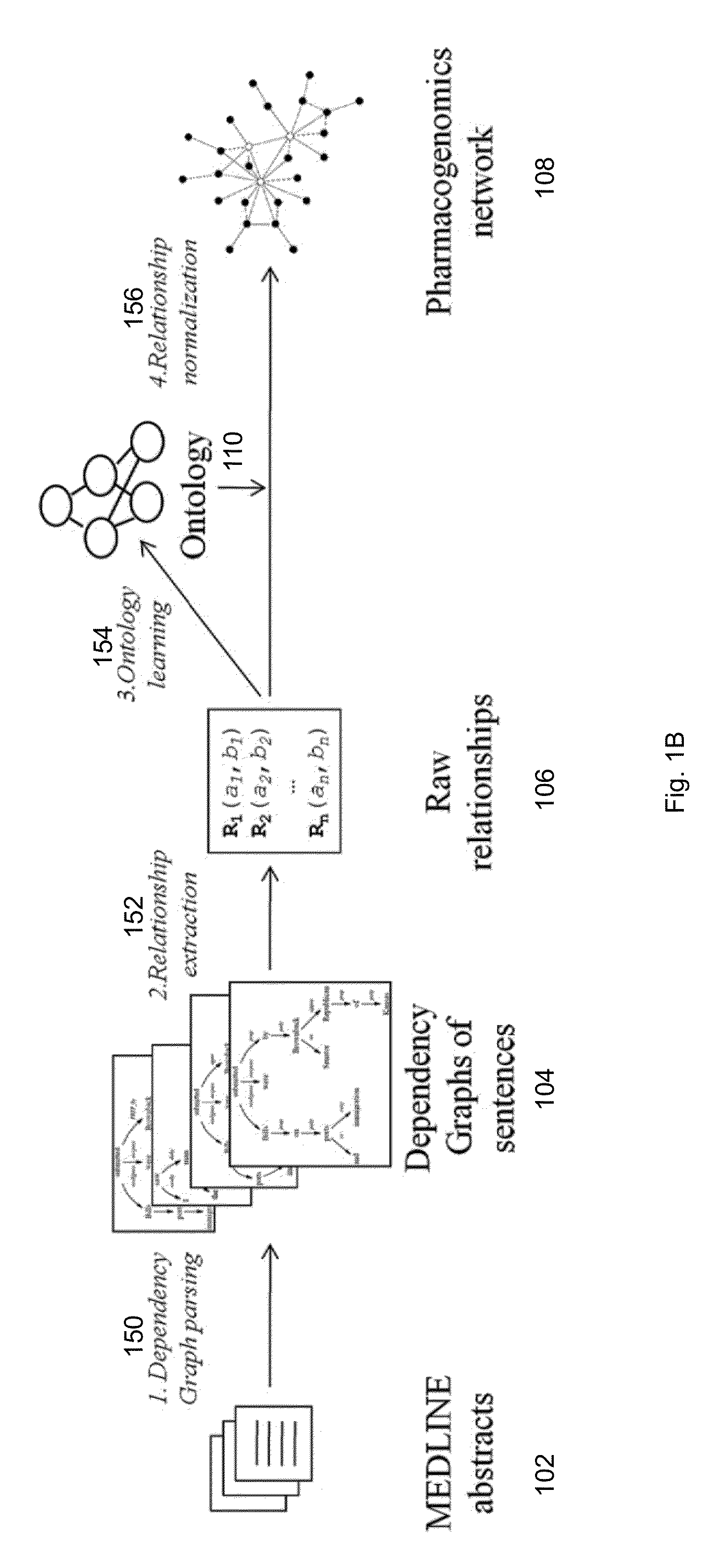
Method And System For Extraction And Normalization Of Relationships Via Ontology Induction
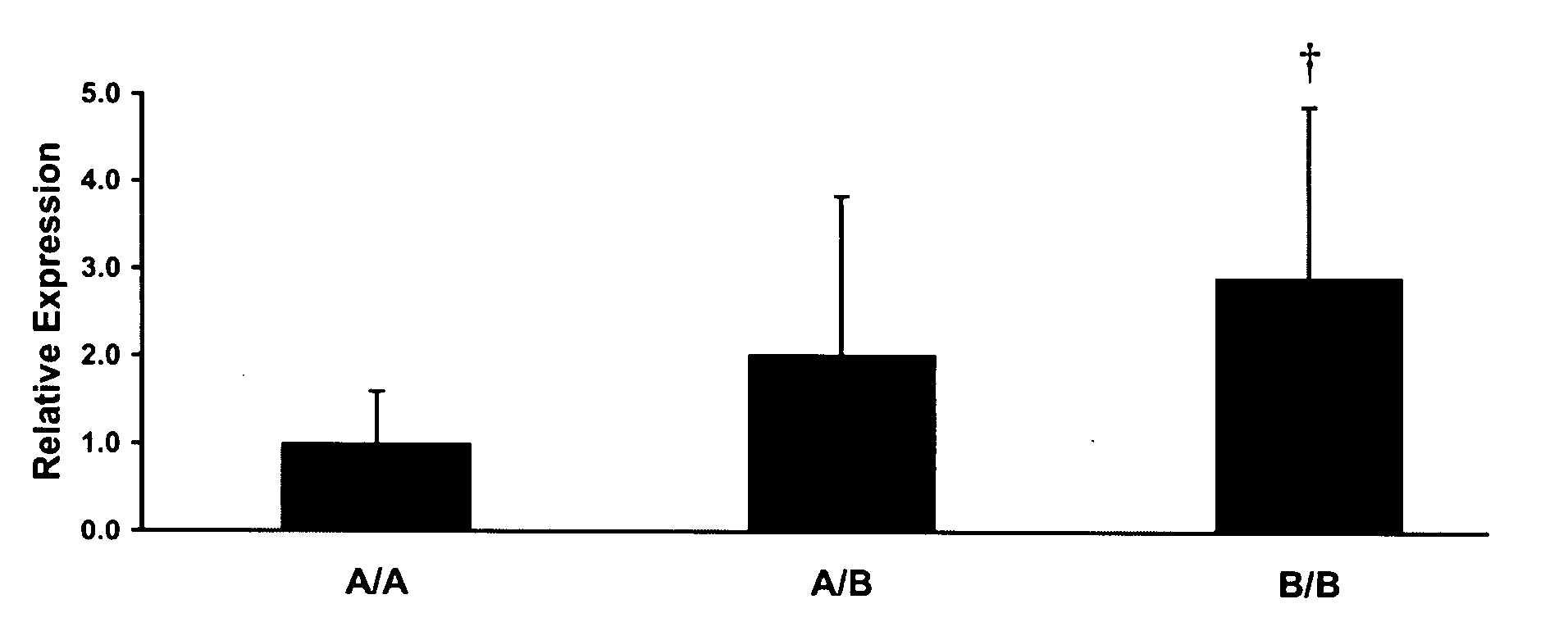
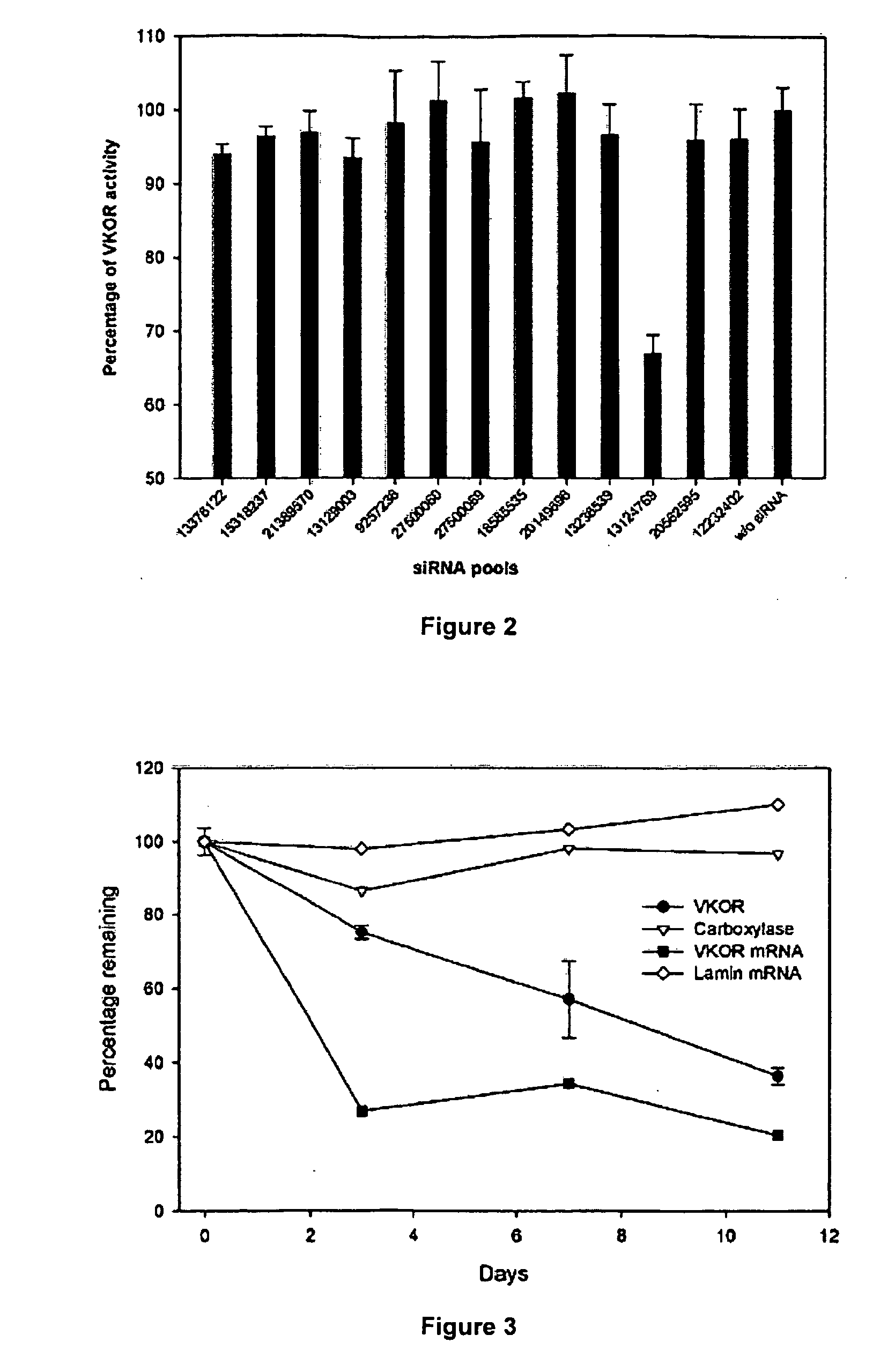
Methods for developing an ontology of pharmacogenomics (PGx) relationships starting from a lexicon of key pharmacogenomic entities and a syntactic parse is described. The syntactic structure of PGx statements is used to systematically extract commonly occurring relationships and to map them to a common schema. In an embodiment, extracted relationships have a 70-87.7% precision and involve not only key PGx entities such as genes, drugs, and phenotypes (e.g., VKORCl, warfarin, clotting disorder), but also critical entities that are frequently modified by these key entities (e.g., VKORCl polymorphism, warfarin response, clotting disorder treatment).
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
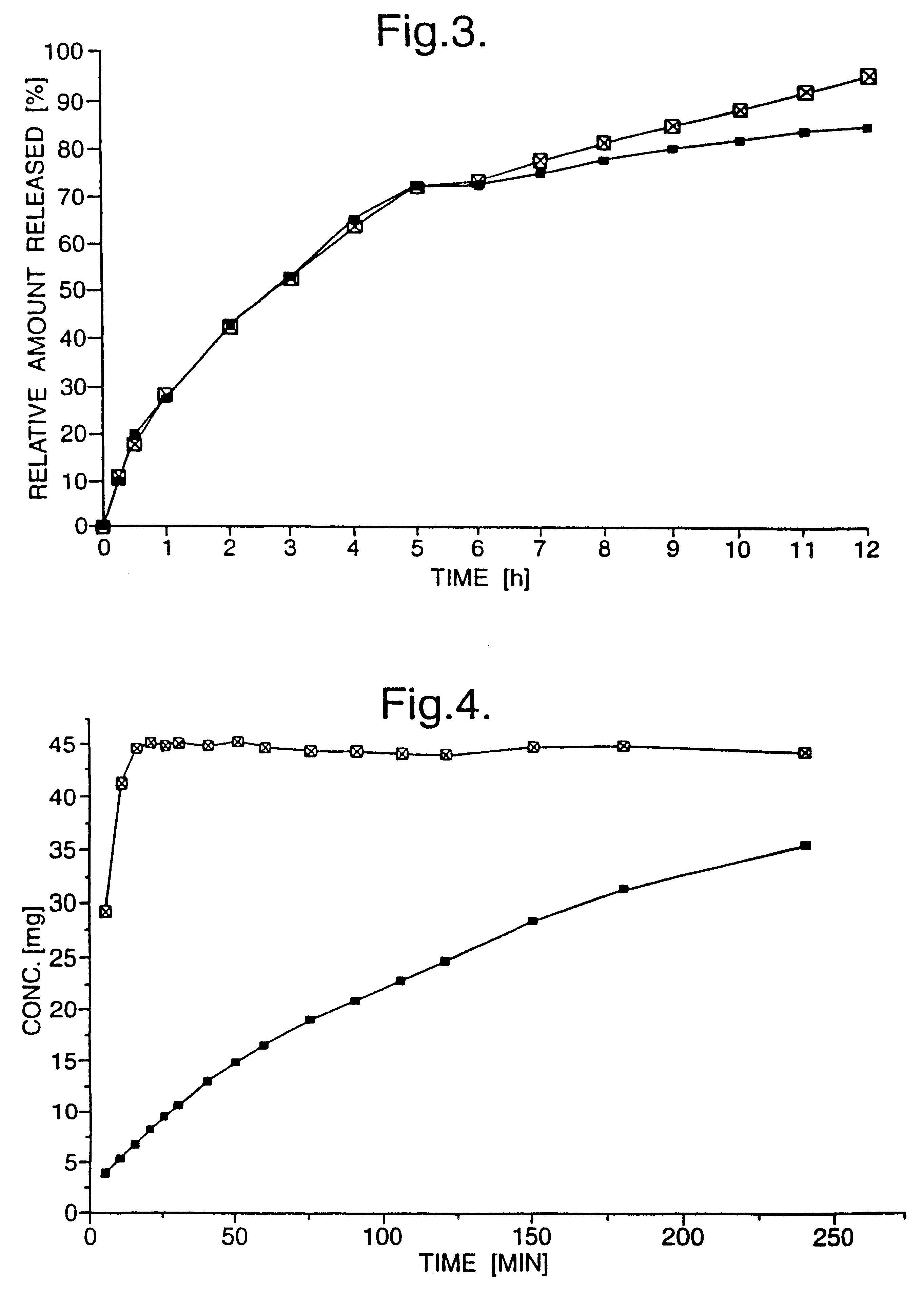
Pharmaceutical drug dosage forms providing different release rates
A pharmaceutical dosage form comprises, in one portion thereof, a substantially single (+)-enantiomer of a chiral drug other than verapamil and, in another, separate portion thereof, a substantially single (-)-enantiomer of the drug wherein, in use, the different enantiomers are released at different rates from the dosage form. The dosage form is useful for administration of chiral drugs where both enantiomers have a valid pharmacological input, and where a clinical benefit may be realised by controlling the release rates of those enantiomers. Examples of such drugs include, in particular, tramadol and warfarin.
Owner:SOSEI R&D LIMITED
Dosage forms
A pharmaceutical dosage form comprises, in one portion thereof, a substantially single (+)-enantiomer of a chiral drug other than verapamil and, in another, separate portion thereof, a substantially single (-)-enantiomer of the drug wherein, in use, the different enantiomers are released at different rates from the dosage form. The dosage form is useful for administration of chiral drugs where both enantiomers have a valid pharmacological input, and where a clinical benefit may be realised by controlling the release rates of those enantiomers. Examples of such drugs include, in particular, tramadol and warfarin.
Owner:SOSEI R&D LIMITED
Primer system for detecting gene SNP (single nucleotide polymorphism) related to warfarin dosage and application of primer system
InactiveCN102690888AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationWarfarinSide effect
The invention discloses a primer system for detecting 7 gene polymorphic sites related to warfarin dosage. A product prepared based on the primer system can be used for realizing the detection of 7 gene polymorphic sites related to warfarin dosage. The product is used so as to detect the genetypes of a warfarin user at the 7 gene polymorphic sites; and the detection result is combined with other clinical indexes so that a reference can be provided for a clinical doctor to reasonably define the warfarin dosage, and the side effect of a medicament is avoided. According to the invention, the primer system can be used for simultaneously detecting the 7 gene polymorphic sites on different genes in one reaction system; and compared with technologies such as sequence measurement and real-time fluorescencent quantitative PCR (polymerase chain reaction), the primer system disclosed by the invention has the advantages of lower cost, simplicity and convenience in operation and improved accuracy and sensitivity.
Owner:毅新兴业(北京)科技有限公司
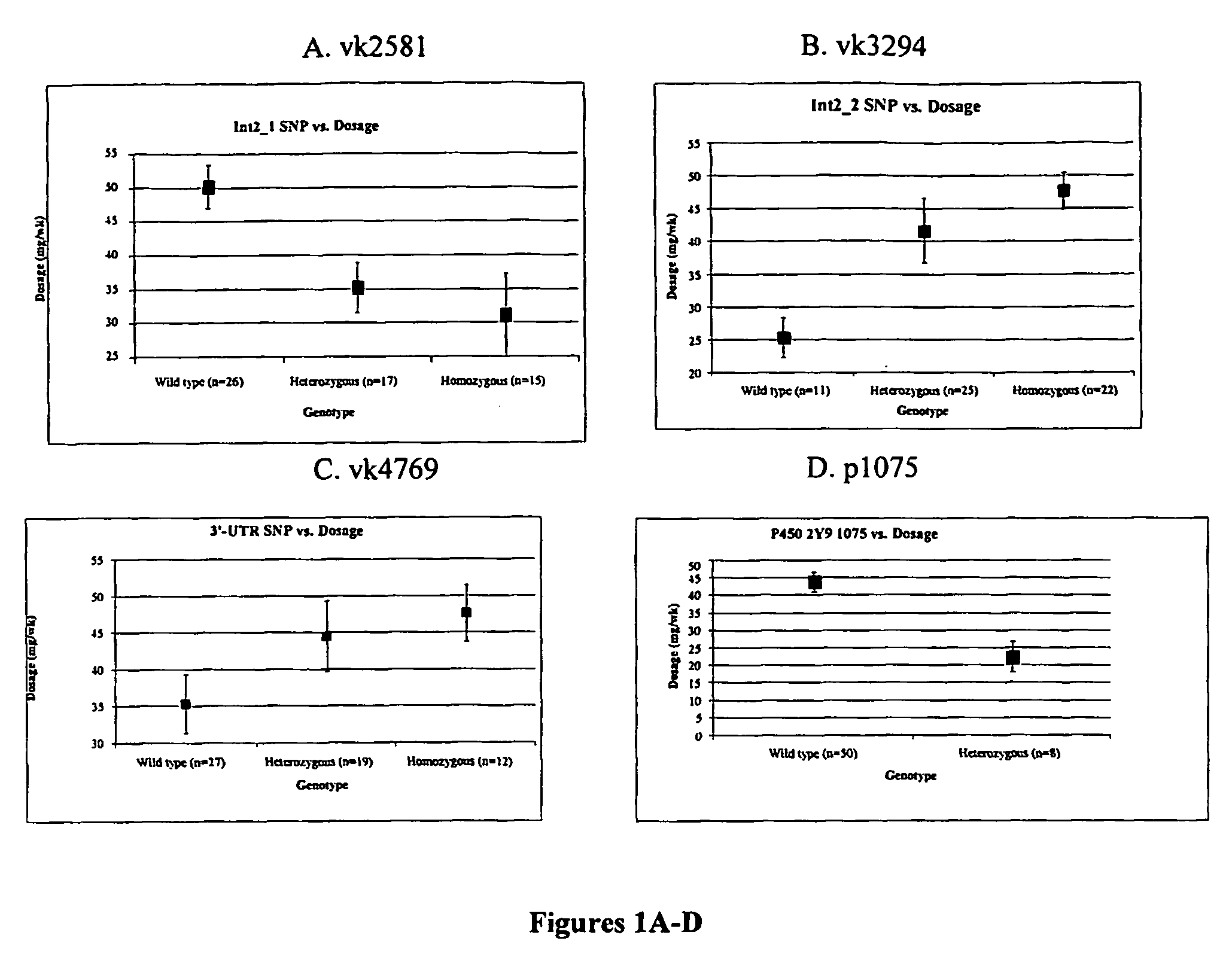
Methods and compositions for the correlation of single nucleotide polymorphisms in the vitamin k epoxide reductase gene and warfarin dosage
InactiveUS20060240440A1Decrease and increase sensitivitySugar derivativesMicrobiological testing/measurementWarfarinFhit gene
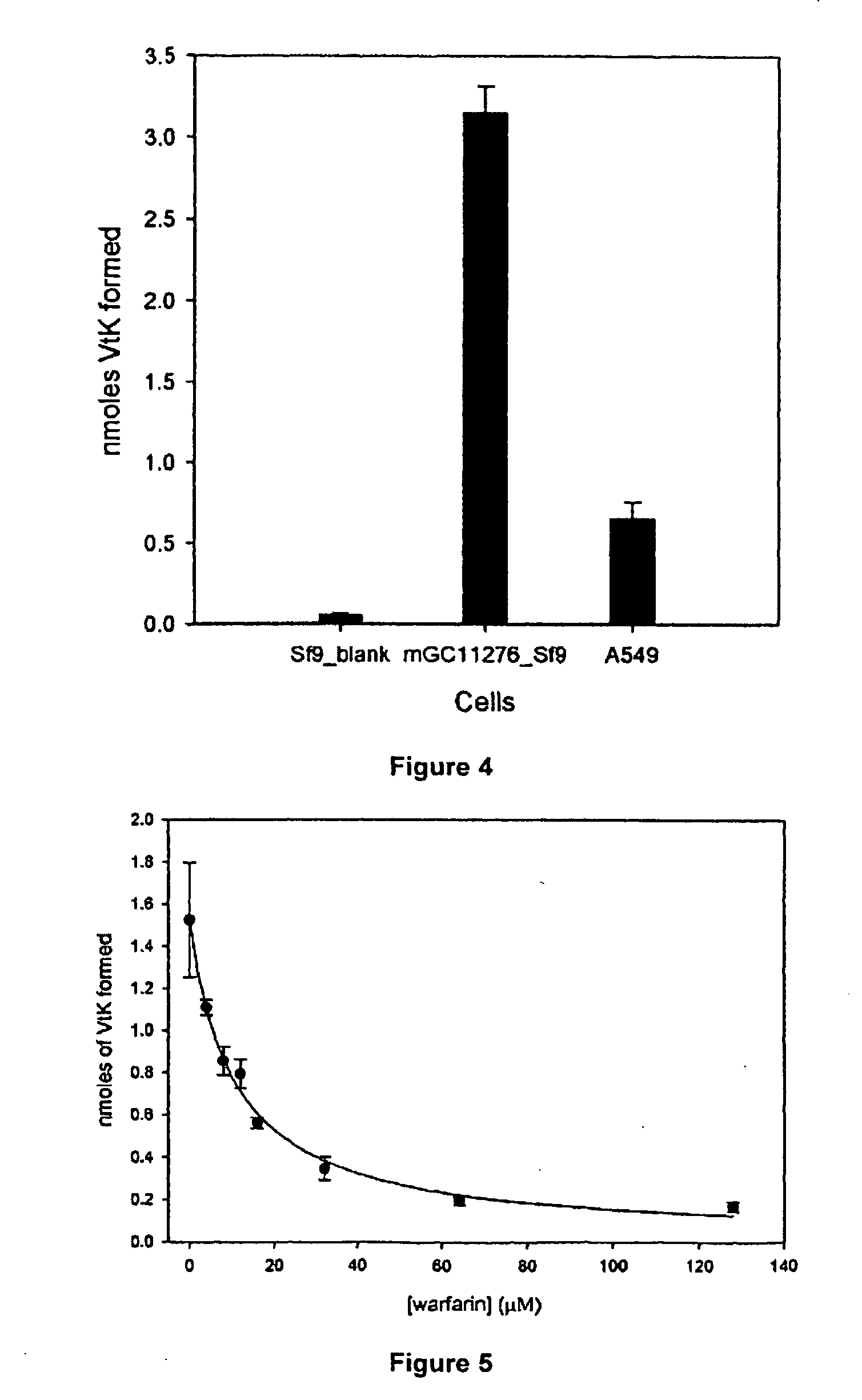
The present invention provides a method of identifying a human subject having increased or decreased sensitivity to warfarin, comprising detecting in the subject the presence of a single nucleotide polymorphism in the VKOR gene, wherein the single nucleotide polymorphism is correlated with increased or decreased sensitivity to warfarin, thereby identifying the subject having increased or decreased sensitivity to warfarin.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
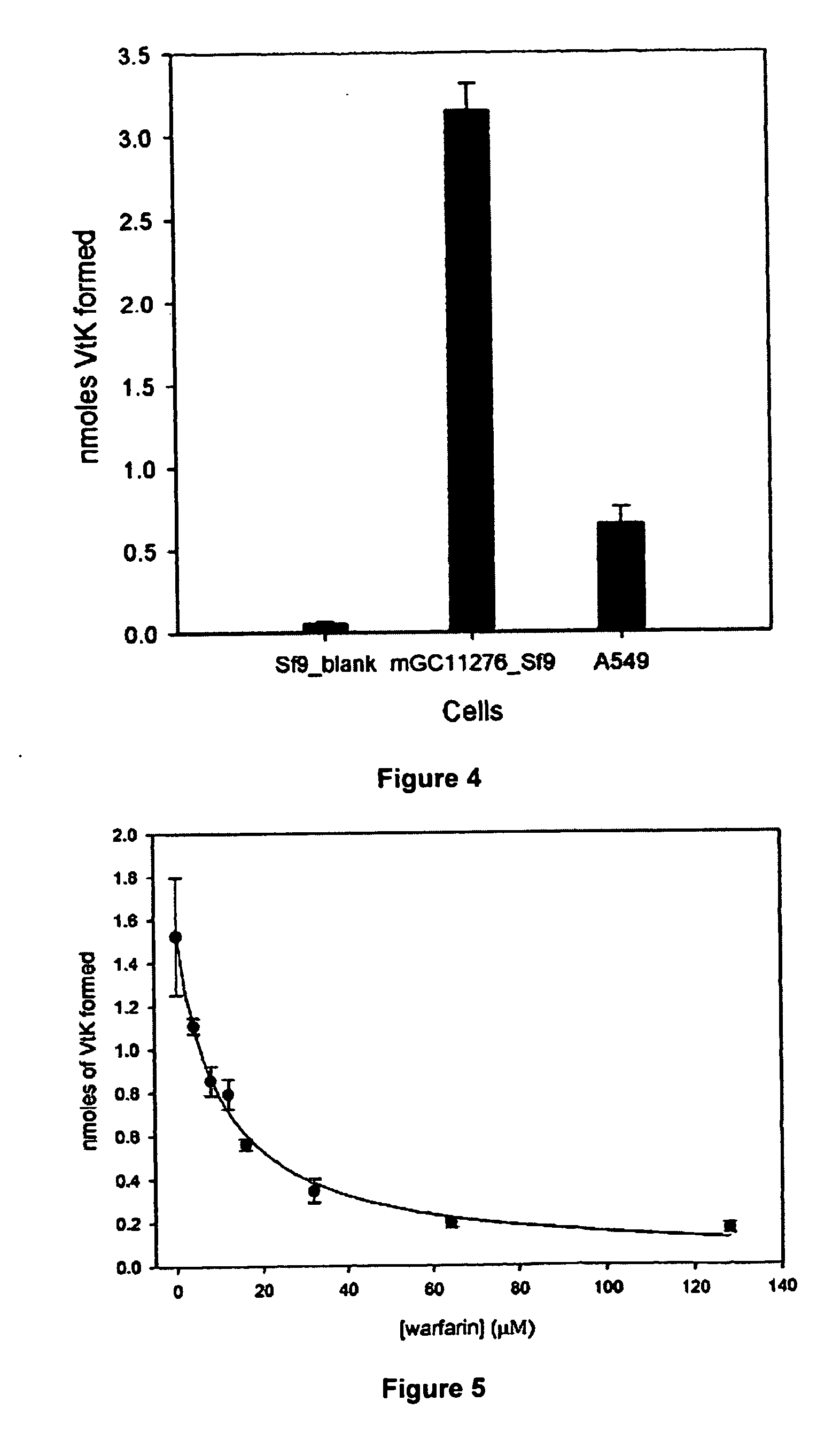
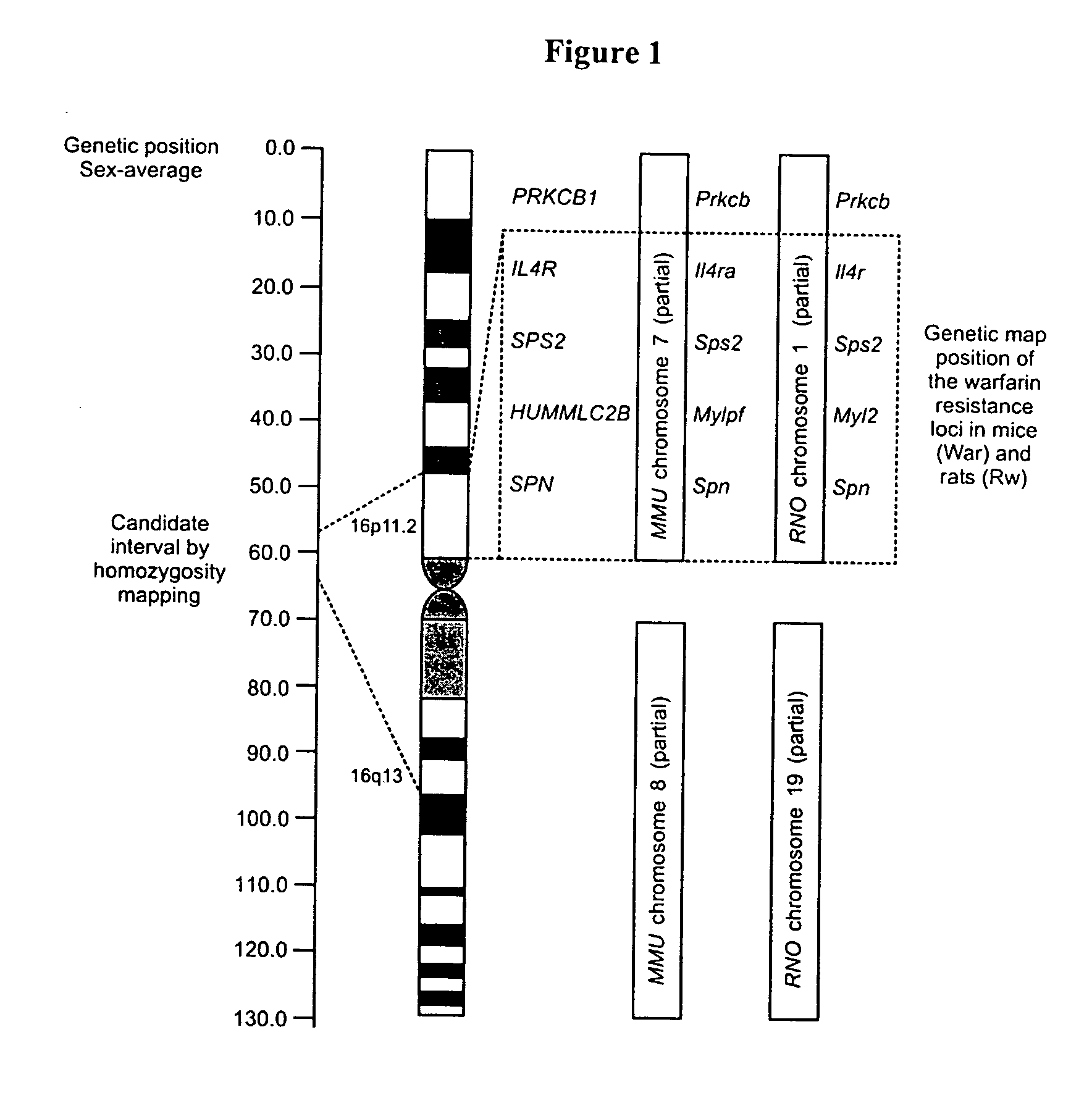
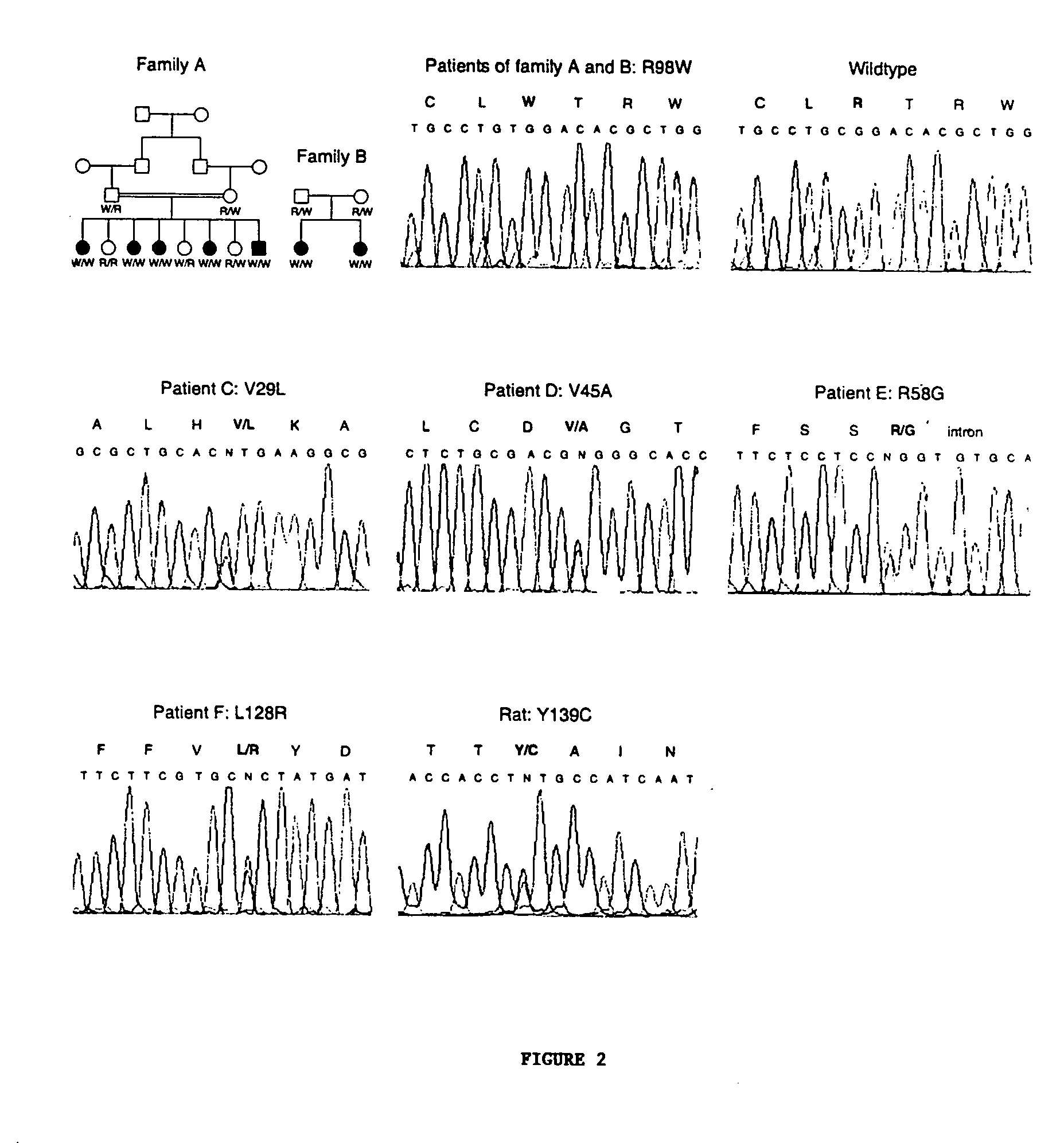
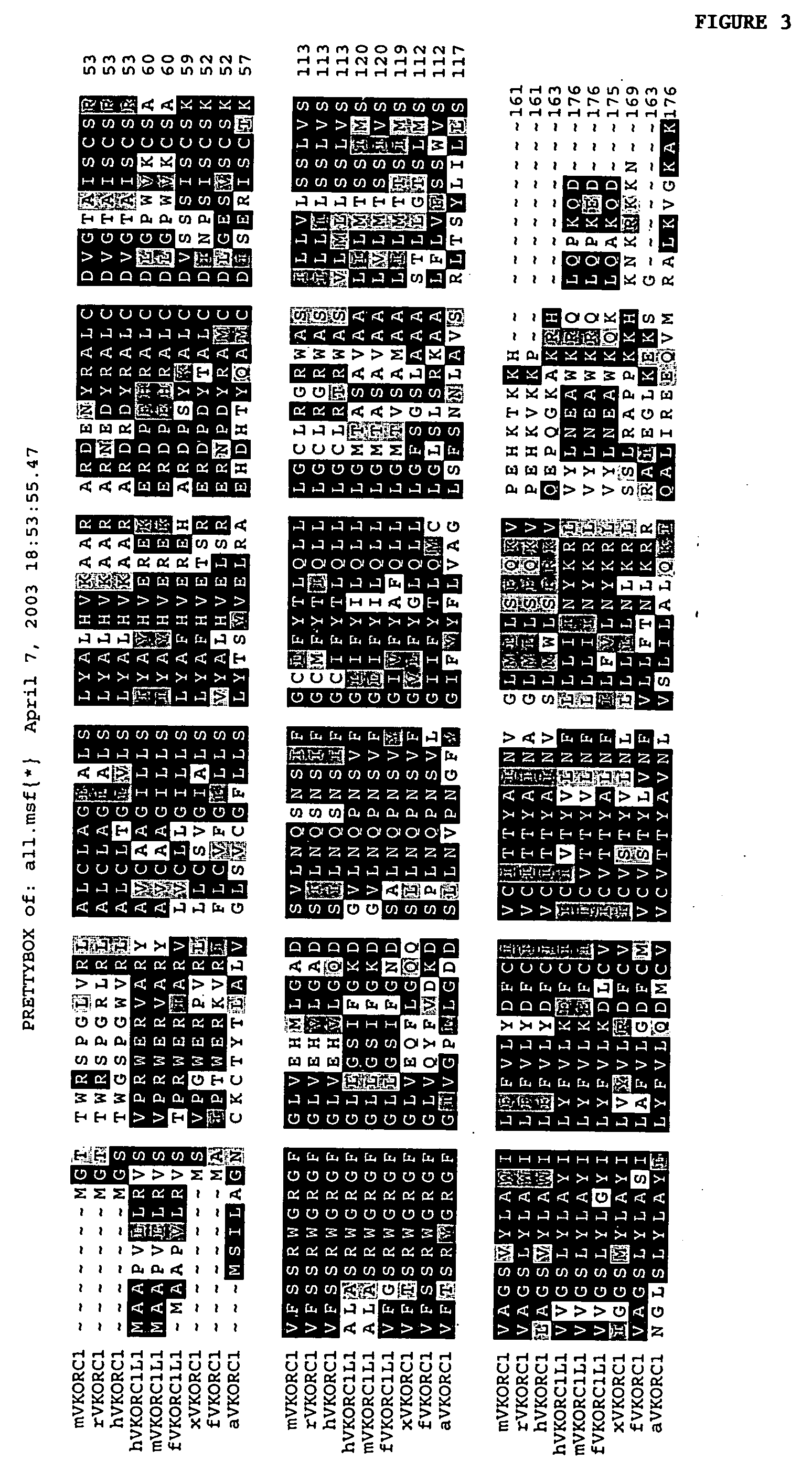
Vitamin K epoxide recycling polypeptide VKORC1, a therapeutic target of coumarin and their derivatives
The invention relates to a novel polypeptide vitamin K epoxide recycling polypeptide (VKORC1) as a target for coumarin and its derivatives. The invention further provides methods for identifying coumarin derivatives, and also claims VKORC1 polypeptides and VKORC1 nucleic acids containing a sequence abnormality associated with a VKORC1 associated deficiency such as warfarin resistance, wherein the VKORC1 polypeptides and VKORC1 nucleic acids can be used for diagnosing these deficiencies. Moreover, the invention relates to methods for identifying coumarin derivatives usable in pest control of rodents.
Owner:BAXALTA GMBH
Methods and compositions for predicting drug responses
InactiveUS20060084081A1Determining responsivenessMicrobiological testing/measurementBiological testingWarfarinGenetics
Owner:UNIV OF WASHINGTON
Detecting method for accurate risk early warning and accurate drug use of cardiovascular and cerebrovascular diseases and specific primer
ActiveCN106893783AStrong specificityReduce experiment costMicrobiological testing/measurementDNA/RNA fragmentationWarfarinSingle strand
The invention discloses a detecting method for accurate risk early warning and accurate drug use of cardiovascular and cerebrovascular diseases and a specific primer. The invention provides a specific primer for detecting SNP site of drug resistance of the cardiovascular and cerebrovascular diseases, which comprises a primer group 1 and a primer group 2; the primer group 1 is composed of primer pair 1-primer pair 13; a primer group 2 is composed of single stranded extended primer 1- single stranded extended primer 13; the method can efficiently detect and complete the accurate early warning of the attack of the cardiovascular and cerebrovascular diseases and the accurate drug use after being attacked, namely, four kinds of cardiovascular and cerebrovascular disease early warning with huge health risk and accurate drug use of four clinical common drugs (aspirin, nitroglycerin, warfarin, and clopidogrel) for prevention and treatment of cardiovascular and cerebrovascular diseases.
Owner:李爱娟
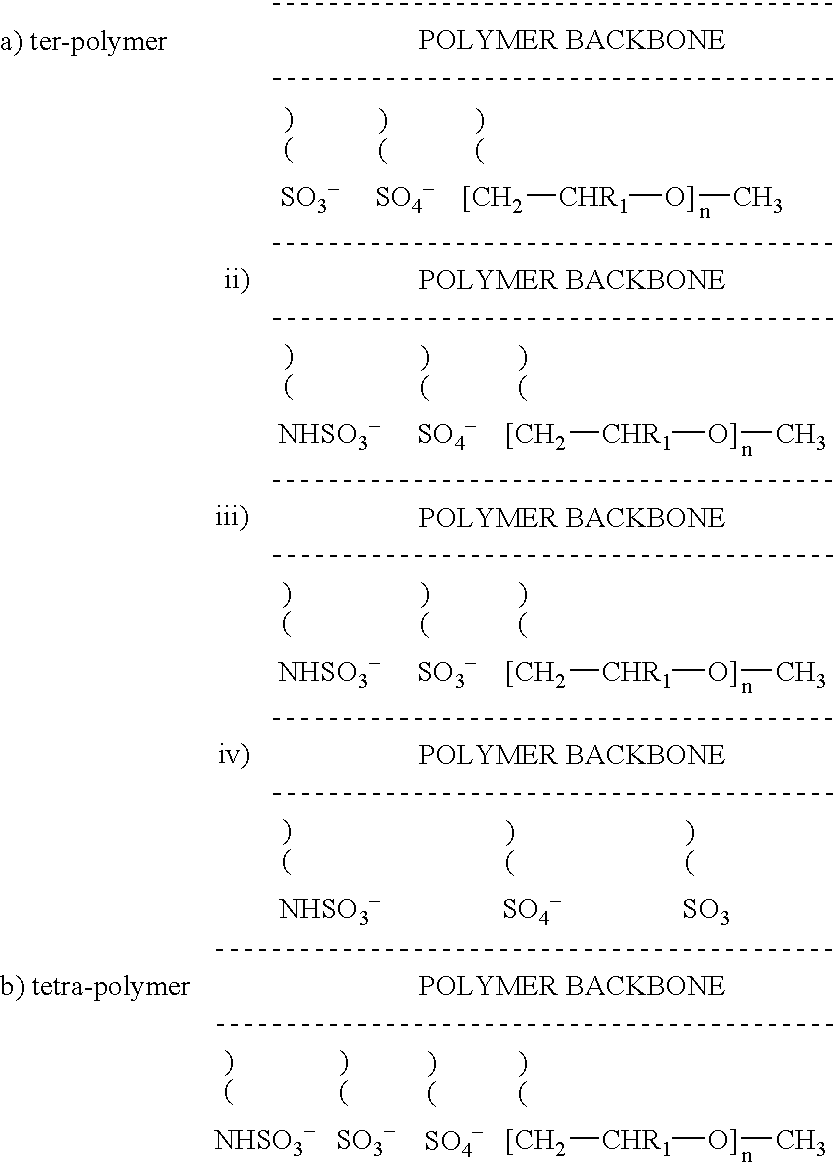
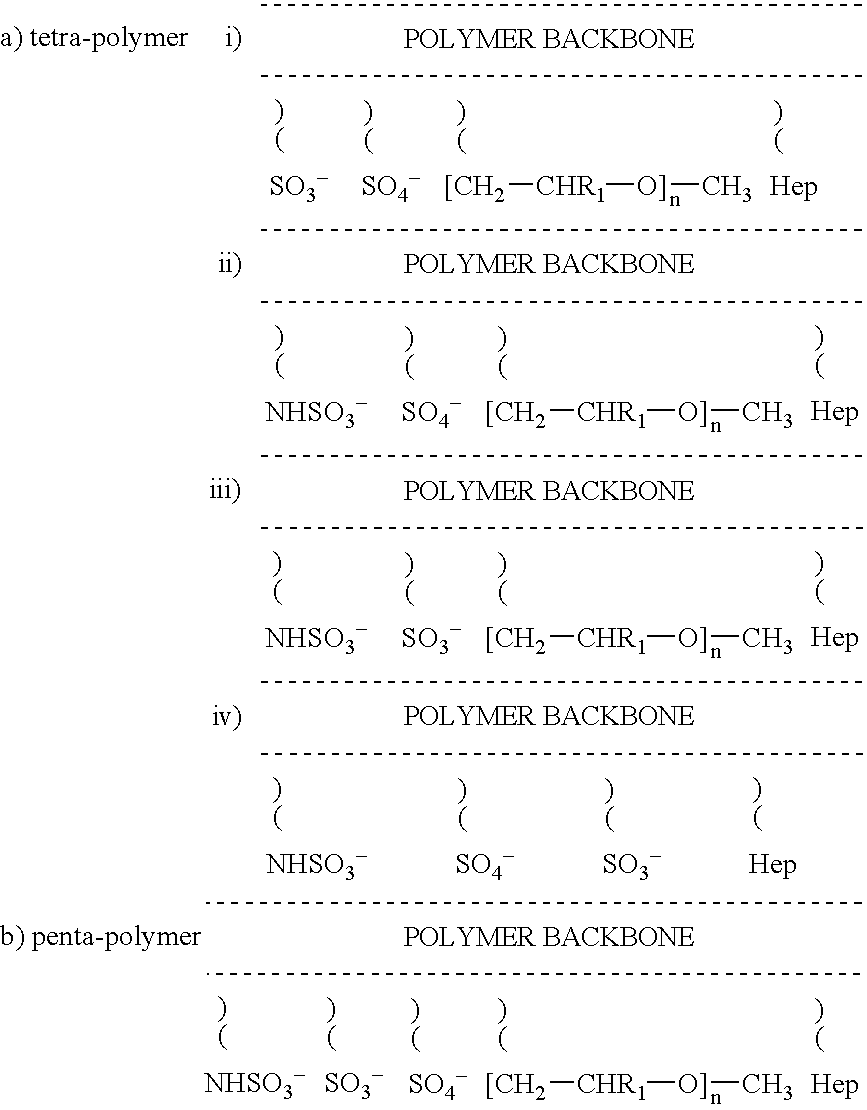
Non-thrombogenic and anti-thrombogenic polymers
InactiveUS7034061B1Improve attachment stabilityPreferential ionic bondingPeptide/protein ingredientsPharmaceutical non-active ingredientsWarfarinPolymer science
Polymers having non-thrombogenic properties can be prepared by copolymerizing monomers of at least three classes selected from (a) monomers having sulphate groups, (b) monomers having sulphonate groups, (c) monomers having sulphamate groups, (d) monomers having polyoxyalkylene ether groups, and (e) monomers having zwitterionic groups. The polymers can additionally be provided with anti-thrombogenic properties by including an additional comonomer having a pendant heparin (or hirudin, warfarin or hyaluronic acid) group. The polymers can be used as coating materials for medical devices, such as tubing or connectors, in order to provide them with non-thrombogenic, and optionally anti-thrombogenic, properties.
Owner:BIOINTERACTIONS
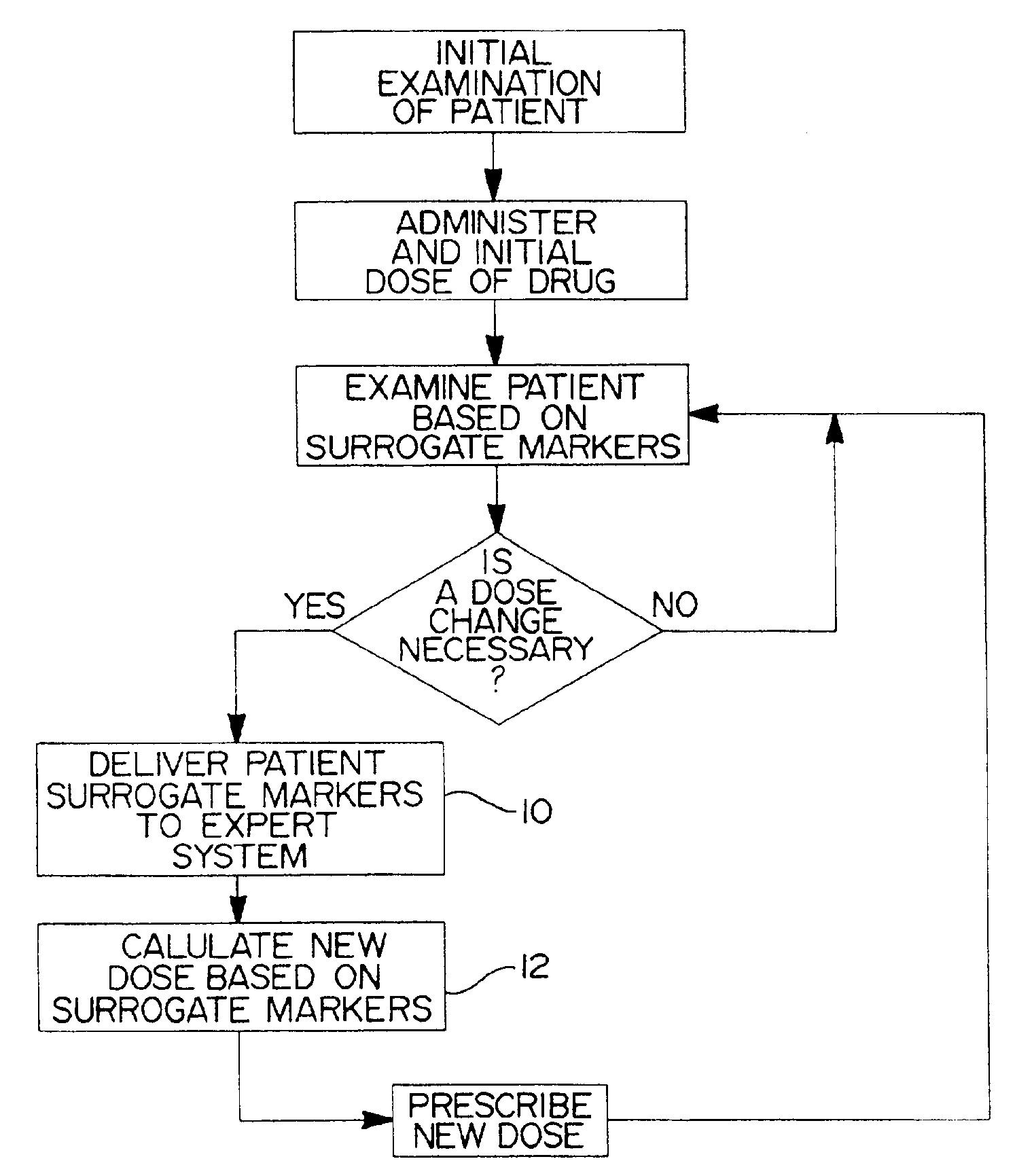
Method and system for use in treating a patient with an anticoagulant to optimize therapy and prevent an adverse drug response
InactiveUS6942614B1Reduce doseMeet growth needsCompounds screening/testingSurgeryBlood levelWarfarin
A method and system for use in treating a patient receiving an anticoagulant or a substance containing warfarin to optimize therapy and prevent an adverse drug response. This system employs surrogate markers or indicators including blood levels of the anticoagulant to determine the next required dose for a patient. Since the surrogate markers are employed as a percent change in status, virtually any indicator can be used. Surrogate markers could include any measure of the effectiveness of the anticoagulant's action. Given the effectiveness of the anticoagulant's action relative to the surrogate markers, a change in anticoagulant dose is calculated by the system. Conversely, by employing this system, one could determine the expected result of the anticoagulant dose change on the surrogate markers.
Owner:THE RXFILES CORP
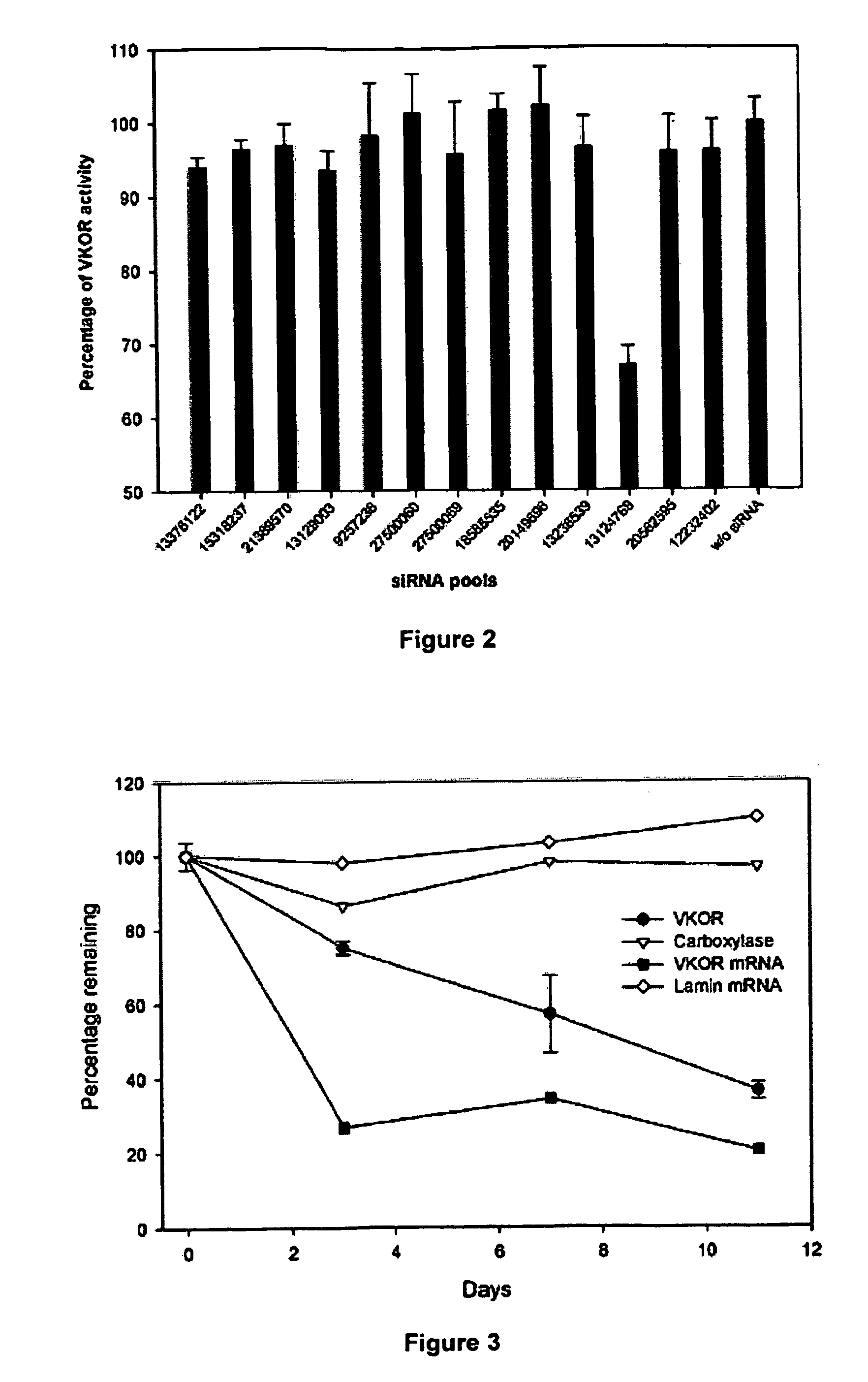
Methods and compositions for vitamin K epoxide reductase
ActiveUS20070009950A1Sugar derivativesMicrobiological testing/measurementWarfarinWARFARIN SENSITIVITY
The present invention provides a method of identifying a human subject having increased or decreased sensitivity to warfarin, comprising detecting in the subject the presence of a single nucleotide polymorphism in the VKOR gene, wherein the single nucleotide polymorphism is correlated with increased or decreased sensitivity to warfarin, thereby identifying the subject having increased or decreased sensitivity to warfarin.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Preparation method of anticoagulant drugs
ActiveCN107019676AAccurate Dosing AchievedProcess stabilityAdditive manufacturing apparatusPharmaceutical non-active ingredientsWarfarinAdjuvant
The invention provides a preparation method of Warfarin anticoagulant drugs. The method comprises the following steps: adding main materials and adjuvants to a 3D printer and conducting 3D modeling by virtue of a computer; then, transmitting 3D modeling data to the 3D printer; conducting hierarchical slicing treatment by virtue of software of the 3D printer and forming corresponding codes; squeezing the main materials and the adjuvant materials out of a nozzle in a mode of hot-melt extrusion; conducting single-layer printing through X-axle and Y-axle motion of a printing head of the 3D printer on plane; and along with layer-by-layer accumulating and mutual bonding of the printer, completing preparation of the Warfarin anticoagulant drugs.
Owner:TRIASTEK INC
Combination therapy for anticoagulation
A combination anticoagulation medicament including vitamin K with warfarin in an oral form is described. Between 50 and 5000 micrograms of vitamin K are combined in a single oral medication with 0.5 to 15 milligrams of warfarin for administration. The combination of vitamin K with warfarin in a single orally dosed form is a novel approach to improving the effectiveness of anticoagulation. The combination allows for broader application of warfarin in medical anticoagulation and reduces the variability of anticoagulation due to the influences of diet, additional medications, nutritional status, changes in physical condition, and potentially other factors. Use of the combination therapy improves the safety of warfarin as an appropriate anticoagulant for many medical conditions.
Owner:SCIENTIA MEDICA DONA A DEO
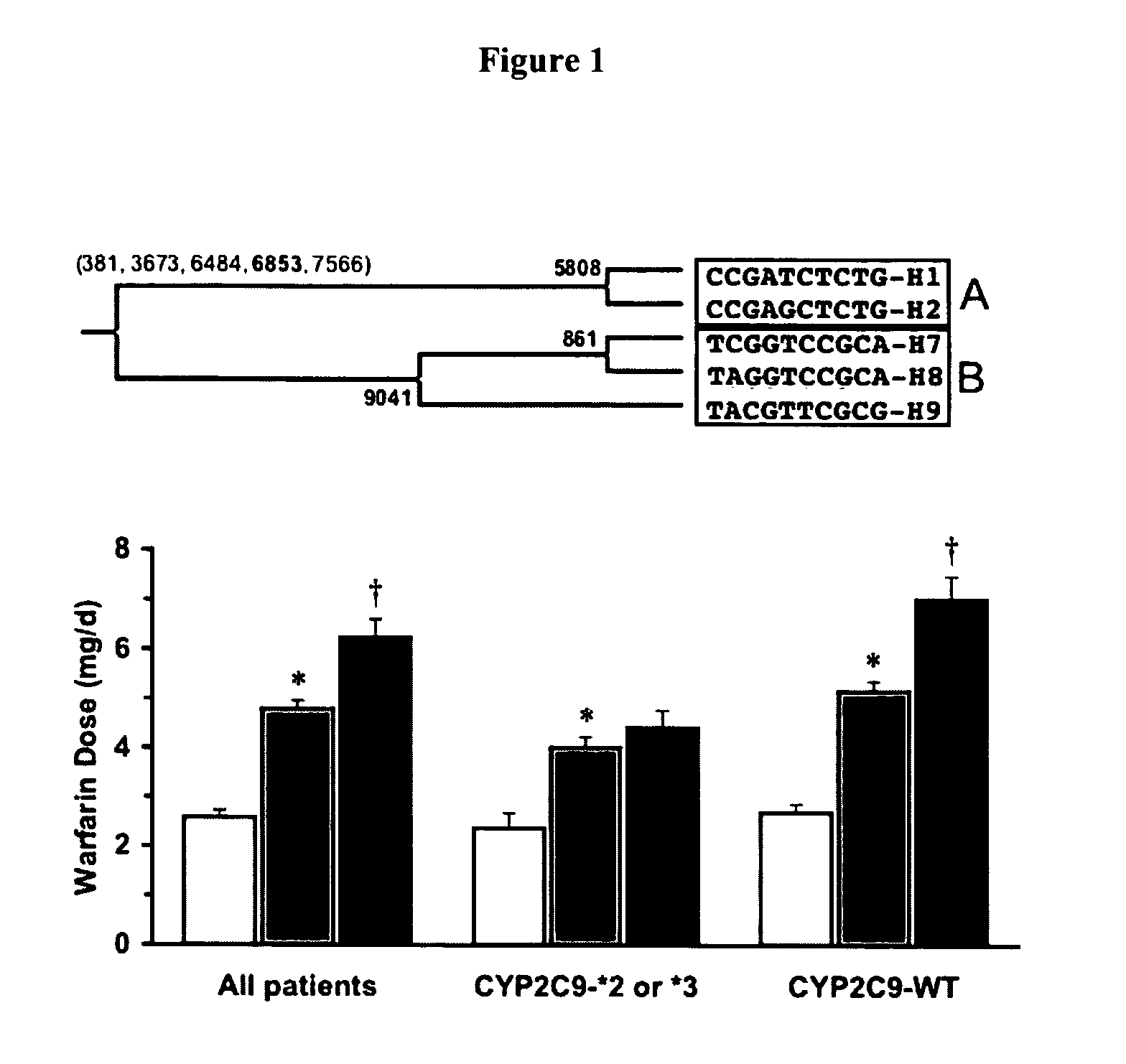
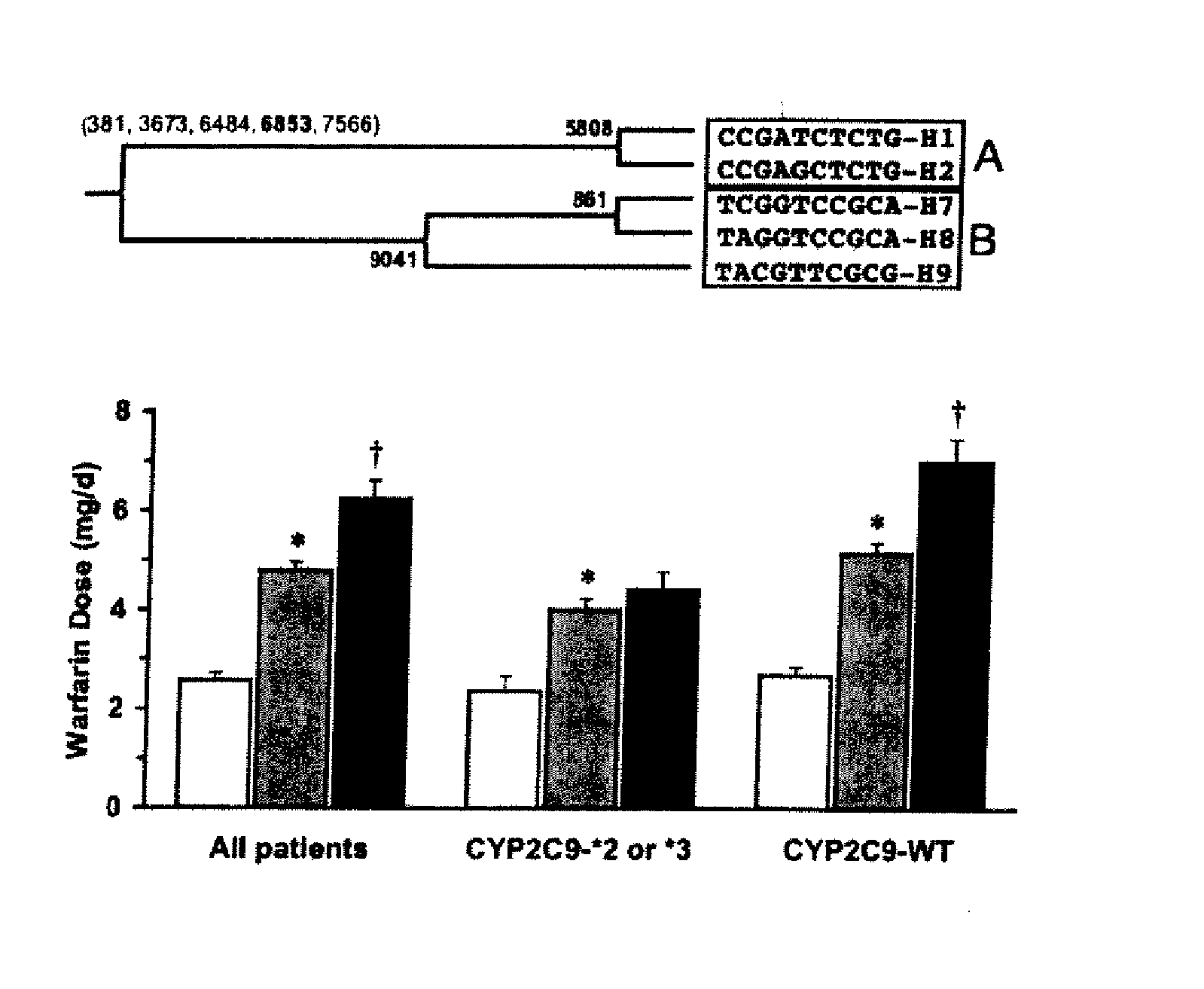
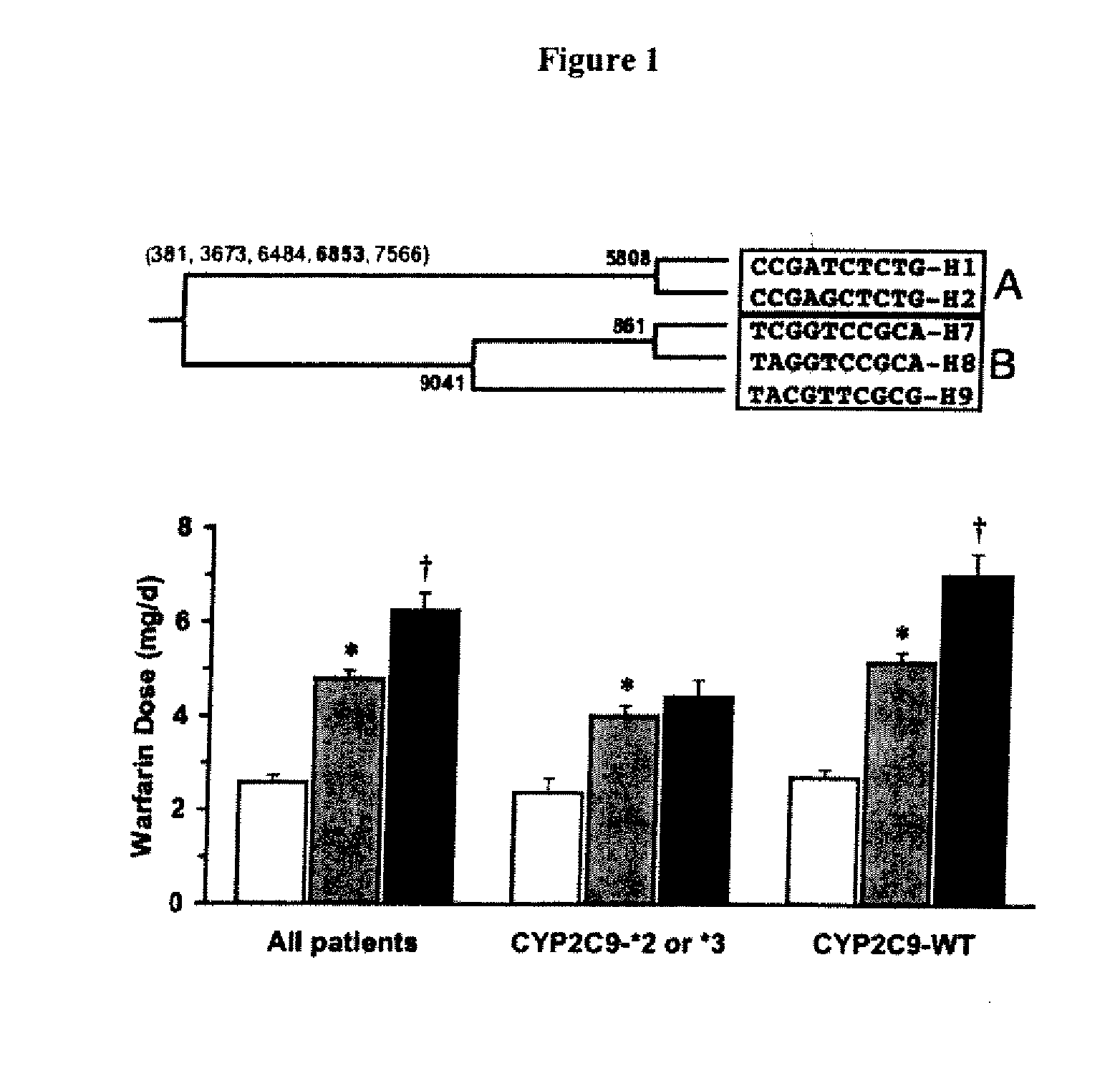
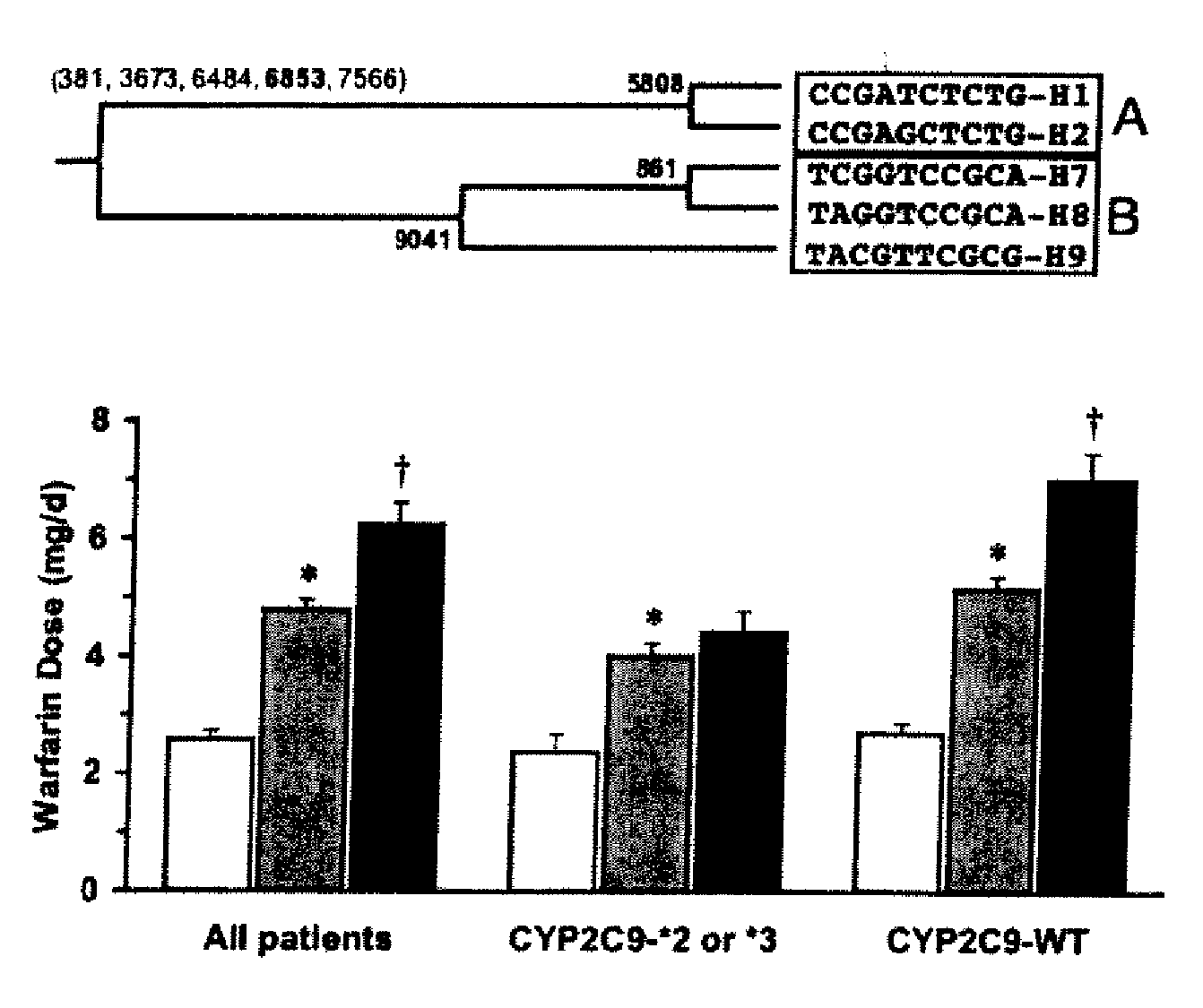
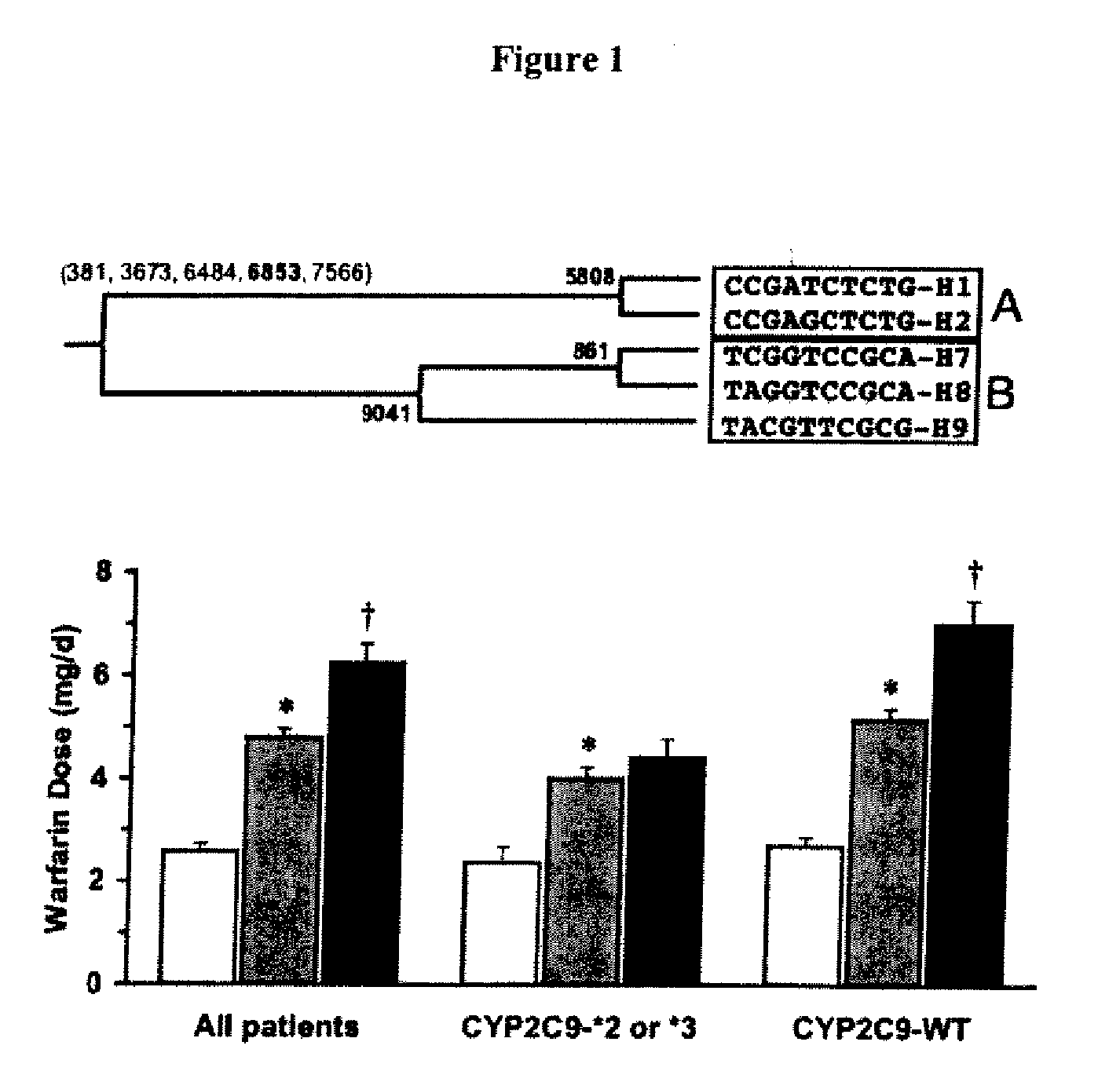
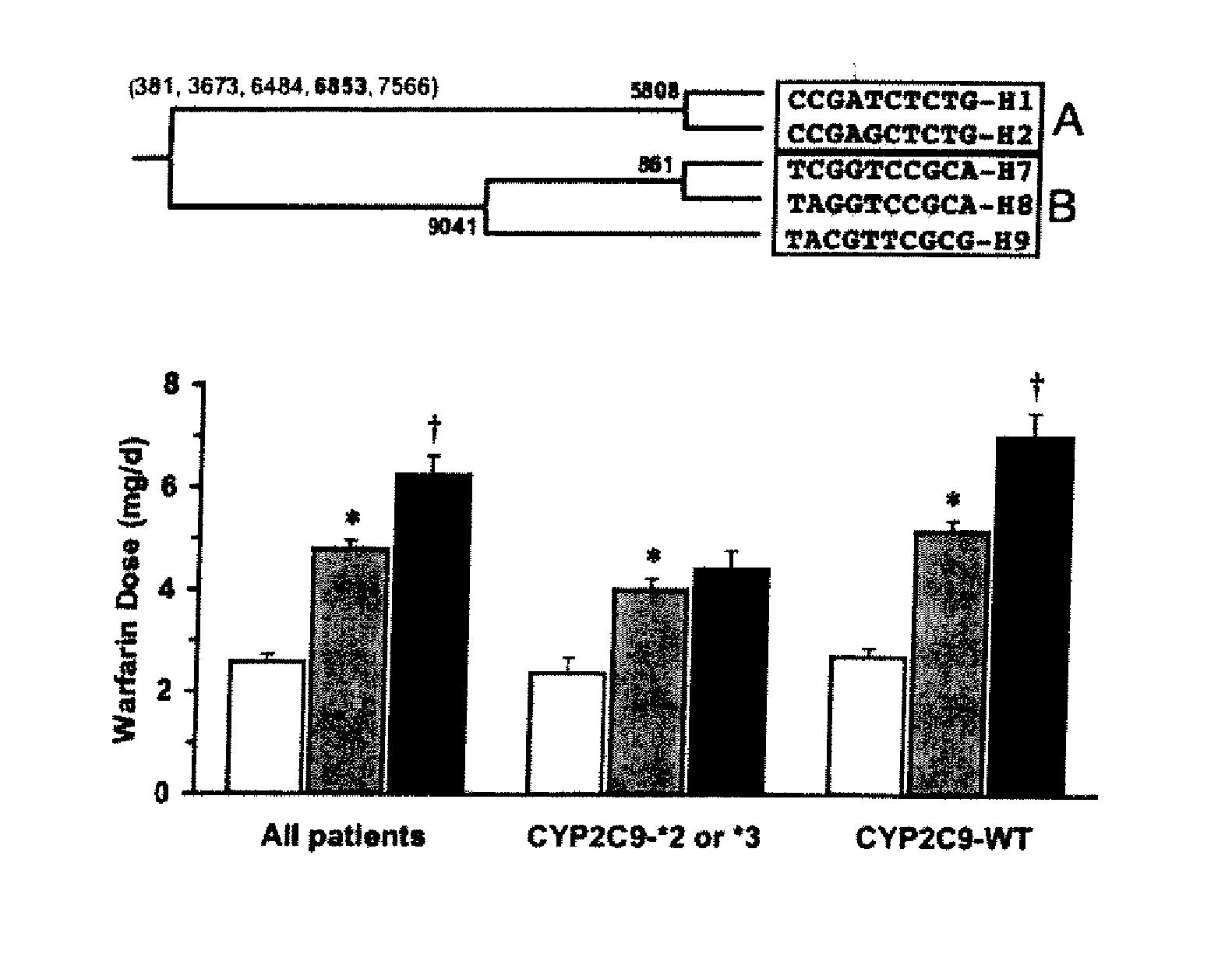
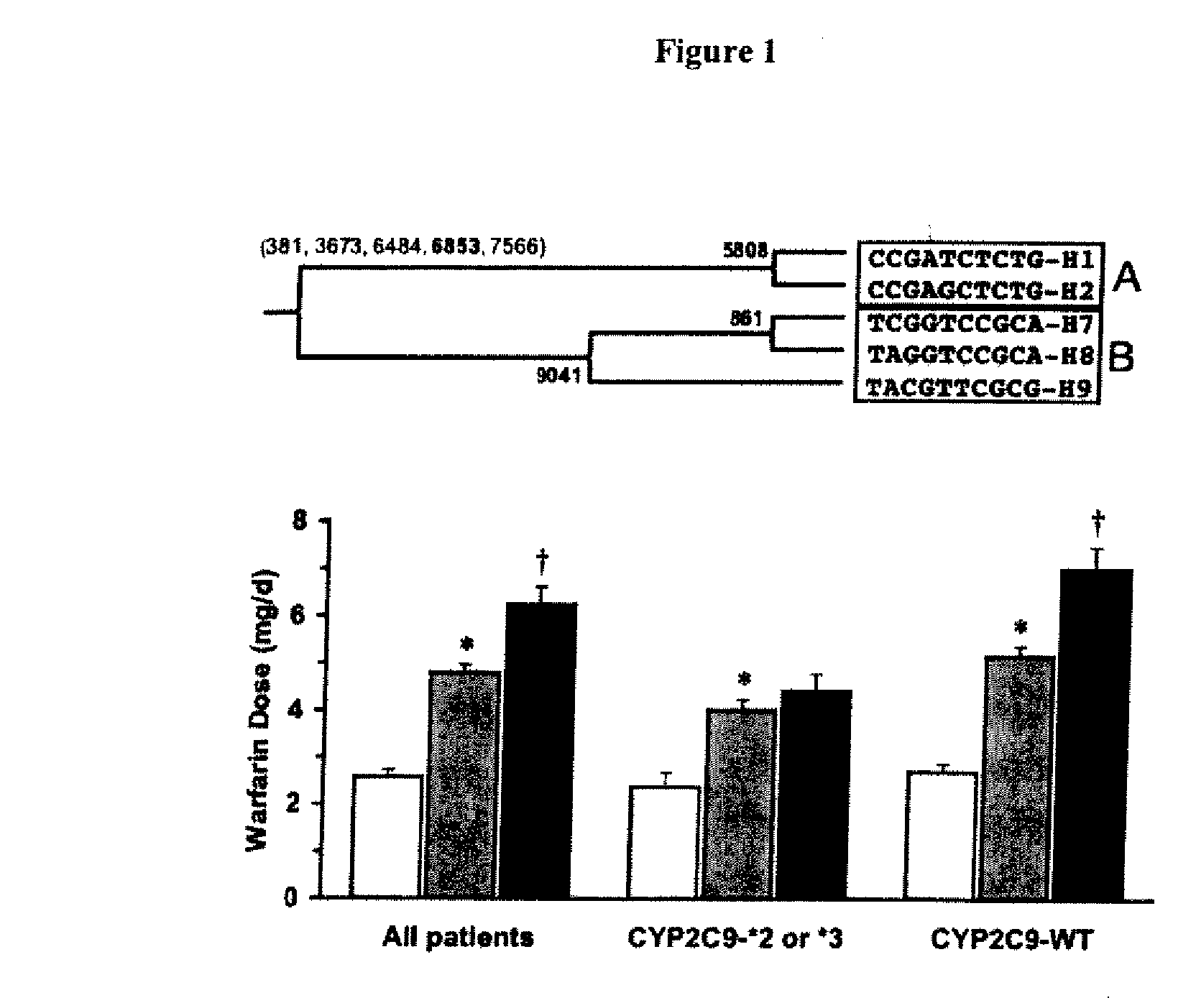
CYP2C9*8 Alleles Correlate With Decreased Warfarin Metabolism And Increased Warfarin Sensitivity
InactiveUS20100130599A1Improve securityLower requirementBiocideMicrobiological testing/measurementWarfarinWARFARIN SENSITIVITY
The present disclosure is related to a method of identifying a subject with increased sensitivity to warfarin. The method includes identifying a CYP2C9*8 polymorphism in the subject, wherein the presence of said polymorphism is indicative of a patient with increased sensitivity to warfarin relative to a subject having the corresponding wild-type allele.
Owner:OSMETECH TECH
Materials and methods for treating coagulation disorders
InactiveUS6864279B2Treatment safetyImprove the quality of lifeBiocideOrganic chemistryWarfarinCoagulation Disorder
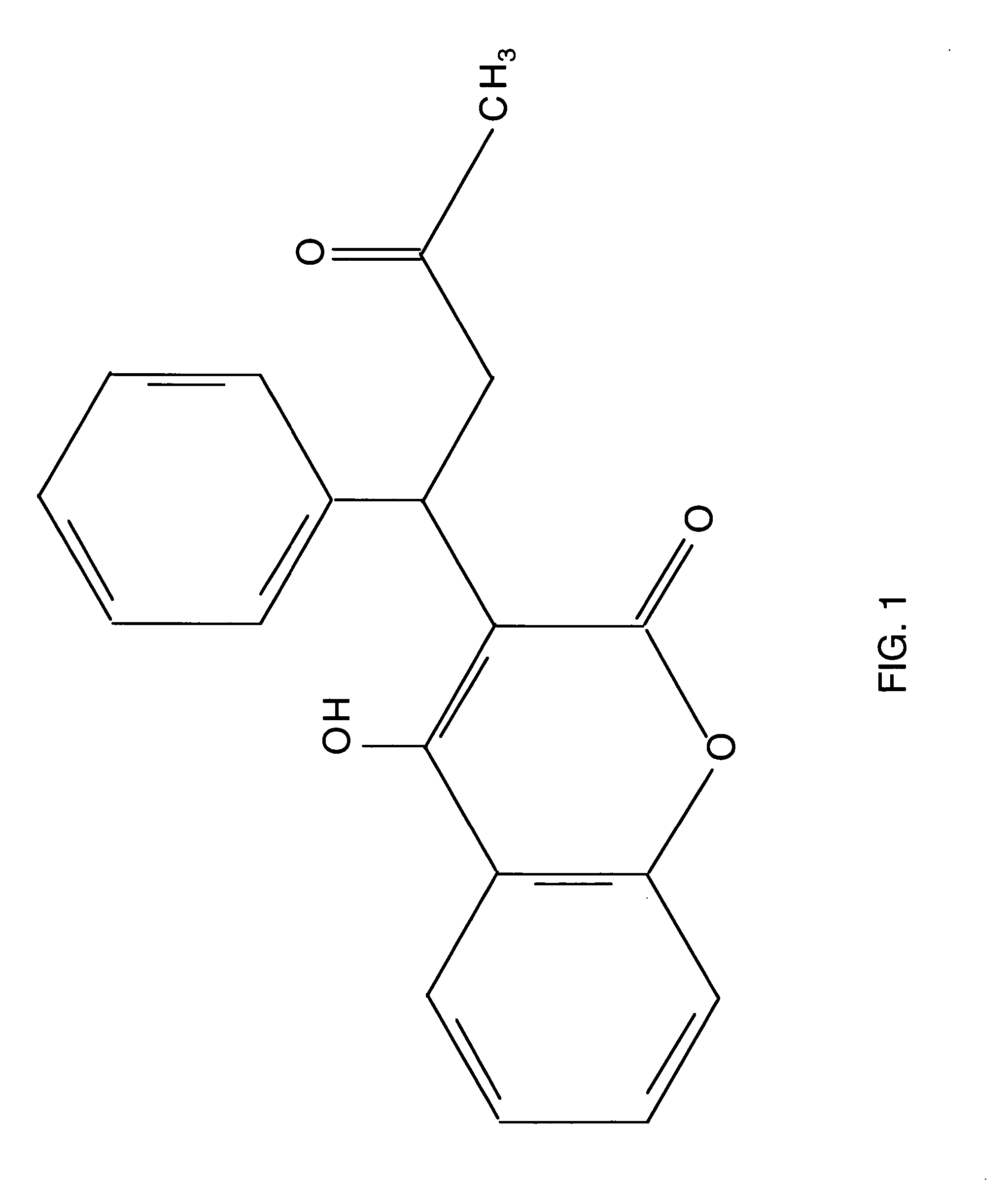
This invention is drawn to compounds which are more easily metabolized by the metabolic drug detoxification systems. Particularly, warfarin analogs which have been designed to include esters within the structure of the compounds are taught. The invention teaches methods of reducing the toxicity of drugs comprising the introduction of ester groups into drugs during the synthesis of the drug. This invention is also drawn to methods of treating coagulation disorders comprising the administration of compounds which have been designed to be metabolized by serum or intracellular hydrolases and esterases. Pharmaceutical compositions of the ester containing warfarin, analogs are also taught.
Owner:ARMETHEON INC
Kit used for detecting polymorphism of genes related to Warfarin and Clopidogrel personalized medication and application thereof
ActiveCN105018583AHigh degree of integrationHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationWarfarinOperability
The invention discloses a kit used for detecting the polymorphism of genes related to Warfarin and Clopidogrel personalized medication and application thereof. The kit comprises a general chip, a first primer combination and a second primer combination, wherein each primer combination unit in the first primer combination is used for identifying one polymorphism site of the genes related to Warfarin medicine resistance, and each primer combination unit in the second primer combination is used for identifying one polymorphism site of the genes related to Clopidogrel medicine resistance. The kit has the advantages of being high in integration degree, sensitivity, specificity, reliability and operability and wide in application range, detection results are stable, a use method is fast and convenient, and automation of the kit is easy to achieve; the kit can be used for detecting gene mutation, analyzing gene polymorphism and the like and is suitable for gene analysis fields such as mutation detection of clinical diseases, pharmacogenomics analysis and medicolegal expertise.
Owner:BOAO BIOLOGICAL CO LTD +2
Preparation method and application of aggregation induced luminescence-molecular imprinting fluorescence sensor for detecting rhodamine B
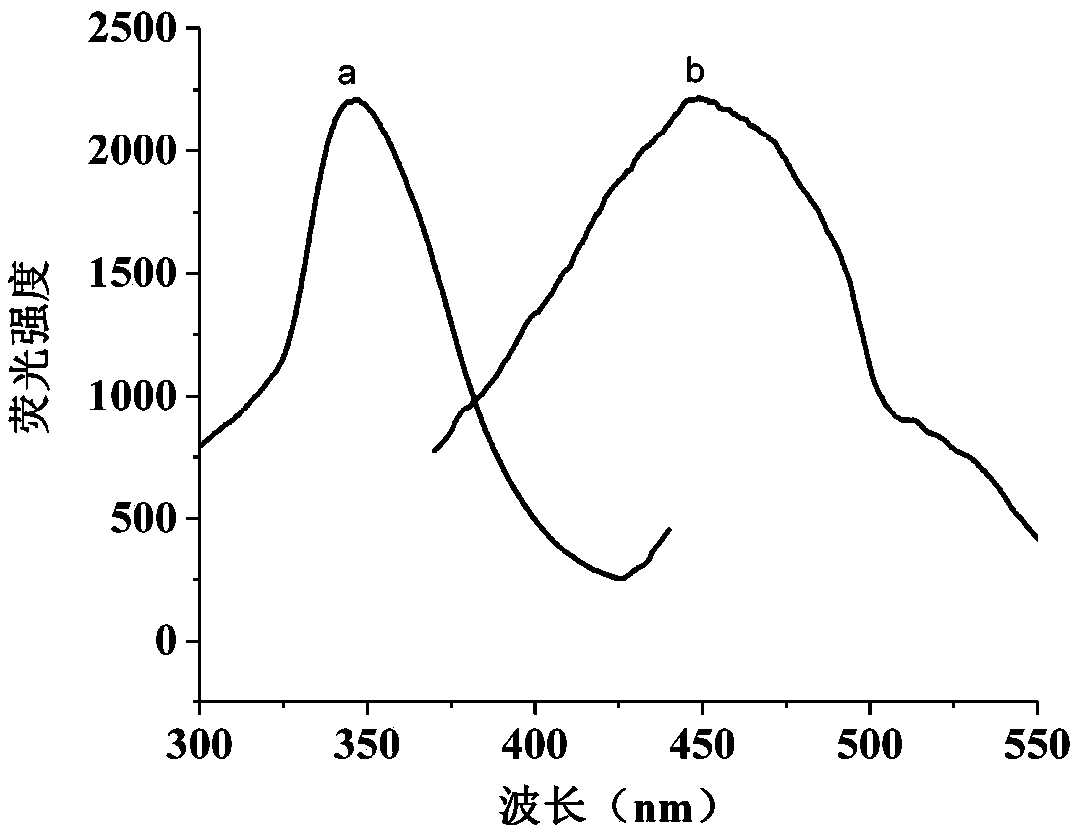
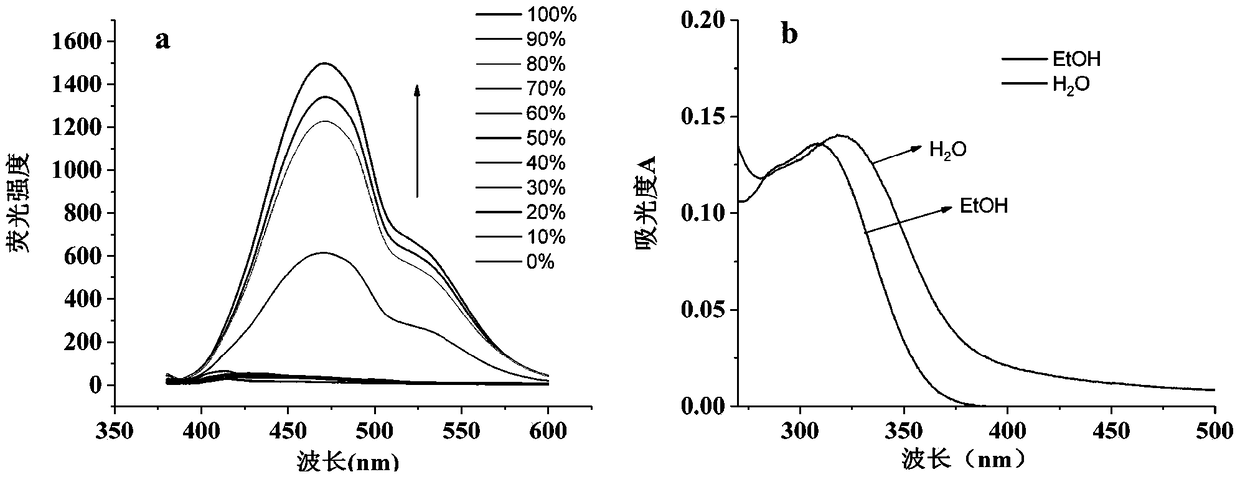
ActiveCN109406474AHigh fluorescence quantum yieldImprove structural stabilityFluorescence/phosphorescenceWarfarinCross-link
The invention discloses a preparation method and application of aggregation induced luminescence-molecular imprinting fluorescence sensor for detecting rhodamine B. According to the method, the molecular imprinting technology and fluorescence detecting technology are combined, warfarin is used as a template molecule, alpha-methacrylic acid is used as a functional monomer, ethylene glycol dimethylacrylate is used as a cross-linking agent, azobisisobutyronitrile is used as an evocating agent, acetonitrile is used as a dissolvant, functionalized AIE molecules are added, and the precipitation polymerization method is adopted to synthesize AIE-MIPs. The AIE-MIPs is simple to operate, organic reagent use is little, ability to recognize the rhodamine B is high, the linear relationship is good inthe concentration range of 1*10<-5>-10*10<-5>mol / L. The AIEMIPs is adopted to carry out standard recovery experiment for papaya dry and Fanta beverage, the results showthat the recovery range of therhodamine B is 96.2%-103.5%, and the relative standard deviation range is 1.5-4.7%. The data indicates that the AIE-MIPs obtained by the combination of fluorescence detecting and molecular imprintingcan be applied to detection of rhodamine B in practical samples.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
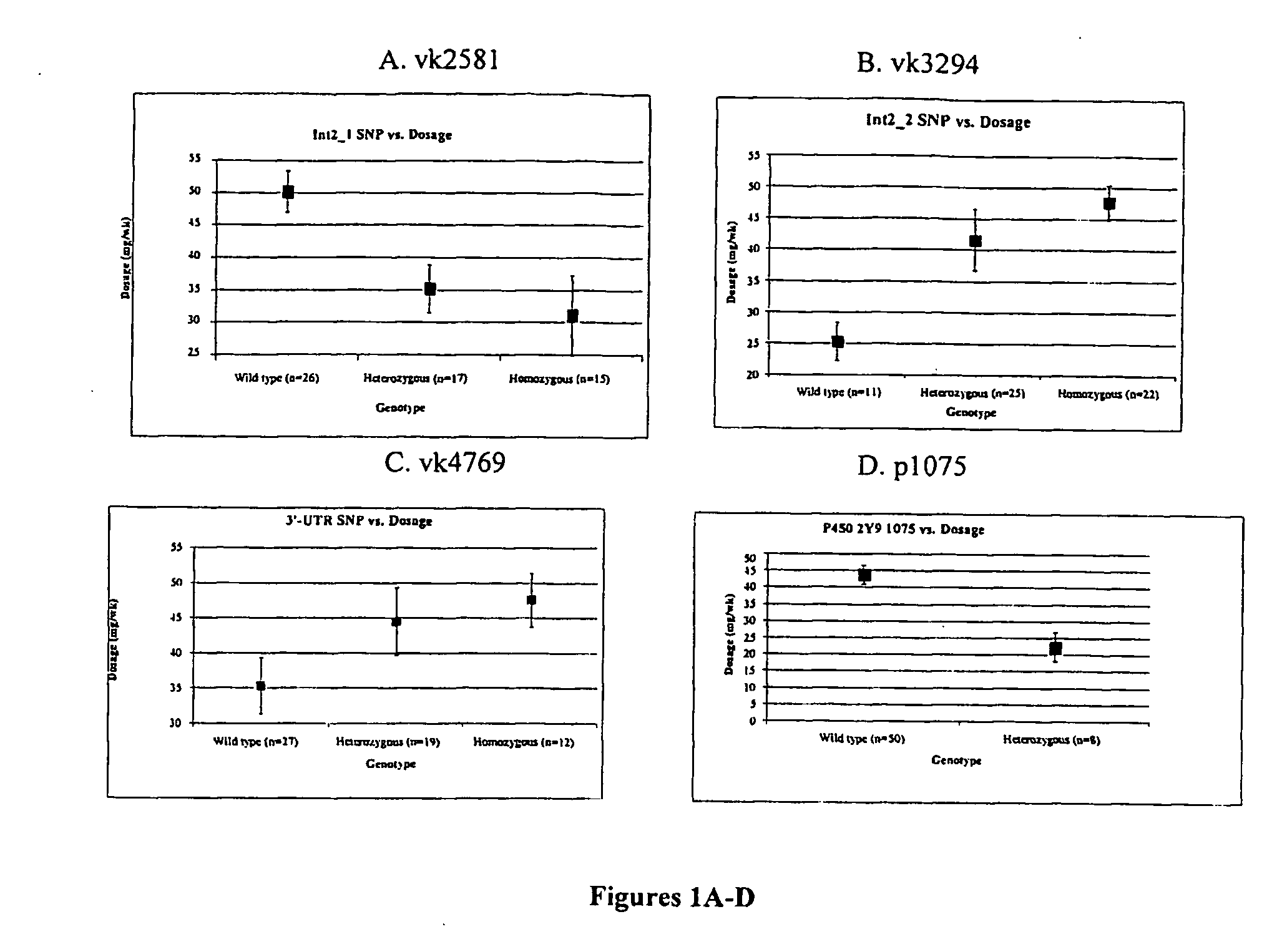
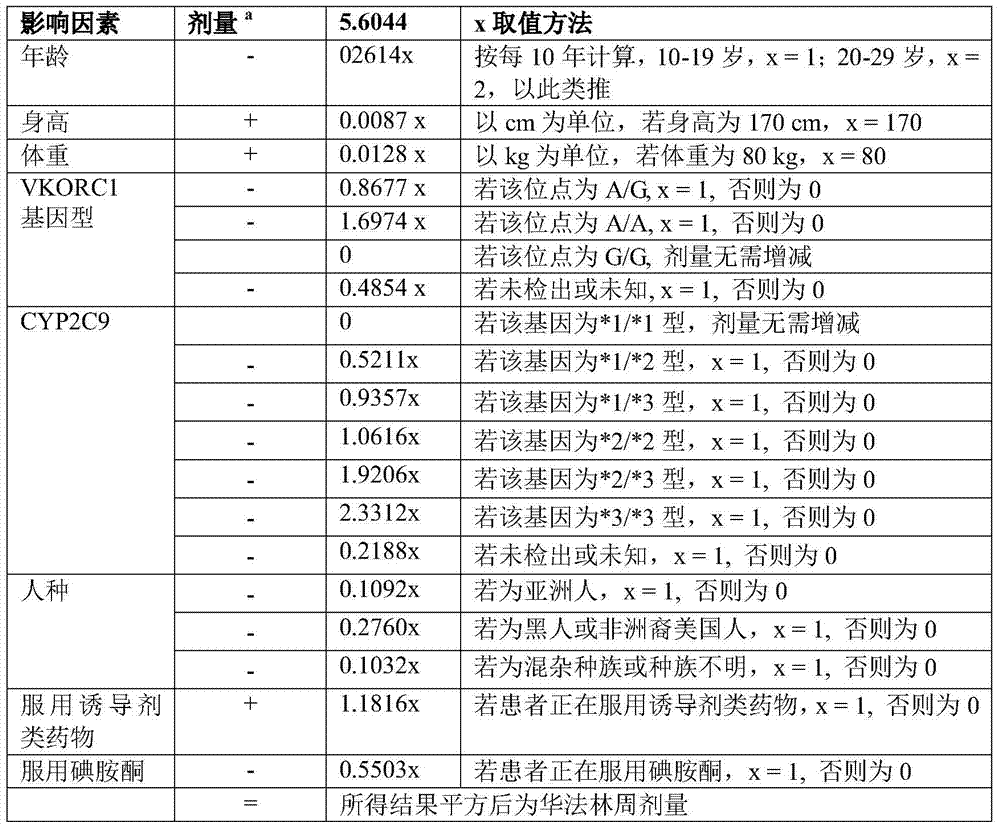
Method for auxiliarily determining warfarin dosage for Chinese Han patients by utilizing single nucleotide polymorphism analysis and biological chip
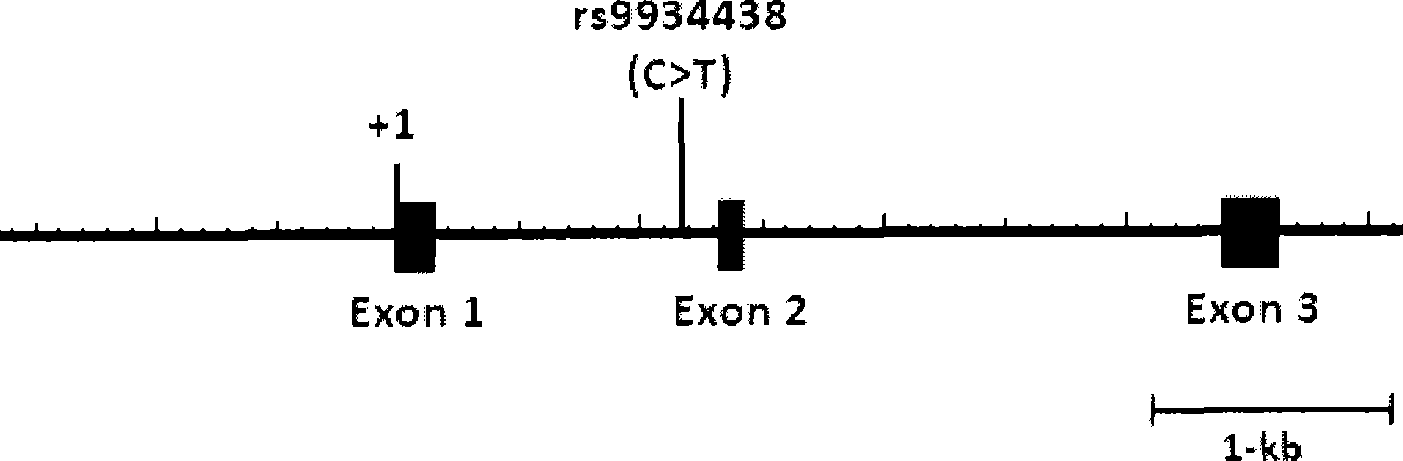
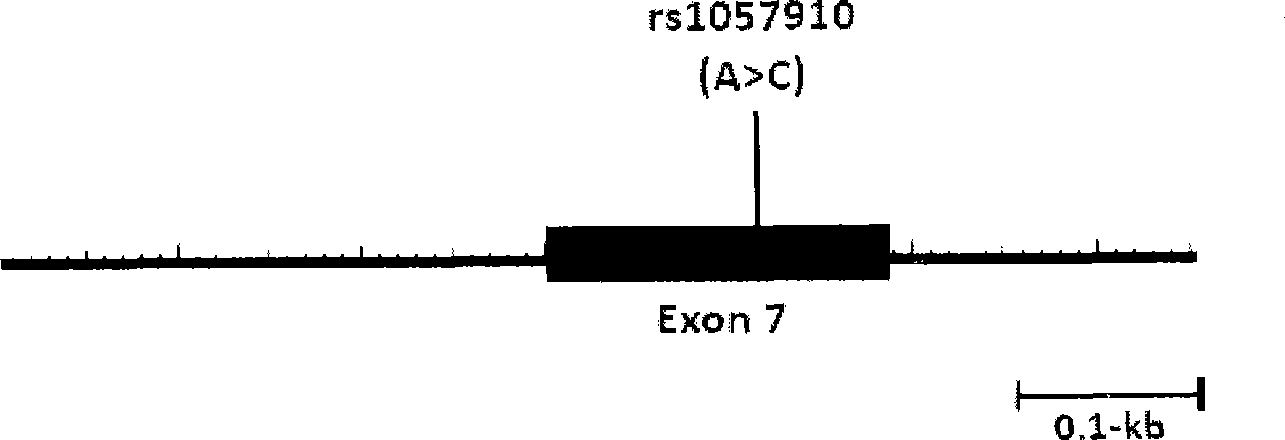
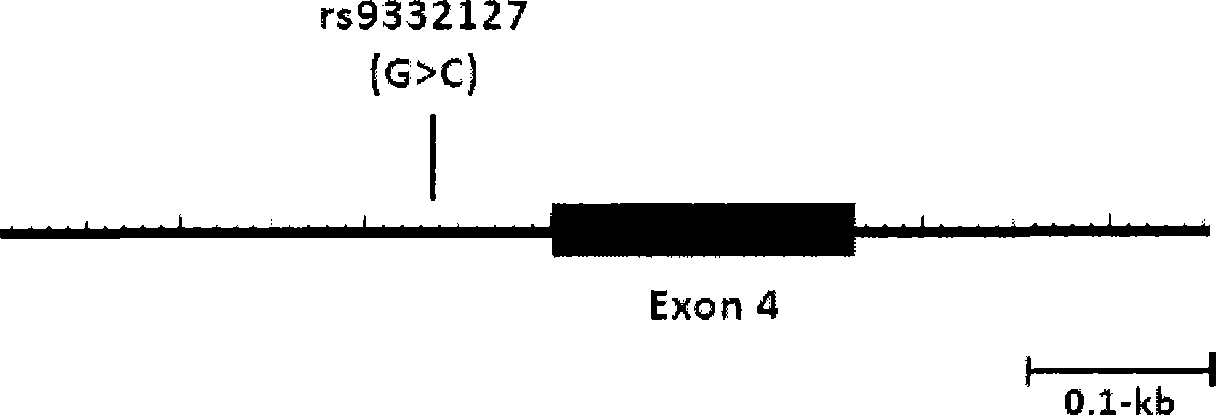
The invention relates to a method for auxiliarily determining warfarin dosage for Chinese Han patients by utilizing single nucleotide polymorphism analysis and a biological chip. In particular, the invention provides a method for acquiring grades useful in determining the warfarin dosage for the Chinese Han patients, which comprises the following steps: (1) acquiring a nucleic acid sample from a patient; (2) detecting the nucleic acid sample aiming at one or more single nucleotide polymorphism sites selected from the following groups: rs9934438 site of VKORC1 gene, rs1057910 site and rs9332127 site of CYP2C9 gene, and rs4653436 site of EPHX1 gene; (3) determining genotypes of the single nucleotide polymorphism sites; and (4) grading the genotypes of the single nucleotide polymorphism sites, wherein the grades obtained in step (4) are helpful in determining the warfarin dosage for the patient. The invention also provides the biological chip used for the method.
Owner:李文正纳米研究院
Methods and compositions for detecting metabolites
InactiveUS20100273203A1Particle separator tubesMicrobiological testing/measurementWarfarinMetabolite
The present invention provides a metabolic profile, a database comprising a metabolic profile, a method for determining a metabolic profile, uses for a metabolic profile, and warfarin metabolites.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Methods and Compositions for Producing Active Vitamin K-Dependent Proteins
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
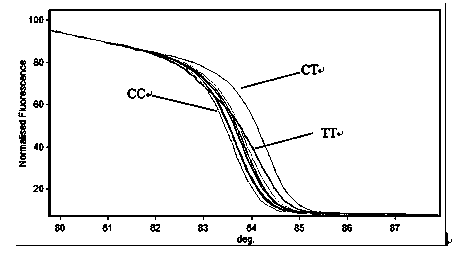
Method for detecting relative gene polymorphism of warfarin personalized medication by using high-resolution melting curve analysis technology
InactiveCN103525911AEasy to operateHigh sensitivityMicrobiological testing/measurementWarfarinSilica gel
The invention discloses a method for detecting relative gene polymorphism of warfarin personalized medication by using a high-resolution melting curve analysis technology. The method is characterized by comprising the following steps: adopting a silica gel adsorption method to extract genome DNA (Deoxyribose Nucleic Acid) of an oral epithelial cell / peripheral blood cell sample of a detected person; designing a detection primer containing a target site; carrying out site typing on a PCR (Polymerase Chain Reaction) amplified gene sequence by using the high-resolution melting curve (HRM) analysis technology. The method disclosed by the invention is high in sensitivity, good in specificity and fast in detection speed, and can be used for providing reference to a clinical medication dosage of warfarin, so as to realize reasonable and effective personalized medication.
Owner:上海中优医药高科技股份有限公司
Kit for detecting polymorphism of VKORC1 and CYP2C9 genes
ActiveCN102329885AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationWarfarinVKORC1
The invention provides a kit for quickly detecting polymorphism of VKORC1 and CYP2C9 genes. The kit is used for guiding the dosage of clinical warfarin. The kit comprises three pairs of primers shown as SEQ ID No.1-6, and can also further comprise one or more of the following reagents: polymerase chain reaction (PCR) reaction liquid, negative control, positive control, PCR sequencing reaction liquid and human peripheral blood genome extraction reagent. The kit is used for detecting the polymorphism of the VKORC1 and CYP2C9 genes by adopting a gene sequencing method with relatively high sensitivity and specificity, the specificity and the sensitivity of the detection result of the kit are remarkably improved, and the kit provides a brand-new, quick, simple and convenient gene diagnosis technology for clinically improving the safety and the validity of warfarin application.
Owner:李艳 +1
Method of controlling zoological and aquatic plant growth
A method of controlling target aquatic microorganism pest populations by exposing the target population to an effective amount of an aquacidal compound. The aquacidal compounds are selected from the group consisting of quinones, anthraquinones, naphthalenediones, quinine, warfarin, coumarins, amphotalide, cyclohexadiene-1,4-dione, phenidione, pirdone, sodium rhodizonate, apirulosin and thymoquinone. The method is particularly effective for treating ballast water of ships or other enclosed volumes of water subject to transport between or among geographic areas to control the relocation of plants, toxic bacteria, and animals contained in the water.
Owner:CUTLER HORACE G +3
Method for administering anticoagulation therapy
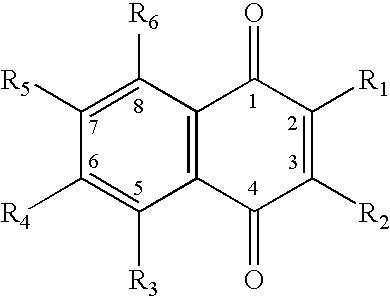
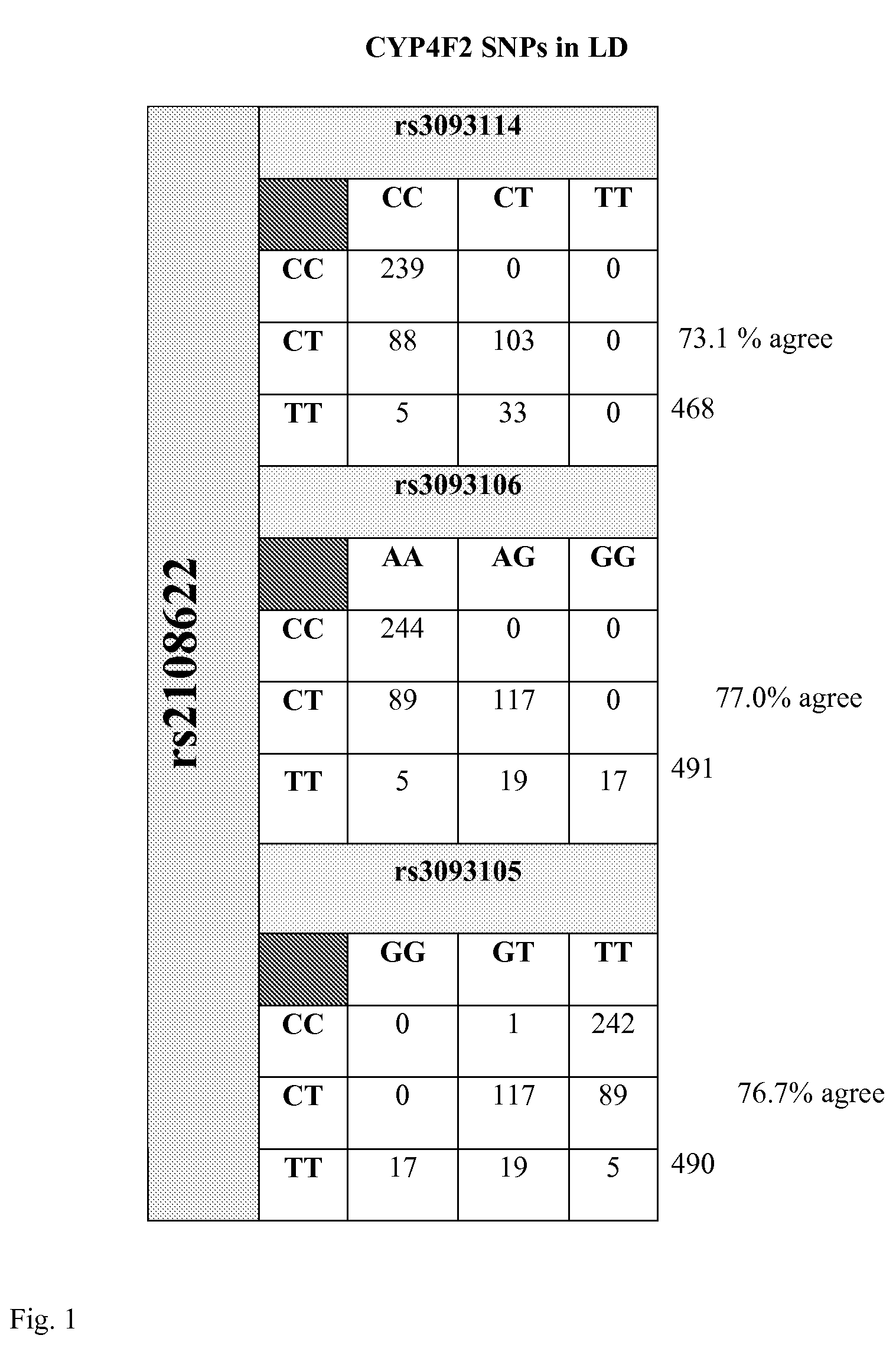
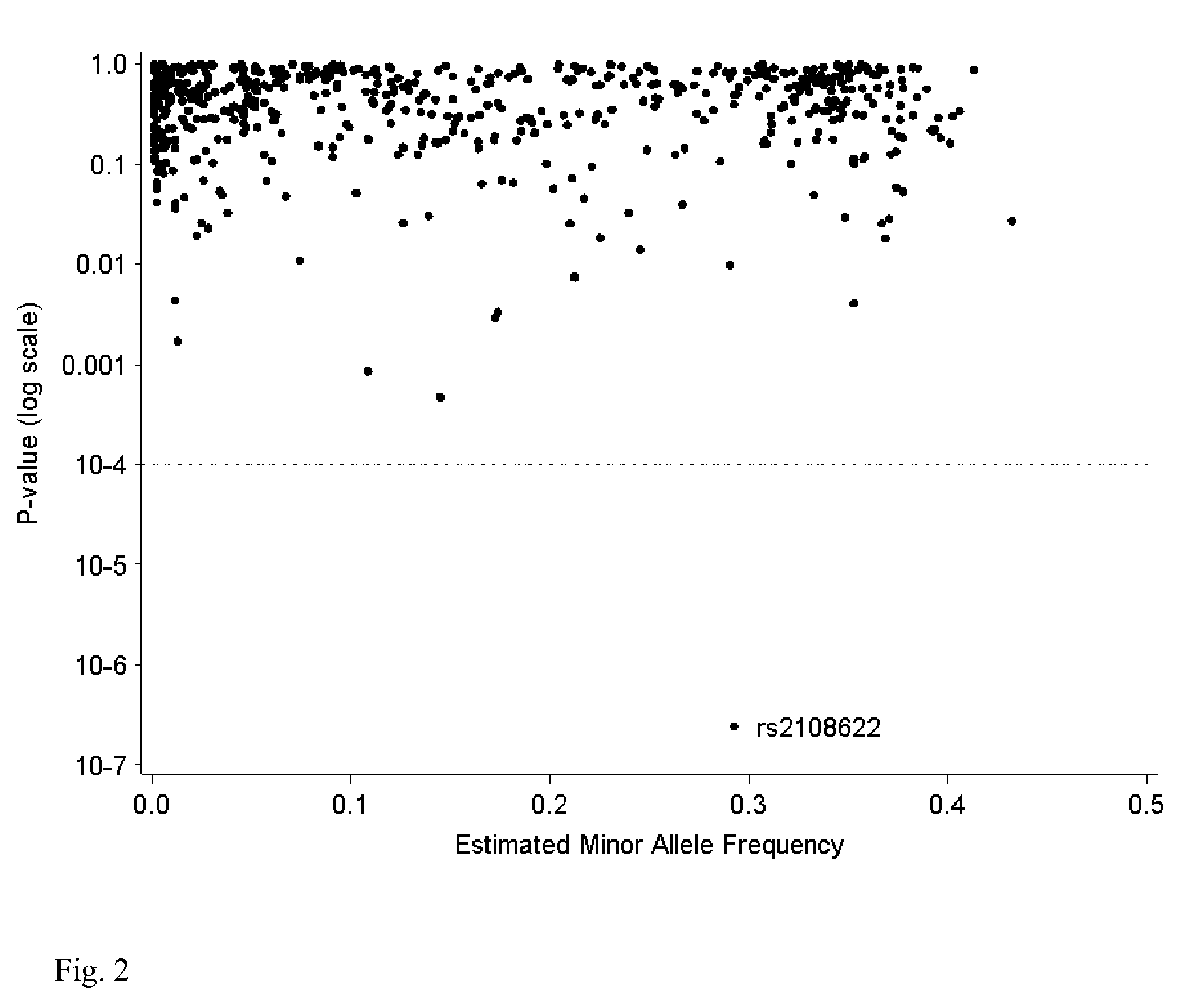
The present invention provides a method for use in treating a patient with an anticoagulant to optimize drug therapy and / or to prevent an adverse drug response. More particularly, the present invention relates to a method and system for use in treating a patient with Coumadin® or a substance containing warfarin. Methods of the present invention utilize variables that include the patient's CYP4F2 genotype.
Owner:MARSHFIELD CLINIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com