Preparation method of anticoagulant drugs
An anticoagulant and drug technology, which is applied in the field of anticoagulant drug preparation, can solve the problems of increased production difficulty, increased drug differentiation, insufficient color, etc., and achieve the effect of stable product process, simple and rapid process, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
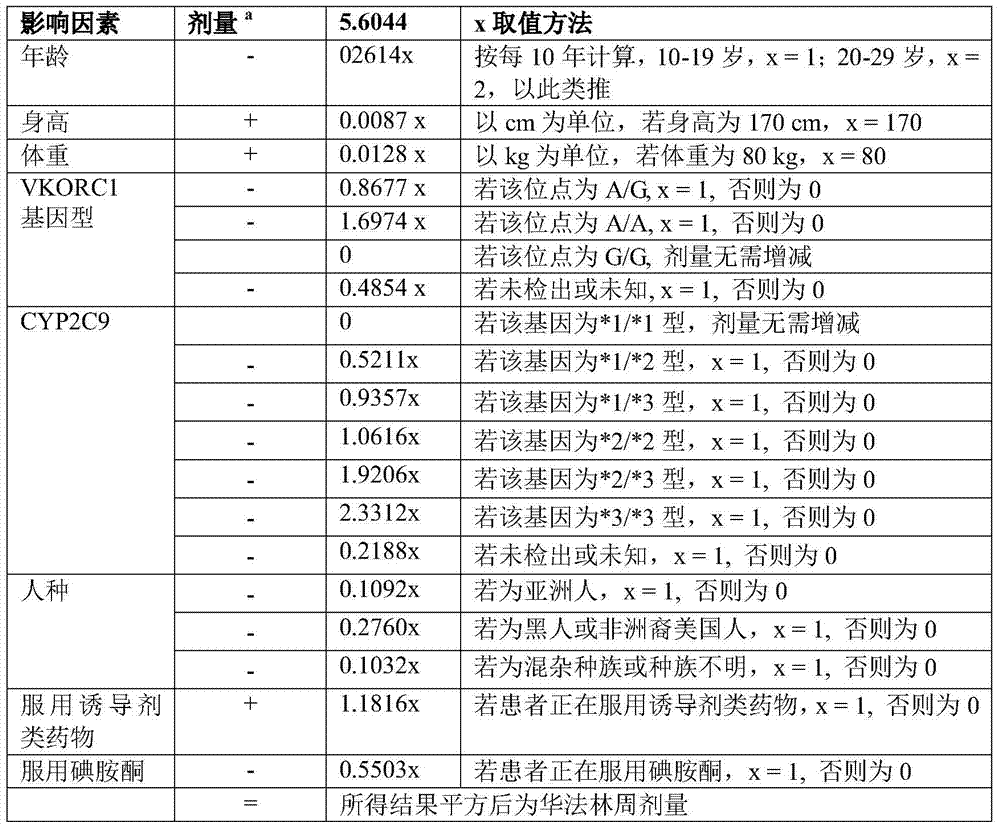
[0036] Take patient A as an example (age 21, height 170cm, weight 60kg, VKORC1 gene type A / G, CYP2C9 gene type *1 / *1, Asian race, not taking inducer drugs, taking iodominone) , Calculated, the required dose is 33.7mg per week, 4.8mg per day.
[0037] Add the pre-mixed raw and auxiliary materials to be printed in the feed hopper of the 3D printer, wherein the content of warfarin sodium is 10%, and the content of poly(vinylpyrrolidone co-vinyl acetate) (vinylpyrrolidone / vinyl acetate=6 / 4) 90%.
[0038] According to the shape (such as cube) and size (length 3mm, width 4mm, height 4mm) of pharmaceutical products, 3D modeling is carried out in the computer through computer-aided design software; the computer transmits the 3D modeling data to the 3D printer, and uses the The software performs layered slice processing and forms corresponding codes (this code is responsible for the control of X, Y, and Z sites).
[0039] Through the control of the print head of the 3D printer, the r...
Embodiment 2
[0042] Taking patient B as an example (age 30, height 180cm, weight 80kg, VKORC1 gene type A / A, CYP2C9 gene type *2 / *2, black, no inducer drugs, no iodarone), calculate So, the required dosage is 19.1mg weekly, 2.7mg daily.
[0043] Add the pre-mixed raw and auxiliary materials to be printed in the feed hopper of the 3D printer, wherein the content of warfarin sodium is 10%, and the content of poly(vinylpyrrolidone co-vinyl acetate) (vinylpyrrolidone / vinyl acetate=6 / 4) 90%.
[0044] According to the shape (such as cube) and size (3mm in length, 3mm in width, and 3mm in height) of pharmaceutical products, 3D modeling is carried out in the computer through computer-aided design software; the computer transmits the 3D modeling data to the 3D printer, and uses the The software performs layered slice processing and forms corresponding codes (this code is responsible for the control of X, Y, and Z sites).
[0045] Through the control of the print head of the 3D printer, the raw an...
Embodiment 3
[0048] Taking patient B as an example (age 30, height 180cm, weight 80kg, VKORC1 gene type A / A, CYP2C9 gene type *2 / *2, black, no inducer drugs, no iodarone), calculate So, the required dosage is 19.1mg weekly, 2.7mg daily.
[0049] Add the pre-mixed raw and auxiliary materials to be printed in the feed hopper of the 3D printer, wherein the content of warfarin sodium is 1%, and the content of poly(vinylpyrrolidone co-vinyl acetate) (vinylpyrrolidone / vinyl acetate=6 / 4) 99%.
[0050] According to the shape (such as cylinder) and size (radius 4.1mm, height 5mm) of pharmaceutical products, 3D modeling is carried out in the computer through computer-aided design software; the computer transmits the 3D modeling data to the 3D printer, and uses the built-in software Hierarchical slice processing, and form corresponding code (this code is responsible for the control of X, Y, Z position).
[0051] Through the control of the print head of the 3D printer, the raw and auxiliary material...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com