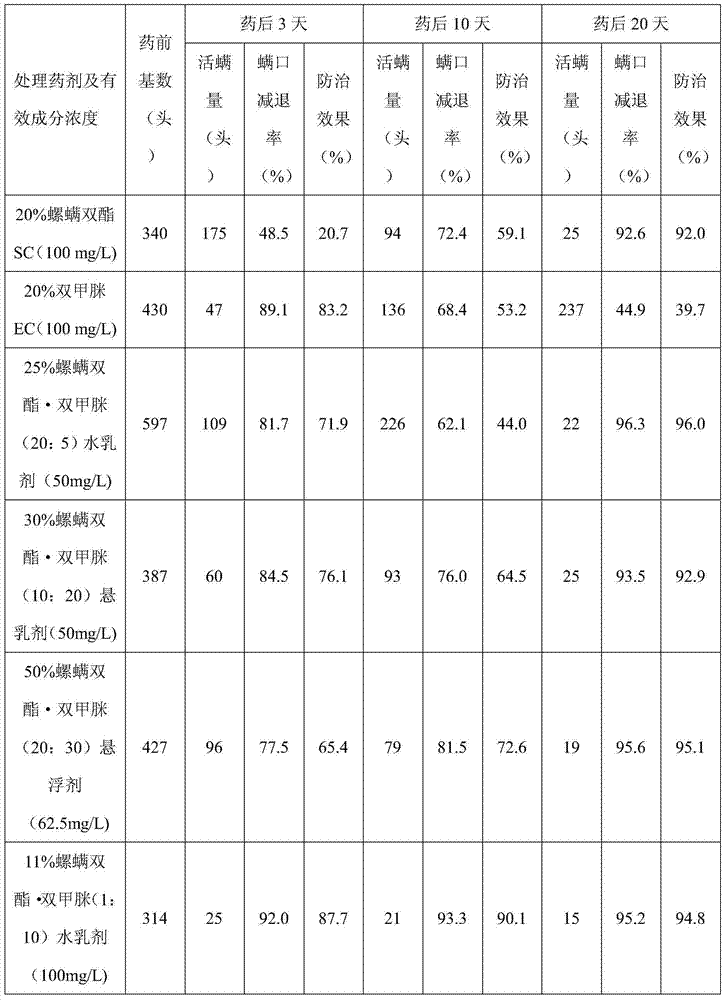
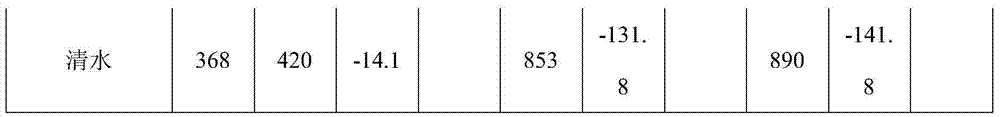
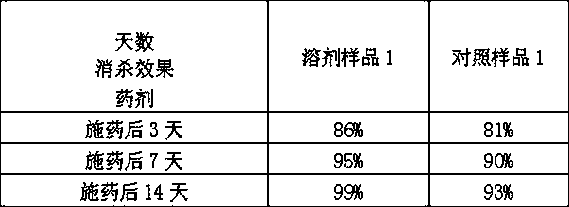
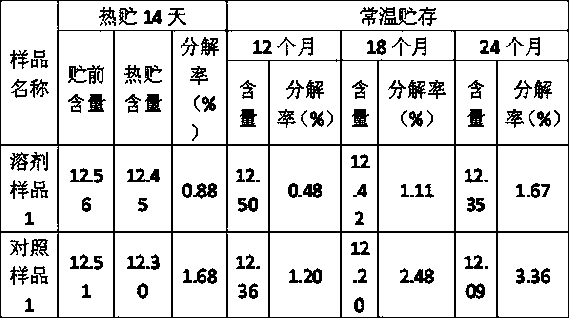
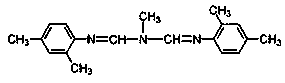Patents
Literature
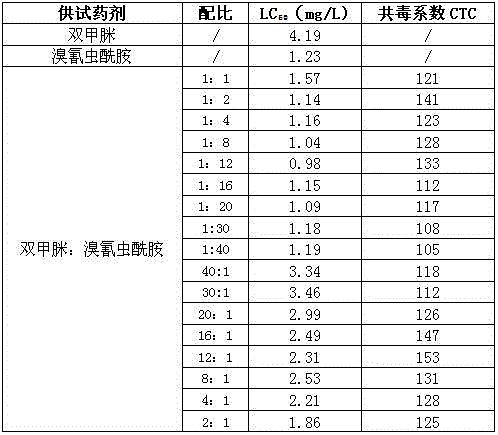
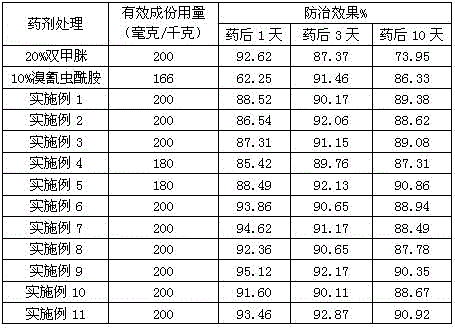
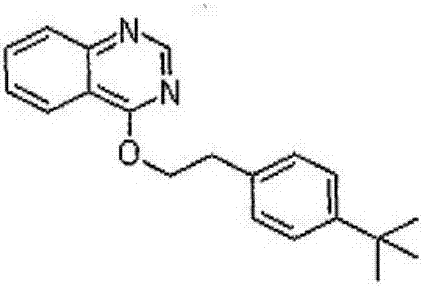
69 results about "Amitraz" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
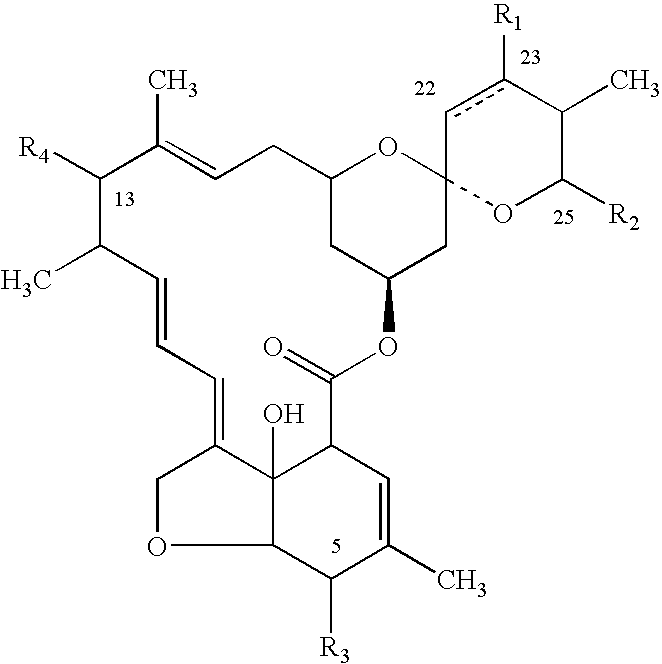
Amitraz (development code BTS27419) is a non-systemic acaricide and insecticide and has also been described as a scabicide. It was first synthesized by the Boots Co. in England in 1969. Amitraz has been found to have an insect repellent effect, works as an insecticide and also as a pesticide synergist. Its effectiveness is traced back on alpha-adrenergic agonist activity, interaction with octopamine receptors of the central nervous system and inhibition of monoamine oxidases and prostaglandin synthesis. Therefore, it leads to overexcitation and consequently paralysis and death in insects. Because amitraz is less harmful to mammals, amitraz is among many other purposes best known as insecticide against mite- or tick-infestation of dogs. It is also widely used in the beekeeping industry as a control for the Varroa destructor mite, although there are recent reports of resistance (driven by overuse and off label use)reference needed here, questionable statement.
Topical formulations comprising 1-N-arylpyrazole derivatives and amitraz
The present invention provides for, inter alia, novel topical formulations comprising at least one 1-N-arypyrazole derivative and amitraz and to methods for treating, controlling, or preventing parasite infestations on mammals or birds The inventive formulations include spot-on, pour-on or spray formulations and may include a further ectoparasiticide, such as an IGR compound, an avermectin or milbemycin derivative, or a pyrethroid insecticides, and anthelmintics, such as benzimidazoles and imidazothiazoles. The inventive formulation provides a larger duration of parasite control at a faster rate of control. The inventive formula remains effective up to three months from the first application. Moreover, the inventive formulations prevent tick attachment to the animal, thereby providing protection against tick borne diseases. The ectoparasites which may be controlled, treated or prevented by the present invention includes ticks, fleas, mites, mange, lice, mosquitoes, flies and cattle grubs.
Owner:MERIAL INC
Topical formulations comprising 1-N-arylpyrazole derivatives and amitraz
ActiveUS20050137244A1Treating and controlling and preventing parasite infestationLong durationBiocideDead animal preservationDiseaseMammal
The present invention provides for, inter alia, novel topical formulations comprising at least one 1-N-arypyrazole derivative and amitraz and to methods for treating, controlling, or preventing parasite infestations on mammals or birds The inventive formulations include spot-on, pour-on or spray formulations and may include a further ectoparasiticide, such as an IGR compound, an avermectin or milbemycin derivative, or a pyrethroid insecticides, and anthelmintics, such as benzimidazoles and imidazothiazoles. The inventive formulation provides a larger duration of parasite control at a faster rate of control. The inventive formula remains effective up to three months from the first application. Moreover, the inventive formulations prevent tick attachment to the animal, thereby providing protection against tick borne diseases. The ectoparasites which may be controlled, treated or prevented by the present invention includes ticks, fleas, mites, mange, lice, mosquitoes, flies and cattle grubs.
Owner:MERIAL INC
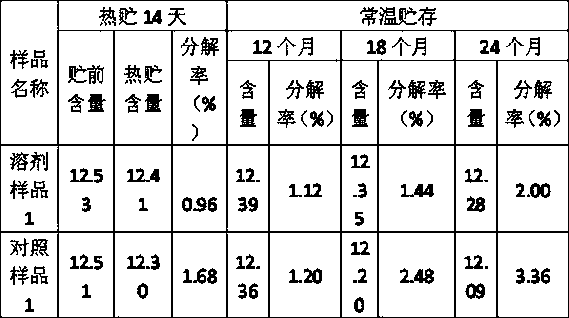
Amitraz compositions
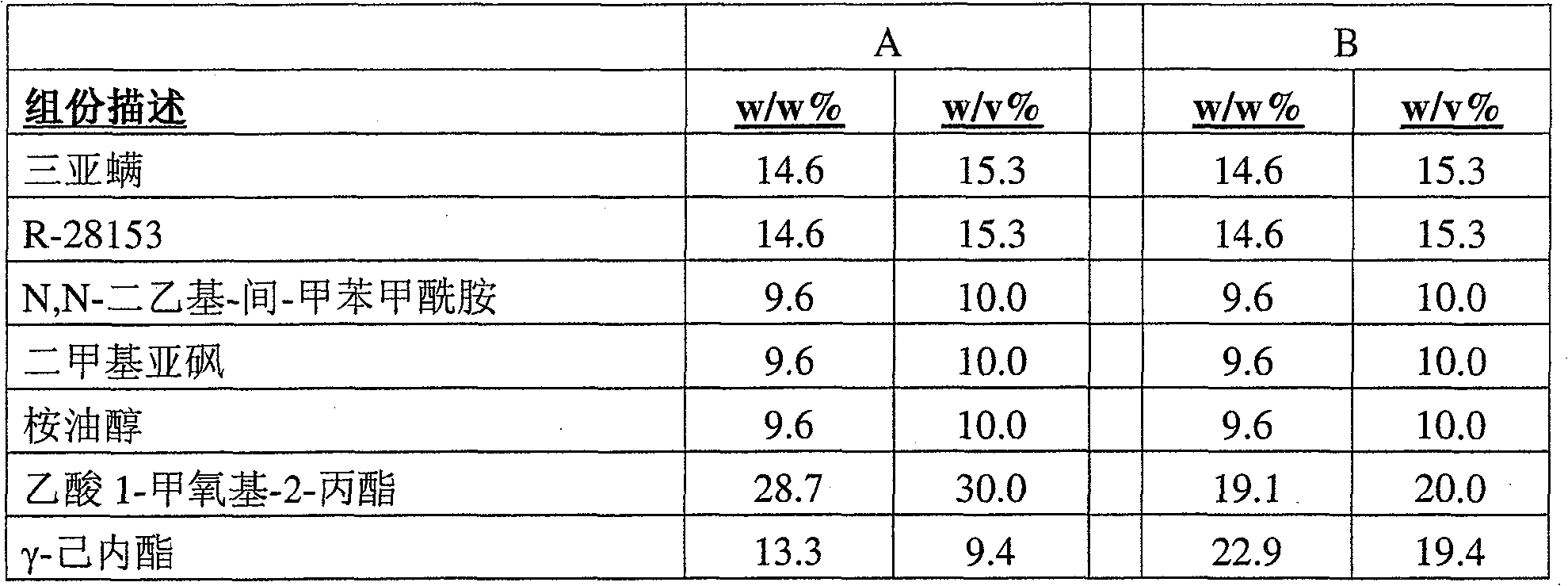
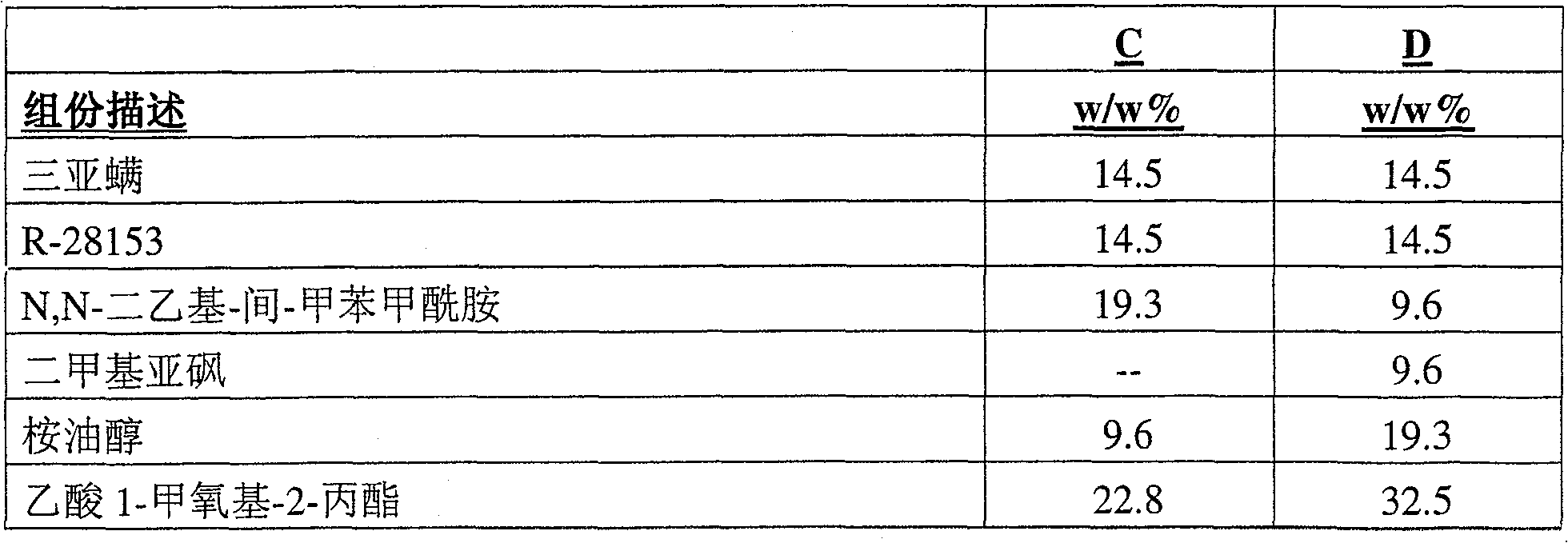
The present invention provides a stable composition which comprises a non-hydroxyl-group-containing solvent mixture comprising N,N-diethyl-m-toluamide and γ-hexalactone, optionally with dimethyl sulfoxide, eucalyptol and 1-methoxy-2-propyl acetate; and an effective amount of each of amitraz and at least one additional parasiticidal compound, such as R-28153. Said composition allows for high concentrations of a mixture of parasiticidal agents in a single application and is useful for treating and controlling parasiticidal infection and infestation in a homeothermic animal.
Owner:NIHON NOHYAKU CO LTD
Acaricidal composition containing spirodiclofen
InactiveCN101647458AReduce dosageOvercoming and delaying drug resistanceBiocideAnimal repellantsFruit treeChemical composition
The invention discloses an insecticidal and acaricidal composition which comprises the active ingredients of spirodiclofen and any one of amitraz and semiamitraz as a formamidine insecticide and acaricide, the ratio of the mass parts of the spirodiclofen to the formamidine insecticide and acaricide is 5:1-1:50. After the spirodiclofen and the amitraz or the semiamitraz are mixed, the insecticidaland acaricidal composition has a marked synergistic function, good quick action and a long lasting period, is helpful for overcoming or delaying the occurrence of drug resistance of harmful acarids, and has the function of secondarily treating various pests. The insecticidal and acaricidal composition can be used for the control of various harmful acarids and pests on agricultural and horticultural crops, and is particularly suitable for the control of acarid pests on crops including fruit trees, cotton, vegetables, flowers and the like.
Owner:DONGGUAN RUIDEFENG BIO TECH
Method for raising and breeding wild boar and special type wild boar
The present invention relates to the raising and propagation method of wild boar and special wild boar, and includes the mating of wild boar and special wild boar. The mating is performed in proper mating period, that is, the female wild boar has 6-7 month age, 60-70 Kg weight and mating requirement. The mating process includes cleaning vulva of female wild boar with 3 % concentration water solution of potassium permanganate, the first mating with male wild boar, the second mating after 6-8 hr, and painting the female wild boar with water solution of amitraz. The said mating process is simple, practical and high in mating success rate.
Owner:姚胜华
Process for producing 20 percent amitraz emulsifiable concentrate
The invention discloses a process for producing 20 percent amitraz emulsifiable concentrate, which relates to the field of production and preparation of pesticides. A formulation of the amitraz emulsifiable concentrate comprises the following components: 202 kilograms per ton of raw amitraz, 120 kilograms per ton of emulsifying agent, 100 kilograms per ton of DMF, and 10 kilograms per ton of potentiating agent; and proper amount of dimethyl benzene is complemented. The amitraz emulsifiable concentrate is amitraz series broad spectrum acaricide, is mainly used for inhibiting the activity of monoamine oxidase, has the functions of contact toxicity, feeding resistance and repelling, and also has certain functions of internal absorption and fumigating. The amitraz emulsifiable concentrate is suitable for mites of various crops, and has better prevention effect on homoptera pests. The amitraz emulsifiable concentrate has a good pesticidal effect, avoids uninterrupted alternated use of pesticides, does not pollute the environment, and improves the quality and sanitation quality safety level of fruits.
Owner:江苏润鸿生物化学有限公司
Amitraz compositions
The present invention provides a stable composition which comprises a non-hydroxyl-group-containing solvent mixture comprising N,N-diethyl-m-toluamide and -hexalactone, optionally with dimethyl sulfoxide, eucalyptol and 1-methoxy-2-propyl acetate; and an effective amount of each of amitraz and at least one additional parasiticidal compound, such as R-28153. Said composition allows for high concentrations of a mixture of parasiticidal agents in a single application and is useful for treating and controlling parasiticidal infection and infestation in a homeothermic animal.
Owner:WYETH LLC
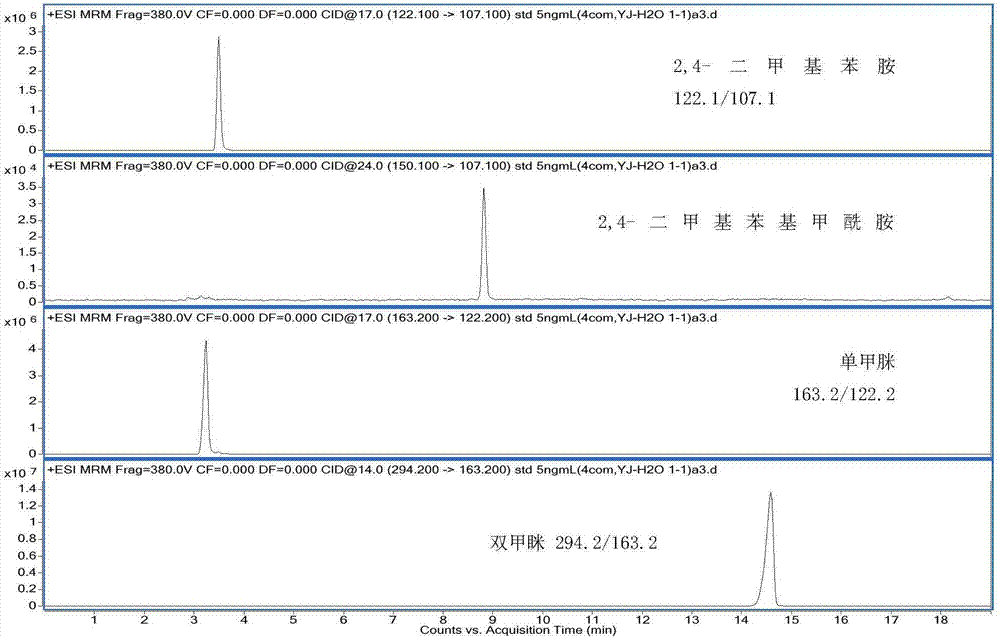
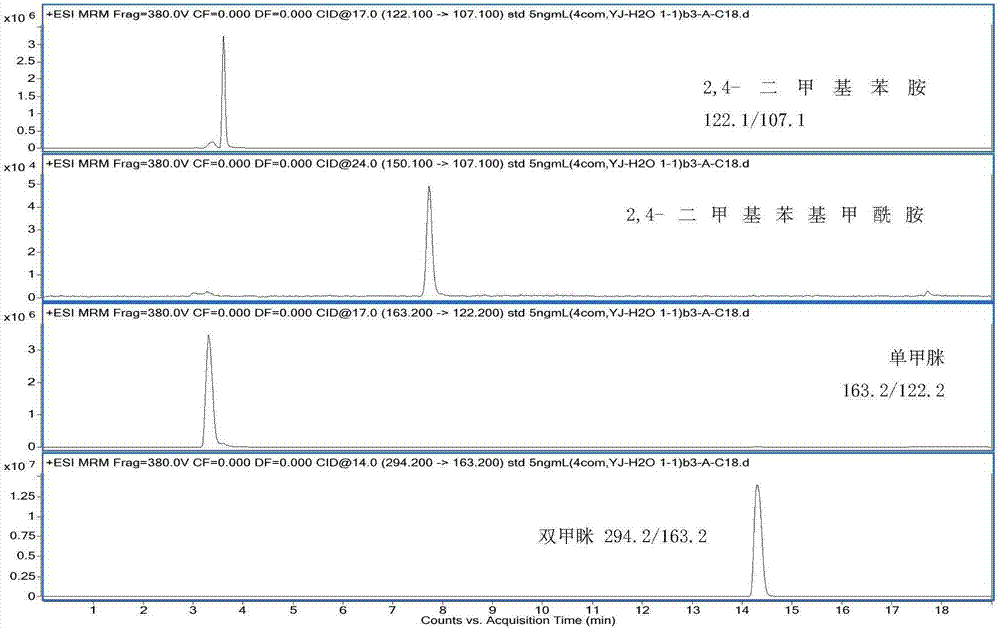
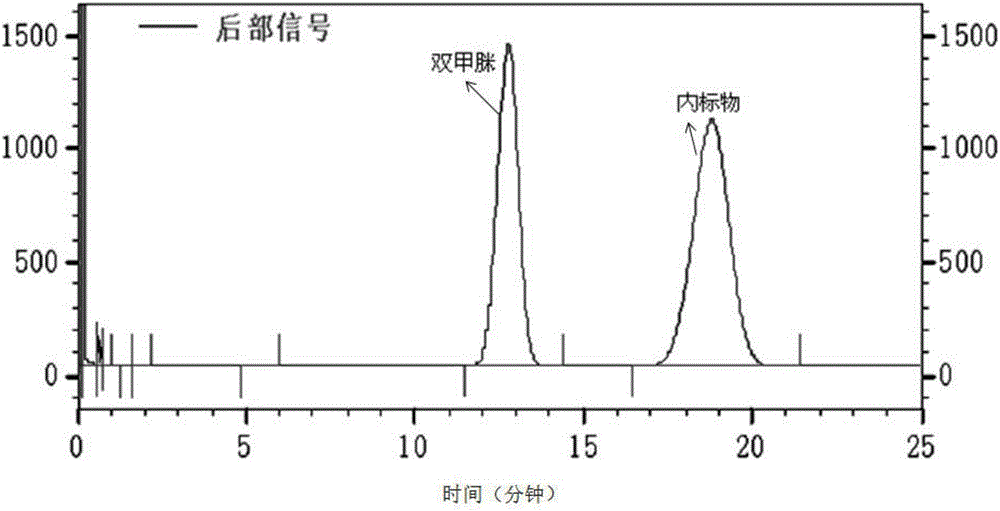
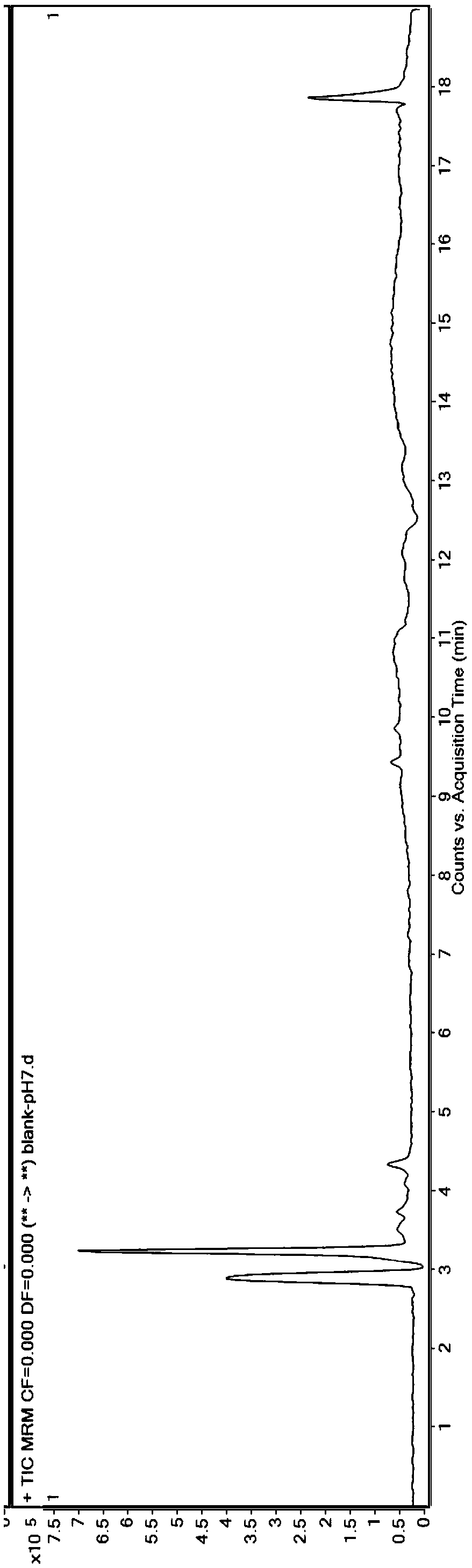
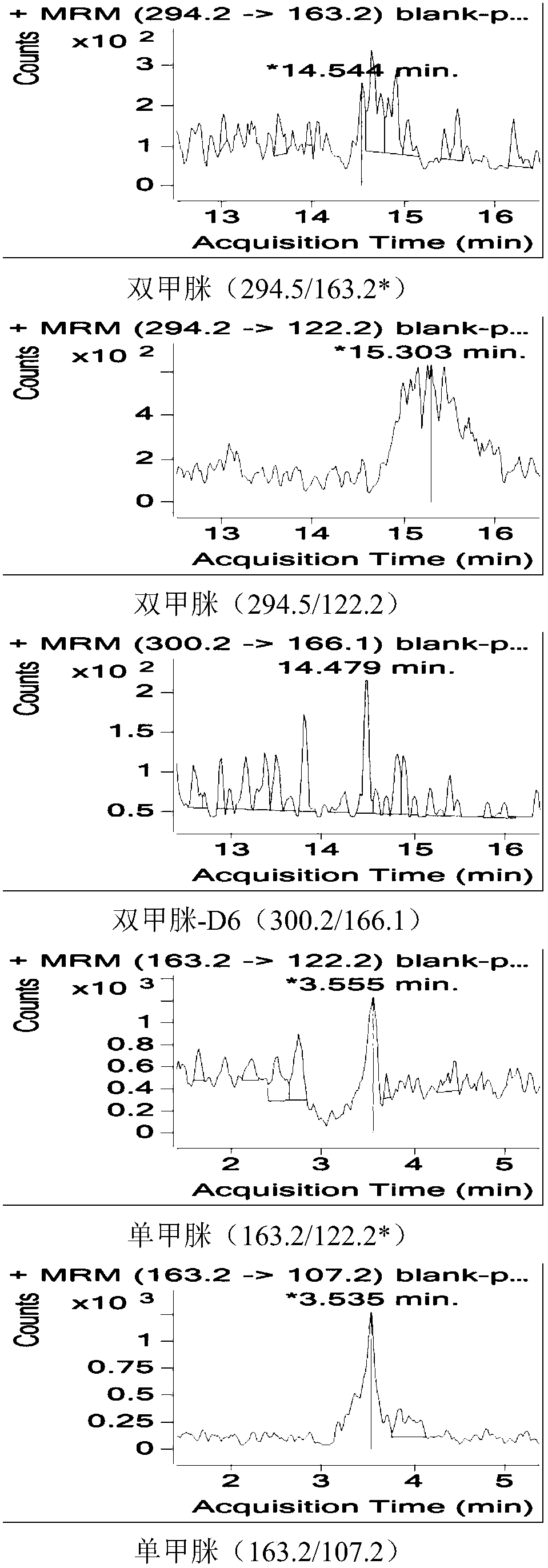
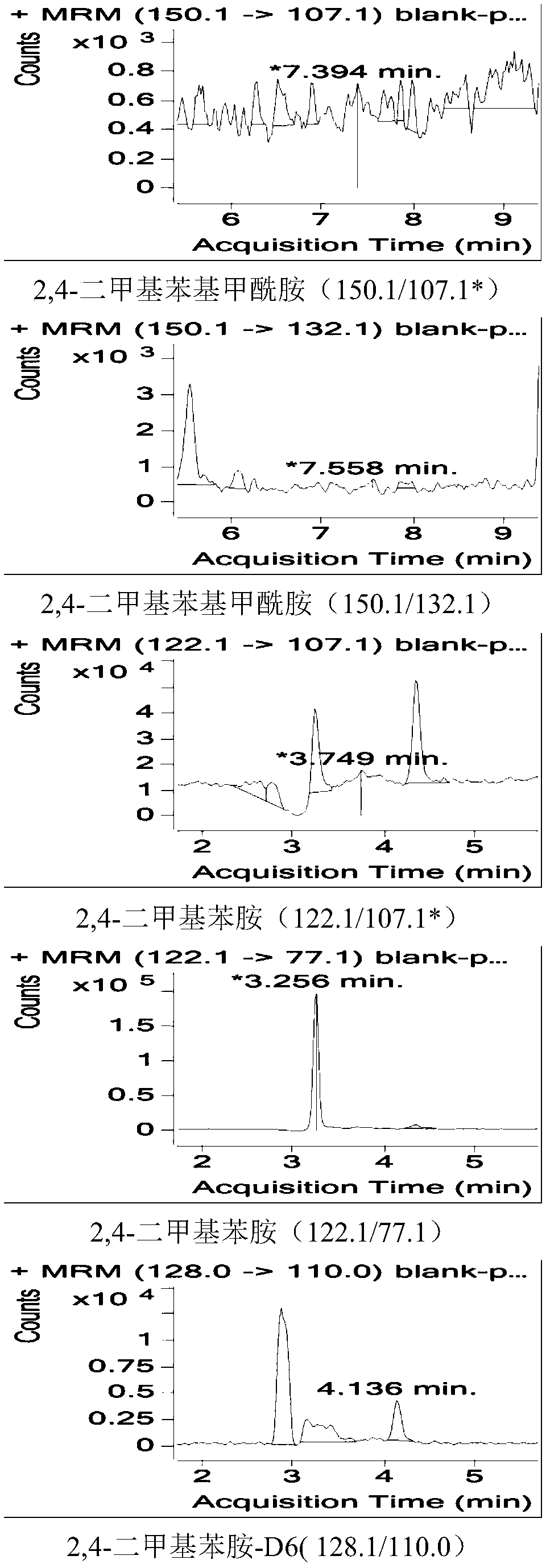
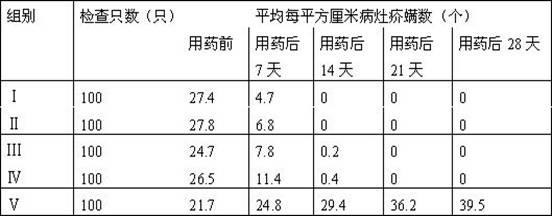
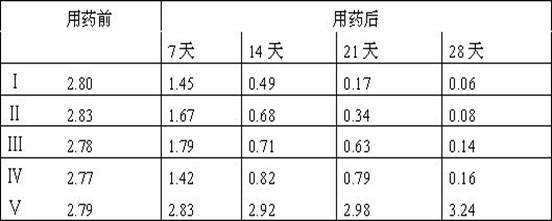
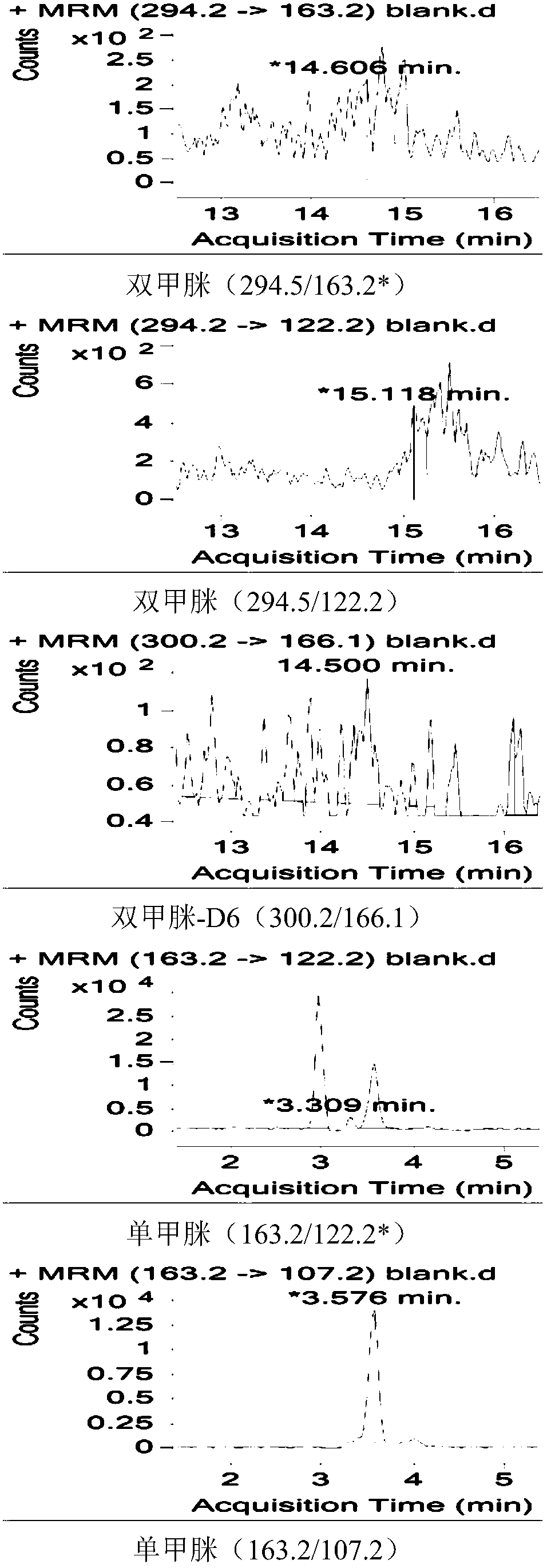
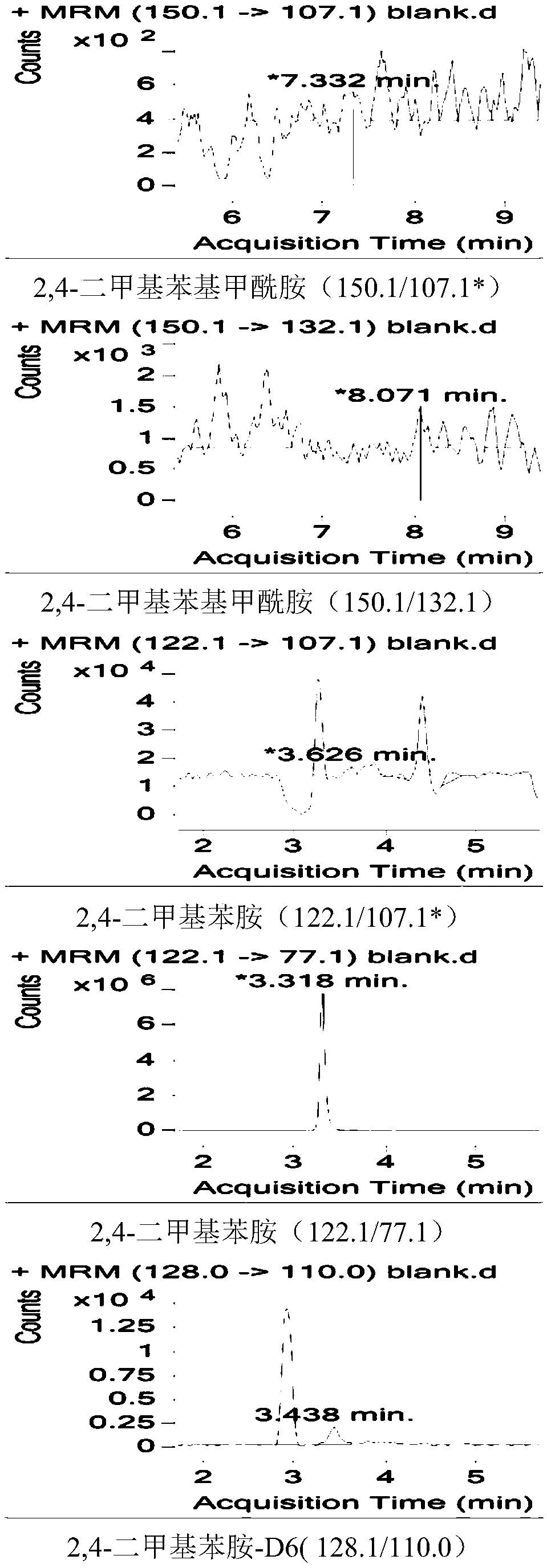
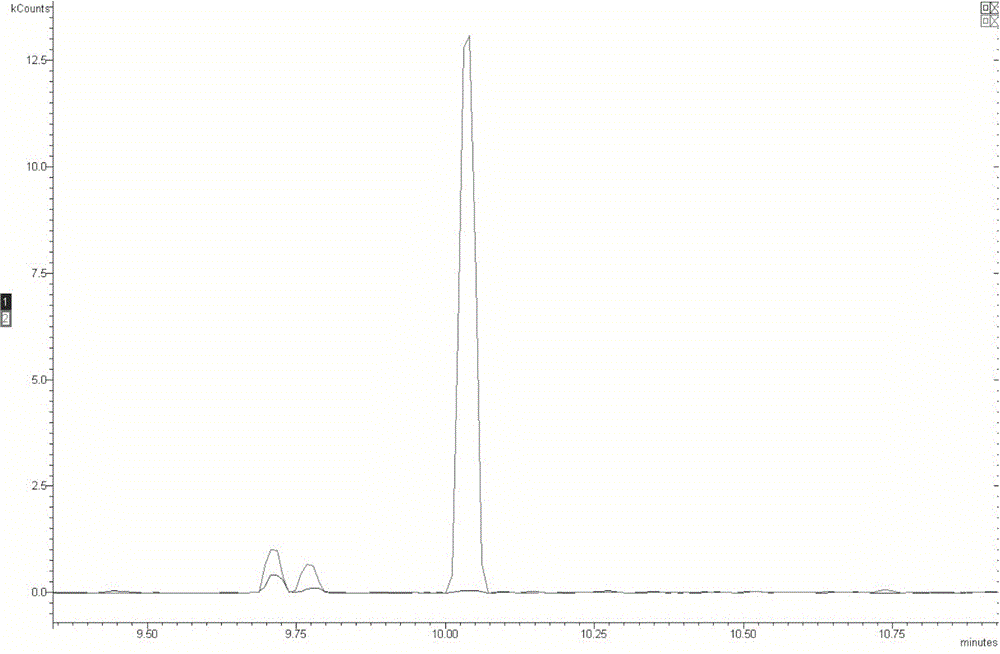
Method for measuring residual quantities of amitraz in royal jelly and metabolites of amitraz by liquid chromatography-mass spectrography/mass spectrometry
ActiveCN107102082AMeet detectionFulfil requirementsComponent separationDimethylaniline N-oxideMetabolite
The invention relates to a detecting method for measuring residual quantities of amitraz in royal jelly and metabolites of the amitraz, in particular to a method for measuring the amitraz, N-(2,4-dimethylphenyl)-N'-methylfomamidine, N-(2,4-dimethylphenyl) formamide and 2,4-dimethylaniline in the royal jelly by liquid chromatography-mass spectrography / mass spectrometry. The method for measuring the residual quantities of the amitraz in the royal jelly and the metabolites of the amitraz by the liquid chromatography-mass spectrography / mass spectrometry comprises the following steps: adding ammoniation water in a test specimen for diluting; then precipitating protein by using ammoniac acetonitrile and extracting the protein; purifying an extracting solution by using a neutral alumina solid phase extraction column; adopting a liquid chromatography-mass spectrography / mass spectrometer for detecting; quantifying the amitraz and the 2,4-dimethylaniline by an internal standard method; quantifying the N-(2,4-dimethylphenyl)-N'-methylfomamidine and the N-(2,4-dimethylphenyl) formamide by an external standard method. The sensitivity of the method can meet detection and verification requirements of the residual quantities of the amitraz in the royal jelly and the metabolites of the amitraz.
Owner:ZHEJIANG ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
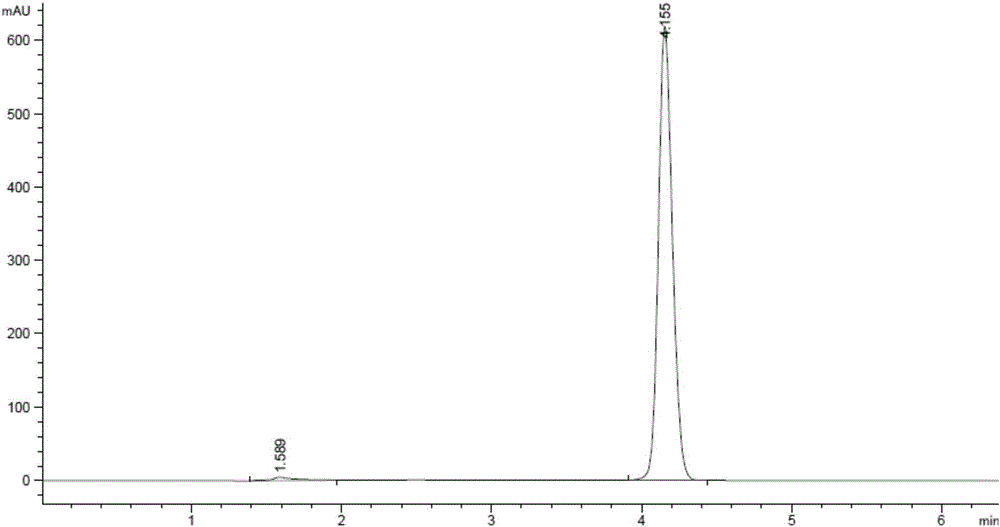
Method for detecting content of amitraz
InactiveCN106093268AReduce usage ratioImprove general performanceComponent separationPhosphoric acidColumn temperature
A method for detecting the content of amitraz includes the following steps that firstly, an amitraz standard substance is taken, standard solutions of a series of concentrations are prepared, detection is carried out with the high performance liquid chromatography, high performance liquid chromatograms of the standard solutions of the series of concentrations are obtained, and an amitraz standard curve is obtained; secondly, a sample to be detected is taken, a sample solution is prepared, detection is carried out with the high performance liquid chromatography, and a high performance liquid chromatogram of the sample solution is obtained; thirdly, the content of amitraz in the sample to be detected is calculated with the external standard method according to the obtained high performance liquid chromatogram of the sample solution and the obtained amitraz standard curve. According to the chromatographic conditions of the high performance liquid chromatography adopted in the first step and the second step, octadecylsilane chemically bonded silica serves as a packed column, an acetonitrile-phosphoric acid solution serves as a mobile phase, the flow velocity of the mobile phase is 0.5-1.5 ml / min, the column temperature is 20-40 DEG C, and the detection wavelength is 250-350 nm. By the adoption of the high performance liquid chromatography, the method for detecting the content of amitraz is easy, convenient and fast to implement, high in university, short in peak appearance time, capable of saving time and labor, economical and environmentally friendly.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
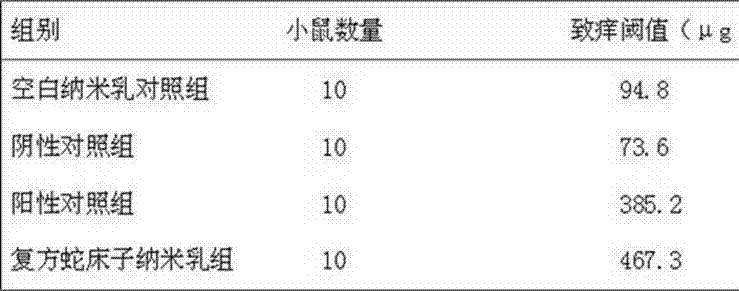
Compound oil-in-water cnidium fruit oil nanoemulsion composition and preparation method thereof
InactiveCN102397307AThe method is simpleSuitable for large-scale productionAntiparasitic agentsAmide active ingredientsBiotechnologyEugenol
The invention discloses a compound oil-in-water cnidium fruit oil nanoemulsion composition. The particle size of the nanoemulsion composition is between 1 nm and 100nm. The nanoemulsion composition comprises the following raw materials in percentage by mass: 0.1 to 9.0 percent of cnidium fruit oil, 0.1 to 3.5 percent of eugenol, 0.1 to 2.0 percent of amitraz, 20.0 to 35.0 percent of surfactant, 0.1 to 6.0 percent of cosurfactant and the balance of distilled water, wherein the total percentage by weight of the raw materials is 100 percent. The compound cnidium fruit oil nanoemulsion has the effects of killing pests, relieving itching and killing bacteria and can be applied to the treatment of parasites (such as sarcoptic mites, psoroptidae, ticks, lice and the like) on body surfaces of animals. Through the compound oil-in-water cnidium fruit oil nanoemulsion composition, the stability and the bioavailability of the medicament are improved, the dosage of the medicament is reduced, the permeability is high, the insecticidal range is wide, the effect is obvious, and the preparation method is simple. Therefore, the compound oil-in-water cnidium fruit oil nanoemulsion composition is convenient to popularize and has wide market prospect in the field of medicaments.
Owner:NORTHWEST A & F UNIV
Agent used for preventing and treating red spiders on florists cineraria
InactiveCN104170835ASimple configuration processEasy to useBiocideAnimal repellantsPericallis × hybridaToxicology
The invention discloses an agent used for preventing and treating red spiders on florists cineraria. The agent comprises the following components in parts by weight: 10-14 parts of 20% fenpropathrin emulsifiable concentrate, 20-26 parts of avermectin, 6-12 parts of amitraz, 10-16 parts of flufenoxuron, 4-7 parts of alkylbenzene sulfonate, 3-6 parts of isopropyl phosphate, 4-8 parts of sodium diglycerol laurate, 3-5 parts of dimethylformamide, 4-8 parts of astragalus polysaccharide, 4-6 parts of essential balm, 4-12 parts of benzalkonium bromide and 40-50 parts of water. A use method comprises the following steps: proportionally allocating and mixing the components and stirring the components uniformly to obtain the agent for prevention and treatment; then spraying the agent around affected plants after diluting the agent for prevention and treatment. The agent has good prevention effects, is convenient to use, reduces the pesticide consumption, reduces environmental pollution and is beneficial to ecological balance.
Owner:SIYANG JUFENG ECOLOGICAL AGRI DEV
Method of measuring residual quantity of amitraz and metabolites in honey by means of QuEChERS-liquid chromatogram-mass spectrum/mass spectrum method
The invention relates to a method of measuring residual quantity of amitraz and three metabolites, in particular to a method of measuring residual quantity of amitraz and metabolites in honey by meansof a QuEChERS-liquid chromatogram-mass spectrum / mass spectrum method. The method comprises the following steps: adding a buffer solution, the pH of which is 7, into a test sample; carrying out extraction and salting out with acetonitrile and sodium chloride; carrying out dispersive solid-phase extraction on an adsorbent containing N-primary secondary amine (PSA), anhydrous magnesium sulfate and C18 powder to purify an extraction solution; carrying out detection by a liquid chromatogram-mass spectrum / mass spectrometer; and carrying out quantification by means of a isotope interior label diluted internal standard method. The method is green and environmentally friendly, simple and rapid, small in consumed resource and low in detection cost, and the sensitivity of the method can meet the detection and confirmation requirements on the residual quantity of amitraz and the three metabolites in honey.
Owner:ZHEJIANG ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
Pesticide composition for eucalyptus
ActiveCN103070195ASynergistic effect is obviousNo pollutionBiocideAnimal repellantsCellulosePolythylene glycol
The invention discloses a pesticide composition for eucalyptus. The pesticide composition for eucalyptus is characterized by comprising 10 to 15 parts by weight of dinotefuran, 15 to 20 parts by weight of N-methyl pyrrolidone, 5 to 15 parts by weight of amitraz, 2 to 5 parts by weight of catechol, 7 to 16 parts by weight of molybdenum disulfide, 3 to 8 parts by weight of methyl silicone oil, 2 to 5 parts by weight of azoxystrobin, 3 to 7 parts by weight of prochloraz, 2 to 7 parts by weight of sodium carboxymethyl cellulose, 1 to 5 parts by weight of polyethylene glycol, 5 to 10 parts by weight of polyoxyethylene, 5 to 8 parts by weight of attapulgite clay and 1 to 5 parts by weight of lignin. The pesticide composition for eucalyptus has the advantages of simple production process flow and good insecticidal effects.
Owner:徐州惠诚银杏产业发展有限公司
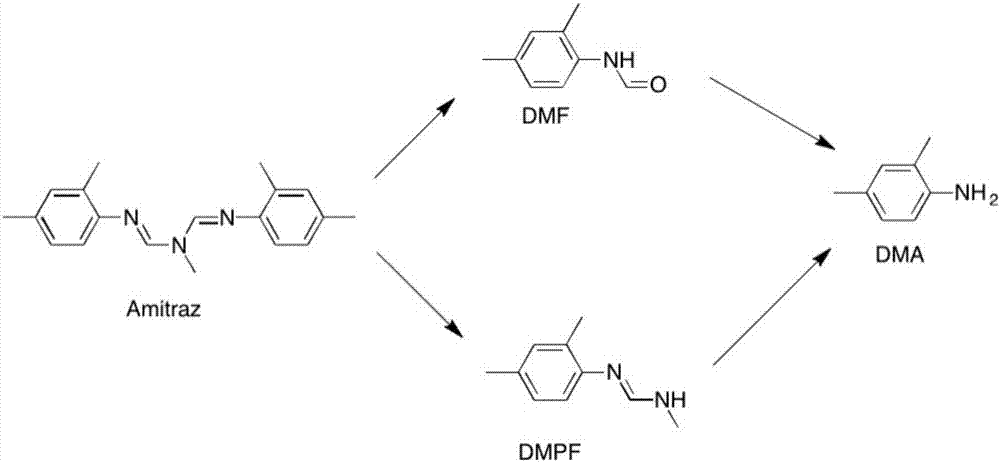
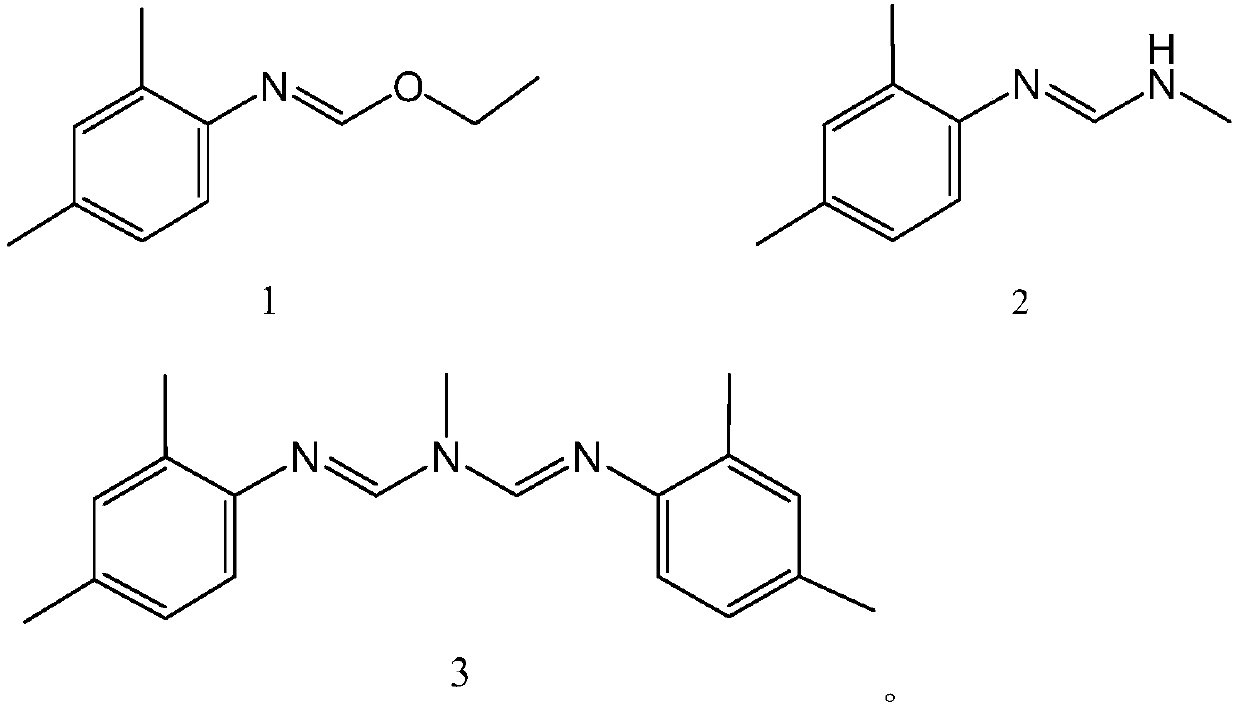
Preparation method of amitraz
ActiveCN107778200ASolve the problem of three wastes treatmentHigh purityOrganic chemistryDimethylaniline N-oxideAlcohol
The invention provides a preparation method of amitraz, which comprises the steps of (1) mixing 2,4-methyl toluidine, N-methyl formamide, triethyl orthoformate and a catalyst to obtain a mixture, (2)heating the mixture to allow the mixture to react, and volatilizing alcohol and ethyl formate to obtain final reaction liquid, and (3) cooling the final reaction liquid to a room temperature, and adding an organic solvent for crystallization to obtain the amitraz. The method has the advantages of greenness, environmental protection, high efficiency, economy, short reaction period, high product purity and the like.
Owner:东莞市东阳光动物保健药品有限公司
Insecticidal composition containing etoxazole and amitraz, and preparation and application thereof
InactiveCN106234376AEffective controlImprove the effect of prevention and controlBiocideDead animal preservationWater dispersibleTherapeutic effect
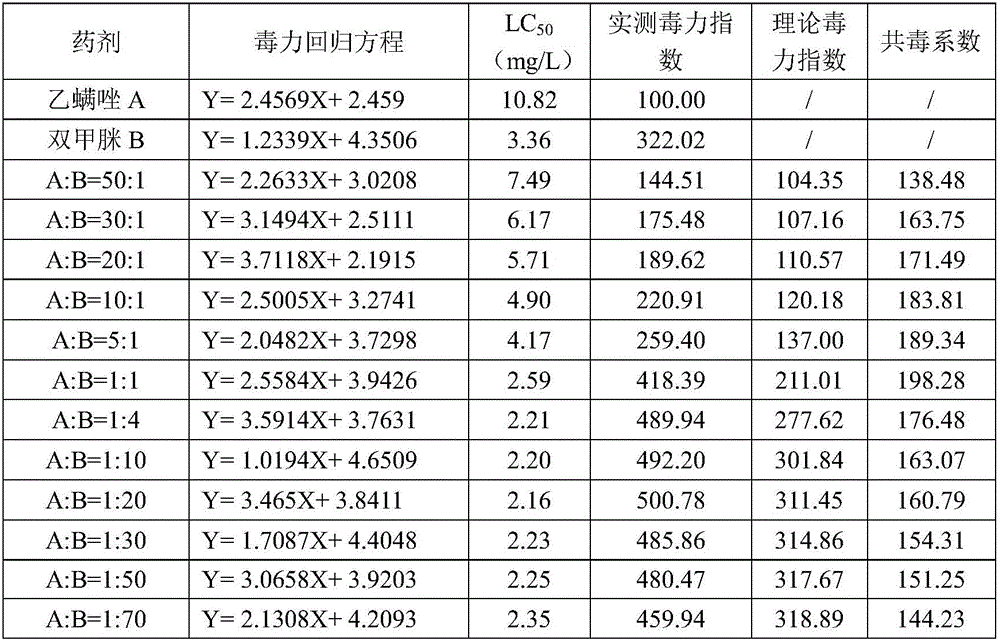
The invention provides an insecticidal composition containing etoxazole and amitraz, wherein the composition includes the etoxazole and the amitraz according to the weight ratio of 50:1-1:70. The invention also provides a preparation of the insecticidal composition, wherein the preparation may include the following agriculturally-acceptable dosages: wettable powders, soluble powders, water-dispersible granules, water emulsions, micro-emulsions, micro-capsule suspensions, suspensions, suspension emulsions or emulsifiable concentrates. The insecticidal composition has reasonable formula and good insecticidal effect, wherein the activities and insecticidal effects of the components are achieved not by only simply adding the activities of all components. Compared with single preparations in the prior art, the composition has significant insecticidal effect and significant synergistic effects, has good safety on crops and satisfies security of pesticide preparations. The insecticidal composition has good prevention and treatment effects on acarid on plants, and especially has significant prevention and treatment effects on panoneychus citri and tetranychus urticae.
Owner:ZHEJIANG XINNONG CHEM CO LTD
Insecticide composition containing SYP-9625 and amitraz
InactiveCN108174852ASynergistic effect is obviousReduce dosageBiocideAnimal repellantsSuspending AgentsAmitraz
The invention relates to the field of pesticide, and discloses an insecticide composition. The active ingredients of the insecticide composition comprise SYP-9625 and amitraz with the mass ratio of (50: 1) to (1: 50), preferably (5: 1) to (1: 2). The insecticide composition disclosed by the invention can be prepared into a suspending agent, wettable powder and water dispersible granules. After thetwo active ingredients of the insecticide composition are compounded, an outstanding synergistic effect is achieved in a certain range, the dosing pesticide can be reduced, and the cost is reduced; the resistance can be retarded; and the insecticide composition is high in safety and environmentally friendly.
Owner:PLANT PROTECTION RES INST OF GUANGDONG ACADEMY OF AGRI SCI
Compound pest/mite killing composition containing spirodiclofen double ester and application of compsition
ActiveCN103783043AGood control effectDelay drug resistanceBiocideAnimal repellantsEmulsionSpirodiclofen
The invention discloses a compound pest / mite killing composition containing spirodiclofen double ester. The compound pest / mite killing composition is preapred from the following components in parts by weight: 1-20 parts of spirodiclofen double ester and 5-30 parts of amitraz, wherein the weight ratio of spirodiclofen double ester to amitraz is 1: (0.2-20). The compound pest / mite killing composition containing spirodiclofen double ester can be prepared into EW (emulsion in water), suspending agents and suspoemulsion, can effectively kill mites or pests such as citrus red mites, two-spotted spider mites, tetranychus cinabarinus Boisdu, pear psylla and diaphorina citri.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Method for analyzing amitraz residual marker
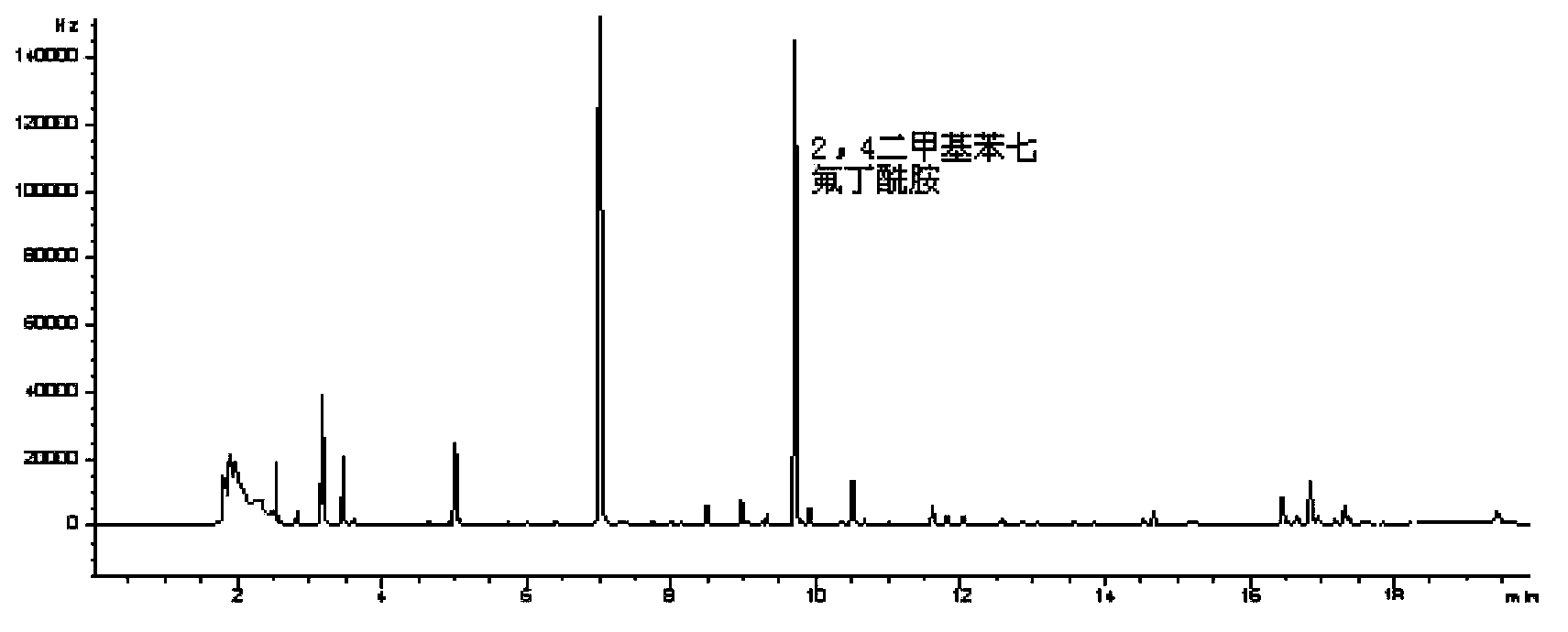
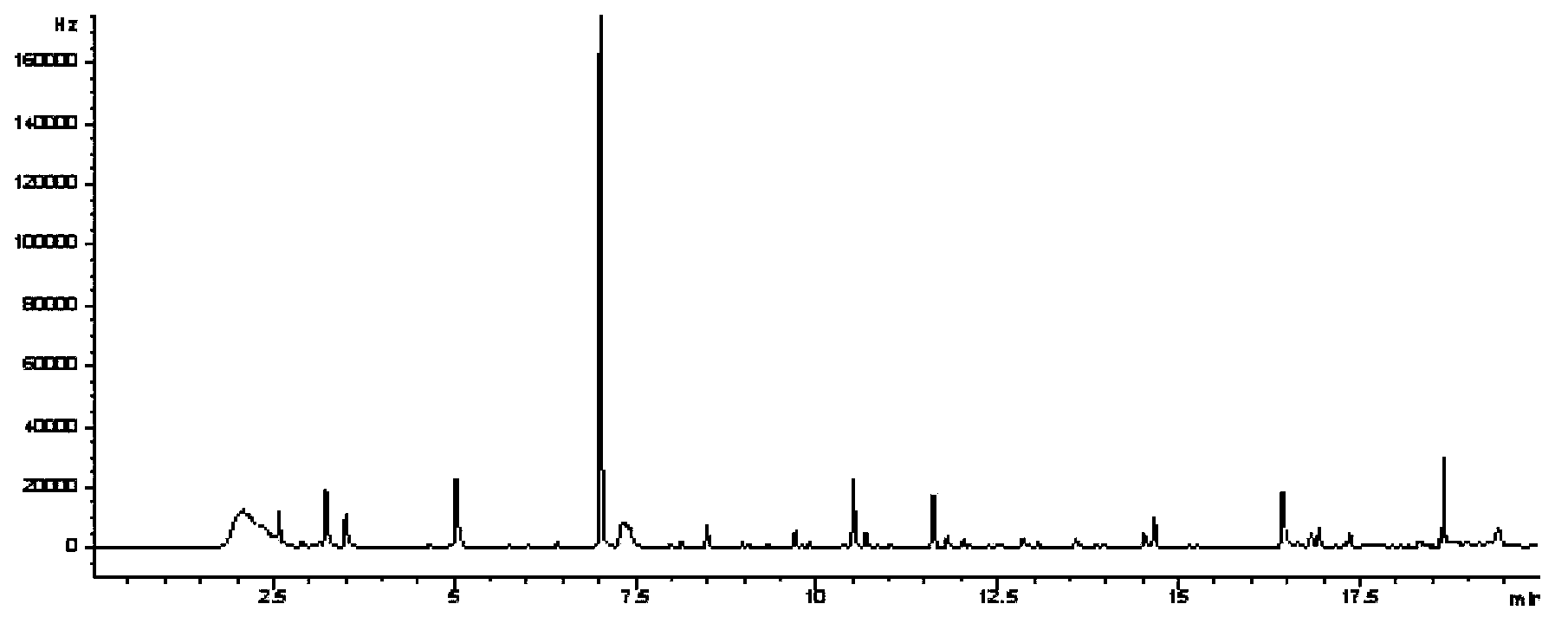
InactiveCN103424484AOvercome the defect that it is not easy to gasifyHigh detection sensitivityComponent separationGas phaseRoom temperature
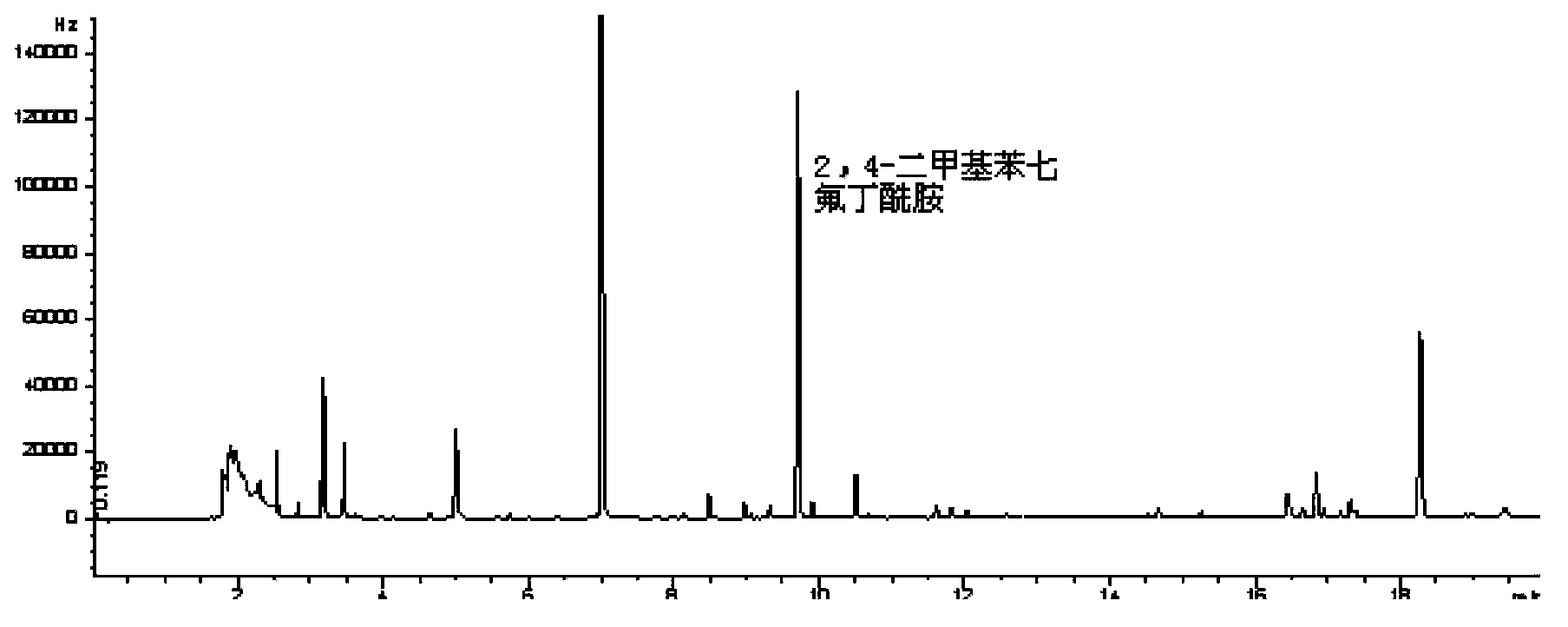
The invention belongs to a method for analyzing an amitraz residual marker, and particularly relates to an analytic method for derivatization reaction of an amitraz residual marker (heptafluorobutyric anhydride) and gas chromatography detection and simultaneous detection. The amitraz residual marker and heptafluorobutyric anhydride can react to obtain a fluorine-containing product which is easier to gasify and has a stronger detection signal. The reaction conditions are optimized, under the alkaline condition, in a thermostatic drying cabinet at the temperature of 65-75 DEG C, an amitraz compound is hydrolyzed for 40-60 min; in a thermostatic drying cabinet at the temperature of 55-75 DEG C, the amitraz hydrolysis product and heptafluorobutyric anhydride react for 80-100 min, after the cooling to the room temperature, saturated NaHCO3 is added for neutralization, and the gas chromatography analysis is directly carried out after the drying. The minimum detectable amount is 5 [mu]g / L, the reproducibility of results is good, three concentrations are obtained, the 5-time independently repeated coefficients of variation are less than 15%, and all indexes can meet the analysis requirement for drug residual quantity.
Owner:HUAZHONG AGRI UNIV
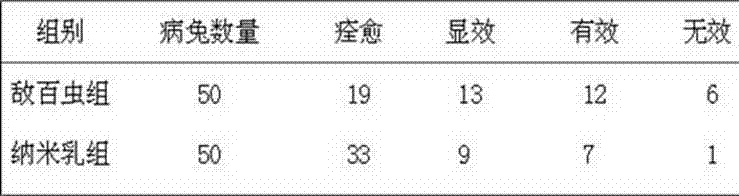
Chinese-western compound spray for treating canine demodicosis infection and preparation method of Chinese-western compound spray for treating canine demodicosis infection
InactiveCN105833141AAvoid secondary infectionAvoid drug resistanceOrganic active ingredientsAerosol deliveryPESTICIDE ADJUVANTSCurative effect
The invention discloses Chinese-western compound spray for treating canine demodicosis infection. The Chinese-western compound spray comprises, by weight, 10-30 parts of alcohol, 0.0125-0.025 part of amitraz, 2-5 parts of pesticide adjuvant 885, 65-80 parts of traditional Chinese medicine composition extract and 2-10 parts of eugeno, wherein the traditional Chinese medicine composition extract is obtained by extracting, by weight, 5-20 parts of Radix Stemonae, 3-10 parts of Herba Schizonepetae, 5-10 parts of Fructus Xanthii, 5-10 parts of Fructus Quisqualis, 2-6 parts of sulphur and 5-10 parts of Fructus Meliae Azedarach. The Chinese-western compound spray for treating canine demodicosis infection is simple in preparation method, convenient to use, remarkably effective and high in safety. The invention further provides a preparation method of the Chinese-western compound spray for treating canine demodicosis infection.
Owner:WUHAN CHOPPER LVYA BIOLOGICAL SCI & TECH CO LTD
Veterinary amitraz solution synergist and preparation method thereof
PendingCN112336706AImprove insecticidal effectReduce dosageAntibacterial agentsOrganic active ingredientsCurative effectVeterinary Drugs
The invention discloses a veterinary amitraz solution synergist and a preparation method thereof, and belongs to the technical field of novel veterinary medicine preparations. The raw material of thesynergist comprises the following components in percentage by weight: 0.01-1% of a bactericide or a bacteriostatic agent, 0.01-3% of an anti-inflammatory agent, 1-10% of a functionalizing agent and 1-10% of a penetration enhancer. The veterinary amitraz solution synergist provided by the invention can be used as a diluent of an amitraz solution, can effectively improve the efficacy of amitraz during treatment of animal ectoparasites, is higher in action speed and wider in action range, and can ensure the same or better curative effect while reducing the use amount of amitraz.
Owner:JIANGSU NANJING AGRI UNIV ANIMAL PHARM CO LTD
Compound clobetasol propionate nano-medicament and preparation method thereof
InactiveCN102327273ASystem stabilityOrganic active ingredientsAntimycoticsPropanoic acidEczematous rash
Owner:NORTHWEST A & F UNIV
Method for simultaneously determining residual quantities of amitraz and metabolites in royal jelly through dispersive solid phase extraction-liquid chromatography-mass spectrometry/mass spectrometry method
InactiveCN109001362AQuality assuranceConsume less resourcesComponent separationEthylenediamineMetabolite
The invention relates to a method for determining residual quantities of amitraz and three metabolites thereof in royal jelly, in particular to a method for simultaneously determining the residual quantities of the amitraz and the metabolites in royal jelly through a dispersive solid phase extraction-liquid chromatography-mass spectrometry / mass spectrometry method. The method includes the steps that after a buffer solution with pH being 9 is added into a sample to conduct diluting, and protein precipitation, extraction, and salt precipitation are carried out through acetonitrile and sodium chloride; dispersive solid phase extraction is conducted through an adsorbent containing N-propyl ethylenediamine (PSA), anhydrous magnesium sulfate and C18 powder to purify an extraction solution, detection is carried out through a liquid chromatography-mass spectrometry / mass spectrometer, and quantifying is carried out through an isotope internal standard dilution method. The method is environmentally friendly, easy, convenient and quick, small in resource consumption, and low in detection cost, and the sensitivity of the method can meet the requirements of detection and confirmation of the residual quantities of the amitraz and the metabolites thereof in the royal jelly.
Owner:ZHEJIANG ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
Preparation method and application of amitraz molecular imprinting monolithic column
ActiveCN104198630AEasy to manufactureFast preparationComponent separationFunctional monomerCross linker
The invention discloses a preparation method and application of an amitraz molecular imprinting monolithic column. The preparation method comprises the following steps: by taking amitraz as a template molecule, dissolving the template molecule into a mixed solution of acetonitrile and dodecyl alcohol, adding a functional monomer, pre-polymerizing at a low temperature, further adding a cross-linking agent and an initiator, introducing nitrogen, filling into a chromatographic column hollow tube of which the lower end is already sealed, sealing the upper end of the chromatographic column hollow tube, and initializing polymerization, thereby obtaining the molecular imprinting monolithic column. After the functional monomer, a pore-foaming agent and the template molecule which are not polymerized are washed off from the column by using a mixed solution of methanol and acetic acid, the molecular imprinting monolithic column which has a good adsorption effect and an enrichment effect on amitraz is obtained. Compared with a conventional body polymerization method, the preparation method has the characteristics that the preparation method is simple and convenient, the aftertreatment is simple, the recognition property is good, the cost is low, required instruments and reaction conditions are easy to achieve, and the like. The molecular imprinting monolithic column prepared by using the preparation method is capable of separating and detecting residual amitraz in foods.
Owner:HENAN UNIV OF SCI & TECH
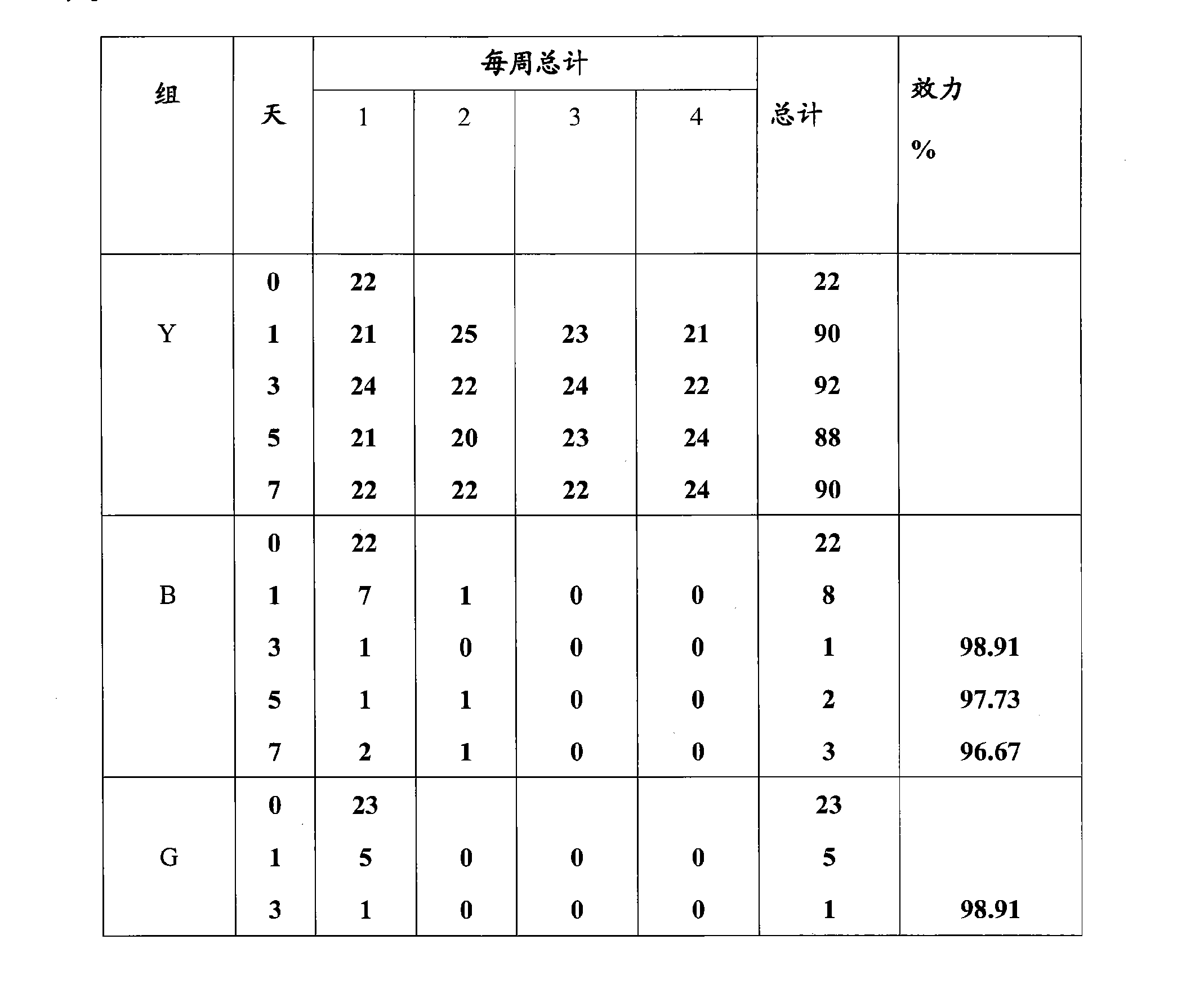
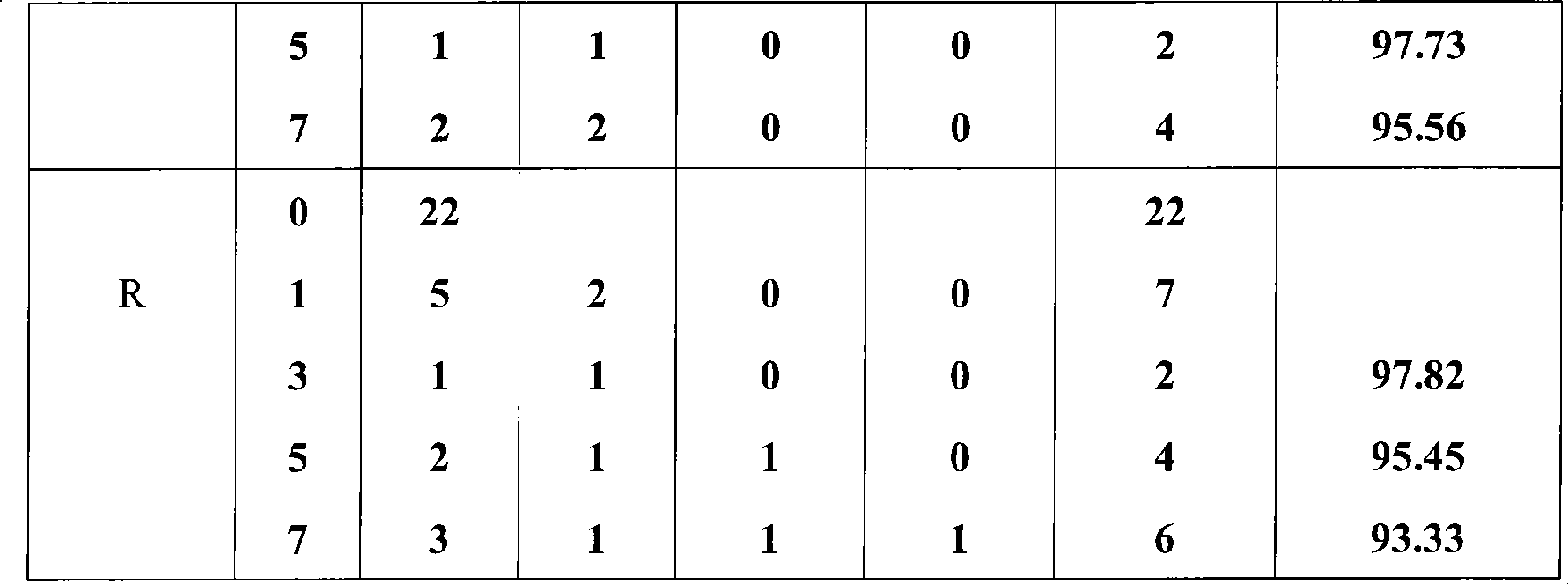
Homoiothermous ectozoon prevention and cure method using amitraz and bifenthrin
The invention relates to a method for controlling parasites (especially ticks and tsetse flies) through amitraz and bifenthrin, which comprises (a) providing a composite of the amitraz and the bifenthrin with cooperative synergic effective quantity and (b) applying the composite to the animals vulnerable to harm by parasites.
Owner:JIANGSU ROTAM CHEM
Water-soluble insecticide with amitraz and method for preparing water-soluble insecticide
The invention discloses a water-soluble insecticide with amitraz and a method for preparing the water-soluble insecticide, and belongs to the technical field of insecticide production. Raw materials for producing the water-soluble insecticide which is a product include, by weight, 10-20% of amitraz, 50-80% of co-solvents, 5-15% of quaternary ammonium salt and 2-8% of synergists. The water-solubleinsecticide and the method are characterized in that the amitraz and the co-solvents are mixed with one another and then are stirred for 20 minutes, then the quaternary ammonium salt and the synergists are added into stirring tanks and are stirred for 20 minutes, and discharge is carried out to obtain the water-soluble insecticide which is the finished product. The water-soluble insecticide and the method have the advantages that the product is non-flammable and non-explosive and is free of environmental pollution; the water-soluble insecticide is low in production cost; obvious synergistic effects can be realized; good insecticidal effects can be realized, and the like.
Owner:江苏康巴特生物工程有限公司
Method for detecting amitraz in food
The invention provides a method for detecting amitraz in food. The method includes the following steps: extracting to obtain extract; hydrolyzing the extract to obtain hydrolysate; deriving to obtain derivative liquid after hydrolysate and derivatizing agent are reacted; purifying derivative liquid to obtain a purification solution; and an instrument determination step of measuring the purification liquid by gas phase-tandem mass spectrometry. In this method, a parent ion and two daughter ions are selected for accurate characterization by GC-MS, which completely avoids the false positives caused by the matrix effect of the sample.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Amitraz-containing high-efficient compounding pesticide composition
InactiveCN106417307AGood control effectAvoid disadvantagesBiocideDead animal preservationPhylloxeraActive component
The invention discloses an amitraz-containing high-efficient compounding pesticide composition, and belongs to the technical field of chemical prevention and treatment of injurious insects. The first active component in the pesticide composition is amitraz and the second active component is cyantraniliprole, wherein the mass ratio of the first active component and the second active component is (1-20): (1-12). The compounding pesticide composition is used for preventing orange phylloxera, the partition ratio of every component is reasonable; the activity and insecticidal efficacy have significant synergism, the drug cost is low; the resistant generation can be released, and the composition is good for crops safety.
Owner:INST OF PLANT PROTECTION JIANGXI ACAD OF AGRI SCI
Fenazaquin-containing pesticide composition
InactiveCN107372522AReduce dosageGood prevention effectBiocideDead animal preservationActive componentBULK ACTIVE INGREDIENT
The invention relates to a fenazaquin-containing pesticide composition and application thereof. The fenazaquin-containing pesticide composition comprises an effective quantity of active components A and active components B, the active components A are fenazaquin, the active components B are any one of semi-amitraz, amitraz or lufenuron, and the weight ratio of the active components A to the active components B is 20:1-1:60. The composition has obvious synergistic effects on pest mites after any one of the fenazaquin, the semi-amitraz, the amitraz or the lufenuron is mixed, the composition is good in fast-acting property and long in lasting period, pesticide resistance of the pest mites can be delayed, and the composition is particularly applicable to control of resistant pest mites.
Owner:江西正邦作物保护股份有限公司
Amitraz compositions
The present invention provides a stable composition which comprises a non-hydroxyl-group-containing solvent mixture comprising N,N-diethyl-m-toluamide and -hexalactone, optionally with dimethyl sulfoxide, eucalyptol and 1-methoxy-2-propyl acetate; and an effective amount of each of amitraz and at least one additional parasiticidal compound, such as R-28153. Said composition allows for high concentrations of a mixture of parasiticidal agents in a single application and is useful for treating and controlling parasiticidal infection and infestation in a homeothermic animal.
Owner:WYETH LLC
Pesticide mixture compound having aphidicidal activity
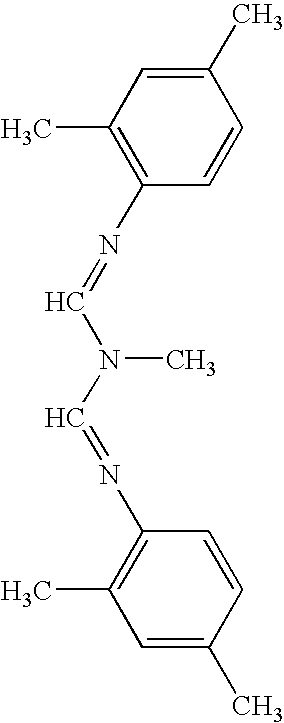
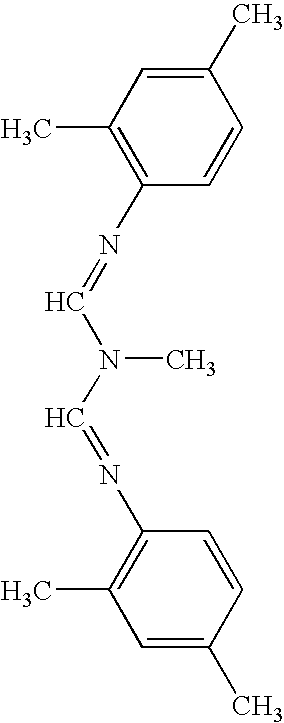
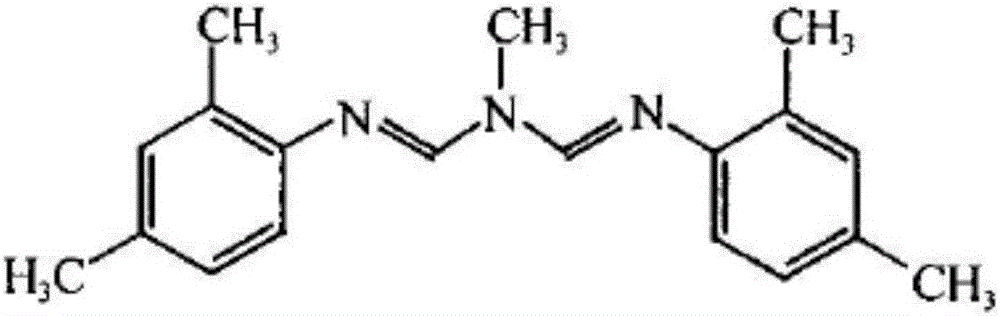
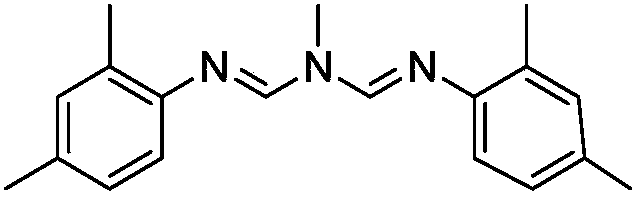
The present invention relates to the active ingredient quinalphos (A: the common name in English is quinalphos, and the chemical name is O, O-diethyl-O-(quinoxalin-2-yl) phosphorothioate) and bis Formamidine (B: English common name is amitraz, chemical name is N′-(2,4-dimethylphenyl)-N-{[(2,4-dimethylphenyl)imino]methyl} -N'-methylmethyleneaminoamine) is a pesticide mixed composition formed with a surfactant and an organic solvent in a reasonable ratio. The pesticide mixed composition is suitable for controlling aphids on vegetables, fruit trees, flowers and other crops, and can also control mites.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com